Applications of Flow Cytometry in Drug Discovery and Translational Research
Abstract
1. Introduction
2. Hit Identification and Lead Optimization
3. Translational Research Informing the Path to the Clinic
4. Quantitative Pharmacokinetic/Pharmacodynamic Evaluation
5. Limitations and Opportunities for Flow Cytometric Techniques to Inform Early Clinical Decision-Making
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKinnon, K.M. Flow Cytometry: An Overview. Curr. Protoc. Immunol. 2018, 120, 5.1.1–5.1.11. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, M.H.; Nolan, G.P. Mass Cytometry: Single Cells, Many Features. Cell 2016, 165, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, D.L.; Reinin, G.; Chua, E. Full Spectrum Flow Cytometry as a Powerful Technology for Cancer Immunotherapy Research. Front. Mol. Biosci. 2021, 7, 612801. [Google Scholar] [CrossRef] [PubMed]
- Rees, P.; Summers, H.D.; Filby, A.; Carpenter, A.E.; Doan, M. Imaging Flow Cytometry. Nat. Rev. Methods Prim. 2022, 2, 86. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Hamers, A.A.J.; Pillai, A.B. CyTOF® for the Masses. Front. Immunol. 2022, 13, 815828. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, P.; Xu, J.; Shao, W.; Yang, C.; Cui, Y. Hydrodynamic Flow Cytometer Performance Enhancement by Two-Dimensional Acoustic Focusing. Biomed. Microdevices 2020, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Tuijnenburg, P.; de Kerk, D.J.A.; Jansen, M.H.; Morris, B.; Lieftink, C.; Beijersbergen, R.L.; van Leeuwen, E.M.; Kuijpers, T.W. High-throughput Compound Screen Reveals MTOR Inhibitors as Potential Therapeutics to Reduce (Auto)Antibody Production by Human Plasma Cells. Eur. J. Immunol. 2020, 50, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Su, Y.; Kang, B.H.; Fan, Z.; Dong, T.; Brown, D.R.; Cheah, J.; Wittrup, K.D.; Chen, J. High-Throughput Phenotypic Screen and Transcriptional Analysis Identify New Compounds and Targets for Macrophage Reprogramming. Nat. Commun. 2021, 12, 773. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Brengdahl, J.; Lindqvist, M.; Gehrmann, U.; Ericson, E.; von Berg, S.; Ripa, L.; Malhotra, R. A Phenotypic Screening Approach Using Human Treg Cells Identified Regulators of Forkhead Box P3 Expression. ACS Chem. Biol. 2019, 14, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Buranda, T.; Gineste, C.; Wu, Y.; Bondu, V.; Perez, D.; Lake, K.R.; Edwards, B.S.; Sklar, L.A. A High-Throughput Flow Cytometry Screen Identifies Molecules That Inhibit Hantavirus Cell Entry. SLAS Discov. 2018, 23, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Schardt, J.S.; Pornnoppadol, G.; Desai, A.A.; Park, K.S.; Zupancic, J.M.; Makowski, E.K.; Smith, M.D.; Chen, H.; Barbosa, M.G.d.M.; Cascalho, M.; et al. Discovery and Characterization of High-Affinity, Potent SARS-CoV-2 Neutralizing Antibodies via Single B Cell Screening. Sci. Rep. 2021, 11, 20738. [Google Scholar] [CrossRef] [PubMed]
- Joslin, J.; Gilligan, J.; Anderson, P.; Garcia, C.; Sharif, O.; Hampton, J.; Cohen, S.; King, M.; Zhou, B.; Jiang, S.; et al. A Fully Automated High-Throughput Flow Cytometry Screening System Enabling Phenotypic Drug Discovery. SLAS Discov. 2018, 23, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Goenaga, A.-L.; Harms, B.D.; Zou, H.; Lou, J.; Conrad, F.; Adams, G.P.; Schoeberl, B.; Nielsen, U.B.; Marks, J.D. Impact of Intrinsic Affinity on Functional Binding and Biological Activity of EGFR Antibodies. Mol. Cancer Ther. 2012, 11, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Floc’h, A.L.; Allinne, J.; Nagashima, K.; Scott, G.; Birchard, D.; Asrat, S.; Bai, Y.; Lim, W.K.; Martin, J.; Huang, T.; et al. Dual Blockade of IL-4 and IL-13 with Dupilumab, an IL-4Rα Antibody, Is Required to Broadly Inhibit Type 2 Inflammation. Allergy 2020, 75, 1188–1204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, K.; Georgiev, P.; Wells, S.; Xu, H.; Lacey, B.M.; Xu, Z.; Laskey, J.; Mcleod, R.; Methot, J.L.; et al. Pharmacological Inhibition of Hematopoietic Progenitor Kinase 1 Positively Regulates T-Cell Function. PLoS ONE 2020, 15, e0243145. [Google Scholar] [CrossRef] [PubMed]
- Linnane, E.; Davey, P.; Zhang, P.; Puri, S.; Edbrooke, M.; Chiarparin, E.; Revenko, A.S.; Macleod, A.R.; Norman, J.C.; Ross, S.J. Differential Uptake, Kinetics and Mechanisms of Intracellular Trafficking of next-Generation Antisense Oligonucleotides across Human Cancer Cell Lines. Nucleic Acids Res. 2019, 47, 4375–4392. [Google Scholar] [CrossRef] [PubMed]
- Revenko, A.; Carnevalli, L.S.; Sinclair, C.; Johnson, B.; Peter, A.; Taylor, M.; Hettrick, L.; Chapman, M.; Klein, S.; Solanki, A.; et al. Direct Targeting of FOXP3 in Tregs with AZD8701, a Novel Antisense Oligonucleotide to Relieve Immunosuppression in Cancer. J. Immunother. Cancer 2022, 10, e003892. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.Y.; Milton, M.N. The Determination and Interpretation of the Therapeutic Index in Drug Development. Nat. Rev. Drug Discov. 2012, 11, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Guo, C.; Li, Y.; Liu, K.; Zhao, Q.; Ouyang, L. Identification of Claudin-6 as a Molecular Biomarker in Pan-Cancer Through Multiple Omics Integrative Analysis. Front. Cell Dev. Biol. 2021, 9, 726656. [Google Scholar] [CrossRef]
- Screnci, B.; Stafford, L.J.; Barnes, T.; Shema, K.; Gilman, S.; Wright, R.; Absi, S.A.; Phillips, T.; Azuelos, C.; Slovik, K.; et al. Antibody Specificity against Highly Conserved Membrane Protein Claudin 6 Driven by Single Atomic Contact Point. iScience 2022, 25, 105665. [Google Scholar] [CrossRef]
- McDermott, M.S.J.; O’Brien, N.A.; Hoffstrom, B.; Gong, K.; Lu, M.; Zhang, J.; Luo, T.; Liang, M.; Jia, W.; Hong, J.; et al. Preclinical Efficacy of the Antibody-Drug-Conjugate CLDN6-23-ADC for the Treatment of CLDN6 Positive Solid Tumors. Clin. Cancer Res. 2023, 29, 2131–2143. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. Properties of FDA-Approved Small Molecule Protein Kinase Inhibitors: A 2023 Update. Pharmacol. Res. 2023, 187, 106552. [Google Scholar] [CrossRef] [PubMed]
- Bendels, S.; Bissantz, C.; Fasching, B.; Gerebtzoff, G.; Guba, W.; Kansy, M.; Migeon, J.; Mohr, S.; Peters, J.-U.; Tillier, F.; et al. Safety Screening in Early Drug Discovery: An Optimized Assay Panel. J. Pharmacol. Toxicol. Methods 2019, 99, 106609. [Google Scholar] [CrossRef] [PubMed]
- Bowes, J.; Brown, A.J.; Hamon, J.; Jarolimek, W.; Sridhar, A.; Waldron, G.; Whitebread, S. Reducing Safety-Related Drug Attrition: The Use of in Vitro Pharmacological Profiling. Nat. Rev. Drug Discov. 2012, 11, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Rhedin, M.; Hendrickx, R.; Berglund, S.; Piras, A.; Blomgran, P.; Cavallin, A.; Collins, M.; Dahl, G.; Dekkak, B.; et al. Characterization of Selective and Potent JAK1 Inhibitors Intended for the Inhaled Treatment of Asthma. Drug Des. Dev. Ther. 2022, 16, 2901–2917. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.; Sun, T.; Durand, S.; Hall, A.; Houston, N.R.; Nett, J.H.; Sharkey, B.; Bobrowicz, B.; Caffry, I.; Yu, Y.; et al. Biophysical Properties of the Clinical-Stage Antibody Landscape. Proc. Natl. Acad. Sci. USA 2017, 114, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Makowski, E.K.; Wu, L.; Desai, A.A.; Tessier, P.M. Highly Sensitive Detection of Antibody Nonspecific Interactions Using Flow Cytometry. mAbs 2021, 13, 1951426. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, M.E.; Patfield, S.; Hughes, A.C.; Hernlem, B.; He, X. A Novel Shiga Toxin 2a Neutralizing Antibody Therapeutic with Low Immunogenicity and High Efficacy. Antimicrob. Agents Chemother. 2024, 68, e0059823. [Google Scholar] [CrossRef] [PubMed]
- Roopenian, D.C.; Akilesh, S. FcRn: The Neonatal Fc Receptor Comes of Age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.; Reynolds, H.M.; Lumicisi, B.; Bryson, C.J. Immunogenicity of Protein Therapeutics: The Key Causes, Consequences and Challenges. SelfNonself 2010, 1, 314–322. [Google Scholar] [CrossRef]
- Ko, S.; Park, S.; Sohn, M.H.; Jo, M.; Ko, B.J.; Na, J.-H.; Yoo, H.; Jeong, A.L.; Ha, K.; Woo, J.R.; et al. An Fc Variant with Two Mutations Confers Prolonged Serum Half-Life and Enhanced Effector Functions on IgG Antibodies. Exp. Mol. Med. 2022, 54, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- Mandrup, O.A.; Ong, S.C.; Lykkemark, S.; Dinesen, A.; Rudnik-Jansen, I.; Dagnæs-Hansen, N.F.; Andersen, J.T.; Alvarez-Vallina, L.; Howard, K.A. Programmable Half-Life and Anti-Tumour Effects of Bispecific T-Cell Engager-Albumin Fusions with Tuned FcRn Affinity. Commun. Biol. 2021, 4, 310. [Google Scholar] [CrossRef]
- Nath, N.; Godat, B.; Zimprich, C.; Dwight, S.J.; Corona, C.; McDougall, M.; Urh, M. Homogeneous Plate Based Antibody Internalization Assay Using PH Sensor Fluorescent Dye. J. Immunol. Methods 2016, 431, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, N.; Luo, L.; Zhang, L.; Chen, J.; DiFilippo, F.P.; Androjna, C.; Fox, D.A.; Ondrejka, S.L.; Hsi, E.D.; Jagadeesh, D.; et al. CD6-Targeted Antibody-Drug Conjugate as a New Therapeutic Agent for T Cell Lymphoma. Leukemia 2023, 37, 2050–2057. [Google Scholar] [CrossRef]
- Yang, M.-C.; Shia, C.-S.; Li, W.-F.; Wang, C.-C.; Chen, I.-J.; Huang, T.-Y.; Chen, Y.-J.; Chang, H.-W.; Lu, C.-H.; Wu, Y.-C.; et al. Preclinical Studies of OBI-999: A Novel Globo H–Targeting Antibody–Drug Conjugate. Mol. Cancer Ther. 2021, 20, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Phanse, Y.; Ramer-Tait, A.E.; Friend, S.L.; Carrillo-Conde, B.; Lueth, P.; Oster, C.J.; Phillips, G.J.; Narasimhan, B.; Wannemuehler, M.J.; Bellaire, B.H. Analyzing Cellular Internalization of Nanoparticles and Bacteria by Multi-Spectral Imaging Flow Cytometry. J. Vis. Exp. 2012, 64, e3884. [Google Scholar] [CrossRef]
- Sharma, S.; Li, Z.; Bussing, D.; Shah, D.K. Evaluation of Quantitative Relationship Between Target Expression and ADC Exposure Inside Cancer Cells. Drug Metab. Dispos. 2020, 48, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody Drug Conjugate: The “Biological Missile” for Targeted Cancer Therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef]
- Kopp, A.; Hofsess, S.; Cardillo, T.M.; Govindan, S.V.; Donnell, J.; Thurber, G.M. Antibody–Drug Conjugate Sacituzumab Govitecan Drives Efficient Tissue Penetration and Rapid Intracellular Drug Release. Mol. Cancer Ther. 2022, 22, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Sharma, S.; Shah, D.K. Quantitative Characterization of in Vitro Bystander Effect of Antibody-Drug Conjugates. J. Pharmacokinet. Pharmacodyn. 2016, 43, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Perry, S.R.; Muniz-Medina, V.; Wang, X.; Wetzel, L.K.; Rebelatto, M.C.; Hinrichs, M.J.M.; Bezabeh, B.Z.; Fleming, R.L.; Dimasi, N.; et al. A Biparatopic HER2-Targeting Antibody-Drug Conjugate Induces Tumor Regression in Primary Models Refractory to or Ineligible for HER2-Targeted Therapy. Cancer Cell 2016, 29, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Maio, M.; Grob, J.-J.; Aamdal, S.; Bondarenko, I.; Robert, C.; Thomas, L.; Garbe, C.; Chiarion-Sileni, V.; Testori, A.; Chen, T.-T.; et al. Five-Year Survival Rates for Treatment-Naive Patients with Advanced Melanoma Who Received Ipilimumab Plus Dacarbazine in a Phase III Trial. J. Clin. Oncol. 2015, 33, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Mosely, S.I.S.; Prime, J.E.; Sainson, R.C.A.; Koopmann, J.-O.; Wang, D.Y.Q.; Greenawalt, D.M.; Ahdesmaki, M.J.; Leyland, R.; Mullins, S.; Pacelli, L.; et al. Rational Selection of Syngeneic Preclinical Tumor Models for Immunotherapeutic Drug Discovery. Cancer Immunol. Res. 2017, 5, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.J.; Elder, M.J.; Yang, C.; Sitnikova, S.I.; Irving, L.; Hansen, A.; Hair, J.; Jones, D.C.; Hasani, S.; Wang, B.; et al. Design and Efficacy of a Monovalent Bispecific PD-1/CTLA4 Antibody That Enhances CTLA4 Blockade on PD-1+ Activated T Cells. Cancer Discov. 2021, 11, 1100–1117. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.A.; Hughes, A.M.; Walton, J.; Coenen-Stass, A.M.L.; Magiera, L.; Mooney, L.; Bell, S.; Staniszewska, A.D.; Sandin, L.C.; Barry, S.T.; et al. Longitudinal Immune Characterization of Syngeneic Tumor Models to Enable Model Selection for Immune Oncology Drug Discovery. J. Immunother. Cancer 2019, 7, 328. [Google Scholar] [CrossRef]
- Leyland, R.; Watkins, A.; Mulgrew, K.A.; Holoweckyj, N.; Bamber, L.; Tigue, N.J.; Offer, E.; Andrews, J.; Yan, L.; Mullins, S.; et al. A Novel Murine GITR Ligand Fusion Protein Induces Antitumor Activity as a Monotherapy That Is Further Enhanced in Combination with an OX40 Agonist. Clin. Cancer Res. 2017, 23, 3416–3427. [Google Scholar] [CrossRef] [PubMed]
- Borodovsky, A.; Barbon, C.M.; Wang, Y.; Ye, M.; Prickett, L.; Chandra, D.; Shaw, J.; Deng, N.; Sachsenmeier, K.; Clarke, J.D.; et al. Small Molecule AZD4635 Inhibitor of A2AR Signaling Rescues Immune Cell Function Including CD103+ Dendritic Cells Enhancing Anti-Tumor Immunity. J. Immunother. Cancer 2020, 8, e000417. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.D.; Pollizzi, K.N.; Heikamp, E.B.; Horton, M.R. Regulation of Immune Responses by MTOR. Annu. Rev. Immunol. 2012, 30, 39–68. [Google Scholar] [CrossRef] [PubMed]
- Langdon, S.; Hughes, A.; Taylor, M.A.; Kuczynski, E.A.; Mele, D.A.; Delpuech, O.; Jarvis, L.; Staniszewska, A.; Cosulich, S.; Carnevalli, L.S.; et al. Combination of Dual MTORC1/2 Inhibition and Immune-Checkpoint Blockade Potentiates Anti-Tumour Immunity. OncoImmunology 2018, 7, e1458810. [Google Scholar] [CrossRef] [PubMed]
- Carnevalli, L.S.; Sinclair, C.; Taylor, M.A.; Gutierrez, P.M.; Langdon, S.; Coenen-Stass, A.M.L.; Mooney, L.; Hughes, A.; Jarvis, L.; Staniszewska, A.; et al. PI3Kα/δ Inhibition Promotes Anti-Tumor Immunity through Direct Enhancement of Effector CD8+ T-Cell Activity. J. Immunother. Cancer 2018, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Sceneay, J.; Sinclair, C. The Future of Immune Checkpoint Combinations with Tumor-Targeted Small Molecule Drugs. Emerg. Top. Life Sci. 2021, 5, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Wichroski, M.; Benci, J.; Liu, S.-Q.; Chupak, L.; Fang, J.; Cao, C.; Wang, C.; Onorato, J.; Qiu, H.; Shan, Y.; et al. DGKα/ζ Inhibitors Combine with PD-1 Checkpoint Therapy to Promote T Cell–Mediated Antitumor Immunity. Sci. Transl. Med. 2023, 15, eadh1892. [Google Scholar] [CrossRef] [PubMed]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Campesato, L.F.; Budhu, S.; Tchaicha, J.; Weng, C.-H.; Gigoux, M.; Cohen, I.J.; Redmond, D.; Mangarin, L.; Pourpe, S.; Liu, C.; et al. Blockade of the AHR Restricts a Treg-Macrophage Suppressive Axis Induced by L-Kynurenine. Nat. Commun. 2020, 11, 4011. [Google Scholar] [CrossRef] [PubMed]
- McGovern, K.; Castro, A.C.; Cavanaugh, J.; Coma, S.; Walsh, M.; Tchaicha, J.; Syed, S.; Natarajan, P.; Manfredi, M.; Zhang, X.M.; et al. Discovery and Characterization of a Novel Aryl Hydrocarbon Receptor Inhibitor, IK-175, and Its Inhibitory Activity on Tumor Immune Suppression. Mol. Cancer Ther. 2022, 21, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Kuczynski, E.A.; Carnevalli, L.; Sinclair, C. Longitudinal Tracking of T Cell Lymphomas in Mice Using Flow Cytometry. STAR Protoc. 2023, 4, 102144. [Google Scholar] [CrossRef] [PubMed]
- Kuczynski, E.A.; Morlino, G.; Peter, A.; Coenen-Stass, A.M.L.; Moss, J.I.; Wali, N.; Delpuech, O.; Reddy, A.; Solanki, A.; Sinclair, C.; et al. A Preclinical Model of Peripheral T-cell Lymphoma GATA3 Reveals DNA Damage Response Pathway Vulnerability. EMBO Mol. Med. 2022, 14, e15816. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, X.; Lavezzi, S.M.; Arshad, U.; Sharma, R. Concept and Application of the Probability of Pharmacological Success (PoPS) as a Decision Tool in Drug Development: A Position Paper. J. Transl. Med. 2023, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, C.K.; Ebrahimi-Nik, H.; Iracheta-Vellve, A.; Hamel, K.M.; Olander, K.E.; Davis, T.G.R.; McGuire, K.A.; Halvorsen, G.T.; Avila, O.I.; Patel, C.H.; et al. The PTPN2/PTPN1 Inhibitor ABBV-CLS-484 Unleashes Potent Anti-Tumour Immunity. Nature 2023, 622, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Casey, K.A.; Guo, X.; Smith, M.A.; Wang, S.; Sinibaldi, D.; Sanjuan, M.A.; Wang, L.; Illei, G.G.; White, W.I. Type I Interferon Receptor Blockade with Anifrolumab Corrects Innate and Adaptive Immune Perturbations of SLE. Lupus Sci. Med. 2018, 5, e000286. [Google Scholar] [CrossRef] [PubMed]
- Kasturi, S.P.; Kozlowski, P.A.; Nakaya, H.I.; Burger, M.C.; Russo, P.; Pham, M.; Kovalenkov, Y.; Silveira, E.L.V.; Havenar-Daughton, C.; Burton, S.L.; et al. Adjuvanting a Simian Immunodeficiency Virus Vaccine with Toll-Like Receptor Ligands Encapsulated in Nanoparticles Induces Persistent Antibody Responses and Enhanced Protection in TRIM5α Restrictive Macaques. J. Virol. 2017, 91, e01844-16. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, P.S.; Scott, M.K.D.; Hagan, T.; Li, C.; Feng, Y.; Wimmers, F.; Grigoryan, L.; Trisal, M.; Edara, V.V.; Lai, L.; et al. Systems Vaccinology of the BNT162b2 MRNA Vaccine in Humans. Nature 2021, 596, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.C.D. Vaccine Applications of Flow Cytometry. Methods 2012, 57, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Singh, S.; Kumari, M.; Gupta, H.; Chauhan, D.; Singh, K.; Eslavath, M.R.; Bhushan, B.; Dogra, V.; Bargotya, M.; et al. Flow Cytometry Profiling of Cellular Immune Response in COVID-19 Infected, Recovered and Vaccinated Individuals. Immunobiology 2023, 228, 152392. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B. Learning Immunology from the Yellow Fever Vaccine: Innate Immunity to Systems Vaccinology. Nat. Rev. Immunol. 2009, 9, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Van de Sande, B.; Lee, J.S.; Mutasa-Gottgens, E.; Naughton, B.; Bacon, W.; Manning, J.; Wang, Y.; Pollard, J.; Mendez, M.; Hill, J.; et al. Applications of Single-Cell RNA Sequencing in Drug Discovery and Development. Nat. Rev. Drug Discov. 2023, 22, 496–520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.; Skrzypczynska, K.M.; Fang, Q.; Zhang, W.; O’Brien, S.A.; He, Y.; Wang, L.; Zhang, Q.; Kim, A.; et al. Single-Cell Analyses Inform Mechanisms of Myeloid-Targeted Therapies in Colon Cancer. Cell 2020, 181, 442–459.e29. [Google Scholar] [CrossRef] [PubMed]
- Martini, E.; Kunderfranco, P.; Peano, C.; Carullo, P.; Cremonesi, M.; Schorn, T.; Carriero, R.; Termanini, A.; Colombo, F.S.; Jachetti, E.; et al. Single-Cell Sequencing of Mouse Heart Immune Infiltrate in Pressure Overload–Driven Heart Failure Reveals Extent of Immune Activation. Circulation 2019, 140, 2089–2107. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Netto, K.G.; Zhou, L.; Liu, X.; Wang, M.; Zhang, G.; Foster, P.S.; Li, F.; Yang, M. Single-Cell Transcriptomic Analysis Reveals the Immune Landscape of Lung in Steroid-Resistant Asthma Exacerbation. Proc. Natl. Acad. Sci. USA 2021, 118, e2005590118. [Google Scholar] [CrossRef] [PubMed]
- Carraro, G.; Langerman, J.; Sabri, S.; Lorenzana, Z.; Purkayastha, A.; Zhang, G.; Konda, B.; Aros, C.J.; Calvert, B.A.; Szymaniak, A.; et al. Transcriptional Analysis of Cystic Fibrosis Airways at Single-Cell Resolution Reveals Altered Epithelial Cell States and Composition. Nat. Med. 2021, 27, 806–814. [Google Scholar] [CrossRef]
- Skånland, S.S. Phospho Flow Cytometry with Fluorescent Cell Barcoding for Single Cell Signaling Analysis and Biomarker Discovery. J. Vis. Exp. 2018, 140, e58386. [Google Scholar] [CrossRef]
- Sinclair, C.; Bains, I.; Yates, A.J.; Seddon, B. Asymmetric Thymocyte Death Underlies the CD4:CD8 T-Cell Ratio in the Adaptive Immune System. Proc. Natl. Acad. Sci. USA 2013, 110, E2905–E2914. [Google Scholar] [CrossRef] [PubMed]
- Hogan, T.; Shuvaev, A.; Commenges, D.; Yates, A.; Callard, R.; Thiebaut, R.; Seddon, B. Clonally Diverse T Cell Homeostasis Is Maintained by a Common Program of Cell-Cycle Control. J. Immunol. 2013, 190, 3985–3993. [Google Scholar] [CrossRef] [PubMed]
- Hogan, T.; Nowicka, M.; Cownden, D.; Pearson, C.F.; Yates, A.J.; Seddon, B. Differential Impact of Self and Environmental Antigens on the Ontogeny and Maintenance of CD4+ T Cell Memory. eLife 2019, 8, e48901. [Google Scholar] [CrossRef] [PubMed]
- Verheijen, M.; Rane, S.; Pearson, C.; Yates, A.J.; Seddon, B. Fate Mapping Quantifies the Dynamics of B Cell Development and Activation throughout Life. Cell Rep. 2020, 33, 108376. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 Programs the Development and Function of CD4+ CD25+ Regulatory T Cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. FOXP3+ Treg as a Therapeutic Target for Promoting Anti-Tumor Immunity. Expert Opin. Ther. Targets 2018, 22, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Wildin, R.S.; Ramsdell, F.; Peake, J.; Faravelli, F.; Casanova, J.-L.; Buist, N.; Levy-Lahad, E.; Mazzella, M.; Goulet, O.; Perroni, L.; et al. X-Linked Neonatal Diabetes Mellitus, Enteropathy and Endocrinopathy Syndrome Is the Human Equivalent of Mouse Scurfy. Nat. Genet. 2001, 27, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.L.; Ochs, H.D. IPEX Is a Unique X-Linked Syndrome Characterized by Immune Dysfunction, Polyendocrinopathy, Enteropathy, and a Variety of Autoimmune Phenomena. Curr. Opin. Pediatr. 2001, 13, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Sarnaik, A.A.; Hamid, O.; Khushalani, N.I.; Lewis, K.D.; Medina, T.; Kluger, H.M.; Thomas, S.S.; Domingo-Musibay, E.; Pavlick, A.C.; Whitman, E.D.; et al. Lifileucel, a Tumor-Infiltrating Lymphocyte Therapy, in Metastatic Melanoma. J. Clin. Oncol. 2021, 39, 2656–2666. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.T.; Maude, S.L.; Porter, D.L.; Frey, N.; Wood, P.; Han, X.; Waldron, E.; Chakraborty, A.; Awasthi, R.; Levine, B.L.; et al. Cellular Kinetics of CTL019 in Relapsed/Refractory B-Cell Acute Lymphoblastic Leukemia and Chronic Lymphocytic Leukemia. Blood 2017, 130, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.T.; Waldron, E.; Grupp, S.A.; Levine, J.E.; Laetsch, T.W.; Pulsipher, M.A.; Boyer, M.W.; August, K.J.; Hamilton, J.; Awasthi, R.; et al. Clinical Pharmacology of Tisagenlecleucel in B-Cell Acute Lymphoblastic Leukemia. Clin. Cancer Res. 2018, 24, 6175–6184. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S. CAR-T Efficacy: Is Conditioning the Key? Blood 2019, 133, 1799–1800. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, J.; Hirayama, A.V.; Purushe, J.; Hay, K.A.; Lymp, J.; Li, D.H.; Yeung, C.C.S.; Sheih, A.; Pender, B.S.; Hawkins, R.M.; et al. Feasibility and Efficacy of CD19-Targeted CAR T Cells with Concurrent Ibrutinib for CLL after Ibrutinib Failure. Blood 2020, 135, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Fraietta, J.A.; Lacey, S.F.; Orlando, E.J.; Pruteanu-Malinici, I.; Gohil, M.; Lundh, S.; Boesteanu, A.C.; Wang, Y.; O’Connor, R.S.; Hwang, W.-T.; et al. Determinants of Response and Resistance to CD19 Chimeric Antigen Receptor (CAR) T Cell Therapy of Chronic Lymphocytic Leukemia. Nat. Med. 2018, 24, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Kvistborg, P.; Shu, C.J.; Heemskerk, B.; Fankhauser, M.; Thrue, C.A.; Toebes, M.; van Rooij, N.; Linnemann, C.; van Buuren, M.M.; Urbanus, J.H.; et al. TIL Therapy Broadens the Tumor-Reactive CD8+ T Cell Compartment in Melanoma Patients. Oncoimmunology 2012, 1, 409–418. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, J.H.; Heemskerk, B.; van Rooij, N.; Gomez-Eerland, R.; Michels, S.; van Zon, M.; de Boer, R.; Bakker, N.A.M.; Jorritsma-Smit, A.; van Buuren, M.M.; et al. Tumor Infiltrating Lymphocytes (TIL) Therapy in Metastatic Melanoma: Boosting of Neoantigen-Specific T Cell Reactivity and Long-Term Follow-Up. J. Immunother. Cancer 2020, 8, e000848. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, L.; Masoumi, E.; Fallah-Mehrjardi, K.; Mirzaei, H.R.; Hadjati, J. Prolonged Persistence of Chimeric Antigen Receptor (CAR) T Cell in Adoptive Cancer Immunotherapy: Challenges and Ways Forward. Front. Immunol. 2020, 11, 702. [Google Scholar] [CrossRef] [PubMed]
- Guzman, G.; Reed, M.R.; Bielamowicz, K.; Koss, B.; Rodriguez, A. CAR-T Therapies in Solid Tumors: Opportunities and Challenges. Curr. Oncol. Rep. 2023, 25, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Bakker, D.S.; van der Wal, M.M.; Heeb, L.E.; Giovannone, B.; Asamoah, M.; Delemarre, E.M.; Drylewicz, J.; Nierkens, S.; Boyman, O.; de Bruin-Weller, M.S.; et al. Early and Long-Term Effects of Dupilumab Treatment on Circulating T-Cell Functions in Patients with Moderate-to-Severe Atopic Dermatitis. J. Investig. Dermatol. 2021, 141, 1943–1953.e13. [Google Scholar] [CrossRef] [PubMed]
- Diks, A.M.; Khatri, I.; Oosten, L.E.; de Mooij, B.; Groenland, R.J.; Teodosio, C.; Perez-Andres, M.; Orfao, A.; Berbers, G.A.M.; Zwaginga, J.J.; et al. Highly Sensitive Flow Cytometry Allows Monitoring of Changes in Circulating Immune Cells in Blood After Tdap Booster Vaccination. Front. Immunol. 2021, 12, 666953. [Google Scholar] [CrossRef]
- Stern, L.; McGuire, H.; Avdic, S.; Rizzetto, S.; Groth, B.F.d.S.; Luciani, F.; Slobedman, B.; Blyth, E. Mass Cytometry for the Assessment of Immune Reconstitution After Hematopoietic Stem Cell Transplantation. Front. Immunol. 2018, 9, 1672. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.; Brown, D.G.; Lennard, S.; Anderton, M.J.; Barrett, J.C.; Eriksson, U.; Fidock, M.; Hamrén, B.; Johnson, A.; March, R.E.; et al. Impact of a Five-Dimensional Framework on R&D Productivity at AstraZeneca. Nat. Rev. Drug Discov. 2018, 17, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.H.; Deng, Q.; Ting, N. Proof of Concept: Drug Selection? Or Dose Selection? Thoughts on Multiplicity Issues. Ther. Innov. Regul. Sci. 2021, 55, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.; Graaf, P.H.V.D.; Arrowsmith, J.; Feltner, D.E.; Drummond, K.S.; Wegner, C.D.; Street, S.D.A. Can the Flow of Medicines Be Improved? Fundamental Pharmacokinetic and Pharmacological Principles toward Improving Phase II Survival. Drug Discov. Today 2012, 17, 419–424. [Google Scholar] [CrossRef]
- Wong, C.H.; Siah, K.W.; Lo, A.W. Estimation of Clinical Trial Success Rates and Related Parameters. Biostatistics 2019, 20, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Schilling, H.-L.; Glehr, G.; Kapinsky, M.; Ahrens, N.; Riquelme, P.; Cordero, L.; Bitterer, F.; Schlitt, H.J.; Geissler, E.K.; Haferkamp, S.; et al. Development of a Flow Cytometry Assay to Predict Immune Checkpoint Blockade-Related Complications. Front. Immunol. 2021, 12, 765644. [Google Scholar] [CrossRef] [PubMed]
- Gadalla, R.; Noamani, B.; MacLeod, B.L.; Dickson, R.J.; Guo, M.; Xu, W.; Lukhele, S.; Elsaesser, H.J.; Razak, A.R.A.; Hirano, N.; et al. Validation of CyTOF Against Flow Cytometry for Immunological Studies and Monitoring of Human Cancer Clinical Trials. Front. Oncol. 2019, 9, 415. [Google Scholar] [CrossRef] [PubMed]
- Fricker, M.; Qin, L.; Niessen, N.; Baines, K.J.; McDonald, V.M.; Scott, H.A.; Simpson, J.L.; Gibson, P.G. Relationship of Sputum Mast Cells with Clinical and Inflammatory Characteristics of Asthma. Clin. Exp. Allergy 2020, 50, 696–707. [Google Scholar] [CrossRef]
- Gonzalez-Vivo, M.; Tiirikainen, M.K.L.; Andreu, M.; Fernandez-Clotet, A.; López-García, A.; Gonzalo, F.M.; Rodriguez, L.A.; de Jesús-Gil, C.; Ruiz-Romeu, E.; Nicolàs, L.S.-D.S.; et al. Memory T Cell Subpopulations as Early Predictors of Remission to Vedolizumab in Ulcerative Colitis. Front. Med. 2022, 9, 837294. [Google Scholar] [CrossRef]
- Liang, M.; Schwickart, M.; Schneider, A.K.; Vainshtein, I.; Nagro, C.D.; Standifer, N.; Roskos, L.K. Receptor Occupancy Assessment by Flow Cytometry as a Pharmacodynamic Biomarker in Biopharmaceutical Development. Cytom. Part B Clin. Cytom. 2016, 90, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Lagoo, A.S. How to Design and Validate a Clinical Flow Cytometry Assay. Clin. Lab. Med. 2023, 43, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Devitt, K.A.; Oldaker, T.; Shah, K.; Illingworth, A. Summary of Validation Considerations with Real-life Examples Using Both Qualitative and Semiquantitative Flow Cytometry Assays. Cytom. Part B Clin. Cytom. 2023, 104, 374–391. [Google Scholar] [CrossRef]
- Audia, A.; Bannish, G.; Bunting, R.; Riveley, C. Flow Cytometry and Receptor Occupancy in Immune-Oncology. Expert Opin. Biol. Ther. 2022, 22, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Cabanski, M.; Oldaker, T.; Stewart, J.J.; Selliah, N.; Eck, S.; Green, C.; Litwin, V.; Vitaliti, A. Flow Cytometric Method Transfer: Recommendations for Best Practice. Cytom. Part B Clin. Cytom. 2021, 100, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Brestoff, J.R.; Frater, J.L. Contemporary Challenges in Clinical Flow Cytometry: Small Samples, Big Data, Little Time. J. Appl. Lab. Med. 2022, 7, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Kalina, T.; Flores-Montero, J.; van der Velden, V.H.J.; Martin-Ayuso, M.; Böttcher, S.; Ritgen, M.; Almeida, J.; Lhermitte, L.; Asnafi, V.; Mendonça, A.; et al. EuroFlow Standardization of Flow Cytometer Instrument Settings and Immunophenotyping Protocols. Leukemia 2012, 26, 1986–2010. [Google Scholar] [CrossRef] [PubMed]
- Krutzik, P.O.; Nolan, G.P. Intracellular Phospho-protein Staining Techniques for Flow Cytometry: Monitoring Single Cell Signaling Events. Cytom. Part A 2003, 55A, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Olson, W.C.; Smolkin, M.E.; Farris, E.M.; Fink, R.J.; Czarkowski, A.R.; Fink, J.H.; Chianese-Bullock, K.A.; Slingluff, C.L. Shipping Blood to a Central Laboratory in Multicenter Clinical Trials: Effect of Ambient Temperature on Specimen Temperature, and Effects of Temperature on Mononuclear Cell Yield, Viability and Immunologic Function. J. Transl. Med. 2011, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Magallon, R.E.; Harmacek, L.D.; Arger, N.K.; Grewal, P.; Powers, L.; Werner, B.R.; Barkes, B.Q.; Li, L.; MacPhail, K.; Gillespie, M.; et al. Standardization of Flow Cytometry and Cell Sorting to Enable a Transcriptomic Analysis in a Multi-Site Sarcoidosis Study. PLoS ONE 2023, 18, e0281210. [Google Scholar] [CrossRef] [PubMed]
- Brestoff, J.R. Full Spectrum Flow Cytometry in the Clinical Laboratory. Int. J. Lab. Hematol. 2023, 45, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Baumgaertner, P.; Sankar, M.; Herrera, F.; Benedetti, F.; Barras, D.; Thierry, A.-C.; Dangaj, D.; Kandalaft, L.E.; Coukos, G.; Xenarios, I.; et al. Unsupervised Analysis of Flow Cytometry Data in a Clinical Setting Captures Cell Diversity and Allows Population Discovery. Front. Immunol. 2021, 12, 633910. [Google Scholar] [CrossRef] [PubMed]
- Kamysheva, A.L.; Fastovets, D.V.; Kruglikov, R.N.; Sokolov, A.A.; Fefler, A.S.; Bolshakova, A.A.; Radko, A.; Krauz, I.E.; Yong, S.T.; Goldberg, M.; et al. Machine Learning (ML)-Enabled Automation for High-Throughput Data Processing in Flow Cytometry. Blood 2023, 142, 905. [Google Scholar] [CrossRef]
- Xu, C.; Yang, J.; Kosters, A.; Babcock, B.R.; Qiu, P.; Ghosn, E.E.B. Comprehensive Multi-Omics Single-Cell Data Integration Reveals Greater Heterogeneity in the Human Immune System. iScience 2022, 25, 105123. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, J.M.; Luke, J.J.; Guglietta, S.; Krieg, C. High Throughput Multi-Omics Approaches for Clinical Trial Evaluation and Drug Discovery. Front. Immunol. 2021, 12, 590742. [Google Scholar] [CrossRef] [PubMed]
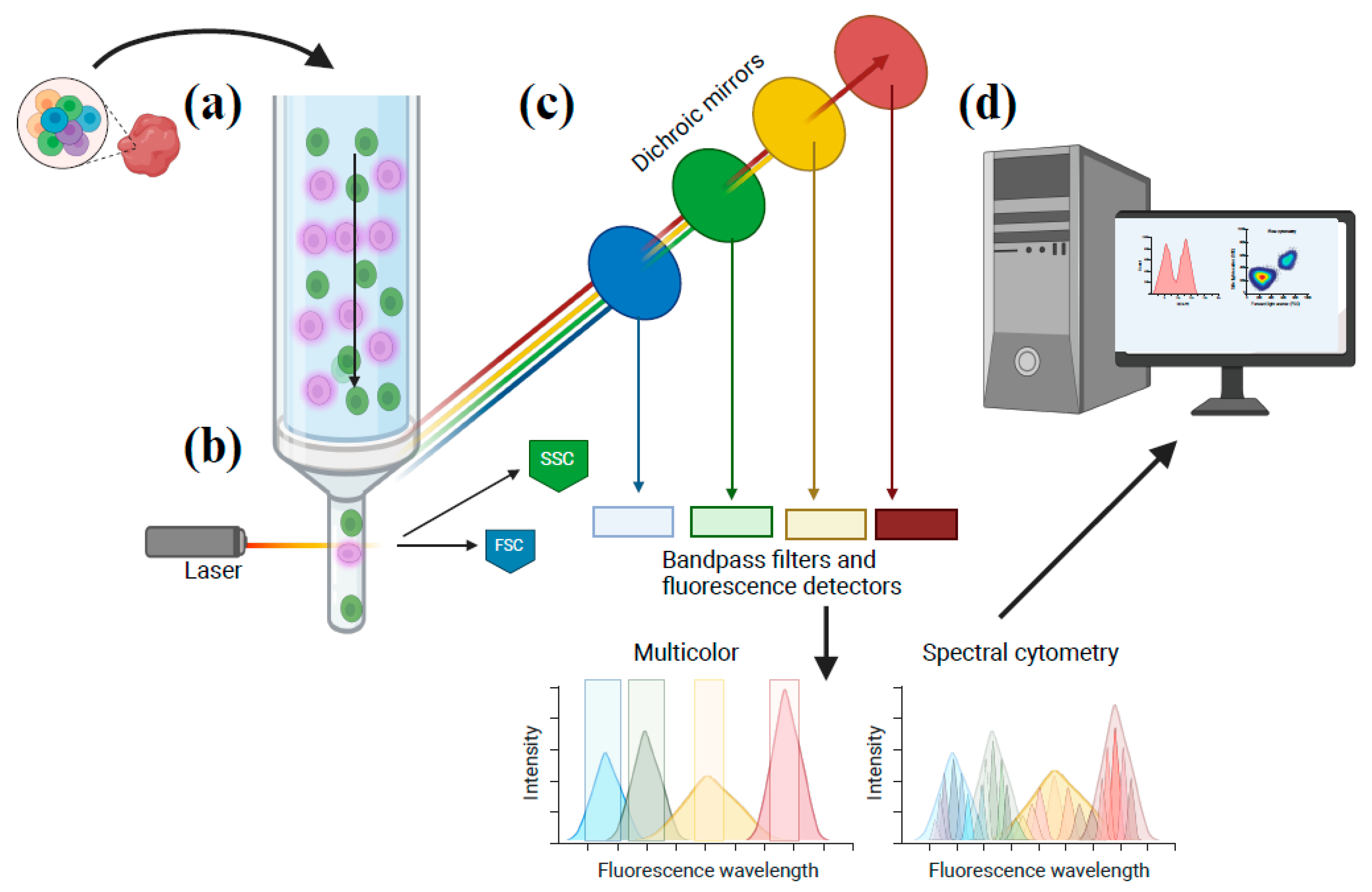
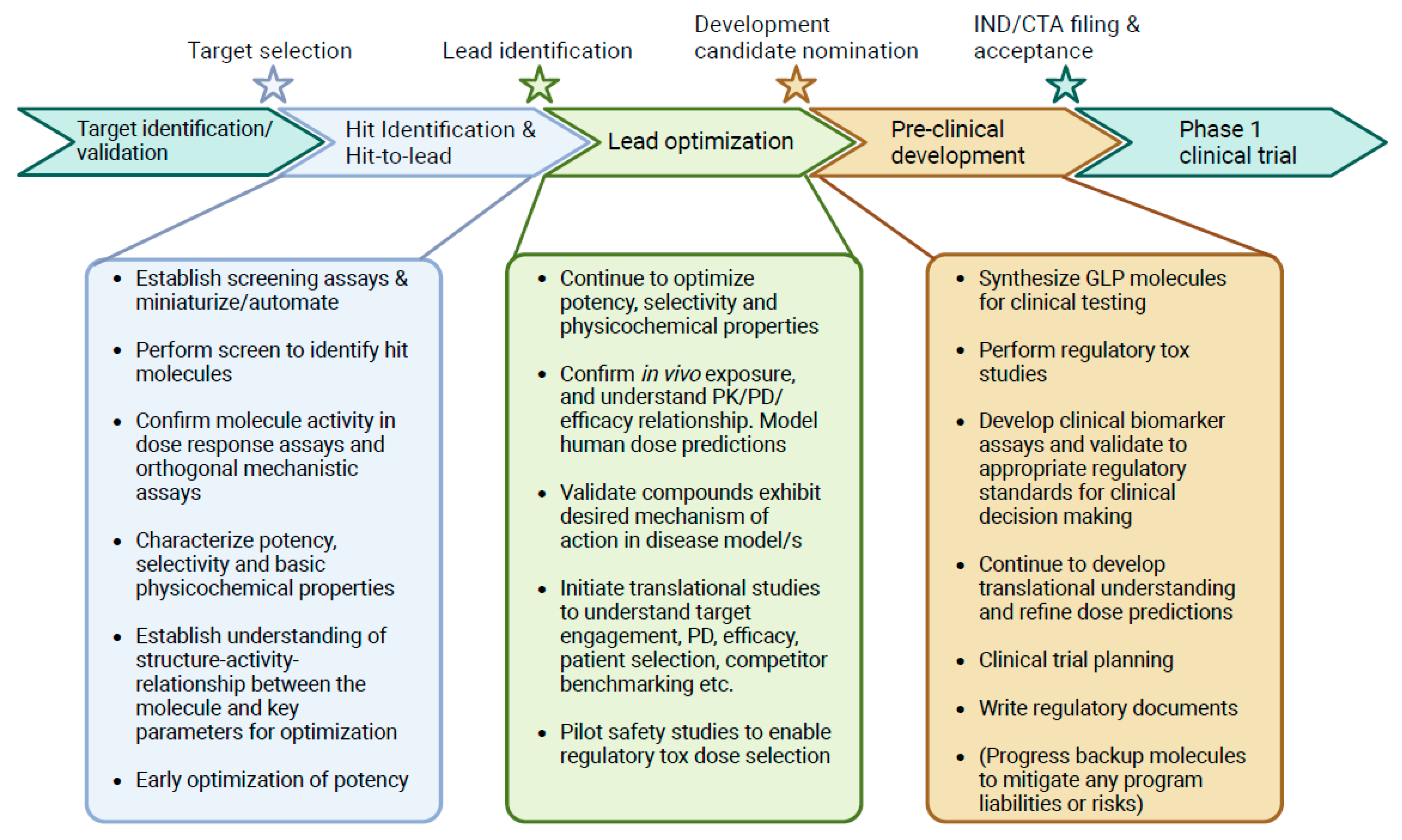
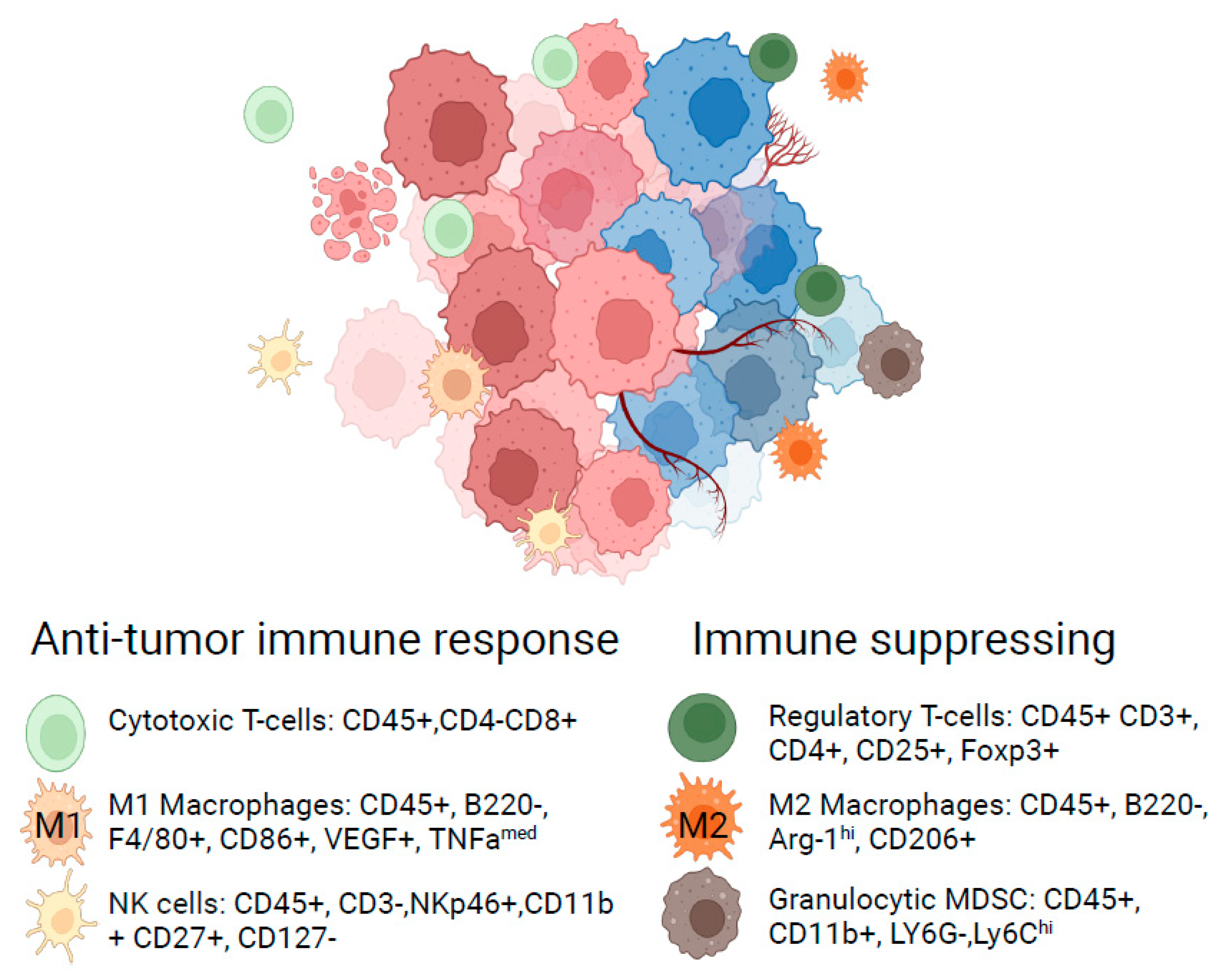
| Therapeutic Property | Examples of Parameters Considered | Objective |
|---|---|---|
| Potency |
| Early screening hits typically lack sufficient potency for clinical activity at a feasible dose and require iterative optimization to improve potency. |
| Safety and selectivity |
| Optimize primary target specificity while minimizing secondary interactions conferring safety risks. |
| Pharmacokinetics and drug exposure |
| Optimization of properties that affect drug exposure in target tissues. These typically include characteristics related to absorption, distribution, metabolism, and excretion (ADME) of a molecule. |
| Therapeutic functionality |
| Properties that affect the molecular mechanism of action. |
| Lead Author | Disease Indication | Target | Modality | Use of Flow Cytometry |
|---|---|---|---|---|
| Tuijnenburg [7] | Auto-immunity | MTOR pathway (via phenotypic screen) | Small molecule | Phenotypic screen for regulators of auto-antibody production |
| Schardt [11] | Virology | SARS-CoV-2 neutralizing antibodies | Antibody | Sorting of memory-specific B cell clones for expansion/antibody production |
| Zhou [13] | Oncology | EGFR | Antibody | Characterization and rank ordering of antibodies based on binding affinity |
| Revenko [17] | Oncology | FOXP3 | ASOs | Potency ranking of ASOs in primary cells |
| McDermott [21] | Oncology | CLDN6 | Antibody/ADC | Characterize selectivity versus related family members |
| Nilsson [25] | Oncology | JAK1 | Small molecule | Characterize selectivity (JAK1 vs. JAK2) |
| Kirkland [28] | Virology | Shiga toxin | Antibody | Evaluate immune cell activation |
| Mandrup [32] | Immuno-Oncology | N/A | Bi-specific antibody | Evaluate the effect of half-life modifications on efficacy |
| Parameswaran [34] | Oncology | CD6 | ADC | Measure internalization efficiency |
| Li [41] | Oncology | HER2 | ADC | ADC payload release versus target and bystander cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullas, S.; Sinclair, C. Applications of Flow Cytometry in Drug Discovery and Translational Research. Int. J. Mol. Sci. 2024, 25, 3851. https://doi.org/10.3390/ijms25073851
Ullas S, Sinclair C. Applications of Flow Cytometry in Drug Discovery and Translational Research. International Journal of Molecular Sciences. 2024; 25(7):3851. https://doi.org/10.3390/ijms25073851
Chicago/Turabian StyleUllas, Sumana, and Charles Sinclair. 2024. "Applications of Flow Cytometry in Drug Discovery and Translational Research" International Journal of Molecular Sciences 25, no. 7: 3851. https://doi.org/10.3390/ijms25073851
APA StyleUllas, S., & Sinclair, C. (2024). Applications of Flow Cytometry in Drug Discovery and Translational Research. International Journal of Molecular Sciences, 25(7), 3851. https://doi.org/10.3390/ijms25073851





