Erythrocyte Glutathione S-Transferase Activity as a Sensitive Marker of Kidney Function Impairment in Children with IgA Vasculitis
Abstract
1. Introduction
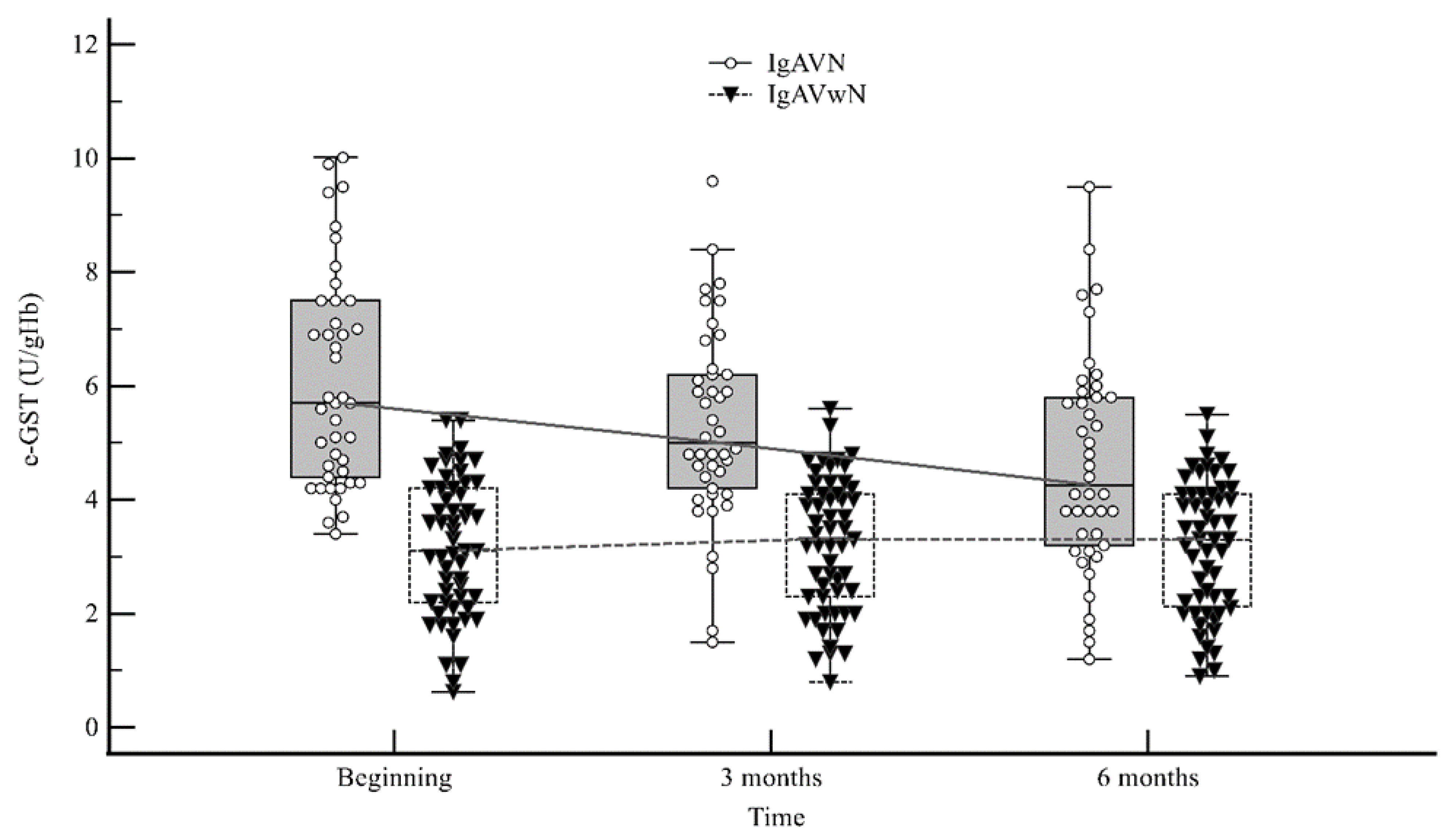
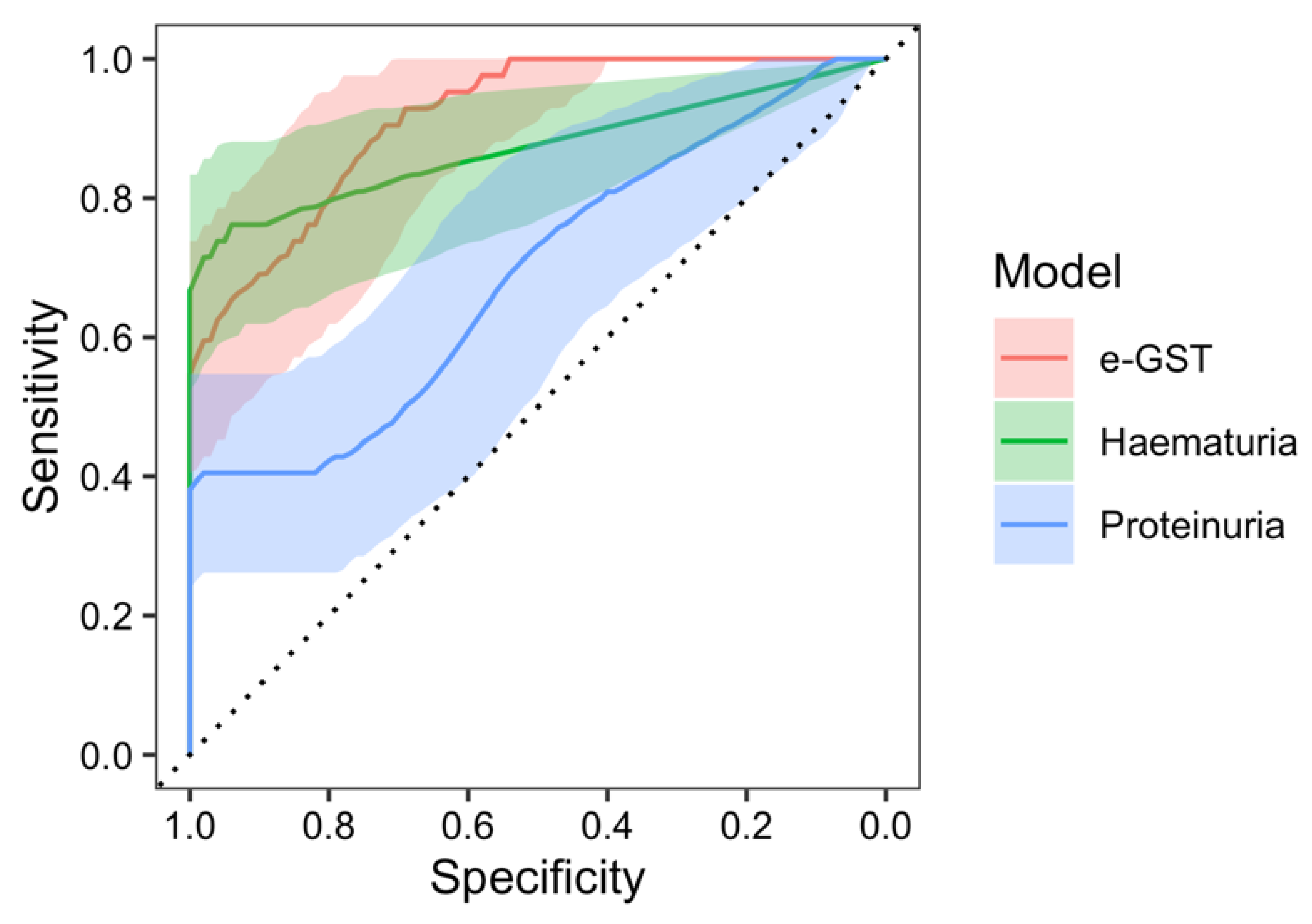
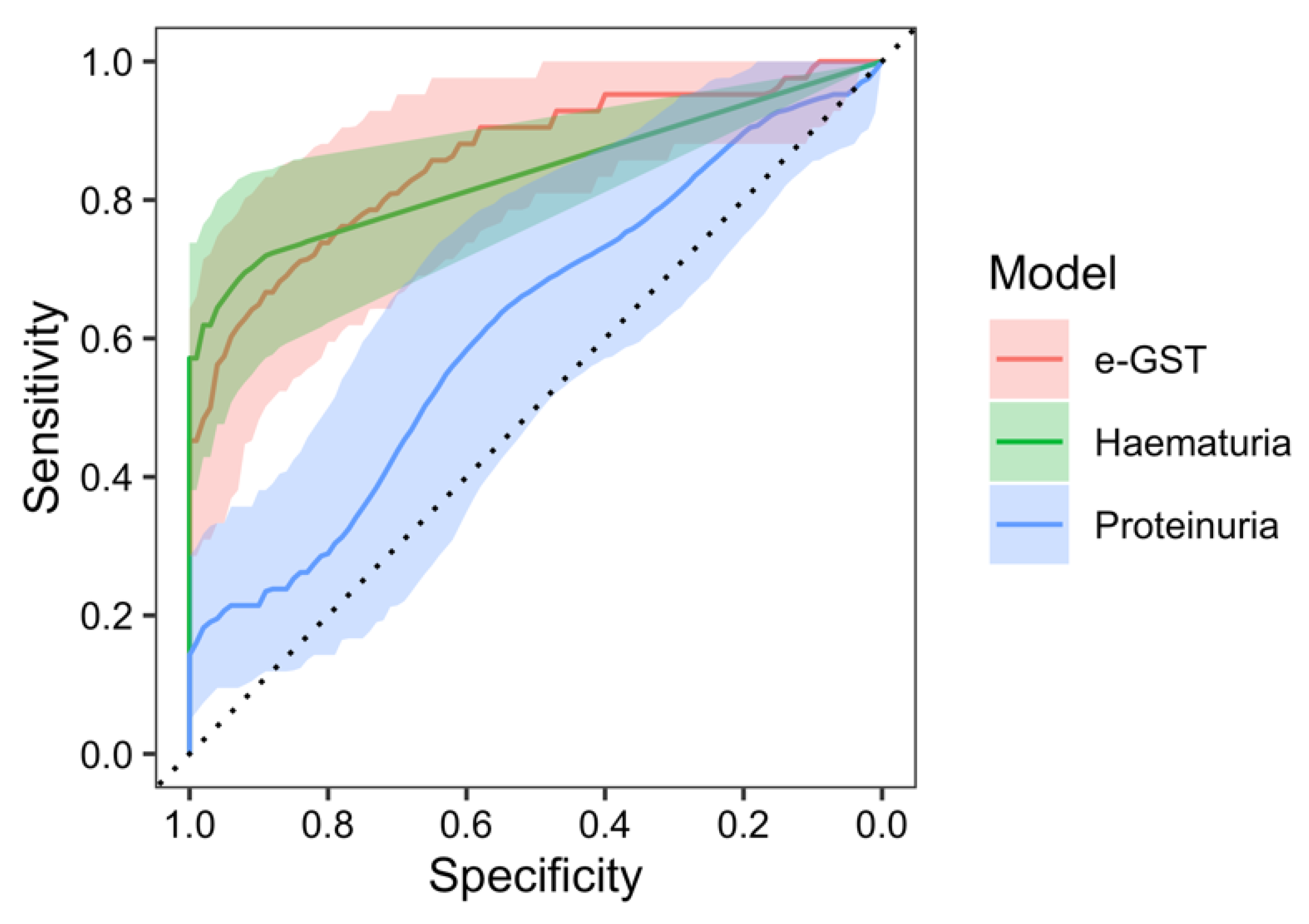
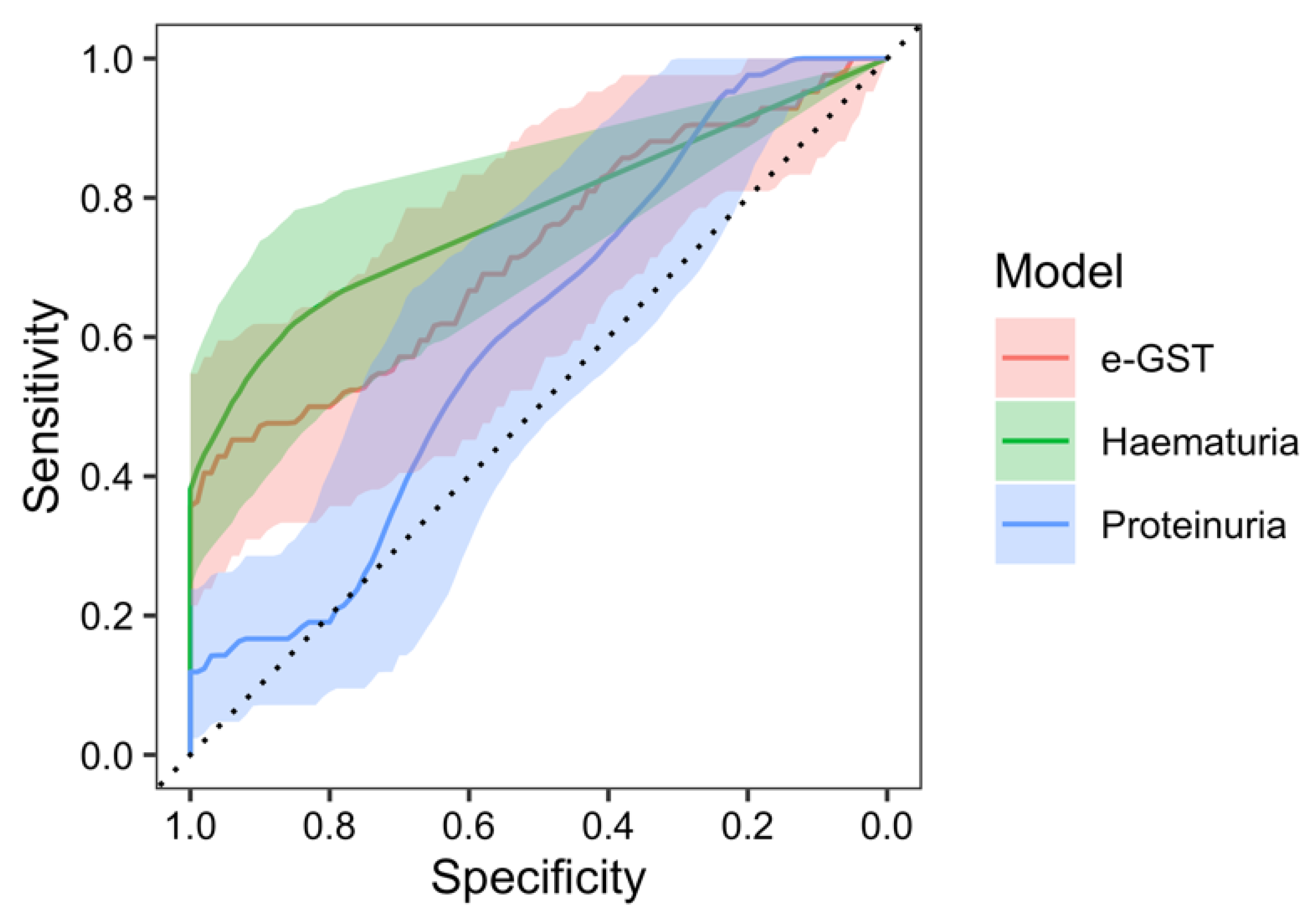
2. Results
3. Discussion
4. Materials and Methods
4.1. Design
4.2. Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heineke, M.H.; Ballering, A.V.; Jamin, A.; Ben Mkaddem, S.; Monteiro, R.C.; Van Egmond, M. New insights in the pathogenesis of immunoglobulin A vasculitis (Henoch-Schönlein purpura). Autoimmun. Rev. 2017, 16, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Ozen, S.; Marks, S.D.; Brogan, P.; Groot, N.; de Graeff, N.; Avcin, T.; Bader-Meunier, B.; Dolezalova, P.; Feldman, B.M.; Kone-Paut, I.; et al. European consensus-based recommendations for diagnosis and treatment of immunoglobulin A vasculitis—The SHARE initiative. Rheumatology 2019, 58, 1607–1616. [Google Scholar] [CrossRef]
- Mizerska-Wasiak, M.; Gajewski, Ł.; Cichoń-Kawa, K.; Małdyk, J.; Dziedzic-Jankowska, K.; Leszczyńska, B.; Rybi-Szumińska, A.; Wasilewska, A.; Pukajło-Marczyk, A.; Zwolińska, D.; et al. Serum GDIgA1 levels in children with IgA nephropathy and Henoch-Schönlein nephritis. Cent. Eur. J. Immunol. 2018, 43, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Sapina, M.; Frkovic, M.; Sestan, M.; Srsen, S.; Ovuka, A.; Batnozic Varga, M.; Kramaric, K.; Brdaric, D.; Milas, K.; Gagro, A.; et al. Geospatial clustering of childhood IgA vasculitis and IgA vasculitis-associated nephritis. Ann. Rheum. Dis. 2021, 80, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Ozen, S.; Pistorio, A.; Iusan, S.M.; Bakkaloglu, A.; Herlin, T.; Brik, R.; Buoncompagni, A.; Lazar, C.; Bilge, I.; Uziel, Y.; et al. Paediatric Rheumatology International Trials Organisation (PRINTO)/EULAR/PRINTO/PRES criteria for Henoch-Schonlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. part II: Final classification criteria. Ann. Rheum. Dis. 2010, 69, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Saulsbury, F.T. Henoch-Schönlein purpura. Curr. Opin. Rheumatol. 2010, 22, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Ghrahani, R.; Ledika, M.A.; Sapartini, G.; Setiabudiawan, B. Age of onset as a risk factor of renal involvement in Henoch-Schönlein purpura. Asia Pac. Allergy 2014, 4, 42–47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Komatsu, H.; Fujimoto, S.; Yoshikawa, N.; Kitamura, H.; Sugiyama, H.; Yokoyama, H. Clinical manifestations of Henoch-Schönlein purpura nephritis and IgA nephropathy: Comparative analysis of data from the Japan Renal Biopsy Registry (J-RBR). Clin. Exp. Nephrol. 2016, 20, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Jelusic, M.; Sestan, M.; Cimaz, R.; Ozen, S. Different histological classifications for Henoch-Schönlein purpura nephritis: Which one should be used? Pediatr. Rheumatol. 2019, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Pillebout, E.; Jamin, A.; Ayari, H.; Housset, P.; Pierre, M.; Sauvaget, V.; Viglietti, D.; Deschenes, G.; Monteiro, R.C.; Berthelot, L.; et al. Biomarkers of IgA vasculitis nephritis in children. PLoS ONE 2017, 12, e0188718. [Google Scholar] [CrossRef] [PubMed]
- Davin, J.C.; Coppo, R. Henoch-Schönlein purpura nephritis in children. Nat. Rev. Nephrol. 2014, 10, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Delbet, J.D.; Hogan, J.; Aoun, B.; Stoica, I.; Salomon, R.; Decramer, S.; Brocheriou, I.; Deschênes, G.; Ulinski, T. Clinical outcomes in children with Henoch-Schönlein purpura nephritis without crescents. Pediatr. Nephrol. 2017, 32, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Koskela, M.; Ylinen, E.; Ukonmaanaho, E.M.; Autio-Harmainen, H.; Heikkilä, P.; Lohi, J.; Jauhola, O.; Ronkainen, J.; Jahnukainen, T.; Nuutinen, M. The ISKDC classification and a new semiquantitative classification for predicting outcomes of HenochSchönlein purpura nephritis. Pediatr. Nephrol. 2017, 32, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Pohl, M. Henoch-Schönlein purpura nephritis. Pediatr. Nephrol. 2015, 30, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y. The pathogenesis and treatment of pediatric Henoch-Schönlein purpura nephritis. Clin. Exp. Nephrol. 2011, 15, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Ono, A.; Ohara, S.; Suzuki, Y.; Suyama, K.; Suzuki, J.; Hosoya, M. Henoch-Schönlein purpura nephritis in childhood: Pathogenesis, prognostic factors and treatment. Fukushima J. Med. Sci. 2013, 59, 15–26. [Google Scholar] [CrossRef] [PubMed]
- González-Gay, M.A.; López-Mejías, R.; Pina, T.; Blanco, R.; Castañeda, S. IgA Vasculitis: Genetics and Clinical and Therapeutic Management. Curr. Rheumatol. Rep. 2018, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Matsumoto, K.; Suzuki, T.; Saito, T.; Kanazawa, N.; Tachibana, S.; Iseri, K.; Sugiyama, M.; Iyoda, M.; Shibata, T. Clinical significance of serum and mesangial galactose-deficient IgA1 in patients with IgA nephropathy. PLoS ONE 2018, 13, e0206865. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Chen, Y.; Dai, X.; Di, Y.; Shen, M.; Ying, Q.; Fu, S.; Li, Y. Urinary Macrophage Migration Inhibitory Factor as a Noninvasive Biomarker in Pediatric Henoch-Schönlein Purpura Nephritis. Am. J. Clin. Oncol. 2017, 23, 258–261. [Google Scholar] [CrossRef]
- Du, Y.; Hou, L.; Guo, J.; Sun, T.; Wang, X.; Wu, Y. Renal neutrophil gelatinaseassociated lipocalin and kidney injury molecule-1 expression in children with acute kidney injury and Henoch-Schönlein purpura nephritis. Exp. Ther. Med. 2014, 7, 1130–1134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ge, W.; Wang, H.L.; Sun, R.P. Pentraxin 3 as a novel early biomarker for the prediction of Henoch-Schönlein purpura nephritis in children. Eur. J. Pediatr. 2014, 173, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Delanghe, S.E.; Speeckaert, M.M.; Segers, H.; Desmet, K.; Vande Walle, J.; Laecke, S.V.; Vanholder, R.; Delanghe, J.R. Soluble transferrin receptor in urine, a new biomarker for IgA nephropathy and Henoch-Schönlein purpura nephritis. Clin. Biochem. 2013, 46, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.B.; Yin, S.S. Association of matrix metalloproteinase-9 level with the risk of renal involvement for Henoch-Schönlein purpura in children. Ren. Fail. 2013, 35, 425–429. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mao, Y.N.; Liu, W.; Li, Y.G.; Jia, G.C.; Zhang, Z.; Guan, Y.J.; Zhou, X.F.; Liu, Y.F. Urinary angiotensinogen levels in relation to renal involvement of Henoch-Schonlein purpura in children. Nephrology 2012, 17, 53–57. [Google Scholar] [CrossRef]
- Zhang, L.; Han, C.; Sun, C.; Meng, H.; Ye, F.; Na, S.; Chen, F.; Zhang, D.; Jin, X. Serum levels of alpha-smooth muscle actin and c-Met as biomarkers of the degree of severity of Henoch-Schonlein purpura nephritis. Transl. Res. 2013, 161, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wu, X.; Zheng, W.; Peng, X.; He, X.; Mo, S. Podocalyxin expression in renal tissues and correlation with the number of urinary podocytes in children with Henoch-Schonlein purpura nephritis. J. Cent. South Univ. Med. Sci. 2012, 37, 161–167. [Google Scholar] [CrossRef]
- Fuentes, Y.; Hernández, A.M.; García-Roca, P.; Valverde, S.; Velásquez-Jones, L.F.; Sosa, G.; Duarte-Durán, U.O.; Ortíz, L.; Maldonado, R.; Faugier, E.; et al. Urinary MCP-1/creatinine in Henoch-Schönlein purpura and its relationship with nephritis. Pediatr. Nephrol. 2014, 29, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Jancova, P.; Anzenbacher, P.; Anzenbacherova, E. Phase II drug metabolizing enzymes. Biomed. Pap. 2010, 154, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Fabrini, R.; Dessì, M.; Bocedi, A.; Santini, S.; Rovella, V.; Pastore, A.; Tesauro, M.; Bernardini, S.; Di Daniele, N.; et al. Erythrocyte glutathione transferase activity: A possible early biomarker for blood toxicity in uremic diabetic patients. Acta Diabetol. 2014, 51, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Tesauro, M.; Nisticò, S.; Noce, A.; Tarantino, A.; Marrone, G.; Costa, A.; Rovella, V.; Di Cola, G.; Campia, U.; Lauro, D.; et al. The possible role of glutathione-S-transferase activity in diabetic nephropathy. Int. J. Immunopathol. Pharmacol. 2015, 28, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Ferrannini, M.; Fabrini, R.; Bocedi, A.; Dessì, M.; Galli, F.; Federici, G.; Palumbo, R.; Di Daniele, N.; Ricci, G. Erythrocyte glutathione transferase: A new biomarker for hemodialysis adequacy, overcoming the Kt/V(urea) dogma? Cell Death Dis. 2012, 3, e377. [Google Scholar] [CrossRef][Green Version]
- Bocedi, A.; Noce, A.; Rovella, V.; Marrone, G.; Cattani, G.; Iappelli, M.; De Paolis, P.; Iaria, G.; Sforza, D.; Gallù, M.; et al. Erythrocyte glutathione transferase in kidney transplantation: A probe for kidney detoxification efficiency. Cell Death Dis. 2018, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Dessì, M.; Noce, A.; Dawood, K.F.; Galli, F.; Taccone-Gallucci, M.; Fabrini, R.; Bocedi, A.; Massoud, R.; Fucci, G.; Pastore, A.; et al. Erythrocyte glutathione transferase: A potential new biomarker in chronic kidney diseases which correlates with plasma homocysteine. Amino Acids 2012, 43, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Fabrini, R.; Rosato, E.; Gigante, A.; Bocedi, A.; Cianci, R.; Barbano, B.; Del Grosso, E.; Ricci, F.; Zingaretti, V.; Salsano, F.; et al. Erythrocyte glutathione transferase: A non-antibody biomarker for systemic sclerosis, which correlates with severity and activity of the disease. Cell Death Dis. 2013, 4, e736. [Google Scholar] [CrossRef]
- Feng, D.; Huang, W.Y.; Hao, S.; Niu, X.L.; Wang, P.; Wu, Y.; Zhu, G.H. A single-center analysis of HenochSchonlein purpura nephritis with nephrotic proteinuria in children. Pediatr. Rheumatol. 2017, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Davin, J.C. Henoch-Schonlein purpura nephritis: Pathophysiology, treatment, and future strategy. Clin. J. Am. Soc. Nephrol. 2011, 6, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Huang, L.; Tang, H.; Li, X.; Zhu, X.; Wang, X. Significance of glomerular fibrinogen deposition in children with Henoch-Schönlein purpura nephritis. Ital. J. Pediatr. 2018, 44, 97. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, L.; Jamin, A.; Viglietti, D.; Chemouny, J.M.; Ayari, H.; Pierre, M.; Housset, P.; Sauvaget, V.; Hurtado-Nedelec, M.; Vrtovsnik, F.; et al. Value of biomarkers for predicting immunoglobulin A vasculitis nephritis outcome in an adult prospective cohort. Nephrol. Dial. Transplant. 2018, 33, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zeng, H.; Wang, N.; Tian, X.; Dou, W.; Shi, P. Beneficial effects of creatine phosphate sodium for the treatment of Henoch-Schönlein purpura in patients with early renal damage detected using urinary kidney injury molecule-1 levels. Eur. J. Pediatr. 2016, 175, 49–55. [Google Scholar] [CrossRef]
- Lau, K.K.; Suzuki, H.; Novak, J.; Wyatt, R.J. Pathogenesis of Henoch-Schönlein purpura nephritis. Pediatr. Nephrol. 2010, 25, 19–26. [Google Scholar] [CrossRef]
- Zaffanello, M.; Brugnara, M.; Franchini, M. Therapy for children with henochschonlein purpura nephritis: A systematic review. Sci. World J. 2007, 7, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Trnka, P. Henoch-Schönlein purpura in children. J. Paediatr. Child Health 2013, 49, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Dalpiaz, A.; Schwamb, R.; Miao, Y.; Gonka, J.; Walzter, W.; Khan, S.A. Urological Manifestations of Henoch-Schonlein Purpura: A Review. Curr. Urol. 2015, 8, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Suh, J.S.; Lee, J.H.; Lee, J.W.; Kim, S.H.; Han, K.H.; Shin, J.I. Advances in our understanding of the patogenesis of Henoch-Schönlein purpura and the implications for improving its diagnosis. Expert Rev. Clin. Immunol. 2013, 9, 1223–1238. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.; Tang, Y.L.; Lv, X.H.; Zhang, G.F.; Wang, M.; Yang, H.P.; Li, Q. Risk Factors Associated with Renal Involvement in Childhood Henoch-Schönlein Purpura: A Meta-Analysis. PLoS ONE 2016, 11, e0167346. [Google Scholar] [CrossRef]
- Knoppova, B.; Reily, C.; Maillard, N.; Rizk, D.V.; Moldoveanu, Z.; Mestecky, J.; Raska, M.; Renfrow, M.B.; Julian, B.A.; Novak, J. The Origin and Activities of IgA1-Containing Immune Complexes in IgA Nephropathy. Front. Immunol. 2016, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Anastasov, A.; Tacheva, T.; Dincheva, V.; Dimov, D.; Habib Ts Peeva, P.; Vlaykova, T. Preliminary study of Erythrocyte glutathione-s-transferase activity in patients with skin melanoma. Trakia J. Sci. 2012, 10, 88–94. [Google Scholar]
- Orhan, H.; Sahin, G. Erythrocyte Glutathione-S-Transferase Activity in Diabetics and Its Association with HBA1c. J. Pharm. Sci. 1999, 24, 127–131. [Google Scholar] [CrossRef]
- Fabrini, R.; Bocedi, A.; Del Grosso, E.; Morici, L.; Federici, G.; Palleschi, A.; Ricci, G. Erythrocyte glutathione transferase: A novel biomarker to check environmental pollution hazardous for humans. Biochem. Biophys. Res. Commun. 2012, 426, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Suvakov, S.; Damjanovic, T.; Stefanovic, A.; Pekmezovic, T.; Savic-Radojevic, A.; Pljesa-Ercegovac, M.; Matic, M.; Djukic, T.; Coric, V.; Jakovljevic, J.; et al. Glutathione S-transferase A1, M1, P1 and T1 null or low-activity genotypes are associated with enhanced oxidative damage among haemodialysis patients. Nephrol. Dial. Transplant. 2013, 28, 202–212. [Google Scholar] [CrossRef] [PubMed]

| IgAVwN | IgAVN | Controls | |
|---|---|---|---|
| Age (months): median (IQR) | 72.0 (58.0–107.0) | 91.0 (56.3–138.0) | 115.0 (76.3–138.5) |
| Gender (M:F) | 36:19 | 26:16 | 36:16 |
| Previous infection, n (%) | 38 (69.1) | 26 (61.9) | - |
| Time between infection and appearance of purpura (days): median (IQR) * | 14.00 (10.00–17.25) | 17.00 (11.75–24.25) | - |
| Purpura: n (%) | 55 (100.0) | 42 (100.0) | - |
| Arthritis/arthralgia: n (%) | 44 (80.0) | 27 (64.3) | - |
| Abdominal pain: n (%) | 22 (40.0) | 16 (38.1) | - |
| Nephritis initially: n (%) | 0 (0.0) | 34 (81.0) | - |
| ESR (mm/h): median (IQR) **,*** | 18.00 (12.00–25.00) | 17.00 (10.00–24.00) | 7.00 (5.00–11.00) |
| CRP (g/L): median (IQR) **,*** | 8.00 (2.03–14.93) | 6.05 (1.58–15.98) | 0.30 (0.30–0.30) |
| Hb (g/L): median (IQR) ** | 127.00 (121.00–132.00) | 130.50 (122.00–136.00) | 132.00 (126.25–138.75) |
| L (×109/L): median (IQR) **,*** | 11.30 (8.58–14.81) | 11.67 (8.93–14.18) | 7.40 (5.93–9.13) |
| Plt (×109/L): median (IQR) **,*** | 342.00 (278.00–427.00) | 388.50 (314.50–441.75) | 294.00 (253.00–329.00) |
| BUN (mmol/L): median (IQR) | 3.90 (3.20–4.70) | 4.15(3.48–5.05) | 4.10 (3.30–4.98) |
| Serum creatinine (μmol/L): median (IQR) *,** | 38.00 (30.00–48.00) | 45.00 (38.25–53.00) | 44.00 (39.00–48.00) |
| Feritin (μg/L) | 65.60 (36.13–102.10) | 68.00 (46.50–93.20) | 59.80 (42.78–98.75) |
| Total serum proteins (g/L): median (IQR) ** | 68.00 (64.00–71.00) | 70.50 (65.00–74.00) | 71.00 (69.00–73.00) |
| IgG (g/L): median (IQR) | 10.03 (8.20–11.78) | 10.10 (8.18–12.41) | 10.32 (9.01–12.01) |
| IgM (g/L): median (IQR) *,** | 0.79 (0.63–1.04) | 0.94 (0.76–1.22) | 0.98 (0.66–1.32) |
| IgA (g/L): median (IQR) *,*** | 1.65 (1.25–2.15) | 2.11 (1.64–2.85) | 1.58 (1.12–2.07) |
| E/mm3 of urine: median (IQR) *,*** | 0.00 (0.00–1.00) | 9.50 (3.50–36.25) | 0.00 (0.00–2.00) |
| 24 h urinary protein excretion (g/dU): median (IQR) *,*** | 0.07 (0.05–0.10) | 0.09 (0.07–0.21) | 0.07 (0.04–0.11) |
| eGFR (mL/min/1.73 m2): median (IQR) * | 121.06 (94.26–138.22) | 99.81 (89.65–118.26) | 111.40 (99.00–120.24) |
| Positive fecal occult blood test: n (%) | 7 (12.7) | 9 (21.4) | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frkovic, M.; Turcic, A.; Gagro, A.; Srsen, S.; Frkovic, S.H.; Rogic, D.; Jelusic, M. Erythrocyte Glutathione S-Transferase Activity as a Sensitive Marker of Kidney Function Impairment in Children with IgA Vasculitis. Int. J. Mol. Sci. 2024, 25, 3795. https://doi.org/10.3390/ijms25073795
Frkovic M, Turcic A, Gagro A, Srsen S, Frkovic SH, Rogic D, Jelusic M. Erythrocyte Glutathione S-Transferase Activity as a Sensitive Marker of Kidney Function Impairment in Children with IgA Vasculitis. International Journal of Molecular Sciences. 2024; 25(7):3795. https://doi.org/10.3390/ijms25073795
Chicago/Turabian StyleFrkovic, Marijan, Ana Turcic, Alenka Gagro, Sasa Srsen, Sanda Huljev Frkovic, Dunja Rogic, and Marija Jelusic. 2024. "Erythrocyte Glutathione S-Transferase Activity as a Sensitive Marker of Kidney Function Impairment in Children with IgA Vasculitis" International Journal of Molecular Sciences 25, no. 7: 3795. https://doi.org/10.3390/ijms25073795
APA StyleFrkovic, M., Turcic, A., Gagro, A., Srsen, S., Frkovic, S. H., Rogic, D., & Jelusic, M. (2024). Erythrocyte Glutathione S-Transferase Activity as a Sensitive Marker of Kidney Function Impairment in Children with IgA Vasculitis. International Journal of Molecular Sciences, 25(7), 3795. https://doi.org/10.3390/ijms25073795





