Substance P Concentration in Gestational Diabetes and Excessive Gestational Weight Gain and Its Impact on Neonatal Anthropometry
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aguayo-Guerrero, J.A.; León-Cabrera, S.; Escobedo, G. Molecular Mechanisms Involved in Fetal Programming and Disease Origin in Adulthood. J. Pediatr. Endocrinol. Metab. 2023, 36, 615–627. [Google Scholar] [CrossRef]
- Edwards, M. The Barker Hypothesis. In Handbook of Famine, Starvation, and Nutrient Deprivation: From Biology to Policy; Preedy, V., Patel, V.B., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–21. [Google Scholar]
- Ross, M.G.; Desai, M. Developmental Programming of Offspring Obesity, Adipogenesis, and Appetite. Clin. Obstet. Gynecol. 2013, 56, 529–536. [Google Scholar] [CrossRef]
- Mazur, D.; Satora, M.; Rekowska, A.K.; Kabała, Z.; Łomża, A.; Kimber-Trojnar, Ż.; Leszczyńska-Gorzelak, B. Influence of Breastfeeding on the State of Meta-Inflammation in Obesity—A Narrative Review. Curr. Issues Mol. Biol. 2023, 45, 9003–9018. [Google Scholar] [CrossRef]
- Lindsay, K.L.; Buss, C.; Wadhwa, P.D.; Entringer, S. The Interplay Between Nutrition and Stress in Pregnancy: Implications for Fetal Programming of Brain Development. Biol. Psychiatry 2019, 85, 135–149. [Google Scholar] [CrossRef]
- Faa, G.; Manchia, M.; Pintus, R.; Gerosa, C.; Marcialis, M.A.; Fanos, V. Fetal Programming of Neuropsychiatric Disorders. Birth Defects Res. C Embryo Today 2016, 108, 207–223. [Google Scholar] [CrossRef]
- Jones, R.H.; Ozanne, S.E. Fetal Programming of Glucose-Insulin Metabolism. Mol. Cell. Endocrinol. 2009, 297, 4–9. [Google Scholar] [CrossRef]
- Alexander, B.T.; Dasinger, J.H.; Intapad, S. Fetal Programming and Cardiovascular Pathology. Compr. Physiol. 2015, 5, 997–1025. [Google Scholar]
- Hjort, L.; Martino, D.; Grunnet, L.G.; Naeem, H.; Maksimovic, J.; Olsson, A.H.; Zhang, C.; Ling, C.; Olsen, S.F.; Saffery, R.; et al. Gestational Diabetes and Maternal Obesity Are Associated with Epigenome-Wide Methylation Changes in Children. JCI Insight 2018, 3, e122572. [Google Scholar] [CrossRef]
- Antoniou, M.-C.; Quansah, D.Y.; Mühlberg, S.; Gilbert, L.; Arhab, A.; Schenk, S.; Lacroix, A.; Stuijfzand, B.; Horsch, A.; Puder, J.J. Maternal and Fetal Predictors of Anthropometry in the First Year of Life in Offspring of Women with GDM. Front. Endocrinol. 2023, 14, 1144195. [Google Scholar] [CrossRef]
- Andersson-Hall, U.K.; Järvinen, E.A.J.; Bosaeus, M.H.; Gustavsson, C.E.; Hårsmar, E.J.; Niklasson, C.A.; Albertsson-Wikland, K.G.; Holmäng, A.B. Maternal Obesity and Gestational Diabetes Mellitus Affect Body Composition through Infancy: The PONCH Study. Pediatr. Res. 2019, 85, 369–377. [Google Scholar] [CrossRef]
- Hufnagel, A.; Dearden, L.; Fernandez-Twinn, D.S.; Ozanne, S.E. Programming of Cardiometabolic Health: The Role of Maternal and Fetal Hyperinsulinaemia. J. Endocrinol. 2022, 253, R47–R63. [Google Scholar] [CrossRef]
- Dessì, A.; Tognazzi, C.; Bosco, A.; Pintus, R.; Fanos, V. Metabolomic Profiles and Microbiota of GDM Offspring: The Key for Future Perspective? Front. Pediatr. 2022, 10, 941800. [Google Scholar] [CrossRef]
- Jennewein, M.F.; Abu-Raya, B.; Jiang, Y.; Alter, G.; Marchant, A. Transfer of Maternal Immunity and Programming of the Newborn Immune System. Semin. Immunopathol. 2017, 39, 605–613. [Google Scholar] [CrossRef]
- Robertson, S.A.; Chin, P.-Y.; Femia, J.G.; Brown, H.M. Embryotoxic Cytokines—Potential Roles in Embryo Loss and Fetal Programming. J. Reprod. Immunol. 2018, 125, 80–88. [Google Scholar] [CrossRef]
- Houde, A.-A.; Hivert, M.-F.; Bouchard, L. Fetal Epigenetic Programming of Adipokines. Adipocyte 2013, 2, 41–46. [Google Scholar] [CrossRef]
- Kelly, A.C.; Powell, T.L.; Jansson, T. Placental Function in Maternal Obesity. Clin. Sci. 2020, 134, 961–984. [Google Scholar] [CrossRef]
- Silva, A.F.; Abruzzese, G.A.; Ferrer, M.J.; Heber, M.F.; Ferreira, S.R.; Cerrone, G.E.; Motta, A.B. Fetal Programming by Androgen Excess Impairs Liver Lipid Content and PPARg Expression in Adult Rats. J. Dev. Orig. Health Dis. 2022, 13, 300–309. [Google Scholar] [CrossRef]
- Minatoya, M.; Itoh, S.; Araki, A.; Tamura, N.; Yamazaki, K.; Miyashita, C.; Kishi, R. Association between Fetal Adipokines and Child Behavioral Problems at Preschool Age: The Hokkaido Study on Environment and Children’s Health. Int. J. Environ. Res. Public Health 2018, 15, 120. [Google Scholar] [CrossRef]
- Thompson, L.P.; Al-Hasan, Y. Impact of Oxidative Stress in Fetal Programming. J. Pregnancy 2012, 2012, 582748. [Google Scholar] [CrossRef]
- Dimas, A.; Politi, A.; Bargiota, A.; Panoskaltsis, T.; Vlahos, N.F.; Valsamakis, G. The Gestational Effects of Maternal Bone Marker Molecules on Fetal Growth, Metabolism and Long-Term Metabolic Health: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 8328. [Google Scholar] [CrossRef]
- Silva, L.; Plösch, T.; Toledo, F.; Faas, M.M.; Sobrevia, L. Adenosine Kinase and Cardiovascular Fetal Programming in Gestational Diabetes Mellitus. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165397. [Google Scholar] [CrossRef]
- Kokabi, F.; Ebrahimi, S.; Mirzavi, F.; Ghiasi Nooghabi, N.; Hashemi, S.F.; Hashemy, S.I. The Neuropeptide Substance P/Neurokinin-1 Receptor System and Diabetes: From Mechanism to Therapy. BioFactors 2023, 49, 534–559. [Google Scholar] [CrossRef]
- Muñoz, M.; Carranza, A.; Pavón, A.; Anderson, G.; Coveñas, R. Immunolocalization of Substance P and NK-1 Receptor in Hofbauer Cells in Human Normal Placenta. Microsc. Res. Tech. 2013, 76, 1310–1313. [Google Scholar] [CrossRef]
- Muñoz, M.; Pavón, A.; Rosso, M.; Salinas, M.V.; Pérez, A.; Carranza, A.; González-Ortega, A. Immunolocalization of NK-1 Receptor and Substance P in Human Normal Placenta. Placenta 2010, 31, 649–651. [Google Scholar] [CrossRef]
- Patro-Malysza, J.; Kimber-Trojnar, Z.; Skorzynska-Dziduszko, K.; Marciniak, B.; Darmochwal-Kolarz, D.; Bartosiewicz, J.; Leszczynska-Gorzelak, B.; Oleszczuk, J. The Impact of Substance P on the Pathogenesis of Insulin Resistance Leading to Gestational Diabetes. CPB 2014, 15, 32–37. [Google Scholar] [CrossRef]
- Fu, J.; Liu, B.; Liu, P.; Liu, L.; Li, G.; Wu, B.; Liu, X. Substance P Is Associated with the Development of Obesity, Chronic Inflammation and Type 2 Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2010, 119, 177–181. [Google Scholar] [CrossRef]
- Karagiannides, I.; Bakirtzi, K.; Kokkotou, E.; Stavrakis, D.; Margolis, K.G.; Thomou, T.; Giorgadze, N.; Kirkland, J.L.; Pothoulakis, C. Role of Substance P in the Regulation of Glucose Metabolism via Insulin Signaling-Associated Pathways. Endocrinology 2011, 152, 4571–4580. [Google Scholar] [CrossRef]
- Bright, F.M.; Byard, R.W.; Vink, R.; Paterson, D.S. Normative Distribution of Substance P and Its Tachykinin Neurokinin-1 Receptor in the Medullary Serotonergic Network of the Human Infant during Postnatal Development. Brain Res. Bull. 2018, 137, 319–328. [Google Scholar] [CrossRef]
- Redkiewicz, P. The Regenerative Potential of Substance P. Int. J. Mol. Sci. 2022, 23, 750. [Google Scholar] [CrossRef]
- Rasmussen, K.M.; Yaktine, A.L.; Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines (Eds.) Weight Gain during Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Bautista, N.M. Transgenerational Epigenetic Programming. In Epigenetics, Development, Ecology and Evolution; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Aiken, C.E.; Ozanne, S.E. Transgenerational developmental programming. Hum. Reprod. Update 2014, 20, 63–75. [Google Scholar] [CrossRef]
- Barnes, R.A.; Wong, T.; Ross, G.P.; Griffiths, M.M.; Smart, C.E.; Collins, C.E.; MacDonald-Wicks, L.; Flack, J.R. Excessive Weight Gain before and during Gestational Diabetes Mellitus Management: What Is the Impact? Diabetes Care 2020, 43, 74–81. [Google Scholar] [CrossRef]
- Marciniak, A.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Marciniak, B.; Oleszczuk, J.; Leszczyńska-Gorzelak, B. Fetal programming of the metabolic syndrome. Taiwan J. Obstet. Gynecol. 2017, 56, 133–138. [Google Scholar] [CrossRef]
- Kwon, E.J.; Kim, Y.J. What is fetal programming?: A lifetime health is under the control of in utero health. Obstet. Gynecol. Sci. 2017, 60, 506–519. [Google Scholar] [CrossRef]
- Pilszyk, A.; Niebrzydowska, M.; Pilszyk, Z.; Wierzchowska-Opoka, M.; Kimber-Trojnar, Ż. Incretins as a potential treatment option for gestational diabetes mellitus. Int. J. Mol. Sci. 2022, 23, 10101. [Google Scholar] [CrossRef]
- Hillier, T.A.; Pedula, K.L.; Ogasawara, K.K.; Vesco, K.K.; Oshiro, C.E.S.; Lubarsky, S.L.; Van Marter, J. A Pragmatic, Randomized Clinical Trial of Gestational Diabetes Screening. N. Engl. J. Med. 2021, 384, 895–904. [Google Scholar] [CrossRef]
- Goławski, K.; Giermaziak, W.; Ciebiera, M.; Wojtyła, C. Excessive Gestational Weight Gain and Pregnancy Outcomes. J. Clin. Med. 2023, 12, 3211. [Google Scholar] [CrossRef]
- Cogswell, M.E.; Serdula, M.K.; Hungerford, D.W.; Yip, R. Gestational weight gain among average-weight and overweight women--what is excessive? Am. J. Obstet. Gynecol. 1995, 172 Pt 1, 705–712. [Google Scholar] [CrossRef]
- Dietz, P.M.; Callaghan, W.M.; Sharma, A.J. High pregnancy weight gain and risk of excessive fetal growth. Am. J. Obstet. Gynecol. 2009, 201, e1–e6. [Google Scholar] [CrossRef]
- Clausen, T.; Burski, T.K.; Øyen, N.; Godang, K.; Bollerslev, J.; Henriksen, T. Maternal anthropometric and metabolic factors in the first half of pregnancy and risk of neonatal macrosomia in term pregnancies. A prospective study. Eur. J. Endocrinol. 2005, 153, 887–894. [Google Scholar] [CrossRef]
- Douglas, S.D.; Leeman, S.E. Neurokinin-1 receptor: Functional significance in the immune system in reference to selected infections and inflammation. Ann. N. Y. Acad. Sci. 2011, 1217, 83–95. [Google Scholar] [CrossRef]
- Baek, S.M.; Kim, K.; Kim, S.; Son, Y.; Hong, H.S.; Yu, S.Y. SP prevents T2DM complications by immunomodulation. Sci. Rep. 2020, 7, 16753. [Google Scholar] [CrossRef]
- Mehboob, R.; Oehme, P.; Pfaff, G. The role of Substance P in the defense line of the respiratory tract and neurological manifestations post COVID-19 infection. Front. Neurol. 2023, 14, 1052811. [Google Scholar] [CrossRef]
- Gellis, S.S.; Hsia, D.Y. The infant of diabetic mother. Am. J. Dis Child. 1959, 97, 1–41. [Google Scholar] [CrossRef]
- Robert, M.F.; Neff, R.K.; Hubbell, J.P.; Taeusch, H.W.; Avery, M.E. Association between Maternal Diabetes and the RespiratoryDistress Syndrome in the Newborn. N. Engl. J. Med. 1976, 294, 357–360. [Google Scholar] [CrossRef]
- Atar, H.; Baatz, J.E.; Ryan, R.M. Molecular Mechanisms of Maternal Diabetes Effects on Fetal and Neonatal Surfactant. Children 2021, 8, 281. [Google Scholar] [CrossRef]
- Bryndina, I.G.; Danilov, G.E. Substance P as a factor enhancing resistance of the surfactant lung system to chronic immobilization stress. Ross. Fiziol. Zhurnal Im. IM Sechenova 2002, 88, 84–89. [Google Scholar]
- Rice, W.R.; Singleton, F.M. Regulation of surfactant secretion from isolated Type II pneumocytes by substance P. BiochimBiophys Acta 1986, 889, 123–127. [Google Scholar] [CrossRef]
- De Angelis, F.; Di Tullio, A.; Del Boccio, P.; Reale, S.; Savelli, G.; Spreti, N. ESI-MS in the study of the activity of alpha-chymotrypsin in aqueous surfactant media. Org. Biomol. Chem. 2003, 1, 3125–3130. [Google Scholar] [CrossRef]
- Bright, F.M.; Vink, R.; Byard, R.W.; Duncan, J.R.; Krous, H.F.; Paterson, D.S. Abnormalities in substance P neurokinin-1 receptor binding in key brainstem nuclei in sudden infant death syndrome related to prematurity and sex. PLoS ONE 2017, 12, e0184958. [Google Scholar] [CrossRef]
- Dziennik Ustaw Rzeczypospolitej Polskiej. Rozporządzenie Ministra Zdrowia z Dnia 16 Sierpnia 2018 r. w Sprawie Standardu Organizacyjnego Opieki Okołoporodowej. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20180001756/O/D20181756.pdf (accessed on 15 December 2023).

| a. Maternal | |||||
| Variables | GDM Group n = 25 A | EGWG n = 25 B | Control Group n = 24 C | p | |
| Age (years) | Median (quartile) | 30 (27–32) | 28 (25–30) | 28 (25–29.25) | p = 0.071 |
| Weight before pregnancy (kg) | Median (quartile) | 73.9 (66–78) | 62 (59–66.5) | 60 (56.75–65.78) | p < 0.001 * A>B,C |
| Weight before delivery (kg) | Median (quartile) | 83.3 (75–95) | 86 (83–92) | 72.35 (70–78.25) | p < 0.001 * B,A>C |
| Glycemia before 10 weeks of pregnancy (mg/dL) | Median (quartile) | 89 (86–90) | 80 (78–84) | 84.5 (79–89.25) | p < 0.001 * A>C>B |
| GWG (kg) | Median (quartile) | 11 (8.5–14) | 24.80 (23–26) | 13 (8–15) | p < 0.001 * B>C,A |
| Maternal weight (g) | Median (quartile) | 3400 (3100–3600) | 3300 (3100–3500) | 3300 (2867.5–3695) | p = 0.719 |
| Maternal GWG (kg) | Median (quartile) | 14 (12–17) | 18 (16–20) | 12.5 (10–15) | p < 0.001 * B>A,C |
| b. Neonatal | |||||
| Variables | GDM Group n = 25 A | EGWG n = 25 B | Control Group n = 24 C | p | |
| Birth weight (g) | Median (quartile) | 3270 (3150–3700) | 3520 (3400–3650) | 3540 (3230–3905) | p = 0.207 |
| Birth length (cm) | Median (quartile) | 54 (53–56) | 55 (54–56) | 55 (55–56) | p = 0.058 |
| PI | Median (quartile) | 2.08 (2–2.3) | 2.1 (2–2.33) | 2.10 (1.94–2.21) | p = 0.84 |
| Head circumference (cm) | Median (quartile) | 34 (34–35) | 34 (34–35) | 34.5 (34–35) | p = 0.441 |
| Chest circumference (cm) | Median (quartile) | 35 (33–35) | 34 (33–35) | 34 (33.75–35) | p = 0.942 |
| Apgar 1’ | Median (quartile) | 10 (9–10) | 10 (10) | 10 (10) | p = 0.764 |
| Apgar 5’ | Median (quartile) | 10 (10) | 10 (10) | 10 (10) | p = 0.223 |
| Parameter | GDM Group n = 25 A | EGWG n = 25 B | Control Group n = 24 C | p | |
|---|---|---|---|---|---|
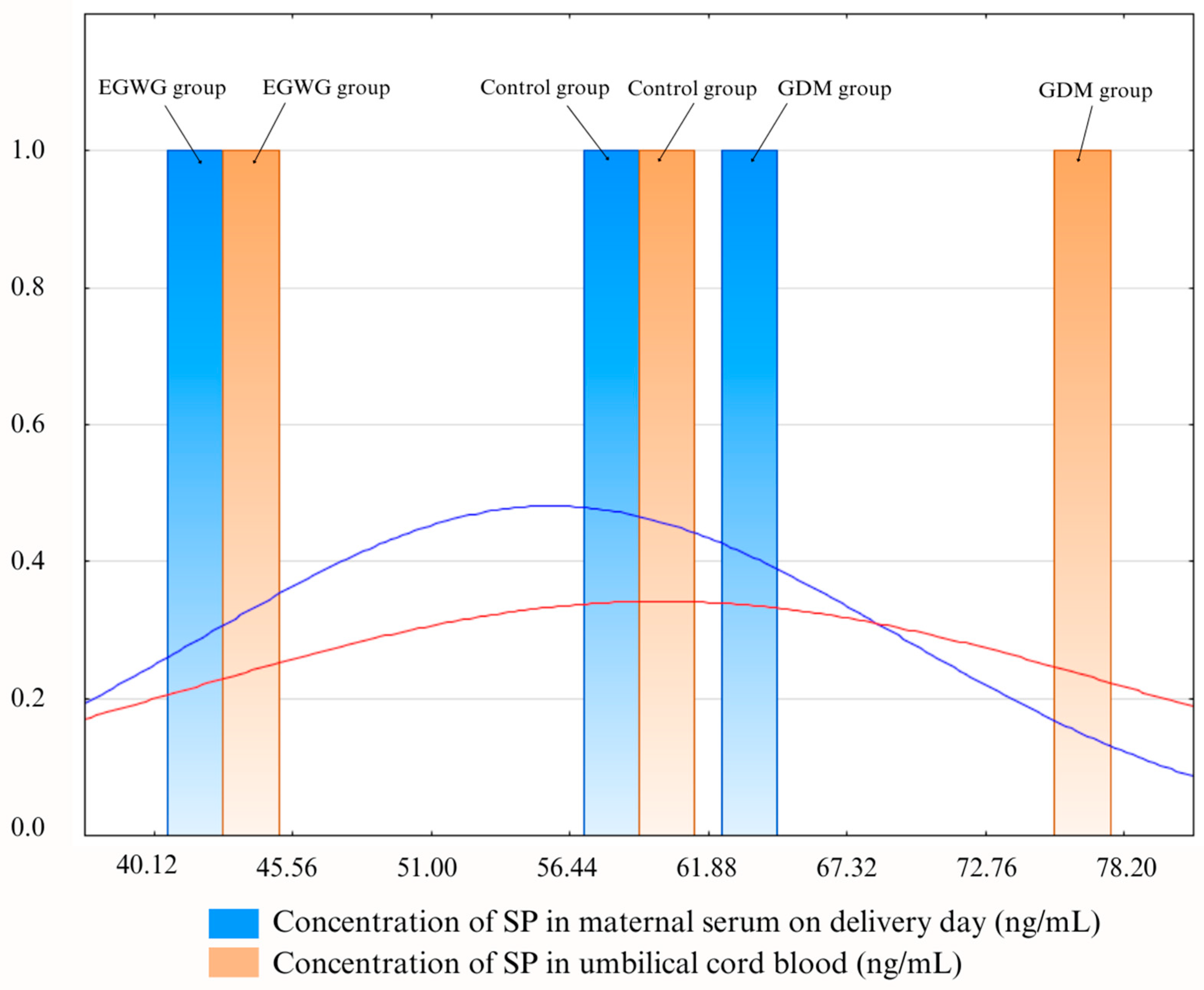
| Concentration of SP in serum on delivery day (ng/mL) | Median (quartile) | 64.94 (48.2–80.4) | 40.12 (24.78–56.9) | 61.85 (36.53–105.89) | p = 0.057 |
| Concentration of SP in umbilical cord blood (ng/mL) | Median (quartile) | 78.2 (53.5–199.45) | 40.2 (34.2–56.51) | 61.19 (34.77–69.59) | p = 0.006 * A>C,B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niebrzydowska-Tatus, M.; Pełech, A.; Bień, K.; Rekowska, A.K.; Domańska, A.; Kimber-Trojnar, Ż.; Leszczyńska-Gorzelak, B.; Trojnar, M. Substance P Concentration in Gestational Diabetes and Excessive Gestational Weight Gain and Its Impact on Neonatal Anthropometry. Int. J. Mol. Sci. 2024, 25, 3759. https://doi.org/10.3390/ijms25073759
Niebrzydowska-Tatus M, Pełech A, Bień K, Rekowska AK, Domańska A, Kimber-Trojnar Ż, Leszczyńska-Gorzelak B, Trojnar M. Substance P Concentration in Gestational Diabetes and Excessive Gestational Weight Gain and Its Impact on Neonatal Anthropometry. International Journal of Molecular Sciences. 2024; 25(7):3759. https://doi.org/10.3390/ijms25073759
Chicago/Turabian StyleNiebrzydowska-Tatus, Magdalena, Aleksandra Pełech, Katarzyna Bień, Anna K. Rekowska, Aleksandra Domańska, Żaneta Kimber-Trojnar, Bożena Leszczyńska-Gorzelak, and Marcin Trojnar. 2024. "Substance P Concentration in Gestational Diabetes and Excessive Gestational Weight Gain and Its Impact on Neonatal Anthropometry" International Journal of Molecular Sciences 25, no. 7: 3759. https://doi.org/10.3390/ijms25073759
APA StyleNiebrzydowska-Tatus, M., Pełech, A., Bień, K., Rekowska, A. K., Domańska, A., Kimber-Trojnar, Ż., Leszczyńska-Gorzelak, B., & Trojnar, M. (2024). Substance P Concentration in Gestational Diabetes and Excessive Gestational Weight Gain and Its Impact on Neonatal Anthropometry. International Journal of Molecular Sciences, 25(7), 3759. https://doi.org/10.3390/ijms25073759








