Bioactive Compounds and Potential Health Benefits through Cosmetic Applications of Cherry Stem Extract
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Characterization
| Peak | RT (min) | m/z Experimental [M-H]− | m/z Theoretical [M-H]− | Error (ppm) | Level of Annotation | Molecular Formula | Proposed Compound | MS/MS Fragments | Rel. Ab. (%) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.91 | 131.0459 | 131.0462 | −2.69 | 2 | C4H8N2O3 | L-Asparagine | 131 | 0.45 | PubChem: 6267 |
| 2 | 0.96 | 181.0717 | 181.0717 | −0.55 | 2 | C6H14O6 | D-Manitol | 59, 71, 85, 89, 101, 110, 181 | 1.63 | PubChem: 5780 |
| 3 | 1.01 | 195.0514 | 195.0510 | 1.73 | 2 | C6H12O7 | D-gluconic acid | 75, 99, 129, 195 | 3.54 | [26] |
| 4 | 1.06 | 133.0146 | 133.0142 | 2.38 | 2 | C4H6O5 | Malic acid isomer 1 | 71, 115, 133 | 0.91 | [31] |
| 5 | 1.11 | 133.0147 | 133.0142 | 3.13 | 2 | C4H6O5 | Malic acid isomer 2 | 71, 115, 133 | 0.91 | [31] |
| 6 | 1.16 | 133.0147 | 133.0142 | 3.13 | 2 | C4H6O5 | Malic acid isomer 3 | 71, 115, 133 | 1.91 | [31] |
| 7 | 1.20 | 191.0215 | 191.0197 | 9.09 | 2 | C6H8O7 | Citric acid | 57, 85, 87, 110, 191 | 1.91 | [31] |
| 8 | 1.30 | 197.8079 | - | - | 2 | - | Unknown | - | 0.82 | - |
| 9 | 1.39 | 125.0000 | 125.0009 | −7.65 | 2 | C2H7O4P | Dimethylphosphate | 62, 78 | 0.76 | HMDB0061734 |
| 10 | 1.60 | 115.0034 | 115.0037 | −2.77 | 2 | C4H4O4 | Maleic acid | 99 | 0.71 | HMDB0000176 |
| 11 | 1.94 | 96.9608 | 96.9601 | 6.81 | 2 | H2O4S | Sulfate | 79, 96 | 0.90 | HMDB0001448 |
| 12 | 2.13 | 358.1142 | 358.1143 | −0.54 | 2 | C15H21NO9 | L-DOPA glucoside | 101, 150, 161, 312 | 0.33 | HMDB0029452 |
| 13 | 2.29 | 299.0765 | 299.0772 | −2.60 | 2 | C13H16O8 | Hydroxybenzoic acid glucoside | 93, 116, 299 | 0.15 | [23] |
| 14 | 2.58 | 315.0730 | 315.0721 | 2.56 | 2 | C13H16O9 | Protocatechuic acid glucoside isomer 1 | 153, 109, 271, 315 | 0.84 | [26] |
| 15 | 2.87 | 315.0729 | 315.0721 | 2.24 | 2 | C13H16O9 | Protocatechuic acid glucoside isomer 2 | 153, 109, 271, 315 | 2.32 | [26] |
| 16 | 3.04 | 313.0934 | 313.0929 | 1.51 | 2 | C14H18O8 | Mandelic acid glucopyranoside isomer 1 | 59, 71, 73, 85, 101, 161, 269, 313 | 0.46 | HMDB0038335 |
| 17 | 3.39 | 313.0933 | 313.0929 | 1.19 | 2 | C14H18O8 | Mandelic acid glucopyranoside isomer 2 | 59, 71, 73, 85, 101, 161, 269, 313 | 1.17 | HMDB0038335 |
| 18 | 3.55 | 353.0878 | 353.0878 | −0.12 | 2 | C16H18O9 | Chlorogenic acid isomer 1 | 85, 87, 93, 134, 135, 179, 191, 353 | 0.37 | [23] |
| 19 | 4.10 | 353.0881 | 353.0878 | −0.12 | 2 | C16H18O9 | Chlorogenic acid isomer 2 | 85, 87, 93, 134, 135, 179, 191, 353 | 0.57 | [23] |
| 20 | 4.35 | 341.0884 | 341.0878 | 1.63 | 2 | C15H18O9 | Caffeic acid glucoside | 133, 161, 179, 281, 341 | 0.22 | [23,31] |
| 21 | 4.47 | 359.1349 | 359.1347 | 0.30 | 2 | C16H24O9 | Junipediol A glucoside | 131, 293, 313, 359 | 0.41 | CNP0148585 |
| 22 | 4.62 | 269.1015 | 269.1030 | −5.94 | 2 | C13H18O6 | Benzyl glucopyranoside | 71, 85, 101, 161, 269 | 0.05 | CNP0261916 |
| 23 | 5.01 | 329.0871 | 329.0878 | −2.26 | 2 | C14H18O9 | Vanillic acid glucoside isomer 1 | 96, 129, 173 | 0.56 | [27] |
| 24 | 5.27 | 329.0870 | 329.0878 | −2.56 | 2 | C14H18O9 | Vanillic acid glucoside isomer 2 | 96, 129, 173 | 0.56 | [27] |
| 25 | 5.47 | 401.1442 | 401.1453 | −2.89 | 2 | C18H26O10 | Benzyl primeveroside isomer 1 | 101, 161, 269, 401 | 0.16 | [26] |
| 26 | 5.59 | 289.0712 | 289.0717 | −2.07 | 2 | C15H14O6 | (Epi)Catechin isomer 1 | 97, 109, 123, 137, 203, 205, 245, 289, 205 | 1.63 | [23,31] |
| 27 | 5.93 | 401.1442 | 401.1453 | −2.89 | 2 | C18H26O10 | Benzyl primeveroside isomer 2 | 101, 161, 269, 401 | 0.48 | [26] |
| 28 | 6.12 | 289.0711 | 289.0717 | −2.42 | 2 | C15H14O6 | (Epi)Catechin isomer 2 | 97, 109, 123, 137, 203, 205, 245, 289 | 3.27 | [23,31] |
| 29 | 6.27 | 325.0907 | 325.0929 | −6.85 | 2 | C15H18O8 | Coumaroyl glucosa isomer 1 | 117, 119, 145, 163, 289, 325 | 0.46 | [23] |
| 30 | 6.48 | 577.1328 | 577.1351 | −4.14 | 2 | C30H26O12 | Procyanidin B isomer 1 | 125, 245, 289, 407, 425, 577, 578 | 0.79 | [26] |
| 31 | 6.58 | 385.1864 | 385.1868 | −1.11 | 2 | C19H30O8 | Roseoside isomer 1 | 59, 71, 89, 101, 385 | 0.33 | CNP0241893 |
| 32 | 6.71 | 385.1864 | 385.1868 | −1.11 | 2 | C19H30O8 | Roseoside isomer 2 | 59, 71, 89, 101, 385 | 0.72 | CNP0241893 |
| 33 | 7.00 | 385.1864 | 385.1868 | −1.11 | 2 | C19H30O8 | Roseoside isomer 3 | 59, 71, 89, 101, 385 | 1.91 | CNP0241893 |
| 34 | 7.14 | 577.1336 | 577.1351 | −2.75 | 2 | C30H26O12 | Procyanidin B isomer 2 | 125, 245, 289, 407, 425, 577, 578 | 0.48 | [26] |
| 35 | 7.42 | 449.1091 | 449.1089 | 0.29 | 2 | C21H22O11 | Aromadendrin glucoside isomer 1 | 125, 151, 287, 449 | 1.25 | [23,31] |
| 36 | 7.61 | 325.0921 | 325.0929 | −2.55 | 2 | C15H18O8 | Coumaroyl glucose isomer 2 | 117, 119, 145, 205, 325, 163 | 1.36 | [23] |
| 37 | 7.73 | 289.0715 | 289.0717 | −1.03 | 2 | C15H14O6 | (Epi)catechin isomer 3 | 97, 109, 123, 137, 203, 205, 245, 289, 205 | 0.87 | [31] |
| 38 | 7.81 | 449.1099 | 449.1089 | 2.07 | 2 | C21H22O11 | Aromadendrin glucoside isomer 2 | 125, 151, 287, 449 | 0.23 | [31] |
| 39 | 8.00 | 415.1590 | 415.1610 | −4.84 | 2 | C19H28O10 | Icariside D1 | 59, 71, 89, 101, 131, 149, 191, 415 | 0.14 | HMDB0303758 |
| 40 | 8.07 | 577.1344 | 577.1351 | −1.36 | 2 | C30H26O12 | Procyanidin B isomer 3 | 125, 245, 289, 407, 425, 577, 578 | 0.83 | [26] |
| 41 | 8.15 | 391.1608 | - | - | - | - | Unknown | - | 0.74 | - |
| 42 | 8.22 | 865.1977 | 865.1985 | −1.01 | 2 | C45H38O18 | Procyanidin C isomer 1 | 125, 161, 243, 289, 287, 407, 577, 695, 713, 865, 866 | 0.71 | HMDB0038370 |
| 43 | 8.27 | 463.2178 | 463.2185 | −1.56 | 2 | C21H36O11 | Linalool oxide primeveroside isomer 1 | 59, 89, 131, 149, 463 | 0.89 | HMDB0035489 |
| 44 | 8.41 | 331.1758 | 331.1762 | −1.40 | 2 | C16H28O7 | Betulalbusida A | 59, 71, 89, 119, 179, 331 | 0.02 | HMDB0035634 |
| 45 | 8.49 | 463.2178 | 463.2185 | −1.56 | 2 | C21H36O11 | Linalool oxide primeveroside isomer 2 | 59, 89, 131, 149, 463 | 0.03 | HMDB0035489 |
| 46 | 8.54 | 865.1972 | 865.1985 | −1.59 | 2 | C45H38O18 | Procyanidin C isomer 2 | 125, 161, 243, 289, 287, 407, 577, 695, 713, 865, 866 | 0.16 | HMDB0038370 |
| 47 | 8.62 | 463.2178 | 463.2185 | −1.56 | 2 | C21H36O11 | Linalool oxide primeveroside isomer 3 | 59, 89, 131, 149, 463 | 0.31 | HMDB0035489 |
| 48 | 8.67 | 519.1729 | 519.1719 | 1.80 | 2 | C22H32O14 | Segetoside A | 147, 161, 325, 473 | 0.22 | CNP0159091 |
| 49 | 8.76 | 441.1408 | 441.1402 | 1.20 | 2 | C20H26O11 | Cinnamoyl arabinosyl glucose | 103, 147, 161, 441 | 0.53 | HMDB0030294 |
| 50 | 8.81 | 865.1988 | 865.1985 | 0.26 | 2 | C45H38O18 | Procyanidin C isomer 3 | 125, 161, 243, 289, 287, 407, 577, 695, 713, 865, 866 | 0.46 | HMDB0038370 |
| 51 | 8.88 | 771.1995 | 771.1989 | 0.69 | 2 | C33H40O21 | Quercetin rutinoside glucoside | 151, 179, 300, 301, 609, 771 | 0.37 | [23,31] |
| 52 | 8.92 | 755.2018 | 755.2040 | −2.98 | 2 | C33H40O20 | Kaempferol rutinoside glucoside | 125, 285, 465, 466, 593, 594, 755 | 0.16 | [23,31] |
| 53 | 8.99 | 465.1050 | 465.1038 | 2.39 | 2 | C21H22O12 | Taxifolin glucoside | 125, 152, 259, 285, 437, 465 | 0.94 | [23,31] |
| 54 | 9.09 | 521.2026 | 521.2028 | −0.52 | 2 | C26H34O11 | Icariside E5 isomer 1 | 329, 341, 359, 521, 567 | 0.16 | HMDB0034749 |
| 55 | 9.14 | 521.2026 | 521.2028 | −0.52 | 2 | C26H34O11 | Icariside E5 isomer 2 | 329, 341, 359, 521, 567 | 0.25 | HMDB0034749 |
| 56 | 9.23 | 431.0990 | 431.0984 | 1.38 | 2 | C21H20O10 | Vitexin | 117, 283, 311, 341, 431 | 0.37 | CNP0212195 |
| 57 | 9.27 | 473.1682 | 473.1664 | 3.62 | 2 | C21H30O12 | Feruloylglucose trihydroxy methylbutylglycoside | 89, 125, 149, 293, 427, 473 | 0.16 | HMDB0036214 |
| 58 | 9.38 | 463.1243 | 463.1246 | −0.70 | 2 | C22H24O11 | Hesperetin glucoside | 109, 165, 257, 301, 343, 435, 463 | 0.42 | [32] |
| 59 | 9.46 | 503.1761 | 503.1770 | −1.89 | 2 | C22H32O13 | Multifidol apiosyl glucoside isomer 1 | 59, 71, 89, 125, 148, 149, 131, 293, 457, 503 | 0.52 | HMDB0039930 |
| 60 | 9.52 | 595.1658 | 595.1668 | −1.70 | 2 | C27H32O15 | Naringenin diglucoside | 271, 433, 595 | 0.01 | [26] |
| 61 | 9.58 | 609.1475 | 609.1461 | 2.22 | 2 | C27H30O16 | Rutin | 255, 271, 300, 301, 609, 610, 611 | 0.74 | [26] |
| 62 | 9.65 | 503.1776 | 503.1770 | 1.09 | 2 | C22H32O13 | Multifidol apiosyl glucoside isomer 2 | 59, 71, 89, 125, 148, 149, 131, 293, 457, 503 | 0.27 | HMDB0039930 |
| 63 | 9.75 | 303.0519 | 303.0510 | 2.76 | 2 | C15H12O7 | Taxifolin | 125, 153, 177, 259, 275, 285, 303 | 0.50 | [32] |
| 64 | 9.82 | 463.0886 | 463.0882 | 0.78 | 2 | C21H20O12 | Isoquercetin | 271, 300, 301 | 0.42 | [31] |
| 65 | 9.90 | 433.1153 | 433.1140 | 2.97 | 2 | C21H22O10 | Naringenin glucoside isomer 1 | 83, 107, 119, 151, 165, 253, 271, 433 | 1.50 | [23,31] |
| 66 | 9.92 | 477.1051 | 477.1038 | 2.54 | 2 | C22H22O12 | Isorhamnetin glucoside | 119, 151, 269, 271, 299, 431 | 0.67 | [26] |
| 67 | 9.99 | 431.0995 | 431.0984 | 2.54 | 2 | C21H20O10 | Cosmosiin isomer 1 | 125, 133, 269, 431 | 0.05 | HMDB0037340 |
| 68 | 10.01 | 593.1520 | 593.1512 | 1.30 | 2 | C27H30O15 | Kaempferol rutinoside isomer 1 | 593, 285, 255, 227, 284 | 0.67 | [31] |
| 69 | 10.05 | 433.1156 | 433.1140 | 3.67 | 2 | C21H22O10 | Naringenin glucoside isomer 2 | 83, 107, 119, 151, 165, 253, 271, 433 | 0.41 | [31] |
| 70 | 10.11 | 307.0464 | 307.0459 | 1.37 | 4 | C14H12O8 | Fulvic acid | 69, 79, 99, 141, 185, 307 | 0.30 | CNP0249768 |
| 71 | 10.17 | 443.1902 | 443.1923 | −4.76 | 2 | C21H32O10 | Dihydrophaseic acid glucopyranoside | 59, 71, 133, 311, 443, 445, 489 | 0.14 | CNP0210117 |
| 72 | 10.20 | 417.1197 | 417.1191 | 1.34 | 2 | C21H22O9 | Liquiritin | 211, 237, 417 | 0.35 | [26] |
| 73 | 10.25 | 431.0973 | 431.0984 | 3.23 | 2 | C21H20O10 | Cosmosiin isomer 2 | 83, 125, 149, 253, 268, 431 | 0.15 | HMDB0037340 |
| 74 | 10.29 | 447.1077 | - | - | 4 | - | Unknown | - | 0.45 | - |
| 75 | 10.40 | 433.1147 | 433.1140 | 1.59 | 2 | C21H22O10 | Naringenin glucoside isomer 3 | 83, 107, 119, 151, 165, 253, 271, 433 | 0.75 | [31] |
| 76 | 10.46 | 329.1609 | 329.1606 | 0.87 | 2 | C16H26O7 | Carboxyethyl dihydroxycyclopentyl oxooctanoic acid | 59, 225, 269, 329 | 0.15 | CNP0150911 |
| 77 | 10.48 | 433.1152 | 433.1140 | 2.74 | 2 | C21H22O10 | Naringenin glucoside isomer 4 | 57, 83, 125, 149, 253, 271, 433 | 0.13 | [31] |
| 78 | 10.51 | 463.2527 | - | - | 4 | - | Unknown | - | 0.19 | - |
| 79 | 10.57 | 575.1200 | 575.1195 | 0.81 | 2 | C30H24O12 | Procyanidin A | 125, 137, 271, 394, 449, 575 | 0.29 | HMDB0037655 |
| 80 | 10.61 | 595.1666 | 595.1668 | −0.47 | 2 | C27H32O15 | Neoeriocitrin | 255, 256, 549, 550 | 0.31 | [32] |
| 81 | 10.65 | 493.2290 | 493.2290 | −0.18 | 2 | C22H38O12 | Rhodioloside B isomer 1 | 101, 131, 161, 315, 447, 493 | 0.29 | CNP0328986 |
| 82 | 10.69 | 493.2325 | 493.2290 | 6.92 | 2 | C22H38O12 | Rhodioloside B isomer 2 | 101, 131, 161, 315, 447, 493 | 0.15 | CNP0328986 |
| 83 | 10.76 | 287.0574 | 287.0561 | 4.36 | 2 | C15H12O6 | Aromadendrin | 125, 243, 259, 260, 287 | 0.20 | HMDB0030847 |
| 84 | 10.81 | 835.2469 | - | - | 4 | - | Unknown | - | 1.10 | - |
| 85 | 10.92 | 831.2151 | - | - | 4 | - | Unknown | - | 1.50 | - |
| 86 | 10.96 | 371.1704 | - | - | 4 | - | Unknown | - | 0.29 | - |
| 87 | 11.09 | 461.1097 | 461.1089 | 1.58 | 2 | C22H22O11 | Chrysoeriol glucoside | 63, 65, 255 | 1.50 | [32] |
| 88 | 11.22 | 447.1293 | 447.1297 | −0.91 | 2 | C22H24O10 | Dihydrowogonin glucoside | 65, 136, 171, 241, 269, 285, 447 | 3.68 | [31] |
| 89 | 11.26 | 491.1209 | 491.1195 | 2.78 | 2 | C23H24O12 | Dimethoxyapigenin glucoside | 268, 269, 270, 283, 285, 432, 447 | 1.06 | HMDB0038825 |
| 90 | 11.38 | 419.1335 | 419.1347 | −3.08 | 2 | C21H24O9 | Trihydroxydihydrochalcone glucoside isomer 1 | 213, 239, 257, 419 | 0.18 | HMDB0037483 |
| 91 | 11.42 | 609.1248 | 609.1250 | −0.35 | 2 | C30H26O14 | Caffeoylastragalin | 161, 179, 227, 255, 285, 323 | 0.12 | HMDB0030239 |
| 92 | 11.61 | 569.1293 | 569.1301 | −1.41 | 2 | C28H26O13 | Cudranian 2 | 125, 259, 285, 541, 569 | 0.12 | CNP0085196 |
| 93 | 11.88 | 301.0721 | 301.0717 | 1.00 | 2 | C16H14O6 | Trihydroxy methoxyflavanone | 110, 137, 165, 194, 258, 286, 301 | 0.09 | CNP0188213 |
| 94 | 11.99 | 593.1315 | 593.1301 | 2.36 | 2 | C30H26O13 | Coumaroyltrifolin | 145, 227, 255, 285, 593 | 0.18 | HMDB0040689 |
| 95 | 12.03 | 327.2180 | 327.2177 | 0.81 | 2 | C18H32O5 | Trihydroxyoctadecadienoic acid | 171, 327, 211 | 1.29 | [27] |
| 96 | 12.24 | 419.1354 | 419.1347 | 1.45 | 2 | C21H24O9 | Trihydroxydihydrochalcone glucoside isomer 2 | 91, 171, 175, 419 | 0.15 | HMDB0037483 |
| 97 | 12.49 | 271.0616 | 271.0612 | 1.35 | 2 | C15H12O5 | Naringenin | 63, 65, 107, 119, 151, 177, 271 | 0.46 | [27] |
| 98 | 12.51 | 329.2332 | 329.2333 | −0.56 | 2 | C18H34O5 | Trihydroxyoctadecenoic acid | 211, 229, 329 | 1.32 | [27] |
| 99 | 13.03 | 269.0461 | 269.0455 | 1.92 | 2 | C15H10O5 | Genistein | 62, 65, 133, 159, 269 | 0.31 | HMDB0003217 |
| 100 | 13.15 | 563.1572 | 563.1559 | 2.27 | 2 | C30H28O11 | Dihydroxyphenyl oxopropenylphenoxy trihydroxyoxanyl methyl hydroxyphenyl propenoate | 145, 211, 307, 563 | 0.13 | CNP0325928 |
| 101 | 13.34 | 521.1458 | 521.1453 | 0.85 | 2 | C28H26O10 | Morusalbanol A | 169, 211, 237, 521 | 0.13 | PubChem: 60166296 |
| 102 | 13.48 | 517.3163 | 517.3171 | −1.57 | 2 | C30H46O7 | Jaligonic acid | 469, 470, 487, 488, 517 | 0.40 | [27] |
| 103 | 13.59 | 519.3362 | - | - | 4 | - | Unknown | - | 0.22 | - |
| 104 | 13.63 | 307.1917 | 307.1915 | 0.59 | 2 | C18H28O4 | Corchorifatty acid D | 65, 121, 185, 235, 289, 307 | 0.22 | HMDB0033243 |
| 105 | 13.76 | 285.0773 | 285.0768 | 1.46 | 2 | C16H14O5 | Methylnaringenin | 65, 82, 110, 137, 165, 270, 285 | 2.59 | [27] |
| 106 | 13.92 | 255.0665 | 255.0663 | 0.71 | 2 | C15H12O4 | Pinocembrin isomer 1 | 65, 169, 211, 255 | 0.18 | [26] |
| 107 | 14.07 | 283.0615 | 283.0612 | 0.94 | 2 | C16H12O5 | narinnin | 109, 163, 184, 268, 283 | 0.15 | CNP0330793 |
| 108 | 14.14 | 597.1626 | 597.1614 | 2.01 | 2 | C30H30O13 | Myrciacitrin V | 241, 269, 286, 327, 536, 551 | 0.09 | CNP0244473 |
| 109 | 14.19 | 255.0664 | 255.0663 | 0.32 | 2 | C15H12O4 | Pinocembrin isomer 2 | 65, 107, 145, 151, 169, 211 | 0.40 | [26] |
| 110 | 14.56 | 253.0510 | 253.0506 | 1.31 | 2 | C15H10O4 | Chrysin | 63, 65, 143, 145, 253 | 0.75 | [26] |
| 111 | 15.07 | 675.3618 | 675.3597 | 3.01 | 2 | C33H56O14 | Gingerglycolipid A | 397, 415, 675, 676, 721 | 0.01 | [17] |
| 112 | 15.49 | 513.2867 | - | - | 4 | - | Unknown | - | 0.23 | - |
| 113 | 15.83 | 293.2114 | 293.2122 | −2.92 | 2 | C18H30O3 | Hydroxyoctadecatrienoic acid | 183, 275, 293 | 0.38 | [26] |
| 114 | 15.85 | 487.3417 | - | - | 4 | - | Unknown | - | 0.37 | - |
| 115 | 15.99 | 537.3289 | - | - | 4 | - | Unknown | - | 0.12 | - |
| 116 | 16.15 | 441.2857 | 441.2858 | −0.26 | 2 | C24H42O7 | Ascorbyl stearate | 163, 181, 277, 441 | 0.03 | HMDB0038242 |
| 117 | 16.22 | 291.1967 | 291.1966 | 0.33 | 2 | C18H28O3 | Oxooctadecatrienoic acid | 121, 185, 291 | 0.40 | CNP0256961 |
| 118 | 16.30 | 494.3206 | 494.3252 | −9.41 | 2 | C24H50NO7P | Palmitoyl phosphatidylcholine | 78, 224, 255, 480 | 0.22 | HMDB0010382 |
| 119 | 16.53 | 295.2279 | 295.2279 | −0.02 | 2 | C18H32O3 | Hydroxy-octadecadienoic acid | 295 | 0.61 | [26] |
| 120 | 16.58 | 417.2865 | - | - | 4 | - | Unknown | - | 0.15 | - |
| 121 | 16.88 | 293.2109 | 293.2122 | −4.62 | 2 | C18H30O3 | Oxooctadecadienoic acid | 293 | 0.76 | CNP0191563 |
| 122 | 17.10 | 469.3316 | 469.3323 | −1.64 | 2 | C30H46O4 | Liquiritic acid | 393, 407, 423, 469 | 0.29 | HMDB0035788 |
| 123 | 17.29 | 565.3598 | - | - | 4 | - | Unknown | - | 0.50 | - |
| 124 | 17.45 | 633.3801 | 633.3797 | 0.61 | 2 | C39H54O7 | Hydroxypyracrenic acid | 117, 145, 633 | 1.50 | HMDB0029780 |
| 125 | 17.53 | 471.3488 | 471.3480 | 1.65 | 2 | C30H48O4 | Hederagenin isomer 1 | 471 | 0.84 | CNP0170966 |
| 126 | 17.70 | 471.3502 | 471.3480 | 4.62 | 2 | C30H48O4 | Hederagenin isomer 2 | 471 | 0.26 | CNP0170966 |
| 127 | 17.79 | 621.3495 | 621.3492 | 0.48 | 3 | C30H54O13 | Fatty acid derivate | - | 0.30 | - |
| 128 | 17.87 | 445.3170 | 445.3171 | −0.26 | 2 | C24H46O7 | Fatty acid derivate | - | 0.74 | - |
| 129 | 18.10 | 607.3702 | 607.3699 | 0.43 | 2 | C30H56O12 | Stearoyl trehalose | 59, 103, 163, 607 | 0.65 | PubChem: 68945547 |
| 130 | 18.60 | 277.2174 | 277.2173 | 0.21 | 2 | C18H30O2 | Linolenic acid | 277 | 1.01 | [26] |
| 131 | 18.74 | 663.3616 | - | - | 4 | - | Unknown | - | 0.49 | - |
| 132 | 18.84 | 499.3790 | 499.3793 | −0.64 | 2 | C32H52O4 | Oleananoic acid acetate | 423, 441, 451, 453, 499 | 0.13 | PubChem: 6708668 |
| 133 | 18.90 | 649.3829 | 649.3746 | 12.74 | 2 | C39H54O8 | Coumaroyloxytormentic acid isomer 1 | 59, 101, 145, 205, 251, 649 | 0.35 | HMDB0040682 |
| 134 | 18.95 | 649.3828 | 649.3746 | 12.58 | 2 | C39H54O8 | Coumaroyloxytormentic acid isomer 2 | 59, 101, 145, 205, 251, 649 | 0.25 | HMDB0040682 |
| 135 | 19.06 | 617.3851 | 617.3848 | 0.49 | 2 | C39H54O6 | Coumaroylalphitolic acid isomer 1 | 117, 145, 617 | 0.23 | CNP0288604 |
| 136 | 19.16 | 617.3867 | 617.3848 | 3.08 | 2 | C39H54O6 | Coumaroylalphitolic acid isomer 2 | 117, 145, 617 | 0.37 | CNP0288604 |
| 137 | 19.21 | 617.3868 | 617.3848 | 3.24 | 2 | C39H54O6 | Coumaroylalphitolic acid isomer 3 | 117, 145, 617 | 0.27 | CNP0288604 |
| 138 | 19.26 | 299.2596 | 299.2592 | 1.32 | 2 | C18H36O3 | Hydroxy octadecanoic acid | 59, 299 | 0.53 | [26] |
| 139 | 19.30 | 279.2334 | 279.2329 | 1.46 | 2 | C18H32O2 | Linoleic acid | 279 | 0.54 | [27] |
| 140 | 19.69 | 691.3918 | 691.3910 | 1.06 | 2 | C34H60O14 | Acetyl octadecanoyl trehalose | 59, 145, 205, 251, 691 | 0.49 | CNP0278012 |
| 141 | 19.89 | 555.2784 | 555.2811 | −4.60 | 2 | C28H44O11 | Picrasinoside F | 100, 555 | 0.90 | PubChem: 14733740 |
| 142 | 20.72 | 935.5802 | 935.5737 | 6.86 | 2 | C51H84O15 | Digalactosyldiacylglycerol | 277, 379, 397, 657, 675, 981 | 0.01 | CNP0130612 |
| 143 | 21.12 | 621.4392 | 621.4372 | 3.17 | 2 | C36H62O8 | Ginsenoside Rh2 | 575, 615 | 2.04 | HMDB0039544 |
| 144 | 21.31 | 429.3026 | 429.3010 | 3.56 | 2 | C27H42O4 | Plastoquinone 1 isomer 1 | 133, 161, 179, 429 | 0.20 | CNP0152651 |
| 145 | 21.41 | 712.5358 | 712.5369 | −1.61 | 2 | C40H75NO9 | Soyacerebroside I isomer 1 | 532, 550, 712 | 0.09 | CNP0245393 |
| 146 | 21.46 | 665.4191 | - | - | 4 | - | Unknown | - | 0.27 | - |
| 147 | 21.53 | 712.5358 | 712.5369 | −1.61 | 2 | C40H75NO9 | Soyacerebroside I isomer 2 | 532, 550, 712 | 0.07 | CNP0245393 |
| 148 | 21.58 | 429.3023 | 429.3010 | 2.87 | 2 | C27H42O4 | Plastoquinone 1 isomer 2 | 133, 161, 179, 429 | 1.16 | CNP0152651 |
| 149 | 21.76 | 773.5240 | 773.5209 | 3.93 | 2 | C45H74O10 | Octadecatrienoyl galactosyl glycerol | 235, 253, 277, 513, 773 | 0.65 | CHEBI:34042 |
| 150 | 22.00 | 959.5959 | - | - | 4 | - | Unknown | - | 0.19 | - |
| 151 | 22.18 | 443.3187 | 443.3167 | 4.47 | 3 | C28H44O4 | Hydroxymethoxyphenyl propenyl octadecenoate | 77, 105, 133, 343, 428, 443 | 0.16 | CNP0271143 |
| 152 | 22.29 | 863.6428 | - | - | 4 | - | Unknown | - | 0.65 | - |
| 153 | 22.41 | 775.5367 | 775.5366 | 0.12 | 2 | C45H76O10 | Octadeca dienoyloxy trihydroxy(hydroxymethyl)oxanyloxypropyl octadecatrienoate | 253, 277, 279, 513 | 0.11 | CNP0110822 |
| 154 | 22.48 | 413.3078 | 413.3061 | 3.98 | 2 | C27H42O3 | Hydroxyphenyl propenyl octadecenoate | 117, 118, 119, 145, 413 | 0.49 | CNP0230032 |
| 155 | 22.54 | 461.3293 | 461.3272 | 4.37 | 3 | C28H46O5 | Polyporusterone F | 57, 125, 461 | 0.13 | HMDB0038499 |
| 156 | 22.64 | 863.6425 | - | - | 4 | - | Unknown | - | 2.32 | - |
| 157 | 22.87 | 531.4077 | - | - | 4 | - | Unknown | - | 0.18 | - |
| 158 | 23.08 | 531.3739 | - | - | 4 | - | Unknown | - | 0.11 | - |
| 159 | 23.16 | 445.3353 | - | - | 4 | - | Unknown | - | 0.54 | - |
| 160 | 23.35 | 919.7022 | - | - | 4 | - | Unknown | - | 0.11 | - |
| 161 | 23.53 | 415.3224 | 415.3218 | 1.43 | 2 | C27H44O3 | Octadecyl coumarate | 117, 118, 119, 145, 163, 415 | 0.63 | CNP0156044 |
| 162 | 23.63 | 489.3605 | 489.3585 | 3.91 | 2 | C30H50O5 | Escinidin | 57, 83, 125, 489 | 0.61 | [17] |
| 163 | 23.75 | 459.3493 | 459.3480 | 2.78 | 2 | C29H48O4 | Eicosanyl caffeate | 133, 161, 179, 459 | 0.40 | PubChem: 5320238 |
| 164 | 24.03 | 659.3641 | - | - | 4 | - | Unknown | - | 0.22 | - |
| 165 | 24.21 | 545.2950 | 545.2909 | 7.52 | 2 | C34H42O6 | Cowagarcinone A | 176, 369, 527, 545 | 0.11 | C00035277 |
| 166 | 24.58 | 824.4111 | - | - | 4 | - | Unknown | - | 0.07 | - |
| 167 | 25.10 | 905.6708 | - | - | 4 | - | Unknown | - | 0.27 | - |
| 168 | 25.14 | 756.5116 | - | - | 4 | - | Unknown | - | 0.07 | - |
| 169 | 25.54 | 887.6596 | - | - | 4 | - | Unknown | - | 0.20 | - |
| 170 | 25.72 | 715.5865 | 715.5882 | −2.45 | 3 | C45H80O6 | Triacylglycerol | 61, 715 | 0.40 | HMDB0047885 |
| 171 | 25.94 | 759.6139 | - | - | 4 | - | Unknown | - | 0.13 | - |
| 172 | 26.21 | 699.5927 | 699.5933 | −0.91 | 2 | C45H80O5 | Diacylglycerol | 61, 699 | 0.11 | HMDB0007660 |
| 173 | 26.33 | 729.6028 | - | - | 4 | - | Unknown | - | 0.59 | - |
| 174 | 26.97 | 743.6174 | 743.6195 | −2.89 | 3 | C47H84O6 | Tetradecenoyl hexadecenoyl tetradecenoyl glycerol | 61, 743 | 0.29 | HMDB0047885 |
2.2. Evaluation of Antioxidant and Antiradical Activities
2.3. Evaluation of Enzyme Inhibition Potential
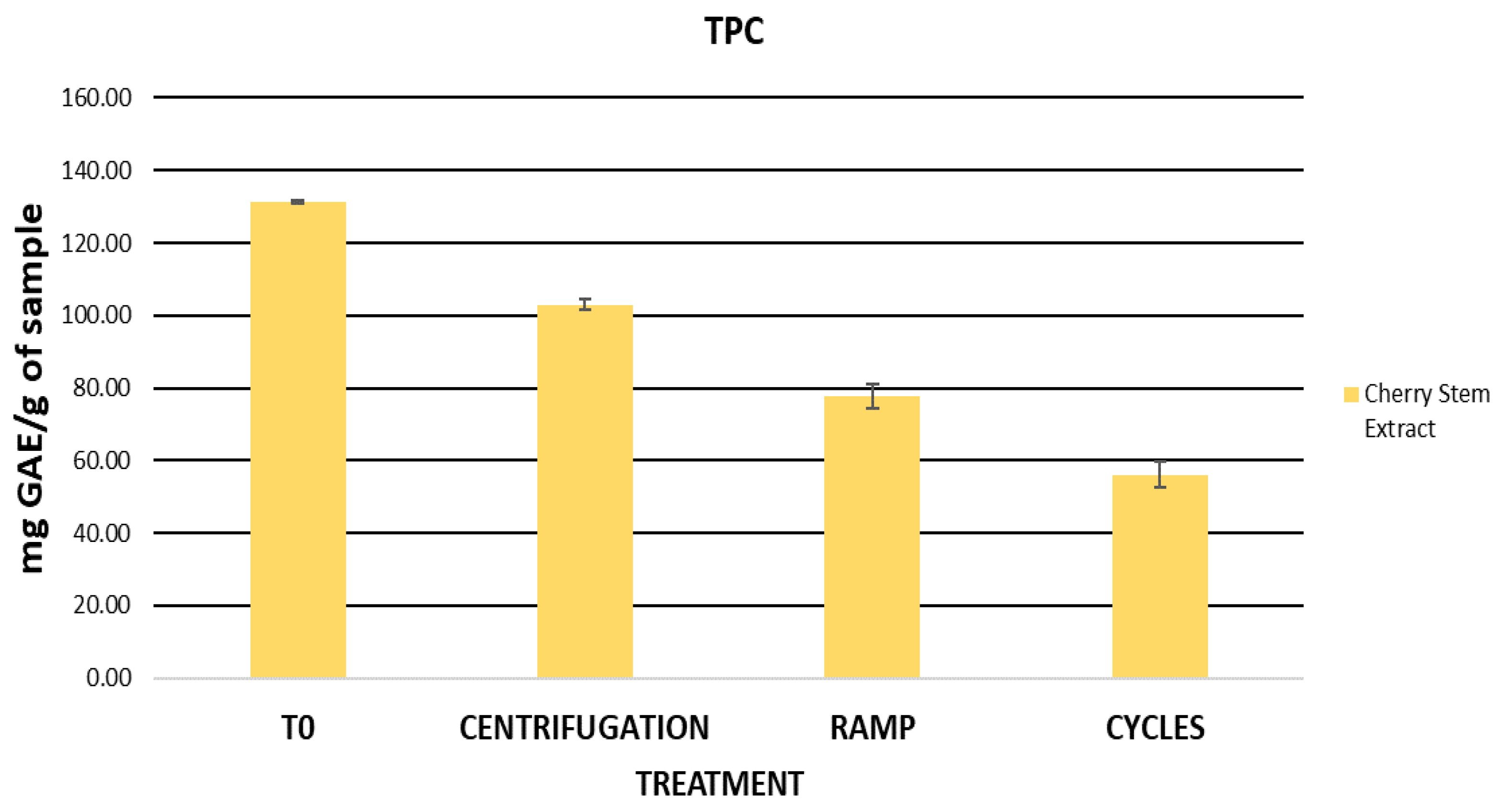
2.4. Assessment of the Stability of the Cherry Stem Extract
2.5. Development of a Cosmetic Formulation and Study of Its Stability
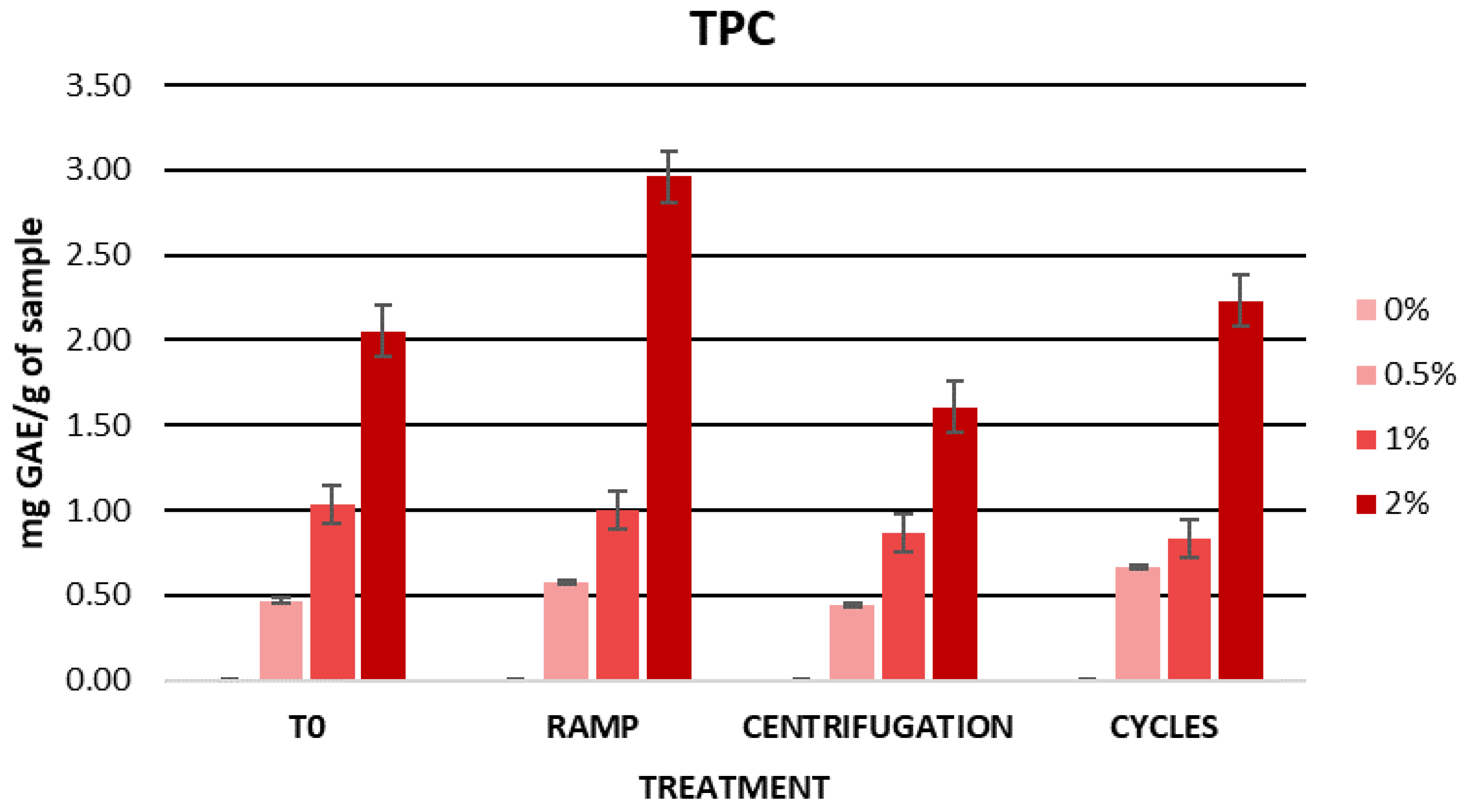
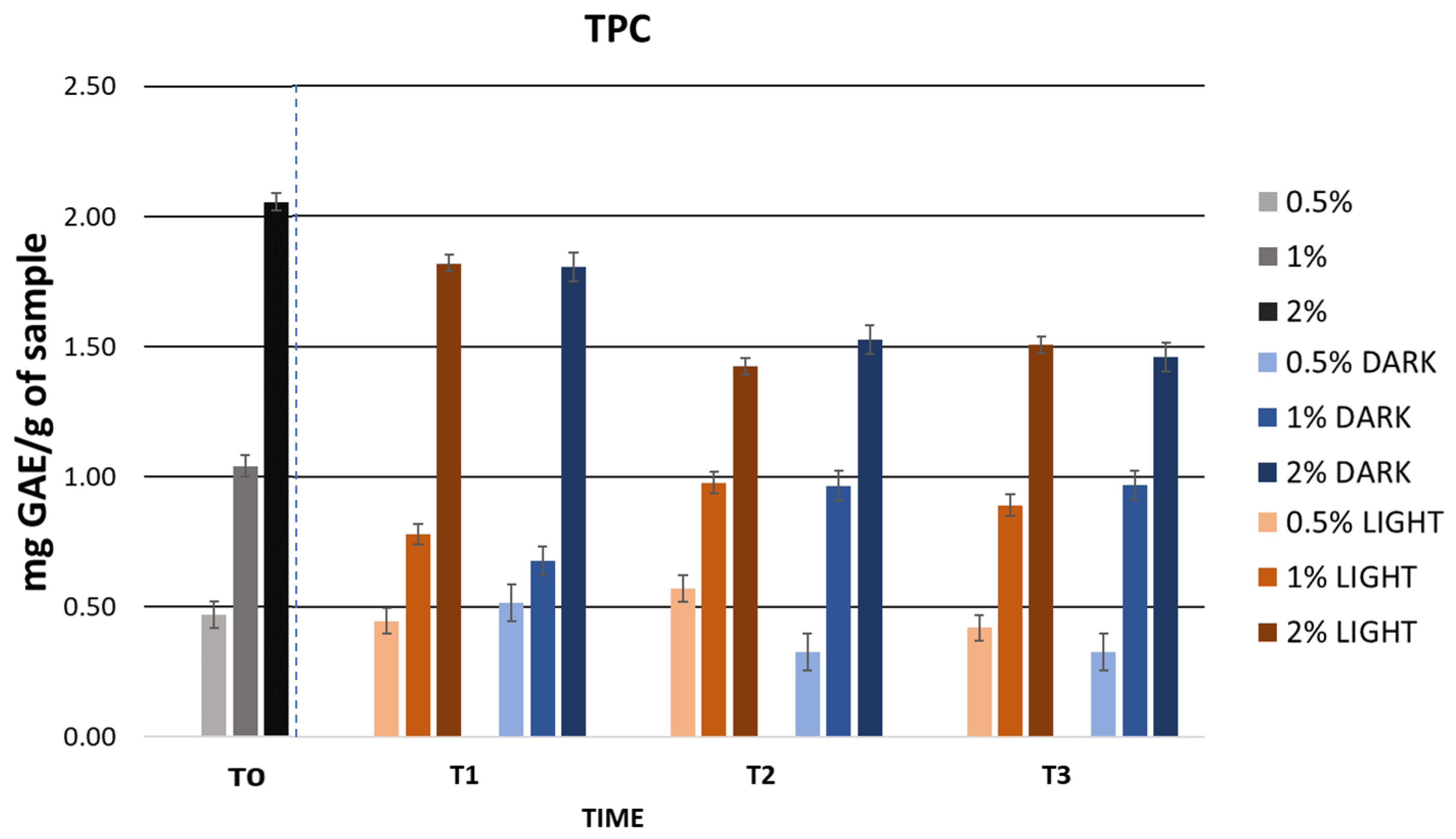
2.5.1. Assessment of Initial Physicochemical Characteristics and Total Phenolic Content (TPC) in Formulations
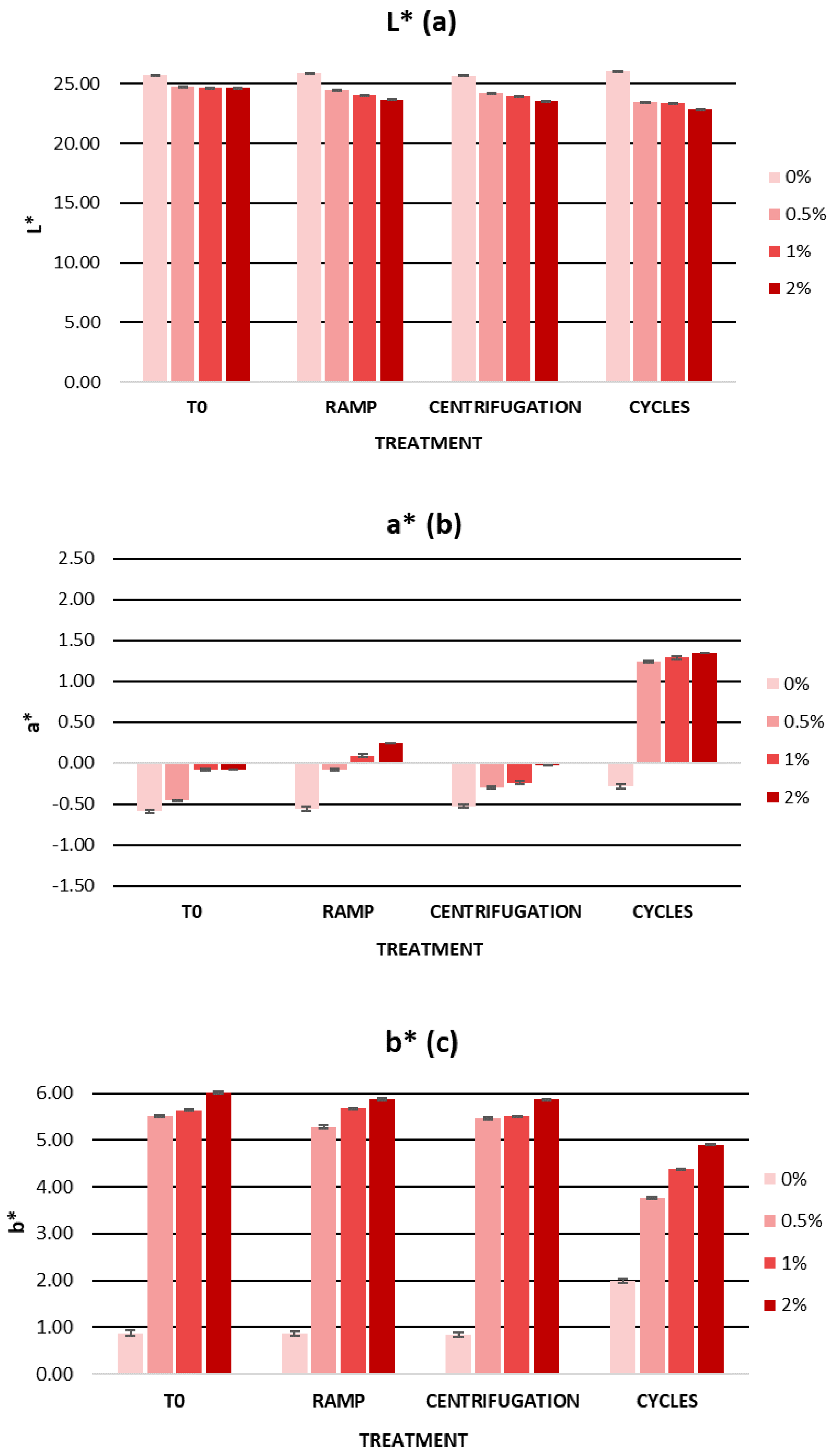
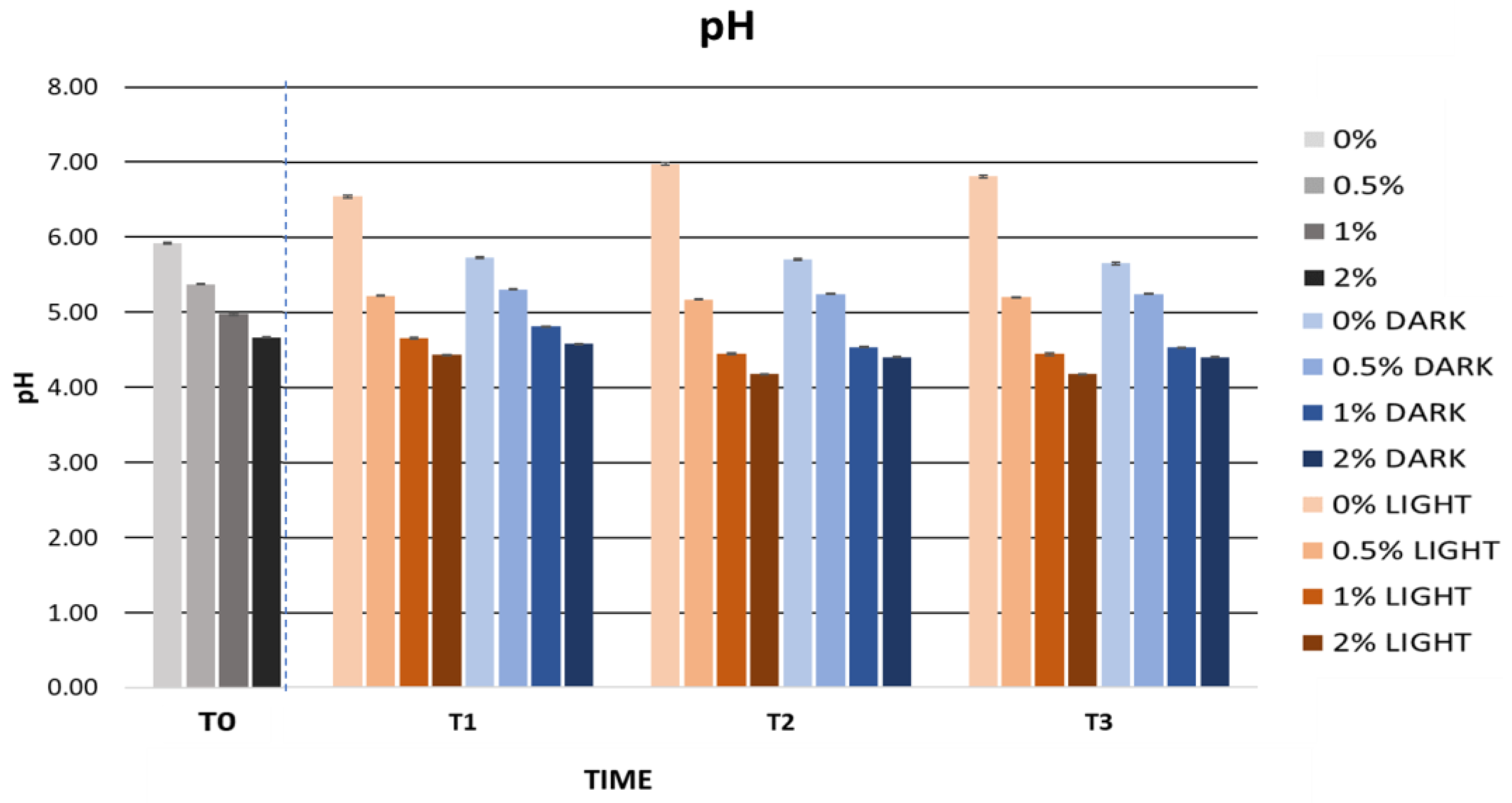
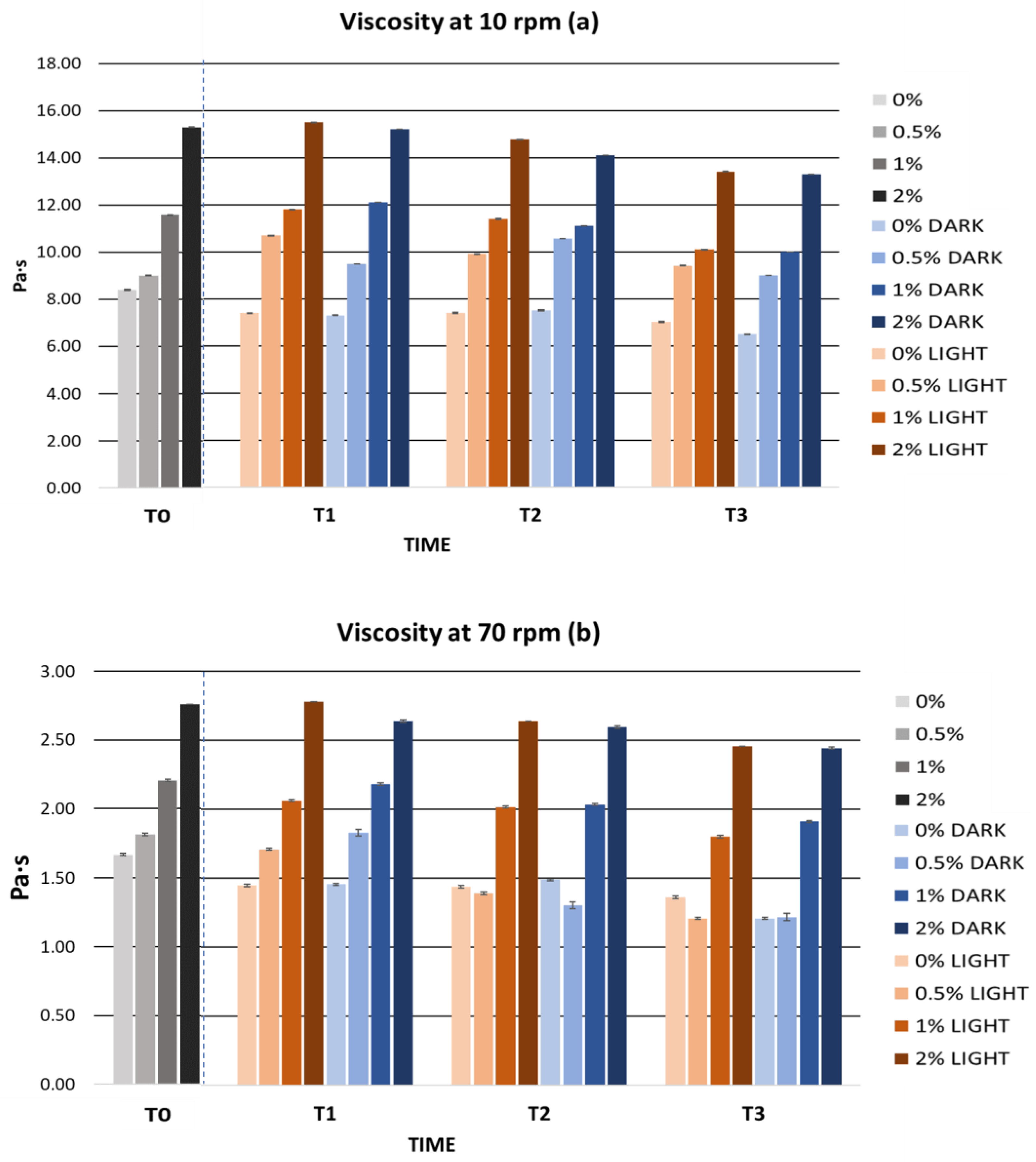
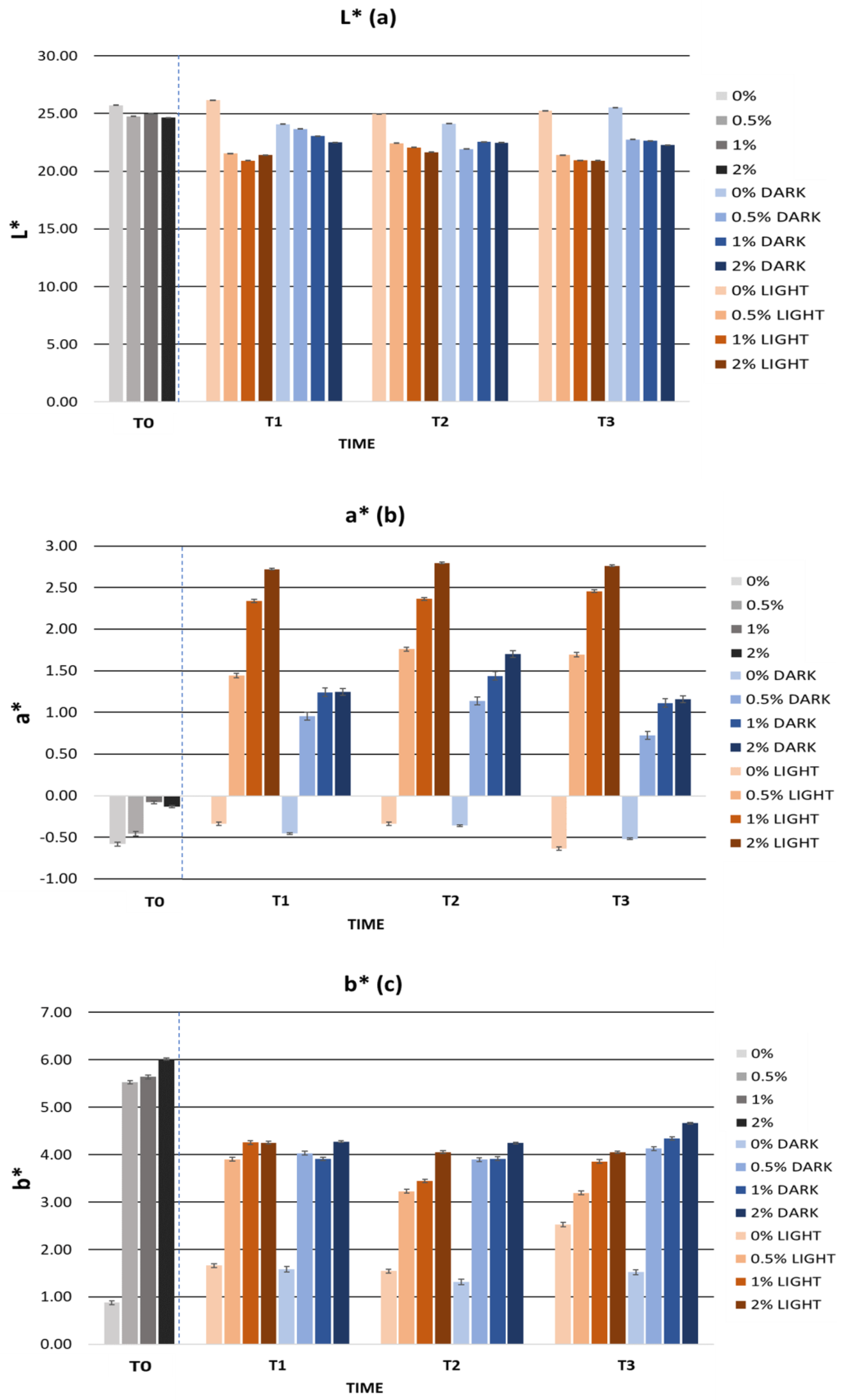
2.5.2. Cosmetic Formulation Stability Study
Accelerated Formulation Stability
Long-Term Formulation Stability
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Sample Preparation
3.3. Analysis HPLC-ESI-qTOF-MS/MS
3.4. In Vitro Antioxidant Activity
3.4.1. Evaluation of Antioxidant and Antiradical Activities
3.4.2. Evaluation of Enzyme Inhibition Potential
3.5. Assessment of the Stability of the Cherry Stem Extract
3.6. Incorporation of the Extract in a Cosmetic Formulation and Study of Its Stability
3.6.1. Cosmetic Formulation
3.6.2. Study of Stability
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and Age-related Diseases: From Mechanisms to Therapeutic Strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive Compounds from Natural Resources against Skin Aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Csekes, E.; Račková, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef]
- Rodrigues, F.; Cádiz-Gurrea, M.D.L.L.; Nunes, M.A.; Pinto, D.; Vinha, A.F.; Linares, I.B.; Oliveira, M.B.P.P.; Carretero, A.S. Cosmetics; Woodhead Publishing: Kidlington, UK, 2018; ISBN 9780128135723. [Google Scholar]
- Tobin, D.J. Introduction to Skin Aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.A.; Mikail, M.A.; Zamakshshari, N.; Abdullah, A.S.H. Natural Anti-Aging Skincare: Role and Potential. Biogerontology 2020, 21, 293–310. [Google Scholar] [CrossRef]
- Amberg, N.; Fogarassy, C. Green Consumer Behaviour in Cosmetic Market. Resources 2019, 8, 137. [Google Scholar] [CrossRef]
- Lazaroiu, G.; Andronie, M.; Uţă, C.; Hurloiu, I. Trust Management in Organic Agriculture: Sustainable Consumption Behavior, Environmentally Conscious Purchase Intention, and Healthy Food Choices. Front. Public Health 2019, 7, 340. [Google Scholar] [CrossRef]
- García-Villegas, A.; Rojas-García, A.; Villegas-Aguilar, M.D.C.; Fernández-Moreno, P.; Fernández-Ochoa, Á.; Cádiz-Gurrea, M.D.L.L.; Arráez-Román, D.; Segura-Carretero, A. Cosmeceutical Potential of Major Tropical and Subtropical Fruit By-Products for a Sustainable Revalorization. Antioxidants 2022, 11, 203. [Google Scholar] [CrossRef]
- Menaa, F.; Menaa, A.; Tréton, J. Polyphenols against Skin Aging. Polyphen. Hum. Health Dis. 2013, 1, 819–830. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Kucharska, E.; Kowalczyk, T.; Zajdel, K.; Cegliński, T.; Zajdel, R. Antioxidant Properties of Plant-Derived Phenolic Compounds and Their Effect on Skin Fibroblast Cells. Antioxidants 2021, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape Seed Extract: Having a Potential Health Benefits. J. Food Sci. Technol. 2020, 57, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Leal, C.; Gouvinhas, I.; Santos, R.A.; Rosa, E.; Silva, A.M.; Saavedra, M.J.; Barros, A.I.R.N.A. Potential Application of Grape (Vitis vinifera L.) Stem Extracts in the Cosmetic and Pharmaceutical Industries: Valorization of a by-Product. Ind. Crops Prod. 2020, 154, 112675. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M. Passion Fruit Seed: Its Antioxidative Extracts and Potency in Protection of Skin Aging, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128186985. [Google Scholar]
- García-Villegas, A.; Fernández-Ochoa, Á.; Rojas-García, A.; Alañón, M.E.; Arráez-Román, D.; Cádiz-Gurrea, M.D.L.L.; Segura-Carretero, A. The Potential of Mangifera indica L. Peel Extract to Be Revalued in Cosmetic Applications. Antioxidants 2023, 12, 1892. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.M.; Gomes, S.M.; Santos, L. A Novel Approach in Skin Care: By-Product Extracts as Natural UV Filters and an Alternative to Synthetic Ones. Molecules 2023, 28, 2037. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.R.; Gonçalves, A.C.; Falcão, A.; Alves, G.; Silva, L.R. Prunus avium L. (Sweet Cherry) by-Products: A Source of Phenolic Compounds with Antioxidant and Anti-Hyperglycemic Properties—A Review. Appl. Sci. 2021, 11, 8516. [Google Scholar] [CrossRef]
- Soares Mateus, A.R.; Pena, A.; Sendón, R.; Almeida, C.; Nieto, G.A.; Khwaldia, K.; Sanches Silva, A. By-Products of Dates, Cherries, Plums and Artichokes: A Source of Valuable Bioactive Compounds. Trends Food Sci. Technol. 2023, 131, 220–243. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Cerdà, V.; Clavijo, S.; Suárez, R.; Mašković, P.; Cvetanović, A.; Delerue-Matos, C.; Carvalho, A.P.; Novakov, V. Bioactive Compounds of Sweet and Sour Cherry Stems Obtained by Subcritical Water Extraction. J. Chem. Technol. Biotechnol. 2018, 93, 1627–1635. [Google Scholar] [CrossRef]
- Nunes, A.R.; Gonçalves, A.C.; Alves, G.; Falcão, A.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L.R. Valorisation of Prunus avium L. By-Products: Phenolic Composition and Effect on Caco-2 Cells Viability. Foods 2021, 10, 1185. [Google Scholar] [CrossRef]
- Peixoto, J.; Álvarez-Rivera, G.; Alves, R.C.; Costa, A.S.G.; Andrade, N.; Moreira, A.; Cifuentes, A.; Martel, F.; Oliveira, M.B.P.P.; Ibáñez, E. Cherry Stem Infusions: Antioxidant Potential and Phenolic Profile by UHPLC-ESI-QTOF-MS. Food Funct. 2020, 11, 3471–3482. [Google Scholar] [CrossRef] [PubMed]
- Demir, T.; Akpınar, Ö.; Kara, H.; Güngör, H. Cherry Stem Phenolic Compounds: Optimization of Extraction Conditions and in Vitro Evaluations of Antioxidant, Antimicrobial, Antidiabetic, Anti-Inflammatory, and Cytotoxic Activities. J. Food Process. Preserv. 2020, 44, e14804. [Google Scholar] [CrossRef]
- Aguilar-Hernández, G.; De Lourdes García-Magaña, M.; De los Ángeles Vivar-Vera, M.; Sáyago-Ayerdi, S.G.; Sánchez-Burgos, J.A.; Morales-Castro, J.; Anaya-Esparza, L.M.; González, E.M. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from Annona Muricata by-Products and Pulp. Molecules 2019, 24, 904. [Google Scholar] [CrossRef] [PubMed]
- Agulló-Chazarra, L.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A.; Micol, V.; Herranz-López, M.; Barrajón-Catalán, E. Sweet Cherry Byproducts Processed by Green Extraction Techniques as a Source of Bioactive Compounds with Antiaging Properties. Antioxidants 2020, 9, 418. [Google Scholar] [CrossRef]
- Nastić, N.; Lozano-Sánchez, J.; Borrás-Linares, I.; Švarc-Gajić, J.; Segura-Carretero, A. New Technological Approaches for Recovering Bioactive Food Constituents from Sweet Cherry (Prunus avium L.) Stems. Phytochem. Anal. 2020, 31, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Chu, M.; Yu, X.; Xie, Y.; Li, Y.; Du, Y.; Liu, X.; Zhang, Z.; Shi, J.; Yan, N. Anthocyanins and Proanthocyanidins: Chemical Structures, Food Sources, Bioactivities, and Product Development. Food Rev. Int. 2023, 39, 4581–4609. [Google Scholar] [CrossRef]
- Nie, F.; Liu, L.; Cui, J.; Zhao, Y.; Zhang, D.; Zhou, D.; Wu, J.; Li, B.; Wang, T.; Li, M.; et al. Oligomeric Proanthocyanidins: An Updated Review of Their Natural Sources, Synthesis, and Potentials. Antioxidants 2023, 12, 1004. [Google Scholar] [CrossRef]
- Afonso, S.; Oliveira, I.V.; Meyer, A.S.; Aires, A.; Saavedra, M.J.; Gonçalves, B. Phenolic Profile and Bioactive Potential of Stems and Seed Kernels of Sweet Cherry Fruit. Antioxidants 2020, 9, 1295. [Google Scholar] [CrossRef]
- Bastos, C.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical Characterisation and Bioactive Properties of Prunus avium L.: The Widely Studied Fruits and the Unexplored Stems. Food Chem. 2015, 173, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Subbiah, V.; Wu, H.; BK, A.; Rauf, A.; Alhumaydhi, F.A.; Suleria, H.A.R. Determination and Characterization of Phenolic Compounds from Australia-Grown Sweet Cherries (Prunus avium L.) and Their Potential Antioxidant Properties. ACS Omega 2021, 6, 34687–34699. [Google Scholar] [CrossRef]
- Babotă, M.; Voştinaru, O.; Păltinean, R.; Mihali, C.; Dias, M.I.; Barros, L.; Ferreira, I.C.F.R.; Mocan, A.; Crişan, O.; Nicula, C.; et al. Chemical Composition, Diuretic, and Antityrosinase Activity of Traditionally Used Romanian Cerasorum Stipites. Front. Pharmacol. 2021, 12, 647947. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic Potential of Flavonoids in Cancer: ROS-Mediated Mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef]
- Perumalla, A.V.S.; Hettiarachchy, N.S. Green Tea and Grape Seed Extracts—Potential Applications in Food Safety and Quality. Food Res. Int. 2011, 44, 827–839. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Troncoso, A.M.; García-Parrilla, M.C. Comparison of Antioxidant Activity of Wine Phenolic Compounds and Metabolites in Vitro. Anal. Chim. Acta 2005, 538, 391–398. [Google Scholar] [CrossRef]
- Bursal, E.; Köksal, E.; Gülçin, I.; Bilsel, G.; Gören, A.C. Antioxidant Activity and Polyphenol Content of Cherry Stem (Cerasus avium L.) Determined by LC-MS/MS. Food Res. Int. 2013, 51, 66–74. [Google Scholar] [CrossRef]
- Ademović, Z.; Hodžić, S.; Halilić Zahirović, Z.; Husejnagić, D.; Džananović, J.; Šarić-Kundalić, B.; Suljagić, J. Phenolic Compounds, Antioxidant and Antimicrobial Properties of the Wild Cherry (Prunus avium L.) Stem. Acta Period. Technol. 2017, 48, 1–13. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative Stress in the Skin: Impact and Related Protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Sánchez-Jiménez, F. Antioxidant enzymes and their implications in pathophysiologic processes José M. Matés and Francisca Sánchez-Jiménez. Front. Biosci. 2000, 4, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.B.; Berto, A.; Chisté, R.C.; Freitas, M.; Visentainer, J.V.; Fernandes, E. Bioactive Compounds and Scavenging Capacity of Extracts from Different Parts of Vismia Cauliflora against Reactive Oxygen and Nitrogen Species. Pharm. Biol. 2015, 53, 1267–1276. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation Events and Skin Aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Tsuruta, D. What Are Reactive Oxygen Species, Free Radicals, and Oxidative Stress in Skin Diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gülçin, I.; Alwasel, S.H. Antioxidant Activity of Taxifolin: An Activity-Structure Relationship. J. Enzyme Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Janeiro, P.; Corduneanu, O.; Brett, A.M.O. Chrysin and (±)-Taxifolin Electrochemical Oxidation Mechanisms. Electroanalysis 2005, 17, 1059–1064. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant Properties of Catechins: Comparison with Other Antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Zorić, M.; Banožić, M.; Aladić, K.; Vladimir-Knežević, S.; Jokić, S. Supercritical CO2 Extracts in Cosmetic Industry: Current Status and Future Perspectives. Sustain. Chem. Pharm. 2022, 27, 100688. [Google Scholar] [CrossRef]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed]
- Domaszewska-Szostek, A.; Puzianowska-Kuźnicka, M.; Kuryłowicz, A. Flavonoids in Skin Senescence Prevention and Treatment. Int. J. Mol. Sci. 2021, 22, 6814. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Silva, T.C.C.; Caviglione, C.V.; Bottura, C.; Fonseca, M.J.V.; Vicentini, F.T.M.C.; Vignoli, J.A.; Baracat, M.M.; et al. Topical Formulation Containing Naringenin: Efficacy against Ultraviolet B Irradiation-Induced Skin Inflammation and Oxidative Stress in Mice. PLoS ONE 2016, 11, e0146296. [Google Scholar] [CrossRef]
- Lim, K.H.; Kim, G.R. Inhibitory Effect of Naringenin on LPS-Induced Skin Senescence by SIRT1 Regulation in HDFs. Biomed. Dermatology 2018, 2, 26. [Google Scholar] [CrossRef]
- Ding, M.; Zhao, J.; Bowman, L.; Lu, Y.; Shi, X. Inhibition of AP-1 and MAPK Signaling and Activation of Nrf2/ARE Pathway by Quercitrin. Int. J. Oncol. 2009, 36, 547–557. [Google Scholar] [CrossRef]
- Kee, H.M.; Anuar, A.; Samat, A.F.; Salled, N.H.M.; Mohamed, A.R. Optimization Studies by Ultrasound Assisted Solvent Extraction and Screening of Phenolic Compounds in Papaya Seeds. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 8, 8–16. [Google Scholar]
- Liu, C.; Liu, H.; Lu, C.; Deng, J.; Yan, Y.; Chen, H.; Wang, Y.; Liang, C.-L.; Wei, J.; Han, L.; et al. Kaempferol Attenuates Imiquimod-Induced Psoriatic Skin Inflammation in a Mouse Model. Clin. Exp. Immunol. 2019, 198, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.R.; Flores-Félix, J.D.; Gonçalves, A.C.; Falcão, A.; Alves, G.; Silva, L.R. Anti-Inflammatory and Antimicrobial Activities of Portuguese Prunus avium L. (Sweet Cherry) By-Products Extracts. Nutrients 2022, 14, 4576. [Google Scholar] [CrossRef] [PubMed]
- Jesus, F.; Gonçalves, A.C.; Alves, G.; Silva, L.R. Exploring the Phenolic Profile, Antioxidant, Antidiabetic and Anti-Hemolytic Potential of Prunus avium Vegetal Parts. Food Res. Int. 2019, 116, 600–610. [Google Scholar] [CrossRef]
- Christabel, P.H.; Nishaa, S.; Vishnupriya, M.; Sasikumar, J.M.; Gopalakrishnan, V.K. In Vitro Antioxidant Studies and the Scavenging Potential of Pulp and Peel of Prunus Persica i. Fruit on Different Solvent Systems. World J. Pharm. Res. 2012, 1, 1371–1386. [Google Scholar]
- Morabbi Najafabad, A.; Jamei, R. Free Radical Scavenging Capacity and Antioxidant Activity of Methanolic and Ethanolic Extracts of Plum (Prunus domestica L.) in Both Fresh and Dried Samples. Avicenna J. Phytomed. 2014, 4, 343–353. [Google Scholar]
- Dhingra, N.; Sharma, R.; Kar, A. Evaluation of the Antioxidant Activities of Prunus Domestica Whole Fruit: An in Vitro Study. Int. J. Pharm. Pharm. Sci. 2014, 6, 271–276. [Google Scholar]
- Cole, M.A.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Extracellular Matrix Regulation of Fibroblast Function: Redefining Our Perspective on Skin Aging. J. Cell Commun. Signal. 2018, 12, 35–43. [Google Scholar] [CrossRef]
- Philips, N.; Auler, S.; Hugo, R.; Gonzalez, S. Beneficial Regulation of Matrix Metalloproteinases for Skin Health. Enzyme Res. 2011, 2011, 427285. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet Radiation and Skin Aging: Roles of Reactive Oxygen Species, Inflammation and Protease Activation, and Strategies for Prevention of Inflammation-induced Matrix Degradation—A Review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Ochocka, R.; Hering, A.; Stefanowicz-Hajduk, J.; Cal, K.; Barańska, H. The Effect of Mangiferin on Skin: Penetration, Permeation and Inhibition of ECM Enzymes. PLoS ONE 2017, 12, e0181542. [Google Scholar] [CrossRef] [PubMed]
- Liyanaarachchi, G.D.; Samarasekera, J.K.R.R.; Mahanama, K.R.R.; Hemalal, K.D.P. Tyrosinase, Elastase, Hyaluronidase, Inhibitory and Antioxidant Activity of Sri Lankan Medicinal Plants for Novel Cosmeceuticals. Ind. Crops Prod. 2018, 111, 597–605. [Google Scholar] [CrossRef]
- Younis, M.M.; Ayoub, I.M.; Mostafa, N.M.; El Hassab, M.A.; Eldehna, W.M.; Al-Rashood, S.T.; Eldahshan, O.A. GC/MS Profiling, Anti-Collagenase, Anti-Elastase, Anti-Tyrosinase and Anti-Hyaluronidase Activities of a Stenocarpus Sinuatus Leaves Extract. Plants 2022, 11, 918. [Google Scholar] [CrossRef]
- Polito, L.; Bortolotti, M.; Battelli, M.G.; Bolognesi, A. Xanthine Oxidoreductase: A Leading Actor in Cardiovascular Disease Drama. Redox Biol. 2021, 48, 102195. [Google Scholar] [CrossRef] [PubMed]
- Kostić, D.A.; Dimitrijević, D.S.; Stojanović, G.S.; Palić, I.R.; Dordević, A.S.; Ickovski, J.D. Xanthine Oxidase: Isolation, Assays of Activity, and Inhibition. J. Chem. 2015, 2015, 294858. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as Active Ingredients for Cosmetic Products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Pessini, A.C.; Takao, T.T.; Cavalheiro, E.C.; Vichnewski, W.; Sampaio, S.V.; Giglio, J.R.; Arantes, E.C. A Hyaluronidase from Tityus Serrulatus Scorpion Venom: Isolation, Characterization and Inhibition by Flavonoids. Toxicon 2001, 39, 1495–1504. [Google Scholar] [CrossRef]
- Li, X.; Xu, R.; Cheng, Z.; Song, Z.; Wang, Z.; Duan, H.; Wu, X.; Ni, T. Comparative Study on the Interaction between Flavonoids with Different Core Structures and Hyaluronidase. Spectrochim. Acta Part A Espectroscopía Mol. Biomol. 2021, 262, 120079. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, G.H. Evaluation of Antioxidant and Inhibitory Activities for Different Subclasses Flavonoids on Enzymes for Rheumatoid Arthritis. J. Food Sci. 2010, 75, H212–H217. [Google Scholar] [CrossRef] [PubMed]
- Ao, C.; Higa, T.; Ming, H.; Ding, Y.T.; Tawata, S. Isolation and Identification of Antioxidant and Hyaluronidase Inhibitory Compounds from Ficus microcarpa L. Fil. Bark. J. Enzyme Inhib. Med. Chem. 2010, 25, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, L.; Ren, L.; Xie, Y. Advances in Structures Required of Polyphenols for Xanthine Oxidase Inhibition. Food Front. 2020, 1, 152–167. [Google Scholar] [CrossRef]
- Madhan, B.; Krishnamoorthy, G.; Rao, J.R.; Nair, B.U. Role of Green Tea Polyphenols in the Inhibition of Collagenolytic Activity by Collagenase. Int. J. Biol. Macromol. 2007, 41, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, Anti-Collagenase and Anti-Elastase Activities of Phyllanthus Emblica, Manilkara Zapota and Silymarin: An in Vitro Comparative Study for Anti-Aging Applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Antognoni, F.; Lianza, M.; Poli, F.; Buccioni, M.; Santinelli, C.; Caprioli, G.; Iannarelli, R.; Lupidi, G.; Maggi, F.; Damiani, E.; et al. Polar Extracts from the Berry-like Fruits of Hypericum androsaemum L. as a Promising Ingredient in Skin Care Formulations. J. Ethnopharmacol. 2017, 195, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Cho, S.H.; Park, D.; Jung, E. Anti-skin Aging Properties of Protocatechuic Acid in Vitro and in Vivo. J. Cosmet. Dermatol. 2020, 19, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yu, J.S.; Phung, H.M.; Lee, J.G.; Kim, K.H.; Kang, K.S. Potential Anti-Skin Aging Effect of (-)-Catechin Isolated from the Root Bark of Ulmus Davidiana Var. Japonica in Tumor Necrosis Factor-α-Stimulated Normal Human Dermal Fibroblasts. Antioxidants 2020, 9, 981. [Google Scholar] [CrossRef] [PubMed]
- Pluemsamran, T.; Onkoksoong, T.; Panich, U. Caffeic Acid and Ferulic Acid Inhibit UVA-Induced Matrix Metalloproteinase-1 through Regulation of Antioxidant Defense System in Keratinocyte HaCaT Cells. Photochem. Photobiol. 2012, 88, 961–968. [Google Scholar] [CrossRef]
- Ferreyra, S.; Bottini, R.; Fontana, A. Temperature and Light Conditions Affect Stability of Phenolic Compounds of Stored Grape Cane Extracts. Food Chem. 2023, 405, 134718. [Google Scholar] [CrossRef]
- Ghafoor, K.; Ahmed, I.A.M.; Doǧu, S.; Uslu, N.; Gbemisola Jamiu, F.; Al Juhaimi, F.; Babiker, E.E.; Özcan, M.M. The Effect of Heating Temperature on Total Phenolic Content, Antioxidant Activity, and Phenolic Compounds of Plum and Mahaleb Fruits. Int. J. Food Eng. 2019, 15, 20170302. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.; Wen, R.H. Kinetic Study of the Thermal Stability of Tea Catechins in Aqueous Systems Using a Microwave Reactor. J. Agric. Food Chem. 2006, 54, 5924–5932. [Google Scholar] [CrossRef]
- Ali, S.M.; Yosipovitch, G. Skin PH: From Basic Science to Basic Skin Care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef]
- Proksch, E. PH in Nature, Humans and Skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural Skin Surface PH Is on Average below 5, Which Is Beneficial for Its Resident Flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef]
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Cosmetics Preservation: A Review on Present Strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef]
- Morávková, T.; Stern, P. Rheological and Textural Properties of Cosmetic Emulsions. Appl. Rheol. 2011, 21, 35200. [Google Scholar] [CrossRef]
- Gaspar, L.R.; Maia Campos, P.M.B.G. Rheological Behavior and the SPF of Sunscreens. Int. J. Pharm. 2003, 250, 35–44. [Google Scholar] [CrossRef]
- Martinez, R.M.; Rosado, C.; Velasco, M.V.R.; Lannes, S.C.S.; Baby, A.R. Main Features and Applications of Organogels in Cosmetics. Int. J. Cosmet. Sci. 2019, 41, 109–117. [Google Scholar] [CrossRef]
- Campanero, M.A. Evaluación De La Estabilidad De Productos Cosméticos: Necesidad Y Procedimiento. Ind. Cosmét. 2019, 10, 48–52. [Google Scholar]
- Lupi, F.R.; Gabriele, D.; Seta, L.; Baldino, N.; de Cindio, B.; Marino, R. Rheological Investigation of Pectin-Based Emulsion Gels for Pharmaceutical and Cosmetic Uses. Rheol. Acta 2015, 54, 41–52. [Google Scholar] [CrossRef]
- Rathod, H.J.; Mehta, D.P. Acta Scientifica International Journal of Pharmaceutical Science. Int. J. Pharm. Sci. 2015, 1, 33–47. [Google Scholar]
- Margarida Silva, A.; Costa, P.C.; Delerue-matos, C.; Rodrigues, F. Mathematical Development and in Vivo Safety and Efficacy Studies of a Topical Formulation Containing Actinidia Arguta Leaves Extract. J. Drug Deliv. Sci. Technol. 2023, 84, 104547. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Gómez-Plaza, E.; Martínez, A.; López-Roca, J.M. Evolution of the CIELAB and Other Spectrophotometric Parameters during Wine Fermentation. Influence of Some Pre and Postfermentative Factors. Food Res. Int. 1997, 30, 699–705. [Google Scholar] [CrossRef]
- Zheng, H.; Lu, H. A Least-Squares Support Vector Machine (LS-SVM) Based on Fractal Analysis and CIELab Parameters for the Detection of Browning Degree on Mango (Mangifera indica L.). Comput. Electron. Agric. 2012, 83, 47–51. [Google Scholar] [CrossRef]
- Liang, Z.; Sang, M.; Fan, P.; Wu, B.; Wang, L.; Yang, S.; Li, S. CIELAB Coordinates in Response to Berry Skin Anthocyanins and Their Composition in Vitis. J. Food Sci. 2011, 76, C490–C497. [Google Scholar] [CrossRef] [PubMed]
- ONUDI. Recomendaciones para el Desarrollo de Estudios de Estabilidad de Productos Cosméticos; Giraldo, H.J.M., Sánchez, M.F., Hernandez, G.N., Eds.; ONUDI: Bogotá, Colombia, 2018; ISBN 9789585985131. [Google Scholar]
- Guaratini, T.; Gianeti, M.D.; Campos, P.M.B.G.M. Stability of Cosmetic Formulations Containing Esters of Vitamins E and A: Chemical and Physical Aspects. Int. J. Pharm. 2006, 327, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Sterry, W.; Lademann, J. Resonance Raman Spectroscopy as an Effective Tool for the Determination of Antioxidative Stability of Cosmetic Formulations. J. Biophotonics 2010, 3, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Dao, H.; Lakhani, P.; Police, A.; Kallakunta, V.; Ajjarapu, S.S.; Wu, K.W.; Ponkshe, P.; Repka, M.A.; Narasimha Murthy, S. Microbial Stability of Pharmaceutical and Cosmetic Products. AAPS PharmSciTech 2018, 19, 60–78. [Google Scholar] [CrossRef]
- Boger, D.V. Demonstration of Upper and Lower Newtonian Fluid Behaviour in a Pseudoplastic Fluid. Nature 1977, 265, 126–128. [Google Scholar] [CrossRef]
- Gore, E.; Picard, C.; Savary, G. Spreading Behavior of Cosmetic Emulsions: Impact of the Oil Phase. Biotribology 2018, 16, 17–24. [Google Scholar] [CrossRef]
- Yang, X.H.; Deng, L.Z.; Mujumdar, A.S.; Xiao, H.W.; Zhang, Q.; Kan, Z. Evolution and Modeling of Colour Changes of Red Pepper (Capsicum annuum L.) during Hot Air Drying. J. Food Eng. 2018, 231, 101–108. [Google Scholar] [CrossRef]
- Sharma, K.; Ko, E.Y.; Assefa, A.D.; Ha, S.; Nile, S.H.; Lee, E.T.; Park, S.W. Temperature-Dependent Studies on the Total Phenolics, Flavonoids, Antioxidant Activities, and Sugar Content in Six Onion Varieties. J. Food Drug Anal. 2015, 23, 243–252. [Google Scholar] [CrossRef]
- Vieira, R.P.; Fernandes, A.R.; Kaneko, T.M.; Consiglieri, V.O.; Pinto, C.A.S.D.O.; Pereira, C.S.C.; Baby, A.R.; Velasco, M.V.R. Physical and Physicochemical Stability Evaluation of Cosmetic Formulations Containing Soybean Extract Fermented by Bifidobacterium Animalis. Braz. J. Pharm. Sci. 2009, 45, 515–525. [Google Scholar] [CrossRef][Green Version]
- Ferreira, S.M.; Falé, Z.; Santos, L. Sustainability in Skin Care: Incorporation of Avocado Peel Extracts in Topical Formulations. Molecules 2022, 27, 1782. [Google Scholar] [CrossRef] [PubMed]
- Huma, S.; Khan, H.M.S.; Sohail, M.; Akhtar, N.; Rasool, F.; Majeed, F.; Daniyal, M. Development, in-Vitro Characterization and Assessment of Cosmetic Potential of Beta Vulgaris Extract Emulsion. J. Herb. Med. 2020, 23, 100372. [Google Scholar] [CrossRef]
- Rodrigues, F.; Gaspar, C.; Palmeira-De-Oliveira, A.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B.P.P. Application of Coffee Silverskin in Cosmetic Formulations: Physical/Antioxidant Stability Studies and Cytotoxicity Effects. Drug Dev. Ind. Pharm. 2016, 42, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B.P.P. Exploring the Antioxidant Potentiality of Two Food By-Products into a Topical Cream: Stability, in Vitro and in Vivo Evaluation. Drug Dev. Ind. Pharm. 2016, 42, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Al-Duais, M.; Müller, L.; Böhm, V.; Jetschke, G. Antioxidant Capacity and Total Phenolics of Cyphostemma Digitatum before and after Processing: Use of Different Assays. Eur. Food Res. Technol. 2009, 228, 813–821. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- De La Luz Cádiz-Gurrea, M.; Fernández-Ochoa, Á.; Leyva-Jiménez, F.J.; Guerrero-Muñoz, N.; Del Carmen Villegas-Aguilar, M.; Pimentel-Moral, S.; Ramos-Escudero, F.; Segura-Carretero, A. LC-MS and Spectrophotometric Approaches for Evaluation of Bioactive Compounds from Peru Cocoa by-Products for Commercial Applications. Molecules 2020, 25, 3177. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-evans, C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Silva, A.M.S.; Santos, C.M.M.; Pinto, D.C.G.A.; Cavaleiro, J.A.S.; Lima, J.L.F.C. 2-Styrylchromones: Novel Strong Scavengers of Reactive Oxygen and Nitrogen Species. Bioorg. Med. Chem. 2007, 15, 6027–6036. [Google Scholar] [CrossRef] [PubMed]
- Nema, N.K.; Maity, N.; Sarkar, B.K.; Mukherjee, P.K. Matrix Metalloproteinase, Hyaluronidase and Elastase Inhibitory Potential of Standardized Extract of Centella Asiatica. Pharm. Biol. 2013, 51, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Nema, N.K.; Maity, N.; Sarkar, B.; Mukherjee, P.K. Cucumis Sativus Fruit-Potential Antioxidant, Anti-Hyaluronidase, and Anti-Elastase Agent. Arch. Dermatol. Res. 2011, 303, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Cádiz-Gurrea, M.D.L.L.; Garcia, J.; Saavedra, M.J.; Freitas, V.; Costa, P.; Sarmento, B.; Delerue-Matos, C.; Rodrigues, F. From Soil to Cosmetic Industry: Validation of a New Cosmetic Ingredient Extracted from Chestnut Shells. Sustain. Mater. Technol. 2021, 29, e00309. [Google Scholar] [CrossRef]
- Berthele, H.; Sella, O.; Lavarde, M.; Mielcarek, C.; Pense-Lheritier, A.M.; Pirnay, S. Determination of the Influence of Factors (Ethanol, PH and a w) on the Preservation of Cosmetics Using Experimental Design. Int. J. Cosmet. Sci. 2014, 36, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ghalleb, S.; De Vaugelade, S.; Sella, O.; Lavarde, M.; Mielcarek, C.; Pense-Lheritier, A.M.; Pirnay, S. Predictive Microbiology for Cosmetics Based on Physicals, Chemicals and Concentration Parameters. Int. J. Cosmet. Sci. 2015, 37, 70–75. [Google Scholar] [CrossRef]
- ISO/TR 18811; Cosmetics-Guidelines on the Stability Testing of Cosmetic Products. International Organization for Standardization: Geneva, Switzerland, 2018.
- Lee, S.H.; Chow, P.S.; Yagnik, C.K. Developing Eco-Friendly Skin Care Formulations with Microemulsions of Essential Oil. Cosmetics 2022, 9, 30. [Google Scholar] [CrossRef]



| Methodology | CS Extract | EPI | GA | KA | ELA | PHE |
|---|---|---|---|---|---|---|
| TPC (mg GAE/g DE) | 131.4 ± 0.4 | - | - | - | - | - |
| FRAP (mmol FeSO4/g DE) | 0.96 ± 0.01 | - | - | - | - | - |
| TEAC (μmol TE/g DE) | 1005.5 ± 0.2 | - | - | - | - | - |
| ORAC (mmol TE/g DE) | 2347 ± 0 | - | - | - | - | - |
| O2·− (mg/L) 1 | 198 ± 6 | 70 ± 5 | 50 ± 3 | - | - | - |
| NO· (mg/L) 1 | 2.3 ± 0.4 | 0.87 ± 0.02 | 1.4 ± 0.3 | - | - | - |
| HOCl (mg/L) 1 | 5.4 ± 0.5 | 0.18 ± 0.01 | 3.8 ± 0.3 | - | - | - |
| Tyrosinase | 690 ± 30 2 | - | - | 49 ± 6 3 | - | - |
| Elastase | 537 ± 16 1 | - | - | - | 53 ± 5 4 | - |
| HYALase (mg/L) | 7.39 ± 0.06 1 | 167 ± 6 5 | 102 ± 4 5 | - | - | - |
| Collagenase (mg/L) 1 | 112 ± 8 | - | - | - | - | 83 ± 2 |
| XOD (mg/L) 1 | 10 ± 1 | 9 ± 1 | - | - | - | - |
| Parameters | 0% | 0.5% | 1% | 2% |
|---|---|---|---|---|
| Viscosity (Pa·s) | ||||
| 10 rpm | 8.40 ± 0.00 | 9.00 ± 0.00 | 11.58 ± 0.03 | 15.30 ± 0.00 |
| 70 rpm | 1.67 ± 0.01 | 1.81 ± 0.00 | 2.21 ± 0.01 | 2.76 ± 0.00 |
| Colour | ||||
| L* | 25.73 ± 0.02 | 24.76 ± 0.04 | 24.67 ± 0.01 | 24.65 ± 0.02 |
| a* | −0.58 ± 0.01 | −0.46 ± 0.01 | −0.08 ± 0.02 | −0.08 ± 0.02 |
| b* | 0.88 ± 0.06 | 5.52 ± 0.04 | 5.64 ± 0.03 | 6.01 ± 0.02 |
| pH | 5.92 ± 0.01 | 5.37 ± 0.01 | 4.97 ± 0.01 | 4.66 ± 0.01 |
| TPC (mg GA/g DE) | 0 | 0.47 ± 0.01 | 1.04 ± 0.23 | 2.05 ± 0.07 |
| Commercial Name | INCI Name | CAS Code | Function | % |
|---|---|---|---|---|
| Demineralized water | Water | 7732-18-5 | Aqueous phase | 89.97, 89.47, 88.97, 87.97 |
| Glycerine | Glycerine | 56-81-5 | Humectant | 5 |
| Vegetal extract | - | - | Active ingredient | 0, 0.5, 1, 2 |
| Xanthan gum | Xanthan gum | 11138-66-2 | Emulsifier/Gelling agent | 2 |
| Ethanol | Alcohol | 64-17-5 | Solvent/Preservative | 3 |
| Citric acid | Citric acid | 77-92-9/5949-29-1 | pH regulator | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Villegas, A.; Fernández-Ochoa, Á.; Alañón, M.E.; Rojas-García, A.; Arráez-Román, D.; Cádiz-Gurrea, M.d.l.L.; Segura-Carretero, A. Bioactive Compounds and Potential Health Benefits through Cosmetic Applications of Cherry Stem Extract. Int. J. Mol. Sci. 2024, 25, 3723. https://doi.org/10.3390/ijms25073723
García-Villegas A, Fernández-Ochoa Á, Alañón ME, Rojas-García A, Arráez-Román D, Cádiz-Gurrea MdlL, Segura-Carretero A. Bioactive Compounds and Potential Health Benefits through Cosmetic Applications of Cherry Stem Extract. International Journal of Molecular Sciences. 2024; 25(7):3723. https://doi.org/10.3390/ijms25073723
Chicago/Turabian StyleGarcía-Villegas, Abigail, Álvaro Fernández-Ochoa, María Elena Alañón, Alejandro Rojas-García, David Arráez-Román, María de la Luz Cádiz-Gurrea, and Antonio Segura-Carretero. 2024. "Bioactive Compounds and Potential Health Benefits through Cosmetic Applications of Cherry Stem Extract" International Journal of Molecular Sciences 25, no. 7: 3723. https://doi.org/10.3390/ijms25073723
APA StyleGarcía-Villegas, A., Fernández-Ochoa, Á., Alañón, M. E., Rojas-García, A., Arráez-Román, D., Cádiz-Gurrea, M. d. l. L., & Segura-Carretero, A. (2024). Bioactive Compounds and Potential Health Benefits through Cosmetic Applications of Cherry Stem Extract. International Journal of Molecular Sciences, 25(7), 3723. https://doi.org/10.3390/ijms25073723











