Cryptomphalus aspersa Egg Extract Protects against Human Stem Cell Stress-Induced Premature Senescence
Abstract
1. Introduction
2. Results
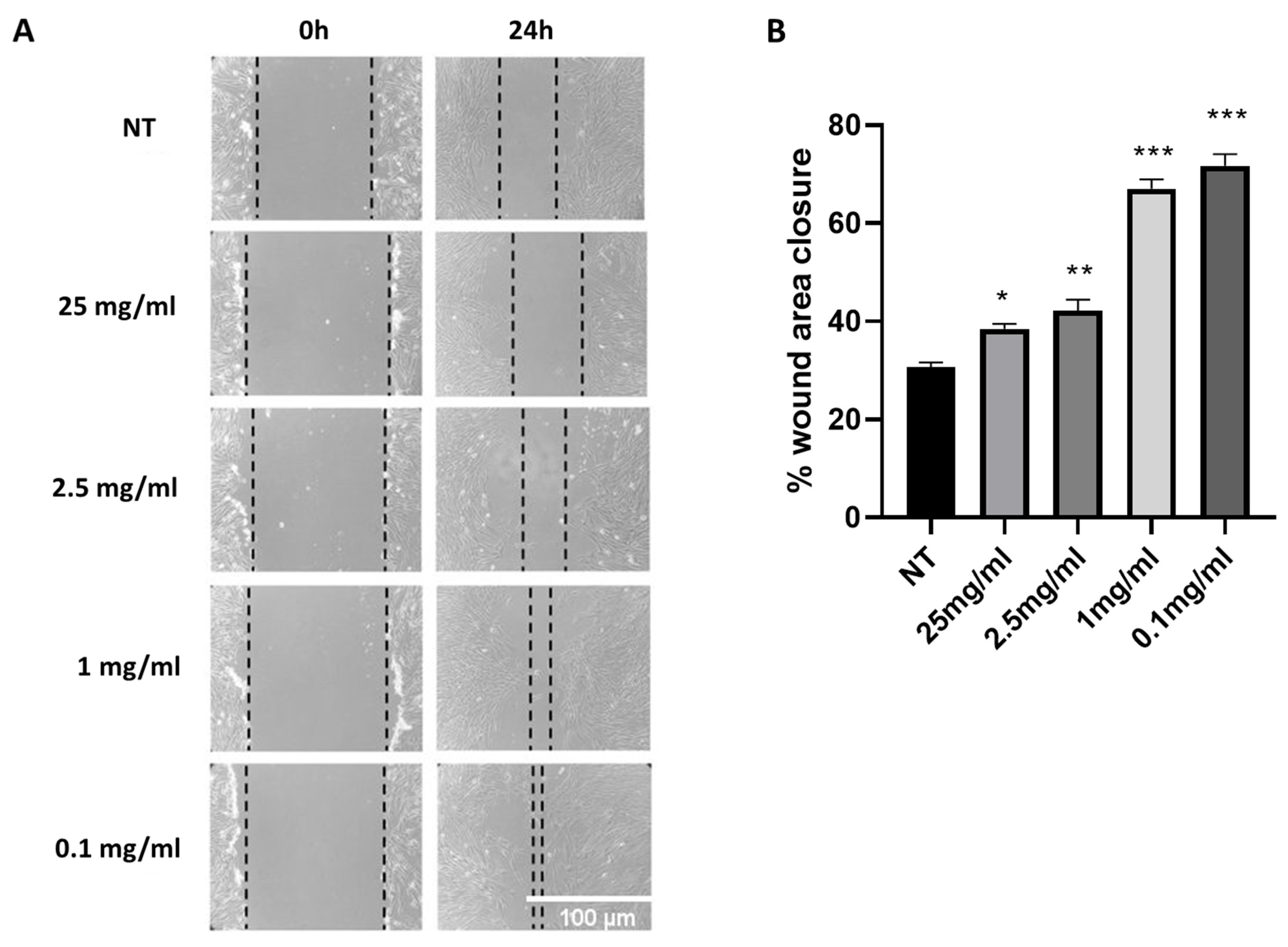
2.1. Cryptomphalus Aspersa Egg Extract (CAEE) Accelerates Wound Healing
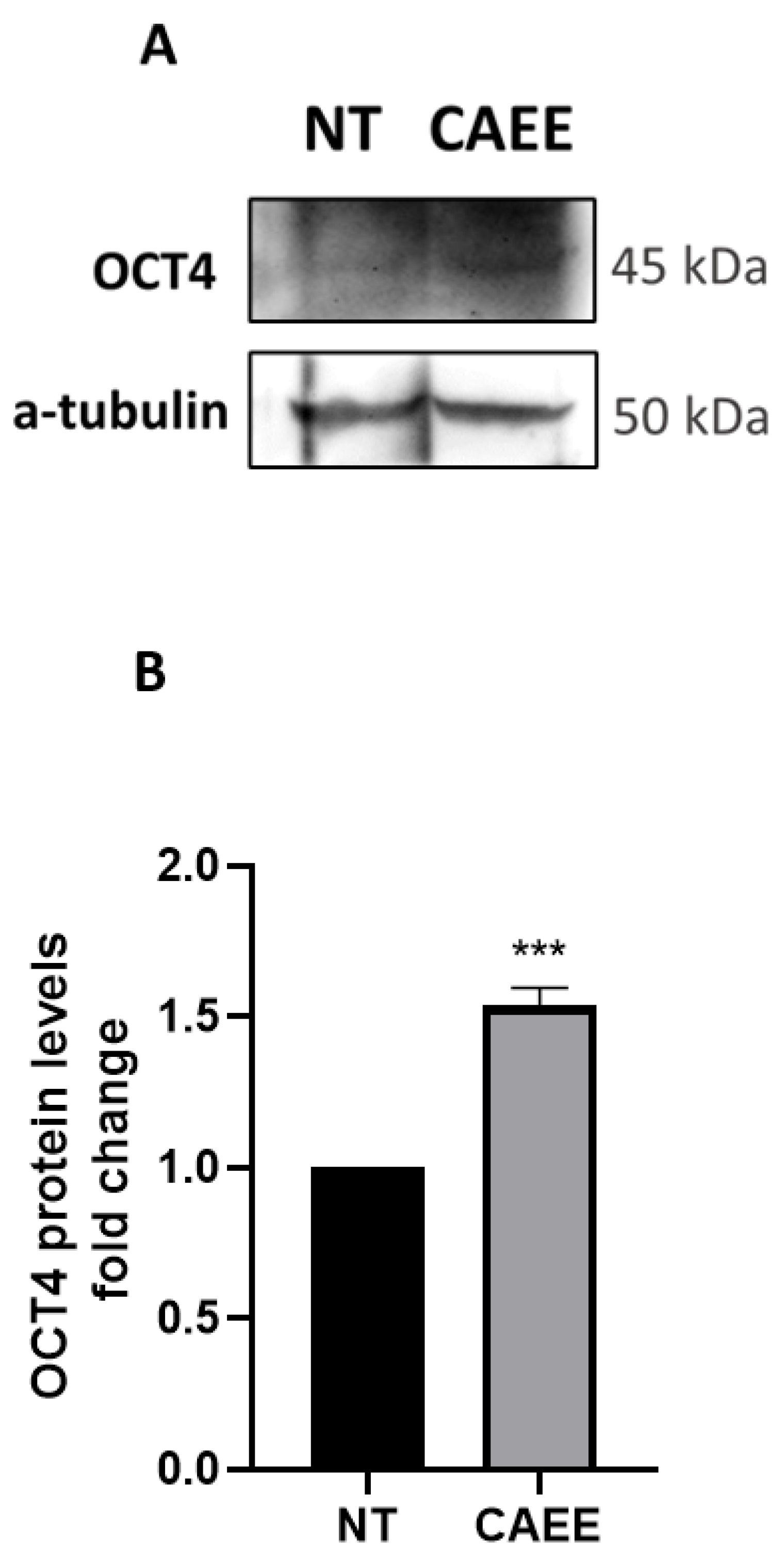
2.2. CAEE Increases the Expression of Stemness Regulator OCT4
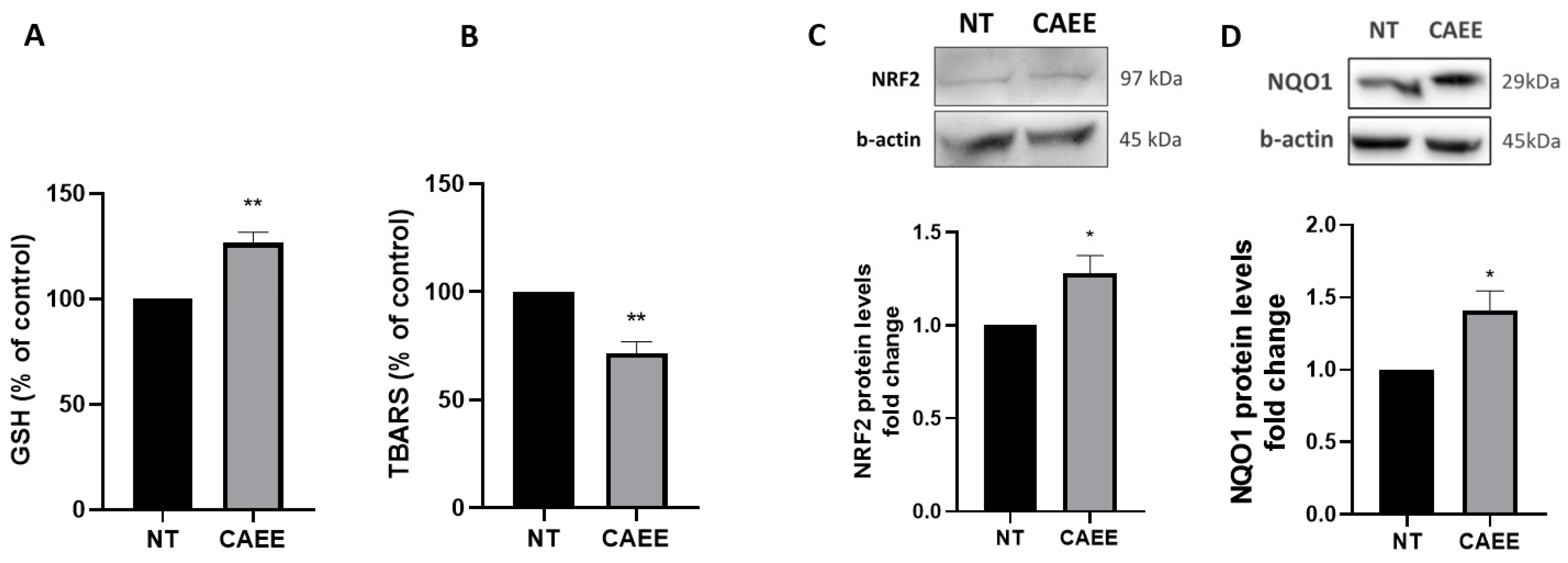
2.3. CAEE Augments the Antioxidant Potential of WJ-MSCs
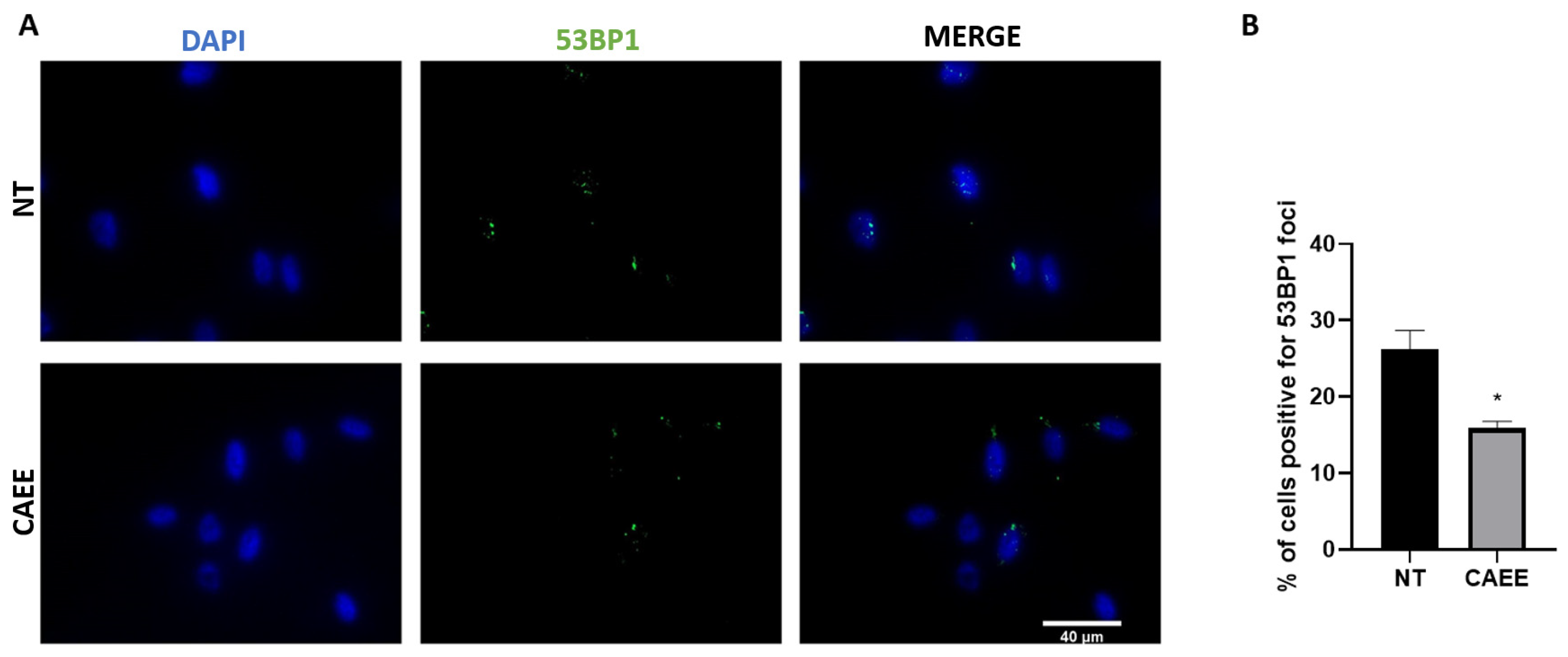
2.4. CAEE Promotes DNA Damage Repair
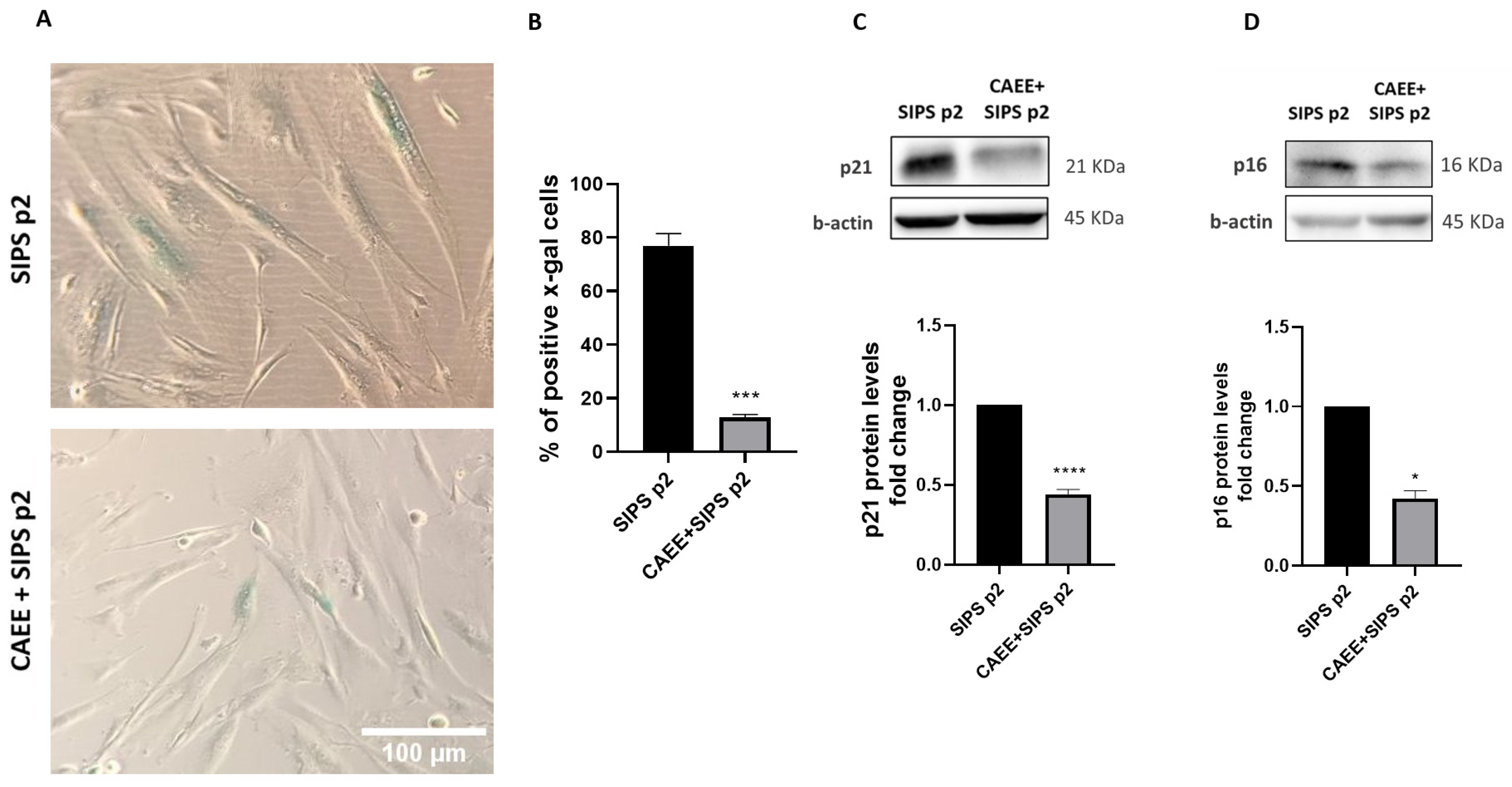
2.5. CAEE Protects against Stress-Induced Premature Senescence (SIPS)
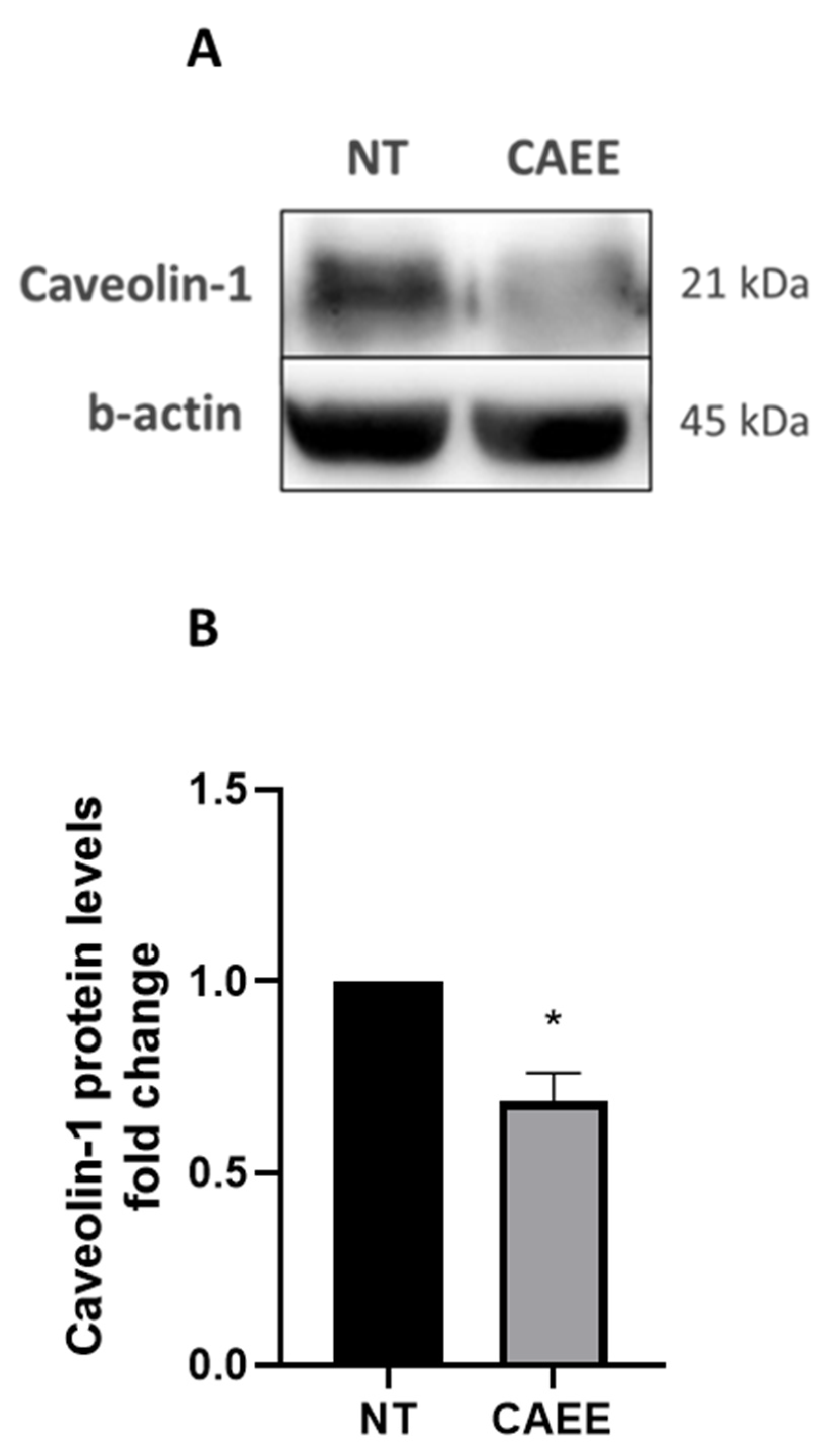
2.6. CAEE Decreased Caveolin-1 Protein Levels
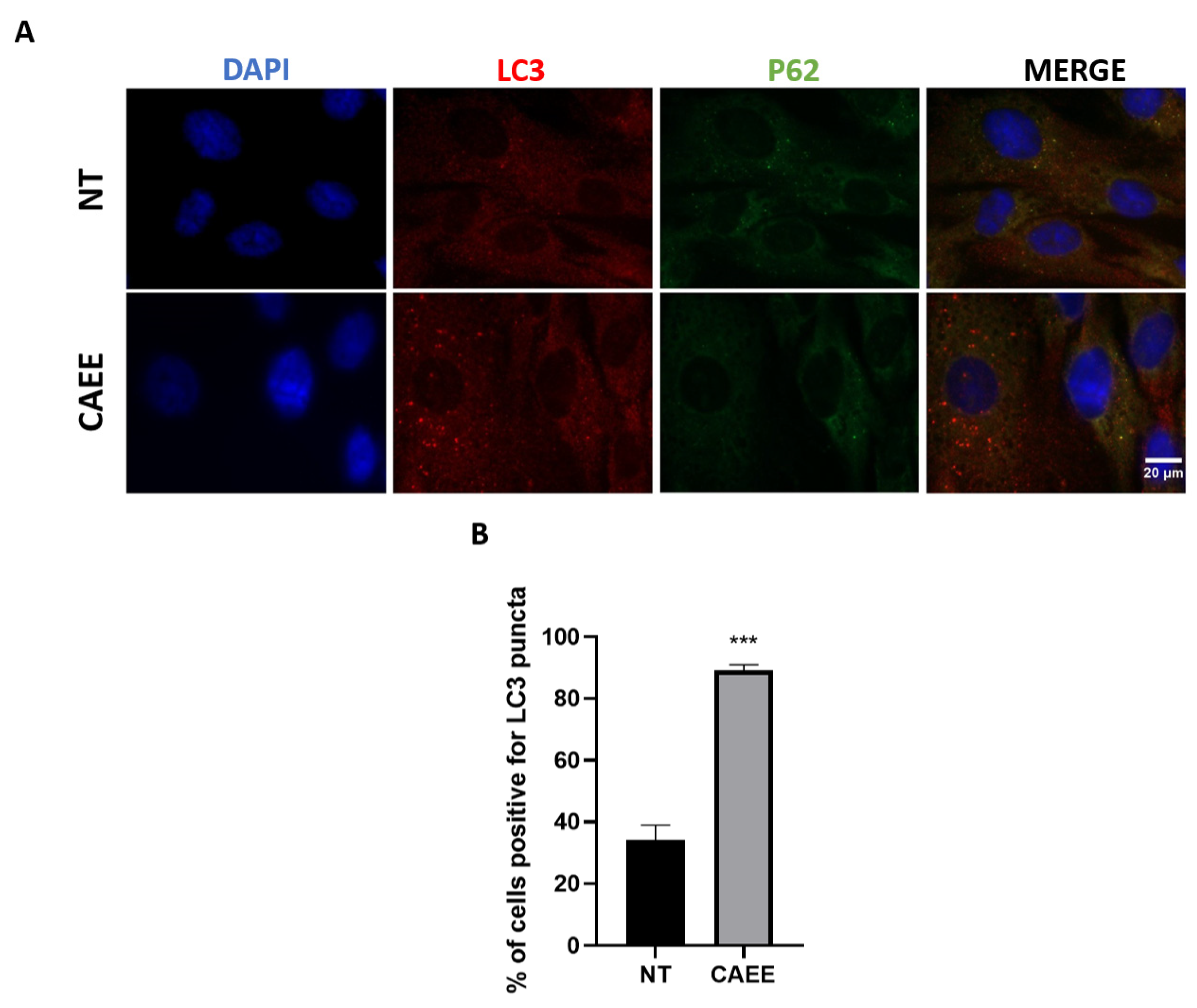
2.7. CAEE Protective Effect against SIPS Is Associated with Autophagy Induction
3. Discussion
4. Material and Methods
4.1. Primary Cultures of Mesenchymal Stem Cells (MSCs)
4.2. Animal Farming and Preparation of the Egg Extracts
4.3. Stress-Induced Premature Senescence (SIPS)
4.4. Protein Extraction and Western Blot Analyses
4.5. Senescence-Associated β-Galactosidase (SA-β-Gal) Staining
4.6. Immunofluorescence (IF)
4.7. Cell-Migration Assay
4.8. Determination of GSH Levels
4.9. TBARS Assay for the Determination of Lipid Peroxidation
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turinetto, V.; Vitale, E.; Giachino, C. Senescence in human mesenchymal stem cells: Functional changes and implications in stem cell-based therapy. Int. J. Mol. Sci. 2016, 17, 1164. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhyan, R.K.; Latsinik, N.V.; Panasyvk, A.F.; Keiliss-Borok, I.V. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues: Cloning in vitro and retransplantation in vivo. Transplantation 1974, 17, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Asakura, A.; Komaki, M.; Rudnicki, M.A. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation 2001, 68, 245–253. [Google Scholar] [CrossRef]
- Pierdomenico, L.; Bonsi, L.; Calvitti, M.; Rondelli, D.; Arpinati, M.; Chirumbolo, G.; Becchetti, E.; Marchionni, C.; Alviano, F.; Fossati, V.; et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 2005, 80, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Marino, L.; Castaldi, M.A.; Rosamilio, R.; Ragni, E.; Vitolo, R.; Fulgione, C.; Castaldi, S.G.; Serio, B.; Bianco, R.; Guida, M.; et al. Mesenchymal stem cells from the Wharton’s jelly of the human umbilical cord: Biological properties and therapeutic potential. Int. J. Stem Cells 2019, 12, 218–226. [Google Scholar] [CrossRef]
- McElreavey, K.D.; Irvine, A.I.; Ennis, K.T.; McLean, W.H.I. Isolation, culture and characterisation of fibroblast-like cells derived from the Wharton’s jelly portion of human umbilical cord. In Biochemical Society Transactions; Portland Press: London, UK, 1991; Volume 19, p. 29S. [Google Scholar]
- Ding, D.C.; Chang, Y.H.; Shyu, W.C.; Lin, S.Z. Human umbilical cord mesenchymal stem cells: A new era for stem cell therapy. Cell Transplant. 2015, 24, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ding, Y.; Liu, Z.; Liang, X. Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms, and Rejuvenation Strategies. Front. Cell Dev. Biol. 2020, 8, 258. [Google Scholar] [CrossRef]
- Denu, R.A.; Hematti, P. Effects of Oxidative Stress on Mesenchymal Stem Cell Biology. Oxid. Med. Cell. Longev. 2016, 2016, 2989076. [Google Scholar] [CrossRef]
- Choo, K.B.; Tai, L.; Hymavathee, K.S.; Wong, C.Y.; Nguyen, P.N.N.; Huang, C.J.; Cheong, S.K.; Kamarul, T. Oxidative stress-induced premature senescence in Wharton’s jelly-derived mesenchymal stem cells. Int. J. Med. Sci. 2014, 11, 1201–1207. [Google Scholar] [CrossRef]
- Vono, R.; Jover Garcia, E.; Spinetti, G.; Madeddu, P. Oxidative Stress in Mesenchymal Stem Cell Senescence: Regulation by Coding and Noncoding RNAs. Antioxid. Redox Signal. 2018, 29, 864–879. [Google Scholar] [CrossRef]
- Varesi, A.; Chirumbolo, S.; Campagnoli, L.I.M.; Pierella, E.; Piccini, G.B.; Carrara, A.; Ricevuti, G.; Scassellati, C.; Bonvicini, C.; Pascale, A. The Role of Antioxidants in the Interplay between Oxidative Stress and Senescence. Antioxidants 2022, 11, 1224. [Google Scholar] [CrossRef]
- Gurău, F.; Baldoni, S.; Prattichizzo, F.; Espinosa, E.; Amenta, F.; Procopio, A.D.; Albertini, M.C.; Bonafè, M.; Olivieri, F. Anti-senescence compounds: A potential nutraceutical approach to healthy aging. Ageing Res. Rev. 2018, 46, 14–31. [Google Scholar] [CrossRef]
- Liu, J.K. Antiaging agents: Safe interventions to slow aging and healthy life span extension. Nat. Prod. Bioprospect. 2022, 12, 18. [Google Scholar] [CrossRef]
- Fumia, A.; Cicero, N.; Gitto, M.; Nicosia, N.; Alesci, A. Role of nutraceuticals on neurodegenerative diseases: Neuroprotective and immunomodulant activity. Nat. Prod. Res. 2022, 36, 5916–5933. [Google Scholar] [CrossRef]
- Hernandez, D.F.; Cervantes, E.L.; Luna-Vital, D.A.; Mojica, L. Food-derived bioactive compounds with anti-aging potential for nutricosmetic and cosmeceutical products. Crit. Rev. Food Sci. Nutr. 2020, 61, 3740–3755. [Google Scholar] [CrossRef]
- Conte, R. Recent advances on nano delivery of Helix mucus pharmacologically active components. Int. J. Nano Dimens. 2016, 7, 181–185. [Google Scholar] [CrossRef]
- Fiorentino, V.; Manganelli, G.; Giusti, F.; Ketmaier, V. Recent expansion and relic survival: Phylogeography of the land snail genus Helix (Mollusca, Gastropoda) from south to north Europe. Mol. Phylogenet. Evol. 2016, 98, 358–372. [Google Scholar] [CrossRef]
- Rygało-Galewska, A.; Zglińska, K.; Niemiec, T. Edible Snail Production in Europe. Animals 2022, 12, 2732. [Google Scholar] [CrossRef]
- Ikauniece, D.; Jemeļjanovs, A.; Šterna, V.; Strazdiņa, V. Evaluation of nutrition value of roman snail’s (Helix pomatia) meat obtained in latvia. In Proceedings of the 9th Baltic Conference on Food Science and Technology “Food for Consumer Well-Being” FOODBALT 2014, Jelgava, Latvia, 8–9 May 2014. [Google Scholar]
- Górka, A.; Oklejewicz, B.; Duda, M. Nutrient Content and Antioxidant Properties of Eggs of the Land Snail Helix aspersa maxima. J. Nutr. Food Sci. 2017, 7, 594. [Google Scholar] [CrossRef]
- Tsoutsos, D.; Kakagia, D.; Tamparopoulos, K. The efficacy of Helix aspersa Müller extract in the healing of partial thickness burns: A novel treatment for open burn management protocols. J. Dermatolog. Treat. 2009, 20, 219–222. [Google Scholar] [CrossRef]
- Pitt, S.J.; Graham, M.A.; Dedi, C.G.; Taylor-Harris, P.M.; Gunn, A. Antimicrobial properties of mucus from the brown garden snail Helix aspersa. Br. J. Biomed. Sci. 2015, 72, 174–181. [Google Scholar] [CrossRef]
- Dolashki, A.; Velkova, L.; Daskalova, E.; Zheleva, N.N.; Topalova, Y.; Atanasov, V.; Voelter, W.; Dolashka, P. Antimicrobial Activities of Different Fractions from Mucus of the Garden Snail Cornu aspersum. Biomedicines 2020, 8, 315. [Google Scholar] [CrossRef]
- Messina, L.; Bruno, F.; Licata, P.; Di Paola, D.; Franco, G.; Marino, Y.; Peritore, A.F.; Cuzzocrea, S.; Gugliandolo, E.; Crupi, R. Snail Mucus Filtrate Reduces Inflammation in Canine Progenitor Epidermal Keratinocytes (CPEK). Animals 2022, 12, 1848. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Bramanti, P.; Mazzon, E. Activation of Nrf2 by Natural Bioactive Compounds: A Promising Approach for Stroke? Int. J. Mol. Sci. 2020, 21, 4875. [Google Scholar] [CrossRef]
- Trapella, C.; Rizzo, R.; Gallo, S.; Alogna, A.; Bortolotti, D.; Casciano, F.; Zauli, G.; Secchiero, P.; Voltan, R. HelixComplex snail mucus exhibits pro-survival, proliferative and pro-migration effects on mammalian fibroblasts. Sci. Rep. 2018, 8, 17665. [Google Scholar] [CrossRef]
- Dhiman, V.; Pant, D. Human health and snails. J. Immunoass. Immunochem. 2021, 42, 211–235. [Google Scholar] [CrossRef]
- Brieva, A.; Philips, N.; Tejedor, R.; Guerrero, A.; Pivel, J.P.; Alonso-Lebrero, J.L.; Gonzalez, S. Molecular basis for the regenerative properties of a secretion of the mollusk Cryptomphalus aspersa. Skin Pharmacol. Physiol. 2008, 21, 15–22. [Google Scholar] [CrossRef]
- Iglesias-De La Cruz, M.C.; Sanz-Rodríguez, F.; Zamarrón, A.; Reyes, E.; Carrasco, E.; González, S.; Juarranz, A. A secretion of the mollusc Cryptomphalus aspersa promotes proliferation, migration and survival of keratinocytes and dermal fibroblasts in vitro. Int. J. Cosmet. Sci. 2012, 34, 183–189. [Google Scholar] [CrossRef]
- Ellijimi, C.; Ben Hammouda, M.; Othman, H.; Moslah, W.; Jebali, J.; Mabrouk, H.B.; Morjen, M.; Haoues, M.; Luis, J.; Marrakchi, N.; et al. Helix aspersa maxima mucus exhibits antimelanogenic and antitumoral effects against melanoma cells. Biomed. Pharmacother. 2018, 101, 871–880. [Google Scholar] [CrossRef]
- Castro, B.; de Paz, N.; González, S.; Rodríguez-Luna, A. SCA® Slows the Decline of Functional Parameters Associated with Senescence in Skin Cells. Int. J. Mol. Sci. 2022, 23, 6538. [Google Scholar] [CrossRef]
- Espada, J.; Matabuena, M.; Salazar, N.; Lucena, S.; Kourani, O.; Carrasco, E.; Calvo, M.; Rodríguez, C.; Reyes, E.; González, S.; et al. Cryptomphalus aspersa mollusc eggs extract promotes migration and prevents cutaneous ageing in keratinocytes and dermal fibroblasts in vitro. Int. J. Cosmet. Sci. 2015, 37, 41–55. [Google Scholar] [CrossRef]
- Juan, L.S.; de Pedro, I.; Rodríguez-Luna, A.; Villalba, M.; Guerrero, A.; González, S.; Gandarillas, A. Cryptomphalus aspersa Eggs Extract Potentiates Human Epidermal Stem Cell Regeneration and Amplification. Cosmetics 2021, 9, 2. [Google Scholar] [CrossRef]
- Matusiewicz, M.; Marczak, K.; Kwiecinska, B.; Kupis, J.; Zglinska, K.; Niemiec, T.; Kosieradzka, I. Effect of extracts from eggs of Helix aspersa maxima and Helix aspersa aspersa snails on Caco-2 colon cancer cells. PeerJ 2022, 10, e13217. [Google Scholar] [CrossRef]
- El Ouar, I.; Braicu, C.; Naimi, D.; Irimie, A.; Berindan-Neagoe, I. Effect of Helix aspersa extract on TNFα, NF-κB and some tumor suppressor genes in breast cancer cell line Hs578T. Pharmacogn. Mag. 2017, 13, 281–285. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investg. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef]
- Alameda, M.T.; Morel, E.; Parrado, C.; González, S.; Juarranz, Á. Cryptomphalus aspersa Mollusc Egg Extract Promotes Regenerative Effects in Human Dermal Papilla Stem Cells. Int. J. Mol. Sci. 2017, 18, 463. [Google Scholar] [CrossRef]
- García-Honduvilla, N.; Cifuentes, A.; Ortega, M.A.; Delgado, A.; González, S.; Bujan, J.; Alvarez-Mon, M. High Sensitivity of Human Adipose Stem Cells to Differentiate into Myofibroblasts in the Presence of C. aspersa Egg Extract. Stem Cells Int. 2017, 2017, 9142493. [Google Scholar] [CrossRef]
- Carlin, R.; Davis, D.; Weiss, M.; Schultz, B.; Troyer, D. Expression of early transcription factors Oct-4, Sox-2 and Nanog by porcine umbilical cord (PUC) matrix cells. Reprod. Biol. Endocrinol. 2006, 4, 8. [Google Scholar] [CrossRef]
- Wollenzien, H.; Voigt, E.; Kareta, M.S. Somatic Pluripotent Genes in Tissue Repair, Developmental Disease, and Cancer. SPG Biomed. 2018, 1. [Google Scholar] [CrossRef]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signalling for an old antioxidant. Front. Pharmacol. 2014, 5, 108999. [Google Scholar] [CrossRef]
- Weng, B.; Zhang, X.; Chu, X.; Gong, X.; Cai, C. Nrf2-Keap1-ARE-NQO1 signaling attenuates hyperoxia-induced lung cell injury by inhibiting apoptosis. Mol. Med. Rep. 2021, 23, 221. [Google Scholar] [CrossRef]
- Li, L.; Dong, H.; Song, E.; Xu, X.; Liu, L.; Song, Y. Nrf2/ARE pathway activation, HO-1 and NQO1 induction by polychlorinated biphenyl quinone is associated with reactive oxygen species and PI3K/AKT signaling. Chem. Biol. Interact. 2014, 209, 56–67. [Google Scholar] [CrossRef]
- Shibata, A.; Conrad, S.; Birraux, J.; Geuting, V.; Barton, O.; Ismail, A.; Kakarougkas, A.; Meek, K.; Taucher-Scholz, G.; Löbrich, M.; et al. Factors determining DNA double-strand break repair pathway choice in G2 phase. EMBO J. 2011, 30, 1079–1092. [Google Scholar] [CrossRef]
- Frippiat, C.; Chen, Q.M.; Zdanov, S.; Magalhaes, J.P.; Remacle, J.; Toussaint, O. Subcytotoxic H2O2 stress triggers a release of transforming growth factor-beta 1, which induces biomarkers of cellular senescence of human diploid fibroblasts. J. Biol. Chem. 2001, 276, 2531–2537. [Google Scholar] [CrossRef]
- Toussaint, O.; Medrano, E.E.; Von Zglinicki, T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp. Gerontol. 2000, 35, 927–945. [Google Scholar] [CrossRef]
- Coppé, J.P.; Rodier, F.; Patil, C.K.; Freund, A.; Desprez, P.Y.; Campisi, J. Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. J. Biol. Chem. 2011, 286, 36396–36403. [Google Scholar] [CrossRef]
- López-Domínguez, J.A.; Rodríguez-López, S.; Ahumada-Castro, U.; Desprez, P.Y.; Konovalenko, M.; Laberge, R.M.; Cárdenas, C.; Villalba, J.M.; Campisi, J. Cdkn1a transcript variant 2 is a marker of aging and cellular senescence. Aging 2021, 13, 13380–13392. [Google Scholar] [CrossRef]
- Goutas, A.; Outskouni, Z.; Papathanasiou, I.; Satra, M.; Koliakos, G.; Trachana, V. Dysregulation of caveolin-1 phosphorylation and nuclear translocation is associated with senescence onset. Cells 2021, 10, 2939. [Google Scholar] [CrossRef]
- Goutas, A.; Papathanasiou, I.; Mourmoura, E.; Tsesmelis, K.; Tsezou, A.; Trachana, V. Oxidative Stress Response Is Mediated by Overexpression and Spatiotemporal Regulation of Caveolin-1. Antioxidants 2020, 9, 766. [Google Scholar] [CrossRef]
- Luo, X.; Wang, D.; Zhu, X.; Wang, G.; You, Y.; Ning, Z.; Li, Y.; Jin, S.; Huang, Y.; Hu, Y.; et al. Autophagic degradation of caveolin-1 promotes liver sinusoidal endothelial cells defenestration article. Cell Death Dis. 2018, 9, 576. [Google Scholar] [CrossRef]
- Shiroto, T.; Romero, N.; Sugiyama, T.; Sartoretto, J.L.; Kalwa, H.; Yan, Z.; Shimokawa, H.; Michel, T. Caveolin-1 is a critical determinant of autophagy, metabolic switching, and oxidative stress in vascular endothelium. PLoS ONE 2014, 9, 87871. [Google Scholar] [CrossRef]
- Chen, Z.H.; Cao, J.F.; Zhou, J.S.; Liu, H.; Che, L.Q.; Mizumura, K.; Li, W.; Choi, A.M.K.; Shen, H.H. Interaction of caveolin-1 with ATG12-ATG5 system suppresses autophagy in lung epithelial cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2014, 306, 1016–1025. [Google Scholar] [CrossRef]
- Goutas, A.; Syrrou, C.; Papathanasiou, I.; Tsezou, A.; Trachana, V. The autophagic response to oxidative stress in osteoarthritic chondrocytes is deregulated. Free Radic. Biol. Med. 2018, 126, 122–132. [Google Scholar] [CrossRef]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef]
- Cook, K.L.; Soto-Pantoja, D.R.; Jin, L.; Abu-Asab, M.; Clarke, R. When is a vesicle not just a vesicle: Mitochondrial spheroids and mitochondrial autophagosomes. Cell Biosci. 2014, 4, 66. [Google Scholar] [CrossRef][Green Version]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural Compounds and Products from an Anti-Aging Perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef]
- Zhuang, W.Z.; Lin, Y.H.; Su, L.J.; Wu, M.S.; Jeng, H.Y.; Chang, H.C.; Huang, Y.H.; Ling, T.Y. Mesenchymal stem/stromal cell-based therapy: Mechanism, systemic safety and biodistribution for precision clinical applications. J. Biomed. Sci. 2021 281 2021, 28, 28. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Macrì, F.; Fusco, R.; Siracusa, R.; D’amico, R.; Cordaro, M.; Peritore, A.F.; Impellizzeri, D.; Genovese, T.; Cuzzocrea, S.; et al. The Protective Effect of Snail Secretion Filtrate in an Experimental Model of Excisional Wounds in Mice. Vet. Sci. 2021, 8, 167. [Google Scholar] [CrossRef]
- Choi, M.; Lee, H.S.; Naidansaren, P.; Kim, H.K.; Eunju, O.; Cha, J.H.; Ahn, H.Y.; Yang, P.I.; Shin, J.C.; Joe, Y.A. Proangiogenic features of Wharton’s jelly-derived mesenchymal stromal/stem cells and their ability to form functional vessels. Int. J. Biochem. Cell Biol. 2013, 45, 560–570. [Google Scholar] [CrossRef]
- Shohara, R.; Yamamoto, A.; Takikawa, S.; Iwase, A.; Hibi, H.; Kikkawa, F.; Ueda, M. Mesenchymal stromal cells of human umbilical cord Wharton’s jelly accelerate wound healing by paracrine mechanisms. Cytotherapy 2012, 14, 1171–1181. [Google Scholar] [CrossRef]
- Arno, A.I.; Amini-Nik, S.; Blit, P.H.; Al-Shehab, M.; Belo, C.; Herer, E.; Tien, C.H.; Jeschke, M.G. Human Wharton’s jelly mesenchymal stem cells promote skin wound healing through paracrine signaling. Stem Cell Res. Ther. 2014, 5, 28. [Google Scholar] [CrossRef]
- Shi, Y.; Shu, B.; Yang, R.; Xu, Y.; Xing, B.; Liu, J.; Chen, L.; Qi, S.; Liu, X.; Wang, P.; et al. Wnt and Notch signaling pathway involved in wound healing by targeting c-Myc and Hes1 separately. Stem Cell Res. Ther. 2015, 6, 120. [Google Scholar] [CrossRef]
- Kumar, V.; Vashishta, M.; Kong, L.; Wu, X.; Lu, J.J.; Guha, C.; Dwarakanath, B.S. The Role of Notch, Hedgehog, and Wnt Signaling Pathways in the Resistance of Tumors to Anticancer Therapies. Front. cell Dev. Biol. 2021, 9, 650772. [Google Scholar] [CrossRef]
- Li, M.; Jiang, Y.; Hou, Q.; Zhao, Y.; Zhong, L.; Fu, X. Potential pre-activation strategies for improving therapeutic efficacy of mesenchymal stem cells: Current status and future prospects. Stem Cell Res. Ther. 2022, 13, 146. [Google Scholar] [CrossRef]
- Ji, S.; Xiong, M.; Chen, H.; Liu, Y.; Zhou, L.; Hong, Y.; Wang, M.; Wang, C.; Fu, X.; Sun, X. Cellular rejuvenation: Molecular mechanisms and potential therapeutic interventions for diseases. Signal Transduct. Target. Ther. 2023, 8, 116. [Google Scholar] [CrossRef]
- Pole, A.; Dimri, M.; Dimri, G.P. Oxidative stress, cellular senescence and ageing. AIMS Mol. Sci. 2016, 3, 300–324. [Google Scholar] [CrossRef]
- Barja, G. The mitochondrial free radical theory of aging. In Progress in Molecular Biology and Translational Science; Academic Press: San Diego, CA, USA, 2014; Volume 127, pp. 1–27. ISBN 9780123946256. [Google Scholar]
- Kwon, Y.; Kim, J.W.; Jeoung, J.A.; Kim, M.-S.; Kang, C. Autophagy Is Pro-Senescence When Seen in Close-Up, but Anti-Senescence in Long-Shot. Moleucles Cells 2017, 40, 607–612. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, C.-J.; Zhuo, R.-H.; Lei, Y.-S.; Han, N.-N.; He, L. Inhibition of autophagy alleviates the senescent state of rat mesenchymal stem cells during long-term culture. Mol. Med. Rep. 2014, 10, 3003–3008. [Google Scholar] [CrossRef]
- Rastaldo, R.; Vitale, E.; Giachino, C. Dual Role of Autophagy in Regulation of Mesenchymal Stem Cell Senescence. Front. Cell Dev. Biol. 2020, 8, 276. [Google Scholar] [CrossRef]
- Bai, X.; Yang, X.; Jia, X.; Rong, Y.; Chen, L.; Zeng, T.; Deng, X.; Li, W.; Wu, G.; Wang, L.; et al. CAV1-CAVIN1-LC3B-mediated autophagy regulates high glucose-stimulated LDL transcytosis. Autophagy 2020, 16, 1111–1129. [Google Scholar] [CrossRef]
- Tsagias, N.; Koliakos, I.; Karagiannis, V.; Eleftheriadou, M.; Koliakos, G.G. Isolation of mesenchymal stem cells using the total length of umbilical cord for transplantation purposes. Transfus. Med. 2011, 21, 253–261. [Google Scholar] [CrossRef]
- Gogas, A.; Laliotis, G.P.; Ladoukakis, E.; Trachana, V. Chemical composition and antioxidant profile of snails (Cornu aspersum aspersum) fed diets with different protein sources under intensive rearing conditions. J. Anim. Feed Sci. 2021, 30, 391–398. [Google Scholar] [CrossRef]
- Ueno, T.; Komatsu, M. Monitoring Autophagy Flux and Activity: Principles and Applications. BioEssays 2020, 42, 2000122. [Google Scholar] [CrossRef]
- Kolonas, A.; Vareltzis, P.; Kiroglou, S.; Goutzourelas, N.; Stagos, D.; Trachana, V.; Tsadila, C.; Mossialos, D.; Mourtakos, S.; Gortzi, O. Antioxidant and Antibacterial Properties of a Functional Sports Beverage Formulation. Int. J. Mol. Sci. 2023, 24, 3558. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Outskouni, Z.; Christodoulou, C.; Goutas, A.; Kyriazis, I.D.; Paraskevopoulou, A.; Laliotis, G.P.; Matsakidou, A.; Gogas, A.; Trachana, V. Cryptomphalus aspersa Egg Extract Protects against Human Stem Cell Stress-Induced Premature Senescence. Int. J. Mol. Sci. 2024, 25, 3715. https://doi.org/10.3390/ijms25073715
Outskouni Z, Christodoulou C, Goutas A, Kyriazis ID, Paraskevopoulou A, Laliotis GP, Matsakidou A, Gogas A, Trachana V. Cryptomphalus aspersa Egg Extract Protects against Human Stem Cell Stress-Induced Premature Senescence. International Journal of Molecular Sciences. 2024; 25(7):3715. https://doi.org/10.3390/ijms25073715
Chicago/Turabian StyleOutskouni, Zozo, Christina Christodoulou, Andreas Goutas, Ioannis D. Kyriazis, Adamantini Paraskevopoulou, George P. Laliotis, Anthia Matsakidou, Athanasios Gogas, and Varvara Trachana. 2024. "Cryptomphalus aspersa Egg Extract Protects against Human Stem Cell Stress-Induced Premature Senescence" International Journal of Molecular Sciences 25, no. 7: 3715. https://doi.org/10.3390/ijms25073715
APA StyleOutskouni, Z., Christodoulou, C., Goutas, A., Kyriazis, I. D., Paraskevopoulou, A., Laliotis, G. P., Matsakidou, A., Gogas, A., & Trachana, V. (2024). Cryptomphalus aspersa Egg Extract Protects against Human Stem Cell Stress-Induced Premature Senescence. International Journal of Molecular Sciences, 25(7), 3715. https://doi.org/10.3390/ijms25073715








