Effect of Probiotics in Stress-Associated Constipation Model in Zebrafish (Danio rerio) Larvae
Abstract
1. Introduction
2. Results
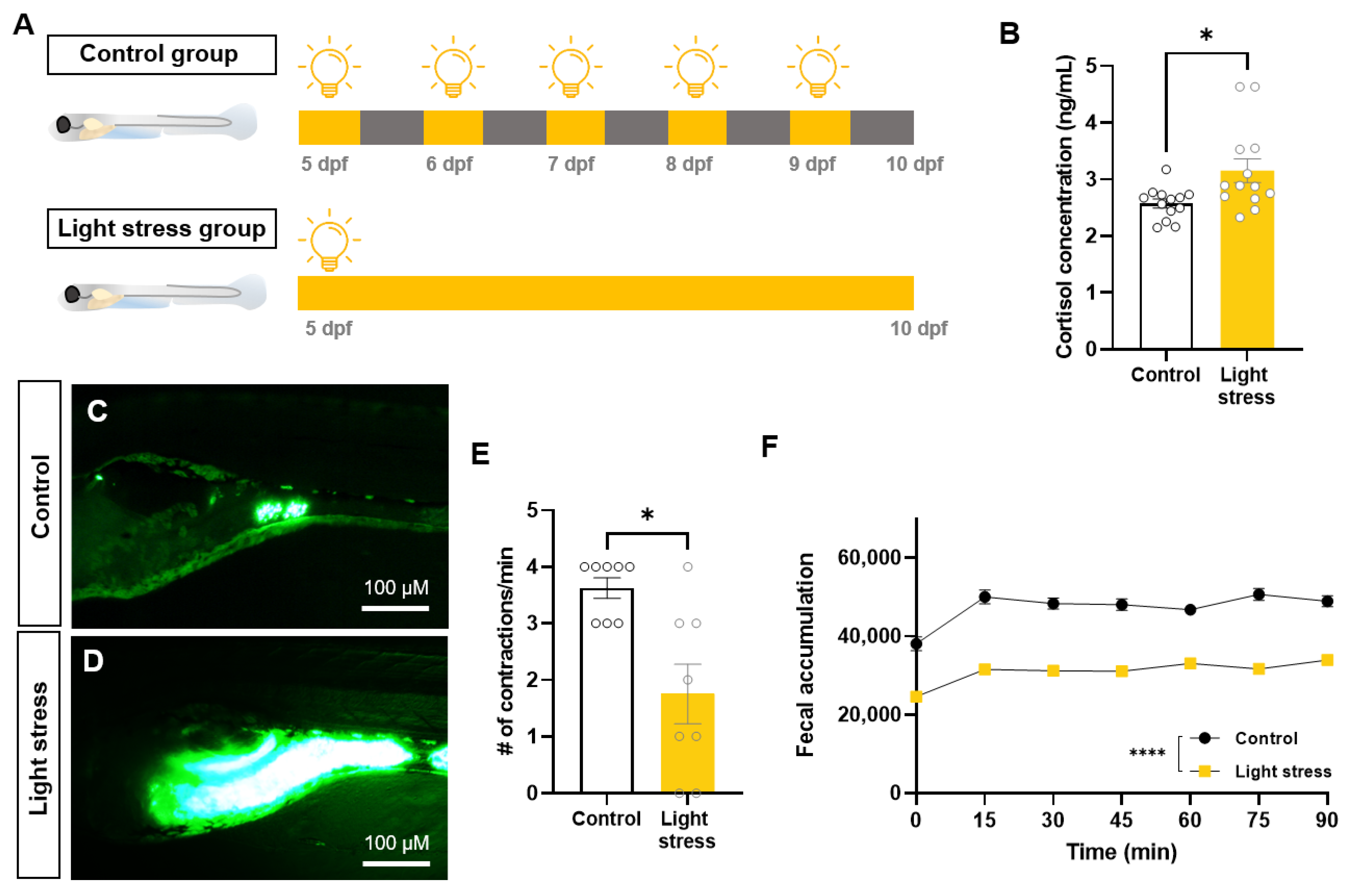
2.1. Changes in Intestinal Motility Caused by Constant-Light-Exposure Stress
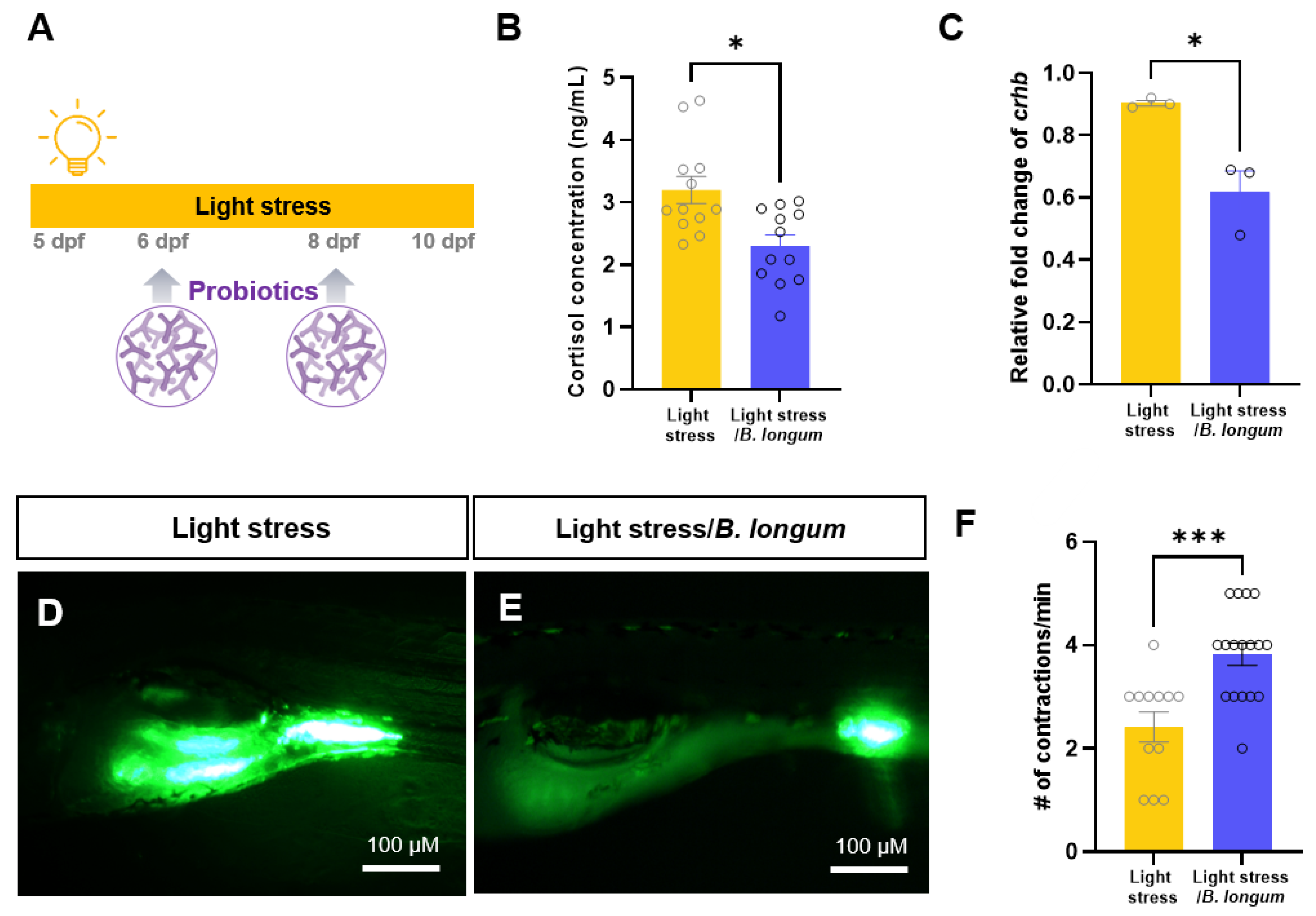
2.2. Effect of Probiotics on Zebrafish Constipation Model Using Light Exposure
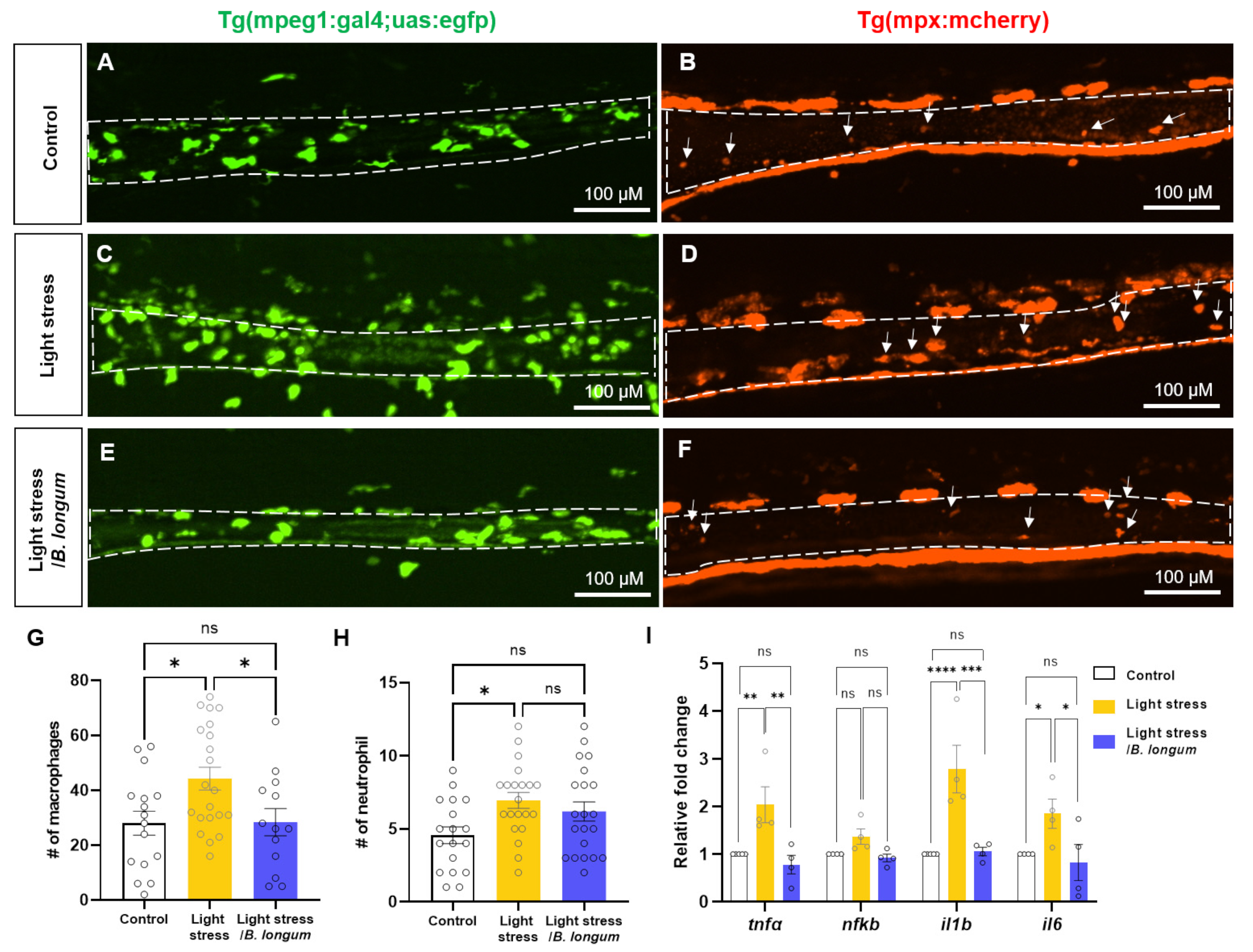
2.3. Probiotic Effect on Intestinal Inflammation in Stress-Induced Constipation Model
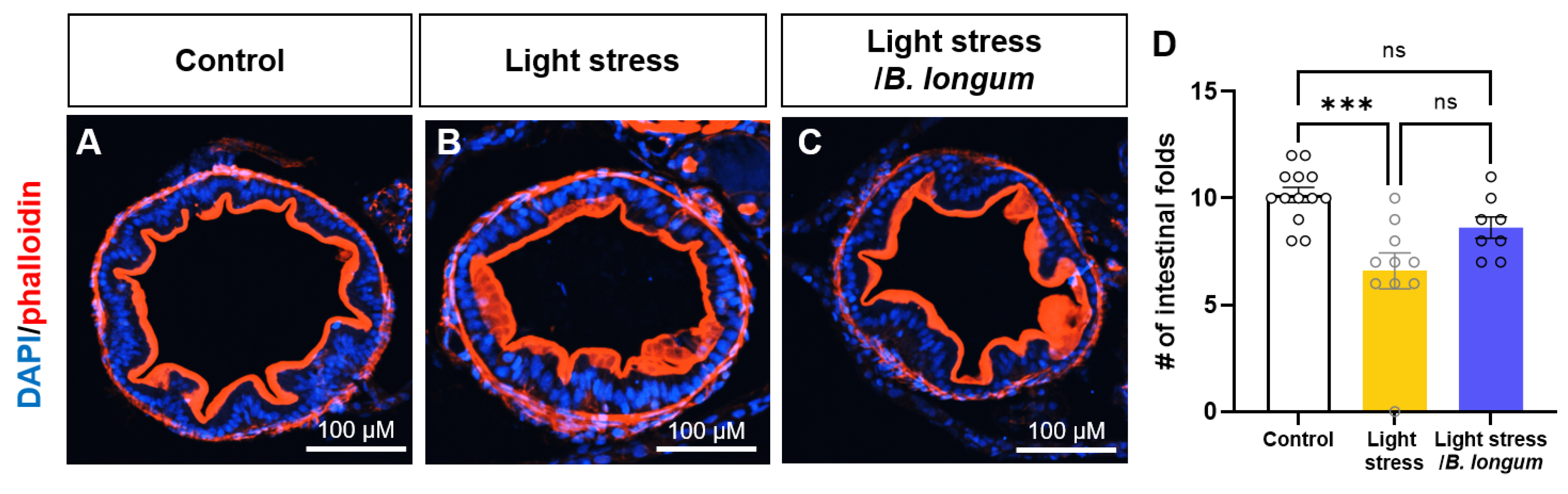
2.4. Probiotic Effect on Intestinal Folds in Stress-Induced Constipation Model
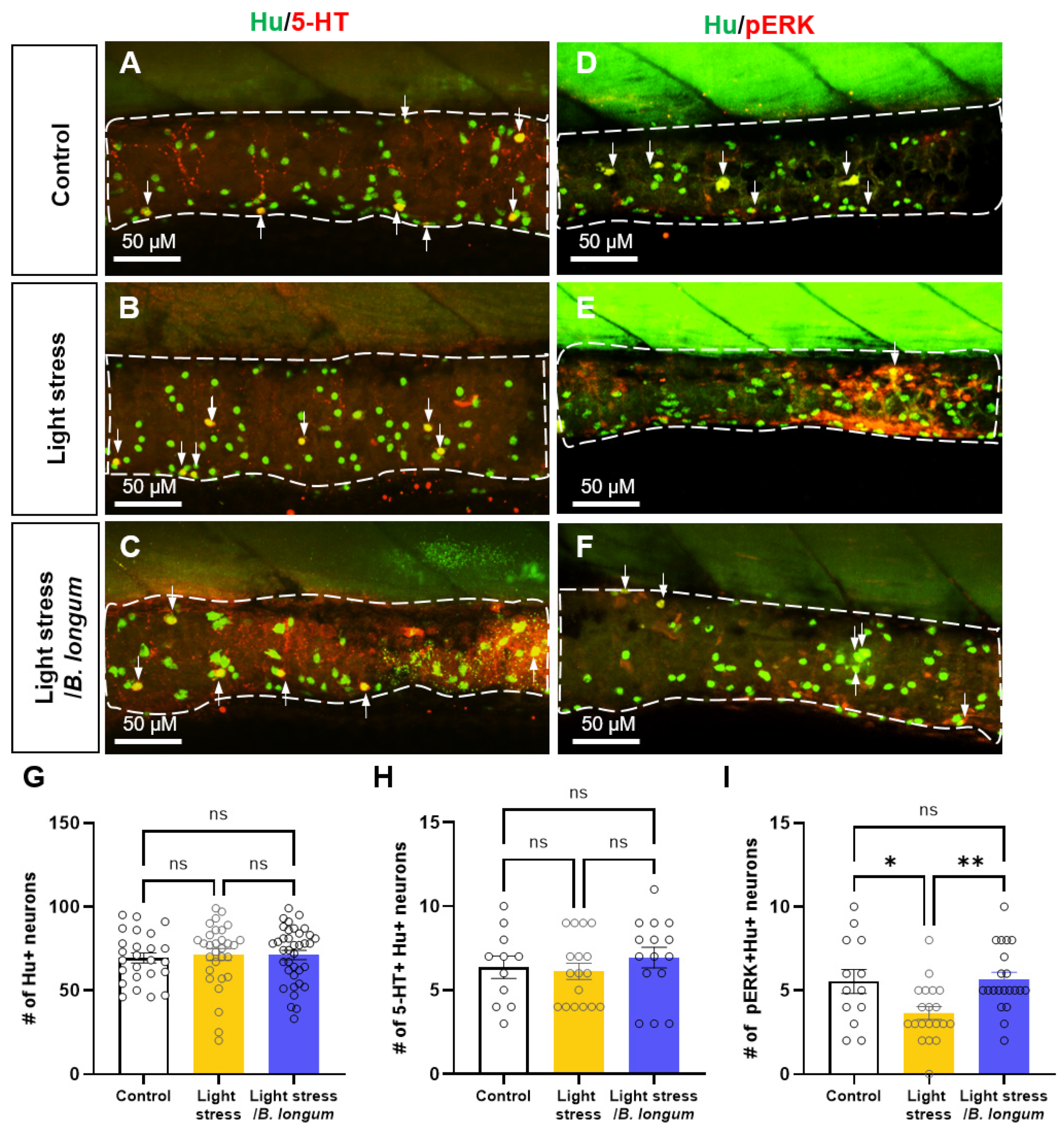
2.5. Probiotic Effect on Enteric Neural Activity in Stress-Induced Constipation Model
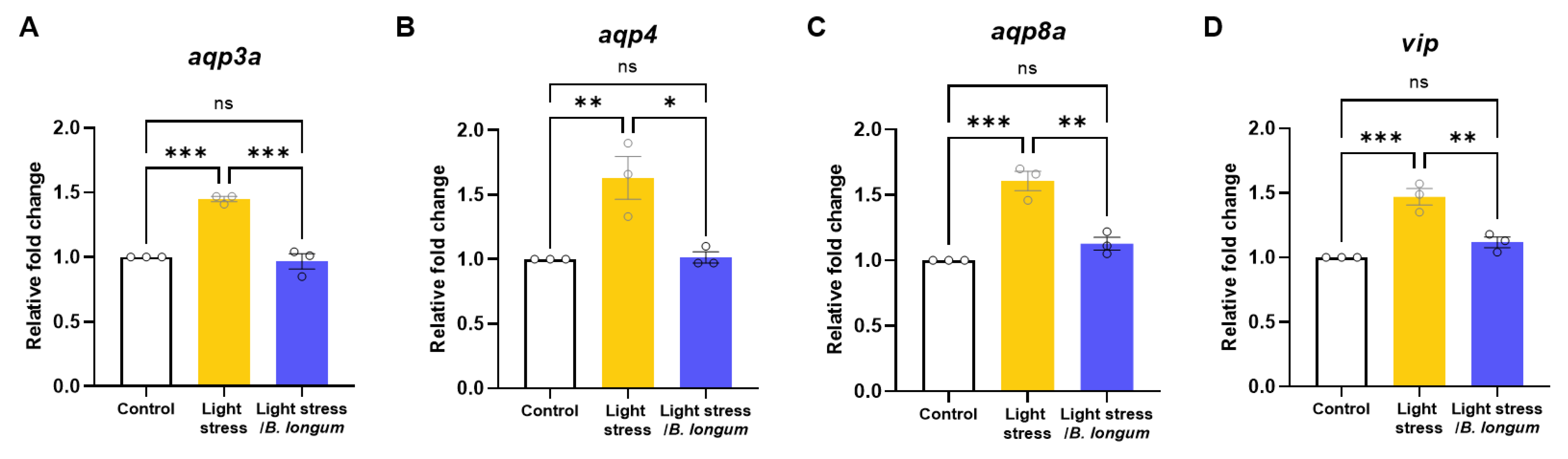
2.6. Stress-Induced-Constipation and Probiotic-Administration-Induced Changes in the Expressions of Aquaporins and Vasoactive Intestinal Peptide (vip)
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Zebrafish Maintenance
4.3. Fluorescent Tracer
4.4. Probiotic-Based Feeding
4.5. Stress Induction with Environmental Stimuli
4.6. Cortisol Assay
4.7. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
4.8. High-Throughput Measurement of Gut Transit Time
4.9. Peristaltic-Movement Measure
4.10. Immunohistochemistry
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef]
- Hendrix, J.; Ranginani, D.; Montero, A.M.; Lockett, C.; Xu, H.; James-Stevenson, T.; Shin, A. Early adverse life events and post-traumatic stress disorder in patients with constipation and suspected disordered defecation. Neurogastroenterol. Motil. 2021, 34, e14195. [Google Scholar] [CrossRef]
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and mental health: A review. Brain Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef]
- Soares, R.L. Irritable bowel syndrome: A clinical review. World J. Gastroenterol. 2014, 20, 12144–12160. [Google Scholar] [CrossRef] [PubMed]
- Fonken, L.K.; Nelson, R.J. The effects of light at night on circadian clocks and metabolism. Endocr. Rev. 2014, 35, 648–670. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.H., 2nd; Walton, J.C.; DeVries, A.C.; Nelson, R.J. Circadian rhythm disruption and mental health. Transl. Psychiatry 2020, 10, 28. [Google Scholar] [CrossRef]
- Konturek, P.C.; Brzozowski, T.; Konturek, S.J. Stress and the gut: Pathophysiology, clinical consequences, diagnostic approach and treatment options. J. Physiol. Pharmacol. 2011, 62, 591–599. [Google Scholar] [PubMed]
- Brugman, S. The zebrafish as a model to study intestinal inflammation. Dev. Comp. Immunol. 2016, 64, 82–92. [Google Scholar] [CrossRef]
- Marks, L.; Beard, E.; Cobey, D.; Moore, N.; Motyer, V.; Valentin, J.-P.; Ewart, L. An evaluation of the non-invasive faecal pellet assessment method as an early drug discovery screen for gastrointestinal liability. J. Pharmacol. Toxicol. Methods 2013, 68, 123–136. [Google Scholar] [CrossRef]
- Harrison, A.; Erlwanger, K.; Elbrønd, V.; Andersen, N.; Unmack, M. Gastrointestinal-tract models and techniques for use in safety pharmacology. J. Pharmacol. Toxicol. Methods 2004, 49, 187–199. [Google Scholar] [CrossRef]
- Field, H.A.; Kelley, K.A.; Martell, L.; Goldstein, A.M.; Serluca, F.C. Analysis of gastrointestinal physiology using a novel intestinal transit assay in zebrafish. Neurogastroenterol. Motil. 2009, 21, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.; Vernier, P.; Wullimann, M.F. The adult central nervous cholinergic system of a neurogenetic model animal, the zebrafish Danio rerio. Brain Res. 2004, 1011, 156–169. [Google Scholar] [CrossRef]
- Alsop, D.; Vijayan, M.M. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am. J. Physiol. Integr. Comp. Physiol. 2008, 294, R711–R719. [Google Scholar] [CrossRef]
- Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Bartels, B.K.; Elkhayat, S.I.; Hart, P.C.; Tien, A.K.; Tien, D.H.; Beeson, E.; Mohnot, S.; et al. Modeling Stress and Anxiety in Zebrafish. In Zebrafish Models in Neurobehavioral Research; Humana: Totowa, NJ, USA, 2011; pp. 73–88. [Google Scholar] [CrossRef]
- Kim, D.; Koun, S.; Kim, S.Y.; Ha, Y.R.; Choe, J.W.; Jung, S.W.; Hyun, J.J.; Jung, Y.K.; Koo, J.S.; Yim, H.J.; et al. Prokinetic effects of diatrizoate meglumine (Gastrografin®) in a zebrafish for opioid-induced constipation model. Anim. Cells Syst. 2021, 25, 264–271. [Google Scholar] [CrossRef]
- Akiho, H.; Ihara, E.; Motomura, Y.; Nakamura, K. Cytokine-induced alterations of gastrointestinal motility in gastrointestinal disorders. World J. Gastrointest. Pathophysiol. 2011, 2, 72–81. [Google Scholar] [CrossRef]
- Nehra, V.; Bruce, B.K.; Rath-Harvey, D.M.; Pemberton, J.H.; Camilleri, M. Psychological disorders in patients with evacuation dis-orders and constipation in a tertiary practice. Am. J. Gastroenterol. 2000, 95, 1755–1758. [Google Scholar] [CrossRef] [PubMed]
- Chapman, G.B.; Tarboush, R.; Connaughton, V.P. The Effects of Rearing Light Level and Duration Differences on the Optic Nerve, Brain, and Associated Structures in Developing Zebrafish Larvae: A Light and Transmission Electron Microscope Study. Anat. Rec. 2012, 295, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Ono, N.; Suzuki, S.; Kawada, K.; Yamaguchi, T.; Azuma, Y.-T. Stress decreases contraction of the colon, and the effects of stress are different among the regions of the colon. J. Vet. Med. Sci. 2022, 84, 1061–1064. [Google Scholar] [CrossRef]
- Julio-Pieper, M.; O’Mahony, C.M.; Clarke, G.; Bravo, J.A.; Dinan, T.G.; Cryan, J.F. Chronic stress-induced alterations in mouse colonic 5-HT and defecation responses are strain dependent. Stress 2011, 15, 218–226. [Google Scholar] [CrossRef]
- Yip, J.L.; Balasuriya, G.K.; Spencer, S.J.; Hill-Yardin, E.L. The Role of Intestinal Macrophages in Gastrointestinal Homeostasis: Heterogeneity and Implications in Disease. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1701–1718. [Google Scholar] [CrossRef]
- Smythies, L.E.; Sellers, M.; Clements, R.H.; Mosteller-Barnum, M.; Meng, G.; Benjamin, W.H.; Orenstein, J.M.; Smith, P.D. Human intestinal macrophages display profound in-flammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Investig. 2005, 115, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Chey, W.D.; Imdad, A.; Almario, C.V.; Bharucha, A.E.; Diem, S.; Greer, K.B.; Hanson, B.; Harris, L.A.; Ko, C.; et al. American Gastroenterological Association-American College of Gastroenterology Clinical Practice Guideline: Pharmacological Management of Chronic Idiopathic Constipation. Gastroenterology 2023, 164, 1086–1106. [Google Scholar] [CrossRef] [PubMed]
- Serra, J.; Pohl, D.; Azpiroz, F.; Chiarioni, G.; Ducrotté, P.; Gourcerol, G.; Hungin, A.P.S.; Layer, P.; Mendive, J.; Pfeifer, J.; et al. European society of neurogastroenterology and motility guidelines on functional constipation in adults. Neurogastroenterol. Motil. 2019, 32, e13762. [Google Scholar] [CrossRef]
- Johanson, J.F.; Kralstein, J. Chronic constipation: A survey of the patient perspective. Aliment. Pharmacol. Ther. 2007, 25, 599–608. [Google Scholar] [CrossRef]
- van der Schoot, A.; Helander, C.; Whelan, K.; Dimidi, E. Probiotics and synbiotics in chronic constipation in adults: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2022, 41, 2759–2777. [Google Scholar] [CrossRef]
- Shang, X.; E, F.-F.; Guo, K.-L.; Li, Y.-F.; Zhao, H.-L.; Wang, Y.; Chen, N.; Nian, T.; Yang, C.-Q.; Yang, K.-H.; et al. Effectiveness and Safety of Probiotics for Patients with Constipation-Predominant Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of 10 Randomized Controlled Trials. Nutrients 2022, 14, 2482. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, C.; Dai, M.; Wang, Q.; Li, C.; Hung, W. Bifidobacterium lactis BL-99 modulates intestinal inflammation and functions in zebrafish models. PLoS ONE 2022, 17, e0262942. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Dai, M.-Z.; Liu, F.-S.; Cao, B.-B.; Guo, J.; Shen, J.-Q.; Li, C.-Q. Probiotics Modulate Intestinal Motility and Inflammation in Zebrafish Models. Zebrafish 2020, 17, 382–393. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Reis, D.J.; Ilardi, S.S.; Punt, S.E.W. The anxiolytic effect of probiotics: A systematic review and meta-analysis of the clinical and preclinical literature. PLoS ONE 2018, 13, e0199041. [Google Scholar] [CrossRef]
- Andrews, C.N.; Storr, M. The pathophysiology of chronic constipation. Can. J. Gastroenterol. 2011, 25, 16b–21b. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Cortes, F.; Turco, F.; Linan-Rico, A.; Soghomonyan, S.; Whitaker, E.; Wehner, S.; Cuomo, R.; Christofi, F.L. Enteric Glial Cells: A New Frontier in Neurogastroen-terology and Clinical Target for Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2016, 22, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-R.; Baba, H.; Brenner, G.J.; Woolf, C.J. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat. Neurosci. 1999, 2, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.J.; Ji, R.R. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009, 2, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Chen, Z.; Jiang, Z. Expression, Distribution and Role of Aquaporin Water Channels in Human and Animal Stomach and Intestines. Int. J. Mol. Sci. 2016, 17, 1399. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, N.; Kon, R.; Sugiyama, K. Aquaporins in the Colon as a New Therapeutic Target in Diarrhea and Constipation. Int. J. Mol. Sci. 2016, 17, 1172. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Wu, X.; Luo, M.; Wei, H.; Xu, D.; Xu, F. Lactobacillus rhamnosus FLRH93 protects against intestinal damage in mice induced by 5-fluorouracil. J. Dairy Sci. 2020, 103, 5003–5018. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Mao, B.; Tang, X.; Zhang, Q.; Zhao, J.; Zhang, H.; Cui, S. Exploring the Dose–Effect Relationship of Bifidobacterium longum in Relieving Loperamide Hydrochloride-Induced Constipation in Rats through Colon-Released Capsules. Int. J. Mol. Sci. 2023, 24, 6585. [Google Scholar] [CrossRef]
- Ellett, F.; Pase, L.; Hayman, J.W.; Andrianopoulos, A.; Lieschke, G.J. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 2011, 117, e49–e56. [Google Scholar] [CrossRef]
- Yoo, S.K.; Deng, Q.; Cavnar, P.J.; Wu, Y.I.; Hahn, K.M.; Huttenlocher, A. Differential Regulation of Protrusion and Polarity by PI(3)K during Neutrophil Motility in Live Zebrafish. Dev. Cell 2010, 18, 226–236. [Google Scholar] [CrossRef]
- Ehsannia, S.; Ahari, H.; Kakoolaki, S.; Anvar, S.A.; Yousefi, S. Effects of probiotics on Zebrafish model infected with Aeromonas hydrophila: Spatial distribution, antimicrobial, and histopathological investigation. BMC Microbiol. 2022, 22, 167. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, X.-L.; Su, Y.-B.; Peng, X.-X.; Li, H. Activation of the TCA Cycle to Provide Immune Protection in Zebrafish Immunized by High Magnesium-Prepared Vibrio alginolyticus Vaccine. Front. Immunol. 2021, 12, 739591. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-J.; Xu, D.; Yang, D.-X.; Li, L.; Peng, X.-X.; Chen, Z.-G.; Li, H. Malate enhances survival of zebrafish against Vibrio alginolyticus infection in the same manner as taurine. Virulence 2020, 11, 349–364. [Google Scholar] [CrossRef]
- Ahi, E.P.; Brunel, M.; Tsakoumis, E.; Chen, J.; Schmitz, M. Appetite regulating genes in zebrafish gut; a gene expression study. PLoS ONE 2022, 17, e0255201. [Google Scholar] [CrossRef]
- Grupp, L.; Wolburg, H.; Mack, A.F. Astroglial structures in the zebrafish brain. J. Comp. Neurol. 2010, 518, 4277–4287. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Huang, X.; Cole, T. High-throughput Measurement of Gut Transit Time Using Larval Zebrafish. J. Vis. Exp. 2018, 23, 58497. [Google Scholar] [CrossRef]
| Gene | Forward Primer (from 5′ to 3′) | Reverse Primer (from 5′ to 3′) | NCBI Accession Number | Reference |
|---|---|---|---|---|
| β-actin | ACCCAGACATCAGGGAGTG | CATCCCAGTTGGTCACAATAC | NM_131031 | Yang J et al., 2021 [43] |
| crhb | TGAATGTAGAGCCATCGAGAGCAG | TGCCGAGCCGGATGAAGTAC | NM_001007379 | |
| il1b | TGGACTTCGCAGCACAAAATG | GTTCACTTCACGCTCTTGGATG | NM_212844 | Yang J et al., 2021 [43] |
| il6 | ATCCGCTCAGAAAACAGTGCT | GTCGCCAAGGAGACTCTTTAC | NM_001261449 | Yang MJ et al., 2020 [44] |
| tnf-α | ATAAGACCCAGGGCAATCAAC | CAGAGTTGTATCCACCTGTTAAATG | NM_212859 | Yang J et al., 2021 [43] |
| nf-kb | GCTCATTCAGATTGCTCTACAC | CGTGTCTCCGTTCTCATCT | NM_001001840 | Yang J et al., 2021 [43] |
| aqp3a | GCAGACTTTCCAAATCCGCAACAAG | GAACCACAGCCAAACATCACCAG | NM_213468 | Ahi EP et al., 2022 [45] |
| aqp 4 | AAGCGTAATGATCTCAAAGGTT | TGGAGAGGACGTCAGCATAG | NM_001003749 | Grupp L et al., 2010 [46] |
| aqp8a | GTCAACTTGCTCCCTTCTGC | GGCTCGTGCAGGATTCATAC | NM_001004661 | |
| vip | CGGGCTCTTCACAAGCGGAT | CTTCATCGGCGCCTGGTCTT | NM_001114553 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.; Kim, S.Y.; Kang, S.; Kang, S.H.; Kim, D.W.; Choe, J.W.; Hyun, J.J.; Jung, S.W.; Jung, Y.K.; Koo, J.S.; et al. Effect of Probiotics in Stress-Associated Constipation Model in Zebrafish (Danio rerio) Larvae. Int. J. Mol. Sci. 2024, 25, 3669. https://doi.org/10.3390/ijms25073669
Lee A, Kim SY, Kang S, Kang SH, Kim DW, Choe JW, Hyun JJ, Jung SW, Jung YK, Koo JS, et al. Effect of Probiotics in Stress-Associated Constipation Model in Zebrafish (Danio rerio) Larvae. International Journal of Molecular Sciences. 2024; 25(7):3669. https://doi.org/10.3390/ijms25073669
Chicago/Turabian StyleLee, Ayoung, Seung Young Kim, Seyoung Kang, Seong Hee Kang, Dong Woo Kim, Jung Wan Choe, Jong Jin Hyun, Sung Woo Jung, Young Kul Jung, Ja Seol Koo, and et al. 2024. "Effect of Probiotics in Stress-Associated Constipation Model in Zebrafish (Danio rerio) Larvae" International Journal of Molecular Sciences 25, no. 7: 3669. https://doi.org/10.3390/ijms25073669
APA StyleLee, A., Kim, S. Y., Kang, S., Kang, S. H., Kim, D. W., Choe, J. W., Hyun, J. J., Jung, S. W., Jung, Y. K., Koo, J. S., Yim, H. J., & Kim, S. (2024). Effect of Probiotics in Stress-Associated Constipation Model in Zebrafish (Danio rerio) Larvae. International Journal of Molecular Sciences, 25(7), 3669. https://doi.org/10.3390/ijms25073669







