Human Rad51 Protein Requires Higher Concentrations of Calcium Ions for D-Loop Formation than for Oligonucleotide Strand Exchange
Abstract
1. Introduction
2. Results
2.1. Optimization of Experimental Conditions
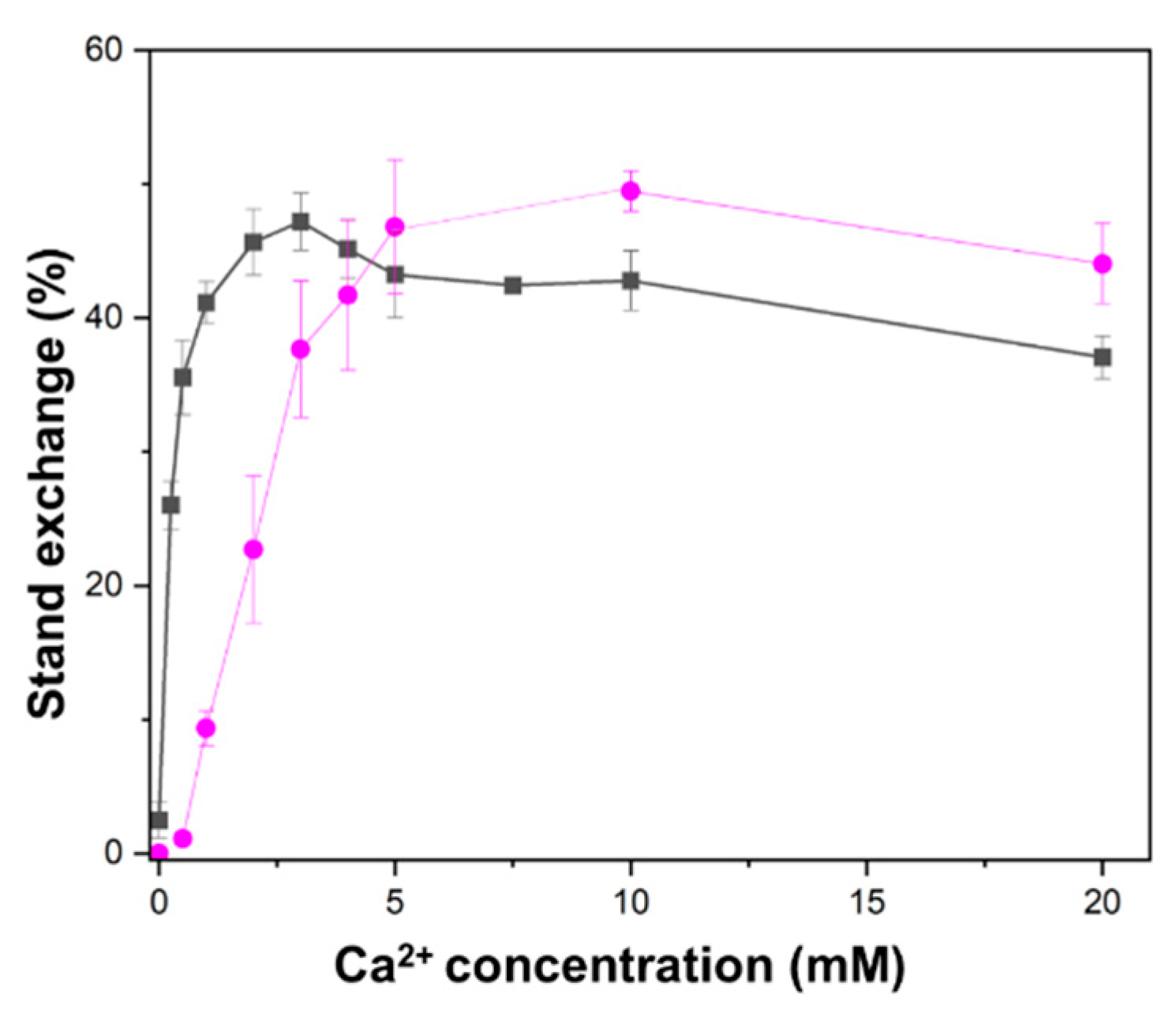
2.2. Higher Ca2+ Concentration Required for D-Loop Formation than for Oligonucleotide Strand Exchange
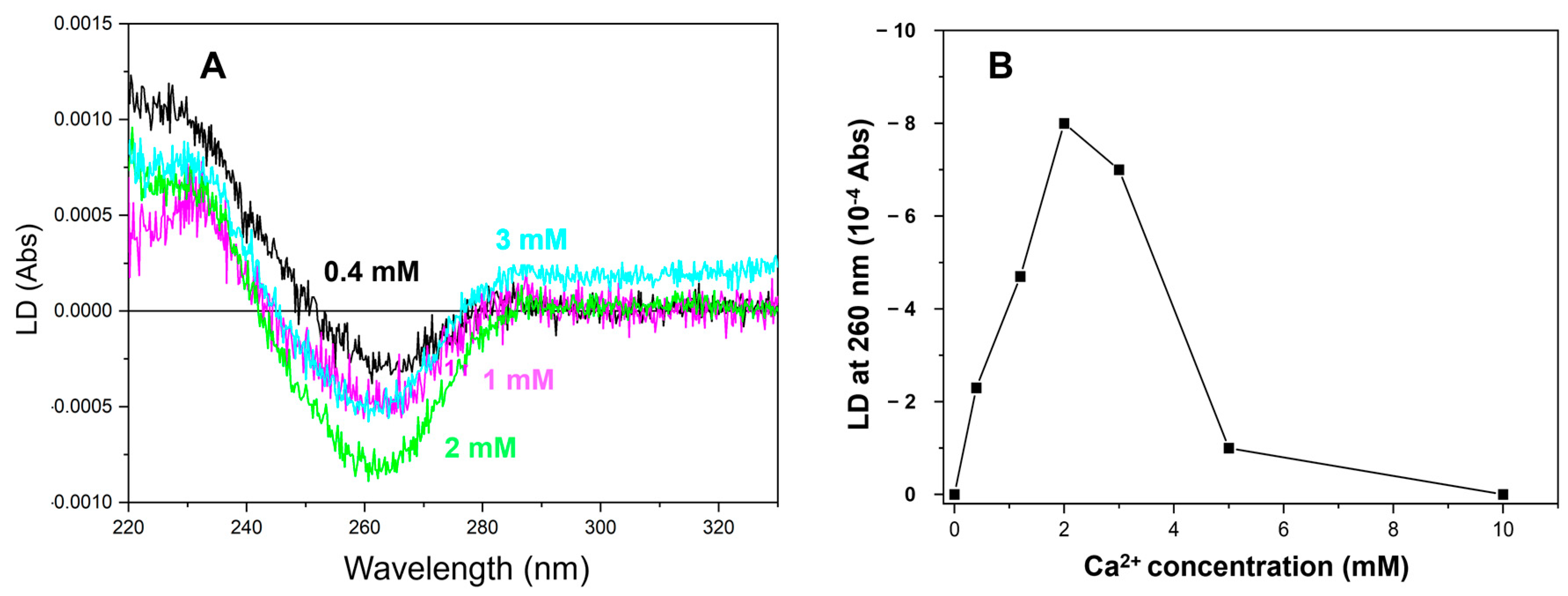
2.3. More than 2 mM of Ca2+ Was Required to Saturate the LD Signal
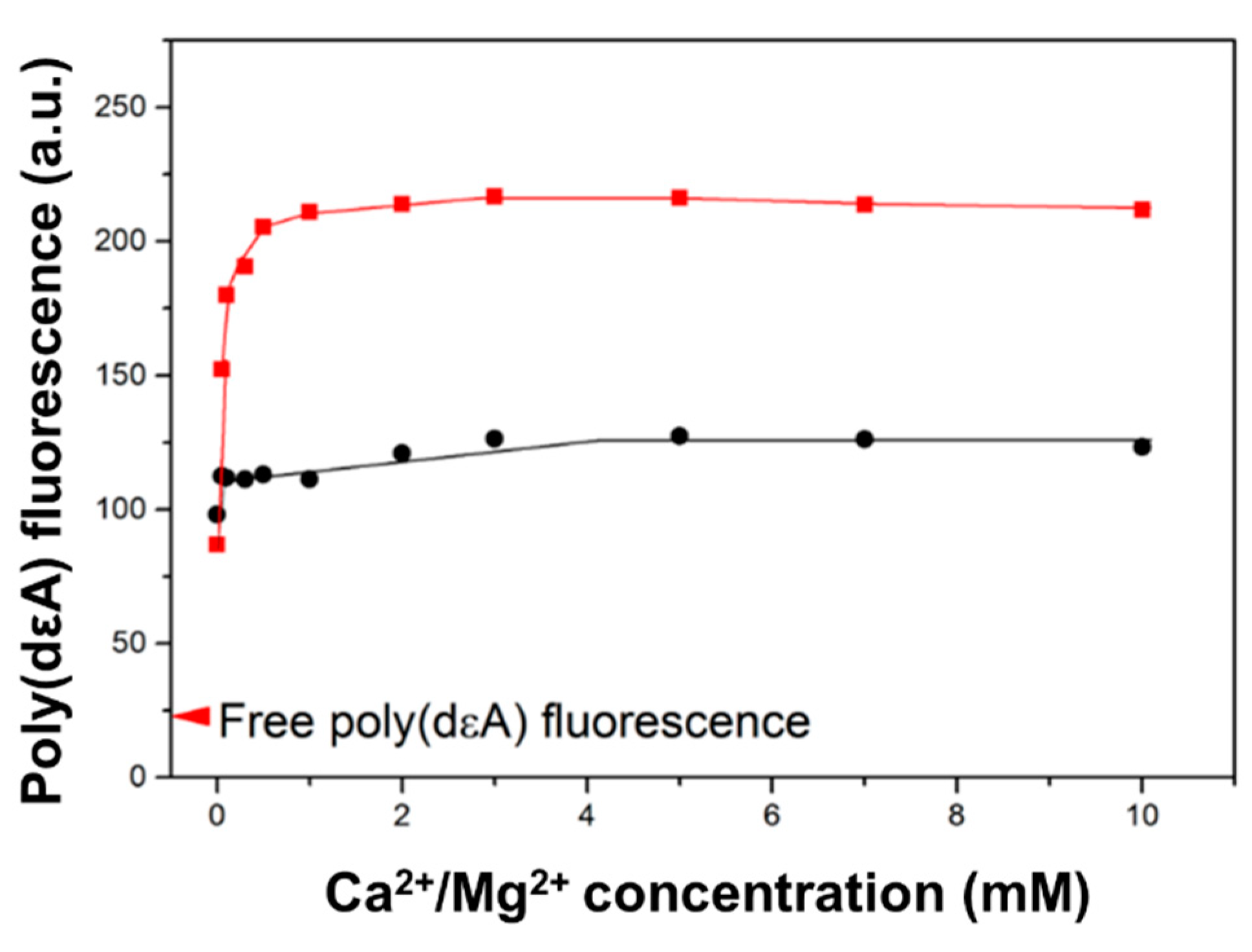
2.4. Saturation of Poly(dεA) Fluorescence Change Requires Less than 1 mM Ca2+
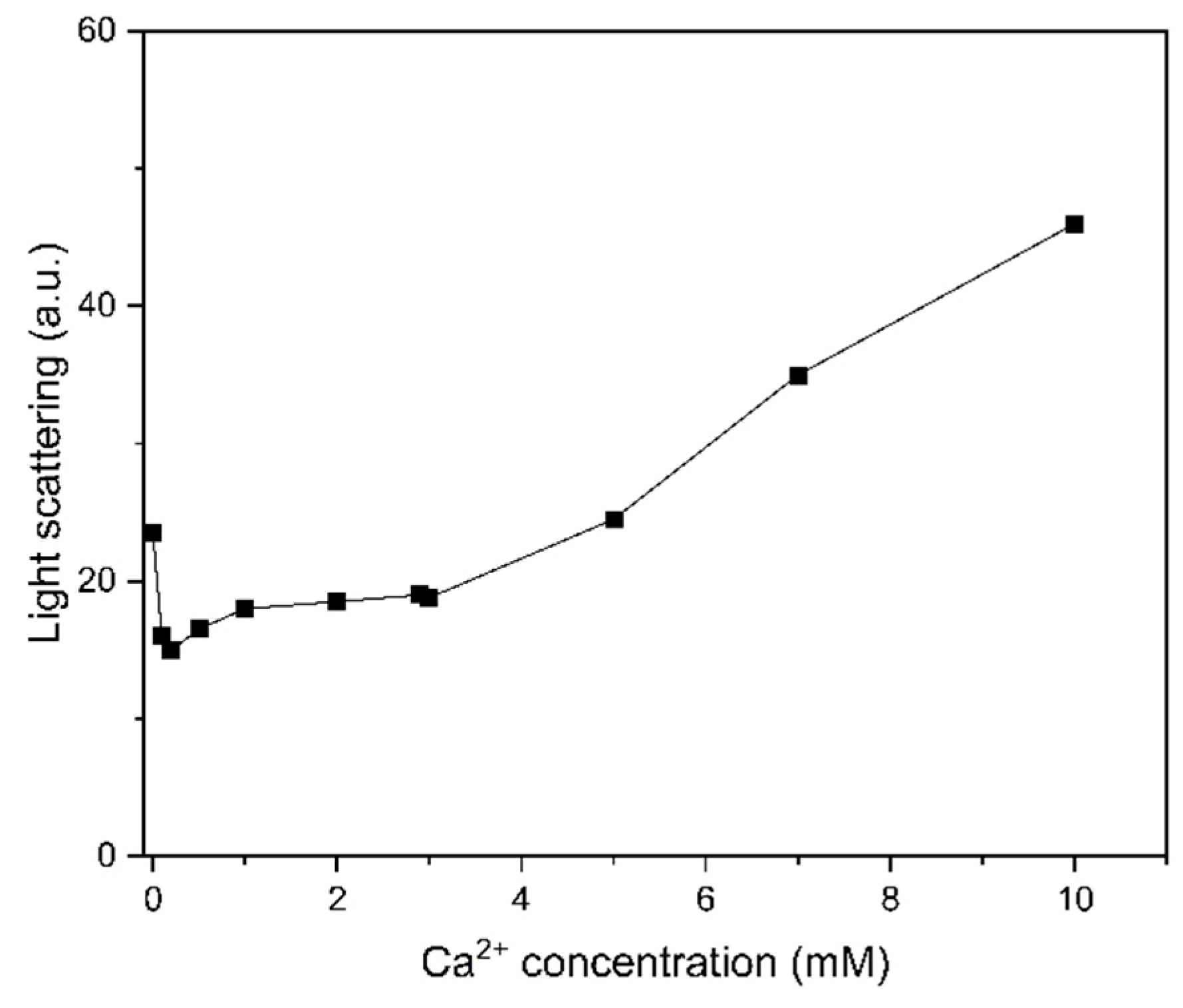
2.5. Light Scattering Promoted at High Ca2+ Concentrations
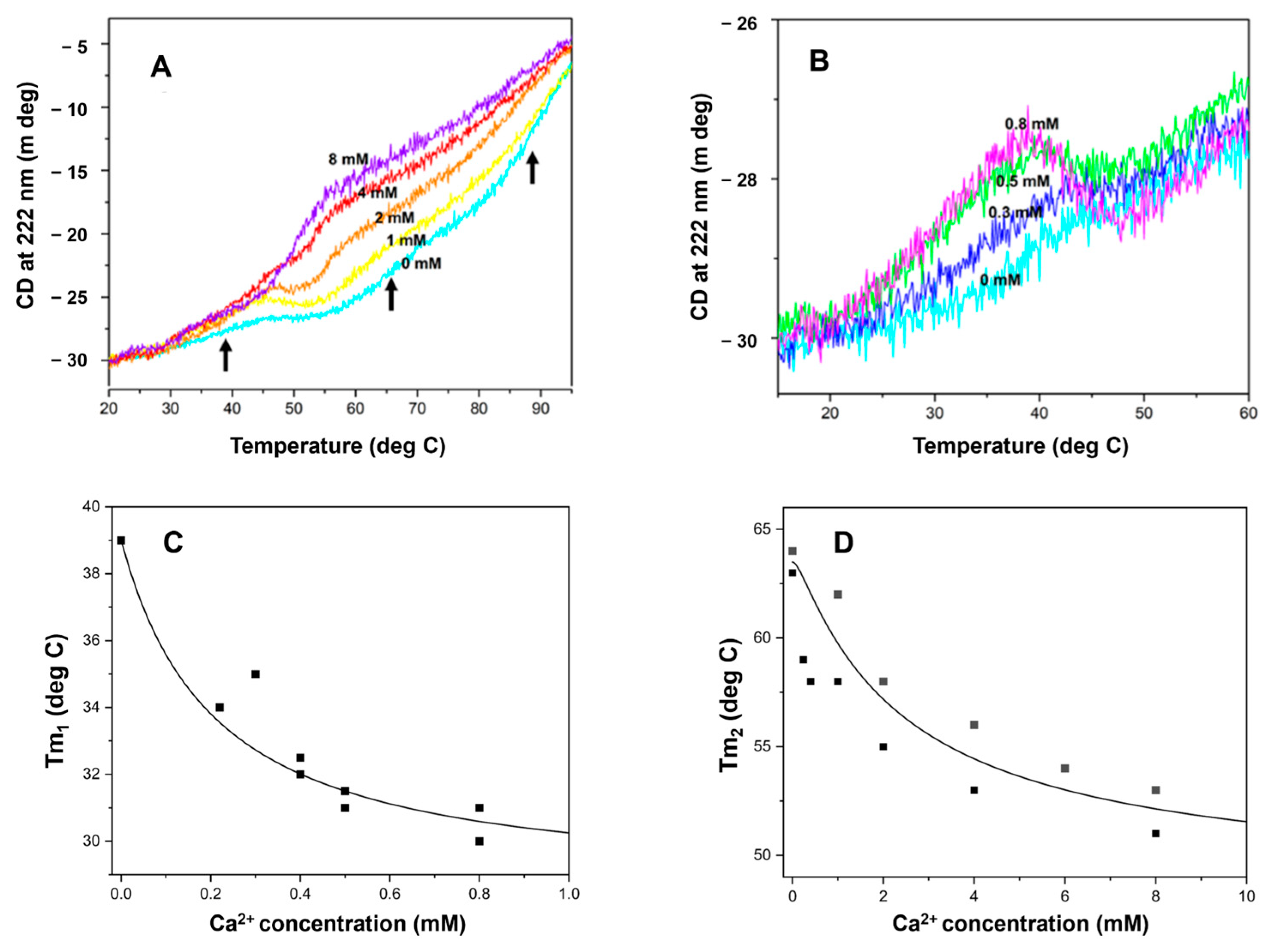
2.6. HsRad51 Binds More than Two Ca2+ Ions: Thermal Denaturation Results
3. Discussion
3.1. Similarity with Strand Exchange Activation by Swi5/Sfr1 Protein
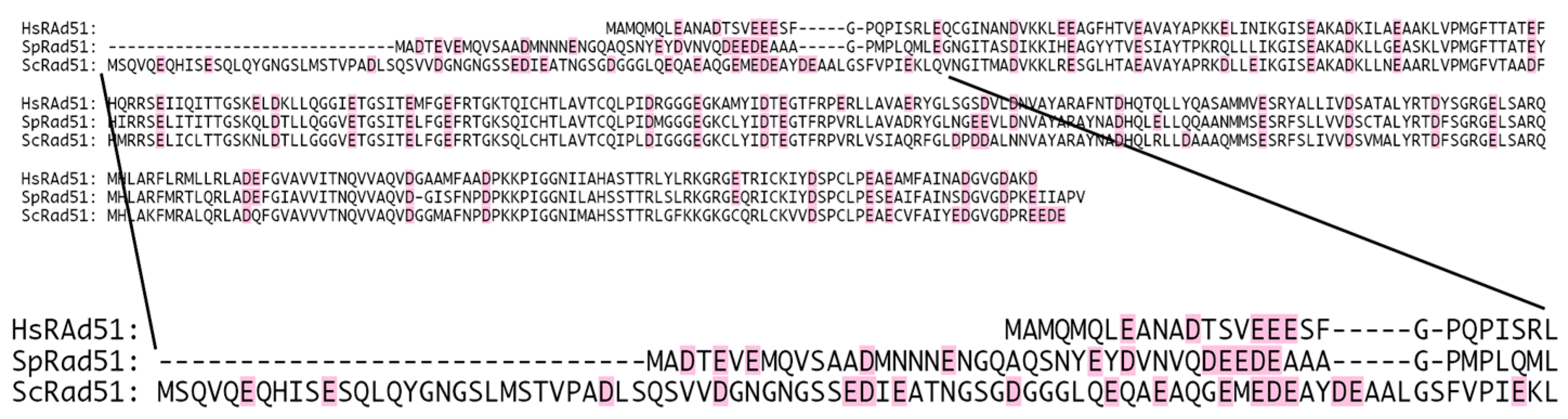
3.2. Potential Ca2+-Binding Site at the N-Terminal Extremity of HsRad51
3.3. Similarity with RecA-Promoted Strand Exchange Activation by Mg2+
4. Materials and Methods
4.1. Materials
4.2. Experimental Conditions
4.3. LD Measurements
4.4. CD Measurements of Thermal Unfolding
4.5. Fluorescence Measurements
4.6. Light Scattering Measurements
4.7. Strand Exchange and D-Loop Formation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shinohara, A.; Ogawa, H.; Matsuda, Y.; Ushio, N.; Ikeo, K.; Ogawa, T. Cloning of human, mouse and fission yeast recombination genes homologous to RAD51 and recA. Nat. Genet. 1993, 4, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Baumann, P.; West, S.C. Role of the human RAD51 protein in homologous recombination and double-stranded-break repair. Trends Biochem. Sci. 1998, 23, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Beach, A.; Li, K.; Haber, J. Rad51-mediated double-strand break repair and mismatch correction of divergent substrates. Nature 2017, 544, 377–380. [Google Scholar] [CrossRef]
- Gerton, J.; Hawley, R. Homologous chromosome interactions in meiosis: Diversity amidst conservation. Nat. Rev. Genet. 2005, 6, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, T.; Fujii, Y.; Sakumi, K.; Tominaga, Y.; Nakao, K.; Sekiguchi, M.; Matsushiro, A.; Yoshimura, Y.; Morita, T. Targeted disruption of the Rad51 gene leads to lethality in embryonic mice. Proc. Natl. Acad. Sci. USA 1996, 93, 6236–6240. [Google Scholar] [CrossRef] [PubMed]
- Sung, P. Function of yeast Rad52 protein as a mediator between replication protein A and the Rad51 recombinase. J. Biol. Chem. 1997, 272, 28194–28197. [Google Scholar] [CrossRef] [PubMed]
- Sung, P. Yeast Rad55 and Rad57 proteins form a heterodimer that functions with replication protein A to promote DNA strand exchange by Rad51 recombinase. Genes. Dev. 1997, 11, 1111–1121. [Google Scholar] [CrossRef]
- Sung, P.; Krejci, L.; Van Komen, S.; Sehorn, M.G. Rad51 recombinase and recombination mediators. J. Biol. Chem. 2003, 278, 42729–42732. [Google Scholar] [CrossRef]
- Jensen, R.B.; Carreira, A.; Kowalczykowski, S.C. Purified human BRCA2 stimulates RAD51-mediated recombination. Nature 2010, 467, 678–683. [Google Scholar] [CrossRef]
- Haruta, N.; Kurokawa, Y.; Murayama, Y.; Akamatsu, Y.; Unzai, S.; Tsutsui, Y.; Iwasaki, H. The Swi5-Sfr1 complex stimulates Rhp51/Rad51- and Dmc1-mediated DNA strand exchange in vitro. Nat. Struct. Mol. Biol. 2006, 13, 823–830. [Google Scholar] [CrossRef]
- Ito, K.; Murayama, Y.; Takahashi, M.; Iwasaki, H. Two three-strand intermediates are processed during Rad51-driven DNA strand exchange. Nat. Struct. Mol. Biol. 2018, 25, 29–36. [Google Scholar] [CrossRef]
- Ito, K.; Murayama, Y.; Kurokawa, Y.; Kanamaru, S.; Kokabu, Y.; Maki, T.; Mikawa, T.; Argunhan, B.; Tsubouchi, H.; Ikeguchi, M.; et al. Real-time tracking reveals catalytic roles for the two DNA binding sites of Rad51. Nat. Commun. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Fornander, L.H.; Frykholm, K.; Reymer, A.; Renodon-Cornière, A.; Takahashi, M.; Nordén, B. Ca2+ improves organization of single-stranded DNA bases in human Rad51 filament, explaining stimulatory effect on gene recombination. Nucleic Acids Res. 2012, 40, 4904–4913. [Google Scholar] [CrossRef]
- Bugreev, D.V.; Mazin, A.V. Ca2+ activates human homologous recombination protein Rad51 by modulating its ATPase activity. Proc. Natl. Acad. Sci. USA 2004, 101, 9988–9993. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, R.; Wang, L.; Yang, Q.; Hu, X.; Fu, Q.; Zhang, X.; Li, W.; Ren, Y. The emerging roles of Rad51 in cancer and Its potential as a therapeutic target. Front. Oncol. 2022, 12, 935593. [Google Scholar] [CrossRef]
- Bindra, R.S.; Schaffer, P.J.; Meng, A.; Woo, J.; Måseide, K.; Roth, M.E.; Lizardi, P.; Hedley, D.W.; Bristow, R.G.; Glazer, P.M. Down-regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol. Cell. Biol. 2004, 24, 8504–8518. [Google Scholar] [CrossRef]
- Bishop, A.J.R.; Schiestl, R.H. Homologous recombination and its role in carcinogenesis. J. Biomed. Biotechnol. 2002, 2, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Heeke, A.L.; Pishvaian, M.J.; Lynce, F.; Xiu, J.; Brody, J.R.; Chen, W.J.; Baker, T.M.; Marshall, J.L.; Isaacs, C. Prevalence of homologous recombination related gene mutations across multiple cancer types. JCO Precis. Oncol. 2018, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.; Weterings, E.; Mahadevan, D.; Sanan, A.; Weinand, M.; Stea, B. Expression levels of RAD51 inversely correlate with survival of glioblastoma patients. Cancers 2021, 13, 5358. [Google Scholar] [CrossRef] [PubMed]
- Qiao, G.B.; Wu, Y.L.; Yang, X.N.; Zhong, W.Z.; Xie, D.; Guan, X.Y.; Fischer, D.; Kolberg, H.C.; Kruger, S.; Stuerzbecher, H.W. High-level expression of Rad51 is an independent prognostic marker of survival in non-small-cell lung cancer patients. Br. J. Cancer 2005, 93, 137–143. [Google Scholar] [CrossRef] [PubMed]
- King, H.O.; Brend, T.; Payne, H.L.; Wright, A.; Ward, T.A.; Patel, K.; Egnuni, T.; Stead, L.F.; Patel, A.; Wurdak, H.; et al. RAD51 is a selective DNA repair target to radiosensitize glioma stem cells. Stem Cell Rep. 2016, 8, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Gachechiladze, M.; Škarda, J.; Soltermann, A.; Joerger, M. RAD51 as a potential surrogate marker for DNA repair capacity in solid malignancies. Int. J. Cancer 2017, 141, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Orhan, E.; Velazquez, C.; Tabet, I.; Sardet, C.; Theillet, C. Regulation of RAD51 at the transcriptional and functional levels: What prospects for cancer therapy? Cancers 2021, 13, 2930. [Google Scholar] [CrossRef] [PubMed]
- Taki, T.; Ohnishi, T.; Yamamoto, A.; Hiraga, S.; Arita, N.; Izumoto, S.; Hayakawa, T.; Morita, T. Antisense inhibition of the RAD51 enhances radiosensitivity. Biochem. Biophys. Res. Commun. 1996, 223, 434–438. [Google Scholar] [CrossRef]
- Ward, A.; Khanna, K.K.; Wiegmans, A.P. Targeting homologous recombination, new pre-clinical and clinical therapeutic combinations inhibiting RAD51. Cancer Treat. Rev. 2014, 41, 35–45. [Google Scholar] [CrossRef]
- Grundy, M.K.; Buckanovich, R.J.; Bernstein, K.A. Regulation and pharmacological targeting of RAD51 in cancer. NAR Cancer 2020, 2, zcaa024. [Google Scholar] [CrossRef] [PubMed]
- Demeyer, A.; Benhelli-Mokrani, H.; Chénais, B.; Weigel, P.; Fleury, F. Inhibiting homologous recombination by targeting RAD51 protein. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188597. [Google Scholar] [CrossRef]
- Fornander, L.H.; Renodon-Corniere, A.; Kuwabara, N.; Ito, K.; Tsutsui, Y.; Shimizu, T.; Iwasaki, H.; Norden, B.; Takahashi, M. Swi5-Sfr1 protein stimulates Rad51-mediated DNA strand exchange reaction through organization of DNA bases in the presynaptic filament. Nucleic Acids Res. 2014, 42, 2358–2365. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Solinger, J.A.; Kiianitsa, K.; Yu, X.; Egelman, E.H.; Heyer, W. Rad51 and Rad54 ATPase activities are both required to modulate Rad51-dsDNA filament dynamics. Nucleic Acids Res. 2007, 35, 4124–4140. [Google Scholar] [CrossRef]
- Barker, B.M.; Vanderkooi, J.; Kallenbach, N.R. Base stacking in a fluorescent dinucleoside monophosphate: εApεA. Biopolymers 1978, 17, 1361–1372. [Google Scholar] [CrossRef]
- Chabbert, M.; Lami, H.; Takahashi, M. Cofactor-induced orientation of the DNA bases in single-stranded DNA complexed with RecA protein. A fluorescence anisotropy and time-decay study. J. Biol. Chem. 1991, 266, 5395–5400. [Google Scholar] [CrossRef]
- Takahashi, M.; Norden, B. Linear dichroism measurements for the study of protein-DNA interactions. Int. J. Mol. Sci. 2023, 24, 16092. [Google Scholar] [CrossRef] [PubMed]
- Minton, A.P. Recent applications of light scattering measurement in the biological and biopharmaceutical sciences. Anal. Biochem. 2016, 501, 4–22. [Google Scholar] [CrossRef]
- Murphy, R.M. Static and dynamic light scattering of biological macromolecules: What can we learn? Curr. Opin. Biotechnol. 1997, 8, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Kanamaru, S.; Mikawa, T.; Prevost, C.; Ishii, K.; Ito, K.; Uchiyama, S.; Oda, M.; Iwasaki, H.; Kim, S.K.; et al. RecA requires two molecules of Mg2+ ions for its optimal strand exchange activity in vitro. Nucleic Acids Res. 2018, 46, 2548–2559. [Google Scholar] [CrossRef] [PubMed]
- Lusetti, S.L.; Shaw, J.J.; Cox, M.M. Magnesium ion-dependent activation of the RecA protein involves the C terminus. J. Biol. Chem. 2003, 278, 16381–16388. [Google Scholar] [CrossRef]
- Fan, H.F.; Su, S. The regulation mechanism of the C-terminus of RecA proteins during DNA strand-exchange process. Biophys. J. 2021, 120, 3166–3179. [Google Scholar] [CrossRef]
- Wilson, J.E.; Chin, A. Chelation of divalent cations by ATP, studied by titration calorimetry. Anal. Biochem. 1991, 193, 16–19. [Google Scholar] [CrossRef]
- Korolev, N.; Lyubartsev, A.P.; Rupprecht, A.; Nordenskiöld, L. Competitive binding of Mg2+, Ca2+, Na+, and K+ ions to DNA in oriented DNA fibers: Experimental and Monte Carlo simulation results. Biophys. J. 1999, 77, 2736–2749. [Google Scholar] [CrossRef]
- Celej, M.S.; Montich, G.G.; Fidelio, G.D. Protein stability induced by ligand binding correlates with changes in protein flexibility. Protein Sci. 2003, 12, 1496–1506. [Google Scholar] [CrossRef]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef]
- Conway, A.B.; Lynch, T.W.; Zhang, Y.; Fortin, G.S.; Fung, C.W.; Symington, L.S.; Rice, P.A. Crystal structure of a Rad51 filament. Nat. Struct. Mol. Biol. 2004, 11, 791–796. [Google Scholar] [CrossRef]
- Pellegrini, L.; Yu, D.S.; Lo, T.; Anand, S.; Lee, M.; Blundell, T.L.; Venkitaraman, A.R. Insights into DNA recombination from the structure of a RAD51–BRCA2 complex. Nature 2002, 420, 287–293. [Google Scholar] [CrossRef]
- Vinograd, J.; Lebowitz, J.; Watson, R. Early and late helix-coil transitions in closed circular DNA the number of superhelical turns in polyoma DNA. J. Mol. Biol. 1968, 33, 173–197. [Google Scholar] [CrossRef]
- Sugaya, N.; Tanaka, S.; Keyamura, K.; Noda, S.; Akanuma, G.; Hishida, T. N-terminal acetyltransferase NatB regulates Rad51-dependent repair of double-strand breaks in Saccharomyces cerevisiae. Genes Genet. Syst. 2023, 98, 61–72. [Google Scholar] [CrossRef]
- Yata, K.; Lloyd, J.; Maslen, S.; Bleuyard, J.Y.; Skehel, M.; Smerdon, S.J.; Esashi, F. Plk1 and CK2 act in concert to regulate Rad51 during DNA double strand break repair. Mol. Cell. 2012, 45, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Appleby, R.; Bollschweiler, D.; Chirgadze, D.Y.; Joudeh, L.; Pellegrini, L. A metal ion-dependent mechanism of RAD51 nucleoprotein filament disassembly. iScience 2023, 26, 106689. [Google Scholar] [CrossRef] [PubMed]
- Nimonkar, A.V.; Dombrowski, C.C.; Siino, J.S.; Stasiak, A.Z.; Stasiak, A.; Kowalczykowski, S.C. Saccharomyces cerevisiae Dmc1 and Rad51 proteins preferentially function with Tid1 and Rad54 proteins, respectively, to promote DNA strand invasion during genetic recombination. J. Biol. Chem. 2012, 287, 28727–28737. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Sakane, I.; Takizawa, Y.; Takahashi, M.; Kurumizaka, H. Roles of the human Rad51 L1 and L2 loops in DNA binding. FEBS J. 2006, 273, 3148–3159. [Google Scholar] [CrossRef] [PubMed]
- Cazenave, C.; Toulme, J.J.; Hélène, C. Binding of RecA protein to single-stranded nucleic acids: Spectroscopic studies using fluorescent polynucleotides. EMBO J. 1983, 2, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Ledneva, R.K.; Razjivin, A.P.; Kost, A.A.; Bogdanov, A.A. Interaction of tobacco mosaic virus protein with synthetic polynucleotides containing a fluorescent label: Optical properties of poly(A,epsilonA) and poly(C,epsilonC) copolymers and energy migration from the tryptophan to 1,N6-ethenoadenine or 3,N4-ethenocytosine residues in RNP. Nucleic Acids Res. 1978, 5, 4225–4243. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renodon-Corniere, A.; Mikawa, T.; Kuwabara, N.; Ito, K.; Levitsky, D.; Iwasaki, H.; Takahashi, M. Human Rad51 Protein Requires Higher Concentrations of Calcium Ions for D-Loop Formation than for Oligonucleotide Strand Exchange. Int. J. Mol. Sci. 2024, 25, 3633. https://doi.org/10.3390/ijms25073633
Renodon-Corniere A, Mikawa T, Kuwabara N, Ito K, Levitsky D, Iwasaki H, Takahashi M. Human Rad51 Protein Requires Higher Concentrations of Calcium Ions for D-Loop Formation than for Oligonucleotide Strand Exchange. International Journal of Molecular Sciences. 2024; 25(7):3633. https://doi.org/10.3390/ijms25073633
Chicago/Turabian StyleRenodon-Corniere, Axelle, Tsutomu Mikawa, Naoyuki Kuwabara, Kentaro Ito, Dmitri Levitsky, Hiroshi Iwasaki, and Masayuki Takahashi. 2024. "Human Rad51 Protein Requires Higher Concentrations of Calcium Ions for D-Loop Formation than for Oligonucleotide Strand Exchange" International Journal of Molecular Sciences 25, no. 7: 3633. https://doi.org/10.3390/ijms25073633
APA StyleRenodon-Corniere, A., Mikawa, T., Kuwabara, N., Ito, K., Levitsky, D., Iwasaki, H., & Takahashi, M. (2024). Human Rad51 Protein Requires Higher Concentrations of Calcium Ions for D-Loop Formation than for Oligonucleotide Strand Exchange. International Journal of Molecular Sciences, 25(7), 3633. https://doi.org/10.3390/ijms25073633






