Comprehensive Molecular Profiling of NPM1-Mutated Acute Myeloid Leukemia Using RNAseq Approach
Abstract
1. Introduction
2. Results
2.1. Patient Cohort
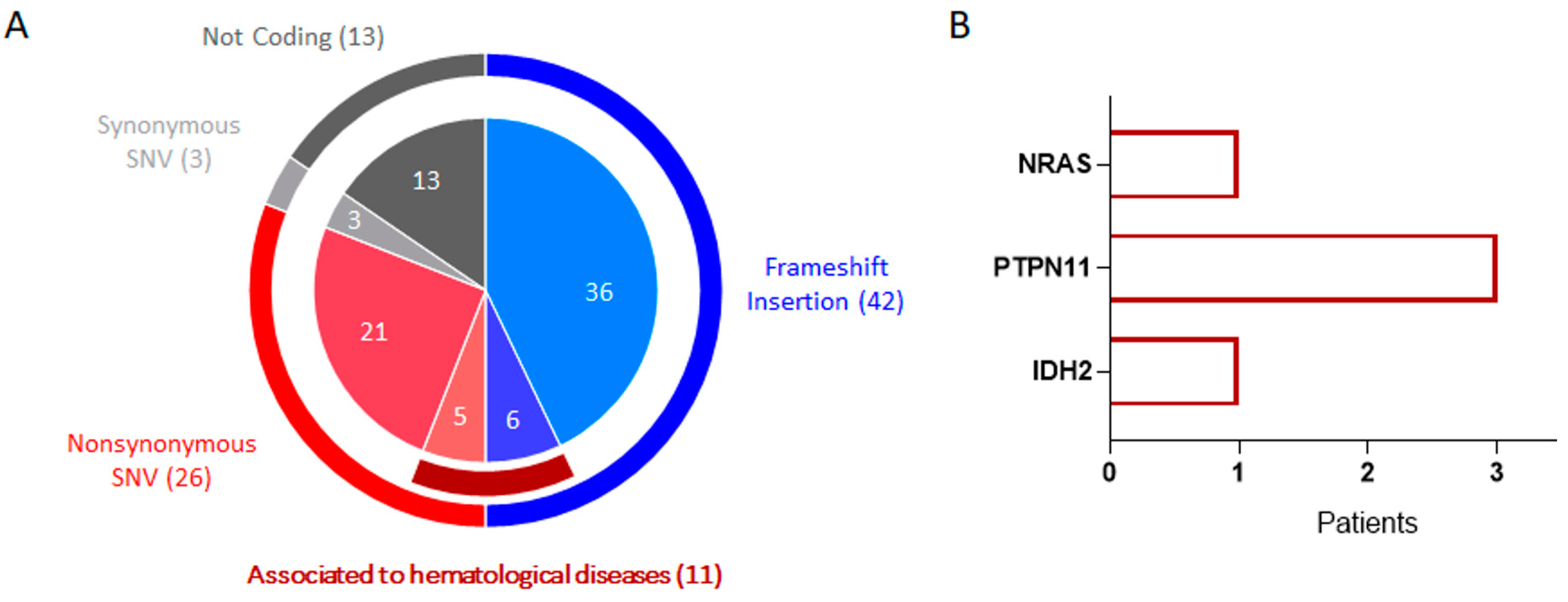
2.2. Targeted DNA Sequencing Analysis Revealed Additional Pathogenic Mutations
2.3. Total RNA Sequencing Analysis Showed the Transcription of Mutated Genes
2.4. Translocation Analysis
3. Discussion
4. Materials and Methods
4.1. Sample Cohort
4.2. NGS Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer.Net. J. Oncol. Pract. 2008, 4, 188. [CrossRef]
- Rau, R.; Brown, P. Nucleophosmin (NPM1) mutations in adult and childhood acute myeloid leukaemia: Towards definition of a new leukaemia entity. Hematol. Oncol. 2009, 27, 171–181. [Google Scholar] [CrossRef]
- Falini, B.; Brunetti, L.; Sportoletti, P.; Martelli, M.P. NPM1-mutated acute myeloid leukemia: From bench to bedside. Blood 2020, 136, 1707–1721. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Testa, U.; Pelosi, E.; Castelli, G. Genetic, Phenotypic, and Clinical Heterogeneity of NPM1-Mutant Acute Myeloid Leukemias. Biomedicines 2023, 11, 1805. [Google Scholar] [CrossRef]
- Sharma, N.; Liesveld, J.L. NPM 1 Mutations in AML-The Landscape in 2023. Cancers 2023, 15, 1177. [Google Scholar] [CrossRef]
- Schneider, F.; Hoster, E.; Unterhalt, M.; Schneider, S.; Dufour, A.; Benthaus, T.; Mellert, G.; Zellmeier, E.; Bohlander, S.K.; Feuring-Buske, M.; et al. NPM1 but not FLT3-ITD mutations predict early blast cell clearance and CR rate in patients with normal karyotype AML (NK-AML) or high-risk myelodysplastic syndrome (MDS). Blood 2009, 113, 5250–5253. [Google Scholar] [CrossRef]
- Dohner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef]
- Dohner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Lo Iacono, M.; Buttigliero, C.; Monica, V.; Bollito, E.; Garrou, D.; Cappia, S.; Rapa, I.; Vignani, F.; Bertaglia, V.; Fiori, C.; et al. Retrospective study testing next generation sequencing of selected cancer-associated genes in resected prostate cancer. Oncotarget 2016, 7, 14394–14404. [Google Scholar] [CrossRef]
- Schubbert, S.; Lieuw, K.; Rowe, S.L.; Lee, C.M.; Li, X.; Loh, M.L.; Clapp, D.W.; Shannon, K.M. Functional analysis of leukemia-associated PTPN11 mutations in primary hematopoietic cells. Blood 2005, 106, 311–317. [Google Scholar] [CrossRef]
- Stasik, S.; Eckardt, J.N.; Kramer, M.; Rollig, C.; Kramer, A.; Scholl, S.; Hochhaus, A.; Crysandt, M.; Brummendorf, T.H.; Naumann, R.; et al. Impact of PTPN11 mutations on clinical outcome analyzed in 1529 patients with acute myeloid leukemia. Blood Adv. 2021, 5, 3279–3289. [Google Scholar] [CrossRef]
- Marensi, V.; Keeshan, K.R.; MacEwan, D.J. Pharmacological impact of FLT3 mutations on receptor activity and responsiveness to tyrosine kinase inhibitors. Biochem. Pharmacol. 2021, 183, 114348. [Google Scholar] [CrossRef] [PubMed]
- Janke, H.; Pastore, F.; Schumacher, D.; Herold, T.; Hopfner, K.P.; Schneider, S.; Berdel, W.E.; Buchner, T.; Woermann, B.J.; Subklewe, M.; et al. Activating FLT3 mutants show distinct gain-of-function phenotypes in vitro and a characteristic signaling pathway profile associated with prognosis in acute myeloid leukemia. PLoS ONE 2014, 9, e89560. [Google Scholar] [CrossRef] [PubMed]
- Kiyoi, H. Flt3 Inhibitors: Recent Advances and Problems for Clinical Application. Nagoya J. Med. Sci. 2015, 77, 7–17. [Google Scholar] [PubMed]
- Petiti, J.; Rosso, V.; Croce, E.; Franceschi, V.; Andreani, G.; Dragani, M.; De Gobbi, M.; Lunghi, M.; Saglio, G.; Fava, C.; et al. Highly Sensitive Detection of IDH2 Mutations in Acute Myeloid Leukemia. J. Clin. Med. 2020, 9, 271. [Google Scholar] [CrossRef]
- Mardis, E.R.; Ding, L.; Dooling, D.J.; Larson, D.E.; McLellan, M.D.; Chen, K.; Koboldt, D.C.; Fulton, R.S.; Delehaunty, K.D.; McGrath, S.D.; et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N. Engl. J. Med. 2009, 361, 1058–1066. [Google Scholar] [CrossRef]
- Marcucci, G.; Maharry, K.; Wu, Y.Z.; Radmacher, M.D.; Mrozek, K.; Margeson, D.; Holland, K.B.; Whitman, S.P.; Becker, H.; Schwind, S.; et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: A Cancer and Leukemia Group B study. J. Clin. Oncol. 2010, 28, 2348–2355. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Ravandi, F.; Agresta, S.; Konopleva, M.; Takahashi, K.; Kadia, T.; Routbort, M.; Patel, K.P.; Mark, B.; Pierce, S.; et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am. J. Hematol. 2015, 90, 732–736. [Google Scholar] [CrossRef]
- Stein, E.M.; DiNardo, C.D.; Pollyea, D.A.; Fathi, A.T.; Roboz, G.J.; Altman, J.K.; Stone, R.M.; DeAngelo, D.J.; Levine, R.L.; Flinn, I.W.; et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017, 130, 722–731. [Google Scholar] [CrossRef]
- Amatangelo, M.D.; Quek, L.; Shih, A.; Stein, E.M.; Roshal, M.; David, M.D.; Marteyn, B.; Farnoud, N.R.; de Botton, S.; Bernard, O.A.; et al. Enasidenib induces acute myeloid leukemia cell differentiation to promote clinical response. Blood 2017, 130, 732–741. [Google Scholar] [CrossRef]
- Kong, G.; Chang, Y.I.; You, X.; Ranheim, E.A.; Zhou, Y.; Burd, C.E.; Zhang, J. The ability of endogenous Nras oncogenes to initiate leukemia is codon-dependent. Leukemia 2016, 30, 1935–1938. [Google Scholar] [CrossRef][Green Version]
- Shahid, S.; Shakeel, M.; Siddiqui, S.; Ahmed, S.; Sohail, M.; Khan, I.A.; Abid, A.; Shamsi, T. Novel Genetic Variations in Acute Myeloid Leukemia in Pakistani Population. Front. Genet. 2020, 11, 560. [Google Scholar] [CrossRef]
- Anwar, N.; Memon, F.A.; Shahid, S.; Shakeel, M.; Irfan, M.; Arshad, A.; Naz, A.; Ujjan, I.D.; Shamsi, T. The Dawn of next generation DNA sequencing in myelodysplastic syndromes- experience from Pakistan. BMC Genom. 2021, 22, 903. [Google Scholar] [CrossRef]
- Vicente-Garces, C.; Maynou, J.; Fernandez, G.; Esperanza-Cebollada, E.; Torrebadell, M.; Catala, A.; Rives, S.; Camos, M.; Vega-Garcia, N. Fusion InPipe, an integrative pipeline for gene fusion detection from RNA-seq data in acute pediatric leukemia. Front. Mol. Biosci. 2023, 10, 1141310. [Google Scholar] [CrossRef]
- Grioni, A.; Fazio, G.; Rigamonti, S.; Bystry, V.; Daniele, G.; Dostalova, Z.; Quadri, M.; Saitta, C.; Silvestri, D.; Songia, S.; et al. A Simple RNA Target Capture NGS Strategy for Fusion Genes Assessment in the Diagnostics of Pediatric B-cell Acute Lymphoblastic Leukemia. Hemasphere 2019, 3, e250. [Google Scholar] [CrossRef]
- Arindrarto, W.; Borras, D.M.; de Groen, R.A.L.; van den Berg, R.R.; Locher, I.J.; van Diessen, S.; van der Holst, R.; van der Meijden, E.D.; Honders, M.W.; de Leeuw, R.H.; et al. Comprehensive diagnostics of acute myeloid leukemia by whole transcriptome RNA sequencing. Leukemia 2021, 35, 47–61. [Google Scholar] [CrossRef]
- Xiong, Z.; Li, M.; Yang, F.; Ma, Y.; Sang, J.; Li, R.; Li, Z.; Zhang, Z.; Bao, Y. EWAS Data Hub: A resource of DNA methylation array data and metadata. Nucleic Acids Res. 2020, 48, D890–D895. [Google Scholar] [CrossRef]
- Domingo-Relloso, A.; Huan, T.; Haack, K.; Riffo-Campos, A.L.; Levy, D.; Fallin, M.D.; Terry, M.B.; Zhang, Y.; Rhoades, D.A.; Herreros-Martinez, M.; et al. DNA methylation and cancer incidence: Lymphatic-hematopoietic versus solid cancers in the Strong Heart Study. Clin. Epigenet. 2021, 13, 43. [Google Scholar] [CrossRef]
- Froehlich, J.; Versapuech, M.; Megrelis, L.; Largeteau, Q.; Meunier, S.; Tanchot, C.; Bismuth, G.; Delon, J.; Mangeney, M. FAM65B controls the proliferation of transformed and primary T cells. Oncotarget 2016, 7, 63215–63225. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Dobin, A.; Stransky, N.; Li, B.; Yang, X.; Tickle, T.; Bankapur, A.; Ganote, C.; Doak, T.G.; Pochet, N.; et al. STAR-Fusion: Fast and Accurate Fusion Transcript Detection from RNA-Seq. bioRxiv 2017, 120295. [Google Scholar] [CrossRef]

| Chr Pos Ref Alt | Representative Amino Acid Change (Transcript) | dbSNP | Cancer (Sequencing Depth > 25) | Leukemia (Sequencing Depth > 25) | Cancer (Sequencing Depth > 100) | Leukemia (Sequencing Depth > 100) |
|---|---|---|---|---|---|---|
| 11 108304736 A T | ATM p.D1853V (NM_000051) | rs1801673 | 2 | |||
| 11 108317431 A T | ATM p.Y2086F (NM_000051) | rs730881380 | 1 | 1 | ||
| 11 108331520 T C | ATM p.M2531T (NM_000051) | rs587781365 | 1 | |||
| 20 56386485 A T | AURKA p.F31I (NM_003600) | rs2273535 | 2 | |||
| 11 119284996 A G | CBL p.M487V (NM_005188) | rs17848896 | 1 | 1 | ||
| 12 57750985 C G | CDK4 p.V154L (NM_000075) | rs563692823 | 1 | |||
| 12 12717864 G T | CDKN1B p.G9W (NM_004064) | . | 1 | 1 | ||
| 14 95099907 C T | DICER1 p.G1360E (NM_001195573) | . | 1 | |||
| 2 25234374 G A | DNMT3A p.R882H (NM_175629) | rs377577594 | 2 | 2 | ||
| 2 25234373 C T | DNMT3A p.R882H (NM_175629) | rs147001633 | 1 | 1 | ||
| 13 28018492 T C | FLT3 p.D839G (NM_004119) | rs991132188 | 1 | 1 | ||
| 11 67585218 A G | GSTP1 p.I105V (NM_000852) | rs1695 | 9 | 9 | ||
| 15 90088702 C T | IDH2 p.R140Q (NM_002168) | rs121913502 | 1 | 1 | 1 | 1 |
| 2 47410166 G A | MSH2 p.V81I (NM_001258281) | rs773125415 | 1 | 1 | ||
| 1 11796321 G A | MTHFR p.A263V (NM_001330358) | rs1801133 | 9 | 1 | ||
| 5 7870860 A G | MTRR p.I22M (NM_001364440) | rs1801394 | 11 | |||
| 1 45332190 C A | MUTYH p.E275D (NM_001048174) | rs863224700 | 1 | |||
| 16 69711242 G A | NQO1 p.P187S (NM_000903) | rs1800566 | 1 | 1 | ||
| 1 114716126 C T | NRAS p.G12D (NM_002524) | rs121913237 | 1 | 1 | 1 | 1 |
| 1 114716124 C G | NRAS p.G13R (NM_002524) | rs121434595 | 1 | 1 | ||
| 7 5995556 C A | PMS2 pR294L (NM_001322006) | . | 1 | |||
| 19 50399016 G C | POLD1 p.E55D (NM_001308632) | . | 1 | |||
| 19 50402013 C T | POLD1 p.E55D (NM_001308632) | rs144770820 | 1 | 1 | ||
| 15 90970444 T C | PRC1 pY511C (NM_003981) | rs12911192 | 1 | |||
| 12 112450395 C T | PTPN11 p.A72V (NM_001330437) | rs121918454 | 1 | 1 | 1 | 1 |
| 12 112450394 G A | PTPN11 p.A72T (NM_001330437) | rs121918453 | 1 | 1 | 1 | 1 |
| 12 112450406 G A | PTPN11 p.E76K (NM_001330437) | rs121918464 | 1 | 1 | 1 | 1 |
| 11 48123823 A C | PTPRJ p.Q276P (NM_001098503) | rs1566734 | 5 | 2 | ||
| 5 132609190 G A | RAD50 p.G968E (NM_005732) | rs199895166 | 1 | |||
| 1 182585422 C T | RNASEL p.R462Q (NM_021133) | rs486907 | 6 | |||
| 17 18328782 G A | SHMT1 p.L474F (NM_004169) | rs1979277 | 2 | |||
| 21 45537880 T C | SLC19A1 p.H27R (NM_001205206) | rs1051266 | 10 | |||
| 17 7676154 G C | TP53 p.P72R (NM_000546) | rs1042522 | 14 | 5 | ||
| 6 96552610 C G | UFL1 p.P705R (NM_015323) | rs199880163 | 1 |
| Chr Fusion | Count | Splice Junctions | Type |
|---|---|---|---|
| C22orf39--IGH@-ext | 1 | INCL_NON_REF_SPLICE | INTERCHROMOSOMAL[chr22--chr14] |
| CTC-786C10.1--RP11-680G10.1 | 1 | ONLY_REF_SPLICE | INTRACHROMOSOMAL[chr16:0.17Mb] |
| HIRA--IGH@-ext | 2 | INCL_NON_REF_SPLICE | INTERCHROMOSOMAL[chr22--chr14] |
| LILRB1--AC010518.2 | 2 | ONLY_REF_SPLICE | INTRACHROMOSOMAL[chr19:0.32Mb] |
| PFKFB3--RP11-563J2.2 | 2 | ONLY_REF_SPLICE | INTRACHROMOSOMAL[chr10:0.04Mb];NEIGHBORS[42142] |
| RP11-367G6.3--FAM65B | 7 | ONLY_REF_SPLICE | INTRACHROMOSOMAL[chr6:0.08Mb];NEIGHBORS[78900] |
| RP11-444D3.1--SOX5 | 1 | ONLY_REF_SPLICE | [“SOX5:Oncogene”];INTRACHROMOSOMAL[chr12:0.26Mb] |
| RP11-632K20.2--RP11-632K20.7 | 4 | INCL_NON_REF_SPLICE | INTRACHROMOSOMAL[chr15:0.02Mb];LOCAL_REARRANGEMENT:−:[19502] |
| RP1-90G24.6--RP1-90G24.10 | 1 | ONLY_REF_SPLICE | INTRACHROMOSOMAL[chr22:0.00Mb];LOCAL_REARRANGEMENT:+:[3754] |
| SLC7A5P1--SMG1 | 2 | INCL_NON_REF_SPLICE | INTRACHROMOSOMAL[chr16:10.69Mb] |
| ST3GAL1--NDRG1 | 1 | ONLY_REF_SPLICE | [“NDRG1:Oncogene”];INTRACHROMOSOMAL[chr8:0.15Mb] |
| # ID ¥ | DNAseq > 100 (NM_) (AF%) | RNAseq Sequencing Depth > 25 (NM_) (TR%) * | Chr Fusion |
|---|---|---|---|
| R12 | RP11-367G6.3--FAM65B | ||
| R13 | PTPN11 p.A72V (NM_001330437) (24%), NQO1 p.P187S (NM_000903) (52%)%), CEBPA p.H59Afs*84 (NM_001287424) (58%) | SLC7A5P1--SMG1; RP11-367G6.3--FAM65B | |
| R14/D1 | NRAS p.G13R (NM_002524) (34%) | NRAS p.G13R (NM_002524) (20%); DNMT3A p.R882H (NM_175629) (47%) | ST3GAL1--NDRG1; SLC7A5P1--SMG1; PFKFB3--RP11-563J2.2 |
| R15/D2 | KRAS (NM_033360) p.Q61H (6%) | DNMT3A p.R882H (NM_175629) (25%) | No fusion |
| R16 | IDH2 p.R140Q (NM_002168) (36%) | RP11-632K20.2--RP11-632K20.7 | |
| R17 | RP11-367G6.3--FAM65B | ||
| R18 | RP11-632K20.2--RP11-632K20.7 | ||
| R20 | RP11-632K20.2--RP11-632K20.7 | ||
| R21 | PTPN11 p.A72T (NM_001330437) (24%), FLT3 p.D839G (NM_004119) (11%); | RP11-367G6.3--FAM65B; RP1-90G24.6--RP1-90G24.10 | |
| R24 | RP11-367G6.3--FAM65B | ||
| R25 | LILRB1--AC010518.2 | ||
| R26/D5 | KRAS p.G13D (NM_033360) (12%) | CBL p.M487V (NM_005188) (39%) | RP11-444D3.1--SOX5; PFKFB3--RP11-563J2.2; CTC-786C10.1--RP11-680G10.1 |
| R5 | NRAS p.G12D (NM_002524) (48%), DNMT3A p.R882H (NM_175629) (36%) | No fusion | |
| R6 | NBN p.S53Cfs*9 (NM_002485) (2%) | RP11-367G6.3--FAM65B; HIRA--IGH@-ext | |
| R7 | PTPN11 p.E76K (NM_001330437) (48%) | LILRB1--AC010518.2 | |
| R9 | RP11-367G6.3--FAM65B; C22orf39--IGH@-ext; RP11-632K20.2--RP11-632K20.7 | ||
| D4 | IDH2 p.R140Q (NM_002168) (6%) | No RNA | No RNA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petiti, J.; Pignochino, Y.; Schiavon, A.; Giugliano, E.; Berrino, E.; Giordano, G.; Itri, F.; Dragani, M.; Cilloni, D.; Lo Iacono, M. Comprehensive Molecular Profiling of NPM1-Mutated Acute Myeloid Leukemia Using RNAseq Approach. Int. J. Mol. Sci. 2024, 25, 3631. https://doi.org/10.3390/ijms25073631
Petiti J, Pignochino Y, Schiavon A, Giugliano E, Berrino E, Giordano G, Itri F, Dragani M, Cilloni D, Lo Iacono M. Comprehensive Molecular Profiling of NPM1-Mutated Acute Myeloid Leukemia Using RNAseq Approach. International Journal of Molecular Sciences. 2024; 25(7):3631. https://doi.org/10.3390/ijms25073631
Chicago/Turabian StylePetiti, Jessica, Ymera Pignochino, Aurora Schiavon, Emilia Giugliano, Enrico Berrino, Giorgia Giordano, Federico Itri, Matteo Dragani, Daniela Cilloni, and Marco Lo Iacono. 2024. "Comprehensive Molecular Profiling of NPM1-Mutated Acute Myeloid Leukemia Using RNAseq Approach" International Journal of Molecular Sciences 25, no. 7: 3631. https://doi.org/10.3390/ijms25073631
APA StylePetiti, J., Pignochino, Y., Schiavon, A., Giugliano, E., Berrino, E., Giordano, G., Itri, F., Dragani, M., Cilloni, D., & Lo Iacono, M. (2024). Comprehensive Molecular Profiling of NPM1-Mutated Acute Myeloid Leukemia Using RNAseq Approach. International Journal of Molecular Sciences, 25(7), 3631. https://doi.org/10.3390/ijms25073631









