The Novel SSTR3 Agonist ITF2984 Exerts Antimitotic and Proapoptotic Effects in Human Non-Functioning Pituitary Neuroendocrine Tumor (NF-PitNET) Cells
Abstract
1. Introduction
2. Results
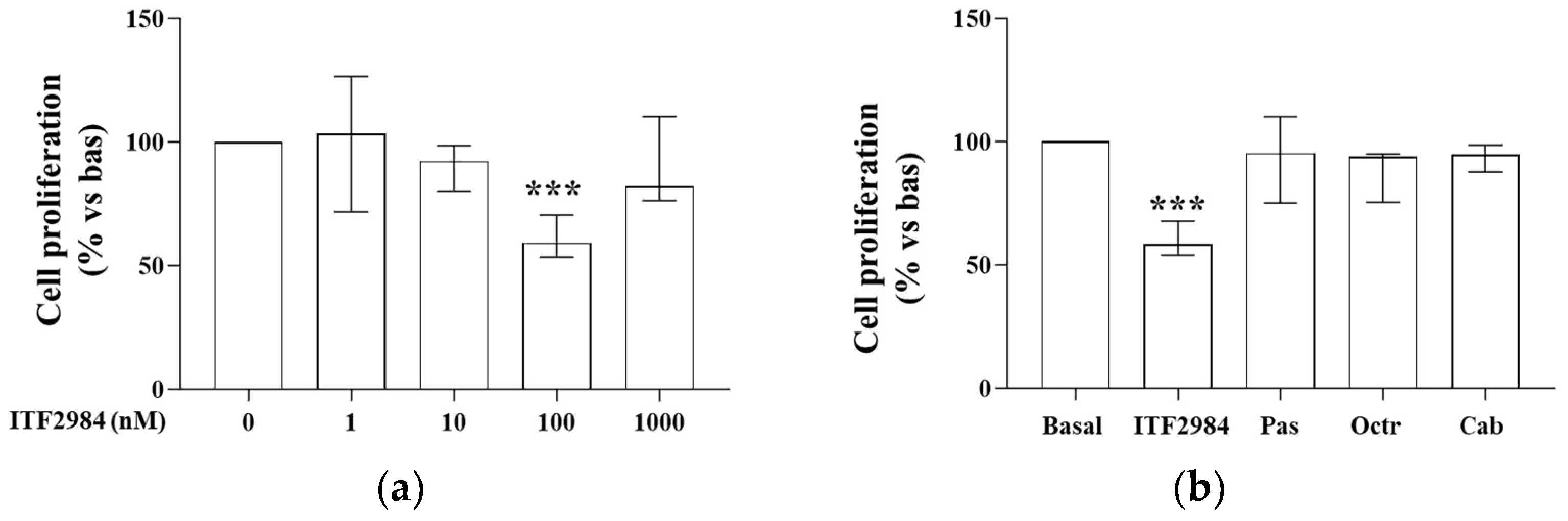
2.1. ITF2984 Effects on Cell Proliferation in Primary Cultured NF-PitNET Cells
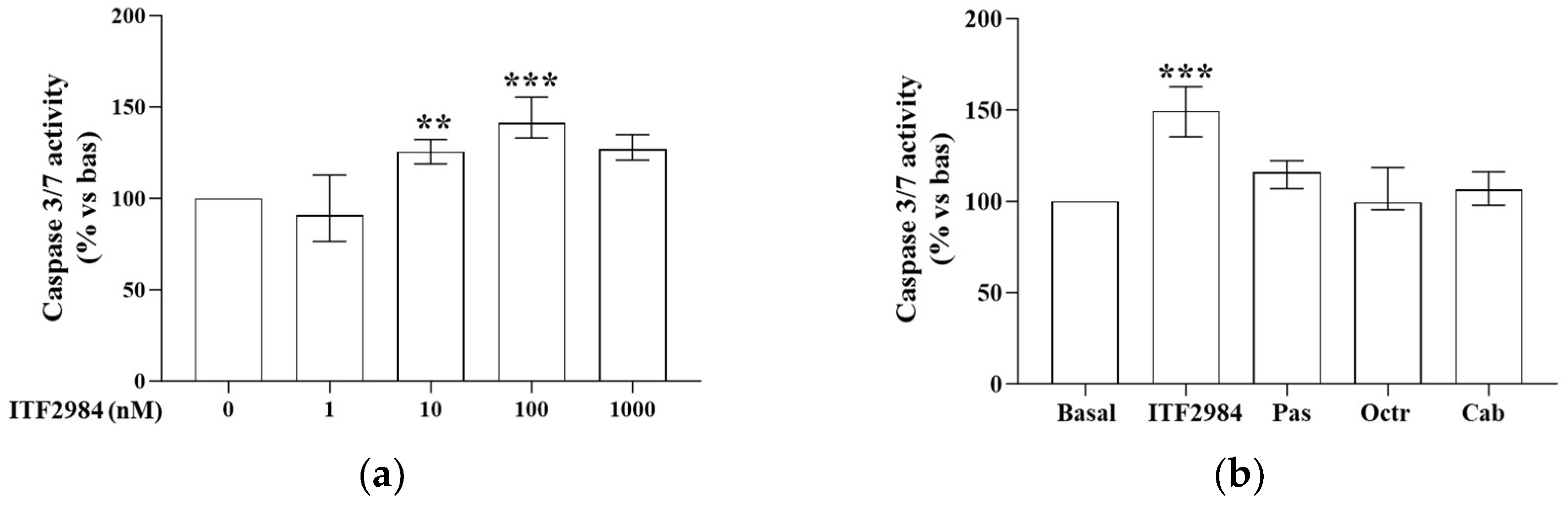
2.2. ITF2984 Effects on Cell Apoptosis in Primary Cultured NF-PitNET Cells
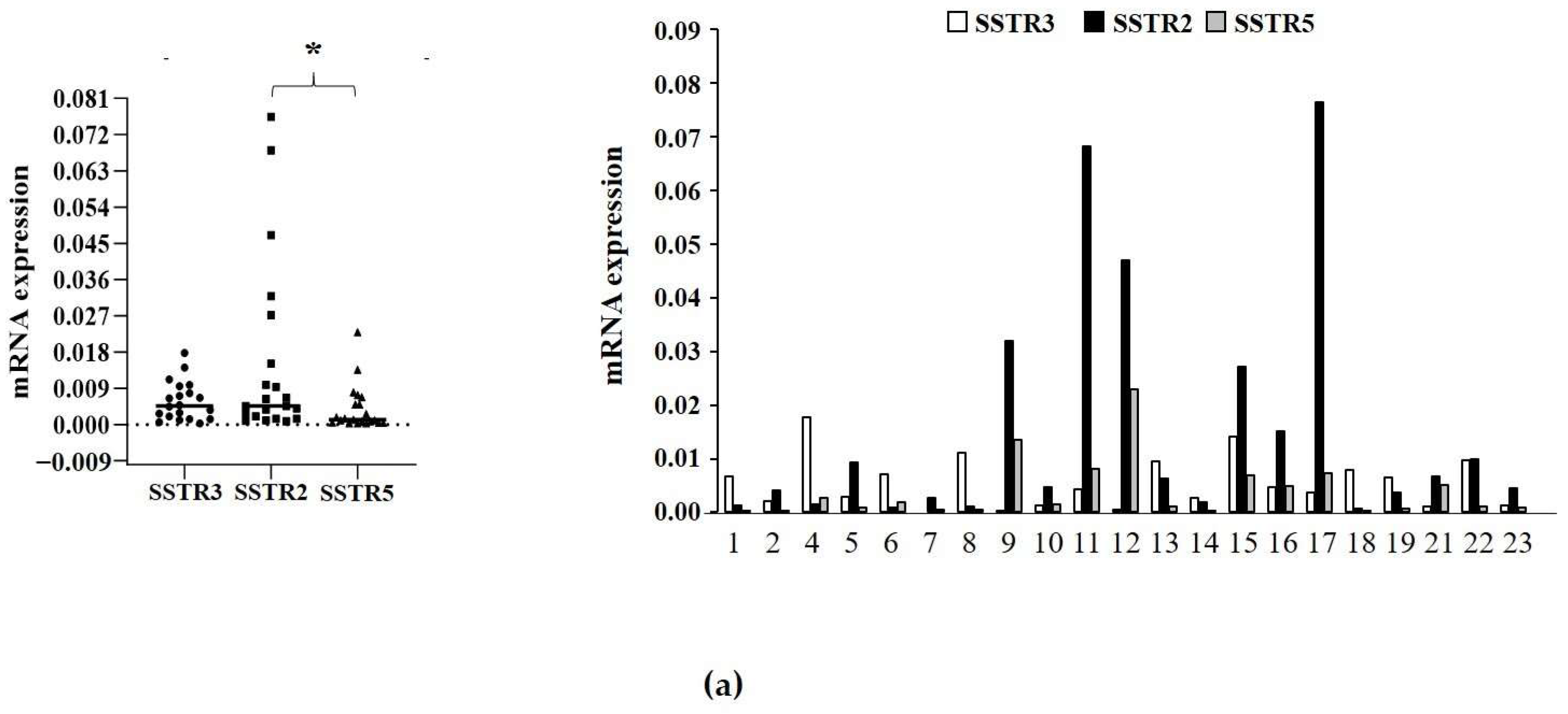
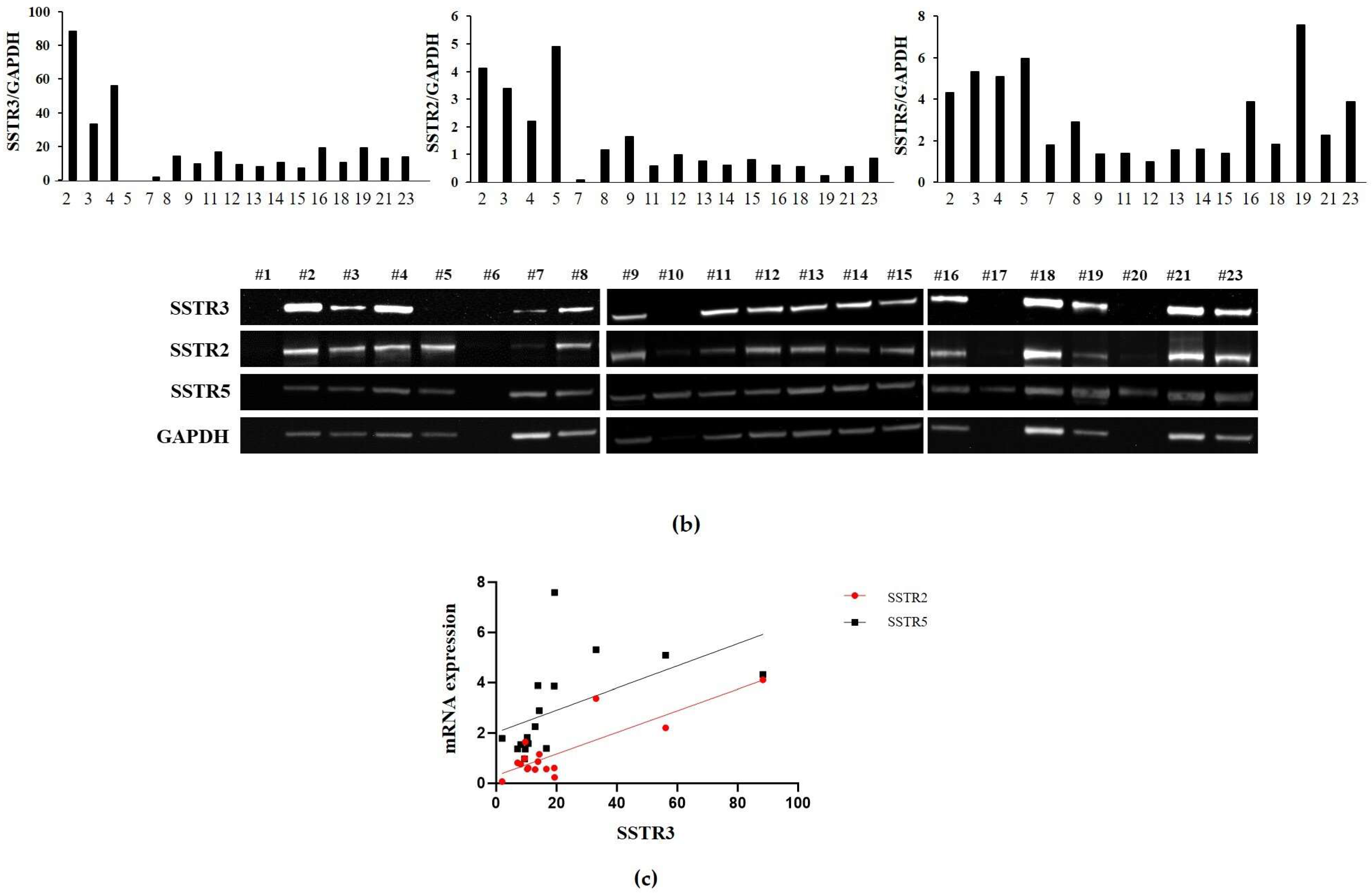
2.3. Analysis of SSTR3, SSTR2, and SSTR5 Expression
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Proliferation Assay
4.3. Apoptosis Assay
4.4. Real-Time Quantitative PCR (RT-qPCR) Analysis
4.5. Western Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molitch, M.E. Nonfunctioning pituitary tumors and pituitary incidentalomas. Endocrinol. Metab. Clin. N. Am. 2008, 37, 151–171. [Google Scholar] [CrossRef]
- Asa, S.L.; Casar-Borota, O.; Chanson, P.; Delgrange, E.; Earls, P.; Ezzat, S.; Grossman, A.; Ikeda, H.; Inoshita, N.; Karavitaki, N.; et al. From pituitary adenoma to pituitary neuroendocrine tumor (PitNET): An International Pituitary Pathology Club proposal. Endocr. Relat. Cancer 2017, 24, C5-8. [Google Scholar] [CrossRef]
- Delgado-López, P.D.; Pi-Barrio, J.; Dueñas-Polo, M.T.; Pascual-Llorente, M.; Gordón-Bolaños, M.C. Recurrent non-functioning pituitary adenomas: A review on the new pathological classification, management guidelines and treatment options. Clin. Transl. Oncol. 2018, 20, 1233–1245. [Google Scholar] [CrossRef]
- Losa, M.; Mortini, P.; Barzagh, R.; Ribotto, P.; Terreni, M.R.; Marzoli, S.B.; Pieralli, S.; Giovanelli, M. Early results of surgery in patients with nonfunctioning pituitary adenomas and analysis of the risk of tumor recurrence. J. Neurosurg. 2008, 108, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Ortega, G.; Gonzalez-Virla, B.; Romero-Gameros, C.A. Pharmacological Treatment of Non-Functioning Pituitary Adenomas. Arch. Med. Res. 2023, 54, 102917. [Google Scholar] [CrossRef]
- Greenman, Y.; Stern, N. Non-functioning pituitary adenomas. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 625–638. [Google Scholar] [CrossRef]
- Peverelli, E.; Treppiedi, D.; Mangili, F.; Catalano, R.; Spada, A.; Mantovani, G. Drug resistance in pituitary tumours: From cell membrane to intracellular signalling. Nat. Rev. Endocrinol. 2021, 9, 560–571. [Google Scholar] [CrossRef]
- Molitch, M.E. Pharmacologic resistance in prolactinoma patients. Pituitary 2005, 8, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Mazziotti, G.; Torri, V.; Spinello, M.; Floriani, I.; Melmed, S. Meta-analysis on the effects of octreotide on tumor mass in acromegaly. PLoS ONE 2012, 7, e36411. [Google Scholar] [CrossRef] [PubMed]
- Møller, L.N.; Stidsen, C.E.; Hartmann, B.; Holst, J.J. Somatostatin receptors. Biochim. Biophys. Acta 2003, 1616, 1–84. [Google Scholar] [CrossRef]
- Sharma, K.; Patel, Y.C.; Srikant, C.B. Subtype-selective induction of wild-type p53 and apoptosis, but not cell cycle arrest, by human somatostatin receptor 3. Mol. Endocrinol. 1996, 10, 1688–1696. [Google Scholar] [CrossRef]
- War, S.A.; Somvanshi, R.K.; Kumar, U. Somatostatin receptor-3 mediated intracellular signaling and apoptosis is regulated by its cytoplasmic terminal. Biochim. Biophys. Acta 2011, 1813, 390–402. [Google Scholar] [CrossRef]
- Vázquez-Borrego, M.C.; Gupta, V.; Ibáñez-Costa, A.; Gahete, M.D.; Venegas-Moreno, E.; Toledano-Delgado, Á.; Cano, D.A.; Blanco-Acevedo, C.; Ortega-Salas, R.; Japón, M.A.; et al. Somatostatin receptor subtype-3 (SST3) peptide agonist shows antitumor effects in experimental models of nonfunctioning pituitary tumors. Clin. Cancer Res. 2020, 26, 957–969. [Google Scholar] [CrossRef]
- Bruns, C.; Lewis, I.; Briner, U.; Meno-Tetang, G.; Weckbecker, G. SOM230: A novel somatostatin eptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur. J. Endocrinol. 2002, 146, 707–716. [Google Scholar] [CrossRef]
- Taboada, G.F.; Luque, R.M.; Bastos, W.; Guimarães, R.F.C.; Marcondes, J.B.; Chimelli, L.M.C.; Fontes, R.; Mata, P.J.P.; Filho, P.N.; Carvalho, D.P.; et al. Quantitative analysis of somatostatin receptor subtype (SSTR1-5) gene expression levels in somatotropinomas and non-functioning pituitary adenomas. Eur. J. Endocrinol. 2007, 156, 65–74. [Google Scholar] [CrossRef]
- Lee, M.; Lupp, A.; Mendoza, N.; Martin, N.; Beschorner, R.; Honegger, J.; Schlegel, J.; Shively, T.; Pulz, E.; Schulz, S.; et al. SSTR3 is a putative target for the medical treatment of gonadotroph adenomas of the pituitary. Endocr. Relat. Cancer 2015, 22, 111–119. [Google Scholar] [CrossRef]
- Florio, T.; Barbieri, F.; Spaziante, R.; Zona, G.; Hofland, L.J.; van Koetsveld, P.M.; Feelders, R.A.; Stalla, G.K.; Theodoropoulou, M.; Culler, M.D.; et al. Efficacy of a dopamine-somatostatin chimeric molecule, BIM-23A760, in the control of cell growth from primary cultures of human non-functioning pituitary adenomas: A multi-center study. Endocr. Relat. Cancer 2008, 15, 583–596. [Google Scholar] [CrossRef]
- Flores-Martinez, Á.; Venegas-Moreno, E.; Dios, E.; Remón-Ruiz, P.; Gros-Herguido, N.; Vázquez-Borrego, M.C.; Madrazo-Atutxa, A.; Japón, M.A.; Kaen, A.; Cárdenas-Valdepeñas, E.; et al. Quantitative Analysis of Somatostatin and Dopamine Receptors Gene Expression Levels in Non-functioning Pituitary Tumors and Association with Clinical and Molecular Aggressiveness Features. J. Clin. Med. 2020, 9, 3052. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Di Somma, C.; Pivonello, R.; Faggiano, A.; Lombardi, G.; Savastano, S. Medical therapy for clinically non-functioning pituitary adenomas. Endocr. Relat. Cancer 2008, 15, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Cooper, O.; Greenman, Y. Dopamine Agonists for Pituitary Adenomas. Front. Endocrinol. 2018, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Porras, M.; Cárdenas-Salas, J.; Álvarez-Escolá, C. Somatostatin Analogs in Clinical Practice: A Review. Int. J. Mol. Sci. 2020, 21, 1682. [Google Scholar] [CrossRef] [PubMed]
- Modena, D.; Moras, M.L.; Sandrone, G.; Stevenazzi, A.; Vergani, B.; Dasgupta, P.; Kliever, A.; Gulde, S.; Marangelo, A.; Schillmaier, M.; et al. Identification of a Novel SSTR3 Full Agonist for the Treatment of Nonfunctioning Pituitary Adenomas. Cancers 2023, 15, 3453. [Google Scholar] [CrossRef] [PubMed]
- Marinoni, I.; Lee, M.; Mountford, S.; Perren, A.; Bravi, I.; Jennen, L.; Feuchtinger, A.; Drouin, J.; Roncaroli, F.; Pellegata, N.S. Characterization of MENX-associated pituitary tumours. Neuropathol. Appl. Neurobiol. 2013, 39, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Pellegata, N.S.; Quintanilla-Martinez, L.; Siggelkow, H.; Samson, E.; Bink, K.; Höfler, H.; Fend, F.; Graw, J.; Atkinson, M.J. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc. Natl. Acad. Sci. USA 2006, 103, 15558–15563. [Google Scholar] [CrossRef] [PubMed]
- Peverelli, E.; Lania, A.G.; Mantovani, G.; Beck-Peccoz, P.; Spada, A. Characterization of intracellular signaling mediated by human somatostatin receptor 5: Role of the DRY motif and the third intracellular loop. Endocrinology 2009, 150, 3169–3176. [Google Scholar] [CrossRef]
- Ibáñez-Costa, A.; Rivero-Cortés, E.; Vázquez-Borrego, M.C.; Gahete, M.D.; Jiménez-Reina, L.; Venegas-Moreno, E.; De La Riva, A.; Arráez, M.Á.; González-Molero, I.; Schmid, H.A.; et al. Octreotide and pasireotide (dis)similarly inhibit pituitary tumor cells in vitro. J. Endocrinol. 2016, 231, 135–145. [Google Scholar] [CrossRef]
- Zatelli, M.C.; Piccin, D.; Vignali, C.; Tagliati, F.; Ambrosio, M.R.; Bondanelli, M.; Cimino, V.; Bianchi, A.; Schmid, H.A.; Scanarini, M.; et al. Pasireotide, a multiple somatostatin receptor subtypes ligand, reduces cell viability in non-functioning pituitary adenomas by inhibiting vascular endothelial growth factor secretion. Endocr. Relat. Cancer 2007, 14, 91–102. [Google Scholar] [CrossRef]
- Peverelli, E.; Olgiati, L.; Locatelli, M.; Magni, P.; Faustini Fustini, M.; Frank, G.; Mantovani, G.; Beck-Peccoz, P.; Spada, A.; Lania, A. The dopamine-somatostatin chimeric compound BIM-23A760 exerts antiproliferative and cytotoxic effects in human non-functioning pituitary tumors by activating ERK1/2 and p38 pathways. Cancer Lett. 2010, 288, 170–176. [Google Scholar] [CrossRef]
- Mangili, F.; Giardino, E.; Treppiedi, D.; Barbieri, A.M.; Catalano, R.; Locatelli, M.; Lania, A.G.; Spada, A.; Arosio, M.; Mantovani, G.; et al. Beta-Arrestin 2 Is Required for Dopamine Receptor Type 2 Inhibitory Effects on AKT Phosphorylation and Cell Proliferation in Pituitary Tumors. Neuroendocrinology 2021, 111, 568–579. [Google Scholar] [CrossRef]
- Gulde, S.; Wiedemann, T.; Schillmaier, M.; Valença, I.; Lupp, A.; Steiger, K.; Yen, H.Y.; Bäuerle, S.; Notni, J.; Luque, R.; et al. Gender-Specific Efficacy Revealed by Head-to-Head Comparison of Pasireotide and Octreotide in a Representative In Vivo Model of Nonfunctioning Pituitary Tumors. Cancers 2021, 13, 3097. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Aini, I.; Gay, S.; Grossrubatscher, E.M.; Mancini, C.; Tarsitano, M.G.; Zamponi, V.; Isidori, A.M.; Colao, A.; Faggiano, A.; et al. Efficacy and tolerability of somatostatin analogues according to gender in patients with neuroendocrine tumors. Rev. Endocr. Metab. Disord. 2023, 25, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Maffezzoni, F.; Frara, S.; Doga, M.; Mazziotti, G.; Giustina, A. New Medical Therapies of Acromegaly. Growth Horm. IGF Res. 2016, 30–31, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Maffezzoni, F.; Formenti, A.M.; Mazziotti, G.; Frara, S.; Giustina, A. Current and Future Medical Treatments for Patients with Acromegaly. Expert. Opin. Pharmacother. 2016, 17, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Muro, G.; Catalano, R.; Treppiedi, D.; Barbieri, A.M.; Mangili, F.; Marra, G.; Di Bari, S.; Esposito, E.; Nozza, E.; Lania, A.G.; et al. The Novel SSTR3 Agonist ITF2984 Exerts Antimitotic and Proapoptotic Effects in Human Non-Functioning Pituitary Neuroendocrine Tumor (NF-PitNET) Cells. Int. J. Mol. Sci. 2024, 25, 3606. https://doi.org/10.3390/ijms25073606
Di Muro G, Catalano R, Treppiedi D, Barbieri AM, Mangili F, Marra G, Di Bari S, Esposito E, Nozza E, Lania AG, et al. The Novel SSTR3 Agonist ITF2984 Exerts Antimitotic and Proapoptotic Effects in Human Non-Functioning Pituitary Neuroendocrine Tumor (NF-PitNET) Cells. International Journal of Molecular Sciences. 2024; 25(7):3606. https://doi.org/10.3390/ijms25073606
Chicago/Turabian StyleDi Muro, Genesio, Rosa Catalano, Donatella Treppiedi, Anna Maria Barbieri, Federica Mangili, Giusy Marra, Sonia Di Bari, Emanuela Esposito, Emma Nozza, Andrea G. Lania, and et al. 2024. "The Novel SSTR3 Agonist ITF2984 Exerts Antimitotic and Proapoptotic Effects in Human Non-Functioning Pituitary Neuroendocrine Tumor (NF-PitNET) Cells" International Journal of Molecular Sciences 25, no. 7: 3606. https://doi.org/10.3390/ijms25073606
APA StyleDi Muro, G., Catalano, R., Treppiedi, D., Barbieri, A. M., Mangili, F., Marra, G., Di Bari, S., Esposito, E., Nozza, E., Lania, A. G., Ferrante, E., Locatelli, M., Modena, D., Steinkuhler, C., Peverelli, E., & Mantovani, G. (2024). The Novel SSTR3 Agonist ITF2984 Exerts Antimitotic and Proapoptotic Effects in Human Non-Functioning Pituitary Neuroendocrine Tumor (NF-PitNET) Cells. International Journal of Molecular Sciences, 25(7), 3606. https://doi.org/10.3390/ijms25073606






