Exercise in Diabetic Nephropathy: Protective Effects and Molecular Mechanism
Abstract
1. Introduction
2. Protective Role of Exercise against DN in Human Studies
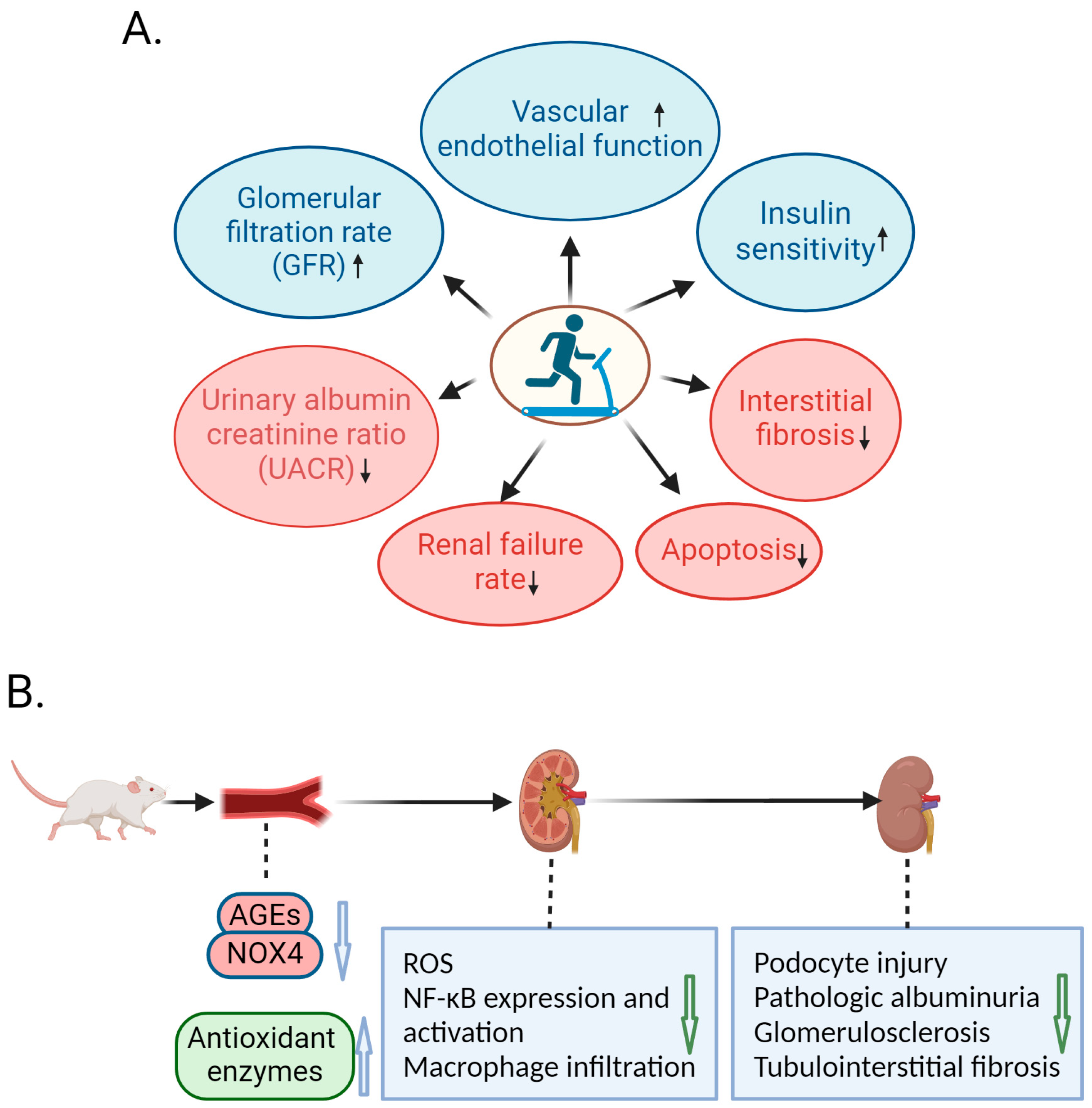
3. Amelioration of DN by Exercise in Animal Studies
4. Molecular Mechanism of Exercise-Mediated Alleviation of DN
4.1. Role of Exercise-Mediated miR-181b Up-Regulation in Amelioration of DN
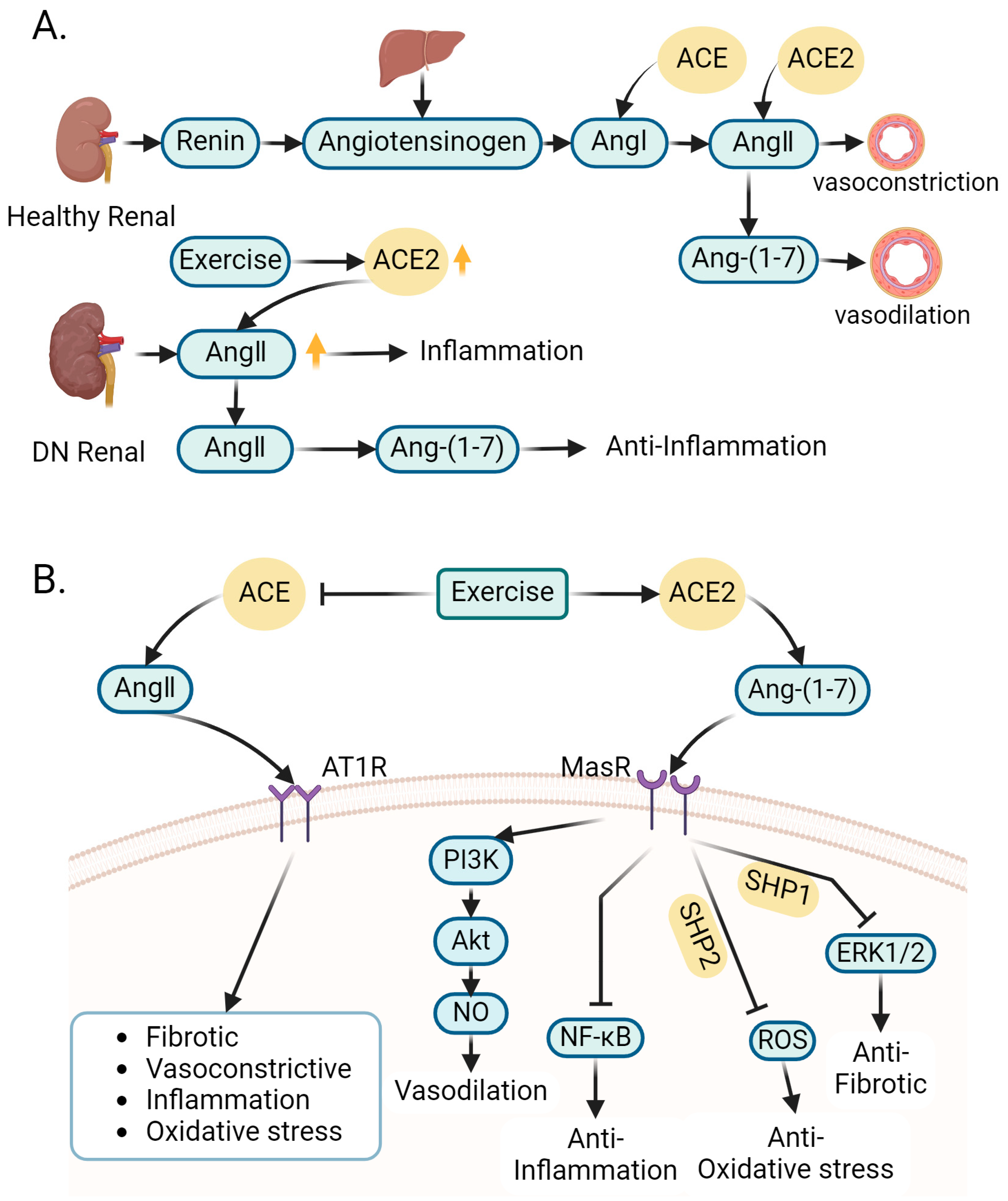
4.2. Role of Exercise-Regulated Renin–Angiotensin System in Amelioration of DN
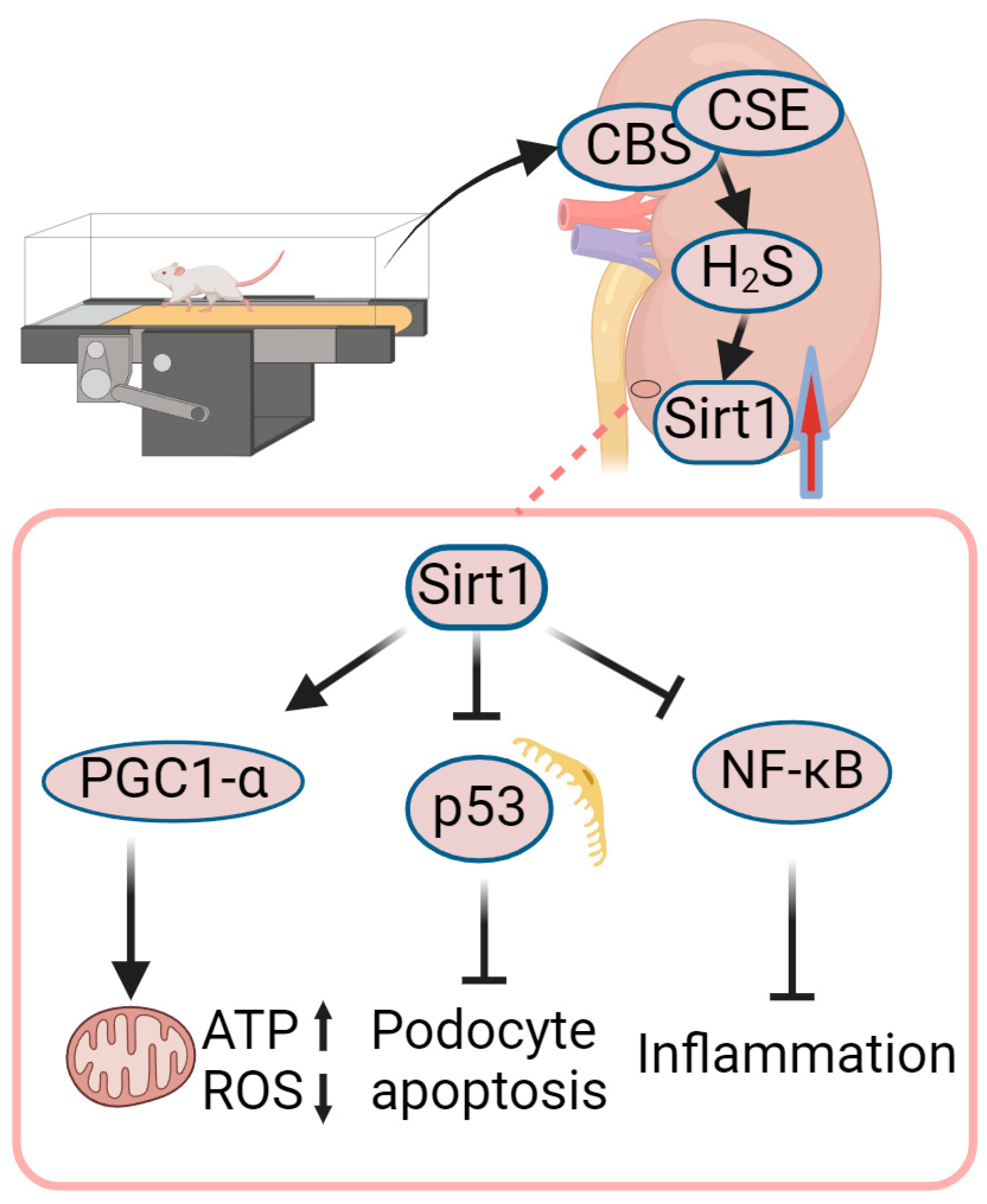
4.3. Role of Exercise-Mediated Increase in Sirt1 in Amelioration of DN
4.4. Role of Exercise-Mediated Increase in NO in Amelioration of DN
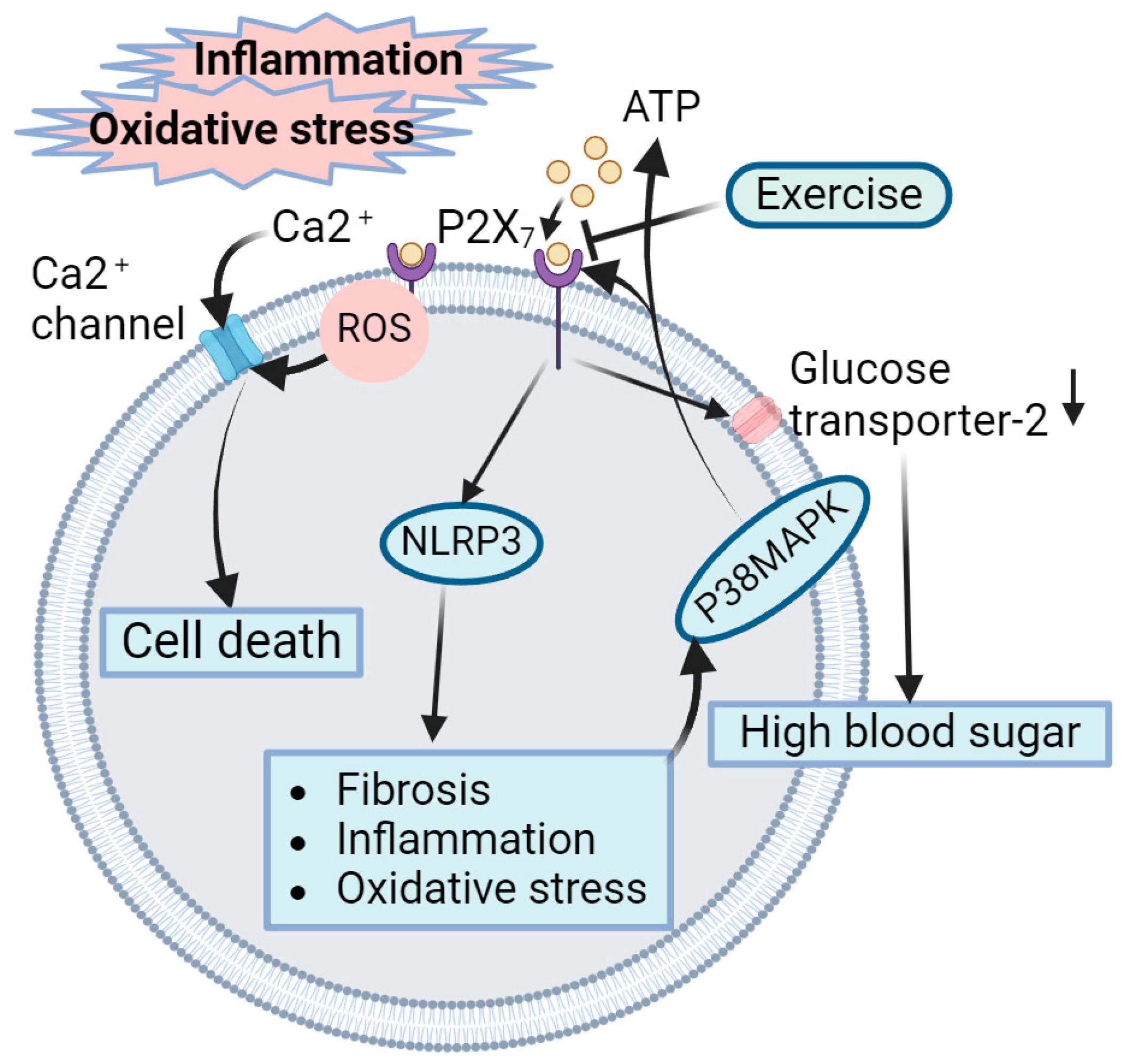
4.5. Role of Exercise-Mediated Inhibition of P2X7 Receptors in Amelioration of DN
4.6. Role of Exercise-Mediated Increase in Heat Shock Protein in Amelioration of DN
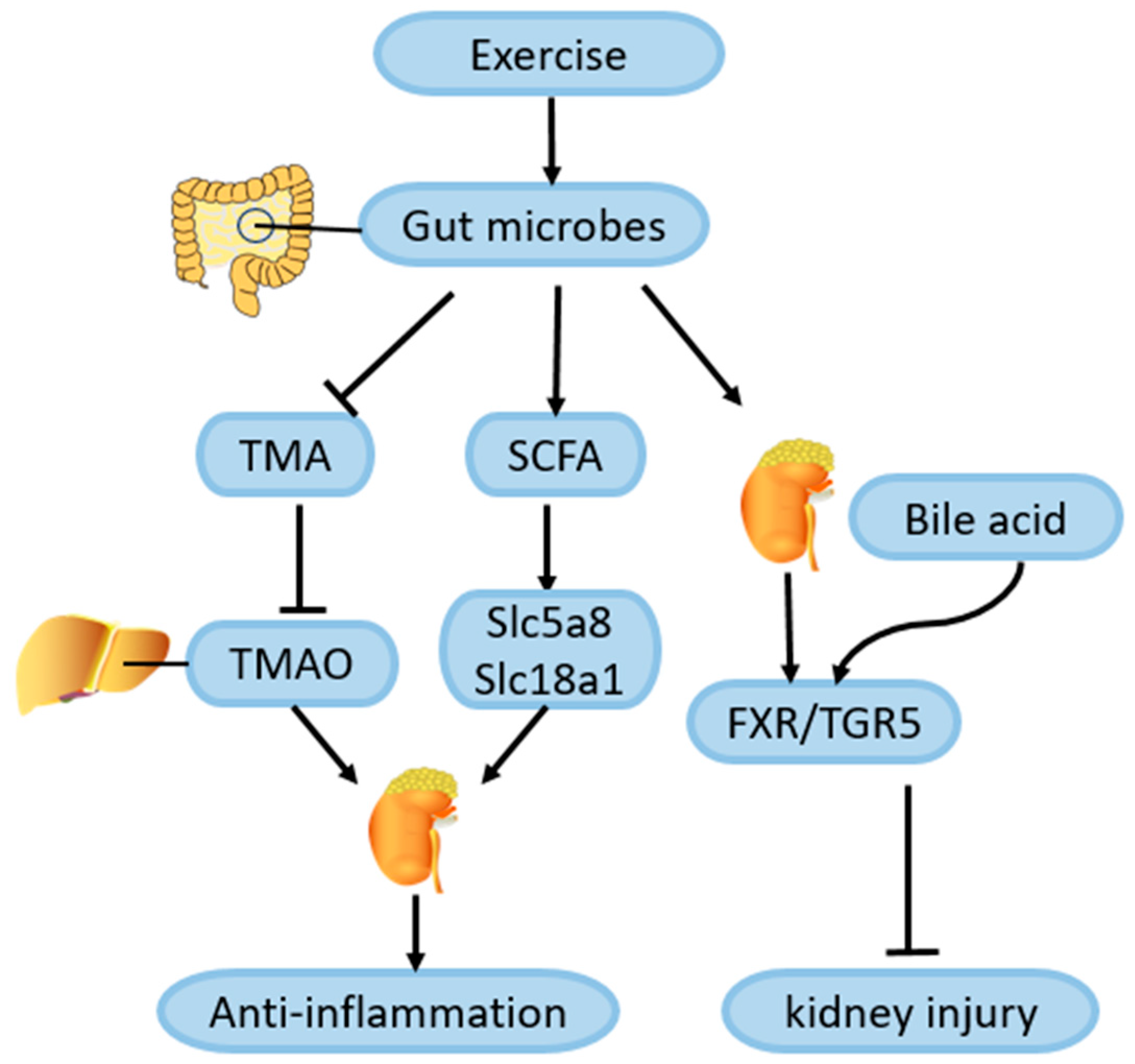
4.7. Role of Exercise-Mediated Gut Microbiota Changes in Amelioration of DN
4.8. Role of Exercise-Induced Hormones and Metabolites in Amelioration of DN
4.8.1. Neuregulin 4 (Nrg4)
4.8.2. Irisin
4.8.3. Metrnl
4.8.4. β-Hydroxybutyrate (BHB)
4.8.5. β-Aminoisobutyric Acid (BAIBA)
4.8.6. Glucagon-like Peptide 1 (GLP-1)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- US Department of Health and Human Services, Centers for Disease Control and Prevention. National Chronic Kidney Disease Fact Sheet: General Information and National Estimates on Chronic Kidney Disease in the United States, 2010; US Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2010.
- Caramori, M.L.; Kim, Y.; Huang, C.; Fish, A.J.; Rich, S.S.; Miller, M.E.; Russell, G.; Mauer, M. Cellular basis of diabetic nephropathy: 1. Study design and renal structural-functional relationships in patients with long-standing type 1 diabetes. Diabetes 2002, 51, 506–513. [Google Scholar] [CrossRef]
- Sagoo, M.K.; Gnudi, L. Diabetic nephropathy: An overview. Diabet. Nephrop. 2020, 2067, 3–7. [Google Scholar]
- Umanath, K.; Lewis, J.B. Update on diabetic nephropathy: Core curriculum 2018. Am. J. Kidney Dis. 2018, 71, 884–895. [Google Scholar] [CrossRef]
- Brellenthin, A.G.; Lanningham-Foster, L.M.; Kohut, M.L.; Li, Y.; Church, T.S.; Blair, S.N.; Lee, D.C. Comparison of the Cardiovascular Benefits of Resistance, Aerobic, and Combined Exercise (CardioRACE): Rationale, design, and methods. Am. Heart J. 2019, 217, 101–111. [Google Scholar] [CrossRef]
- Bohm, M.; Schumacher, H.; Werner, C.; Teo, K.K.; Lonn, E.M.; Mahfoud, F.; Speer, T.; Mancia, G.; Redon, J.; Schmieder, R.E.; et al. Association between exercise frequency with renal and cardiovascular outcomes in diabetic and non-diabetic individuals at high cardiovascular risk. Cardiovasc. Diabetol. 2022, 21, 12. [Google Scholar] [CrossRef]
- Cai, Z.; Yang, Y.; Zhang, J. Effects of physical activity on the progression of diabetic nephropathy: A meta-analysis. Biosci. Rep. 2021, 41, BSR20203624. [Google Scholar] [CrossRef] [PubMed]
- Kotake, H.; Yamada, S.; Ogura, Y.; Watanabe, S.; Inoue, K.; Ichikawa, D.; Sugaya, T.; Ohata, K.; Natsuki, Y.; Hoshino, S.; et al. Endurance Exercise Training-Attenuated Diabetic Kidney Disease with Muscle Weakness in Spontaneously Diabetic Torii Fatty Rats. Kidney Blood Press. Res. 2022, 47, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Calle, P.; Hotter, G. Macrophage Phenotype and Fibrosis in Diabetic Nephropathy. Int. J. Mol. Sci. 2020, 21, 2806. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Butler, A.E. Aerobic exercise can modulate the underlying mechanisms involved in the development of diabetic complications. J. Cell. Physiol. 2019, 234, 12508–12515. [Google Scholar] [CrossRef]
- Chu, D.J.; Ahmed, A.M.; Qureshi, W.T.; Brawner, C.A.; Keteyian, S.J.; Nasir, K.; Blumenthal, R.S.; Blaha, M.J.; Ehrman, J.K.; Cainzos-Achirica, M.; et al. Prognostic Value of Cardiorespiratory Fitness in Patients with Chronic Kidney Disease: The FIT (Henry Ford Exercise Testing) Project. Am. J. Med. 2022, 135, 67–75.e1. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Lessard, S.J. Exercise training-induced improvements in insulin action. Acta Physiol. 2008, 192, 127–135. [Google Scholar] [CrossRef]
- Weiner, D.E.; Liu, C.K.; Miao, S.; Fielding, R.; Katzel, L.I.; Giffuni, J.; Well, A.; Seliger, S.L. Effect of Long-term Exercise Training on Physical Performance and Cardiorespiratory Function in Adults With CKD: A Randomized Controlled Trial. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2023, 81, 59–66. [Google Scholar] [CrossRef]
- Fox, C.S.; Matsushita, K.; Woodward, M.; Bilo, H.J.; Chalmers, J.; Heerspink, H.J.; Lee, B.J.; Perkins, R.M.; Rossing, P.; Sairenchi, T.; et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: A meta-analysis. Lancet 2012, 380, 1662–1673. [Google Scholar] [CrossRef]
- Pongrac Barlovic, D.; Harjutsalo, V.; Groop, P.H. Exercise and nutrition in type 1 diabetes: Insights from the FinnDiane cohort. Front. Endocrinol. 2022, 13, 1064185. [Google Scholar] [CrossRef] [PubMed]
- Paffenbarger, R.S., Jr.; Jung, D.L.; Leung, R.W.; Hyde, R.T. Physical activity and hypertension: An epidemiological view. Ann. Med. 1991, 23, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Jerums, G.; MacIsaac, R.J. Diabetic nephropathy: How does exercise affect kidney disease in T1DM? Nat. Rev. Endocrinol. 2015, 11, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Pongrac Barlovic, D.; Tikkanen-Dolenc, H.; Groop, P.H. Physical Activity in the Prevention of Development and Progression of Kidney Disease in Type 1 Diabetes. Curr. Diabetes Rep. 2019, 19, 41. [Google Scholar] [CrossRef] [PubMed]
- Baião, V.M.; Cunha, V.A.; Duarte, M.P. Effects of Exercise on Inflammatory Markers in Individuals with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Metabolites 2023, 13, 795. [Google Scholar] [CrossRef] [PubMed]
- Helge, J.W.; Stallknecht, B.; Pedersen, B.K.; Galbo, H.; Kiens, B.; Richter, E.A. The effect of graded exercise on IL-6 release and glucose uptake in human skeletal muscle. J. Physiol. 2003, 546, 299–305. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Fischer, C.P. Physiological roles of muscle-derived interleukin-6 in response to exercise. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Y.; Guo, Y.Y.; Xiao, G.; Guo, L.; Tang, Q.Q. SERPINA3C ameliorates adipose tissue inflammation through the Cathepsin G/Integrin/AKT pathway. Mol. Metab. 2022, 61, 101500. [Google Scholar] [CrossRef] [PubMed]
- Steensberg, A.; Fischer, C.P.; Keller, C.; Møller, K.; Pedersen, B.K. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E433–E437. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Luo, S.; Yang, J.; Chen, W.; He, L.; Liu, D.; Zhao, L.; Wang, X. Myokines: Novel therapeutic targets for diabetic nephropathy. Front. Endocrinol. 2022, 13, 1014581. [Google Scholar] [CrossRef]
- Costanti-Nascimento, A.C.; Brelaz-Abreu, L.; Bragança-Jardim, E.; Pereira, W.O.; Camara, N.O.S.; Amano, M.T. Physical exercise as a friend not a foe in acute kidney diseases through immune system modulation. Front. Immunol. 2023, 14, 1212163. [Google Scholar] [CrossRef]
- Wilkinson, T.J.; Shur, N.F.; Smith, A.C. “Exercise as medicine” in chronic kidney disease. Scand. J. Med. Sci. Sports 2016, 26, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Karstoft, K.; Winding, K.; Knudsen, S.H.; Nielsen, J.S.; Thomsen, C.; Pedersen, B.K.; Solomon, T.P. The effects of free-living interval-walking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients: A randomized, controlled trial. Diabetes Care 2013, 36, 228–236. [Google Scholar] [CrossRef]
- Pechter, U.; Maaroos, J.; Mesikepp, S.; Veraksits, A.; Ots, M. Regular low-intensity aquatic exercise improves cardio-respiratory functional capacity and reduces proteinuria in chronic renal failure patients. Nephrol. Dial. Transplant. 2003, 18, 624–625. [Google Scholar] [CrossRef]
- Pechter, U.; Ots, M.; Mesikepp, S.; Zilmer, K.; Kullissaar, T.; Vihalemm, T.; Zilmer, M.; Maaroos, J. Beneficial effects of water-based exercise in patients with chronic kidney disease. Int. J. Rehabil. Res. 2003, 26, 153–156. [Google Scholar]
- Ikeda, T.; Gomi, T.; Sasaki, Y. Effects of swim training on blood pressure, catecholamines and prostaglandins in spontaneously hypertensive rats. Jpn. Heart J. 1994, 35, 205–211. [Google Scholar] [CrossRef][Green Version]
- Afolayan, A.J.; Sunmonu, T.O. Protective role of Artemisia afra aqueous extract on tissue antioxidant defense systems in streptozotocin-induced diabetic rats. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2012, 10, 15–20. [Google Scholar] [CrossRef]
- da Cunha, M.J.; da Cunha, A.A.; Ferreira, G.K.; Baladão, M.E.; Savio, L.E.; Reichel, C.L.; Kessler, A.; Netto, C.A.; Wyse, A.T. The effect of exercise on the oxidative stress induced by experimental lung injury. Life Sci. 2013, 92, 218–227. [Google Scholar] [CrossRef]
- Yamakoshi, S.; Nakamura, T.; Xu, L.; Kohzuki, M.; Ito, O. Exercise Training Ameliorates Renal Oxidative Stress in Rats with Chronic Renal Failure. Metabolites 2022, 12, 836. [Google Scholar] [CrossRef]
- Dounousi, E.; Papavasiliou, E.; Makedou, A.; Ioannou, K.; Katopodis, K.P.; Tselepis, A.; Siamopoulos, K.C.; Tsakiris, D. Oxidative stress is progressively enhanced with advancing stages of CKD. Am. J. Kidney Dis. 2006, 48, 752–760. [Google Scholar] [CrossRef]
- Souza, C.S.; de Sousa Oliveira, B.S.; Viana, G.N.; Correia, T.M.L.; de Braganca, A.C.; Canale, D.; Oliveira, M.V.; de Magalhaes, A.C.M.; Volpini, R.A.; de Brito Amaral, L.S.; et al. Preventive effect of exercise training on diabetic kidney disease in ovariectomized rats with type 1 diabetes. Exp. Biol. Med. 2019, 244, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Gohda, T.; Tanimoto, M.; Omote, K.; Furukawa, M.; Yamaguchi, S.; Murakoshi, M.; Hagiwara, S.; Horikoshi, S.; Funabiki, K.; et al. Effect of exercise on kidney function, oxidative stress, and inflammation in type 2 diabetic KK-A(y) mice. Exp. Diabetes Res. 2012, 2012, 702948. [Google Scholar] [CrossRef] [PubMed]
- Oberg, B.P.; McMenamin, E.; Lucas, F.L.; McMonagle, E.; Morrow, J.; Ikizler, T.A.; Himmelfarb, J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004, 65, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Y.; Li, B.Y.; Xiao, G.; Liu, Y. Cdo1 promotes PPARγ-mediated adipose tissue lipolysis in male mice. Nat. Metab. 2022, 4, 1352–1368. [Google Scholar] [CrossRef] [PubMed]
- Cachofeiro, V.; Goicochea, M.; de Vinuesa, S.G.; Oubiña, P.; Lahera, V.; Luño, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. Suppl. 2008, 74, S4–S9. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, J.Y. Cdo1-Camkk2-AMPK axis confers the protective effects of exercise against NAFLD in mice. Nat. Commun. 2023, 14, 8391. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.S.; Wilcox, C.S. NADPH oxidases in the kidney. Antioxid. Redox Signal. 2006, 8, 1597–1607. [Google Scholar] [CrossRef]
- Cepas, V.; Collino, M. Redox Signaling and Advanced Glycation Endproducts (AGEs) in Diet-Related Diseases. Antioxidants 2020, 9, 142. [Google Scholar] [CrossRef]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef]
- Boor, P.; Celec, P.; Behuliak, M.; Grancic, P.; Kebis, A.; Kukan, M.; Pronayova, N.; Liptaj, T.; Ostendorf, T.; Sebekova, K. Regular moderate exercise reduces advanced glycation and ameliorates early diabetic nephropathy in obese Zucker rats. Metab. Clin. Exp. 2009, 58, 1669–1677. [Google Scholar] [CrossRef]
- Hamada, Y.; Araki, N.; Koh, N.; Nakamura, J.; Horiuchi, S.; Hotta, N. Rapid formation of advanced glycation end products by intermediate metabolites of glycolytic pathway and polyol pathway. Biochem. Biophys. Res. Commun. 1996, 228, 539–543. [Google Scholar] [CrossRef]
- Amaral, L.S.d.B.; Silva, F.A.; Correia, V.B.; Andrade, C.E.F.; Dutra, B.A.; Oliveira, M.V.; Magalhães, A.C.M.d.; Volpini, R.A.; Seguro, A.C.; Coimbra, T.M.; et al. Beneficial effects of previous exercise training on renal changes in streptozotocin-induced diabetic female rats. Exp. Biol. Med. 2015, 241, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Hafner, M.; Landthaler, M.; Burger, L.; Khorshid, M.; Hausser, J.; Berninger, P.; Rothballer, A.; Ascano, M., Jr.; Jungkamp, A.C.; Munschauer, M.; et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 2010, 141, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Mahtal, N.; Lenoir, O. MicroRNAs in kidney injury and disease. Nat. Rev. Nephrol. 2022, 18, 643–662. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Witkowski, M.; Saffarzadeh, M.; Friebel, J.; Tabaraie, T.; Ta Bao, L.; Chakraborty, A.; Dörner, A.; Stratmann, B.; Tschoepe, D.; et al. Vascular miR-181b controls tissue factor-dependent thrombogenicity and inflammation in type 2 diabetes. Cardiovasc. Diabetol. 2020, 19, 20. [Google Scholar] [CrossRef]
- Ishii, H.; Kaneko, S.; Yanai, K.; Aomatsu, A.; Hirai, K.; Ookawara, S.; Morishita, Y. MicroRNA Expression Profiling in Diabetic Kidney Disease. Transl. Res. J. Lab. Clin. Med. 2021, 237, 31–52. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.K.; Shang, W.; Liu, J.; Cheang, W.S. Activation of AMPK/miR-181b Axis Alleviates Endothelial Dysfunction and Vascular Inflammation in Diabetic Mice. Antioxidants 2022, 11, 1137. [Google Scholar] [CrossRef]
- Formigari, G.P.; Datilo, M.N.; Vareda, B.; Bonfante, I.L.P.; Cavaglieri, C.R.; Lopes de Faria, J.M.; Lopes de Faria, J.B. Renal protection induced by physical exercise may be mediated by the irisin/AMPK axis in diabetic nephropathy. Sci. Rep. 2022, 12, 9062. [Google Scholar] [CrossRef]
- Han, Y.C.; Tang, S.Q.; Liu, Y.T.; Li, A.M. AMPK agonist alleviate renal tubulointerstitial fibrosis via activating mitophagy in high fat and streptozotocin induced diabetic mice. Cell Death Dis. 2021, 12, 925. [Google Scholar] [CrossRef]
- Downes, C.P.; Ross, S.; Maccario, H.; Perera, N.; Davidson, L.; Leslie, N.R. Stimulation of PI 3-kinase signaling via inhibition of the tumor suppressor phosphatase, PTEN. Adv. Enzym. Regul. 2007, 47, 184–194. [Google Scholar] [CrossRef]
- DiNitto, J.P.; Cronin, T.C.; Lambright, D.G. Membrane recognition and targeting by lipid-binding domains. Sci. STKE Signal Transduct. Knowl. Environ. 2003, 2003, re16. [Google Scholar] [CrossRef]
- Cardone, M.H.; Roy, N.; Stennicke, H.R.; Salvesen, G.S.; Franke, T.F.; Stanbridge, E.; Frisch, S.; Reed, J.C. Regulation of cell death protease caspase-9 by phosphorylation. Science 1998, 282, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; Mackman, N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J. Biol. Chem. 2002, 277, 32124–32132. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, M.; Gohda, T.; Tanimoto, M.; Tomino, Y. Pathogenesis and novel treatment from the mouse model of type 2 diabetic nephropathy. Sci. World J. 2013, 2013, 928197. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017, 94, 317–325. [Google Scholar] [CrossRef] [PubMed]
- de Alcantara Santos, R.; Guzzoni, V.; Silva, K.A.S.; Aragão, D.S.; de Paula Vieira, R.; Bertoncello, N.; Schor, N.; Aimbire, F.; Casarini, D.E.; Cunha, T.S. Resistance exercise shifts the balance of renin-angiotensin system toward ACE2/Ang 1–7 axis and reduces inflammation in the kidney of diabetic rats. Life Sci. 2021, 287, 120058. [Google Scholar] [CrossRef]
- Santos, R.A.S.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D. The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar] [CrossRef]
- Xu, P.; Sriramula, S.; Lazartigues, E. ACE2/ANG-(1-7)/Mas pathway in the brain: The axis of good. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R804–R817. [Google Scholar] [CrossRef] [PubMed]
- Deshotels, M.R.; Xia, H.; Sriramula, S.; Lazartigues, E.; Filipeanu, C.M. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension 2014, 64, 1368–1375. [Google Scholar] [CrossRef]
- Qi, W.; Hu, C.; Zhao, D.; Li, X. SIRT1-SIRT7 in Diabetic Kidney Disease: Biological Functions and Molecular Mechanisms. Front. Endocrinol. 2022, 13, 801303. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Y.; Peng, W.Q.; Liu, Y.; Guo, L.; Tang, Q.Q. HIGD1A links SIRT1 activity to adipose browning by inhibiting the ROS/DNA damage pathway. Cell Rep. 2023, 42, 112731. [Google Scholar] [CrossRef] [PubMed]
- Chuang, P.Y.; Xu, J.; Dai, Y.; Jia, F.; Mallipattu, S.K.; Yacoub, R.; Gu, L.; Premsrirut, P.K.; He, J.C. In vivo RNA interference models of inducible and reversible Sirt1 knockdown in kidney cells. Am. J. Pathol. 2014, 184, 1940–1956. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, D.Y.; Sha, W.G.; Shen, L.; Lu, G.Y.; Yin, X.; Wang, M.J. High glucose induces renal tubular epithelial injury via Sirt1/NF-kappaB/microR-29/Keap1 signal pathway. J. Transl. Med. 2015, 13, 352. [Google Scholar] [CrossRef]
- Huang, K.; Gao, X.; Wei, W. The crosstalk between Sirt1 and Keap1/Nrf2/ARE anti-oxidative pathway forms a positive feedback loop to inhibit FN and TGF-β1 expressions in rat glomerular mesangial cells. Exp. Cell Res. 2017, 361, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.J.; Parikh, S.M. Targeting energy pathways in kidney disease: The roles of sirtuins, AMPK, and PGC1-α. Kidney Int. 2021, 99, 828–840. [Google Scholar] [CrossRef]
- Tran, M.; Tam, D.; Bardia, A.; Bhasin, M.; Rowe, G.C.; Kher, A.; Zsengeller, Z.K.; Akhavan-Sharif, M.R.; Khankin, E.V.; Saintgeniez, M.; et al. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J. Clin. Investig. 2011, 121, 4003–4014. [Google Scholar] [CrossRef]
- Tang, L.X.; Wang, B.; Wu, Z.K. Aerobic Exercise Training Alleviates Renal Injury by Interfering with Mitochondrial Function in Type-1 Diabetic Mice. Med. Sci. Monit. 2018, 24, 9081–9089. [Google Scholar] [CrossRef]
- Yang, L.; Li, D.X.; Cao, B.Q.; Liu, S.J.; Xu, D.H.; Zhu, X.Y. Exercise training ameliorates early diabetic kidney injury by regulating the H(2) S/SIRT1/p53 pathway. FASEB J. 2021, 35, e21823. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Kao, H.H.; Wu, C.H. Exercise training upregulates SIRT1 to attenuate inflammation and metabolic dysfunction in kidney and liver of diabetic db/db mice. Nutr. Metab. 2019, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lin, F.; Ruan, Y.; Zhao, S.; Yuan, R.; Ning, J.; Jiang, K.; Xie, J.; Li, H.; Li, C.; et al. miR-132-3p promotes the cisplatin-induced apoptosis and inflammatory response of renal tubular epithelial cells by targeting SIRT1 via the NF-κB pathway. Int. Immunopharmacol. 2021, 99, 108022. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, Y.; Liu, Y.; Tang, C.; Cai, J.; Chen, G.; Dong, Z. p53/sirtuin 1/NF-κB Signaling Axis in Chronic Inflammation and Maladaptive Kidney Repair After Cisplatin Nephrotoxicity. Front. Immunol. 2022, 13, 925738. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell. Signal. 2013, 25, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiol. Rev. 2019, 99, 311–379. [Google Scholar] [CrossRef] [PubMed]
- Carlström, M. Nitric oxide signalling in kidney regulation and cardiometabolic health. Nat. Rev. Nephrol. 2021, 17, 575–590. [Google Scholar] [CrossRef]
- Carlstrom, M.; Montenegro, M.F. Therapeutic value of stimulating the nitrate-nitrite-nitric oxide pathway to attenuate oxidative stress and restore nitric oxide bioavailability in cardiorenal disease. J. Intern. Med. 2019, 285, 2–18. [Google Scholar] [CrossRef]
- Anavi, S.; Tirosh, O. iNOS as a metabolic enzyme under stress conditions. Free Radic. Biol. Med. 2020, 146, 16–35. [Google Scholar] [CrossRef]
- Fujihara, C.K.; Mattar, A.L.; Vieira, J.M., Jr.; Malheiros, D.M.; Noronha Ide, L.; Gonçalves, A.R.; De Nucci, G.; Zatz, R. Evidence for the existence of two distinct functions for the inducible NO synthase in the rat kidney: Effect of aminoguanidine in rats with 5/6 ablation. J. Am. Soc. Nephrol. JASN 2002, 13, 2278–2287. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, S.V.; Morrishow, A.M.; Dharia, N.; Gross, S.S.; Goligorsky, M.S. Glucose scavenging of nitric oxide. Am. J. Physiol. Ren. Physiol. 2001, 280, F480–F486. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Bergamaschi, C.T.; Araujo, R.C.; Mouro, M.G.; Rosa, T.S.; Higa, E.M. Effects of training and nitric oxide on diabetic nephropathy progression in type I diabetic rats. Exp. Biol. Med. 2011, 236, 1180–1187. [Google Scholar] [CrossRef]
- Szabó, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Cao, P.; Kakihana, T.; Sato, E.; Suda, C.; Muroya, Y.; Ogawa, Y.; Hu, G.; Ishii, T.; Ito, O.; et al. Chronic Running Exercise Alleviates Early Progression of Nephropathy with Upregulation of Nitric Oxide Synthases and Suppression of Glycation in Zucker Diabetic Rats. PLoS ONE 2015, 10, e0138037. [Google Scholar] [CrossRef]
- Kalyanaraman, B. Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biol. 2013, 1, 244–257. [Google Scholar] [CrossRef]
- Mori, T.; O’Connor, P.M.; Abe, M.; Cowley, A.W., Jr. Enhanced superoxide production in renal outer medulla of Dahl salt-sensitive rats reduces nitric oxide tubular-vascular cross-talk. Hypertension 2007, 49, 1336–1341. [Google Scholar] [CrossRef]
- Tinken, T.M.; Thijssen, D.H.; Hopkins, N.; Dawson, E.A.; Cable, N.T.; Green, D.J. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 2010, 55, 312–318. [Google Scholar] [CrossRef]
- Ito, D.; Ito, O.; Mori, N.; Cao, P.; Suda, C.; Muroya, Y.; Hao, K.; Shimokawa, H.; Kohzuki, M. Exercise training upregulates nitric oxide synthases in the kidney of rats with chronic heart failure. Clin. Exp. Pharmacol. Physiol. 2013, 40, 617–625. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef]
- Solini, A.; Menini, S.; Rossi, C.; Ricci, C.; Santini, E.; Blasetti Fantauzzi, C.; Iacobini, C.; Pugliese, G. The purinergic 2X7 receptor participates in renal inflammation and injury induced by high-fat diet: Possible role of NLRP3 inflammasome activation. J. Pathol. 2013, 231, 342–353. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Bergamaschi, C.T.; Fernandes, M.J.; Paredes-Gamero, E.J.; Buri, M.V.; Ferreira, A.T.; Araujo, S.R.; Punaro, G.R.; Maciel, F.R.; Nogueira, G.B.; et al. P2X(7) receptor in the kidneys of diabetic rats submitted to aerobic training or to N-acetylcysteine supplementation [corrected]. PLoS ONE 2014, 9, e97452. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wu, B.; Jiang, F.; Xiong, S.; Zhang, B.; Li, G.; Liu, S.; Gao, Y.; Xu, C.; Tu, G.; et al. High fatty acids modulate P2X(7) expression and IL-6 release via the p38 MAPK pathway in PC12 cells. Brain Res. Bull. 2013, 94, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Trueblood, K.E.; Mohr, S.; Dubyak, G.R. Purinergic regulation of high-glucose-induced caspase-1 activation in the rat retinal Müller cell line rMC-1. Am. J. Physiol. Cell Physiol. 2011, 301, C1213–C1223. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fei, M.; Zhang, G.; Liang, W.C.; Lin, W.; Wu, Y.; Piskol, R.; Ridgway, J.; McNamara, E.; Huang, H.; et al. Blockade of the Phagocytic Receptor MerTK on Tumor-Associated Macrophages Enhances P2X7R-Dependent STING Activation by Tumor-Derived cGAMP. Immunity 2020, 52, 357–373.e9. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Qian, C.; Xie, K.; Fan, Q.; Yan, Y.; Lu, R.; Wang, L.; Zhang, M.; Wang, Q.; Mou, S.; et al. P2X7 receptor signaling promotes inflammation in renal parenchymal cells suffering from ischemia-reperfusion injury. Cell Death Dis. 2021, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Bourzac, J.F.; L’Ériger, K.; Larrivée, J.F.; Arguin, G.; Bilodeau, M.S.; Stankova, J.; Gendron, F.P. Glucose transporter 2 expression is down regulated following P2X7 activation in enterocytes. J. Cell. Physiol. 2013, 228, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Kurop, M.K.; Huyen, C.M.; Kelly, J.H.; Blagg, B.S.J. The heat shock response and small molecule regulators. Eur. J. Med. Chem. 2021, 226, 113846. [Google Scholar] [CrossRef] [PubMed]
- Neuhofer, W.; Lugmayr, K.; Fraek, M.L.; Beck, F.X. Regulated overexpression of heat shock protein 72 protects Madin-Darby canine kidney cells from the detrimental effects of high urea concentrations. J. Am. Soc. Nephrol. JASN 2001, 12, 2565–2571. [Google Scholar] [CrossRef]
- Archer, A.E.; Von Schulze, A.T.; Geiger, P.C. Exercise, heat shock proteins and insulin resistance. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160529. [Google Scholar] [CrossRef]
- Henstridge, D.C.; Bruce, C.R.; Drew, B.G.; Tory, K.; Kolonics, A.; Estevez, E.; Chung, J.; Watson, N.; Gardner, T.; Lee-Young, R.S.; et al. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes 2014, 63, 1881–1894. [Google Scholar] [CrossRef]
- Lappalainen, J.; Oksala, N.K.J.; Laaksonen, D.E.; Khanna, S.; Kokkola, T.; Kaarniranta, K.; Sen, C.K.; Atalay, M. Suppressed heat shock protein response in the kidney of exercise-trained diabetic rats. Scand. J. Med. Sci. Sports 2018, 28, 1808–1817. [Google Scholar] [CrossRef]
- Mao, H.; Li, Z.; Zhou, Y.; Li, Z.; Zhuang, S.; An, X.; Zhang, B.; Chen, W.; Nie, J.; Wang, Z.; et al. HSP72 attenuates renal tubular cell apoptosis and interstitial fibrosis in obstructive nephropathy. Am. J. Physiol. Ren. Physiol. 2008, 295, F202–F214. [Google Scholar] [CrossRef]
- Wu, I.W.; Lin, C.Y.; Chang, L.C.; Lee, C.C.; Chiu, C.Y.; Hsu, H.J.; Sun, C.Y.; Chen, Y.C.; Kuo, Y.L.; Yang, C.W.; et al. Gut Microbiota as Diagnostic Tools for Mirroring Disease Progression and Circulating Nephrotoxin Levels in Chronic Kidney Disease: Discovery and Validation Study. Int. J. Biol. Sci. 2020, 16, 420–434. [Google Scholar] [CrossRef]
- Rukavina Mikusic, N.L.; Kouyoumdzian, N.M.; Choi, M.R. Gut microbiota and chronic kidney disease: Evidences and mechanisms that mediate a new communication in the gastrointestinal-renal axis. Pflügers Arch. Eur. J. Physiol. 2020, 472, 303–320. [Google Scholar] [CrossRef]
- Yao, T.; Wang, H.; Lin, K.; Wang, R.; Guo, S.; Chen, P.; Wu, H.; Liu, T.; Wang, R. Exercise-induced microbial changes in preventing type 2 diabetes. Sci. China. Life Sci. 2023. [Google Scholar] [CrossRef]
- Zhu, H.; Cao, C.; Wu, Z.; Zhang, H.; Sun, Z.; Wang, M.; Xu, H.; Zhao, Z.; Wang, Y.; Pei, G.; et al. The probiotic L. casei Zhang slows the progression of acute and chronic kidney disease. Cell Metab. 2021, 33, 1926–1942.e8. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Ni, Y.; Cheung, C.K.Y.; Lam, K.S.L.; Wang, Y.; Xia, Z.; Ye, D.; Guo, J.; Tse, M.A.; et al. Gut Microbiome Fermentation Determines the Efficacy of Exercise for Diabetes Prevention. Cell Metab. 2020, 31, 77–91.e5. [Google Scholar] [CrossRef]
- Wertheim, B.C.; Martínez, M.E.; Ashbeck, E.L.; Roe, D.J.; Jacobs, E.T.; Alberts, D.S.; Thompson, P.A. Physical activity as a determinant of fecal bile acid levels. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1591–1598. [Google Scholar] [CrossRef]
- Wang, X.X.; Wang, D.; Luo, Y.; Myakala, K.; Dobrinskikh, E.; Rosenberg, A.Z.; Levi, J.; Kopp, J.B.; Field, A.; Hill, A.; et al. FXR/TGR5 Dual Agonist Prevents Progression of Nephropathy in Diabetes and Obesity. J. Am. Soc. Nephrol. JASN 2018, 29, 118–137. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Liu, N.; Zheng, B.; Guo, F.; Zeng, X.; Huang, X.; Ouyang, D. Roles of Gut Microbial Metabolites in Diabetic Kidney Disease. Front. Endocrinol. 2021, 12, 636175. [Google Scholar] [CrossRef]
- Argyridou, S.; Bernieh, D.; Henson, J. Associations between physical activity and trimethylamine N-oxide in those at risk of type 2 diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001359. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.L.; Malin, S.K. Effects of Lifestyle Intervention on Plasma Trimethylamine N-Oxide in Obese Adults. Nutrients 2019, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Shahrbanian, S.; Hackney, A.C. Comparison of the effects of 12 weeks of three types of resistance training (traditional, circular and interval) on the levels of neuregulin 4, adiponectin and leptin in non-athletic men with obesity. Arch. Med. Deporte 2021, 38, 389–396. [Google Scholar] [CrossRef]
- Ding, S.; Yang, Y.; Zheng, Y.; Xu, J.; Cheng, Y.; Wei, W.; Yu, F.; Li, L.; Li, M.; Wang, M.; et al. Diagnostic Value of the Combined Measurement of Serum HCY and NRG4 in Type 2 Diabetes Mellitus with Early Complicating Diabetic Nephropathy. J. Pers. Med. 2023, 13, 556. [Google Scholar] [CrossRef]
- Kralisch, S.; Hoffmann, A.; Klöting, N.; Frille, A.; Kuhn, H.; Nowicki, M.; Paeschke, S.; Bachmann, A.; Blüher, M.; Zhang, M.Z.; et al. The brown fat-secreted adipokine neuregulin 4 is decreased in human and murine chronic kidney disease. Eur. J. Endocrinol. 2019, 181, 151–159. [Google Scholar] [CrossRef]
- Shi, J.; Xu, W.; Zheng, R.; Miao, H.; Hu, Q. Neuregulin 4 attenuate tubulointerstitial fibrosis and advanced glycosylation end products accumulation in diabetic nephropathy rats via regulating TNF-R1 signaling. Am. J. Transl. Res. 2019, 11, 5501–5513. [Google Scholar]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Belén Crujeiras, A.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef]
- Liu, S.; Cui, F.; Ning, K.; Wang, Z.; Fu, P.; Wang, D.; Xu, H. Role of irisin in physiology and pathology. Front. Endocrinol. 2022, 13, 962968. [Google Scholar] [CrossRef]
- Lai, W.; Luo, D. Irisin ameliorates diabetic kidney disease by restoring autophagy in podocytes. FASEB J. 2023, 37, e23175. [Google Scholar] [CrossRef]
- Jung, T.W.; Lee, S.H.; Kim, H.C.; Bang, J.S.; Abd El-Aty, A.M. METRNL attenuates lipid-induced inflammation and insulin resistance via AMPK or PPARδ-dependent pathways in skeletal muscle of mice. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Zheng, S.L.; Li, Z.Y.; Song, J.; Liu, J.M.; Miao, C.Y. Metrnl: A secreted protein with new emerging functions. Acta Pharmacol. Sin. 2016, 37, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hu, D.; Zhao, X.; Hu, W. Correlation of serum meteorin-like concentrations with diabetic nephropathy. Diabetes Res. Clin. Pract. 2020, 169, 108443. [Google Scholar] [CrossRef]
- Ruas, J.L.; White, J.P.; Rao, R.R.; Kleiner, S.; Brannan, K.T.; Harrison, B.C.; Greene, N.P.; Wu, J.; Estall, J.L.; Irving, B.A.; et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 2012, 151, 1319–1331. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. β-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, M.; Li, X.; Wang, Y.; Du, R. Exercise Ameliorates Atherosclerosis via Up-Regulating Serum β-Hydroxybutyrate Levels. Int. J. Mol. Sci. 2022, 23, 3788. [Google Scholar] [CrossRef] [PubMed]
- Knol, M.G.E.; Bais, T.; Geertsema, P.; Connelly, M.A.; Bakker, S.J.L.; Gansevoort, R.T.; van Gastel, M.D.A.; Gansevoort, R.T.; Drenth, J.P.H.; Peters, D.J.M.; et al. Higher beta-hydroxybutyrate ketone levels associated with a slower kidney function decline in ADPKD. Nephrol. Dial. Transplant. 2023. [Google Scholar] [CrossRef]
- Luo, S.; Yang, M.; Han, Y.; Zhao, H.; Jiang, N.; Li, L.; Chen, W.; Li, C.; Yang, J.; Liu, Y.; et al. β-Hydroxybutyrate against Cisplatin-Induced acute kidney injury via inhibiting NLRP3 inflammasome and oxidative stress. Int. Immunopharmacol. 2022, 111, 109101. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, B.; Gong, A.Y.; Malhotra, D.K.; Gupta, R.; Dworkin, L.D.; Gong, R. The ketone body β-hydroxybutyrate mitigates the senescence response of glomerular podocytes to diabetic insults. Kidney Int. 2021, 100, 1037–1053. [Google Scholar] [CrossRef]
- Kamei, Y.; Hatazawa, Y.; Uchitomi, R.; Yoshimura, R.; Miura, S. Regulation of Skeletal Muscle Function by Amino Acids. Nutrients 2020, 12, 261. [Google Scholar] [CrossRef]
- Jung, T.W.; Park, H.S.; Choi, G.H.; Kim, D.; Lee, T. β-aminoisobutyric acid attenuates LPS-induced inflammation and insulin resistance in adipocytes through AMPK-mediated pathway. J. Biomed. Sci. 2018, 25, 27. [Google Scholar] [CrossRef]
- Audzeyenka, I.; Szrejder, M.; Rogacka, D.; Angielski, S.; Saleem, M.A.; Piwkowska, A. β-Aminoisobutyric acid (L-BAIBA) is a novel regulator of mitochondrial biogenesis and respiratory function in human podocytes. Sci. Rep. 2023, 13, 766. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Zhao, X.; Xing, C.; Sun, B. β-Aminoisobutyric acid ameliorates the renal fibrosis in mouse obstructed kidneys via inhibition of renal fibroblast activation and fibrosis. J. Pharmacol. Sci. 2017, 133, 203–213. [Google Scholar] [CrossRef]
- Meier, J.J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2012, 8, 728–742. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, M.; Xie, Y.; Lang, H.; Li, T.; Yi, L.; Zhang, Q.; Mi, M. Dihydromyricetin Enhances Exercise-Induced GLP-1 Elevation through Stimulating cAMP and Inhibiting DPP-4. Nutrients 2022, 14, 4583. [Google Scholar] [CrossRef]
- Greco, E.V.; Russo, G.; Giandalia, A.; Viazzi, F.; Pontremoli, R.; De Cosmo, S. GLP-1 Receptor Agonists and Kidney Protection. Medicina 2019, 55, 233. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet. Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Winiarska, A.; Knysak, M.; Nabrdalik, K. Inflammation and Oxidative Stress in Diabetic Kidney Disease: The Targets for SGLT2 Inhibitors and GLP-1 Receptor Agonists. Int. J. Mol. Sci. 2021, 22, 10822. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.-Y.; Guo, L. Exercise in Diabetic Nephropathy: Protective Effects and Molecular Mechanism. Int. J. Mol. Sci. 2024, 25, 3605. https://doi.org/10.3390/ijms25073605
Li R-Y, Guo L. Exercise in Diabetic Nephropathy: Protective Effects and Molecular Mechanism. International Journal of Molecular Sciences. 2024; 25(7):3605. https://doi.org/10.3390/ijms25073605
Chicago/Turabian StyleLi, Ruo-Ying, and Liang Guo. 2024. "Exercise in Diabetic Nephropathy: Protective Effects and Molecular Mechanism" International Journal of Molecular Sciences 25, no. 7: 3605. https://doi.org/10.3390/ijms25073605
APA StyleLi, R.-Y., & Guo, L. (2024). Exercise in Diabetic Nephropathy: Protective Effects and Molecular Mechanism. International Journal of Molecular Sciences, 25(7), 3605. https://doi.org/10.3390/ijms25073605




