Assessment of Blood and Semen Detection and DNA Collection from Swabs up to Three Months after Deposition on Five Different Cloth Materials
Abstract
1. Introduction
2. Results
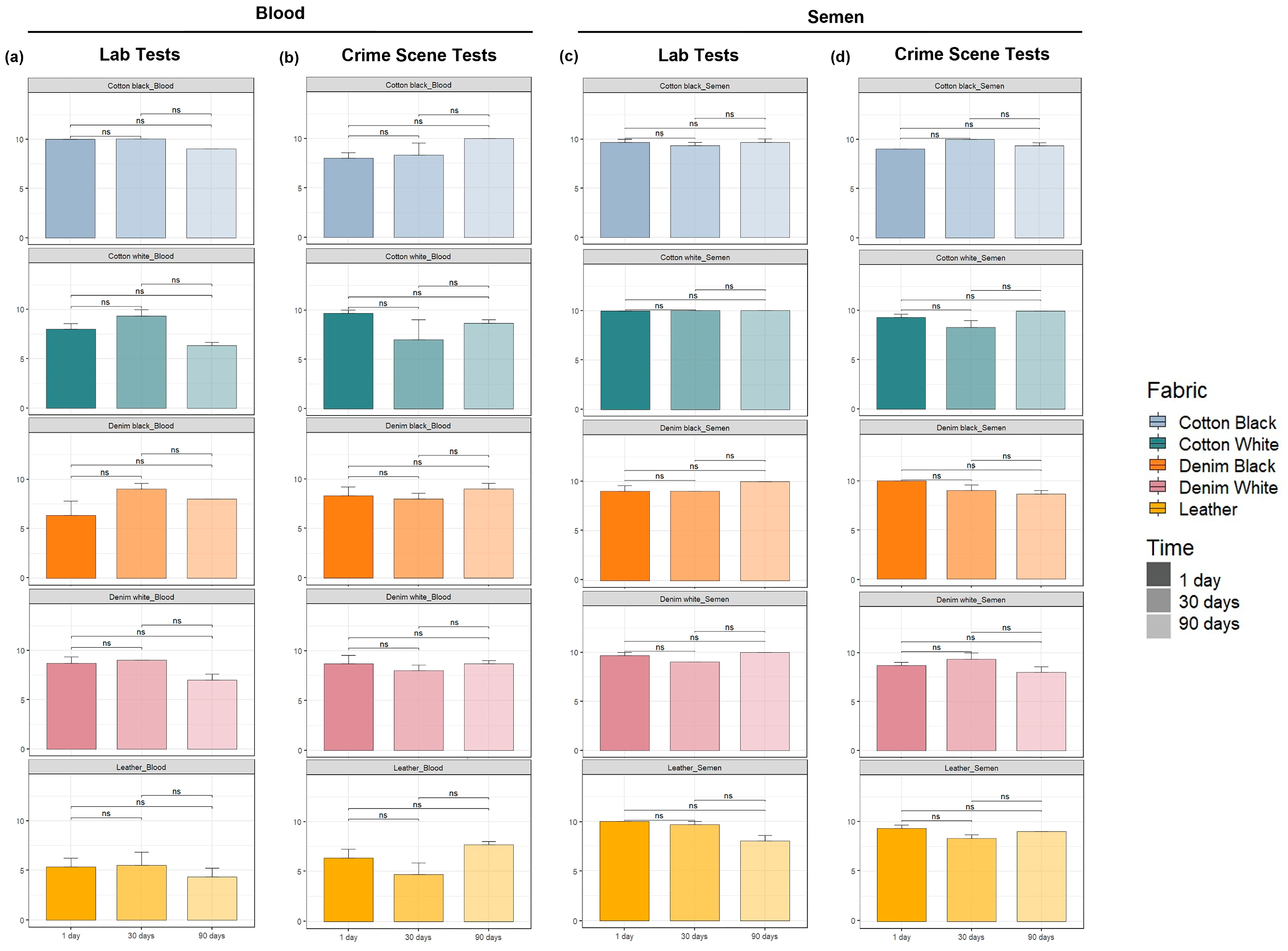
2.1. Immunochromatographic Test Results
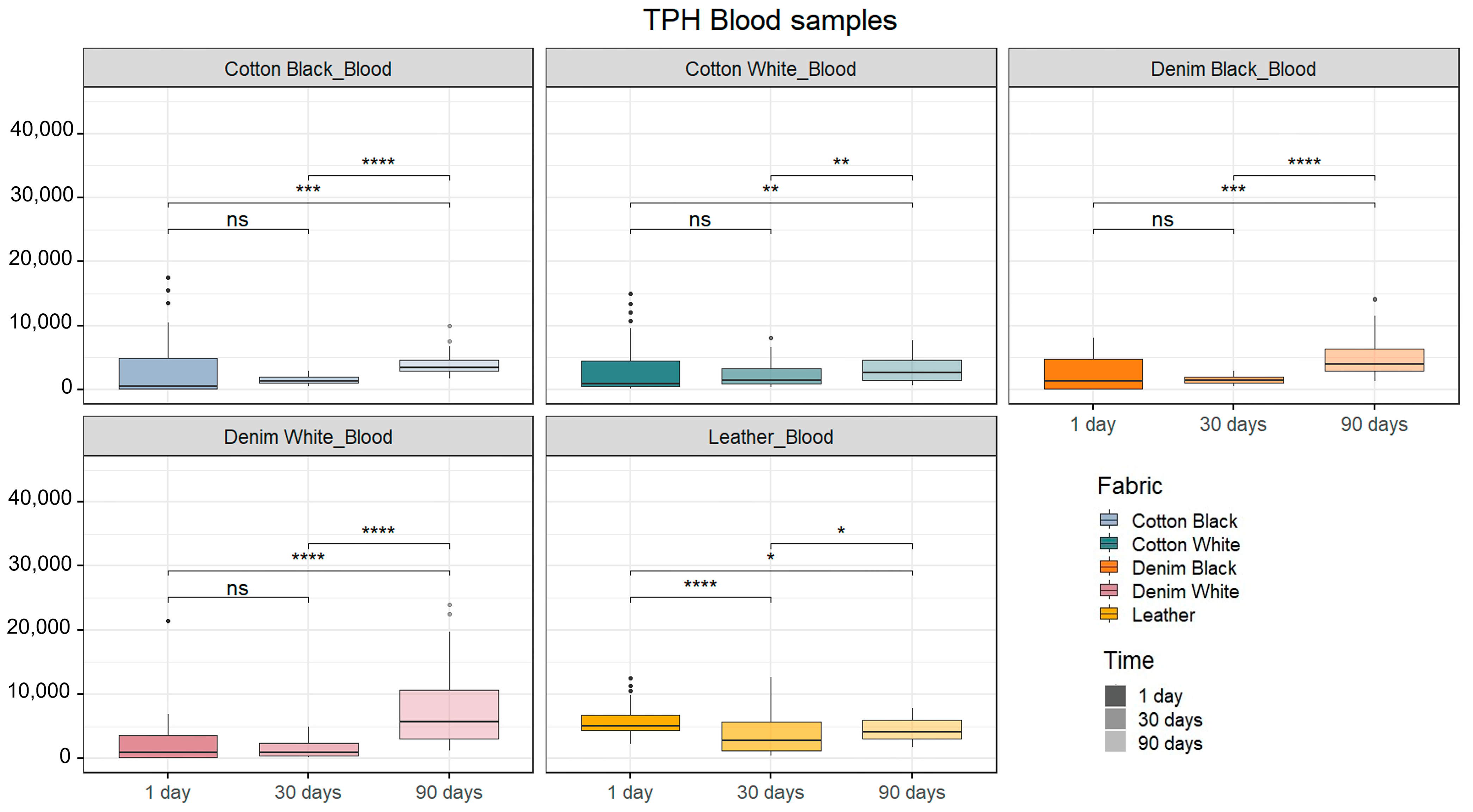
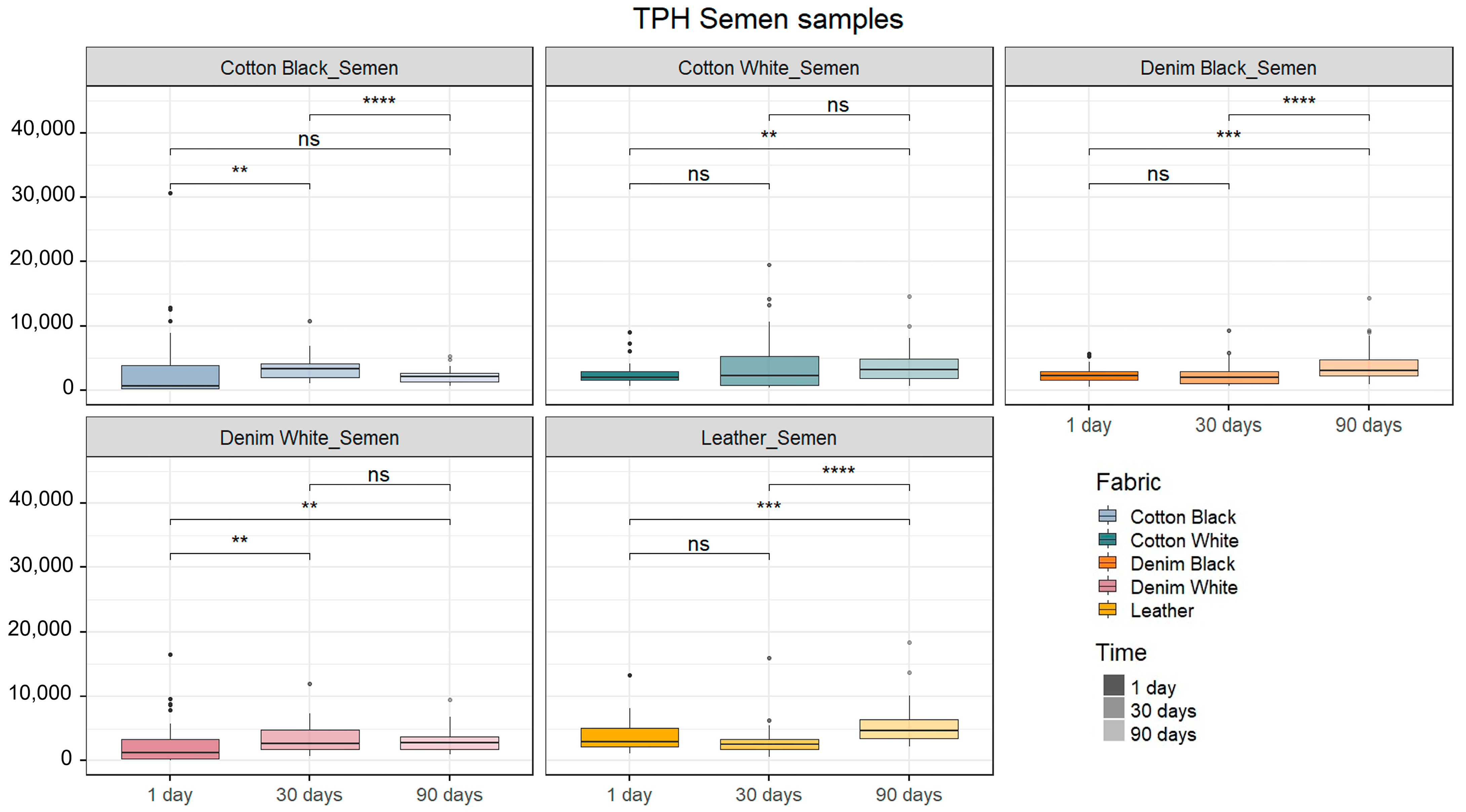
2.2. Human DNA Quantification
2.3. DNA Profiling
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Experimental Design
4.3. DNA Extraction
4.4. Total and Human DNA Quantification
4.5. Nuclear DNA Profiling
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virkler, K.; Lednev, I.K. Analysis of body fluids for forensic purposes: From laboratory testing to non-destructive rapid confirmatory identification at a crime scene. Forensic Sci. Int. 2009, 188, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Harbison, S.; Fleming, R. Forensic body fluid identification: State of the art. Res. Rep. Forensic Med. Sci. 2016, 6, 11–23. [Google Scholar] [CrossRef]
- Misencik, A.; Laux, D.L. Validation Study of the Seratec HemDirect Hemoglobin Assay for the Forensic Identification of Human Blood. MAFS Newsl. 2007, 18–26. [Google Scholar]
- Healy, D.A.; Hayes, C.J.; Leonard, P.; McKenna, L.; O’kennedy, R. Biosensor developments: Application to prostate-specific antigen detection. Trends Biotechnol. 2007, 25, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Sensabaugh, G.F.; Crim, D. Isolation and Characterization of a Semen-Specific Protein from Human Seminal Plasma: A Potential New Marker for Semen Identification. J. Forensic Sci. 1971, 23, 106–115. [Google Scholar] [CrossRef]
- Khaldi, N.; Miras, A.; Botti, K.; Benali, L.; Gromb, S. Evaluation of Three Rapid Detection Methods for the Forensic Identification of Seminal Fluid in Rape Cases. J. Forensic Sci. 2004, 49, 1–5. [Google Scholar] [CrossRef]
- Pang, B.; Cheung, B. Identification of human semenogelin in membrane strip test as an alternative method for the detection of semen. Forensic Sci. Int. 2007, 169, 27–31. [Google Scholar] [CrossRef]
- Sato, I.; Kojima, K.; Yamasaki, T.; Yoshida, K.; Yoshiike, M.; Takano, S.; Mukai, T.; Iwamoto, T. Rapid detection of semenogelin by one-step immunochromatographic assay for semen identification. J. Immunol. Methods 2004, 287, 137–145. [Google Scholar] [CrossRef]
- Weber, A.R.; Lednev, I.K. Crime clock—Analytical studies for approximating time since deposition of bloodstains. Forensic Chem. 2020, 19, 100248. [Google Scholar] [CrossRef]
- Schneider, T.D.; Roschitzki, B.; Grossmann, J.; Kraemer, T.; Steuer, A.E. Determination of the Time since Deposition of Blood Traces Utilizing a Liquid Chromatography–Mass Spectrometry-Based Proteomics Approach. Anal. Chem. 2022, 94, 10695–10704. [Google Scholar] [CrossRef]
- Schneider, T.D.; Kraemer, T.; Steuer, A.E. Determination of the Time since Deposition of blood-traces in a forensic context: Application of untargeted LC-HR-MS/MS metabolomics profiling. Drug Test. Anal. 2023, 15, 840–852. [Google Scholar] [CrossRef]
- Doty, K.C.; McLaughlin, G.; Lednev, I.K. A Raman “spectroscopic clock” for bloodstain age determination: The first week after deposition. Anal. Bioanal. Chem. 2016, 408, 3993–4001. [Google Scholar] [CrossRef] [PubMed]
- Stotesbury, T.; Cossette, M.-L.; Newell-Bell, T.; Shafer, A.B.A. An Exploratory Time Since Deposition Analysis of Whole Blood Using Metrics of DNA Degradation and Visible Absorbance Spectroscopy. Pure Appl. Geophys. 2020, 178, 735–743. [Google Scholar] [CrossRef]
- Laber, T.L.; Epstein, B.P. Substrate Effects on the Clotting time of Human Blood. Can. Soc. Forensic Sci. J. 2001, 34, 209–214. [Google Scholar] [CrossRef]
- Greenfield, A.; Sloan, M.A. Identification of blood body fluids. In Forensic Science: An Introduction to Scientific and Investigative Techniques; James, S.H., Nordby, J.J., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 203–220. [Google Scholar]
- Conte, J.; Ruddy, A.; Domonoski, L.; Shanahan, A.; Daley, N.; McDevitt, C.; Roca, G. Recovery of DNA from SERATEC(R) Immunochromatographic PSA and saliva test strips. J. Forensic Sci. 2022, 67, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Zapico, S.C.; Dytso, A.; Rubio, L.; Roca, G. The Perfect Match: Assessment of Sample Collection Efficiency for Immunological and Molecular Findings in Different Types of Fabrics. Int. J. Mol. Sci. 2022, 23, 10686. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Inokuchi, S.; Kitayama, T.; Nakahara, H.; Mizuno, N.; Sekiguchi, K. A comparison of DNA extraction using AutoMate Express and EZ1 advanced XL from liquid blood, bloodstains, and semen stains. J. Forensic Sci. 2013, 58, 981–988. [Google Scholar] [CrossRef]
- Davis, C.P.; King, J.L.; Budowle, B.; Eisenberg, A.J.; Turnbough, M.A. Extraction platform evaluations: A comparison of AutoMate Express, EZ1(R) Advanced XL, and Maxwell(R) 16 Bench-top DNA extraction systems. Leg. Med. 2012, 14, 36–39. [Google Scholar] [CrossRef]
- Bond, M.M.; Richards-Kortum, R.R. Drop-to-drop variation in the cellular components of fingerprick blood: Implications for point-of-care diagnostic development. Am. J. Clin. Pathol. 2015, 144, 885–894. [Google Scholar] [CrossRef]
- Akane, A.; Matsubara, K.; Nakamura, H.; Takahashi, S.; Kimura, K. Identification of the Heme Compound Copurified with Deoxyribonucleic Acid (DNA) from Bloodstains, a Major Inhibitor of Polymerase Chain Reaction (PCR) Amplification. J. Forensic Sci. 1994, 39, 362–372. [Google Scholar] [CrossRef]
- Sidstedt, M.; Steffen, C.R.; Kiesler, K.M.; Vallone, P.M.; Rådström, P.; Hedman, J. The impact of common PCR inhibitors on forensic MPS analysis. Forensic Sci. Int. Genet. 2019, 40, 182–191. [Google Scholar] [CrossRef]
- Zapico, S.C.; Roca, G. Making the Most of Lateral Flow Immunochromatographic Tests: An Efficient Protocol to Recover DNA. Methods Protoc. 2024, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Nam, Y.-H.; Lee, J.H.; Kim, H.S.; Kim, E. Efficacy of reduced-size short tandem repeat PCR analysis for degraded DNA samples. Genes Genom. 2021, 43, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Hanson, E.K.; Ballantyne, J. A blue spectral shift of the hemoglobin soret band correlates with theage (time since deposition) of dried bloodstains. PLoS ONE 2010, 5, e12830. [Google Scholar] [CrossRef] [PubMed]
- Mayes, C.; Houston, R.; Seashols-Williams, S.; LaRue, B.; Hughes-Stamm, S. The stability and persistence of blood and semen mRNA and miRNA targets for body fluid identification in environmentally challenged and laundered samples. Leg. Med. 2019, 38, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Hady, R.H.A.; Thabet, H.Z.; Ebrahem, N.E.; Yassa, H.A. Thermal Effects on DNA Degradation in Blood and Seminal Stains: Forensic View. Acad. Forensic Pathol. 2021, 11, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Twanabasu, B. Degradation of Seminal Components in Different Environmental Condition. Master’s Thesis, Boston University, Boston, MA, USA, 2021. [Google Scholar]
- Srettabunjong, S.; Betset, P.; Limawongpranee, S.; Ekpo, P. The Stability of Prostate-Specific Antigen in Semen Under Various Temperatures. J. Forensic Sci. 2015, 60, 1577–1581. [Google Scholar] [CrossRef]
- Jimenez-Verdejo, A.; Osuna, E.; Garcia-Olivares, E.; Luna, A. Study of the enzymatic activity of GGT, LDH, PAP and PSA in semen stains: Application to age calculation. Forensic Sci. Int. 1994, 68, 7–15. [Google Scholar] [CrossRef]
- SERATEC®. In-Vitro Diagnostic Test for Professional Forensic Use for the Detection of Seminal Fluid by the Semi-Quantitative Determination of PSA (Prostate-Specific Antigen); SERATEC: Göttingen, Germany, 2011. [Google Scholar]
- Garriga, J.A.; Ubelaker, D.H.; Zapico, S.C. Evaluation of macroscopic changes and the efficiency of DNA profiling from burnt teeth. Sci. Justice 2016, 56, 437–442. [Google Scholar] [CrossRef]
- R Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- R Team. RStudio: Integrated Development for R 2020; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Paz, F.; Kuba, B.; Kryvorutsky, E.; Roca, G.; Zapico, S.C. Assessment of Blood and Semen Detection and DNA Collection from Swabs up to Three Months after Deposition on Five Different Cloth Materials. Int. J. Mol. Sci. 2024, 25, 3522. https://doi.org/10.3390/ijms25063522
Medina-Paz F, Kuba B, Kryvorutsky E, Roca G, Zapico SC. Assessment of Blood and Semen Detection and DNA Collection from Swabs up to Three Months after Deposition on Five Different Cloth Materials. International Journal of Molecular Sciences. 2024; 25(6):3522. https://doi.org/10.3390/ijms25063522
Chicago/Turabian StyleMedina-Paz, Francisco, Brandon Kuba, Emily Kryvorutsky, Gabriela Roca, and Sara C. Zapico. 2024. "Assessment of Blood and Semen Detection and DNA Collection from Swabs up to Three Months after Deposition on Five Different Cloth Materials" International Journal of Molecular Sciences 25, no. 6: 3522. https://doi.org/10.3390/ijms25063522
APA StyleMedina-Paz, F., Kuba, B., Kryvorutsky, E., Roca, G., & Zapico, S. C. (2024). Assessment of Blood and Semen Detection and DNA Collection from Swabs up to Three Months after Deposition on Five Different Cloth Materials. International Journal of Molecular Sciences, 25(6), 3522. https://doi.org/10.3390/ijms25063522








