Differential Expression of CKLF-like MARVEL Transmembrane Domain-Containing Protein 6 and Programmed Cell Death Ligand 1 as Prognostic Biomarkers in Upper Tract Urothelial Carcinoma
Abstract
1. Introduction
2. Results
2.1. Patient and Tumor Characteristics
2.2. Morphological Evaluation
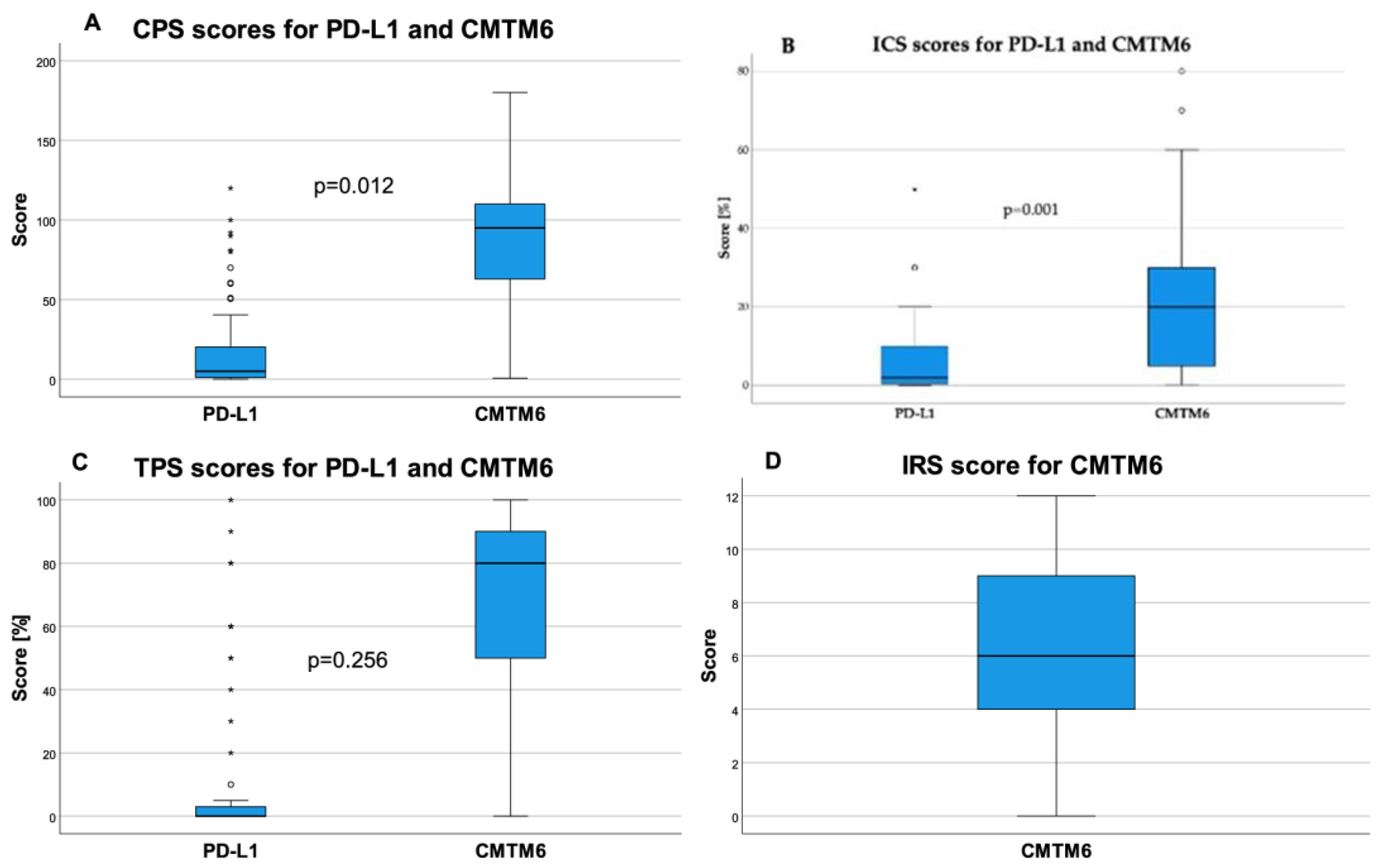
2.3. CMTM6 and PD-L1 Expression and Correlation Analysis
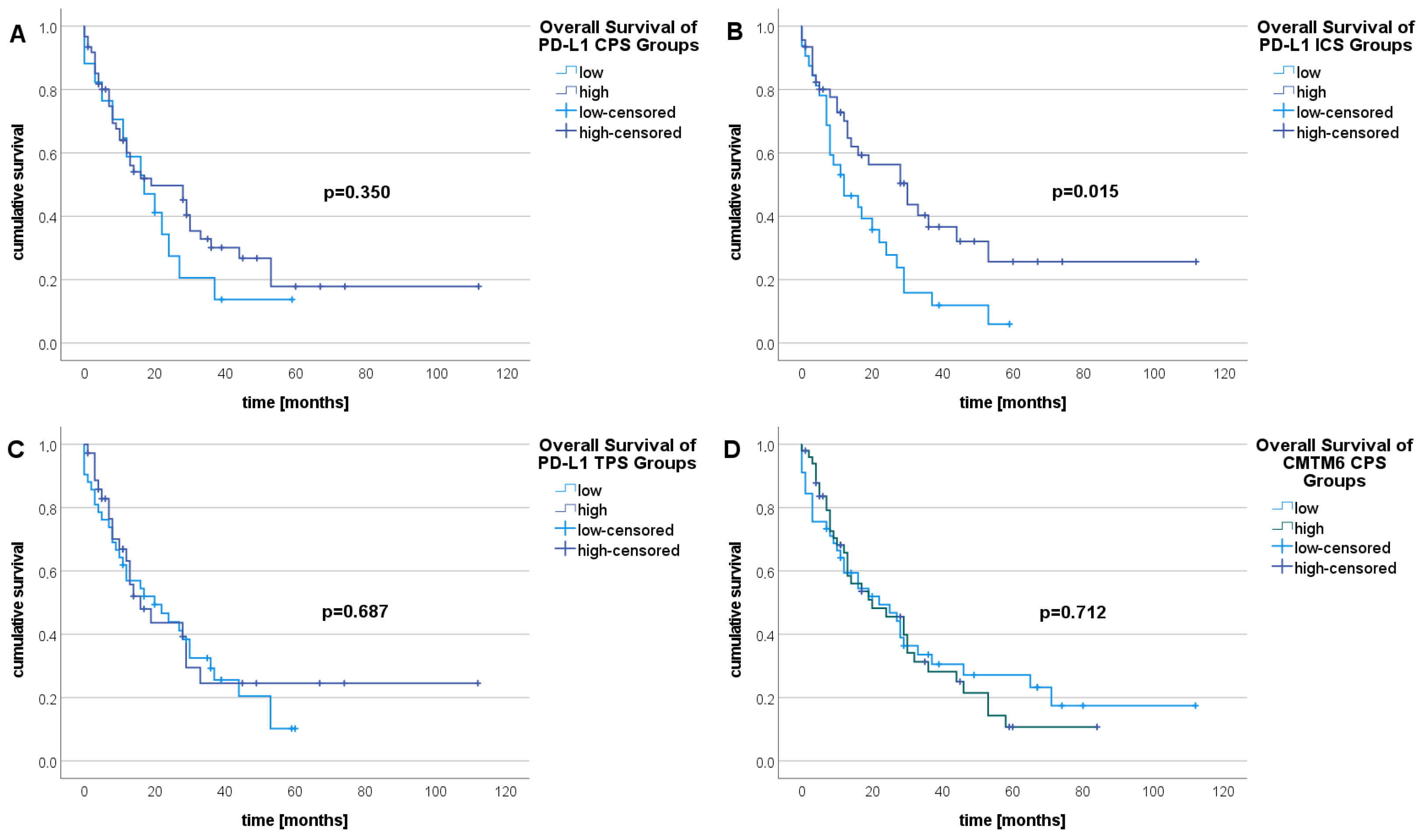
2.4. Survival Analysis
3. Discussion
4. Materials and Methods
4.1. Study Population and Clinicopathological Data Assessment
4.2. Morphology Evaluation
4.3. Immunohistochemistry
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Zigeuner, R.; Shariat, S.F.; van Rhijn, B.W.G.; Compérat, E.; Sylvester, R.J.; Kaasinen, E.; Böhle, A.; Palou Redorta, J.; et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update 2013. Eur. Urol. 2013, 64, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, M.; Palou, J.; Gaya, J.M.; Breda, A.; Rodriguez-Faba, O.; Villavicencio-Mavrich, H. Upper urinary tract urothelial cell carcinoma: Location as a predictive factor for concomitant bladder carcinoma. World J. Urol. 2013, 31, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Xylinas, E.; Rink, M.; Margulis, V.; Karakiewicz, P.; Novara, G.; Shariat, S.F. Multifocal carcinoma in situ of the upper tract is associated with high risk of bladder cancer recurrence. Eur. Urol. 2012, 61, 1069–1070. [Google Scholar] [CrossRef]
- Li, W.-M.; Shen, J.-T.; Li, C.-C.; Ke, H.-L.; Wei, Y.-C.; Wu, W.-J.; Chou, Y.-H.; Huang, C.-H. Oncologic outcomes following three different approaches to the distal ureter and bladder cuff in nephroureterectomy for primary upper urinary tract urothelial carcinoma. Eur. Urol. 2010, 57, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Margulis, V.; Shariat, S.F.; Matin, S.F.; Kamat, A.M.; Zigeuner, R.; Kikuchi, E.; Lotan, Y.; Weizer, A.; Raman, J.D.; Wood, C.G. Outcomes of radical nephroureterectomy: A series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009, 115, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Maruschke, M.; Kram, W.; Zimpfer, A.; Kundt, G.; Hakenberg, O.W. Upper Urinary Tract Tumors: Which Diagnostic Methods Are Needed? Urol. Int. 2017, 98, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Rouprêt, M.; Seisen, T.; Birtle, A.J.; Capoun, O.; Compérat, E.M.; Dominguez-Escrig, J.L.; Gürses Andersson, I.; Liedberg, F.; Mariappan, P.; Hugh Mostafid, A.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2023 Update. Eur. Urol. 2023, 84, 49–64. [Google Scholar] [CrossRef]
- Zganjar, A.J.; Thiel, D.D.; Lyon, T.D. Diagnosis, workup, and risk stratification of upper tract urothelial carcinoma. Transl. Androl. Urol. 2023, 12, 1456–1468. [Google Scholar] [CrossRef]
- Qi, N.; Zhang, J.; Chen, Y.; Wen, R.; Li, H. Microscopic hematuria predicts lower stage in patients with upper tract urothelial carcinoma. Cancer Manag. Res. 2018, 10, 4929–4933. [Google Scholar] [CrossRef]
- Rouprêt, M.; Babjuk, M.; Compérat, E.; Zigeuner, R.; Sylvester, R.J.; Burger, M.; Cowan, N.C.; Gontero, P.; van Rhijn, B.W.G.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2017 Update. Eur. Urol. 2018, 73, 111–122. [Google Scholar] [CrossRef]
- Potretzke, A.M.; Knight, B.A.; Vetter, J.M.; Anderson, B.G.; Hardi, A.C.; Bhayani, S.B.; Figenshau, R.S. Diagnostic Utility of Selective Upper Tract Urinary Cytology: A Systematic Review and Meta-analysis of the Literature. Urology 2016, 96, 35–43. [Google Scholar] [CrossRef]
- Janisch, F.; Shariat, S.F.; Baltzer, P.; Fajkovic, H.; Kimura, S.; Iwata, T.; Korn, P.; Yang, L.; Glybochko, P.V.; Rink, M.; et al. Diagnostic performance of multidetector computed tomographic (MDCTU) in upper tract urothelial carcinoma (UTUC): A systematic review and meta-analysis. World J. Urol. 2020, 38, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Caoili, E.M.; Cohan, R.H.; Inampudi, P.; Ellis, J.H.; Shah, R.B.; Faerber, G.J.; Montie, J.E. MDCT urography of upper tract urothelial neoplasms. AJR Am. J. Roentgenol. 2005, 184, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Millán-Rodríguez, F.; Palou, J.; de La Torre-Holguera, P.; Vayreda-Martija, J.M.; Villavicencio-Mavrich, H.; Vicente-Rodríguez, J. Conventional CT signs in staging transitional cell tumors of the upper urinary tract. Eur. Urol. 1999, 35, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Froemming, A.; Potretzke, T.; Takahashi, N.; Kim, B. Upper tract urothelial cancer. Eur. J. Radiol. 2018, 98, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Rouprêt, M.; Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Cowan, N.C.; Dominguez-Escrig, J.L.; Gontero, P.; Hugh Mostafid, A.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur. Urol. 2021, 79, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Birtle, A.; Johnson, M.; Chester, J.; Jones, R.; Dolling, D.; Bryan, R.T.; Harris, C.; Winterbottom, A.; Blacker, A.; Catto, J.W.F.; et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): A phase 3, open-label, randomised controlled trial. Lancet 2020, 395, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Cancer Research Institute. FDA Approval Timeline of Active Immunotherapies: CRI. Available online: https://www.cancerresearch.org/?s=FDA+Approval+Timeline+of+Active+Immunotherapies (accessed on 23 March 2023).
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the Pd-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Schildhaus, H.-U. Der prädiktive Wert der PD-L1-Diagnostik. Pathologe 2018, 39, 498–519. [Google Scholar] [CrossRef]
- Lu, Y.; Kang, J.; Luo, Z.; Song, Y.; Tian, J.; Li, Z.; Wang, X.; Liu, L.; Yang, Y.; Liu, X. The Prevalence and Prognostic Role of PD-L1 in Upper Tract Urothelial Carcinoma Patients Underwent Radical Nephroureterectomy: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 1400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yu, W.; Feng, X.; Zhao, Z.; Fan, Y.; Meng, Y.; Hu, S.; Cui, Y.; He, Q.; Zhang, H.; et al. Prognostic significance of PD-L1 expression on tumor cells and tumor-infiltrating mononuclear cells in upper tract urothelial carcinoma. Med. Oncol. 2017, 34, 94. [Google Scholar] [CrossRef] [PubMed]
- Krabbe, L.-M.; Heitplatz, B.; Preuss, S.; Hutchinson, R.C.; Woldu, S.L.; Singla, N.; Boegemann, M.; Wood, C.G.; Karam, J.A.; Weizer, A.Z.; et al. Prognostic Value of PD-1 and PD-L1 Expression in Patients with High Grade Upper Tract Urothelial Carcinoma. J. Urol. 2017, 198, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Tomita, Y.; Nasroulah, F.; Li, J.; Collette, S.; Valderrama, B.P.; Grimm, M.-O.; et al. Disease-free Survival Analysis for Patients with High-risk Muscle-invasive Urothelial Carcinoma from the Randomized CheckMate 274 Trial by PD-L1 Combined Positive Score and Tumor Cell Score. Eur. Urol. 2023, 83, 432–440. [Google Scholar] [CrossRef]
- Nakanishi, J.; Wada, Y.; Matsumoto, K.; Azuma, M.; Kikuchi, K.; Ueda, S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol. Immunother. 2007, 56, 1173–1182. [Google Scholar] [CrossRef]
- Xylinas, E.; Robinson, B.D.; Kluth, L.A.; Volkmer, B.G.; Hautmann, R.; Küfer, R.; Zerbib, M.; Kwon, E.; Thompson, R.H.; Boorjian, S.A.; et al. Association of T-cell co-regulatory protein expression with clinical outcomes following radical cystectomy for urothelial carcinoma of the bladder. Eur. J. Surg. Oncol. 2014, 40, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; O’Donnell, P.H.; Massard, C.; Arkenau, H.-T.; Friedlander, T.W.; Hoimes, C.J.; Lee, J.L.; Ong, M.; Sridhar, S.S.; Vogelzang, N.J.; et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results from a Phase 1/2 Open-label Study. JAMA Oncol. 2017, 3, e172411. [Google Scholar] [CrossRef]
- Apolo, A.B.; Infante, J.R.; Balmanoukian, A.; Patel, M.R.; Wang, D.; Kelly, K.; Mega, A.E.; Britten, C.D.; Ravaud, A.; Mita, A.C.; et al. Avelumab, an Anti-Programmed Death-Ligand 1 Antibody, in Patients with Refractory Metastatic Urothelial Carcinoma: Results from a Multicenter, Phase Ib Study. J. Clin. Oncol. 2017, 35, 2117–2124. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef]
- Ribas, A.; Hu-Lieskovan, S. What does PD-L1 positive or negative mean? J. Exp. Med. 2016, 213, 2835–2840. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.-A.; Reed, K.; et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Burr, M.L.; Sparbier, C.E.; Chan, Y.-C.; Williamson, J.C.; Woods, K.; Beavis, P.A.; Lam, E.Y.N.; Henderson, M.A.; Bell, C.C.; Stolzenburg, S.; et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature 2017, 549, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Zugazagoitia, J.; Liu, Y.; Toki, M.; McGuire, J.; Ahmed, F.S.; Henick, B.S.; Gupta, R.; Gettinger, S.N.; Herbst, R.S.; Schalper, K.A.; et al. Quantitative Assessment of CMTM6 in the Tumor Microenvironment and Association with Response to PD-1 Pathway Blockade in Advanced-Stage Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2019, 14, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, Q.-C.; Li, Y.-C.; Yang, L.-L.; Liu, J.-F.; Li, H.; Xiao, Y.; Bu, L.-L.; Zhang, W.-F.; Sun, Z.-J. Targeting CMTM6 Suppresses Stem Cell-Like Properties and Enhances Antitumor Immunity in Head and Neck Squamous Cell Carcinoma. Cancer Immunol. Res. 2020, 8, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhao, F.; Yang, K.; Lu, Y.; Zhang, Y.; Wang, W.; Xie, H.; Deng, K.; Yang, C.; Rong, Z.; et al. An immunophenotyping of renal clear cell carcinoma with characteristics and a potential therapeutic target for patients insensitive to immune checkpoint blockade. J. Cell. Biochem. 2019, 120, 13330–13341. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Wang, S.-S.; Zhang, M.; Jiang, J.; Fan, H.-Y.; Wu, J.-S.; Wang, H.-F.; Liang, X.-H.; Tang, Y.-L. OSCC cell-secreted exosomal CMTM6 induced M2-like macrophages polarization via ERK1/2 signaling pathway. Cancer Immunol. Immunother. 2021, 70, 1015–1029. [Google Scholar] [CrossRef] [PubMed]
- Evmorfopoulos, K.; Mitrakas, L.; Karathanasis, A.; Zachos, I.; Tzortzis, V.; Vlachostergios, P.J. Upper Tract Urothelial Carcinoma: A Rare Malignancy with Distinct Immuno-Genomic Features in the Era of Precision-Based Therapies. Biomedicines 2023, 11, 1775. [Google Scholar] [CrossRef] [PubMed]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef]
- Campedel, L.; Compérat, E.; Cancel-Tassin, G.; Varinot, J.; Pfister, C.; Delcourt, C.; Gobet, F.; Roumiguié, M.; Patard, P.-M.; Daniel, G.; et al. Prognostic value of programmed death ligand-1 and programmed death-1 expression in patients with upper tract urothelial carcinoma. BJU Int. 2023, 132, 581–590. [Google Scholar] [CrossRef]
- Skala, S.L.; Liu, T.-Y.; Udager, A.M.; Weizer, A.Z.; Montgomery, J.S.; Palapattu, G.S.; Siddiqui, J.; Cao, X.; Fields, K.; Abugharib, A.E.; et al. Programmed Death-ligand 1 Expression in Upper Tract Urothelial Carcinoma. Eur. Urol. Focus 2017, 3, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef]
- Tulchiner, G.; Brunner, A.; Schmidinger, M.; Staudacher, N.; Orme, J.J.; Thurnher, M.; Horninger, W.; Culig, Z.; Pichler, R. CMTM6 expression as a potential biomarker for immunotherapy in metastatic renal cell carcinoma. BJU Int. 2021, 128, 29–32. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Hussain, M.; Gschwend, J.E.; Albers, P.; Oudard, S.; Castellano, D.; Daneshmand, S.; Nishiyama, H.; Majchrowicz, M.; Degaonkar, V.; et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 525–537. [Google Scholar] [CrossRef]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.-O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, N.; Kikuchi, E.; Mikami, S.; Fukumoto, K.; Oya, M. The Role of PD-1 Positivity in the Tumour Nest on Clinical Outcome in Upper Tract Urothelial Carcinoma Patients Treated with Radical Nephroureterectomy. Clin. Oncol. (R. Coll. Radiol.) 2018, 30, e1–e8. [Google Scholar] [CrossRef]
- Fernandez, A.I.; Gaule, P.; Rimm, D.L. Tissue Age Affects Antigenicity and Scoring for the 22C3 Immunohistochemistry Companion Diagnostic Test. Mod. Pathol. 2023, 36, 100159. [Google Scholar] [CrossRef] [PubMed]
- Karpathiou, G.; Vincent, M.; Dumollard, J.M.; Mobarki, M.; Péoc’h, M. PD-L1 expression in head and neck cancer tissue specimens decreases with time. Pathol. Res. Pract. 2022, 237, 154042. [Google Scholar] [CrossRef]
- Wu, X.; Lan, X.; Hu, W.; Zhang, W.; Lai, X.; Xu, S.; Li, J.; Qiu, W.; Wang, W.; Xiao, J.; et al. CMTM6 expression in M2 macrophages is a potential predictor of PD-1/PD-L1 inhibitor response in colorectal cancer. Cancer Immunol. Immunother. 2021, 70, 3235–3248. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, M.; Pu, H.; Guo, S.; Zhang, S.; Wang, Y. Prognostic Implications of Pan-Cancer CMTM6 Expression and Its Relationship with the Immune Microenvironment. Front. Oncol. 2020, 10, 585961. [Google Scholar] [CrossRef]
- Guan, X.; Zhang, C.; Zhao, J.; Sun, G.; Song, Q.; Jia, W. CMTM6 overexpression is associated with molecular and clinical characteristics of malignancy and predicts poor prognosis in gliomas. eBioMedicine 2018, 35, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.-S.; Kluge, C.; Schofeld, C.; Zimpfer, A.H.; Schneider, B.; Strüder, D.; Redwanz, C.; Ribbat-Idel, J.; Idel, C.; Maletzki, C. Identifying Predictive Biomarkers for Head and Neck Squamous Cell Carcinoma Response. Cancers 2023, 15, 5597. [Google Scholar] [CrossRef] [PubMed]
- Mostofi, F.K.; Sobin, L.H.; Torloni, H. Histologic typing of urinary bladders. In International Histological Classification of Tumors; World Health Organization: Geneva, Switzerland, 1973; pp. 15–17. [Google Scholar]
- Williamson, S.R.; Al-Ahmadie, H.A.; Cheng, L.; Downes, M.R.; Lopez-Beltran, A.; Narumi, Y.; Panebianco, V.; Paner, G.P.; Raspolllini, M.R.; Ro, J.Y.; et al. Urinary and Male Genital Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2022; pp. 158–159. [Google Scholar]



| Feature | Characteristics/Values | |
|---|---|---|
| Number of patients | 102 | |
| Age and gender n (%) | mean: 72 yrs, range 42–91 yrs 66 (65.0) males, 36 females (35.0) | |
| Localization n (%) total n = 103 tumors | renal pelvis ureter renal pelvis and ureter | 69 (67.0) 30 (29.1) 4 (3.9) |
| 1973 WHO grading n (%) total n = 103 | G2 G3 | 25 (24.3) 78 (75.7) |
| 2022 WHO grading n (%) total n = 103 | low grade high grade | 2 (1.9) 101 (98.1) |
| Tumor size (mm) total n = 83 | mean ± SD range | 40 ± 21.6 10–120 |
| pT n (%) total n = 103 | 1 2/3/4 | 22 (21.4) 10 (9.7)/51 (49.5)/20 (19.4) |
| pN n (%) total n = 88 | 0 1/2 | 59 (67.0) 7 (8.0)/22 (25.0) |
| cM n (%) total n = 99 | 0 1 | 80 (80.8) 19 (19.2) |
| Morphology n (%) total n = 101 | papillary solid papillary-solid/ inverted | 5 (4.9) 44 (43.6) 52 (51.5) |
| L n (%) total n = 102 | 0 1 | 47 (46.1) 55 (53.9) |
| V n (%) total n = 102 | 0 1/2 | 66 (64.7) 33 (32.4)/3 (2.9) |
| Pn n (%) total n = 91 | 0 1 | 57 (62.6) 34 (37.4) |
| Resection status * n (%) total n = 95 | 0 1/2 | 70 (73.7) 17 (17.9)/8 (8.4) |
| Necrosis n (%) total n = 103 | absent present (≥10%) | 25 (24.3) 78 (75.7) |
| Acute inflammation | 0 1/2 | 36 (35.0) 6 (5.8)/61 (59.2) |
| Chronic inflammation n (%) total n = 103 | absent lymphocytic lymphofollicular | 18 (17.4) 29 (28.2) 56 (54.4) |
| PD-L1 | CMTM6 | |||||
|---|---|---|---|---|---|---|
| Staining Intensity Category | High [%] | Threshold | Low [%] | High [%] | Threshold | Low [%] |
| CPS | 76.2 | 1.0 | 23.8 | 51.5 | 90.0 | 48.5 |
| ICS | 58.3 | 2.0% | 41.7 | 51.5 | 20.0% | 48.5 |
| TPS | 45.2 | 0.5% | 54.8 | 55.4 | 70.0% | 44.6 |
| IRS | - | - | - | 63.4 | 6 | 36.6 |
| PD-L1 CPS Low/High | PD-L1 ICS Low/High | PD-L1 TPS Low/High | CMTM6 IRS Low/High | CMTM6 CPS Low/High | CMTM6 ICS Low/High | CMTM6 TPS Low/High | |
|---|---|---|---|---|---|---|---|
| pT | 0.100 | 0.020 | 0.319 ** | 0.246 * | 0.218 * | 0.208 * | 0.154 |
| WHO grade | 0.294 ** | 0.115 | 0.339 ** | 0.273 ** | 0.145 | 0.229 * | 0.083 |
| survival | 0.181 | 0.294 ** | 0.155 | 0.015 | 0.021 | 0.105 | 0.018 |
| inflammation | 0.111 | 0.162 | 0.183 | 0.025 | 0.079 | 0.234 * | 0.040 |
| necrosis | 0.294 ** | 0.115 | 0.339 ** | 0.203 * | 0.261 ** | 0.249 * | 0.155 |
| stromal reaction | 0.119 | 0.104 | 0.148 | 0.152 | 0.106 | 0.002 | 0.144 |
| pN | 0.000 | 0.038 | 0.170 | 0.112 | 0.074 | 0.051 | 0.054 |
| cM | 0.004 | 0.119 | 0.128 | 0.209 * | 0.147 | 0.145 | 0.012 |
| L | 0.093 | 0.069 | 0.180 | 0.201 * | 0.115 | 0.056 | 0.008 |
| V | 0.026 | 0.250 * | 0.095 | 0.002 | 0.003 | 0.113 | 0.007 |
| Pn | 0.195 | 0.011 | 0.219 | 0.008 | 0.014 | 0.002 | 0.041 |
| resection status | 0.218 | 0.266 * | 0.099 | 0.020 | 0.031 | 0.128 | 0.027 |
| PD-L1 CPS low/high | – | 0.640 ** | 0.491 ** | 0.355 ** | 0.306 ** | 0.365 ** | 0.156 |
| PD-L1 ICS low/high | 0.640 ** | – | 0.283 ** | 0.147 | 0.161 | 0.309 ** | 0.046 |
| PD-L1 TPS low/high | 0.491 ** | 0.283 ** | – | 0.234 * | 0.170 | 0.283 ** | 0.077 |
| CMTM6 IRS low/high | 0.355 ** | 0.147 | 0.234 * | – | 0.717 ** | 0.331 ** | 0.683 ** |
| CMTM6 CPS low/high | 0.306 ** | 0.161 | 0.170 | 0.717 ** | – | 0.465 ** | 0.743 ** |
| CMTM6 ICS low/high | 0.365 ** | 0.309 ** | 0.283 ** | 0.331 ** | 0.465 ** | – | 0.206 * |
| CMTM6 TPS low/high | 0.156 | 0.046 | 0.077 | 0.683 ** | 0.743 ** | 0.206 * | – |
| pT | WHO Grade | Inflammation Degree | Acute Inflammation Type | Chronic Inflammation Type | Necrosis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| αadj = 0.008 | αadj = 0.008 | αadj = 0.008 | αadj = 0.02 | αadj = 0.02 | αadj = 0.02 | ||||||
| 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | none vs. weak | none vs. moderate | none vs. strong | none vs. GR | none vs. LC | none vs. LF | none vs. low | none vs. high | |
| PD-L1 CPS | – | – | 0.005 | 0.003 | <0.001 | 0.004 | <0.001 | 0.001 | <0.001 | <0.001 | 0.005 |
| PD-L1 ICS | – | – | 0.040 | – | 0.001 | – | 0.017 | 0.002 | 0.003 | 0.008 | – |
| PD-L1 TPS | 0.005 | 0.004 | 0.002 | – | – | – | 0.001 | – | – | 0.005 | 0.004 |
| CMTM6 CPS | 0.004 | – | – | – | – | – | – | – | – | 0.008 | – |
| CMTM6 ICS | – | – | 0.007 | 0.007 | 0.002 | 0.001 | 0.003 | 0.004 | <0.001 | 0.003 | – |
| Parameter | Univariable Regression | Multivariable Regression | ||||
|---|---|---|---|---|---|---|
| p-Value | 95% CI | HR | p-Value | 95% CI | Adj. HR | |
| Population | ||||||
| Age * ≤60 yrs vs. >60 yrs | 0.892 | 0.547–1.999 | 1.046 | |||
| Sex * m vs. f | 0.348 | 0.460–1.315 | 0.777 | |||
| PD-L1 CPS: * low vs. high | 0.360 | 0.406–1.387 | 0.750 | |||
| PD-L1 ICS: * low vs. high | 0.019 | 0.303–0.899 | 0.522 | 0.851 | 0.390–2.178 | 0.921 |
| PD-L1 TPS: * low vs. high | 0.692 | 0.514–1.555 | 0.894 | |||
| CMTM6 IRS: * low vs. high | 0.980 | 0.604–1.635 | 0.994 | |||
| CMTM6 CPS: * low vs. high | 0.716 | 0.674–1.776 | 1.094 | |||
| CMTM6 ICS: * low vs. high | 0.790 | 0.579–1.515 | 0.937 | |||
| CMTM6 TPS: * low vs. high | 0.647 | 0.689–1.822 | 1.120 | |||
| Growth pattern | 0.323 | |||||
| * papillary vs. Solid | 0.650 | 0.400–4.323 | 1.318 | |||
| * papillary vs. papillary-solid/inverted | 0.920 | 0.286–3.092 | 0.941 | |||
| Necrosis: * absent vs. present | 0.068 | 0.958–3.300 | 1.778 | |||
| Stromal reaction: * low vs. high | 0.001 | 1.372–3.696 | 2.252 | 0.047 | 1.008–4.315 | 2.086 |
| Inflammation: | ||||||
| * no inflammation vs. chronic lymphofollicular inflammation | 0.774 | 0.459–1.785 | 0.905 | |||
| Chronic lymphocytic inflammation: | ||||||
| * absent vs. Present | 0.498 | 0.619–2.681 | 1.228 | |||
| Inflammation intensity: | ||||||
| * low vs. High | 0.003 | 0.075–0.590 | 0.211 | 0.019 | 0.012–0.670 | 0.090 |
| WHO grading: * G2 vs. G3 | 0.008 | 1.276–4.943 | 2.511 | 0.389 | 0.520–5.360 | 1.669 |
| T-stage: | 0.003 | 0.083 | 0.954–2.165 | 1.437 | ||
| * pT1 vs. pT2 | 0.471 | 0.548–3.668 | 1.418 | |||
| * pT1 vs. pT3 | 0.121 | 0.866–3.433 | 1.724 | |||
| * pT1 vs. pT4 | 0.001 | 1.617–7.475 | 3.476 | |||
| Nodal status: * pN0 vs. pN1 or pN2 | 0.022 | 1.099–3.273 | 1.896 | 0.655 | 0.556–2.541 | 1.189 |
| Metastasis: * cM0 vs. cM1 | 0.027 | 1.075–3.378 | 1.906 | 0.797 | 0.356–2.209 | 0.887 |
| Lymphatic invasion: * L0 vs. L1 | 0.007 | 1.204–3.276 | 1.986 | 0.160 | 0.212–1.292 | 0.523 |
| Venous invasion: * V0 vs. V1 or V2 | 0.036 | 1.036–2.769 | 1.694 | 0.516 | 0.583–2.930 | 1.307 |
| Perineural invasion: * Pn0 vs. Pn1 | 0.393 | 0.749–2.090 | 1.251 | |||
| Residual status: * R0 vs. R1 or R2 | <0.001 | 1.512–4.637 | 2.648 | 0.325 | 0.682–3.175 | 1.471 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kdimati, S.; Christoph, C.; Glass, Ä.; Engel, N.; Dräger, D.-L.; Maletzki, C.; Becker, A.-S.; Zimpfer, A. Differential Expression of CKLF-like MARVEL Transmembrane Domain-Containing Protein 6 and Programmed Cell Death Ligand 1 as Prognostic Biomarkers in Upper Tract Urothelial Carcinoma. Int. J. Mol. Sci. 2024, 25, 3492. https://doi.org/10.3390/ijms25063492
Kdimati S, Christoph C, Glass Ä, Engel N, Dräger D-L, Maletzki C, Becker A-S, Zimpfer A. Differential Expression of CKLF-like MARVEL Transmembrane Domain-Containing Protein 6 and Programmed Cell Death Ligand 1 as Prognostic Biomarkers in Upper Tract Urothelial Carcinoma. International Journal of Molecular Sciences. 2024; 25(6):3492. https://doi.org/10.3390/ijms25063492
Chicago/Turabian StyleKdimati, Said, Clemens Christoph, Änne Glass, Nadja Engel, Desiree-Louise Dräger, Claudia Maletzki, Anne-Sophie Becker, and Annette Zimpfer. 2024. "Differential Expression of CKLF-like MARVEL Transmembrane Domain-Containing Protein 6 and Programmed Cell Death Ligand 1 as Prognostic Biomarkers in Upper Tract Urothelial Carcinoma" International Journal of Molecular Sciences 25, no. 6: 3492. https://doi.org/10.3390/ijms25063492
APA StyleKdimati, S., Christoph, C., Glass, Ä., Engel, N., Dräger, D.-L., Maletzki, C., Becker, A.-S., & Zimpfer, A. (2024). Differential Expression of CKLF-like MARVEL Transmembrane Domain-Containing Protein 6 and Programmed Cell Death Ligand 1 as Prognostic Biomarkers in Upper Tract Urothelial Carcinoma. International Journal of Molecular Sciences, 25(6), 3492. https://doi.org/10.3390/ijms25063492







