Aberrantly Glycosylated GLUT1 as a Poor Prognosis Marker in Aggressive Bladder Cancer
Abstract
1. Introduction
2. Results
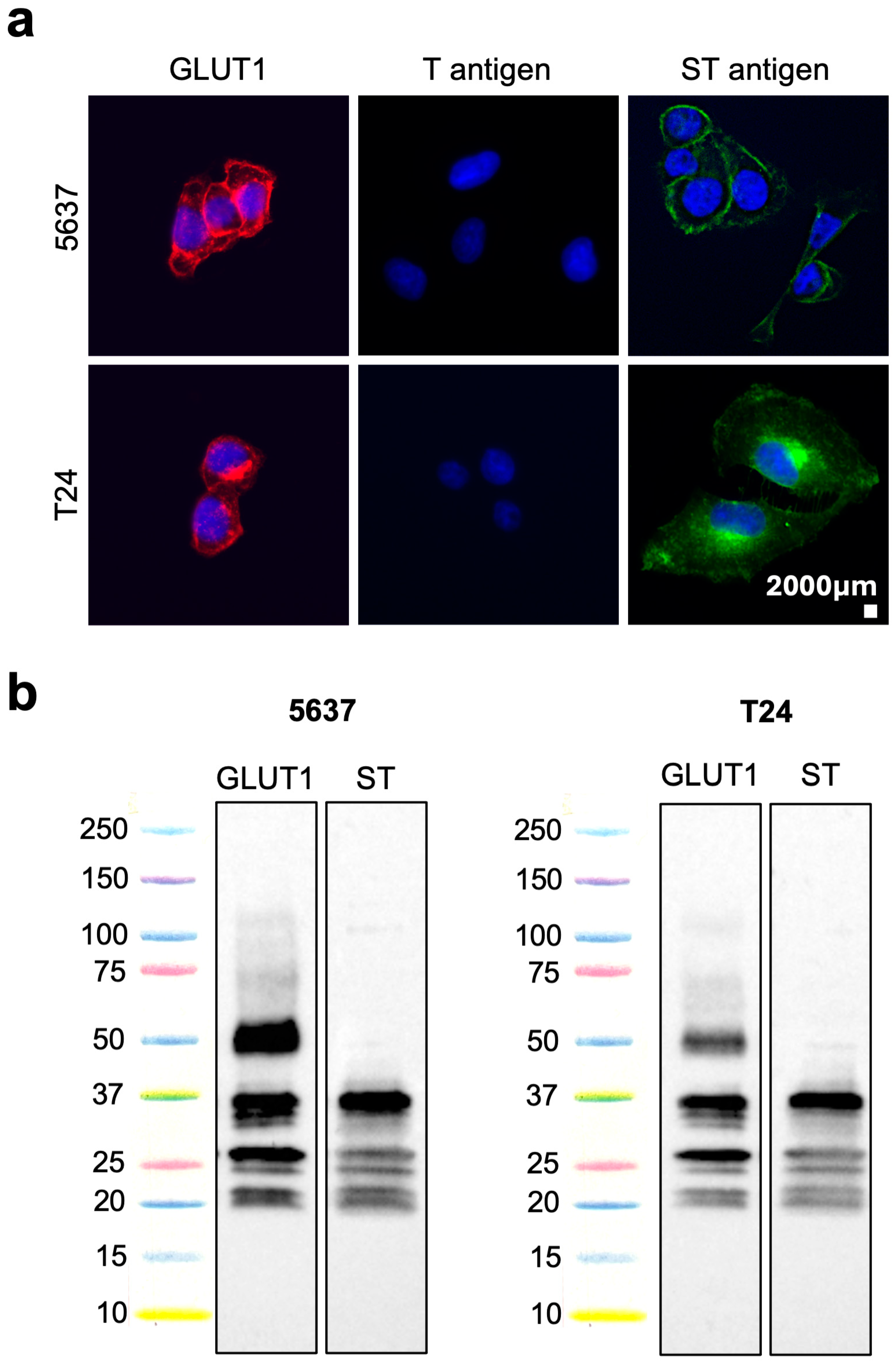
2.1. GLUT1 Glycosylation in Cell Models
2.2. GLUT1 Expression in Bladder Cancer
2.3. GLUT1 Glycosylation in Bladder Cancer
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Immunocytochemistry
4.3. Immunoprecipitation and Western Blot
4.4. Patient Samples
| n (%) | |
|---|---|
| Non-muscle-invasive | 53 (50.9) |
| Muscle-invasive | 51 (49.1) |
| Stage | |
| Ta low grade | 19 (18.3) |
| T1 high grade | 34 (32.7) |
| T2 | 12 (11.5) |
| T3 | 25 (24.0) |
| T4 | 14 (13.5) |
| Lymph node metastasis (N) | |
| No | 13 (12.5) |
| Yes | 15 (14.4) |
| Missing information | 76 (73.1) |
| Distant metastasis (M) | |
| M0 | 48 (46.2) |
| M1 | 2 (1.9) |
| Missing information | 54 (51.9) |
4.5. Bladder Tumours Glycoproteomics
4.6. Tissue Expressions of GLUT1, STn and ST Antigens
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Minoli, M.; Kiener, M.; Thalmann, G.N.; Kruithof-de Julio, M.; Seiler, R. Evolution of Urothelial Bladder Cancer in the Context of Molecular Classifications. Int. J. Mol. Sci. 2020, 21, 5670. [Google Scholar] [CrossRef]
- Kamat, A.M.; Hahn, N.M.; Efstathiou, J.A.; Lerner, S.P.; Malmstrom, P.U.; Choi, W.; Guo, C.C.; Lotan, Y.; Kassouf, W. Bladder cancer. Lancet 2016, 388, 2796–2810. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Callahan, M.K.; Bono, P.; Kim, J.; Spiliopoulou, P.; Calvo, E.; Pillai, R.N.; Ott, P.A.; de Braud, F.; Morse, M.; et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): A multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016, 17, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Wilczak, M.; Surman, M.; Przybyło, M. Altered Glycosylation in Progression and Management of Bladder Cancer. Molecules 2023, 28, 3436. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.; Videira, P.A.; Lima, L.; Pereira, S.; Silva, M.; Carrascal, M.; Severino, P.F.; Fernandes, E.; Almeida, A.; Costa, C.; et al. Overexpression of tumour-associated carbohydrate antigen sialyl-Tn in advanced bladder tumours. Mol. Oncol. 2013, 7, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Videira, P.A.; Correia, M.; Malagolini, N.; Crespo, H.J.; Ligeiro, D.; Calais, F.M.; Trindade, H.; Dall’Olio, F. ST3Gal.I sialyltransferase relevance in bladder cancer tissues and cell lines. BMC Cancer 2009, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, A.; Ferreira, D.; Azevedo, R.; Freitas, R.; Fernandes, E.; Relvas-Santos, M.; Gaiteiro, C.; Soares, J.; Cotton, S.; Teixeira, B.; et al. Glycoproteomics identifies HOMER3 as a potentially targetable biomarker triggered by hypoxia and glucose deprivation in bladder cancer. J. Exp. Clin. Cancer Res. 2021, 40, 191. [Google Scholar] [CrossRef]
- Lima, L.; Neves, M.; Oliveira, M.I.; Dieguez, L.; Freitas, R.; Azevedo, R.; Gaiteiro, C.; Soares, J.; Ferreira, D.; Peixoto, A.; et al. Sialyl-Tn identifies muscle-invasive bladder cancer basal and luminal subtypes facing decreased survival, being expressed by circulating tumor cells and metastases. Urol. Oncol. 2017, 35, 675.e1–675.e8. [Google Scholar] [CrossRef]
- Yeagle, P.L. Chapter 13—Membrane Transport. In The Membranes of Cells, 3rd ed.; Yeagle, P.L., Ed.; Academic Press: Boston, MA, USA, 2016. [Google Scholar]
- Yu, M.; Yongzhi, H.; Chen, S.; Luo, X.; Lin, Y.; Zhou, Y.; Jin, H.; Hou, B.; Deng, Y.; Tu, L.; et al. The prognostic value of GLUT1 in cancers: A systematic review and meta-analysis. Oncotarget 2017, 8, 43356–43367. [Google Scholar] [CrossRef]
- Berlth, F.; Mönig, S.; Pinther, B.; Grimminger, P.; Maus, M.; Schlösser, H.; Plum, P.; Warnecke-Eberz, U.; Harismendy, O.; Drebber, U.; et al. Both GLUT-1 and GLUT-14 are Independent Prognostic Factors in Gastric Adenocarcinoma. Ann. Surg. Oncol. 2015, 22 (Suppl. S3), S822–S831. [Google Scholar] [CrossRef]
- Kang, S.S.; Chun, Y.K.; Hur, M.H.; Lee, H.K.; Kim, Y.J.; Hong, S.R.; Lee, J.H.; Lee, S.G.; Park, Y.K. Clinical significance of glucose transporter 1 (GLUT1) expression in human breast carcinoma. Jpn. J. Cancer Res. 2002, 93, 1123–1128. [Google Scholar] [CrossRef]
- Haber, R.S.; Rathan, A.; Weiser, K.R.; Pritsker, A.; Itzkowitz, S.H.; Bodian, C.; Slater, G.; Weiss, A.; Burstein, D.E. GLUT1 glucose transporter expression in colorectal carcinoma: A marker for poor prognosis. Cancer 1998, 83, 34–40. [Google Scholar] [CrossRef]
- Cantuaria, G.; Fagotti, A.; Ferrandina, G.; Magalhaes, A.; Nadji, M.; Angioli, R.; Penalver, M.; Mancuso, S.; Scambia, G. GLUT-1 expression in ovarian carcinoma: Association with survival and response to chemotherapy. Cancer 2001, 92, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Massari, F.; Ciccarese, C.; Santoni, M.; Iacovelli, R.; Mazzucchelli, R.; Piva, F.; Scarpelli, M.; Berardi, R.; Tortora, G.; Lopez-Beltran, A.; et al. Metabolic phenotype of bladder cancer. Cancer Treat. Rev. 2016, 45, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, P.J.; Sibtain, A.; Daley, F.M.; Wilson, G.D. GLUT1 and CAIX as intrinsic markers of hypoxia in bladder cancer: Relationship with vascularity and proliferation as predictors of outcome of ARCON. Br. J. Cancer 2003, 89, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M.; Boado, R.J.; Farrell, C.R. Brain-type glucose transporter (GLUT-1) is selectively localized to the blood-brain barrier. Studies with quantitative western blotting and in situ hybridization. J. Biol. Chem. 1990, 265, 18035–18040. [Google Scholar] [CrossRef] [PubMed]
- Krzeslak, A.; Wojcik-Krowiranda, K.; Forma, E.; Jozwiak, P.; Romanowicz, H.; Bienkiewicz, A.; Brys, M. Expression of GLUT1 and GLUT3 Glucose Transporters in Endometrial and Breast Cancers. Pathol. Oncol. Res. 2012, 18, 721–728. [Google Scholar] [CrossRef]
- Julien, S.; Videira, P.A.; Delannoy, P. Sialyl-tn in cancer: (how) did we miss the target? Biomolecules 2012, 2, 435–466. [Google Scholar] [CrossRef]
- David, L.; Nesland, J.M.; Clausen, H.; Carneiro, F.; Sobrinho-Simões, M. Simple mucin-type carbohydrate antigens (Tn, sialosyl-Tn and T) in gastric mucosa, carcinomas and metastases. APMIS Suppl. 1992, 27, 162–172. [Google Scholar]
- Powles, T.; Bellmunt, J.; Comperat, E.; De Santis, M.; Huddart, R.; Loriot, Y.; Necchi, A.; Valderrama, B.P.; Ravaud, A.; Shariat, S.F.; et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 244–258. [Google Scholar] [CrossRef]
- Fassan, M.; Trabulsi, E.J.; Gomella, L.G.; Baffa, R. Targeted therapies in the management of metastatic bladder cancer. Biologics 2007, 1, 393–406. [Google Scholar]
- Gaiteiro, C.; Soares, J.; Relvas-Santos, M.; Peixoto, A.; Ferreira, D.; Paulo, P.; Brandão, A.; Fernandes, E.; Azevedo, R.; Palmeira, C.; et al. Glycoproteogenomics characterizes the CD44 splicing code associated with bladder cancer invasion. Theranostics 2022, 12, 3150–3177. [Google Scholar] [CrossRef]
- Pan, S.; Chen, R.; Aebersold, R.; Brentnall, T.A. Mass spectrometry based glycoproteomics—From a proteomics perspective. Mol. Cell. Proteom. 2011, 10, R110.003251. [Google Scholar] [CrossRef]
- Severino, P.F.; Silva, M.; Carrascal, M.; Malagolini, N.; Chiricolo, M.; Venturi, G.; Astolfi, A.; Catera, M.; Videira, P.A.; Dall’Olio, F. Expression of sialyl-Tn sugar antigen in bladder cancer cells affects response to Bacillus Calmette Guérin (BCG) and to oxidative damage. Oncotarget 2017, 8, 54506–54517. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carrascal, M.A.; Severino, P.F.; Guadalupe Cabral, M.; Silva, M.; Ferreira, J.A.; Calais, F.; Quinto, H.; Pen, C.; Ligeiro, D.; Santos, L.L.; et al. Sialyl Tn-expressing bladder cancer cells induce a tolerogenic phenotype in innate and adaptive immune cells. Mol. Oncol. 2014, 8, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Jiang, Y.; Liang, L.; Wu, J.; Cao, L.; Zhou, X.; Song, Z.; Ye, Z.; Zhao, Z.; Feng, H.; et al. Dysregulation and prometastatic function of glycosyltransferase C1GALT1 modulated by cHP1BP3/miR-1-3p axis in bladder cancer. J. Exp. Clin. Cancer Res. 2022, 41, 228. [Google Scholar] [CrossRef] [PubMed]
- Andreia, P.; Dylan, F.; Andreia, M.; Marta, R.-S.; Rui, F.; Tim, S.V.; Andreia, B.; Eduardo, F.; Paula, P.; Marta, C.; et al. Multilevel Plasticity and Altered Glycosylation Drive Aggressiveness in Hypoxic and Glucose-Deprived Bladder Cancer Cells. bioRxiv, 2023; perprint. [Google Scholar] [CrossRef]
- Temre, M.K.; Kumar, A.; Singh, S.M. An appraisal of the current status of inhibition of glucose transporters as an emerging antineoplastic approach: Promising potential of new pan-GLUT inhibitors. Front. Pharmacol. 2022, 13, 1035510. [Google Scholar] [CrossRef] [PubMed]
- Cotton, S.; Ferreira, D.; Soares, J.; Peixoto, A.; Relvas-Santos, M.; Azevedo, R.; Piairo, P.; Diéguez, L.; Palmeira, C.; Lima, L.; et al. Target Score-A Proteomics Data Selection Tool Applied to Esophageal Cancer Identifies GLUT1-Sialyl Tn Glycoforms as Biomarkers of Cancer Aggressiveness. Int. J. Mol. Sci. 2021, 22, 1664. [Google Scholar] [CrossRef]
- Klinger, M.; Farhan, H.; Just, H.; Drobny, H.; Himmler, G.; Loibner, H.; Mudde, G.C.; Freissmuth, M.; Sexl, V. Antibodies directed against Lewis-Y antigen inhibit signaling of Lewis-Y modified ErbB receptors. Cancer Res. 2004, 64, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Woods, E.C.; Vukojicic, P.; Bertozzi, C.R. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc. Natl. Acad. Sci. USA 2016, 113, 10304–10309. [Google Scholar] [CrossRef] [PubMed]
- Anderluh, M.; Berti, F.; Bzducha-Wróbel, A.; Chiodo, F.; Colombo, C.; Compostella, F.; Durlik, K.; Ferhati, X.; Holmdahl, R.; Jovanovic, D.; et al. Recent advances on smart glycoconjugate vaccines in infections and cancer. FEBS J. 2022, 289, 4251–4303. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Degliangeli, F.; Palitzsch, B.; Gerlitzki, B.; Kunz, H.; Schmitt, E.; Fiammengo, R.; Westerlind, U. Glycopeptide-functionalized gold nanoparticles for antibody induction against the tumor associated mucin-1 glycoprotein. Bioorg. Med. Chem. 2016, 24, 1132–1135. [Google Scholar] [CrossRef]
- Diniz, F.; Lamas, S.; Osório, H.; Aguiar, P.; Freitas, D.; Gärtner, F.; Sarmento, B.; Reis, C.A.; Gomes, J. Nanoparticles targeting Sialyl-Tn for efficient tyrosine kinase inhibitor delivery in gastric cancer. Acta Biomater. 2023, 170, 142–154. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, E.; Ferreira, D.; Relvas-Santos, M.; Freitas, R.; Soares, J.; Azevedo, R.; Afonso, L.P.; Lima, L.; Santos, B.; Gonçalves, M.; et al. Aberrantly Glycosylated GLUT1 as a Poor Prognosis Marker in Aggressive Bladder Cancer. Int. J. Mol. Sci. 2024, 25, 3462. https://doi.org/10.3390/ijms25063462
Ferreira E, Ferreira D, Relvas-Santos M, Freitas R, Soares J, Azevedo R, Afonso LP, Lima L, Santos B, Gonçalves M, et al. Aberrantly Glycosylated GLUT1 as a Poor Prognosis Marker in Aggressive Bladder Cancer. International Journal of Molecular Sciences. 2024; 25(6):3462. https://doi.org/10.3390/ijms25063462
Chicago/Turabian StyleFerreira, Eduardo, Dylan Ferreira, Marta Relvas-Santos, Rui Freitas, Janine Soares, Rita Azevedo, Luís Pedro Afonso, Luís Lima, Beatriz Santos, Martina Gonçalves, and et al. 2024. "Aberrantly Glycosylated GLUT1 as a Poor Prognosis Marker in Aggressive Bladder Cancer" International Journal of Molecular Sciences 25, no. 6: 3462. https://doi.org/10.3390/ijms25063462
APA StyleFerreira, E., Ferreira, D., Relvas-Santos, M., Freitas, R., Soares, J., Azevedo, R., Afonso, L. P., Lima, L., Santos, B., Gonçalves, M., Silva, A. M. N., Santos, L. L., Peixoto, A., & Ferreira, J. A. (2024). Aberrantly Glycosylated GLUT1 as a Poor Prognosis Marker in Aggressive Bladder Cancer. International Journal of Molecular Sciences, 25(6), 3462. https://doi.org/10.3390/ijms25063462









