A Current Synopsis of the Emerging Role of Extracellular Vesicles and Micro-RNAs in Pancreatic Cancer: A Forward-Looking Plan for Diagnosis and Treatment
Abstract
1. Introduction
2. A Brief Analysis of the EV and miRNA Biogenesis Mechanism
2.1. EV Biogenesis
2.2. MiRNA Biogenesis
3. An Overview of the Implication of miRNAs and Other Non-Coding RNAs in PDAC
3.1. OncomiRs
- ►
- miR-21: This miRNA is highly expressed in serum or tissue biopsies. The expression levels of miR-21 are closely related to the regulation of tumor-suppressor genes that are implicated in pivotal cell functions and pathways, such as apoptosis and Ras-Raf-MEK-ERK pathways or/and epidermal growth factor receptor (EGFR), or/and PI3K/AKT signaling pathways, respectively [36,37]. More particularly, miR-21 overexpression induces the growth and proliferation of pancreatic cancer cells, while it concomitantly inhibits their apoptosis, resulting in a decontrolled cell cycle [36,37].
- ►
- miR 186: The levels of miR-186 are also highly found in PDAC, which also induces proliferation and metastasis via targeting the Nuclear Receptor Subfamily 5 Group A Member 2 (NR5A2) gene (encodes the transcription factor NR5A2), leading to several deregulations in gene expression [38].
- ►
- miR-17-5p: Its overexpressed levels are closely implicated in the cell cycle deregulation, via interrupting the expression of RBL2/E2F4 repressing complexes [39].
- ►
- miR-196b suppresses the apoptotic mechanism via targeting CADM1 [40].
- ►
- miR-18a, which is a member of the oncogenic miR-17-92 cluster, is highly expressed in PDAC, whereas its levels are significantly reduced after surgical treatment. This miRNA is highly implicated in proliferation and MYC-induced transcriptional activation [41].
- ►
- miR-191 is implicated in the modification of the extracellular matrix and the promotion of distant tumor cell dissemination [42].
- ►
- ►
- miR-301a-3p and miR-374 also have an oncogenic role in PDAC via inducing migration and increasing the invasiveness of pancreatic cancer cells, with the former targeting SMAD4 expression [46], whereas the latter does so via deregulating Secernin 1 (SRCIN1), leading to its low expression, and to EMT and PDAC progression [47].
- ►
- miR-1469-5p is correlated to the over-proliferation of PDAC cells, via interacting with the NDRG1/NF-κB/E-cadherin pathway [48].
- ►
- miR-205 is implicated with the Wnt signaling pathway, increasing the proliferation via targeting the suppressive gene that encodes Adenomatous polyposis coli (APC) [49].
- ►
- miR-10b: Its overexpression is closely involved in PDAC invasive behavior and progression via inhibiting TIP30 expression and promoting EGF and TGF-β effects, leading to a generally poor prognosis [50].
3.2. Τumor-Suppressive miRNAs in PDAC
- ►
- miR-506: Enhanced levels of miR-506 were closely related to the suppression of pancreatic cancer cell growth and chemosensitivity; however, it is usually detected in low levels. More particularly, its downregulated levels are correlated to chemoresistance via sphingosine kinase 1 (SPHK1)/Akt/NF-κB signaling [51].
- ►
- miR-34 also induces tumor suppression, when its levels are reinstated, leading to the inhibition of the cancer stem cells [52].
- ►
- miR-142: It is reported that miR-142 restored levels are closely related to the limitation of the tumor invasive behavior and growth via regulating the expression of hypoxia-inducible factor 1-alpha (HIF-1a), which is a transcription factor. However, this miRNA is usually presented in low levels in PDAC cases [53].
- ►
- miR-216b: Increased expression of miR-216b has tumor-suppressive effects via its implication in the expression of translationally controlled 1 tumor proteins (TC1TP) as well as via KRAS inhibition [54].
- ►
- miR-30c: The enhancement of its levels has also shown PDAC suppression via targeting TWF1, which has a key role in cell cycle regulation (G1) and in programmed cell death [55].
- ►
- miR-143-3p: Increased miR-143-3p expression levels lead to the suppression of MERK/ERK signaling pathway and limit the pancreatic cell dysplasia [56].
- ►
- miR-519-3d suppresses hypoxia-related carcinogenesis by regulating programmed death ligand 1 (PD-L1) [57].
- ►
- miR-1181 induces the suppression of STAT3, limiting the invasiveness and progression of PDAC [58].
- ►
- miR-375 induces increased PDAC cell apoptosis, as well as limits the lymphatic spread and distant metastasis [59].
- ►
- ►
- miR-340: The enhancement of its expression levels also limits tumor progression and proliferation via targeting the expression Bicaudal-D2 (BICD2) [62].
- ►
- miR-203a-3p also reduces PDAC progression and invasion via its implication in fibroblast growth factor 2 (FGF2) expression, leading to EMT limitation [63].
3.3. A Brief Review of the Role of Other Non-Coding RNAs in PDAC
3.3.1. Oncogenic lncRNAs Implicated in PDAC
- ►
- HOTTIP is correlated with chemoresistance, which is widely expressed in PDAC tissues, compared to normal non-malignant ones, promoting PDAC progression and GEM-resistance [68]. On the other hand, its suppression could be a future druggable target, as it is reported that the knockdown of this lncRNA suppresses tumor growth and cells sensitize to GEM [68].
- ►
- PVT1, HOTTIP, and HOTAIR: Based on the current study that was conducted by Jiang XY et al. (2023) several lncRNAs are implicated in PDAC proliferation, migration, and chemoresistance, which can be utilized also as druggable targets and diagnostic biomarkers, including plasmacytoma variant translocation 1 (PVT1), HOTTIP, and HOTAIR [69]. These molecules are closely implicated in PDAC progression via inducing EMT, interacting with signaling pathways, and binding several miRNAs. More particularly, PVT1 is closely related to PDAC progression and GEM-resistance, as it acts as a “sponge” for the miR-619-5p that induces autophagy activation and is implicated in Pygopus2 increased expression. HOTAIR is considered quite oncogenic, with its expression levels being negatively correlated with PDAC prognosis, overall survival, and lymphatic dissemination [69].
- ►
- GSTM3TV2 contributes in GEM resistance by increasing the expression levels of L-type amino acid transporter 2 (LAT2) and oxidized low-density lipoprotein receptor 1 (OLR1) by sponging let-7 [70].
- ►
- XLOC_006390, HOTTIP-005, and RP11-567G11.1 are notably increased in PDAC samples, implying its potential as diagnostic tools in PDAC [71].
- ►
- linc00511 is highly expressed in PDAC tissues and it is closely associated with worrisome prognosis, PDAC progression, and neoangiogenesis by inducing upregulation of VEGFA. The aforementioned phenomenon is mediated via competing the binding activity of has-miR-29b-3p towards its partially complementary mRNA, which encodes VEGFA protein. Linc00511 constitutes a novel prognostic biomarker, as well as a possible druggable target via its knockdown [72].
- ►
- MALAT-1, AFAP1-AS1, AF339813, and H19 are tumor-promoting lncRNAs, which are overexpressed in PDAC cells and patient samples [73]. More specifically, lncRNA AF339813 upregulates NFUF2 mRNA translation in PDAC cell lines, while its knockdown could serve as a future druggable target [73,74]. Moreover, MALAT1 induces activation of the autophagy pathway. PDAC constitutes a malignancy that presents overregulation of autophagy that needs to be suppressed, compared to other malignancies, in which autophagy inhibition could lead to oncogenesis [35,73,75,76]. Furthermore, AFAP1-AS1 promotes PDAC growth and invasion by upregulating the IGF1R oncogene via miR-133a sequestration, which regulates its translation [77]. LncRNA H19 acts as a sponge of several specific miRNAs such as let-7b, miR-107, miR-874, miR-130a, and miR-200, as well as miR-675 and miR-194, leading to the deregulation of the expression levels of several proteins and signaling pathway, while its expression levels are higher in PDAC patients samples, like saliva [78,79]. Meanwhile, its knockdown demonstrated tumor suppression in xenografts. In addition, the lncRNA regulator of reprogramming (ROR) competes with miR-145 and induces its sponging [80], leading to the downregulation of Nanog expression in Capan-1 and BxPC-3 cell lines, which is a phenomenon that promotes pancreatic cell proliferation [80].
- ►
- ENST00000480739 is another lncRNA that is negatively associated with the PDAC patient prognosis, by up-regulating osteosarcoma amplified-9 (OS-9) that interacts with hypoxia-inducible factor 1 (HIF-1), leading to hypoxia-related adaption, invasion, and metastatic dissemination [81].
- ►
- NUTF2P3-001 is induced by hypoxia, facilitates Panc-1 and BXPC-3 cell line proliferation in Panc-1, and is correlated with KRAS overexpression [82].
3.3.2. Tumor-Suppressive lncRNAs
- ►
- GAS5 is downregulated in PDAC, whereas its high levels suppress PDAC cell proliferation [83].
- ►
- BC008363 is usually downregulated in PDAC; however, when it is overexpressed, it is notably correlated to better survival levels, implying its utilization as a prognostic PDAC biomarker [84].
3.3.3. CircRNAs Implicated in PDAC
4. The Effect of PDAC-Derived EV-miRNAs and EV-Proteins
4.1. Implication of EV-miRNAs in Glucose Homeostasis and PDAC-Related DM
- ►
- Exosomal miR-197-3p, miR-6796-3p, miR-4750-3p, and miR-6763-5p: These exosomes that are derived from PDAC cells deregulate glucose homeostasis by altering the expression of two major peptides, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulin tropic peptide (GIP), in vitro [91].
- ►
- Exosomal miR-125b-5p, miR-450b-3p, miR-666-3p, miR-883b-5p, and miR-540-3p: These exosomes that are derived from PDAC cells induce insulin resistance in C2C12 myotube cells by interrupting PI3K/Akt/FoxO1 axis [91].
- ►
- Exosomal hsa-miR-3133, hsa-miR-144-5p, and hsa-miR-3148 were proposed as candidate markers and their potential role in the development of insulin resistance and/or DM-associated PDAC, was also demonstrated, when they were exposed to normal pancreatic islets [93].
- ►
- EV-miR-19a was proven to alter insulin production, via interacting with the expression of the gene for Neurod1 protein, which constitutes an important transcription factor, implicated in the β-cells development and differentiation. The aberrations in NeuroD1 are closely implicated with DM and β-cells regulation, leading to reduced insulin secretion in DM-associated PDAC [94].
4.2. Implication of EV-miRNAs in PDAC Progression and TME Modification
- ►
- EV-miR-222: Its levels are correlated to the stage and size of PDAC [95].
- ►
- EV-miR-155: Its increased levels are closely associated with suppression of apoptosis in PDAC cells, GEM chemoresistance, as well as induction of EV-miR-155 secretion by the other PDAC cells [96].
- ►
- EV-miR-125b-5p: It induces TME alterations and PDAC progression via the activation of the MEK/ERK pathway that leads to PDAC invasion, EMT, and metastatic dissemination [97].
- ►
- EV-miR-27a: secreted by PDAC cells, significantly increases angiogenesis through B-cell translocation gene 2 (BTG2), which has anti-proliferative properties, as well as induces PDAC invasion, human microvascular endothelial cells (HMVEC) angiogenesis progression, and metastatic dissemination. The aforementioned phenomenon implies the potential role of miR-27a suppression as a druggable target [98].
4.3. ΕV-Proteins Derived from PDAC Cells
- ►
- EV-CKAP4: An interesting observation in surgically treated PDAC patients was the significantly decreased post-operative levels of EV-containing cytoskeleton-associated protein 4 (CKAP4), which were increased before the operation. These EVs are implicated in PDAC progression by interfering with the Wnt signaling pathway [100].
- ►
- Circulating EV-O-glycan-binding lectin were also notably overexpressed in PDAC patients before the surgical treatment, whereas were significantly decreased after pancreatectomy [101].
- ►
- EV-β2-microglobulin (B2M): The phenomenon of tumor escape was significantly correlated with the increased levels of EV-β2-microglobulin (B2M) [102].
- ►
- EV-Epidermal Growth Factor Receptor (EGFR), EV-KRAS, and EV-CD44: Their increased levels have been correlated with pancreatic oncogenesis and PDAC progression [102].
- ►
- ►
- EV-Caveolin-1 (CAV1) and EV-Clusterin (CLU) are closely implicated in the over-proliferation and the impaired apoptosis of pancreatic cancer cells [103].
- ►
- ►
- ►
- EVs with Tspan8, Integrins, and CD151 induce stromal changes, promoting cell motility and increasing their invasion and metastatic capacity [107].
- EV-proteins implicated in Pre-metastatic niche formation
- ►
- ►
- ►
- ►
- ►
- EVs containing STAT14, LAMP1, and Lin28B are implicated in metastasis [102].
- ►
- EV-macrophage migration inhibitory factor (MIF) is implicated in the pre-metastatic niche formation in the liver and finally in the development of the liver metastatic lesions. More particularly, MIF induces the secretion of fibronectin and TGFβ by the hepatic stellate cells and Kupffer cells, respectively. The increased levels of MIF have been closely correlated with the early stages of PDAC, with a concomitant increase in cytokine levels in patients of stage I, who eventually presented liver metastasis [110,111].
- Poor prognosis and survival, lesion differentiation
- ►
- EV-Glypican-1 (GPC1): The increased circulating amount of EV-GPC1, which is attributed to epigenetic alterations in PDAC, is related to poor prognosis and decreased survival. Additionally, its increased levels can differentiate PDAC patients from healthy individuals, or PDAC patients from those who present benign pancreatic diseases, with the latter differentiation being controversial in other recent studies [113]. More specifically, another study demonstrated that glycoprotein 2 (GP2) and GPC1+ EVs are not enough for the proper differentiation between malignant pancreatic lesions and benign ones [114].
- ►
- EV-EphA2: Its increased levels have also been correlated with the tumor stage, although it was proven that they present limited sensitivity in PDAC early stages. Additionally, their levels have been related to the prediction for neoadjuvant treatment response. The increased levels of EV-EphA2 have been related to favorable responses to neoadjuvant therapy, implying their possible use as a monitoring tool for treatment response [115].
- ►
- ►
- EVs containing HIST2H2BE, CD151, CLDN4, LGALS3BP, and EpCAM: Another enlightening study was conducted by Castillo et al., which analyzed the surface protein markers of PDAC exosomes, the so-called “surfaceome”, such as HIST2H2BE, CD151, and CLDN4, as well as LGALS3BP and EpCAM [118]. The authors identified that 73% of exosomes with the selected markers had KRAS mutations, while from the whole sample population, KRAS was detected in 44.1% [118].
- ►
- EV-zinc transporter (ZIP4): Its levels have been found upregulated in highly malignant PDAC, compared to moderate ones and healthy controls. It was demonstrated that EV-ZIP4 has an oncogenic potential and it could be utilized in the future as a druggable target [119].
- ►
- EV-Adrenomedullin: it has a pivotal role in DM-related PDAC, while it could be potentially utilized as a marker for β-cell destruction in this malignancy [120].
5. The Implication of EVs in the PDAC Microenvironment and Tumor Escape Phenomenon
5.1. PDAC-Derived EVs That Are Implicated in TME Modification
- Implication in immune cell functionality
- ►
- EV-Integrins are correlated with reduced anti-cancer immune response, which is attributed to the decreased expression of INF-γ, CD107a, and TNF-α in NK cells [125].
- Neoangiogenesis, lymphangiogenesis, and metastatic dissemination
- ►
- EV-ANXA1, which is accompanied by increased ANXA1 levels has a pivotal pro-angiogenic role in TME, as it significantly modifies the function of endothelial cells and fibroblasts. More particularly, it was demonstrated that ANXA1-EVs activate the formyl peptide receptors (FPRs) and subsequently induce the modification of ECs and fibroblasts, implying the potential role of these EVs as diagnostic and prognostic tools [109]. It is also reported that the aforementioned EVs can induce neoangiogenesis by interacting with ECs and being implicated in the Akt/ERK pathway [127].
- ►
- EV-miR-27a promotes lymphatic metastasis and lymphangiogenesis by targeting BTG2 expression [98].
- ►
- EV-SUMOylated heterogeneous nuclear ribonucleoprotein A1 (hnENPA1) promotes lymphatic metastasis and lymphangiogenesis [98].
- Modification of non-malignant pancreatic cells
- ►
- EV-palmitic acid: These EVs are capable of inducing ER stress in physiological pancreatic cells [129].
- Interactions with TAMs and PSCs in TME
- ►
- EV-Ezrin (EZR) interacts with TAMs and they can potentially alter the polarity of macrophage and promote tumor metastatic dissemination [130]. This phenomenon can potentially open up new therapeutic chances by targeting the EZR-EVs [130]. Moreover, in the aforementioned study by Chang YT et al. (2020), circulating levels of EZR levels and plasma EZR-EVs in PDAC patients were found higher in comparison to healthy controls [130]. Additionally, they conclude that patients with a higher amount of the aforementioned EVs had a reduced overall survival compared to the group with low levels of EZR.
- ►
- EV-miR-155-5p induces the alteration of macrophages from M1 to M2 immunosuppressive state via interacting with the EHF/Akt/NF-kB pathway [131].
- ►
- EV-lin28B: These vesicles induce overexpression of PDGFB in the recipient cells, which leads to metastatic dissemination and pancreatic stellate cells (PSCs) recruitment [132].
- ►
- Exosomes containing FGD5-AS1: It was demonstrated that when these EVs are co-cultured with M2 macrophages, they promote tumor progression via the activation of STAT3/NF-κB pathway, which worsens the prognosis in PDAC patients [133].
- Promotion of PDAC invasion, migration, and dissemination
- ►
- EV-miR-125b-5p, which can activate the MEK/ERK pathway, induces PDAC invasion, EMT, as well as metastatic dissemination [97]. It has to be underlined that these EVs can accelerate PDAC development by preventing the targeting of StAR-related lipid transfer protein domain 3 (STARD13), which is a protein family with a crucial role in cancer development and the regulation of inter-organelle cholesterol transportation [90,134]. This phenomenon is derived from the fact that the metabolism of cancer cells is quite accelerated, requiring a high need for cholesterol to fulfill their continuous proliferation and membrane restoration [90,134,135].
- ►
- EV-LDL receptor-related proteins activate Yes1-associated transcriptional regulators (YAP) [136].
5.2. CAFs-Derived EVs
- PDAC progression and chemoresistance
- ►
- EV-miR-21: These EVs are correlated with GEM-chemoresistance, a phenomenon that is attributed to their implication in PTEN expression and its inhibition [137,138]. However, the study by Richards et al. (2022) demonstrates the favorable effects of exosome inhibitor GW4869 in vivo and in vitro to restore the expression of PTEN, which is commonly lost in PDAC [138].
- ►
- EV-miR-3173-5p: In the study (ChiCTR2200061320) by Qi et al. (2023), the CAF-derived miRNAs were analyzed, and it was demonstrated that EV-miR-3173-5p promoted chemoresistance to GEM in a xenograft PDAC mouse model via interacting with Acyl-CoA Synthetase Long-Chain Family Member 4 (ACSL4) gene, as well as by inducing suppression of ferroptosis in the recipient PDAC cells, which is a pivotal process of programmed cell death in cases of excessive lipid peroxidation [139]. The aforementioned phenomenon opens up new therapeutic strategies for the management of GEM-resistant tumors via targeting EV-miR-3173-5p [139].
- ►
- EV-ANXA6: In the study by Nigri et al. (2022), it was demonstrated that CAF-derived EVs containing ANXA6-EVs promote PDAC aggressive behavior via the overexpression of CD9 on the surface of these EVs, which induces MAPK pathway activity, increases EMT, and promotes PDAC expansion. Inhibiting these CD9-positive ANXA6-EVs could potentially reduce stromal modification and tumor progression [140].
- ►
5.3. MDSC-Derived Evs
5.4. Total Blood EVs and Leukocyte- and NK-Derived EVs
5.5. Pancreatic-Stellate EVs
- ►
- EV-miR-451a or miR-21-5p: The study of Takikawa et al. demonstrated that PSC-EVs that contain miR-451a or miR-21-5p significantly promote the PDAC progression and expansion via promoting the expression of CCL1 and CCL2 [145].
5.6. TAMs-Derived EVs
5.7. Stromal EVs and Stromal Modification
6. The Effects of EVs in PDAC-Associated Thrombosis
- ►
- EV-tissue factor (TF): TF constitutes a pro-coagulant protein that can be contained in the EVs that are derived from several types of host cells under pathological conditions, including cancer [153]. It has been demonstrated that TF-EVs promote thrombosis in PDAC patients, while baseline TF levels at the start point of chemotherapy constitute a predictive factor of cancer-related thromboembolism, as was demonstrated in Japanese PDAC patient cohorts [154]. It was also demonstrated in the study of Kobayashi et al. (2021) that TF levels above or equal to 100 pg/Ml constitute an independent predictive factor for cancer-associated thromboembolism, while it was also proposed that D-dimers and microvesicle tissue factor (MV-TF) could be utilized as biomarkers for venous thromboembolism in PDAC and may facilitate the identification of patients for whom thromboprophylaxis has to be administrated [154,155].
7. EV Secretion and Hypoxia in PDAC
- ►
- Exosomal-circPDK1: Lin et al. (2022) suggested that the induction of exosomal circPDK1 by HIF1A under hypoxia leads to PDAC cell survival and migration via c-myc stimulation. The induction of c-myc promotes glycolysis in vivo, as well as in vitro by miR-628-3p sponging that leads to Bromodomain and PHD Finger-Containing Transcription Factor (BPTF) deregulated expression, which has a key role in the regulation of gene encoding [158]. On the other hand, the elimination of these EVs leads to a reduction in tumor migration and proliferation, as well as to shorter PDAC cell survival [158].
8. A Summary of EV-Mediated Chemoresistance
- ►
- EVs containing miR-155 or CAT or SOD2 induce GEM resistance when they are added to pancreatic cell cultures [95].
- ►
- EV-miR-210, which is derived from BxR cancer stem cells (CSCs), induces GEM resistance [160].
- ►
- EV-ATP-binding cassette (ABC) superfamily G member 2 (ABCG2), which is derived from GIPC-depleted PANC1 or AsPC-1, induces GEM resistance [161].
- ►
- ►
- ►
- EV-MMP14, which is derived from BxPC3-Gem cells, induces GEM resistance [162].
- ►
- ►
- EV-fibronectin and chitinase 3-like-1 (CHI3L1), which are derived from TAMs, are closely implicated in the responses to PDAC treatment via inducing PDAC resistance to GEM [164,165]. However, the inhibition of these molecules by pirfenidone and pentoxifylline for FN1 and CHI3L1, respectively, was demonstrated. Their inhibition partially restored the sensitivity of PDAC cells to GEM, implying the potential role of the aforementioned proteins as druggable targets for PDAC adjuvant treatment [165].
9. EV-Mediated Cachexia
10. EVs as Diagnostic Tools in PDAC
10.1. EVs in Pancreatic Juice
- ►
- EVs with P-glycoprotein (MDR1), mucin (MUC) 1, MUC16, MUC5AC, and MUC6, as well as MUC4 and Cystic Fibrosis Transmembrane Conductance Regulator (CFTR), which are isolated from PJ, are considered diagnostic for PDAC [169].
- ►
- EVs with moesin, CD55, and Ras proteins were found increased in the PJ of PDAC patients who were in the early stages (I–II), while patients in late stages (III-V) had an increased amount of EV-Ras [170].
- ►
- EVs with ADP-ribosylation factor 3, moesin, olfactomedin-4, pyruvate kinase, mucins, and Ras proteins, as well as CD55 and lipopolysaccharide-induced tumor necrosis factor: They are members of a panel that includes 89 overexpressed proteins based on the study by Inoue H et al. (2022). These EVs are isolated from PJ that is collected via fine needle aspiration (FNA) samples guided by EUS. Their overexpression is considered diagnostic for PDAC among a population of patients with PDAC and Autoimmune pancreatitis (AIP) [171]. Meanwhile, in the aforementioned study, 64 EV-proteins were significantly reduced in PDAC patients, in comparison with the controls (AIP patients).
- ►
- EVs with miR-155 and miR-21 were notably elevated in patients with PDAC, compared to patients with chronic pancreatitis (CP), as also demonstrated in the aforementioned study [171].
10.2. Circulating Blood EVs for Diagnosis and Screening
- Serum
- ►
- PLT-EVs (CD61 and CD41-positive) and CD63-positive EVs: Their levels were proved diagnostic for PDAC [172]. It was proved that the levels of CD61, CD63, and CD41-positive EV in serum were higher in PDAC patients than in healthy controls, presenting an AUC of 0.846 [172]. Moreover, the performance of CA19-9 alone was compared to the EV (CD41+, CD63+, and CD61+) levels, as well as their combination, aiming the identification of the most diagnostic tool among them [172]. More particularly, the diagnostic accuracy of CA 19-9 alone, exhibited a lower one (AUC: 0.842) for PDAC discrimination from healthy patients, in comparison to serum CD61, CD63, and CD41+ levels [172]. However, their combination (CD63+ and CA19-9) showed a notably higher AUC of 0.903, which implies their potential role as an early-stage I–II diagnostic tool, compared to CA-19-9 alone (AUC: 0.814) [172]. Nevertheless, the above EVs had similar accuracy in the early (I–II) and late (III–IV) tumor stages. Furthermore, their post-operative levels were also studied, which were notably reduced in both cases, implying their proportional increase with the tumor growth and progression [172].
- ►
- EV with CD82+, GPC1, and levels of CA19-9: This panel was studied by Xiao D et al. for its diagnostic accuracy in the early stages of PDAC in the Chinese population [173]. Favorably, the diagnostic accuracy of the aforementioned panel as a screening tool was significantly high (AUC = 0.942) [173]. Additionally, these researchers also studied the controversial role of EV-GPC1 alone as a screening biomarker [173].
- ►
- EVs with proto-oncogene mesenchymal–epithelial transition factor (c-met) present a specificity of 85% and sensitivity of 70% [174].
- ►
- EVs A Disintegrin And Metalloproteinase (ADAM) 8 (ADAM8): Its high levels are correlated with pre-malignant pancreatic lesion and PDAC [175].
- ►
- EV-ANXA6: Its levels present a great AUC of 0.979 for detecting PDAC patients [176].
- ►
- EV-ZIP4: Its high levels present a great diagnostic efficacy (AUC of 0.893) [119].
- ►
- EV-GPC1 and LRG-1: This panel presents a great diagnostic performance, even for early-stage PDAC tumors with an AUC of 0.95 [177].
- ►
- EVs with RAS-associated protein RaB5 and D63 that are isolated by ExoChip are considered potent diagnostic tools in serum samples [178].
- ►
- EVs with miR-21 and miR-17-5p: EV-miR-21 has a AUC of 0.897 and EV-miR-17-5p has an AUC of 0.887 [179]. These EVs are found in high levels in PDAC patients.
- ►
- EV-miR-1226-3p has an AUC of 0.74 and it is downregulated in PDAC [180].
- ►
- EVs with miR-451a, miR-191, and miR-21 are found notably overexpressed in cases of IPMNs and PDAC, while they have an AUC of 0.759, 0.788, and 0.826, respectively [181].
- ►
- EVs with miR-4306, miR-1246, miR-3976, and miR-4644: This panel presented an adequate specificity (AUC: 0.80), with their levels found increased in the majority of PDAC patients (83%) [182].
- ►
- EV-GPC-1 mRNA have expressed levels in PDAC patients, independently of their tumor stage (AUC: 1.00) [183].
- ►
- EVs with CRNDE or MALAT-1 (lncRNAs) were significantly increased in PDAC patients [176].
- ►
- EV-HULC (lncRNA): Its levels were increased in IPMN or PDAC patients, in comparison with healthy controls, with great specificity (AUC: 0.92) [184].
- Plasma
- ►
- EV-miR-10b: Its level has been increased in PDAC patients, compared to healthy controls or CP patients (AUC: 0.81) [185].
- ►
- EVs with miR-205-5p, miR-122-5p, and miR-125b-3p: This panel tested these EVs, which were found downregulated in Brazilians with PDAC. These EVs had an AUC of 0.857, 0.814, and 0.782, respectively [186].
- ►
- EVs with miR-451a, and miR-196a: This panel has an AUC of 0.81, while the EVs have been tested as diagnostic biomarkers in several studies [187,188]. The former has been tested in early-stage PDAC (I–II) and has been proven that its level is statistically significant for the discrimination between stage I and II (p-value of 0.041) [187], while the latter, EV-miR-196a, has also been tested in patients at early disease stages (I–II stages) [188].
- ►
- EVs with miR-30c, EV-miR-10 b, miR-let7a, miR-21, and miR-181: This panel was superior to the one of GPC1 [189]. Their levels have been significantly altered, including the low expression of EV-miR-let7a and the upregulation of the others, while it presented a great specificity in differentiating the healthy patients, or those with benign pancreatic disease (CP) from the ones with PDAC (AUC: 1.00) [189].
- ►
- EVs with miR-409 and mRNAs (CK18, CD63), combined by CA-19-9 and cell-free DNA concentration levels: This multianalyte panel by Yang Z et al. (2020) has a great specificity, sensitivity, and accuracy of 95%, 88%, and 92%, respectively [190]. Meanwhile, plasma levels of EV-miR-409 constitute a potential diagnostic tool for PDAC with an AUC of 0.93. Additionally, the levels of EV-CK18 and EV-CD63 mRNAs were also increased in PDAC cases, with an AUC of 0.93 [190].
- ►
- EVs with long RNAs (TIMP1, FGA, HIST1H2BK, CLDN1, and ITIH2, as well as MAL2, MARCH 2, and KRT19): The panel by Yu et al. (2019) that is based on long RNA sequencing presents a great AUC of 0.949 [191].
- ►
- EV-circ-IARS: Its level have been overexpressed in PDAC tissues [192].
- ►
- EVs with EpCAM, GCP-1, and CD44V6: This panel presents great specificity and an AUC of 1.00 [193].
- ►
- EVs with mutant proteins KRAS and/or P53 were detected in early-stage PDAC patients [194].
- ►
- EV-GPC1 alone did not have a high AUC (0.59) for PDAC detection [114].
- ►
- EVs with EphA2, EpCAM, and MIF: This panel constitute great diagnostic tools for PDAC detection [195].
- ►
- EVs with WNT2, EpCAM, MUC1, GPC1, and EGFR: This panel constitute great diagnostic tools for PDAC detection, presenting a specificity and a sensitivity of 81% and 86%, respectively [196].
- ►
- EV-alkaline phosphatase placental-like 2 (ALPPL2) constitutes another diagnostic biomarker for PDAC, as was demonstrated in PDAC cell culture media [197].
- Total Blood
- ►
- EVs with CD63 and GPC1 are increased in PDAC blood samples, with a high sensitivity of detecting PDAC (99%) and a specificity of 82% [198].
10.3. EVs in Saliva
- ►
- EVs with miR-1246 and miR-4644 are significantly elevated in the saliva of PDAC patients (AUC 0.814 for miR-1246 and 0.763 for miR-4644), implying the potential use of this panel as a non-invasive diagnostic procedures [199].
- ►
- EVs mRNAs (Incenp, Apbb1ip, BCO31781, as well as Foxp1, Aspn, Daf2, and Gng2) were significantly increased in PDAC mice models [200].
- ►
- EVs with DNA that contain KRAS and TP53 mutations: The PCR-mediated identification of EVs that contain several mutations such as KRAS and TP53, which are embedded with DNA molecules that present the aforementioned genetic aberrations, is a diagnostic method that could differentiate the health controls or CP patients from PDAC ones [202].
11. EVs as Prognostic Tools in PDAC
- Plasma and serum
- ►
- Serum EpCAM-positive EVs, the total plasma EV concentration, and the levels of EV-DNA (KRAS) did not increase the mortality non-resectable PDAC patients, implying that the detection of these biomarkers is not correlated with the resectability-based survival and mortality [204].
- ►
- Exosomes with phosphatidylethanolamine and miR-45 levels have been correlated to the resectability of PDAC [204].
- ►
- Exosomes with EpCAM, miR-200b, mir-222, and miR-451a: This panel can be used in non-resectable cases for the selection of these patients, for whose systemic treatment plans could be advantageous [204].
- ►
- EV-Integrin α6: Its high levels in PDAC patients have been closely associated with clinical recurrence, even months before the time of recurrence, whereas it has also been demonstrated that these levels were notably decreased postoperatively [205].
- Plasma
- ►
- Plasma EV-Sox2ot (lncRNA) levels have been significantly associated with PDAC progression, related to vascular and lymphatic dissemination of cancer cells [206].
- ►
- Plasma EV-Circ-PDE8A levels were significantly overexpressed in PDAC [207].
- ►
- Plasma EV-circ-IARS overexpressed levels have been detected in advanced (metastatic) PDAC cases (p-value of 0.002) [208].
- ►
- Plasma EV-miR-222 has been associated with tumor invasion and metastasis in PDAC culture media, as well as the survival of PDAC cancer cells by down-regulating p27 and suppressing PPP2R2A expression, with the latter leading to AKT pathway activation [95]. The aforementioned EV-miRNA could be utilized as a prognostic factor for survival, tumor stage, and size [95].
- ►
- Plasma EVs contacting MIF, GPC1, EpCAM, and CD44V6 [204].
- ►
- Plasma EV-MIF levels are found increased in PDAC patients that do not present liver metastasis [209].
- ►
- Plasma EV-GPC-1 levels are correlated to tumor burden and size [201].
- ►
- Plasma EVs with EpCAM and CD44V6 also have a prognostic role in PDAC, with the former’s levels being associated with the treatment response during systemic, palliative chemotherapy in advanced PDAC cases, as well as with the tumor stage [210], whereas the latter’s are only related to the tumor stage [193].
- Serum
- ►
- Serum EVs with C1QBP and CD44V6 were found increased in PDAC patients, and they are associated with prognosis and liver metastatic disease [211].
12. EVs as Therapeutic Tools in PDAC
12.1. EVs as Vectors for Bioactive Molecules in PDAC Treatment
12.2. EVs as Drug Vectors in PDAC Treatment
12.3. EVs as Targets
12.4. EV-Based Photodynamic Therapy and EV-Based Immunotherapy
13. Pros and Cons of EV-Utilization in Research
14. Future Perspectives of EV-Based Machine Learning-Based Algorithms
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANXA | Annexin A |
| APC | Adenomatous polyposis coli |
| AUC | Area under the curve |
| Ago2 | Argonaute RISC Catalytic Component 2 |
| ARRDC1 | Arrestin domain-containing protein-1 |
| AI | Artificial Intelligence |
| AIP | Autoimmune pancreatitis |
| BICD2 | Bicaudal-D2 |
| B2M | β2-microglobulin |
| BM-MSC | Bone marrow mesenchymal stem cell |
| BTG2 | B-cell translocation gene 2 |
| BTG2 | B-cell translocation gene 2 |
| Bregs | B-regulatory cells |
| CAFs | Cancer-associated fibroblasts |
| CAV1 | Caveolin-1 |
| CCL5 | Chemokine (C-C motif) ligand 5 |
| CHI3L1. | Chitinase 3-like-1 |
| CP | Chronic Pancreatitis |
| circRNAs | Circular RNAs |
| CLDN1 | Claudin-1 |
| CLU | Clusterin |
| CFTR | Cystic Fibrosis Transmembrane Conductance Regulator |
| CKAP4, | Cytoskeleton-associated protein 4 |
| DCs | Dendritic cells |
| DM | Diabetes Mellitus |
| DGCR8 | DiGeorge Syndrome Critical Region 8 |
| DOXO | Doxorubicin |
| ER | Endoplasmic reticulum |
| EUS, | Endoscopic Ultrasound |
| ESCRT | Endosomal sorting complex |
| ECs | Endothelial |
| EGFR, | Epidermal growth factor receptor |
| EMT | Epithelial–mesenchymal transition |
| EVMAP, | Extracellular vesicle machine learning analysis platform |
| EVs | Extracellular vesicles |
| EZR | Ezrin |
| FGF2 expression | Fibroblast growth factor 2 |
| FPRs | Formyl peptide receptors |
| GEM | GEM |
| GLP-1 | Glucagon-like peptide-1 |
| GIP | Glucose-dependent insulin tropic peptide |
| GP2 | Glycoprotein 2 |
| GPC1 | Glypican-1 |
| HMVEC | Human microvascular endothelial cells |
| HPC-4 | Human PDAC cell lines |
| HUVEC | Human umbilical vein endothelial cells |
| HIF-1a | Hypoxia-inducible factor 1-alpha |
| ICIs | Immune checkpoint inhibitors |
| IGFR | Insulin-like growth factor 1 receptor |
| ITGΒ5, | Integrin Beta-5 |
| LRNAs | Long RNAs |
| lncRNAs | Long non-coding RNAs |
| LAT2 | L-type amino acid transporter 2 |
| ML | Machine learning |
| MIF | Macrophage migration inhibitory factor |
| MHC-I | Major histocompatibility Complex-I |
| mRNAs | Messenger RNAs |
| miRISC | miRNARISC |
| MUC | Mucin |
| MDSCs | Myeloid-derived suppressor cells |
| NK cells | Natural killer |
| OncomiRs | Oncogenic miRNAs |
| OXA | Oxaliplatin |
| OLR1 | Oxidized low-density lipoprotein receptor 1 |
| PDAC | Pancreatic ductal adenocarcinoma |
| PJ | Pancreatic juice |
| PSCs | Pancreatic stellate cells |
| PVT1 | Plasmacytoma variant translocation 1 |
| PODX | Podocalyxin-like protein |
| pre-miRNA | Precursor miRNAs |
| pri-miRNA | Primary miRNA |
| MDR1 | P-glycoprotein |
| Ran-GTP | RAS-related nuclear protein–guanosine-5′-triphosphate |
| RBP | RNA-binding protein |
| RNAi Pathway | RNA Interference Pathway |
| RISC | RNA-induced silencing complex |
| SRCIN1 | Secernin 1 |
| micro-RNAs or miRs | Short non-coding RNA molecules |
| SNAREs | Soluble N-ethylmaleimide-sensitive factor attachment protein receptors |
| SLE | Systemic Lupus Erythematosus |
| F3 | Tissue factor 3 |
| TF | Tissue factor |
| TGF β | Tumor growth factor β |
| TME | Tumor microenvironment |
| TAMs | Tumor-associated macrophages |
| TILs | Tumor-infiltrating lymphocytes |
| Treg | T-regulatory |
| UPR | Unfolded protein response |
| VEGF | Vascular endothelial growth factor |
| YAP | Yes1-associated transcriptional regulators |
| ZIP4 | Zinc transporter |
| FNA | Fine needle aspiration |
References
- Hu, J.-X.; Zhao, C.-F.; Chen, W.-B.; Liu, Q.-C.; Li, Q.-W.; Lin, Y.-Y.; Gao, F. Pancreatic Cancer: A Review of Epidemiology, Trend, and Risk Factors. World J. Gastroenterol. 2021, 27, 4298–4321. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Klein, A.P. Pancreatic Cancer Epidemiology: Understanding the Role of Lifestyle and Inherited Risk Factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef]
- Takikawa, T.; Kikuta, K.; Hamada, S.; Kume, K.; Miura, S.; Yoshida, N.; Tanaka, Y.; Matsumoto, R.; Ikeda, M.; Kataoka, F.; et al. Clinical Features and Prognostic Impact of Asymptomatic Pancreatic Cancer. Sci. Rep. 2022, 12, 4262. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, W. Pancreatic Cancer: A Review of Risk Factors, Diagnosis, and Treatment. Technol. Cancer Res. Treat. 2020, 19, 153303382096211. [Google Scholar] [CrossRef] [PubMed]
- Saba, E.; Farhat, M.; Daoud, A.; Khashan, A.; Forkush, E.; Menahem, N.H.; Makkawi, H.; Pandi, K.; Angabo, S.; Kawasaki, H.; et al. Oral Bacteria Accelerate Pancreatic Cancer Development in Mice. Gut 2024, gutjnl-2023-330941. [Google Scholar] [CrossRef]
- Seo, M.-S.; Yeo, J.; Hwang, I.C.; Shim, J.-Y. Risk of Pancreatic Cancer in Patients with Systemic Lupus Erythematosus: A Meta-Analysis. Clin. Rheumatol. 2019, 38, 3109–3116. [Google Scholar] [CrossRef] [PubMed]
- Korsse, S.E.; Harinck, F.; van Lier, M.G.F.; Biermann, K.; Offerhaus, G.J.A.; Krak, N.; Looman, C.W.N.; van Veelen, W.; Kuipers, E.J.; Wagner, A.; et al. Pancreatic Cancer Risk in Peutz-Jeghers Syndrome Patients: A Large Cohort Study and Implications for Surveillance. J. Med. Genet. 2013, 50, 59–64. [Google Scholar] [CrossRef]
- Liu, J.; Mroczek, M.; Mach, A.; Stępień, M.; Aplas, A.; Pronobis-Szczylik, B.; Bukowski, S.; Mielczarek, M.; Gajewska, E.; Topolski, P.; et al. Genetics, Genomics and Emerging Molecular Therapies of Pancreatic Cancer. Cancers 2023, 15, 779. [Google Scholar] [CrossRef]
- Gbolahan, O.B.; Tong, Y.; Sehdev, A.; O’Neil, B.; Shahda, S. Overall Survival of Patients with Recurrent Pancreatic Cancer Treated with Systemic Therapy: A Retrospective Study. BMC Cancer 2019, 19, 468. [Google Scholar] [CrossRef]
- Principe, D.R.; Underwood, P.W.; Korc, M.; Trevino, J.G.; Munshi, H.G.; Rana, A. The Current Treatment Paradigm for Pancreatic Ductal Adenocarcinoma and Barriers to Therapeutic Efficacy. Front. Oncol. 2021, 11, 688377. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Brennan, M.Á.; Lötvall, J.; Breakefield, X.O.; EL Andaloussi, S. Advances in Therapeutic Applications of Extracellular Vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [CrossRef] [PubMed]
- Rupaimoole, R.; Slack, F.J. MicroRNA Therapeutics: Towards a New Era for the Management of Cancer and Other Diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Dixson, A.C.; Dawson, T.R.; Di Vizio, D.; Weaver, A.M. Context-Specific Regulation of Extracellular Vesicle Biogenesis and Cargo Selection. Nat. Rev. Mol. Cell Biol. 2023, 24, 454–476. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, M.; Comandatore, A.; Centra, C.; Blandino, G.; Morelli, L.; Giovannetti, E. Thinking Small to Win Big? A Critical Review on the Potential Application of Extracellular Vesicles for Biomarker Discovery and New Therapeutic Approaches in Pancreatic Cancer. Semin. Cancer Biol. 2023, 97, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Nandwani, A.; Rathore, S.; Datta, M. LncRNAs in Cancer: Regulatory and Therapeutic Implications. Cancer Lett. 2021, 501, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Pisignano, G.; Michael, D.C.; Visal, T.H.; Pirlog, R.; Ladomery, M.; Calin, G.A. Going Circular: History, Present, and Future of circRNAs in Cancer. Oncogene 2023, 42, 2783–2800. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Hou, D.; Wei, Z.; Zheng, S.; Zhang, Y.; Li, J. Extracellular and Intracellular microRNAs in Pancreatic Cancer: From Early Diagnosis to Reducing Chemoresistance. ExRNA 2019, 1, 17. [Google Scholar] [CrossRef]
- Han, Q.-F.; Li, W.-J.; Hu, K.-S.; Gao, J.; Zhai, W.-L.; Yang, J.-H.; Zhang, S.-J. Exosome Biogenesis: Machinery, Regulation, and Therapeutic Implications in Cancer. Mol. Cancer 2022, 21, 207. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, G.; Zhang, Z.; Yu, Y.; Zeng, L.; Xu, Z.; Weng, J.; Xia, J.; Li, J.; Pathak, J.L. Apoptotic Bodies: Bioactive Treasure Left behind by the Dying Cells with Robust Diagnostic and Therapeutic Application Potentials. J. Nanobiotechnology 2023, 21, 218. [Google Scholar] [CrossRef]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and Biogenesis of Shed Microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef]
- Menck, K.; Sivaloganathan, S.; Bleckmann, A.; Binder, C. Microvesicles in Cancer: Small Size, Large Potential. Int. J. Mol. Sci. 2020, 21, 5373. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The Exosome Journey: From Biogenesis to Uptake and Intracellular Signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Vietri, M.; Radulovic, M.; Stenmark, H. The Many Functions of ESCRTs. Nat. Rev. Mol. Cell Biol. 2020, 21, 25–42. [Google Scholar] [CrossRef]
- Williams, R.L.; Urbé, S. The Emerging Shape of the ESCRT Machinery. Nat. Rev. Mol. Cell Biol. 2007, 8, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.H.; Emr, S.D. THE ESCRT COMPLEXES: Structure and Mechanism of a Membrane-Trafficking Network. Annu. Rev. Biophys. Biomol. Struct. 2006, 35, 277–298. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 388354. [Google Scholar] [CrossRef] [PubMed]
- Achkar, N.P.; Cambiagno, D.A.; Manavella, P.A. MiRNA Biogenesis: A Dynamic Pathway. Trends Plant Sci. 2016, 21, 1034–1044. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Lee, H.; Kim, H.; Kim, V.N.; Roh, S.-H. Structure of the Human DICER–Pre-miRNA Complex in a Dicing State. Nature 2023, 615, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef]
- Kawamata, T.; Seitz, H.; Tomari, Y. Structural Determinants of miRNAs for RISC Loading and Slicer-Independent Unwinding. Nat. Struct. Mol. Biol. 2009, 16, 953–960. [Google Scholar] [CrossRef]
- Hang, Q.; Zeng, L.; Wang, L.; Nie, L.; Yao, F.; Teng, H.; Deng, Y.; Yap, S.; Sun, Y.; Frank, S.J.; et al. Non-Canonical Function of DGCR8 in DNA Double-Strand Break Repair Signaling and Tumor Radioresistance. Nat. Commun. 2021, 12, 4033. [Google Scholar] [CrossRef] [PubMed]
- Stavast, C.; Erkeland, S. The Non-Canonical Aspects of MicroRNAs: Many Roads to Gene Regulation. Cells 2019, 8, 1465. [Google Scholar] [CrossRef] [PubMed]
- Koustas, E.; Trifylli, E.-M.; Sarantis, P.; Papadopoulos, N.; Papanikolopoulos, K.; Aloizos, G.; Damaskos, C.; Garmpis, N.; Garmpi, A.; Karamouzis, M.V. The Emerging Role of MicroRNAs and Autophagy Mechanism in Pancreatic Cancer Progression: Future Therapeutic Approaches. Genes 2022, 13, 1868. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, S.; Zhu, Z.; Yu, L.; Ren, Y.; Jiang, M.; Weng, J.; Li, B. miR-21 Promotes EGF-Induced Pancreatic Cancer Cell Proliferation by Targeting Spry2. Cell Death Dis. 2018, 9, 1157. [Google Scholar] [CrossRef]
- Stanciu, S.; Ionita-Radu, F.; Stefani, C.; Miricescu, D.; Stanescu-Spinu, I.-I.; Greabu, M.; Ripszky Totan, A.; Jinga, M. Targeting PI3K/AKT/mTOR Signaling Pathway in Pancreatic Cancer: From Molecular to Clinical Aspects. Int. J. Mol. Sci. 2022, 23, 10132. [Google Scholar] [CrossRef]
- Zhang, Z.-L.; Bai, Z.-H.; Wang, X.-B.; Bai, L.; Miao, F.; Pei, H.-H. MiR-186 and 326 Predict the Prognosis of Pancreatic Ductal Adenocarcinoma and Affect the Proliferation and Migration of Cancer Cells. PLoS ONE 2015, 10, e0118814. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, J.; Li, Y.; Peng, C.; Shi, M.; Wang, X.; Wei, G.; Ge, O.; Wang, D.; Zhang, B.; et al. MiR-17-5p Enhances Pancreatic Cancer Proliferation by Altering Cell Cycle Profiles via Disruption of RBL2/E2F4-Repressing Complexes. Cancer Lett. 2018, 412, 59–68. [Google Scholar] [CrossRef]
- Wang, H.-L.; Zhou, R.; Liu, J.; Chang, Y.; Liu, S.; Wang, X.-B.; Huang, M.-F.; Zhao, Q. MicroRNA-196b Inhibits Late Apoptosis of Pancreatic Cancer Cells by Targeting CADM1. Sci. Rep. 2017, 7, 11467. [Google Scholar] [CrossRef]
- Shen, K.; Cao, Z.; Zhu, R.; You, L.; Zhang, T. The Dual Functional Role of MicroRNA-18a (miR-18a) in Cancer Development. Clin. Transl. Med. 2019, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Mortoglou, M.; Tabin, Z.K.; Arisan, E.D.; Kocher, H.M.; Uysal-Onganer, P. Non-Coding RNAs in Pancreatic Ductal Adenocarcinoma: New Approaches for Better Diagnosis and Therapy. Transl. Oncol. 2021, 14, 101090. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, A.A.; Azmi, A.S.; Philip, P.A. MiRNA and Gene Expression in Pancreatic Ductal Adenocarcinoma. Am. J. Pathol. 2019, 189, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Mok, E.T.Y.; Chitty, J.L.; Cox, T.R. miRNAs in Pancreatic Cancer Progression and Metastasis. Clin. Exp. Metastasis 2024. [Google Scholar] [CrossRef]
- Sarkar, S.; Dubaybo, H.; Ali, S.; Goncalves, P.; Kollepara, S.L.; Sethi, S.; Philip, P.A.; Li, Y. Down-Regulation of miR-221 Inhibits Proliferation of Pancreatic Cancer Cells through up-Regulation of PTEN, P27(Kip1), P57(Kip2), and PUMA. Am. J. Cancer Res. 2013, 3, 465. [Google Scholar] [PubMed]
- Xia, X.; Zhang, K.; Cen, G.; Jiang, T.; Cao, J.; Huang, K.; Huang, C.; Zhao, Q.; Qiu, Z. MicroRNA-301a-3p Promotes Pancreatic Cancer Progression via Negative Regulation ofSMAD4. Oncotarget 2015, 6, 21046–21063. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Shao, Z.; Zhao, Y. MicroRNA-374a Promotes Pancreatic Cancer Cell Proliferation and Epithelial to Mesenchymal Transition by Targeting SRCIN1. Pathol. Res. Pract. 2019, 215, 152382. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, C.; Zhang, L.; Lu, H.; Wang, Z.; Lv, J.; Fan, C. MicroRNA-1469-5p Promotes the Invasion and Proliferation of Pancreatic Cancer Cells via Direct Regulating the NDRG1/NF-κB/E-Cadherin Axis. Hum. Cell 2020, 33, 1176–1185. [Google Scholar] [CrossRef]
- Chauhan, N.; Dhasmana, A.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. MiR-205: A Potential Biomedicine for Cancer Therapy. Cells 2020, 9, 1957. [Google Scholar] [CrossRef]
- Ouyang, H.; Gore, J.; Deitz, S.; Korc, M. microRNA-10b Enhances Pancreatic Cancer Cell Invasion by Suppressing TIP30 Expression and Promoting EGF and TGF-β Actions. Oncogene 2014, 33, 4664–4674. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Li, W.; Yin, L.; Guo, S.; Xu, X.; Ouyang, Y.; Zhao, Z.; Liu, S.; Tian, Y.; et al. Downregulated miR-506 Expression Facilitates Pancreatic Cancer Progression and Chemoresistance via SPHK1/Akt/NF-κB Signaling. Oncogene 2016, 35, 5501–5514. [Google Scholar] [CrossRef]
- Li, S.; Wei, X.; He, J.; Cao, Q.; Du, D.; Zhan, X.; Zeng, Y.; Yuan, S.; Sun, L. The Comprehensive Landscape of miR-34a in Cancer Research. Cancer Metastasis Rev. 2021, 40, 925–948. [Google Scholar] [CrossRef]
- Lu, Y.; Ji, N.; Wei, W.; Sun, W.; Gong, X.; Wang, X. MiR-142 Modulates Human Pancreatic Cancer Proliferation and Invasion by Targeting Hypoxia-Inducible Factor 1 (HIF-1α) in the Tumor Microenvironments. Biol. Open 2017, 6, 252–259. [Google Scholar] [CrossRef]
- Wu, X.; Chen, W.; Cai, H.; Hu, J.; Wu, B.; Jiang, Y.; Chen, X.; Sun, D.; An, Y. MiR-216b Inhibits Pancreatic Cancer Cell Progression and Promotes Apoptosis by down-Regulating KRAS. Arch. Med. Sci. 2018, 14, 1321–1332. [Google Scholar] [CrossRef]
- Sun, L.-L.; Cheng, M.; Xu, X.-D. MicroRNA-30c Inhibits Pancreatic Cancer Cell Proliferation by Targeting Twinfilin 1 and Indicates a Poor Prognosis. World J. Gastroenterol. 2019, 25, 6311–6321. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Li, C.; Zhang, X.; Peng, W.; Wen, T. MiR-143-3p Suppresses Tumorigenesis in Pancreatic Ductal Adenocarcinoma by Targeting KRAS. Biomed. Pharmacother. 2019, 119, 109424. [Google Scholar] [CrossRef]
- Nong, K.; Zhang, D.; Chen, C.; Yang, Y.; Yang, Y.; Liu, S.; Cai, H. MicroRNA-519 Inhibits Hypoxia-induced Tumorigenesis of Pancreatic Cancer by Regulating Immune Checkpoint PD-L1. Oncol. Lett. 2019, 19, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X.-J.; Ding, Y.-M.; Jiang, J.-X. miR-1181 Inhibits Invasion and Proliferation via STAT3 in Pancreatic Cancer. World J. Gastroenterol. 2017, 23, 1594. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhou, J.; He, S.; Zhu, D.; Zhang, Z.; Zhao, H.; Wang, Y.I.; Li, D. Expression Levels of MicroRNA-375 in Pancreatic Cancer. Biomed. Rep. 2013, 1, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Zhu, Q.; Han, Z.; Tan, J.; Liu, M.; Liu, W.; Chen, W.; Chen, X.; Chen, X.; Deng, J.; et al. MiR-455-3p Functions as a Tumor Suppressor by Restraining Wnt/β-Catenin Signaling via TAZ in Pancreatic Cancer. Cancer Manag. Res. 2020, 12, 1483–1492. [Google Scholar] [CrossRef]
- Cao, Z.; Qiu, J.; Yang, G.; Liu, Y.; Luo, W.; You, L.; Zheng, L.; Zhang, T. MiR-135a Biogenesis and Regulation in Malignancy: A New Hope for Cancer Research and Therapy. Cancer Biol. Med. 2020, 17, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Z.; Ding, Y.; Zhang, P.; Wang, J. WITHDRAWN: MicroRNA-340 Suppresses Pancreatic Cancer Growth by Targeting BICD2. Pancreatology 2019. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.-F.; Zhao, H.-C.; Yang, C.-L.; Chen, C.-Z.; Wang, K.; Gao, F.; Tian, Y.-Z.; Zhao, H.-L. MicroRNA-203-3p Inhibits the Proliferation, Invasion and Migration of Pancreatic Cancer Cells by Downregulating Fibroblast Growth Factor 2. Oncol. Lett. 2021, 22, 626. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long Non-Coding RNAs: Definitions, Functions, Challenges and Recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Rabiee, N.; Kumar, A.P.; Sethi, G.; Zarrabi, A.; Wang, Y. Long Noncoding RNAs (lncRNAs) in Pancreatic Cancer Progression. Drug Discov. Today 2022, 27, 2181–2198. [Google Scholar] [CrossRef] [PubMed]
- Misir, S.; Wu, N.; Yang, B.B. Specific Expression and Functions of Circular RNAs. Cell Death Differ. 2022, 29, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xue, M.; Du, S.; Feng, W.; Zhang, K.; Zhang, L.; Liu, H.; Jia, G.; Wu, L.; Hu, X.; et al. Competitive Endogenous RNA Is an Intrinsic Component of EMT Regulatory Circuits and Modulates EMT. Nat. Commun. 2019, 10, 1637. [Google Scholar] [CrossRef]
- Dalmasso, B.; Ghiorzo, P. Long Non-Coding RNAs and Metabolic Rewiring in Pancreatic Cancer. Cancers 2023, 15, 3486. [Google Scholar] [CrossRef]
- Jiang, X.-Y.; Zhu, Q.-C.; Zhang, X.-J.; Duan, T.; Feng, J.; Sui, X.-B.; Sun, X.-N.; Mou, Y.-P. Roles of lncRNAs in Pancreatic Ductal Adenocarcinoma: Diagnosis, Treatment, and the Development of Drug Resistance. Hepatobiliary Pancreat. Dis. Int. 2023, 22, 128–139. [Google Scholar] [CrossRef]
- Xiong, G.; Liu, C.; Yang, G.; Feng, M.; Xu, J.; Zhao, F.; You, L.; Zhou, L.; Zheng, L.; Hu, Y.; et al. Long Noncoding RNA GSTM3TV2 Upregulates LAT2 and OLR1 by Competitively Sponging Let-7 to Promote GEM Resistance in Pancreatic Cancer. J. Hematol. Oncol. 2019, 12, 97. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Zheng, S.; Zhou, Y.; Zhao, L.; Ye, H.; Zhao, X.; Gao, W.; Fu, Z.; Zhou, Q.; et al. Expression Profile of Long Non-Coding RNAs in Pancreatic Cancer and Their Clinical Significance as Biomarkers. Oncotarget 2015, 6, 35684–35698. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Li, Z.; Zheng, S.; Wang, Z.; Li, W.; Bi, Z.; Li, L.; Jiang, Y.; Luo, Y.; et al. Linc00511 Acts as a Competing Endogenous RNA to Regulate VEGFA Expression through Sponging hsa-miR-29b-3p in Pancreatic Ductal Adenocarcinoma. J. Cell. Mol. Med. 2018, 22, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Ramya Devi, K.T.; Karthik, D.; Mahendran, T.; Jaganathan, M.K.; Hemdev, S.P. Long Noncoding RNAs: Role and Contribution in Pancreatic Cancer. Transcription 2021, 12, 12–27. [Google Scholar] [CrossRef]
- Chio, I.I.C.; Jafarnejad, S.M.; Ponz-Sarvise, M.; Park, Y.; Rivera, K.; Palm, W.; Wilson, J.; Sangar, V.; Hao, Y.; Öhlund, D.; et al. NRF2 Promotes Tumor Maintenance by Modulating mRNA Translation in Pancreatic Cancer. Cell 2016, 166, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lin, X.; Wang, L.; Sun, T.; Zhao, Q.; Ma, Q.; Zhou, Y. LncRNA MALAT1 Enhances Ox-LDL-Induced Autophagy through the SIRT1/MAPK/NF-κB Pathway in Macrophages. Curr. Vasc. Pharmacol. 2020, 18, 652–662. [Google Scholar] [CrossRef]
- Piffoux, M.; Eriau, E.; Cassier, P.A. Autophagy as a Therapeutic Target in Pancreatic Cancer. Br. J. Cancer 2021, 124, 333–344. [Google Scholar] [CrossRef]
- Chen, B.; Li, Q.; Zhou, Y.; Wang, X.; Zhang, Q.; Wang, Y.; Zhuang, H.; Jiang, X.; Xiong, W. The Long Coding RNA AFAP1-AS1 Promotes Tumor Cell Growth and Invasion in Pancreatic Cancer through Upregulating the IGF1R Oncogene via Sequestration of miR-133a. Cell Cycle 2018, 17, 1949–1966. [Google Scholar] [CrossRef]
- Yang, J.; Qi, M.; Fei, X.; Wang, X.; Wang, K. LncRNA H19: A Novel Oncogene in Multiple Cancers. Int. J. Biol. Sci. 2021, 17, 3188–3208. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Chen, X.; Li, J.; Guo, Y.; Li, H.; Pan, X.; Jiang, J.; Liu, H.; Wu, B. Salivary HOTAIR and PVT1 as Novel Biomarkers for Early Pancreatic Cancer. Oncotarget 2016, 7, 25408–25419. [Google Scholar] [CrossRef]
- Fu, Z.; Li, G.; Li, Z.; Wang, Y.; Zhao, Y.; Zheng, S.; Ye, H.; Luo, Y.; Zhao, X.; Wei, L.; et al. Endogenous miRNA Sponge LincRNA-ROR Promotes Proliferation, Invasion and Stem Cell-like Phenotype of Pancreatic Cancer Cells. Cell Death Discov. 2017, 3, 17004. [Google Scholar] [CrossRef]
- Sun, Y.-W.; Chen, Y.-F.; Li, J.; Huo, Y.-M.; Liu, D.-J.; Hua, R.; Zhang, J.-F.; Liu, W.; Yang, J.-Y.; Fu, X.-L.; et al. A Novel Long Non-Coding RNA ENST00000480739 Suppresses Tumour Cell Invasion by Regulating OS-9 and HIF-1α in Pancreatic Ductal Adenocarcinoma. Br. J. Cancer 2014, 111, 2131–2141. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.-J.; Zhu, S.; Jin, Y.; Cui, S.-P.; Chen, J.-Y.; Xiang, C.; Li, Q.-Y.; He, C.; Zhao, S.-F.; et al. Hypoxia-Induced lncRNA-NUTF2P3-001 Contributes to Tumorigenesis of Pancreatic Cancer by Derepressing the miR-3923/KRAS Pathway. Oncotarget 2016, 7, 6000–6014. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.-Q.; Wang, J.-F.; Chen, D.-H.; Ma, X.-S.; Wu, Y.; Tang, Z.; Dang, X.-W. Long Non-Coding RNA GAS5 Suppresses Pancreatic Cancer Metastasis through Modulating miR-32-5p/PTEN Axis. Cell Biosci. 2017, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Turanli, B.; Yildirim, E.; Gulfidan, G.; Arga, K.Y.; Sinha, R. Current State of “Omics” Biomarkers in Pancreatic Cancer. J. Pers. Med. 2021, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, W.; Li, Q.; Wang, Z.; Yang, X. Identification of m6A/m5C/m1A-Associated LncRNAs for Prognostic Assessment and Immunotherapy in Pancreatic Cancer. Sci. Rep. 2023, 13, 3661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, X.; Wang, L.; Li, X.; Feng, D.; Liu, M.; Li, J.; Cheng, M.; Song, N.; Yang, X.; et al. Circular RNA Hsa_circ_0007367 Promotes the Progression of Pancreatic Ductal Adenocarcinoma by Sponging miR-6820-3p and Upregulating YAP1 Expression. Cell Death Dis. 2022, 13, 736. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Li, G.; Lu, J.; Li, L. Crosstalk between circRNAs and the PI3K/AKT Signaling Pathway in Cancer Progression. Signal Transduct. Target. Ther. 2021, 6, 400. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Jun, E.; Okugawa, Y.; Toiyama, Y.; Borazanci, E.; Bolton, J.; Taketomi, A.; Kim, S.C.; Shang, D.; Von Hoff, D.; et al. A Circulating Panel of circRNA Biomarkers for the Noninvasive and Early Detection of Pancreatic Ductal Adenocarcinoma. Gastroenterology 2024, 166, 178–190.e16. [Google Scholar] [CrossRef]
- Cavallari, C.; Camussi, G.; Brizzi, M.F. Extracellular Vesicles in the Tumour Microenvironment: Eclectic Supervisors. Int. J. Mol. Sci. 2020, 21, 6768. [Google Scholar] [CrossRef]
- Zou, X.; Huang, Z.; Guan, C.; Shi, W.; Gao, J.; Wang, J.; Cui, Y.; Wang, M.; Xu, Y.; Zhong, X. Exosomal miRNAs in the Microenvironment of Pancreatic Cancer. Clin. Chim. Acta 2023, 544, 117360. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, S.; Li, P.; Chen, Q.; Li, Y.; Zhou, Y.; Wang, L.; Kang, M.; Zhang, B.; Yang, B.; et al. Pancreatic Cancer-Derived Exosomes Suppress the Production of GIP and GLP-1 from STC-1 Cells in Vitro by down-Regulating the PCSK1/3. Cancer Lett. 2018, 431, 190–200. [Google Scholar] [CrossRef]
- Andersen, D.K.; Korc, M.; Petersen, G.M.; Eibl, G.; Li, D.; Rickels, M.R.; Chari, S.T.; Abbruzzese, J.L. Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer. Diabetes 2017, 66, 1103–1110. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Park, J.; Park, E.Y.; Kim, S.-M.; Lee, S.-Y. Analysis of MicroRNA Signature Differentially Expressed in Pancreatic Islet Cells Treated with Pancreatic Cancer-Derived Exosomes. Int. J. Mol. Sci. 2023, 24, 14301. [Google Scholar] [CrossRef]
- Su, J.; Pang, W.; Zhang, A.; Li, L.; Yao, W.; Dai, X. Exosomal miR-19a Decreases Insulin Production by Targeting Neurod1 in Pancreatic Cancer Associated Diabetes. Mol. Biol. Rep. 2022, 49, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tao, Y.; Wang, X.; Jiang, P.; Li, J.; Peng, M.; Zhang, X.; Chen, K.; Liu, H.; Zhen, P.; et al. Tumor-Secreted Exosomal miR-222 Promotes Tumor Progression via Regulating P27 Expression and Re-Localization in Pancreatic Cancer. Cell. Physiol. Biochem. 2018, 51, 610–629. [Google Scholar] [CrossRef]
- Mikamori, M.; Yamada, D.; Eguchi, H.; Hasegawa, S.; Kishimoto, T.; Tomimaru, Y.; Asaoka, T.; Noda, T.; Wada, H.; Kawamoto, K.; et al. MicroRNA-155 Controls Exosome Synthesis and Promotes GEM Resistance in Pancreatic Ductal Adenocarcinoma. Sci. Rep. 2017, 7, 42339. [Google Scholar] [CrossRef]
- Wu, M.; Tan, X.; Liu, P.; Yang, Y.; Huang, Y.; Liu, X.; Meng, X.; Yu, B.; Wu, Y.; Jin, H. Role of Exosomal MicroRNA-125b-5p in Conferring the Metastatic Phenotype among Pancreatic Cancer Cells with Different Potential of Metastasis. Life Sci. 2020, 255, 117857. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Xie, C.; Hu, J.; Tan, J.; Yuan, Y.; Liu, Z.; Yang, Z. Pancreatic Cancer Cell–Derived Exosomal microRNA-27a Promotes Angiogenesis of Human Microvascular Endothelial Cells in Pancreatic Cancer via BTG2. J. Cell. Mol. Med. 2020, 24, 588–604. [Google Scholar] [CrossRef]
- Ansari, D.; Torén, W.; Zhou, Q.; Hu, D.; Andersson, R. Proteomic and Genomic Profiling of Pancreatic Cancer. Cell Biol. Toxicol. 2019, 35, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Yamamoto, H.; Harada, T.; Fumoto, K.; Osugi, Y.; Sada, R.; Maehara, N.; Hikita, H.; Mori, S.; Eguchi, H.; et al. CKAP4, a DKK1 Receptor, Is a Biomarker in Exosomes Derived from Pancreatic Cancer and a Molecular Target for Therapy. Clin. Cancer Res. 2019, 25, 1936–1947. [Google Scholar] [CrossRef]
- Yokose, T.; Kabe, Y.; Matsuda, A.; Kitago, M.; Matsuda, S.; Hirai, M.; Nakagawa, T.; Masugi, Y.; Hishiki, T.; Nakamura, Y.; et al. O-Glycan-Altered Extracellular Vesicles: A Specific Serum Marker Elevated in Pancreatic Cancer. Cancers 2020, 12, 2469. [Google Scholar] [CrossRef]
- Emmanouilidi, A.; Paladin, D.; Greening, D.W.; Falasca, M. Oncogenic and Non-malignant Pancreatic Exosome Cargo Reveal Distinct Expression of Oncogenic and Prognostic Factors Involved in Tumor Invasion and Metastasis. Proteomics 2019, 19, e1800158. [Google Scholar] [CrossRef]
- Waters, A.M.; Der, C.J. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a031435. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Meckes, D.G., Jr. Extracellular Vesicle Integrins Distinguish Unique Cancers. Proteomes 2019, 7, 14. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, Y.; Liu, S.; Chen, Q.; Liu, Y. ITGA3 Serves as a Diagnostic and Prognostic Biomarker for Pancreatic Cancer. Onco Targets Ther. 2019, 12, 4141–4152. [Google Scholar] [CrossRef]
- Eurola, A.; Ristimäki, A.; Mustonen, H.; Nurmi, A.-M.; Hagström, J.; Haglund, C.; Seppänen, H. Impact of Histological Response after Neoadjuvant Therapy on Podocalyxin as a Prognostic Marker in Pancreatic Cancer. Sci. Rep. 2021, 11, 9896. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Mu, W.; Erb, U.; Zöller, M. The Tetraspanins CD151 and Tspan8 Are Essential Exosome Components for the Crosstalk between Cancer Initiating Cells and Their Surrounding. Oncotarget 2015, 6, 2366–2384. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, K.; Qu, C.; Peng, J.; Yang, L. Non-Coding RNAs Derived from Extracellular Vesicles Promote Pre-Metastatic Niche Formation and Tumor Distant Metastasis. Cancers 2023, 15, 2158. [Google Scholar] [CrossRef] [PubMed]
- Novizio, N.; Belvedere, R.; Pessolano, E.; Tosco, A.; Porta, A.; Perretti, M.; Campiglia, P.; Filippelli, A.; Petrella, A. Annexin A1 Released in Extracellular Vesicles by Pancreatic Cancer Cells Activates Components of the Tumor Microenvironment, through Interaction with the Formyl-Peptide Receptors. Cells 2020, 9, 2719. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, H.; Yang, Y.M.; Pandol, S.J.; Seki, E. Exosome Migration Inhibitory Factor as a Marker and Therapeutic Target for Pancreatic Cancer. Gastroenterology 2016, 150, 1033–1035. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Wang, Q.; Song, Y.; Liu, Y.; Liu, Y.; Yang, S.; Li, D.; Zhang, Y.; Zhu, C. MIF Inhibitor, ISO-1, Attenuates Human Pancreatic Cancer Cell Proliferation, Migration and Invasion in Vitro, and Suppresses Xenograft Tumour Growth in Vivo. Sci. Rep. 2020, 10, 6741. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, A.; Kadoi, Y.; Tsuruda, T.; Kim, Y.-S.; Miyoshi, M.; Nomoto, Y.; Nakata, Y.; Miyake, M.; Miyashita, K.; Shimizu, K.; et al. Exosomes in Ascites from Patients with Human Pancreatic Cancer Enhance Remote Metastasis Partially through Endothelial-Mesenchymal Transition. Pancreatology 2023, 23, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 Identifies Cancer Exosomes and Detects Early Pancreatic Cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef]
- Lucien, F.; Lac, V.; Billadeau, D.D.; Borgida, A.; Gallinger, S.; Leong, H.S. Glypican-1 and Glycoprotein 2 Bearing Extracellular Vesicles Do Not Discern Pancreatic Cancer from Benign Pancreatic Diseases. Oncotarget 2019, 10, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wei, Q.; Koay, E.J.; Liu, Y.; Ning, B.; Bernard, P.W.; Zhang, N.; Han, H.; Katz, M.H.; Zhao, Z.; et al. Chemoresistance Transmission via Exosome-Mediated EphA2 Transfer in Pancreatic Cancer. Theranostics 2018, 8, 5986–5994. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; You, L.; Dai, M.; Zhao, Y. Mucins in Pancreatic Cancer: A Well-established but Promising Family for Diagnosis, Prognosis and Therapy. J. Cell. Mol. Med. 2020, 24, 10279–10289. [Google Scholar] [CrossRef]
- Wang, C.; Wu, N.; Pei, B.; Ma, X.; Yang, W. Claudin and Pancreatic Cancer. Front. Oncol. 2023, 13, 1136227. [Google Scholar] [CrossRef]
- Castillo, J.; Bernard, V.; San Lucas, F.A.; Allenson, K.; Capello, M.; Kim, D.U.; Gascoyne, P.; Mulu, F.C.; Stephens, B.M.; Huang, J.; et al. Surfaceome Profiling Enables Isolation of Cancer-Specific Exosomal Cargo in Liquid Biopsies from Pancreatic Cancer Patients. Ann. Oncol. 2018, 29, 223–229. [Google Scholar] [CrossRef]
- Jin, H.; Liu, P.; Wu, Y.; Meng, X.; Wu, M.; Han, J.; Tan, X. Exosomal Zinc Transporter ZIP4 Promotes Cancer Growth and Is a Novel Diagnostic Biomarker for Pancreatic Cancer. Cancer Sci. 2018, 109, 2946–2956. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Koustas, E.; Trifylli, E.-M.; Sarantis, P.; Papadopoulos, N.; Papanikolopoulos, K.; Aloizos, G.; Damaskos, C.; Garmpis, N.; Garmpi, A.; Matthaios, D.; et al. Exploiting Autophagy-Dependent Neoantigen Presentation in Tumor Microenvironment. Genes 2023, 14, 474. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.-Z.; Jin, W.-L. The Updated Landscape of Tumor Microenvironment and Drug Repurposing. Signal Transduct Target. Ther. 2020, 5, 166. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, J.; Dye, D.; Razak, N.B.A.; Metharom, P.; Falasca, M. Immune Evasion on the Nanoscale: Small Extracellular Vesicles in Pancreatic Ductal Adenocarcinoma Immunity. Semin. Cancer Biol. 2023, 96, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Huang, Z.; Shi, C.; Pu, X.; Xu, X.; Wu, Z.; Ding, G.; Cao, L. Pancreatic Cancer-derived Exosomes Induce Apoptosis of T Lymphocytes through the P38 MAPK-mediated Endoplasmic Reticulum Stress. FASEB J. 2020, 34, 8442–8458. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Schlößer, H.A.; Wang, Z.; Qin, J.; Li, J.; Popp, F.; Popp, M.C.; Alakus, H.; Chon, S.-H.; Hansen, H.P.; et al. Tumor-Derived Extracellular Vesicles Inhibit Natural Killer Cell Function in Pancreatic Cancer. Cancers 2019, 11, 874. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Pauklin, S. Extracellular Vesicles in Pancreatic Cancer Progression and Therapies. Cell Death Dis. 2021, 12, 973. [Google Scholar] [CrossRef]
- Chiba, M.; Kubota, S.; Sato, K.; Monzen, S. Exosomes Released from Pancreatic Cancer Cells Enhance Angiogenic Activities via Dynamin-Dependent Endocytosis in Endothelial Cells in Vitro. Sci. Rep. 2018, 8, 11972. [Google Scholar] [CrossRef]
- Armacki, M.; Polaschek, S.; Waldenmaier, M.; Morawe, M.; Ruhland, C.; Schmid, R.; Lechel, A.; Tharehalli, U.; Steup, C.; Bektas, Y.; et al. Protein Kinase D1, Reduced in Human Pancreatic Tumors, Increases Secretion of Small Extracellular Vesicles from Cancer Cells That Promote Metastasis to Lung in Mice. Gastroenterology 2020, 159, 1019–1035.e22. [Google Scholar] [CrossRef]
- Hinzman, C.P.; Singh, B.; Bansal, S.; Li, Y.; Iliuk, A.; Girgis, M.; Herremans, K.M.; Trevino, J.G.; Singh, V.K.; Banerjee, P.P.; et al. A Multi-omics Approach Identifies Pancreatic Cancer Cell Extracellular Vesicles as Mediators of the Unfolded Protein Response in Normal Pancreatic Epithelial Cells. J. Extracell. Vesicles 2022, 11, e12232. [Google Scholar] [CrossRef]
- Chang, Y.T.; Peng, H.Y.; Hu, C.M.; Huang, S.C.; Tien, S.C.; Jeng, Y.M. Pancreatic Cancer-Derived Small Extracellular Vesical Ezrin Regulates Macrophage Polarization and Promotes Metastasis. Am. J. Cancer Res. 2020, 10, 12–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, Y. Pancreatic Cancer Cell-Derived microRNA-155-5p-Containing Extracellular Vesicles Promote Immune Evasion by Triggering EHF-Dependent Activation of Akt/NF-κB Signaling Pathway. Int. Immunopharmacol. 2021, 100, 107990. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Zhou, Y.-Z.; Zhang, B.; Huang, S.-F.; Li, P.-P.; He, X.-M.; Cao, G.-D.; Kang, M.-X.; Dong, X.; Wu, Y.-L. Pancreatic Cancer-Derived Exosomes Promoted Pancreatic Stellate Cells Recruitment by Pancreatic Cancer. J. Cancer 2019, 10, 4397–4407. [Google Scholar] [CrossRef]
- He, Z.; Wang, J.; Zhu, C.; Xu, J.; Chen, P.; Jiang, X.; Chen, Y.; Jiang, J.; Sun, C. Exosome-Derived FGD5-AS1 Promotes Tumor-Associated Macrophage M2 Polarization-Mediated Pancreatic Cancer Cell Proliferation and Metastasis. Cancer Lett. 2022, 548, 215751. [Google Scholar] [CrossRef] [PubMed]
- Pfrieger, F.W.; Vitale, N. Thematic Review Series: Exosomes and Microvesicles: Lipids as Key Components of Their Biogenesis and Functions, Cholesterol and the Journey of Extracellular Vesicles. J. Lipid Res. 2018, 59, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Fendt, S.-M. The Metabolism of Cancer Cells during Metastasis. Nat. Rev. Cancer 2021, 21, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, A.; Negri, M.; Paratore, M.; Vitale, F.; Ainora, M.E.; Nista, E.C.; Gasbarrini, A.; Zocco, M.A.; Zileri Dal Verme, L. Diagnostic and Prognostic Role of Extracellular Vesicles in Pancreatic Cancer: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 885. [Google Scholar] [CrossRef] [PubMed]
- De Rubis, G.; Bebawy, M. Extracellular Vesicles in Chemoresistance. In Subcellular Biochemistry; Springer International Publishing: Cham, Switzerland, 2021; Volume 97, pp. 211–245. [Google Scholar]
- Richards, K.; Xiao, W.; Hill, R.; on behalf of the USC Pancreas Research Team. Cancer-Associated Fibroblasts Confer GEM Resistance to Pancreatic Cancer Cells through PTEN-Targeting miRNAs in Exosomes. Cancers 2022, 14, 2812. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Bai, Y.; Li, K.; Liu, N.; Xu, Y.; Dal, E.; Wang, Y.; Lin, R.; Wang, H.; Liu, Z.; et al. Cancer-Associated Fibroblasts Suppress Ferroptosis and Induce GEM Resistance in Pancreatic Cancer Cells by Secreting Exosome-Derived ACSL4-Targeting miRNAs. Drug Resist Updat. 2023, 68, 100960. [Google Scholar] [CrossRef]
- Nigri, J.; Leca, J.; Tubiana, S.-S.; Finetti, P.; Guillaumond, F.; Martinez, S.; Lac, S.; Iovanna, J.L.; Audebert, S.; Camoin, L.; et al. CD9 Mediates the Uptake of Extracellular Vesicles from Cancer-Associated Fibroblasts That Promote Pancreatic Cancer Cell Aggressiveness. Sci. Signal. 2022, 15, eabg8191. [Google Scholar] [CrossRef]
- Fang, Y.; Zhou, W.; Rong, Y.; Kuang, T.; Xu, X.; Wu, W.; Wang, D.; Lou, W. Exosomal miRNA106b from Cancer-Associated Fibroblast Promotes GEM Resistance in Pancreatic Cancer. Exp. Cell Res. 2019, 383, 111543. [Google Scholar] [CrossRef]
- Sagar, S.K. miR-106b as an Emerging Therapeutic Target in Cancer. Genes Dis. 2022, 9, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; He, D.; Li, L.; Zhang, S.; Wang, L.; Fan, Z.; Wang, Y. Extracellular Vesicles in Pancreatic Cancer Immune Escape: Emerging Roles and Mechanisms. Pharmacol. Res. 2022, 183, 106364. [Google Scholar] [CrossRef] [PubMed]
- Brocco, D.; De Bellis, D.; Di Marino, P.; Simeone, P.; Grassadonia, A.; De Tursi, M.; Grottola, T.; Di Mola, F.F.; Di Gregorio, P.; Zappacosta, B.; et al. High Blood Concentration of Leukocyte-Derived Extracellular Vesicles Is Predictive of Favorable Clinical Outcomes in Patients with Pancreatic Cancer: Results from a Multicenter Prospective Study. Cancers 2022, 14, 4748. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, T.; Masamune, A.; Yoshida, N.; Hamada, S.; Kogure, T.; Shimosegawa, T. Exosomes Derived from Pancreatic Stellate Cells: MicroRNA Signature and Effects on Pancreatic Cancer Cells. Pancreas 2017, 46, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Ma, T.; Huang, B.; Lin, L.; Zhou, Y.; Yan, J.; Zou, Y.; Chen, S. Macrophage-Derived Exosomal microRNA-501-3p Promotes Progression of Pancreatic Ductal Adenocarcinoma through the TGFBR3-Mediated TGF-β Signaling Pathway. J. Exp. Clin. Cancer Res. 2019, 38, 310. [Google Scholar] [CrossRef] [PubMed]
- Hanks, B.A.; Holtzhausen, A.; Evans, K.S.; Jamieson, R.; Gimpel, P.; Campbell, O.M.; Hector-Greene, M.; Sun, L.; Tewari, A.; George, A.; et al. Type III TGF-β Receptor Downregulation Generates an Immunotolerant Tumor Microenvironment. J. Clin. Invest 2013, 123, 3925–3940. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Wu, Y.; Wu, Y.; Wang, P.; Vadgama, J.V. Tumor-Derived Exosomes in Tumor-Induced Immune Suppression. Int. J. Mol. Sci. 2022, 23, 1461. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, W.; Wang, Y.; Wang, H.; Liu, S. Extracellular Vesicle-Mediated Crosstalk between Pancreatic Cancer and Stromal Cells in the Tumor Microenvironment. J. Nanobiotechnology 2022, 20, 208. [Google Scholar] [CrossRef]
- Boomgarden, A.C.; Sheehan, C.; D’Souza-Schorey, C. Extracellular Vesicles in the Tumor Microenvironment: Various Implications in Tumor Progression. In Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; Volume 1259, pp. 155–170. [Google Scholar]
- Hussain, Z.; Nigri, J.; Tomasini, R. The Cellular and Biological Impact of Extracellular Vesicles in Pancreatic Cancer. Cancers 2021, 13, 3040. [Google Scholar] [CrossRef]
- Nannan, L.; Oudart, J.-B.; Monboisse, J.C.; Ramont, L.; Brassart-Pasco, S.; Brassart, B. Extracellular Vesicle-Dependent Cross-Talk in Cancer—Focus on Pancreatic Cancer. Front. Oncol. 2020, 10, 1456. [Google Scholar] [CrossRef]
- Hisada, Y.; Sachetto, A.T.A.; Mackman, N. Circulating Tissue Factor-positive Extracellular Vesicles and Their Association with Thrombosis in Different Diseases. Immunol. Rev. 2022, 312, 61–75. [Google Scholar] [CrossRef]
- Kobayashi, S.; Koizume, S.; Takahashi, T.; Ueno, M.; Oishi, R.; Nagashima, S.; Sano, Y.; Fukushima, T.; Tezuka, S.; Morimoto, M.; et al. Tissue Factor and Its Procoagulant Activity on Cancer-associated Thromboembolism in Pancreatic Cancer. Cancer Sci. 2021, 112, 4679–4691. [Google Scholar] [CrossRef]
- Cosmi, B.; Legnani, C.; Libra, A.; Palareti, G. D-Dimer in Diagnosis and Prevention of Venous Thrombosis: Recent Advances and Their Practical Implications. Pol. Arch. Med. Wewn. 2023, 133, 16604. [Google Scholar] [CrossRef] [PubMed]
- Baj-Krzyworzeka, M.; Mytar, B.; Weglarczyk, K.; Szatanek, R.; Kijowski, J.; Siedlar, M. Protumorogenic Potential of Pancreatic Adenocarcinoma-Derived Extracellular Vesicles. Folia Biol. 2020, 66, 104–110. [Google Scholar] [CrossRef]
- Patton, M.C.; Zubair, H.; Khan, M.A.; Singh, S.; Singh, A.P. Hypoxia Alters the Release and Size Distribution of Extracellular Vesicles in Pancreatic Cancer Cells to Support Their Adaptive Survival. J. Cell. Biochem. 2020, 121, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, X.; Zhai, S.; Shi, M.; Peng, C.; Deng, X.; Fu, D.; Wang, J.; Shen, B. Hypoxia-Induced Exosomal circPDK1 Promotes Pancreatic Cancer Glycolysis via c-Myc Activation by Modulating miR-628-3p/BPTF Axis and Degrading BIN1. J. Hematol. Oncol. 2022, 15, 128. [Google Scholar] [CrossRef]
- Marzan, A.L.; Stewart, S.E. Elucidating the Role of Extracellular Vesicles in Pancreatic Cancer. Cancers 2021, 13, 5669. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, N.; Cui, J.; Wu, H.; Xiong, J.; Peng, T. Exosomes Derived from Cancer Stem Cells of GEM -Resistant Pancreatic Cancer Cells Enhance Drug Resistance by Delivering miR-210. Cell. Oncol. 2020, 43, 123–136. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Pal, K.; Sharma, A.K.; Dutta, S.K.; Lau, J.S.; Yan, I.K.; Wang, E.; Elkhanany, A.; Alkharfy, K.M.; Sanyal, A.; et al. GAIP Interacting Protein C-Terminus Regulates Autophagy and Exosome Biogenesis of Pancreatic Cancer through Metabolic Pathways. PLoS ONE 2014, 9, e114409. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tang, H.; Li, Q.; Chen, G.; Li, D. Exosomes and Their Roles in the Chemoresistance of Pancreatic Cancer. Cancer Med. 2022, 11, 4979–4988. [Google Scholar] [CrossRef]
- Patel, G.K.; Khan, M.A.; Bhardwaj, A.; Srivastava, S.K.; Zubair, H.; Patton, M.C.; Singh, S.; Khushman, M.; Singh, A.P. Exosomes Confer Chemoresistance to Pancreatic Cancer Cells by Promoting ROS Detoxification and miR-155-Mediated Suppression of Key GEM -Metabolising Enzyme, DCK. Br. J. Cancer 2017, 116, 609–619. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Q.; Liao, Q. Tumor-Associated Macrophages in Pancreatic Ductal Adenocarcinoma: Origin, Polarization, Function, and Reprogramming. Front. Cell Dev. Biol. 2021, 8, 607209. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.P.R.; Castro, I.; Caires, H.R.; Ferreira, D.; Cavadas, B.; Pereira, L.; Santos, L.L.; Oliveira, M.J.; Vasconcelos, M.H. Chitinase 3-like-1 and Fibronectin in the Cargo of Extracellular Vesicles Shed by Human Macrophages Influence Pancreatic Cancer Cellular Response to GEM. Cancer Lett. 2021, 501, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, J.; Büchler, M.W.; Friess, H.; Martignoni, M.E. Cachexia in Patients with Chronic Pancreatitis and Pancreatic Cancer: Impact on Survival and Outcome. Nutr. Cancer 2013, 65, 827–833. [Google Scholar] [CrossRef]
- Han, L.; Zhao, Z.; Yang, K.; Xin, M.; Zhou, L.; Chen, S.; Zhou, S.; Tang, Z.; Ji, H.; Dai, R. Application of Exosomes in the Diagnosis and Treatment of Pancreatic Diseases. Stem Cell Res. Ther. 2022, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, M.; Medici, N.; Migliaccio, A.; Castoria, G.; Giovannelli, P. Exosomes: Emerging Modulators of Pancreatic Cancer Drug Resistance. Cancers 2023, 15, 4714. [Google Scholar] [CrossRef] [PubMed]
- Osteikoetxea, X.; Benke, M.; Rodriguez, M.; Pálóczi, K.; Sódar, B.W.; Szvicsek, Z.; Szabó-Taylor, K.; Vukman, K.V.; Kittel, Á.; Wiener, Z.; et al. Detection and Proteomic Characterization of Extracellular Vesicles in Human Pancreatic Juice. Biochem. Biophys. Res. Commun. 2018, 499, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Eguchi, A.; Kobayashi, Y.; Usugi, E.; Yamada, R.; Tsuboi, J.; Akuta, T.; Horiki, N.; Iwasa, M.; Takei, Y. Extracellular Vesicles from Pancreatic Ductal Adenocarcinoma Endoscopic Ultrasound-fine Needle Aspiration Samples Contain a Protein Barcode. J. Hepatobiliary Pancreat. Sci. 2022, 29, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Nesteruk, K.; Levink, I.J.M.; de Vries, E.; Visser, I.J.; Peppelenbosch, M.P.; Cahen, D.L.; Fuhler, G.M.; Bruno, M.J. Extracellular Vesicle-Derived microRNAs in Pancreatic Juice as Biomarkers for Detection of Pancreatic Ductal Adenocarcinoma. Pancreatology 2022, 22, 626–635. [Google Scholar] [CrossRef]
- Odaka, H.; Hiemori, K.; Shimoda, A.; Akiyoshi, K.; Tateno, H. CD63-Positive Extracellular Vesicles Are Potential Diagnostic Biomarkers of Pancreatic Ductal Adenocarcinoma. BMC Gastroenterol. 2022, 22, 153. [Google Scholar] [CrossRef]
- Xiao, D.; Dong, Z.; Zhen, L.; Xia, G.; Huang, X.; Wang, T.; Guo, H.; Yang, B.; Xu, C.; Wu, W.; et al. Combined Exosomal GPC1, CD82, and Serum CA19-9 as Multiplex Targets: A Specific, Sensitive, and Reproducible Detection Panel for the Diagnosis of Pancreatic Cancer. Mol. Cancer Res. 2020, 18, 300–310. [Google Scholar] [CrossRef]
- Lux, A.; Kahlert, C.; Grützmann, R.; Pilarsky, C. C-Met and PD-L1 on Circulating Exosomes as Diagnostic and Prognostic Markers for Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 3305. [Google Scholar] [CrossRef]
- Verel-Yilmaz, Y.; Fernández, J.P.; Schäfer, A.; Nevermann, S.; Cook, L.; Gercke, N.; Helmprobst, F.; Jaworek, C.; Pogge von Strandmann, E.; Pagenstecher, A.; et al. Extracellular Vesicle-Based Detection of Pancreatic Cancer. Front. Cell Dev. Biol. 2021, 9, 697939. [Google Scholar] [CrossRef]
- Zhang, W.; Campbell, D.H.; Walsh, B.J.; Packer, N.H.; Liu, D.; Wang, Y. Cancer-Derived Small Extracellular Vesicles: Emerging Biomarkers and Therapies for Pancreatic Ductal Adenocarcinoma Diagnosis/Prognosis and Treatment. J. Nanobiotechnology 2022, 20, 446. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Chen, S.; Duan, W.; Kong, X.; Wang, Y.; Zhou, L.; Li, P.; Zhang, C.; Du, L.; et al. Highly Sensitive Exosome Detection for Early Diagnosis of Pancreatic Cancer Using Immunoassay Based on Hierarchical Surface-enhanced Raman Scattering Substrate. Small Methods 2022, 6, e2200154. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, S.S.; Dunlay, C.J.; Simeone, D.M.; Nagrath, S. Microfluidic Device (ExoChip) for on-Chip Isolation, Quantification and Characterization of Circulating Exosomes. Lab. Chip 2014, 14, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Que, R.; Ding, G.; Chen, J.; Cao, L. Analysis of Serum Exosomal microRNAs and Clinicopathologic Features of Patients with Pancreatic Adenocarcinoma. World J. Surg. Oncol. 2013, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Cui, W.; Liu, Y.; Zhou, H.; Wang, Y.; Chen, X.; Chen, X.; Wang, Z. Serum Exosomal miRNA1226 as Potential Biomarker of Pancreatic Ductal Adenocarcinoma. Onco Targets Ther. 2021, 14, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Fujiya, M.; Konishi, H.; Sasajima, J.; Fujibayashi, S.; Hayashi, A.; Utsumi, T.; Sato, H.; Iwama, T.; Ijiri, M.; et al. An Elevated Expression of Serum Exosomal microRNA-191, − 21, −451a of Pancreatic Neoplasm Is Considered to Be Efficient Diagnostic Marker. BMC Cancer 2018, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, B.; Yue, S.; Galli, U.; Rana, S.; Gross, W.; Müller, M.; Giese, N.A.; Kalthoff, H.; Becker, T.; Büchler, M.W.; et al. Combined Evaluation of a Panel of Protein and miRNA Serum-exosome Biomarkers for Pancreatic Cancer Diagnosis Increases Sensitivity and Specificity. Int. J. Cancer 2015, 136, 2616–2627. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Sheng, Y.; Kwak, K.J.; Shi, J.; Yu, B.; Lee, L.J. A Signal-Amplifiable Biochip Quantifies Extracellular Vesicle-Associated RNAs for Early Cancer Detection. Nat. Commun. 2017, 8, 1683. [Google Scholar] [CrossRef]
- Takahashi, K.; Ota, Y.; Kogure, T.; Suzuki, Y.; Iwamoto, H.; Yamakita, K.; Kitano, Y.; Fujii, S.; Haneda, M.; Patel, T.; et al. Circulating Extracellular Vesicle-encapsulated HULC Is a Potential Biomarker for Human Pancreatic Cancer. Cancer Sci. 2020, 111, 98–111. [Google Scholar] [CrossRef]
- Joshi, G.K.; Deitz-McElyea, S.; Liyanage, T.; Lawrence, K.; Mali, S.; Sardar, R.; Korc, M. Label-Free Nanoplasmonic-Based Short Noncoding RNA Sensing at Attomolar Concentrations Allows for Quantitative and Highly Specific Assay of MicroRNA-10b in Biological Fluids and Circulating Exosomes. ACS Nano 2015, 9, 11075–11089. [Google Scholar] [CrossRef] [PubMed]
- Marin, A.M.; Mattar, S.B.; Amatuzzi, R.F.; Chammas, R.; Uno, M.; Zanette, D.L.; Aoki, M.N. Plasma Exosome-Derived microRNAs as Potential Diagnostic and Prognostic Biomarkers in Brazilian Pancreatic Cancer Patients. Biomolecules 2022, 12, 769. [Google Scholar] [CrossRef] [PubMed]
- Takahasi, K.; Iinuma, H.; Wada, K.; Minezaki, S.; Kawamura, S.; Kainuma, M.; Ikeda, Y.; Shibuya, M.; Miura, F.; Sano, K. Usefulness of Exosome-encapsulated MicroRNA-451a as a Minimally Invasive Biomarker for Prediction of Recurrence and Prognosis in Pancreatic Ductal Adenocarcinoma. J. Hepatobiliary Pancreat. Sci. 2018, 25, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-F.; Hannafon, B.N.; Zhao, Y.D.; Postier, R.G.; Ding, W.-Q. Plasma Exosome miR-196a and miR-1246 Are Potential Indicators of Localized Pancreatic Cancer. Oncotarget 2017, 8, 77028–77040. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wang, M.; McElyea, S.D.; Sherman, S.; House, M.; Korc, M. A microRNA Signature in Circulating Exosomes Is Superior to Exosomal Glypican-1 Levels for Diagnosing Pancreatic Cancer. Cancer Lett. 2017, 393, 86–93. [Google Scholar] [CrossRef]
- Yang, Z.; LaRiviere, M.J.; Ko, J.; Till, J.E.; Christensen, T.; Yee, S.S.; Black, T.A.; Tien, K.; Lin, A.; Shen, H.; et al. A Multianalyte Panel Consisting of Extracellular Vesicle miRNAs and mRNAs, cfDNA, and CA19-9 Shows Utility for Diagnosis and Staging of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2020, 26, 3248–3258. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, Y.; Liao, Z.; Wang, Z.; Wang, Z.; Li, Y.; Qian, L.; Zhao, J.; Zong, H.; Kang, B.; et al. Plasma Extracellular Vesicle Long RNA Profiling Identifies a Diagnostic Signature for the Detection of Pancreatic Ductal Adenocarcinoma. Gut 2020, 69, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ren, Y.; Lu, Z. Potential Diagnostic and Therapeutic Roles of Exosomes in Pancreatic Cancer. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188414. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Li, D.; Campbell, D.H.; Walsh, B.J.; Packer, N.H.; Dong, Q.; Wang, E.; Wang, Y. Phenotypic Profiling of Pancreatic Ductal Adenocarcinoma Plasma-Derived Small Extracellular Vesicles for Cancer Diagnosis and Cancer Stage Prediction: A Proof-of-Concept Study. Anal. Methods 2022, 14, 2255–2265. [Google Scholar] [CrossRef]
- Armstrong, E.A.; Beal, E.W.; Chakedis, J.; Paredes, A.Z.; Moris, D.; Pawlik, T.M.; Schmidt, C.R.; Dillhoff, M.E. Exosomes in Pancreatic Cancer: From Early Detection to Treatment. J. Gastrointest. Surg. 2018, 22, 737–750. [Google Scholar] [CrossRef]
- Jia, E.; Ren, N.; Shi, X.; Zhang, R.; Yu, H.; Yu, F.; Qin, S.; Xue, J. Extracellular Vesicle Biomarkers for Pancreatic Cancer Diagnosis: A Systematic Review and Meta-Analysis. BMC Cancer 2022, 22, 573. [Google Scholar] [CrossRef]
- Yang, K.S.; Im, H.; Hong, S.; Pergolini, I.; del Castillo, A.F.; Wang, R.; Clardy, S.; Huang, C.-H.; Pille, C.; Ferrone, S.; et al. Multiparametric Plasma EV Profiling Facilitates Diagnosis of Pancreatic Malignancy. Sci. Transl. Med. 2017, 9, eaal3226. [Google Scholar] [CrossRef]
- Shin, H.-S.; Jung, S.B.; Park, S.; Dua, P.; Lee, D.k. ALPPL2 Is a Potential Diagnostic Biomarker for Pancreatic Cancer-Derived Extracellular Vesicles. Mol. Ther. Methods Clin. Dev. 2019, 15, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.M.; Vyas, A.D.; Qiu, Y.; Messer, K.S.; White, R.; Heller, M.J. Integrated Analysis of Exosomal Protein Biomarkers on Alternating Current Electrokinetic Chips Enables Rapid Detection of Pancreatic Cancer in Patient Blood. ACS Nano 2018, 12, 3311–3320. [Google Scholar] [CrossRef] [PubMed]
- Machida, T.; Tomofuji, T.; Maruyama, T.; Yoneda, T.; Ekuni, D.; Azuma, T.; Miyai, H.; Mizuno, H.; Kato, H.; Tsutsumi, K.; et al. miR-1246 and miR-4644 in Salivary Exosome as Potential Biomarkers for Pancreatobiliary Tract Cancer. Oncol. Rep. 2016, 36, 2375–2381. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Kim, Y.; Chia, D.; Spielmann, N.; Eibl, G.; Elashoff, D.; Wei, F.; Lin, Y.-L.; Moro, A.; Grogan, T.; et al. Role of Pancreatic Cancer-Derived Exosomes in Salivary Biomarker Development. J. Biol. Chem. 2013, 288, 26888–26897. [Google Scholar] [CrossRef] [PubMed]
- Frampton, A.E.; Prado, M.M.; López-Jiménez, E.; Fajardo-Puerta, A.B.; Jawad, Z.A.R.; Lawton, P.; Giovannetti, E.; Habib, N.A.; Castellano, L.; Stebbing, J.; et al. Glypican-1 Is Enriched in Circulating-Exosomes in Pancreatic Cancer and Correlates with Tumor Burden. Oncotarget 2018, 9, 19006–19013. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.; Yang, K.S.; Zelga, P.; Liss, A.S.; Carlson, J.C.T.; del Castillo, C.F.; Weissleder, R. Single-EV Analysis (sEVA) of Mutated Proteins Allows Detection of Stage 1 Pancreatic Cancer. Sci. Adv. 2022, 8, eabm3453. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Lan, H.; Jin, K.; Qian, J. Pancreatic Cancer and Exosomes: Role in Progression, Diagnosis, Monitoring, and Treatment. Front. Oncol. 2023, 13, 1149551. [Google Scholar] [CrossRef]
- Bunduc, S.; Gede, N.; Váncsa, S.; Lillik, V.; Kiss, S.; Juhász, M.F.; Erőss, B.; Szakács, Z.; Gheorghe, C.; Mikó, A.; et al. Exosomes as Prognostic Biomarkers in Pancreatic Ductal Adenocarcinoma—A Systematic Review and Meta-Analysis. Transl. Res. 2022, 244, 126–136. [Google Scholar] [CrossRef]
- Asada, T.; Nakahata, S.; Fauzi, Y.R.; Ichikawa, T.; Inoue, K.; Shibata, N.; Fujii, Y.; Imamura, N.; Hiyoshi, M.; Nanashima, A.; et al. Integrin α6A (ITGA6A)-Type Splice Variant in Extracellular Vesicles Has a Potential as a Novel Marker of the Early Recurrence of Pancreatic Cancer. Anticancer Res. 2022, 42, 1763–1775. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, P.; Li, J.; Peng, M.; Zhao, X.; Zhang, X.; Chen, K.; Zhang, Y.; Liu, H.; Gan, L.; et al. Tumor-Derived Exosomal Lnc-Sox2ot Promotes EMT and Stemness by Acting as a ceRNA in Pancreatic Ductal Adenocarcinoma. Oncogene 2018, 37, 3822–3838. [Google Scholar] [CrossRef]
- Li, Z.; Yanfang, W.; Li, J.; Jiang, P.; Peng, T.; Chen, K.; Zhao, X.; Zhang, Y.; Zhen, P.; Zhu, J.; et al. Tumor-Released Exosomal Circular RNA PDE8A Promotes Invasive Growth via the miR-338/MACC1/MET Pathway in Pancreatic Cancer. Cancer Lett. 2018, 432, 237–250. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Gao, Y.; Lin, C.; An, Q.; Feng, Y.; Liu, Y.; Liu, D.; Luo, H.; Wang, D. The Biology and Function of Extracellular Vesicles in Cancer Development. Front. Cell Dev. Biol. 2021, 9, 777441. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic Cancer Exosomes Initiate Pre-Metastatic Niche Formation in the Liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Giampieri, R.; Piva, F.; Occhipinti, G.; Bittoni, A.; Righetti, A.; Pagliaretta, S.; Murrone, A.; Bianchi, F.; Amantini, C.; Giulietti, M.; et al. Clinical Impact of Different Exosomes’ Protein Expression in Pancreatic Ductal Carcinoma Patients Treated with Standard First Line Palliative Chemotherapy. PLoS ONE 2019, 14, e0215990. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Gao, Y.; Ho, C.; Li, L.; Jin, C.; Wang, X.; Zou, C.; Mao, Y.; Wang, X.; Li, Q.; et al. Exosome-Delivered CD44v6/C1QBP Complex Drives Pancreatic Cancer Liver Metastasis by Promoting Fibrotic Liver Microenvironment. Gut 2022, 71, 568–579. [Google Scholar] [CrossRef]
- Couto, N.; Elzanowska, J.; Maia, J.; Batista, S.; Pereira, C.E.; Beck, H.C.; Carvalho, A.S.; Strano Moraes, M.C.; Carvalho, C.; Oliveira, M.; et al. IgG+ Extracellular Vesicles Measure Therapeutic Response in Advanced Pancreatic Cancer. Cells 2022, 11, 2800. [Google Scholar] [CrossRef]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes Facilitate Therapeutic Targeting of Oncogenic KRAS in Pancreatic Cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Raposo, L.; Lourenço, A.P.; Nascimento, D.S.; Cerqueira, R.; Cardim, N.; Leite-Moreira, A. Human Umbilical Cord Tissue-Derived Mesenchymal Stromal Cells as Adjuvant Therapy for Myocardial Infarction: A Review of Current Evidence Focusing on Pre-Clinical Large Animal Models and Early Human Trials. Cytotherapy 2021, 23, 974–979. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, Y.; Chen, X.; Ning, T.; Chen, H.; Guo, Q.; Zhang, Y.; Liu, P.; Zhang, Y.; Li, C.; et al. Pancreatic Cancer-Targeting Exosomes for Enhancing Immunotherapy and Reprogramming Tumor Microenvironment. Biomaterials 2021, 268, 120546. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Wu, J.-Y.; Wang, J.-M.; Hu, X.-B.; Cai, J.-X.; Xiang, D.-X. Gemcitabine Loaded Autologous Exosomes for Effective and Safe Chemotherapy of Pancreatic Cancer. Acta Biomater. 2020, 101, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, Y.; Li, Z.; Liang, J.; Zhang, Y.; Zhou, S.; Zhang, Y.; Fan, Z.; Shen, Y.; Liu, Y.; et al. Pirfenidone-Loaded Exosomes Derived from Pancreatic Ductal Adenocarcinoma Cells Alleviate Fibrosis of Premetastatic Niches to Inhibit Liver Metastasis. Biomater. Sci. 2022, 10, 6614–6626. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Guo, R.; Wang, Y.; Li, J.-X.; He, J.; Li, M.; He, Q. Curbing Exosome Communications via Introducing Artificial Membrane Receptors for Metastatic Pancreatic Cancer Therapy. Adv. Mater. 2023, 35, e2303736. [Google Scholar] [CrossRef]
- Wang, C.-A.; Li, C.-F.; Huang, R.-C.; Li, Y.-H.; Liou, J.-P.; Tsai, S.-J. Suppression of Extracellular Vesicle VEGF-C–Mediated Lymphangiogenesis and Pancreatic Cancer Early Dissemination by a Selective HDAC1/2 Inhibitor. Mol. Cancer Ther. 2021, 20, 1550–1560. [Google Scholar] [CrossRef]
- Richards, K.E.; Zeleniak, A.E.; Fishel, M.L.; Wu, J.; Littlepage, L.E.; Hill, R. Cancer-Associated Fibroblast Exosomes Regulate Survival and Proliferation of Pancreatic Cancer Cells. Oncogene 2017, 36, 1770–1778. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, H.; Yoon, S.; Lee, H.; Hwang, J.; Jung, J.; Chang, J.H.; Choi, J.; Kim, H. Exosome-Based Photoacoustic Imaging Guided Photodynamic and Immunotherapy for the Treatment of Pancreatic Cancer. J. Control. Release 2021, 330, 293–304. [Google Scholar] [CrossRef]
- Trifylli, E.-M.; Kriebardis, A.G.; Koustas, E.; Papadopoulos, N.; Vasileiadi, S.; Fortis, S.P.; Tzounakas, V.L.; Anastasiadi, A.T.; Sarantis, P.; Papageorgiou, E.G.; et al. The Arising Role of Extracellular Vesicles in Cholangiocarcinoma: A Rundown of the Current Knowledge Regarding Diagnostic and Therapeutic Approaches. Int. J. Mol. Sci. 2023, 24, 15563. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wijerathne, H.; Godwin, A.K.; Soper, S.A. Isolation and Analysis Methods of Extracellular Vesicles (EVs). Extracell. Vesicles Circ. Nucleic Acids 2021, 2, 80. [Google Scholar] [CrossRef]
- Cao, X.-H.; Liang, M.-X.; Wu, Y.; Yang, K.; Tang, J.-H.; Zhang, W. Extracellular Vesicles as Drug Vectors for Precise Cancer Treatment. Nanomedicine 2021, 16, 1519–1537. [Google Scholar] [CrossRef]
- van der Koog, L.; Gandek, T.B.; Nagelkerke, A. Liposomes and Extracellular Vesicles as Drug Delivery Systems: A Comparison of Composition, Pharmacokinetics, and Functionalization. Adv. Healthc. Mater. 2021, 11, e2100639. [Google Scholar] [CrossRef]
- Sun, H.; Burrola, S.; Wu, J.; Ding, W.-Q. Extracellular Vesicles in the Development of Cancer Therapeutics. Int. J. Mol. Sci. 2020, 21, 6097. [Google Scholar] [CrossRef]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and Directions in Studying Cell–Cell Communication by Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Soori, M.; Arezoo, B.; Dastres, R. Artificial Intelligence, Machine Learning and Deep Learning in Advanced Robotics, a Review. Cogn. Robot. 2023, 3, 54–70. [Google Scholar] [CrossRef]
- Hinestrosa, J.P.; Sears, R.C.; Dhani, H.; Lewis, J.M.; Schroeder, G.; Balcer, H.I.; Keith, D.; Sheppard, B.C.; Kurzrock, R.; Billings, P.R. Development of a Blood-Based Extracellular Vesicle Classifier for Detection of Early-Stage Pancreatic Ductal Adenocarcinoma. Commun. Med. 2023, 3, 146. [Google Scholar] [CrossRef] [PubMed]
- Paproski, R.J.; Pink, D.; Sosnowski, D.L.; Vasquez, C.; Lewis, J.D. Building Predictive Disease Models Using Extracellular Vesicle Microscale Flow Cytometry and Machine Learning. Mol. Oncol. 2023, 17, 407–421. [Google Scholar] [CrossRef] [PubMed]
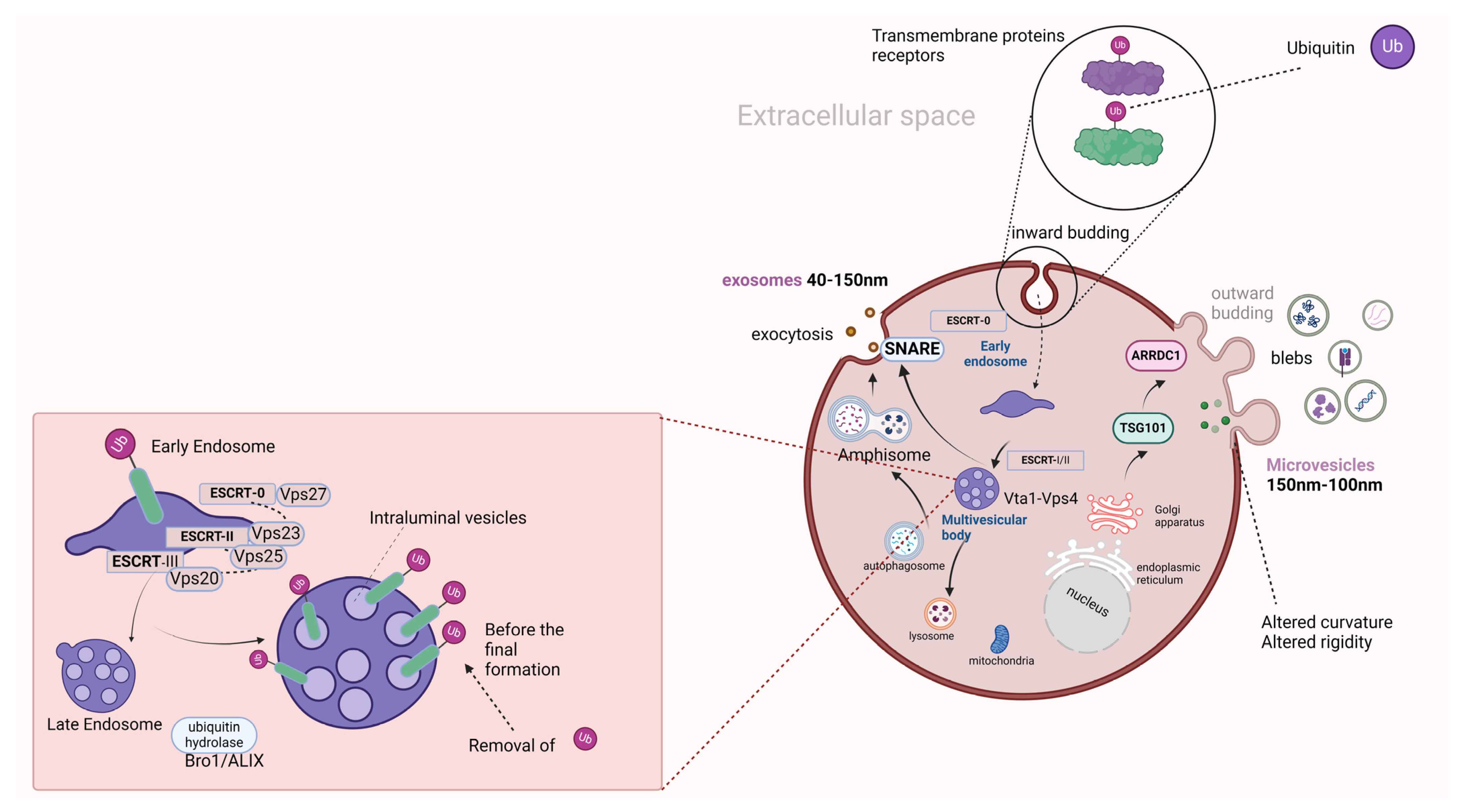
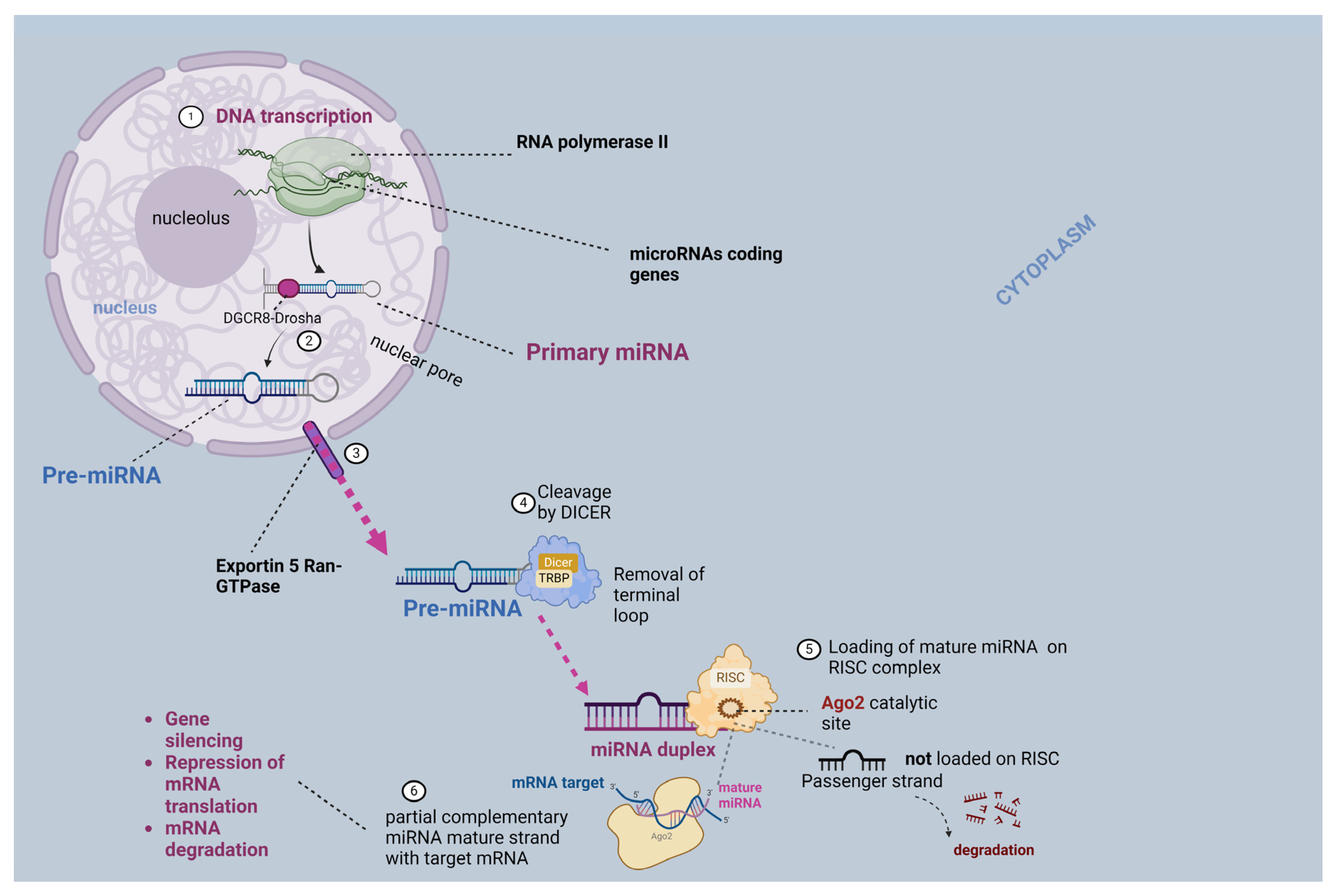
| miRNA | Role in PDAC | Expression Levels |
|---|---|---|
| miR-21 [36,37] | ↑ proliferation via targeting Ras-Raf-MEK-ERK PI3K/AKT EGFR signaling pathways ↓ apoptosis | Increased |
| miR-186 [38] | ↑ proliferation via targeting NR5A2 | Increased |
| miR-17-5p [39] | Decontrolled cell cycle ↑ proliferation via targeting RBL2/E2F4 repressing complexes | Increased |
| miR-196b [40] | ↑ proliferation Apoptosis suppression via targeting CADM1 | Increased |
| mir-18a [41] | Higher expression in pancreatic cancer tissues Lower in postoperative samples Member of miR-17–92 cluster ↑ genome proliferation Transcriptional activation by MYC | Increased |
| miR-191 [42] | Extracellular matrix modification ↑ metastasis | Increased |
| miR-29a [43,44] | Migration and invasion | Increased |
| miR-221 [45] | ↑ proliferation and ↓ apoptosis Metastatic dissemination | Increased |
| mir-301a-3p [46] | ↑ migration and invasion via targeting SMAD4 | Increased |
| miR-374a [47] | ↑ migration and proliferation, EMT via ↓ SRCIN1 | Increased |
| miR-1469-5p [48] | ↑ proliferation and progression via targeting NDRG1/NF-κB/E-cadherin axis | Increased |
| mir-205 [49] | ↑ proliferation Targets APC Implicated in Wnt/β-catenin signaling pathway | Increased |
| miR-10b [50] | ↑ invasive behavior, progression TIP30 expression and ↑ EGF and TGF-β effects | Increased |
| Tumor-Suppressive miRNAs | Role in PDAC | Expression Levels |
|---|---|---|
| miR-506 [51] | ↓ tumor growth and progression | decreased |
| miR-34 [52] | inhibition of the cancer stem cells | decreased |
| miR-142 [53] | ↓ tumor growth and invasion via regulation of HIF-1a expression | decreased |
| miR-216b [54] | Regulation of TC1TP expression KRAS inhibition | decreased |
| miR-30c [55] | ↓ tumor growth and progression G1 phase and apoptosis regulation | decreased |
| miR-143-3p [56] | ↓ malignant transformation of pancreatic cells MERK/ERK signaling suppression | decreased |
| miR-519-3d [57] | ↓ hypoxia-induced tumorigenesis by regulating ICI, PD-L1 | decreased |
| miR-1181 [58] | ↓ tumor invasion and progression | decreased |
| miR-375 [59] | ↑ apoptosis, ↓ lymphatic spread ↓ metastasis | decreased |
| miR-455-3p [60] | ↓ growth, ↑ apoptosis, EMT, regulation of TAZ expression | decreased |
| miR-135a [61] | Growth and progression bmi1 targeting | decreased |
| miR-340 [62] | ↓ tumor growth and progression, regulation of BICD2 expression | decreased |
| miR-203a-3p [63] | ↓ growth, invasion, limitation of EMT, regulation of FGF2 expression | decreased |
| Parental Cell | Cargo | Effect |
|---|---|---|
| PDAC | miR-197-3p miR-6796-3p miR-4750-3p miR-6763-5p | Deregulation of glucose metabolism via GIP and GLP1 altered expression [91] |
| miR-125b-5p miR-450b-3p miR-666-3p miR-883b-5p | Interrupting PI3K/Akt/FoxO1 axis Insulin resistance in C2C12 Myotube cells [91] | |
| miR-540-3p | ||
| hsa-miR-3133 hsa-miR-144-5p hsa-miR-3148 | Insulin resistance and/or DM-associated PDAC [93] | |
| miR-19a | Altered insulin production, via interacting with the Neurod1 protein implicated in DM-associated PDAC [94] | |
| miR-222 | Promotes cancer proliferation, which is correlated with the size and stage of tumor [95] | |
| miR-155 | Suppression of apoptosis GEM chemoresistance Promotion of cancer cell proliferation [96] | |
| miR-125b-5p | Activates the MEK/ERK pathway, promotes PDAC invasion, EMT, metastatic dissemination [97]. | |
| miR-27a | ↑ angiogenesis through BTG2 PDAC invasion, human microvascular endothelial cells (HMVEC) angiogenesis progression and metastasis [98] | |
| CKAP4 O-glycan-binding lectin | ↑ pre-operative levels ↓ post-operative [100,101] | |
| B2M | Tumor escape phenomenon [102] | |
| EGFR KRAS CD44 | PDAC progression Shorter overall and progression-free survival (↑ EV-KRAS) [102] | |
| CAV1 CLU | Over-proliferation Impaired apoptosis of pancreatic cancer cells [102] | |
| ITGA3 PODX | PDAC progression, migration, invasion of surrounding tissue [102,103,104,105,106] | |
| Tspan8 Integrins CD151 | Stromal changes, promoting cell motility, invasion and metastatic capacity [107] | |
| ITGΒ5 S100A4 ANXA1 F3 | Facilitate the metastatic dissemination [102,109] (pre-metastatic niche) | |
| STAT14 LAMP1 Lin28B | [102] | |
| MIF GFP + EVs | ↑ MIF in early stages of PDAC progression and ↑ cytokines in patients at stage I, who will develop liver metastasis [110,111]. Distant metastasis in mice Increased vascular permeability and induction of HUVEC cells in vitro [112]. | |
| GPC1 | Poor prognosis and decreased survival [113,114]. | |
| EphA2 | ↑ proportional to the tumor stage Prediction for neoadjuvant treatment response and possible monitoring tool [115] | |
| MUC1 CLDN1 | PDAC progression and poor prognosis [102,116,117] | |
| HIST2H2BE CD151 CLDN4 LGALS3BP EpCAM | Overall, 73% of exosomes with the selected markers had KRAS mutation [118] | |
| ZIP4 | Oncogenic potential for non-malignant cells found upregulated in highly malignant PDAC [119] | |
| Adrenomedullin | Marker for β-cell destruction in PDAC [120] |
| EV-Origin | Cargo | Recipient Cells | Effect on PDAC TME |
|---|---|---|---|
| PDAC | EVs | T cells | T cell death↑ via p38 MAPK pathway and the initiation of Endoplasmic reticulum stress [124] |
| Integrins | Nk cells | ↓ functionality of NK cells via ↓ expression of INF-γ, CD107a, and TNF-α in NK cells [125] | |
| A3 adenosine receptors | Mast cells | Induction of MAP and ERK1/2 kinases, PDAC progression [126] | |
| ANXA1 | ESCs fibroblasts | Angiogenesis, activation of FPRs [109] Implicated in the Akt/ERK pathway, neoangiogenesis [127] | |
| MiR-27a | ESCs | BTG2 expression—increased proliferation and angiogenesis [98] | |
| hnENPA1 | Lymphangiogenesis and lymphatic metastasis [98] | ||
| EVs | non-malignant pancreatic cells | UPR and ER stress in 24 h via targeting the expression of DDIT3 [129] | |
| palmitic acid | ER stress in physiological pancreatic cells [129] | ||
| EZR | TAMs | Altered macrophage polarization and PDAC progression [130] | |
| miR-155-5p | Interaction with EHF/Akt/NF-kB pathway—altered M2 phenotype [131] | ||
| FGD5-AS1. | Activation of the STAT3/NF-κB pathway, worsening the prognosis in PDAC patients [133] | ||
| Lin28B | PSCs | Recruitment of PSCs via overexpression of PDGFB, promotion of metastasis [132] | |
| miR-125b-5p | stromal | MEK/ERK pathway induces PDAC invasion, EMT, as well as metastatic dissemination [97] | |
| Suppression of STARD13 targeting—deregulation of regulation of interorganelle cholesterol transportation PDAC progression | |||
| LDL receptor-related proteins | Activation of YAP PDAC progression [136] | ||
| CAFs | miR-21 | PDAC cells | Chemoresistance and PDAC progression PTEN inhibited expression [137,138] |
| miR-3173-5p | PDAC cells | GEM resistance in xenograft PDAC mouse model, via ACSL4 gene Suppression of ferroptosis in PDAC cells [139] | |
| ANXA6 | PDAC aggressive behavior via the overexpression of CD9 on the EV surface Induction of MAPK pathway activity, increases EMT, and promotes PDAC expansion [140] | ||
| MiR-106b | GEM resistance via its interaction with TP53INP1 [141,142] | ||
| Blood | EVs | ↑ levels, independently interrelated with increased progression-free survival and PDAC control rate for patients under chemotherapy [144] | |
| WBC | EVs | ↑ levels in unresectable or borderline resectable PDAC patients—an independent factor for increased overall survival [144] | |
| PSCs | miR-451a miR-21-5p | Promotion of the PDAC progression and expansion via promoting the expression of CCL1, and CCL2 [145] | |
| TAMs | miR-21a-5p miR-501-3p miR-301a-3p | PDAC progression [90] Interaction with TGFBR3 via the activation of the TGF-β pathway [146,147], M2 phenotypic state promotes PDAC progression to metastasis via the PTEN/PI3Kγ pathway [148] | |
| PDAC | TF | Promotion of thrombosis in PDAC patients [154] | |
| circPDK1 | HIF1A induces their secretion, PDAC cell survival, and migration via c-myc stimulation, promoting glycolysis in vivo [158] | ||
| BxR-CSCs | miR-210 | PANC-1/BxS | Resistance to GEM [160] |
| GIPC-depleted PANC1 or AsPC-1 | ABCG2 | PDAC | Resistance to GEM [161] |
| PANC1 | EphA2 | BxPC-3/MiaPaCa2 | Resistance to GEM [115,162] |
| PANC1, MiaPaCa2 | miR-155 | Resistance to GEM [96,162] | |
| BxPC-3-Gem cells | MMP14 | BxPC-3/MiaPaCa2 | Resistance to GEM [162] |
| CAF | Snail-Mrna miR-106b | ECs | resistance to GEM [138,162] |
| PDAC GEM-treated | miR-155 CAT SOD2 | resistance to GEM in vitro [95,168] | |
| PDAC | miR-155 | PDAC cells | Implicated in GEM metabolism and inactivation, via suppressing the GEM-metabolizing enzyme DCK in PDAC cells [165] |
| ZIP4 | Leading to cachexia [166], induce PDAC progression [119] |
| Role | Sample | Cargo Family | Cargo | Characteristics |
|---|---|---|---|---|
| Diagnosis | PJ | Proteins | MDR1, MUC 1, MUC16, MUC5AC, MUC6, MUC4, CFTR | Differentiation of PDAC from other benign and premalignant pancreatic diseases (diagnostic accuracy of up to 91%) [169] |
| PJ | Proteins | Overregulated ARF3, MSN, OLFM4, PK, MUC, Ras, LITAF | Panel from EV-proteins from PJ collected by EUS-FNA. In the early PDAC stages (I–II), MSN, CD55, and Ras proteins were notably overexpressed, with Ras being overexpression in PDAC stages III and IV compared to AIP patients and HC [170] | |
| PJ | MiRNA | miR-155 miR-21 | Notably elevated in patients with PDAC, compared to patients with CP [171] | |
| Diagnosis | Serum | Proteins | CD61, CD41, CD63 | ↑ levels of PLT-EVs, CD61+, CD41+, and CD63+ EVs are higher in PDAC patients than in HC (AUC 0.846) Combination with CA-19-9 (AUC 0.903 versus CA19-9 alone AUC: 0.814) [172] |
| CD82+, GPC1 | Combined with CA19-9 for early diagnosis (AUC: 0.942) [173] | |||
| Proteins | c-met | ↑ EV-c-met: specificity of 85% and sensitivity of 70% for PDAC diagnosis [174] | ||
| ADAM8 | ↑ EV-ADAM8 correlated with pre-malignant pancreatic lesions and PDAC vs. HC [175] | |||
| ANXA6 | ↑ EV-ANXA6 has AUC of 0.979 for detecting PDAC [176] | |||
| ZIP4 | ↑ EV-ZIP4 has great diagnostic efficacy (AUC: 0.893) [176] | |||
| GPC1, LRG-1 | Presents a great diagnostic performance, even for early-stage PDAC tumors with an AUC of 0.95 [177] | |||
| Rab5, D63. | Detected by ExoChip, as potent PDAC diagnostic tools [178] | |||
| Serum | miRNA | miR-21 miR-17-5p miR-1226-3p miR-451a miR-191 miR-21 | ↑ EV-miR-21 for PDAC detection with AUC: 0.897 ↑ EV-miR-17-5p for PDAC detection (AUC: 0.887) [179] ↓ EV-miR-1226-3p for PDAC detection with AUC: 0.74 [180]. ↑ EV-miR-451a (AUC: 0.759), ↑ EV-miR-191(AUC: 0.788) and ↑ EV-miR-21 (AUC: 0.826) for PDAC and IPMN detection [181] | |
| miR-4306, miR-1246, miR-3976, miR-4644 | Increased level of the panel EV-miRNAs in the majority of PDAC patients (83%) with an AUC: 0.80 [182] | |||
| mRNA | GPC-1 mRNA | ↑ EVs-GPC-1 mRNA in PDAC patients, independently of the stage (AUC: 1.00) [183] | ||
| lncRNA | CRNDE MALAT-1 HULC | ↑ EV-lncRNA with CRNDE or MALAT-1 were significantly increased in PDAC patients [176] ↑ EV-lncRNA HULC in IPMN or PDAC patients, in comparison with healthy controls, with great specificity (AUC: 0.92) [184] | ||
| Diagnosis | Plasma | miRNA | miR-205-5p miR-122-5p miR-125b-3p miR-10b miR-451a, miR-196a miR-451a | ↑ EV-miR-205-5 p for PDAC detection with AUC of 0.857 ↑ EV-miR-122-5p for PDAC detection with AUC of 0.814 ↑ EV-miR-125b-3p for PDAC detection with AUC of 0.782 ↑ EV-miR-10b for PDAC detection with AUC of 0.81; former has been increased in PDAC patients, compared to healthy controls or CP patients [186] Increased levels for PDAC detection (AUC: 0.81) ↑ EV-miR-451a n PDAC patients, compared to HC or CP patients in early-stage PDAC (I–II) and has proven statistically significant for the discrimination between stage I and II (p-value of 0.041) [187,188] |
| Diagnosis | Plasma | miRNA | miR-196a | ↑ EV-miR-196a in early-stage PDAC patients (I–II stages) [188] |
| miR-30c, miR-10 b, miR-let7a, miR-21, miR-181 | Up-regulated, except EV-miR-let7a, differentiating the benign pancreatic disease (CP) and healthy donors from PDAC patients (AUC: 1.00) [189] | |||
| Plasma | miRNAs, mRNAs | CK18 mRNA CD63 mRNA EV-miR.409 | Multianalyte panel consisting of CA-19-9, cell-free DNA, EV-miRNAs, and EV-mRNAs has a great specificity, sensitivity, and accuracy of 95%, 88%, and 92%, respectively [190]. | |
| miRNA | has-miR-409 | Diagnostic tool for PDAC with an AUC of 0.93 [190] | ||
| mRNAs | CK18 and CD63 mRNAs | Increased in PDAC cases with an AUC of 0.93 [190] | ||
| Plasma | Long RNAs | TIMP1, FGA, HIST1H2BK, CLDN1, ITIH2, MAL2, MARCH 2, KRT19 | Presented in PDAC with AUC of 0.949 [191] | |
| circRNA | IARS | Overexpressed in PDAC tissues [192] | ||
| Protein | EpCAM, GCP-1, CD44V6 | Panel for PDAC detection with AUC of 1.00 [193] | ||
| Mutant protein | Mutant KRAS and/or P53 | Mutant proteins KRAS and/or P53 in EVs were detected in early-stage PDAC patients [194] | ||
| Plasma | Protein | GPC1 | EV-GPC1 alone do not have high AUC (0.59) for PDAC detection [114] | |
| Plasma | EphA2 EpCAM MIF WNT2, EpCAM, MUC1, GPC1 and EGFR | Detection of PDAC from HC [195] Panel presenting a specificity and sensitivity of 81% and 86%, respectively [196] | ||
| ALPPL2 | Diagnostic biomarker for PDAC, as it was demonstrated in PDAC cell culture media [197] | |||
| Blood | Protein | CD63, GPC1 | ↑ EV-CD63 and ↑ EV-GPC1 levels in PDAC blood samples, with a high sensitivity of detecting PDAC (99%) and a specificity of 82% [198] | |
| Saliva | miRNA | miR-1246 miR-4644 | ↑ EV-miR-1246 levels in PDAC patients with AUC of 0.814 ↑ EV-miR-4644 levels in PDAC patients (AUC of 0.763) [199] | |
| Saliva | mRNA | Incenp, Apbb1ip, BCO31781, Foxp1, Aspn, Daf2, Gng2 | Significantly increased in PDAC mice models [200] | |
| Prognosis | Serum and plasma | Protein and DNA Lipid and miRNA | EpCAM (serum) KRAS- DNA (plasma) phosphatidylethanolamine miR-45 | The levels of these biomarkers did not increase the mortality in non-resectable PDAC patients The levels of these biomarkers were correlated with increased mortality risk in resectable PDAC cases, facilitating the stratification of these patients, in which systemic treatment is advantageous [204] |
| Protein and miRNA | EpCAM, miR-200b, mir-222, miR-451a | ↑ pre-operative expression levels t are correlated with ↓ survival [204] | ||
| KRAS | KRAS MAF ≥ 1% level during chemotherapy is correlated with PDAC progression before the increase in CA19-9 or the presence of radiological findings (≈50 days before) [204] | |||
| Blood | protein | Integrin α6 | Increased levels in clinical recurrence (even months before the time of recurrence) and decreased post-operatively [205] | |
| Prognosis | Plasma | lncRNA | Sox2ot | ↑ EV-lncRNA Sox2ot in PDAC progression, vascular, and lymphatic dissemination [206] |
| circRNA | PDE8A | Overexpressed in PDAC [207] | ||
| Plasma | circRNA | IARS | Increased in advanced (metastatic) PDAC cases (p-value of 0.002) [208] | |
| miRNA | miR-222 | EV-miR-222 from PDAC patients promote tumor invasion and metastatic dissemination, and ↓ survival in PDAC cell cultures [95] | ||
| protein | MIF GPC1, EpCAM EV-CD44V6 | ↑ EV-MIF are correlated to PDAC patients with no liver metastasis [209] ↑ EV-GPC-1 levels are correlated to tumor burden/size [201] ↑ EV-EpCAM levels are associated with the treatment response during systemic, palliative chemotherapy in advanced PDAC stage [210] ↑ EV-CD44V6 are associated with tumor size [193] | ||
| Plasma | Protein | EpCAM, GCP-1, CD44V6 | Plasma panel for prognosis (tumor stage) and diagnosis (AUC: 1.00) [193] | |
| Serum | C1QBP, CD44V6 | ↑ EV-C1QBP and EV-CD44V6 have been closely associated with prognosis in PDAC patients and for liver metastatic disease [211] |
| EV-Based Modality | Cargoes | Therapeutic Target | Characteristics |
|---|---|---|---|
| Vector for bioactive molecules | Short hairpin or interfering RNA molecule | Mutant GTPase KRAS, | Engineered EVs from physiological fibroblast-like loaded with a short hairpin or interfering RNA molecule for KRAS mutation. PDAC suppression was reported in xenografts (mice) [213]. |
| SiRNA (KRASG12D-targeting siRNA) or therapeutic agents (DOXO) | PDAC cells with KRASG12D expression | Mesenchymal stromal cells of the human umbilical cord (UC-MSC-derived EVs) that target the PDAC cells, resulting in a notable decrease in the expression of KRASG12D. The uptake of KRASG12D siRNA EVs by the cell lines induced the apoptosis of the lines that were expressing KRASG12D (the LS180 and PANC-1). Improvement of survival and enhancement of the anti-cancer immune response [214]. | |
| galectin-9 siRNA and OXA | TAMs | Bone marrow mesenchymal stem cell (BM-MSC) exosomes, which can be loaded with galectin-9 siRNA and prodrug oxaliplatin (OXA). Inhibition of the immunosuppressive effect of TAMs interrupting the axis of galectin-9/dectin [215]. OXA–related deregulation of Tregs and recruitment T-cytotoxic cells in the TME (reprogramming) [215]. | |
| Drug vector | GEM | PDAC cells | GEM-loaded autologous exosomes. PDAC growth was significantly inhibited. Mice survival was elongated, with dose-related results, and more targeted and less toxic treatment [216]. |
| Pirfenidone | Anti-fibrotic agent in pre-metastatic niches. Delivery of Pirfenidone-loaded exosomes derived from PDAC cells. Significantly suppresses liver metastasis [217]. | ||
| EV as target | Agents for EV targeting: tetraacetylated N-azidoacetyl-d-mannosamine-loaded nanoparticles and modified nanoparticles (dibenzyl-cyclootyne). Selective HDAC1/2 inhibitor (B390) | PDAC cells EV-VEGF-C | Tetraacetylated N-azidoacetyl-d-mannosamine-loaded nanoparticles target and tag with azides the PDAC cells and their EVs [218]. Modified nanoparticles (dibenzyl-cyclootyne) inhibit the proliferation of PDAC cells and EV secretion [218]. Suppression of EV-VEGF-C expression by selective HDAC1/2 inhibitor (B390), and suppression of lymphangiogenesis and lymphatic tumor dissemination [219]. |
| Exosome inhibitor GW4869 | EV-mir-21 | PTEN expression restored in vivo and in vitro suppression of PDAC progression and chemoresistance [220]. | |
| Inhibition of CD9-positive ANXA6-EVs | ANXA6-EVs | Reduced the stromal modification and tumor progression [140]. | |
| Photodynamic therapy and immunotherapy | clorin e6 | PDAC cells | PDAC-derived CeR-R exo increases the photosensitivity of PDAC [221]. Stimulatory effect on immune cells, via inducing cytokine release by immune cells. Increase in reactive oxygen species in PDAC cells, under the effect of the irradiation laser [220]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifylli, E.M.; Kriebardis, A.G.; Koustas, E.; Papadopoulos, N.; Fortis, S.P.; Tzounakas, V.L.; Anastasiadi, A.T.; Sarantis, P.; Vasileiadi, S.; Tsagarakis, A.; et al. A Current Synopsis of the Emerging Role of Extracellular Vesicles and Micro-RNAs in Pancreatic Cancer: A Forward-Looking Plan for Diagnosis and Treatment. Int. J. Mol. Sci. 2024, 25, 3406. https://doi.org/10.3390/ijms25063406
Trifylli EM, Kriebardis AG, Koustas E, Papadopoulos N, Fortis SP, Tzounakas VL, Anastasiadi AT, Sarantis P, Vasileiadi S, Tsagarakis A, et al. A Current Synopsis of the Emerging Role of Extracellular Vesicles and Micro-RNAs in Pancreatic Cancer: A Forward-Looking Plan for Diagnosis and Treatment. International Journal of Molecular Sciences. 2024; 25(6):3406. https://doi.org/10.3390/ijms25063406
Chicago/Turabian StyleTrifylli, Eleni Myrto, Anastasios G. Kriebardis, Evangelos Koustas, Nikolaos Papadopoulos, Sotirios P. Fortis, Vassilis L. Tzounakas, Alkmini T. Anastasiadi, Panagiotis Sarantis, Sofia Vasileiadi, Ariadne Tsagarakis, and et al. 2024. "A Current Synopsis of the Emerging Role of Extracellular Vesicles and Micro-RNAs in Pancreatic Cancer: A Forward-Looking Plan for Diagnosis and Treatment" International Journal of Molecular Sciences 25, no. 6: 3406. https://doi.org/10.3390/ijms25063406
APA StyleTrifylli, E. M., Kriebardis, A. G., Koustas, E., Papadopoulos, N., Fortis, S. P., Tzounakas, V. L., Anastasiadi, A. T., Sarantis, P., Vasileiadi, S., Tsagarakis, A., Aloizos, G., Manolakopoulos, S., & Deutsch, M. (2024). A Current Synopsis of the Emerging Role of Extracellular Vesicles and Micro-RNAs in Pancreatic Cancer: A Forward-Looking Plan for Diagnosis and Treatment. International Journal of Molecular Sciences, 25(6), 3406. https://doi.org/10.3390/ijms25063406










