Vascular Endothelial Cell-Derived Exosomal Sphingosylphosphorylcholine Attenuates Myocardial Ischemia–Reperfusion Injury through NR4A2-Mediated Mitophagy
Abstract
1. Introduction
2. Results
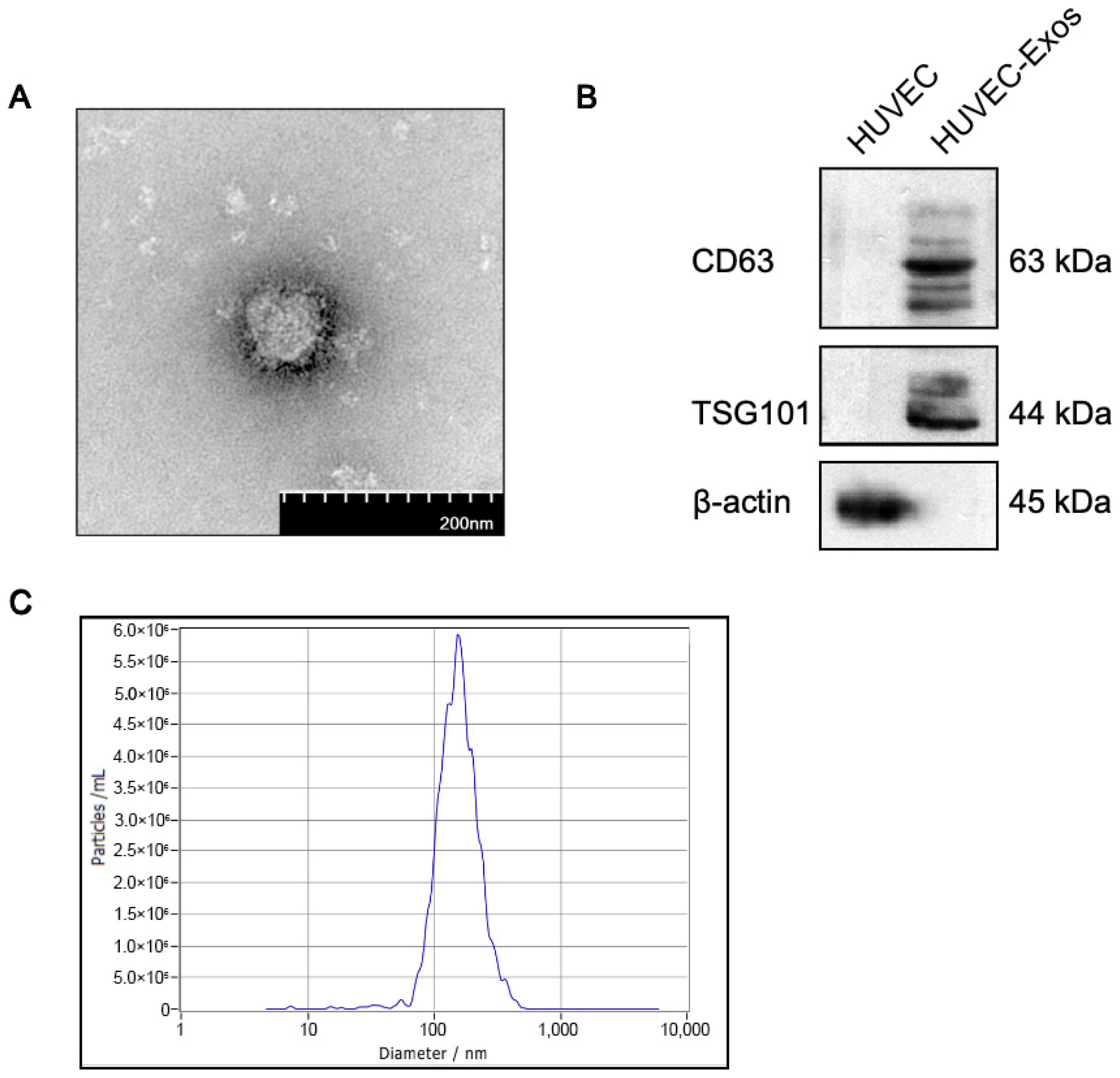
2.1. Isolation and Characterization of Human Umbilical Vascular Endothelial Cell-Derived Exosomes (HUVEC-Exos)
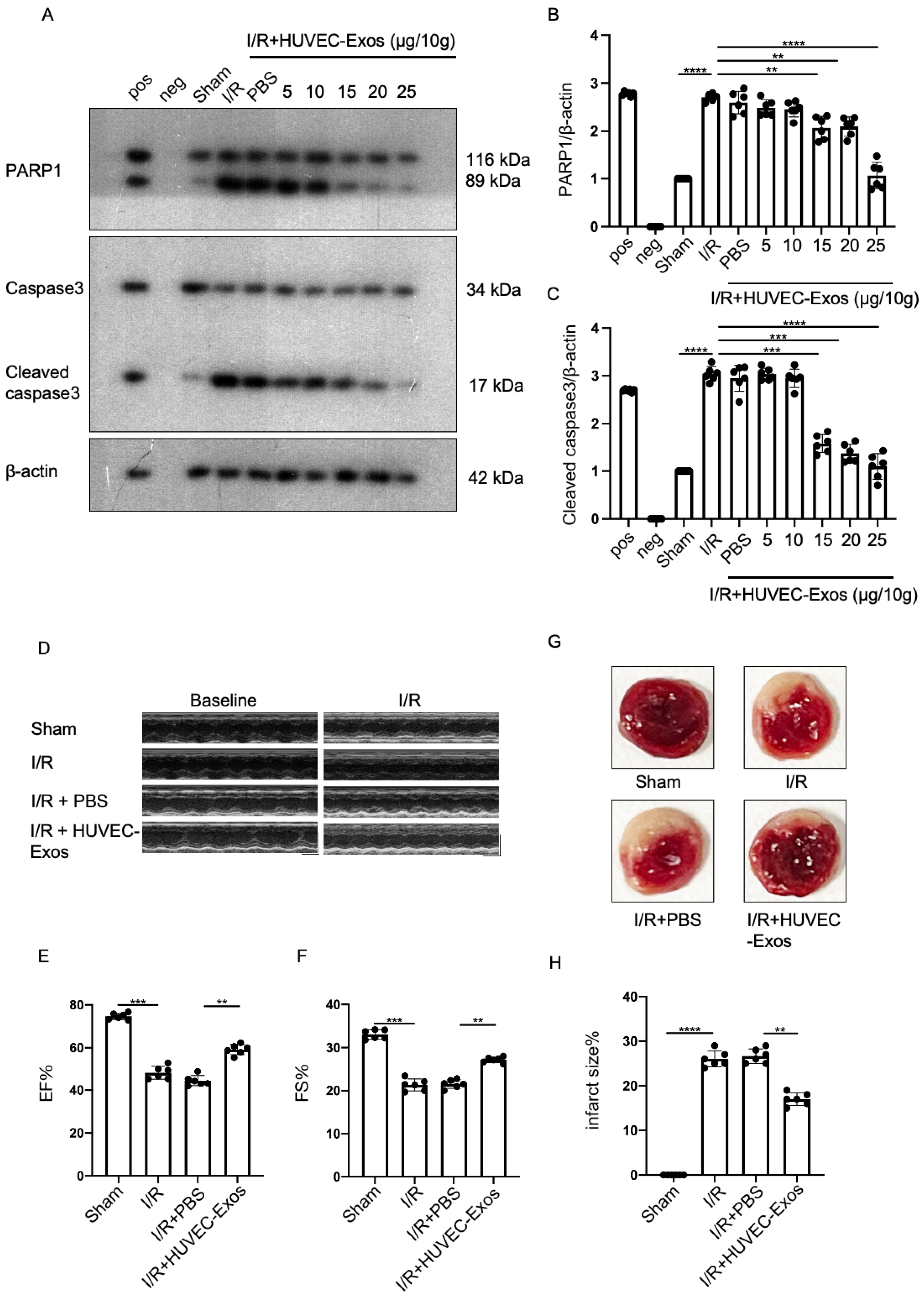
2.2. HUVEC-Exos Inhibit Myocardial Ischemia/Reperfusion (I/R) Injury
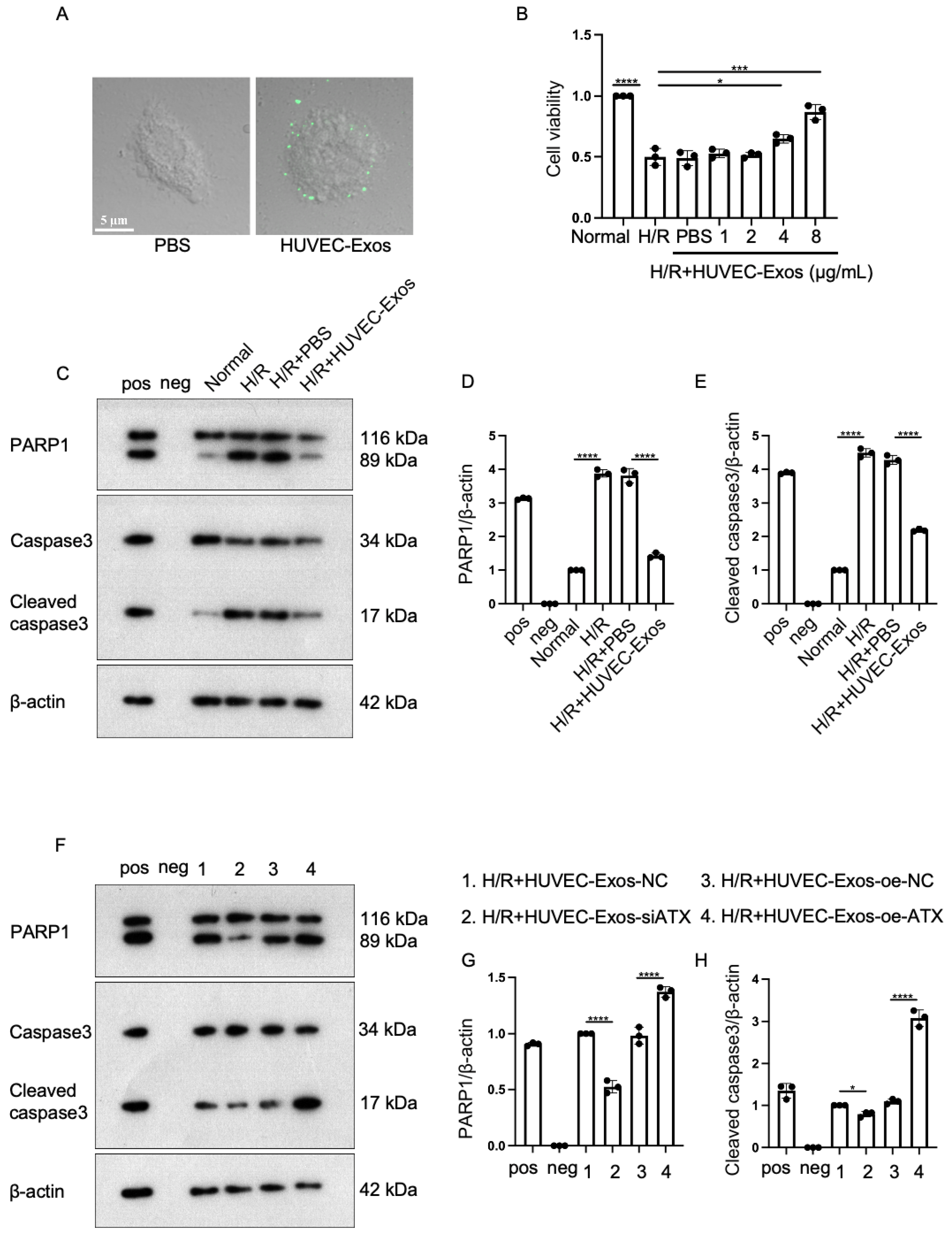
2.3. Effect of Sphingosylphosphorylcholine (SPC) Upregulation and Downregulation in HUVEC-Exos
2.4. Exosomal SPC Inhibits Apoptosis in the Hypoxia/Reoxygenation (H/R)-Induced Cardiomyocyte Injury Model
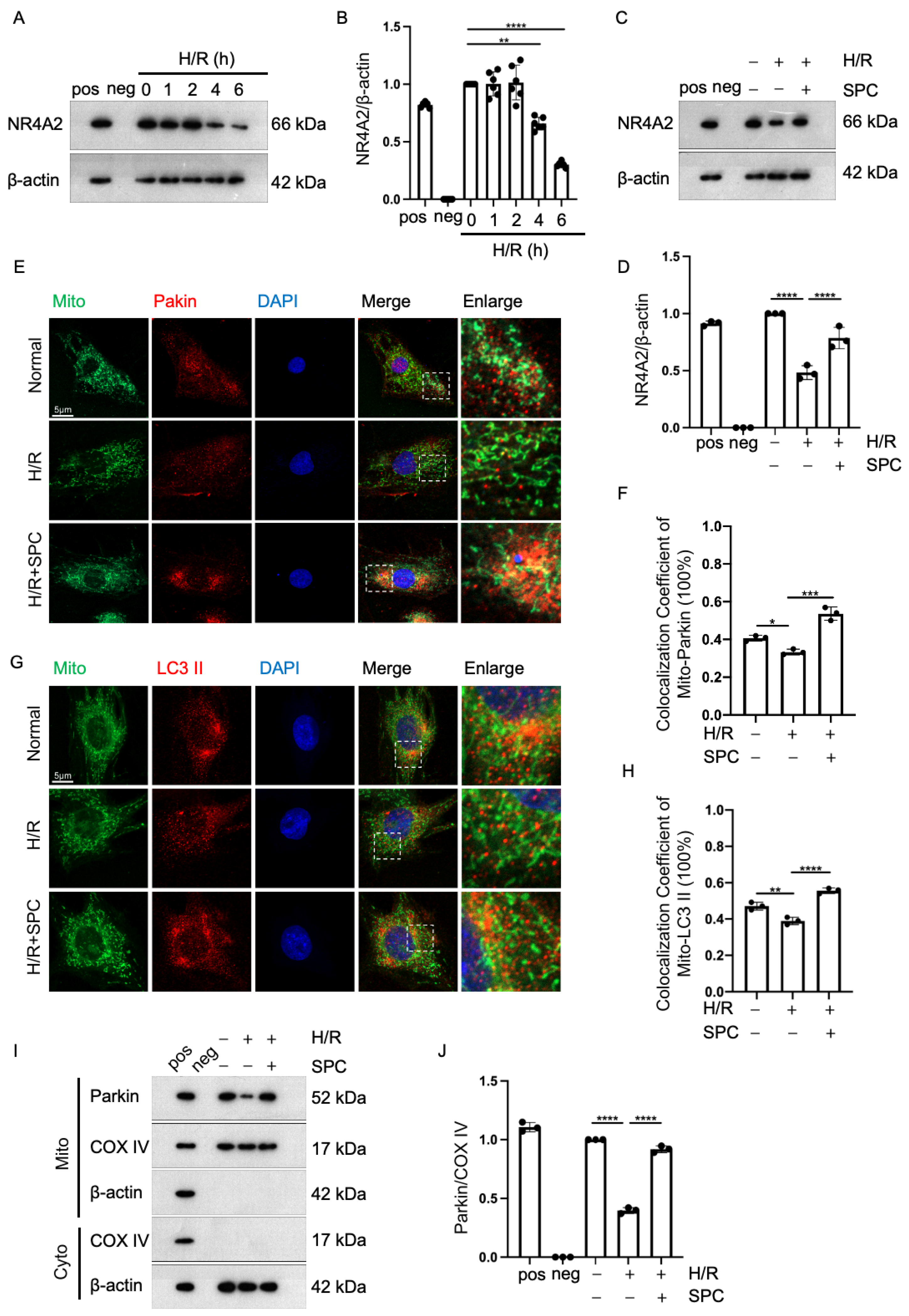
2.5. SPC Exerts Cardioprotective Effects against I/R Injury by Promoting Mitophagy through NR4A2
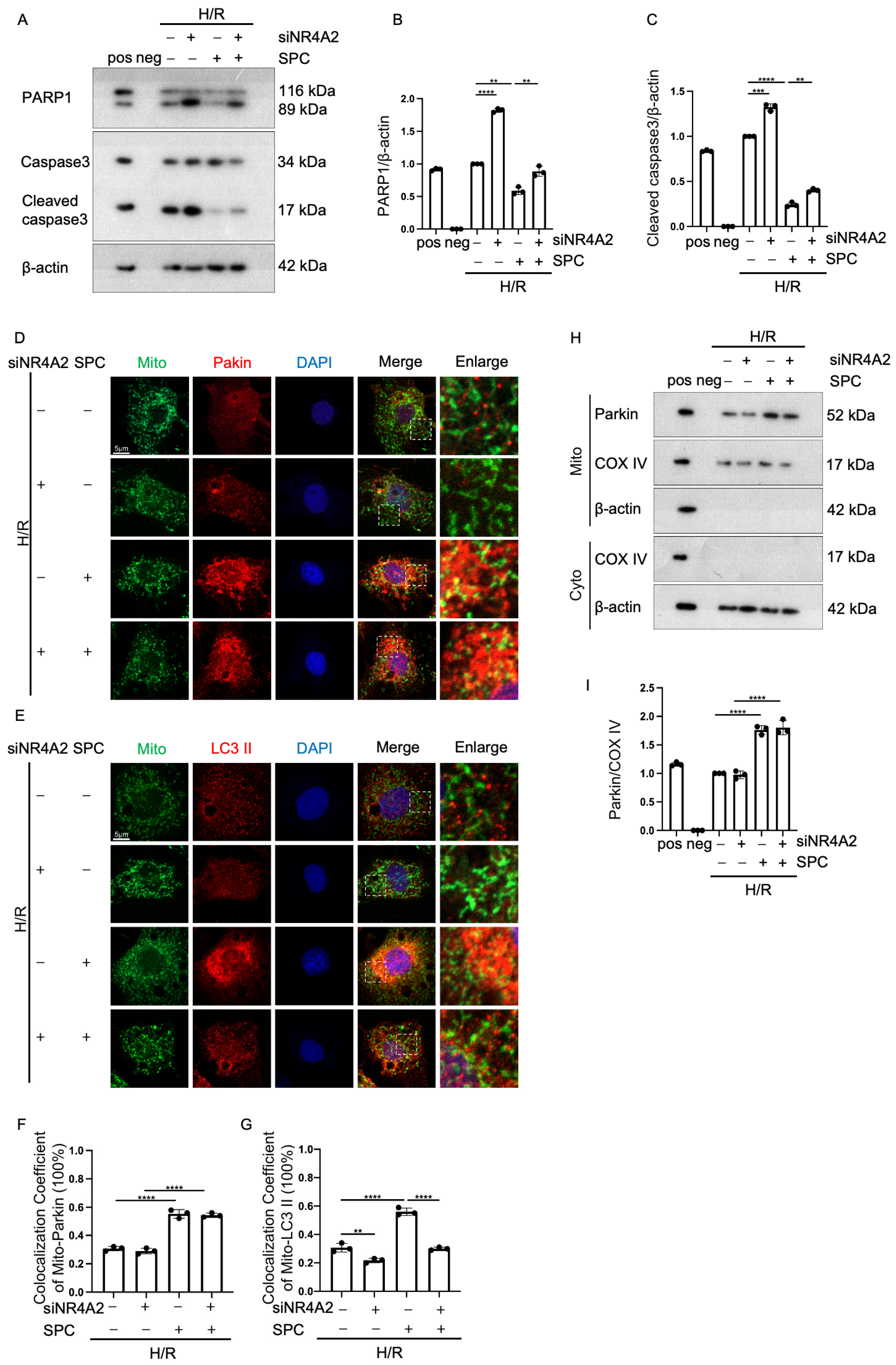
2.6. NR4A2 Knockdown Mitigates the Cardioprotective Effect of SPC
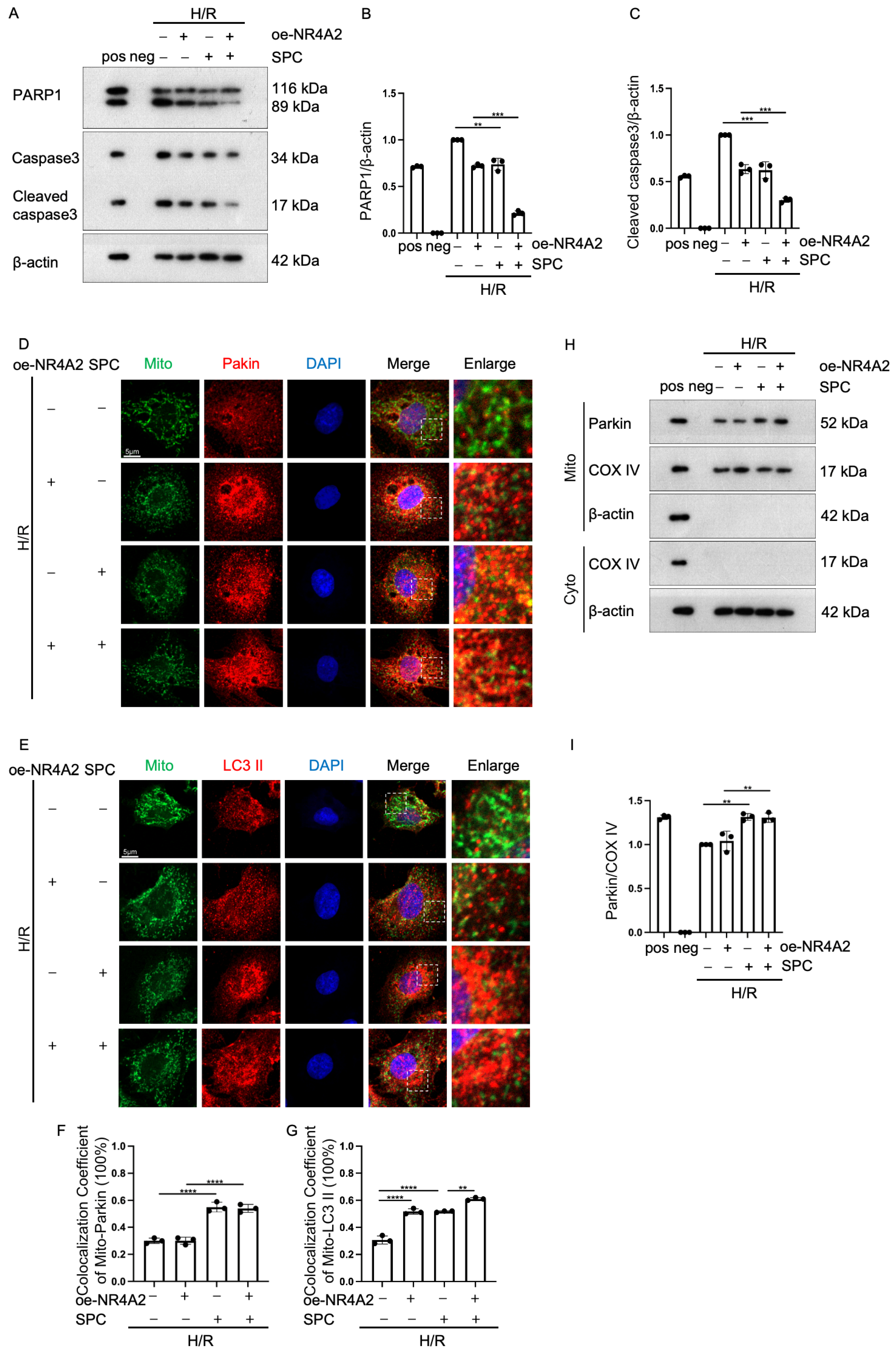
2.7. NR4A2 Overexpression Potentiates SPC-Mediated Protective Effects
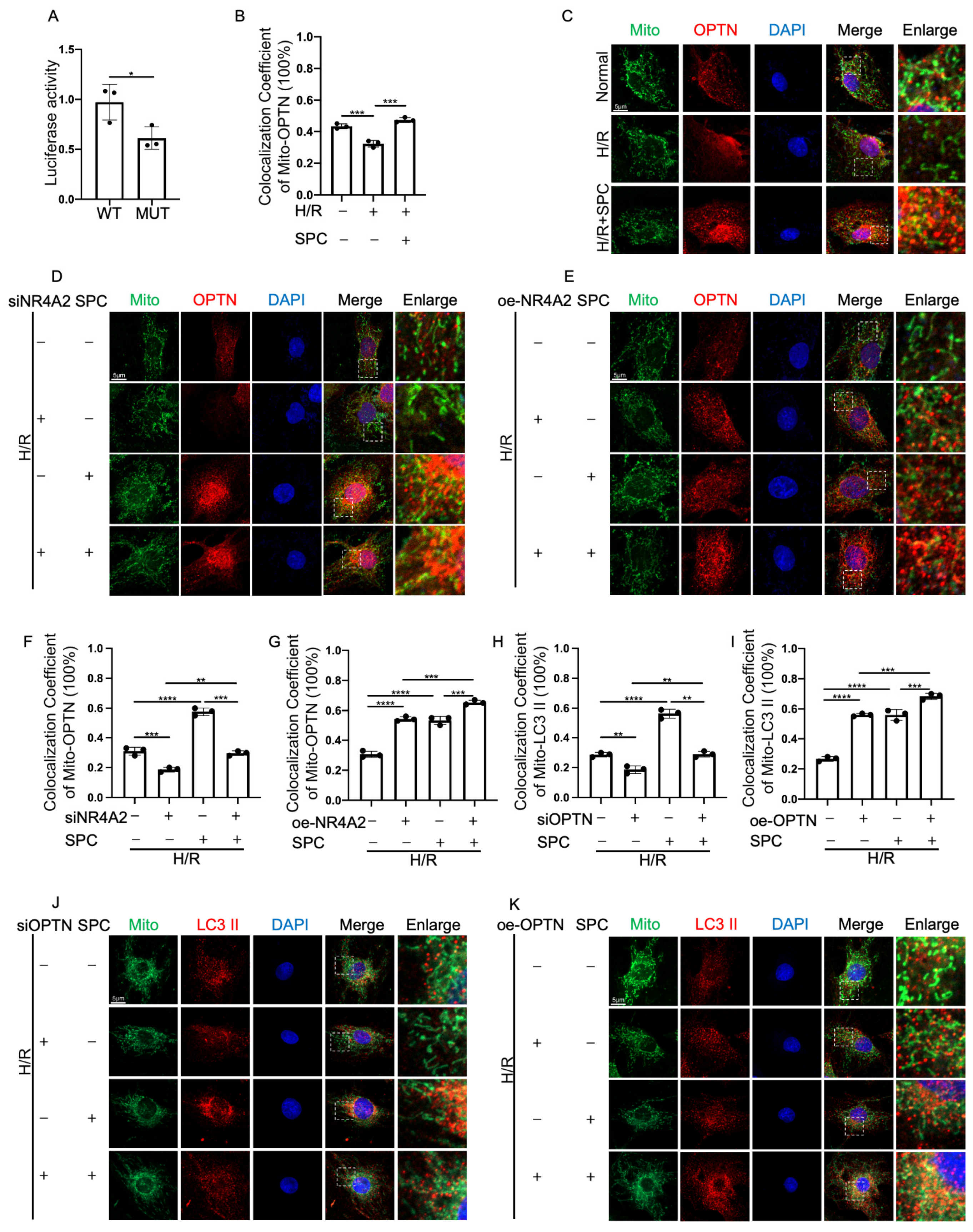
2.8. NR4A2 Promotes Mitophagy through OPTN
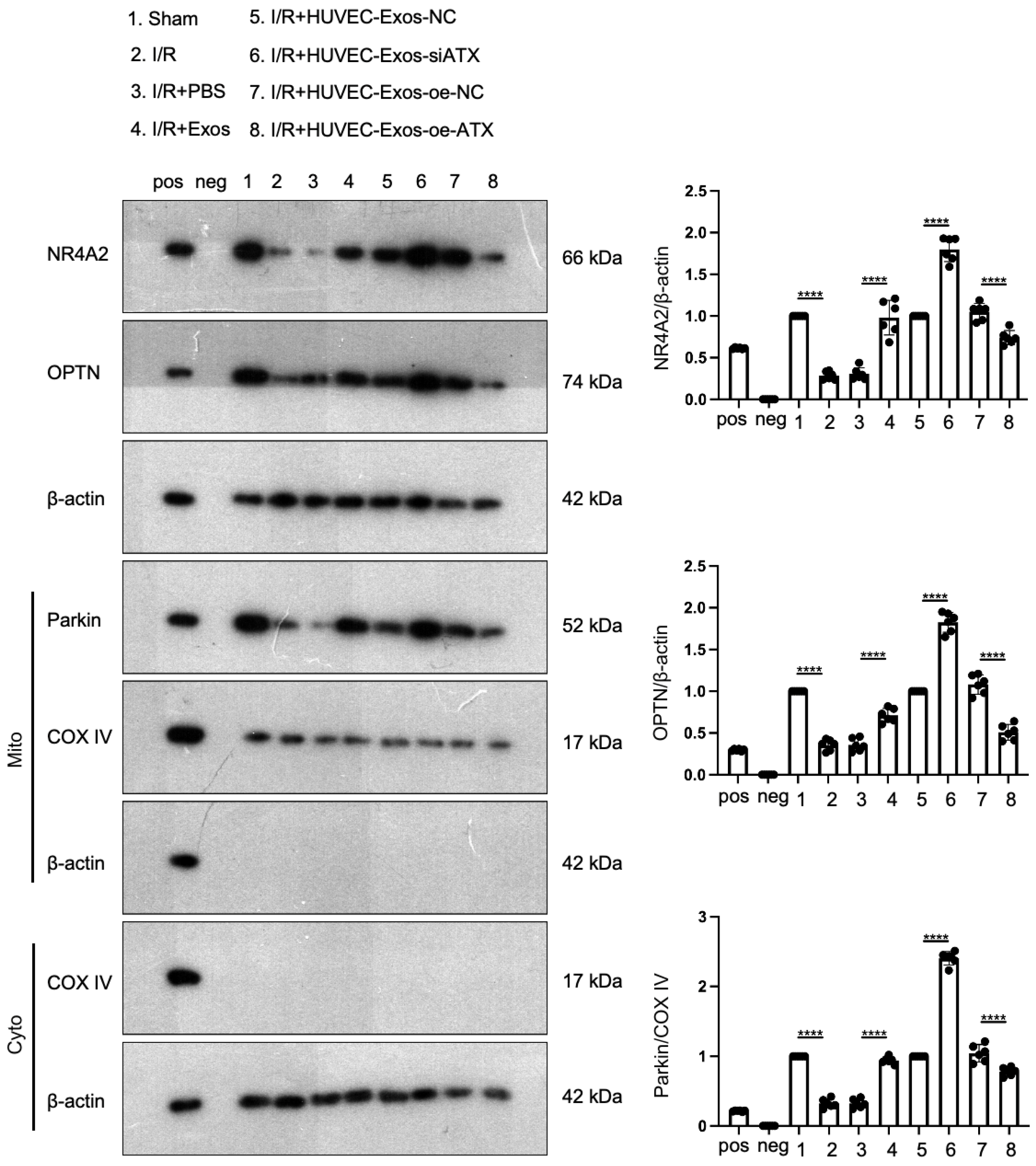
2.9. SPC in HUVEC-Exos Upregulates the Expression of NR4A2, OPTN, and Parkin in the Myocardial Tissues of I/R Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Construction of the Myocardial I/R Injury Mice Model
4.4. Tetrazolium Chloride (TTC) Staining
4.5. Echocardiography
4.6. Establishment of the H/R Cell Model
4.7. Isolation and Identification of HUVEC-Exos
4.8. Cardiac Lipid Extraction
4.9. Purification of SPC Using HPLC
4.10. Western Blotting Analysis
4.11. Mitophagy Assay
4.12. Dual-Luciferase Reporter Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATX | Autotaxin |
| H/R | Hypoxia/reoxygenation |
| H9c2 | Rat cardiomyoblasts cell line |
| HeLa | Henrietta lacks cells |
| HUVEC | Human umbilical vein endothelial cells |
| I/R | Ischemia/reperfusion |
| LVEF | Left ventricular ejection fraction |
| LVFS | Left ventricular fraction shortening |
| NR4A2 | Nuclear receptor subfamily 4 group A member 2 |
| OPTN | Optineurin |
| SPC | Sphingosylphosphorylcholine |
| TEM | Transmission electron microscopy |
References
- Lindahl, B.; Mills, N.L. A new clinical classification of acute myocardial infarction. Nat. Med. 2023, 29, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, L.; Wang, Z.; Zhang, J.; Zhou, Z. Myocardial ischemia-reperfusion injury; Molecular mechanisms and prevention. Microvasc. Res. 2023, 149, 104565. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Lu, Y.; Xin, L.; Gao, J.; Shang, C.; Jiang, Z.; Lin, H.; Fang, X.; Qu, Y.; Wang, Y.; et al. Role of Oxidative Stress in Reperfusion following Myocardial Ischemia and Its Treatments. Oxid. Med. Cell Longev. 2021, 2021, 6614009. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.; Cordaro, M.; Crupi, R.; Siracusa, R.; Campolo, M.; Bruschetta, G.; Fusco, R.; Pugliatti, P.; Esposito, E.; Cuzzocrea, S. Protective Effects of Ultramicronized Palmitoylethanolamide (PEA-um) in Myocardial Ischaemia and Reperfusion Injury in VIVO. Shock 2016, 46, 202–213. [Google Scholar] [CrossRef]
- Soppert, J.; Lehrke, M.; Marx, N.; Jankowski, J.; Noels, H. Lipoproteins and lipids in cardiovascular disease: From mechanistic insights to therapeutic targeting. Adv. Drug Deliv. Rev. 2020, 159, 4–33. [Google Scholar] [CrossRef]
- Pardhi, E.; Yadav, R.; Chaurasiya, A.; Madan, J.; Guru, S.K.; Singh, S.B.; Mehra, N.K. Multifunctional targetable liposomal drug delivery system in the management of leukemia: Potential, opportunities, and emerging strategies. Life Sci. 2023, 325, 121771. [Google Scholar] [CrossRef] [PubMed]
- Saez, V.; Souza, I.D.L.; Mansur, C.R.E. Lipid nanoparticles (SLN & NLC) for delivery of vitamin E: A comprehensive review. Int. J. Cosmet. Sci. 2018, 40, 103–116. [Google Scholar] [CrossRef]
- Wang, H.; Ordoubadi, M.; Connaughton, P.; Lachacz, K.; Carrigy, N.; Tavernini, S.; Martin, A.R.; Finlay, W.H.; Lechuga-Ballesteros, D.; Vehring, R. Spray Dried Rugose Lipid Particle Platform for Respiratory Drug Delivery. Pharm. Res. 2022, 39, 805–823. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Wang, H.; Xie, Y.; Salvador, A.M.; Zhang, Z.; Chen, K.; Li, G.; Xiao, J. Exosomes: Multifaceted Messengers in Atherosclerosis. Curr. Atheroscler. Rep. 2020, 22, 57. [Google Scholar] [CrossRef] [PubMed]
- Kudo, K.; Miki, Y.; Carreras, J.; Nakayama, S.; Nakamoto, Y.; Ito, M.; Nagashima, E.; Yamamoto, K.; Higuchi, H.; Morita, S.Y.; et al. Secreted phospholipase A2 modifies extracellular vesicles and accelerates B cell lymphoma. Cell Metab. 2022, 34, 615–633.e8. [Google Scholar] [CrossRef] [PubMed]
- Ghadami, S.; Dellinger, K. The lipid composition of extracellular vesicles: Applications in diagnostics and therapeutic delivery. Front. Mol. Biosci. 2023, 10, 1198044. [Google Scholar] [CrossRef] [PubMed]
- Aswad, H.; Forterre, A.; Wiklander, O.P.; Vial, G.; Danty-Berger, E.; Jalabert, A.; Lamaziere, A.; Meugnier, E.; Pesenti, S.; Ott, C.; et al. Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice. Diabetologia 2014, 57, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Mu, J.; Tseng, M.; Wattenberg, B.; Zhuang, X.; Egilmez, N.K.; Wang, Q.; Zhang, L.; Norris, J.; Guo, H.; et al. Enterobacteria-secreted particles induce production of exosome-like S1P-containing particles by intestinal epithelium to drive Th17-mediated tumorigenesis. Nat. Commun. 2015, 6, 6956. [Google Scholar] [CrossRef]
- Xiang, X.; Poliakov, A.; Liu, C.; Liu, Y.; Deng, Z.B.; Wang, J.; Cheng, Z.; Shah, S.V.; Wang, G.J.; Zhang, L.; et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int. J. Cancer 2009, 124, 2621–2633. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Yue, H.W.; Liu, H.H.; Zhao, J. Emerging roles of sphingosylphosphorylcholine in modulating cardiovascular functions and diseases. Acta Pharmacol. Sin. 2018, 39, 1830–1836. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, S.Y.; Kim, S.D.; Shim, J.W.; Kim, H.J.; Jung, Y.S.; Kwon, J.Y.; Baek, S.H.; Chung, J.; Bae, Y.S. Sphingosylphosphorylcholine stimulates CCL2 production from human umbilical vein endothelial cells. J. Immunol. 2011, 186, 4347–4353. [Google Scholar] [CrossRef]
- Lee, H.Y.; Cho, K.M.; Kim, M.K.; Lee, M.; Kim, H.; Choi, C.Y.; Kim, K.K.; Park, J.S.; Kim, H.H.; Bae, Y.S. Sphingosylphosphorylcholine blocks ovariectomy-induced bone loss by suppressing Ca2+/calmodulin-mediated osteoclast differentiation. J. Cell Mol. Med. 2021, 25, 473–483. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, Y.; Li, Z.; Wang, Z.; Kong, W.; Zhao, J. Sphingosylphosphorylcholine ameliorates doxorubicin-induced cardiotoxicity in zebrafish and H9c2 cells by reducing excessive mitophagy and mitochondrial dysfunction. Toxicol. Appl. Pharmacol. 2022, 452, 116207. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, J.; Lu, C.; Sun, W.; Kong, W.; Zhao, J. MicroRNA-155-5p/EPAS1/interleukin 6 pathway participated in the protection function of sphingosylphosphorylcholine to ischemic cardiomyocytes. Life Sci. 2021, 264, 118692. [Google Scholar] [CrossRef] [PubMed]
- Herzog, C.; Schmitz, M.; Levkau, B.; Herrgott, I.; Mersmann, J.; Larmann, J.; Johanning, K.; Winterhalter, M.; Chun, J.; Muller, F.U.; et al. Intravenous sphingosylphosphorylcholine protects ischemic and postischemic myocardial tissue in a mouse model of myocardial ischemia/reperfusion injury. Mediat. Inflamm. 2010, 2010, 425191. [Google Scholar] [CrossRef] [PubMed]
- Odagiu, L.; May, J.; Boulet, S.; Baldwin, T.A.; Labrecque, N. Role of the Orphan Nuclear Receptor NR4A Family in T-Cell Biology. Front. Endocrinol. 2020, 11, 624122. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Taegtmeyer, H.; Harmancey, R. Prolonged cardiac NR4A2 activation causes dilated cardiomyopathy in mice. Basic. Res. Cardiol. 2022, 117, 33. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Li, X.; Zhou, C.; Liang, Y.; Li, D.; Ji, Q. NR4A2 alleviates cardiomyocyte loss and myocardial injury in rats by transcriptionally suppressing CCR5 and inducing M2 polarization of macrophages. Microvasc. Res. 2022, 140, 104279. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.S.; Rezq, S.; Alsemeh, A.E.; Mahmoud, M.F. Moxonidine ameliorates cardiac injury in rats with metabolic syndrome by regulating autophagy. Life Sci. 2023, 312, 121210. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Xie, Y. Propofol protects H9C2 cells against hypoxia/reoxygenation injury through miR-449a and NR4A2. Exp. Ther. Med. 2021, 22, 1181. [Google Scholar] [CrossRef]
- Zhai, X.; Liu, R.; Li, J.; Wang, F.; Liu, L.; Wei, S.; Bian, Y.; Pang, J.; Xue, M.; Qin, D.; et al. LincRNA-p21 Upregulates Nuclear Orphan Receptor Nr4a2 and Aggravates Myocardial Ischemia/Reperfusion Injury via Targeting MiR-466i-5p. Int. Heart J. 2022, 63, 1004–1014. [Google Scholar] [CrossRef]
- Liu, H.; Liu, P.; Shi, X.; Yin, D.; Zhao, J. NR4A2 protects cardiomyocytes against myocardial infarction injury by promoting autophagy. Cell Death Discov. 2018, 4, 27. [Google Scholar] [CrossRef]
- Bourgoin, S.G.; Zhao, C. Autotaxin and lysophospholipids in rheumatoid arthritis. Curr. Opin. Investig. Drugs 2010, 11, 515–526. [Google Scholar]
- Wang, C.; Li, Z.; Liu, Y.; Yuan, L. Exosomes in atherosclerosis: Performers, bystanders, biomarkers, and therapeutic targets. Theranostics 2021, 11, 3996–4010. [Google Scholar] [CrossRef]
- Morelli, M.B.; Shu, J.; Sardu, C.; Matarese, A.; Santulli, G. Cardiosomal microRNAs Are Essential in Post-Infarction Myofibroblast Phenoconversion. Int. J. Mol. Sci. 2019, 21, 201. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Pan, Y.; Zhang, L.; Shen, C.; Qin, G.; Ashraf, M.; Weintraub, N.; Ma, G.; Tang, Y. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem. Biophys. Res. Commun. 2013, 431, 566–571. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Li, Y.; Chen, L.; Wang, X.; Guo, W.; Zhang, X.; Qin, G.; He, S.H.; Zimmerman, A.; et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int. J. Cardiol. 2015, 192, 61–69. [Google Scholar] [CrossRef]
- Youn, S.W.; Li, Y.; Kim, Y.M.; Sudhahar, V.; Abdelsaid, K.; Kim, H.W.; Liu, Y.; Fulton, D.J.; Ashraf, M.; Tang, Y.; et al. Modification of Cardiac Progenitor Cell-Derived Exosomes by miR-322 Provides Protection against Myocardial Infarction through Nox2-Dependent Angiogenesis. Antioxidants 2019, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Huang, F.; Zhu, P. Endothelial exosomes work as a functional mediator to activate macrophages. Front. Immunol. 2023, 14, 1169471. [Google Scholar] [CrossRef]
- Su, Q.; Lv, X.W.; Xu, Y.L.; Cai, R.P.; Dai, R.X.; Yang, X.H.; Zhao, W.K.; Kong, B.H. Exosomal LINC00174 derived from vascular endothelial cells attenuates myocardial I/R injury via p53-mediated autophagy and apoptosis. Mol. Ther. Nucleic Acids 2021, 23, 1304–1322. [Google Scholar] [CrossRef] [PubMed]
- Varzideh, F.; Jankauskas, S.S.; Kansakar, U.; Mone, P.; Gambardella, J.; Santulli, G. Sortilin drives hypertension by modulating sphingolipid/ceramide homeostasis and by triggering oxidative stress. J. Clin. Investig. 2022, 132, e156624. [Google Scholar] [CrossRef] [PubMed]
- Valikeserlis, I.; Athanasiou, A.A.; Stakos, D. Cellular mechanisms and pathways in myocardial reperfusion injury. Coron. Artery Dis. 2021, 32, 567–577. [Google Scholar] [CrossRef]
- Thomas, G.D.; Snetkov, V.A.; Patel, R.; Leach, R.M.; Aaronson, P.I.; Ward, J.P. Sphingosylphosphorylcholine-induced vasoconstriction of pulmonary artery: Activation of non-store-operated Ca2+ entry. Cardiovasc. Res. 2005, 68, 56–64. [Google Scholar] [CrossRef][Green Version]
- Snetkov, V.A.; Thomas, G.D.; Teague, B.; Leach, R.M.; Shaifta, Y.; Knock, G.A.; Aaronson, P.I.; Ward, J.P. Low concentrations of sphingosylphosphorylcholine enhance pulmonary artery vasoreactivity: The role of protein kinase C delta and Ca2+ entry. Hypertension 2008, 51, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Jeon, E.S.; Kang, Y.J.; Song, H.Y.; Im, D.S.; Kim, H.S.; Ryu, S.H.; Kim, Y.K.; Kim, J.H. Sphingosylphosphorylcholine generates reactive oxygen species through calcium-, protein kinase Cdelta- and phospholipase D-dependent pathways. Cell. Signal. 2005, 17, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Jeon, E.S.; Lee, M.J.; Sung, S.M.; Kim, J.H. Sphingosylphosphorylcholine induces apoptosis of endothelial cells through reactive oxygen species-mediated activation of ERK. J. Cell. Biochem. 2007, 100, 1536–1547. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Jing, Q.; Meng, N.; Su, L.; Zhang, Y.; Zhang, S.; Miao, J.; Zhao, J. Regulation of apoptosis and autophagy by sphingosylphosphorylcholine in vascular endothelial cells. J. Cell. Physiol. 2011, 226, 2827–2833. [Google Scholar] [CrossRef]
- Bhattarai, S.; Sharma, S.; Subedi, U.; Ara, H.; Shum, A.; Milena, M.; Bhuiyan, M.S.; Kidambi, S.; Sun, H.; Miriyala, S.; et al. The ATX-LPA Axis Regulates Vascular Permeability during Cerebral Ischemic-Reperfusion. Int. J. Mol. Sci. 2022, 23, 4138. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Polo, F.; Borza, R.; Matsoukas, M.T.; Marsais, F.; Jagerschmidt, C.; Waeckel, L.; Moolenaar, W.H.; Ford, P.; Heckmann, B.; Perrakis, A. Autotaxin facilitates selective LPA receptor signaling. Cell Chem. Biol. 2023, 30, 69–84.e14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hasse, S.; Zhao, C.; Bourgoin, S.G. Targeting the autotaxin—Lysophosphatidic acid receptor axis in cardiovascular diseases. Biochem. Pharmacol. 2019, 164, 74–81. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Guo, Y.; Yu, H.; Bao, Y.; Xin, X.; Yang, H.; Ni, X.; Wu, N.; Jia, D. Astaxanthin Attenuates Hypertensive Vascular Remodeling by Protecting Vascular Smooth Muscle Cells from Oxidative Stress-Induced Mitochondrial Dysfunction. Oxid. Med. Cell Longev. 2020, 2020, 4629189. [Google Scholar] [CrossRef]
- Tripathi, H.; Al-Darraji, A.; Abo-Aly, M.; Peng, H.; Shokri, E.; Chelvarajan, L.; Donahue, R.; Levitan, B.M.; Gao, E.; Hernandez, G.; et al. Autotaxin inhibition reduces cardiac inflammation and mitigates adverse cardiac remodeling after myocardial infarction. J. Mol. Cell Cardiol. 2020, 149, 95–114. [Google Scholar] [CrossRef]
- Yuyama, K.; Sun, H.; Sakai, S.; Mitsutake, S.; Okada, M.; Tahara, H.; Furukawa, J.; Fujitani, N.; Shinohara, Y.; Igarashi, Y. Decreased amyloid-β pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice. J. Biol. Chem. 2014, 289, 24488–24498. [Google Scholar] [CrossRef]
- Weng, X.; Tan, H.; Huang, Z.; Chen, J.; Zhang, N.; Wang, Q.; Li, Q.; Gao, J.; Sun, D.; Yakufu, W.; et al. Targeted delivery and ROS-responsive release of Resolvin D1 by platelet chimeric liposome ameliorates myocardial ischemia-reperfusion injury. J. Nanobiotechnology 2022, 20, 454. [Google Scholar] [CrossRef]
- Liu, C.; Su, C. Design strategies and application progress of therapeutic exosomes. Theranostics 2019, 9, 1015–1028. [Google Scholar] [CrossRef]
- Li, S.; Li, F.; Wang, Y.; Li, W.; Wu, J.; Hu, X.; Tang, T.; Liu, X. Multiple delivery strategies of nanocarriers for myocardial ischemia-reperfusion injury: Current strategies and future prospective. Drug Deliv. 2024, 31, 2298514. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zhao, Z.; Meng, Q.; Yu, Y.; Sun, J.; Yang, Z.; Chen, Y.; Li, J.; Ma, T.; et al. Engineered Exosomes With Ischemic Myocardium-Targeting Peptide for Targeted Therapy in Myocardial Infarction. J. Am. Heart Assoc. 2018, 7, e008737. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Zhang, L.; Jin, Z.; Kasumov, T.; Chen, Y.R. Mitochondrial redox regulation and myocardial ischemia-reperfusion injury. Am. J. Physiol. Cell Physiol. 2022, 322, C12–C23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, C.; Wang, J.; Liu, L.; He, Y.; Chen, Q. Mitophagy in Cardiomyocytes and in Platelets: A Major Mechanism of Cardioprotection Against Ischemia/Reperfusion Injury. Physiology 2018, 33, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.Y.; Callegari, S.; Cobbold, S.A.; Cotton, T.R.; Mlodzianoski, M.J.; Schubert, A.F.; Geoghegan, N.D.; Rogers, K.L.; Leis, A.; Dewson, G.; et al. Activation mechanism of PINK1. Nature 2022, 602, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Alewijnse, A.E.; Peters, S.L.; Michel, M.C. Cardiovascular effects of sphingosine-1-phosphate and other sphingomyelin metabolites. Br. J. Pharmacol. 2004, 143, 666–684. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.N.S.; Hamasaki, M.; Kawabata, T.; Youle, R.J.; Yoshimori, T. The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 2023, 24, 167–185. [Google Scholar] [CrossRef]
- Yan, C.; Gong, L.; Chen, L.; Xu, M.; Abou-Hamdan, H.; Tang, M.; Desaubry, L.; Song, Z. PHB2 (prohibitin 2) promotes PINK1-PRKN/Parkin-dependent mitophagy by the PARL-PGAM5-PINK1 axis. Autophagy 2020, 16, 419–434. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Hong, C.G.; Hu, W.B.; Chen, M.L.; Duan, R.; Li, H.M.; Yue, T.; Cao, J.; Wang, Z.X.; Chen, C.Y.; et al. Autophagy receptor OPTN (optineurin) regulates mesenchymal stem cell fate and bone-fat balance during aging by clearing FABP3. Autophagy 2021, 17, 2766–2782. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Li, Z.; Yan, X.; Li, Y.; Liu, S. microRNA-130a-5p suppresses myocardial ischemia reperfusion injury by downregulating the HMGB2/NF-κB axis. BMC Cardiovasc. Disord. 2021, 21, 121. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, H.; Zhang, T.; Wang, Z.; Zhang, J.; Lu, Y.; Li, Z.; Kong, W.; Zhao, J. MORN4 protects cardiomyocytes against ischemic injury via MFN2-mediated mitochondrial dynamics and mitophagy. Free Radic. Biol. Med. 2023, 196, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wu, B.; Zhong, B.; Lin, L.; Ding, Y.; Jin, X.; Huang, Z.; Lin, M.; Wu, H.; Xu, D. Naringenin alleviates myocardial ischemia/reperfusion injury by regulating the nuclear factor-erythroid factor 2-related factor 2 (Nrf2) /System xc-/ glutathione peroxidase 4 (GPX4) axis to inhibit ferroptosis. Bioengineered 2021, 12, 10924–10934. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Cao, K.; Zeng, M.; Fu, X.; Zheng, A.; Zhang, F.; Gao, F.; Zou, X.; Li, H.; et al. Cardiac disruption of SDHAF4-mediated mitochondrial complex II assembly promotes dilated cardiomyopathy. Nat. Commun. 2022, 13, 3947. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qi, Q.; Yang, W.C.; Zhang, T.L.; Lu, C.C.; Yao, Y.J.; Kong, W.H.; Zhao, J. Sphingosylphosphorylcholine alleviates hypoxia-caused apoptosis in cardiac myofibroblasts via CaM/p38/STAT3 pathway. Apoptosis 2020, 25, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhu, K.; Li, Z.; Xiao, C. Adiponectin Protects Hypoxia/Reoxygenation-Induced Cardiomyocyte Injury by Suppressing Autophagy. J. Immunol. Res. 2022, 2022, 8433464. [Google Scholar] [CrossRef]
- Hou, S.; Song, Y.; Sun, D.; Zhu, S.; Wang, Z. Xanthohumol-Induced Rat Glioma C6 Cells Death by Triggering Mitochondrial Stress. Int. J. Mol. Sci. 2021, 22, 4506. [Google Scholar] [CrossRef]
- Yang, Q.; Fang, Y.; Zhang, C.; Liu, X.; Wu, Y.; Zhang, Y.; Yang, J.; Yong, K. Exposure to zinc induces lysosomal-mitochondrial axis-mediated apoptosis in PK-15 cells. Ecotoxicol. Environ. Saf. 2022, 241, 113716. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, S.; Tang, H.; Fang, W.; Chen, K.; Chen, X. CoQ10 protects against acetaminophen-induced liver injury by enhancing mitophagy. Toxicol. Appl. Pharmacol. 2021, 410, 115355. [Google Scholar] [CrossRef]
- Guo, W.; Liu, J.; Sun, J.; Gong, Q.; Ma, H.; Kan, X.; Cao, Y.; Wang, J.; Fu, S. Butyrate alleviates oxidative stress by regulating NRF2 nuclear accumulation and H3K9/14 acetylation via GPR109A in bovine mammary epithelial cells and mammary glands. Free Radic. Biol. Med. 2020, 152, 728–742. [Google Scholar] [CrossRef] [PubMed]


| Primer | Sequence (5′ → 3′) |
|---|---|
| siATX-F | AUCGACAAAAUUGUGGGGCTT |
| siATX-R | GCCCCACAAUUUUGUCGAUTT |
| NC-ATX-F | UUCUCCGAACGUGUCACGUTT |
| NC-ATX-R | ACGUGACACGUUCGGAGAATT |
| siNR4A2-F | AAGCGCCGCCGAAAUCGUUGU |
| siNR4A2-R | ACAACGAUUUCGGCGGCGCUU |
| NC-NR4A2-F | UAUCGGAACCCUAGGUUCCTT |
| NC-NR4A2-R | GGAACCUAGGGUUCCGAUATT |
| siOPTN-F | GGAAACACUGAGCAUUCAATT |
| siOPTN-R | UUGAAUGCUCAGUGUUUCCTT |
| NC-OPTN-F | UUCUCCGAACGUGUCACGUTT |
| NC-OPTN-R | ACGUGACACGUUCGGAGAATT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Li, Z.; Cai, Y.; Guo, J.; Lin, Y.; Zhao, J. Vascular Endothelial Cell-Derived Exosomal Sphingosylphosphorylcholine Attenuates Myocardial Ischemia–Reperfusion Injury through NR4A2-Mediated Mitophagy. Int. J. Mol. Sci. 2024, 25, 3305. https://doi.org/10.3390/ijms25063305
Yu Y, Li Z, Cai Y, Guo J, Lin Y, Zhao J. Vascular Endothelial Cell-Derived Exosomal Sphingosylphosphorylcholine Attenuates Myocardial Ischemia–Reperfusion Injury through NR4A2-Mediated Mitophagy. International Journal of Molecular Sciences. 2024; 25(6):3305. https://doi.org/10.3390/ijms25063305
Chicago/Turabian StyleYu, Yifan, Zhiliang Li, Yuqing Cai, Jiahui Guo, Yushuang Lin, and Jing Zhao. 2024. "Vascular Endothelial Cell-Derived Exosomal Sphingosylphosphorylcholine Attenuates Myocardial Ischemia–Reperfusion Injury through NR4A2-Mediated Mitophagy" International Journal of Molecular Sciences 25, no. 6: 3305. https://doi.org/10.3390/ijms25063305
APA StyleYu, Y., Li, Z., Cai, Y., Guo, J., Lin, Y., & Zhao, J. (2024). Vascular Endothelial Cell-Derived Exosomal Sphingosylphosphorylcholine Attenuates Myocardial Ischemia–Reperfusion Injury through NR4A2-Mediated Mitophagy. International Journal of Molecular Sciences, 25(6), 3305. https://doi.org/10.3390/ijms25063305




