Targeting KRAS G12C Mutation in Colorectal Cancer, A Review: New Arrows in the Quiver
Abstract
1. Introduction
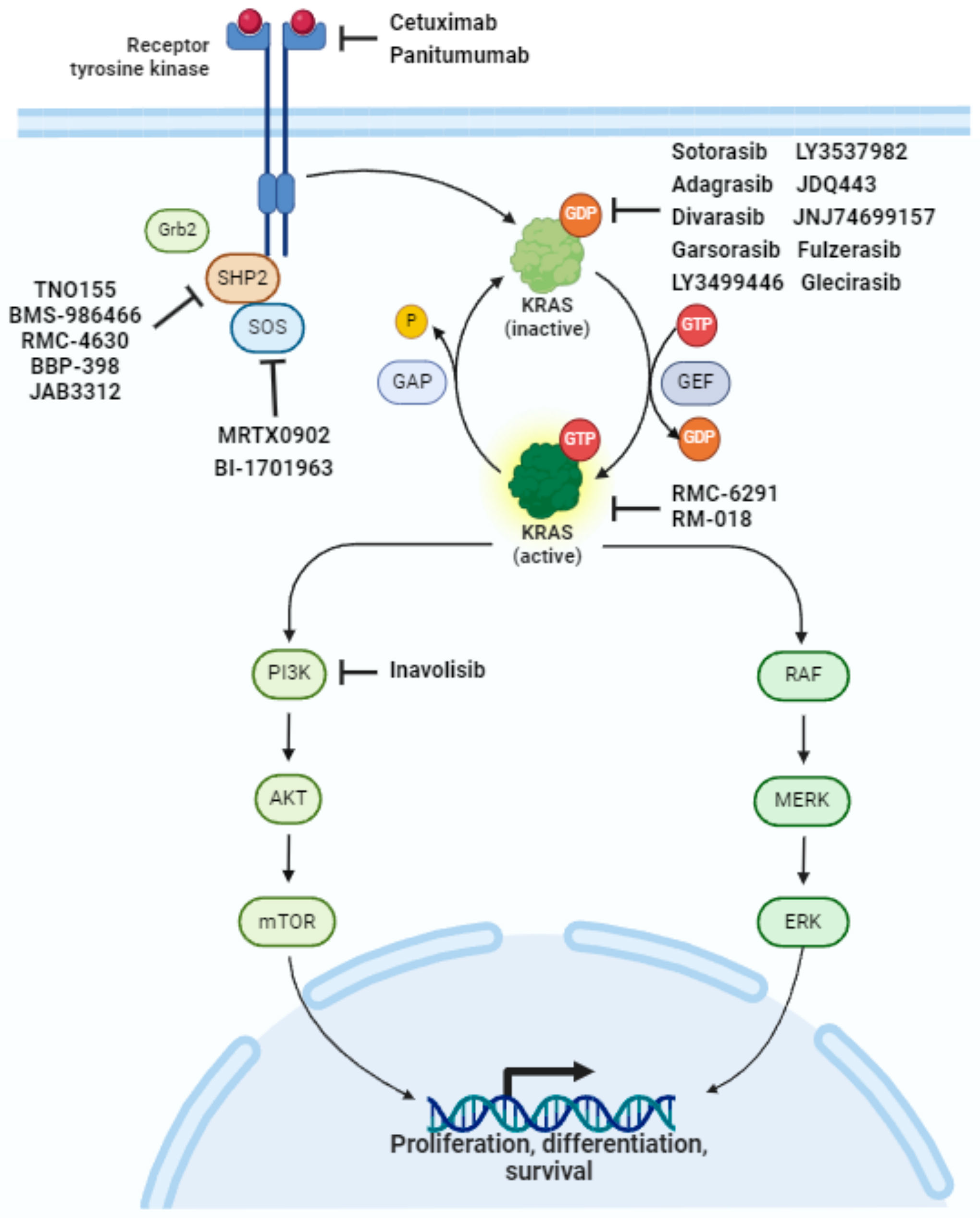
2. The RAS Pathway and Downstream Signaling
3. Development of KRAS G12C Inhibitors
3.1. KRAS Inhibitors in Monotherapy
3.1.1. Sotorasib
3.1.2. Adagrasib
3.1.3. Divarasib
3.1.4. Other Inhibitors
3.2. A Step Forward in Boosting Antitumor Activity: Combining KRAS G12C Inhibitors with Anti-EGFR
3.2.1. Adagrasib Cetuximab
3.2.2. Sotorasib Panitumumab
3.2.3. Divarasib Cetuximab
3.3. Ongoing Clinical Trials and New KRAS G12C Inhibitors in Colorectal Cancer
4. Mechanisms of Resistance
4.1. EGFR-Mediated Adaptive Feedback Reactivation of the RAS-MAPK Pathway
4.2. Acquired Genomic Events
4.3. KRAS Switch-II Pocket Mutations
4.4. Histological Switch
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Muzny, D.M.; Bainbridge, M.N.; Chang, K.; Dinh, H.H.; Drummond, J.A.; Fowler, G.; Kovar, C.L.; Lewis, L.R.; Morgan, M.B.; Newsham, I.F.; et al. Comprehensive Molecular Characterization of Human Colon and Rectal Cancer. Nature 2012, 487, 330. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic Colorectal Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-up ☆. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.J.; Van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. Randomized Trial of TAS-102 for Refractory Metastatic Colorectal Cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Prager, G.W.; Taieb, J.; Fakih, M.; Ciardiello, F.; Van Cutsem, E.; Elez, E.; Cruz, F.M.; Wyrwicz, L.; Stroyakovskiy, D.; Pápai, Z.; et al. Trifluridine–Tipiracil and Bevacizumab in Refractory Metastatic Colorectal Cancer. N. Engl. J. Med. 2023, 388, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib Monotherapy for Previously Treated Metastatic Colorectal Cancer (CORRECT): An International, Multicentre, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.T.; Coker, O.; Chowdhury, S.; Shen, J.P.; Morris, V.K.; Dasari, A.; Raghav, K.; Nusrat, M.; Kee, B.; Parseghian, C.; et al. Comprehensive Clinical and Molecular Characterization of KRAS G12C -Mutant Colorectal Cancer. JCO Precis. Oncol. 2021, 6, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.P.; Sutton, P.A.; Evans, J.P.; Clifford, R.; McAvoy, A.; Lewis, J.; Rousseau, A.; Mountford, R.; McWhirter, D.; Malik, H.Z. Specific Mutations in KRAS Codon 12 Are Associated with Worse Overall Survival in Patients with Advanced and Recurrent Colorectal Cancer. Br. J. Cancer 2017, 116, 923–929. [Google Scholar] [CrossRef]
- Lee, J.K.; Sivakumar, S.; Schrock, A.B.; Madison, R.; Fabrizio, D.; Gjoerup, O.; Ross, J.S.; Frampton, G.M.; Napalkov, P.; Montesion, M.; et al. Comprehensive Pan-Cancer Genomic Landscape of KRAS Altered Cancers and Real-World Outcomes in Solid Tumors. NPJ Precis. Oncol. 2022, 6, 91. [Google Scholar] [CrossRef]
- Nassar, A.H.; Adib, E.; Kwiatkowski, D.J. Distribution of KRAS G12C Somatic Mutations across Race, Sex, and Cancer Type. N. Engl. J. Med. 2021, 384, 185–187. [Google Scholar] [CrossRef]
- Schirripa, M.; Nappo, F.; Cremolini, C.; Salvatore, L.; Rossini, D.; Bensi, M.; Businello, G.; Pietrantonio, F.; Randon, G.; Fucà, G.; et al. KRAS G12C Metastatic Colorectal Cancer: Specific Features of a New Emerging Target Population. Clin. Color. Cancer 2020, 19, 219–225. [Google Scholar] [CrossRef]
- Ciardiello, D.; Chiarazzo, C.; Famiglietti, V.; Damato, A.; Pinto, C.; Zampino, M.G.; Castellano, G.; Gervaso, L.; Zaniboni, A.; Oneda, E.; et al. Clinical Efficacy of Sequential Treatments in KRASG12C-Mutant Metastatic Colorectal Cancer: Findings from a Real-Life Multicenter Italian Study (CRC-KR GOIM). ESMO Open 2022, 7, 100567. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.; Tu, H.; Hsu, H.; Aggarwal, S.; Chan, E.; Rehn, M.; Chia, V.; Kopetz, S. Real-World Study of Characteristics and Treatment Outcomes Among Patients with KRAS p.G12C-Mutated or Other KRAS Mutated Metastatic Colorectal Cancer. Oncologist 2022, 27, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Van de Haar, J.; Ma, X.; Ooft, S.N.; van der Helm, P.W.; Hoes, L.R.; Mainardi, S.; Pinato, D.J.; Sun, K.; Salvatore, L.; Tortora, G.; et al. Codon-Specific KRAS Mutations Predict Survival Benefit of Trifluridine/Tipiracil in Metastatic Colorectal Cancer. Nat. Med. 2023, 29, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Zeissig, M.N.; Ashwood, L.M.; Kondrashova, O.; Sutherland, K.D. Next Batter up! Targeting Cancers with KRAS-G12D Mutations. Trends Cancer 2023, 9, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Hammond, D.E.; Mageean, C.J.; Rusilowicz, E.V.; Wickenden, J.A.; Clague, M.J.; Prior, I.A. Differential Reprogramming of Isogenic Colorectal Cancer Cells by Distinct Activating KRAS Mutations. J. Proteome Res. 2015, 14, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, D.K.; Paulo, J.A.; Sheth, S.; Gygi, S.P.; Lauffenburger, D.A.; Haigis, K.M. Proteogenomic Network Analysis of Context-Specific KRAS Signaling in Mouse-to-Human Cross-Species Translation. Cell Syst. 2019, 9, 258–270. [Google Scholar] [CrossRef]
- Kwan, A.K.; Piazza, G.A.; Keeton, A.B.; Leite, C.A. The Path to the Clinic: A Comprehensive Review on Direct KRASG12C Inhibitors. J. Exp. Clin. Cancer Res. 2022, 41, 27. [Google Scholar] [CrossRef]
- Ciardiello, D.; Maiorano, B.A.; Martinelli, E. Targeting KRASG12C in Colorectal Cancer: The Beginning of a New Era. ESMO Open 2023, 8, 100745. [Google Scholar] [CrossRef]
- Ostrem, J.M.; Peters, U.; Sos, M.L.; Wells, J.A.; Shokat, K.M. K-Ras(G12C) Inhibitors Allosterically Control GTP Affinity and Effector Interactions. Nature 2013, 503, 548–551. [Google Scholar] [CrossRef]
- Kargbo, R.B. Inhibitors of G12C Mutant Ras Proteins for the Treatment of Cancers. ACS Med. Chem. Lett. 2019, 10, 10. [Google Scholar] [CrossRef]
- Lito, P.; Solomon, M.; Li, L.S.; Hansen, R.; Rosen, N. Cancer Therapeutics: Allele-Specific Inhibitors Inactivate Mutant KRAS G12C by a Trapping Mechanism. Science 2016, 351, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Patricelli, M.P.; Janes, M.R.; Li, L.S.; Hansen, R.; Peters, U.; Kessler, L.V.; Chen, Y.; Kucharski, J.M.; Feng, J.; Ely, T.; et al. Selective Inhibition of Oncogenic KRAS Output with Small Molecules Targeting the Inactive State. Cancer Discov. 2016, 6, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Canon, J.; Rex, K.; Saiki, A.Y.; Mohr, C.; Cooke, K.; Bagal, D.; Gaida, K.; Holt, T.; Knutson, C.G.; Koppada, N.; et al. The Clinical KRAS(G12C) Inhibitor AMG 510 Drives Anti-Tumour Immunity. Nature 2019, 575, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Fakih, M.G.; Strickler, J.H.; Desai, J.; Durm, G.A.; Shapiro, G.I.; Falchook, G.S.; Price, T.J.; Sacher, A.; Denlinger, C.S.; et al. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N. Engl. J. Med. 2020, 383, 1207. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.G.; Kopetz, S.; Kuboki, Y.; Kim, T.W.; Munster, P.N.; Krauss, J.C.; Falchook, G.S.; Han, S.W.; Heinemann, V.; Muro, K.; et al. Sotorasib for Previously Treated Colorectal Cancers with KRASG12C Mutation (CodeBreaK100): A Prespecified Analysis of a Single-Arm, Phase 2 Trial. Lancet Oncol. 2022, 23, 115–124. [Google Scholar] [CrossRef]
- Dy, G.K.; Govindan, R.; Velcheti, V.; Falchook, G.S.; Italiano, A.; Wolf, J.; Sacher, A.G.; Takahashi, T.; Ramalingam, S.S.; Dooms, C.; et al. Long-Term Outcomes and Molecular Correlates of Sotorasib Efficacy in Patients With Pretreated KRAS G12C-Mutated Non–Small-Cell Lung Cancer: 2-Year Analysis of CodeBreaK 100. J. Clin. Oncol. 2023, 41, 3311. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.H.I.; Jänne, P.A.; Leal, T.A.; Rybkin, I.I.; Sabari, J.K.; Barve, M.A.; Bazhenova, L.; Johnson, M.L.; Velastegui, K.L.; Cilliers, C.; et al. First-in-Human Phase I/IB Dose-Finding Study of Adagrasib (MRTX849) in Patients with Advanced KRAS G12CSolid Tumors (KRYSTAL-1). J. Clin. Oncol. 2022, 40, 2530–2538. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, R.; Weiss, J.; Pelster, M.S.; Spira, A.I.; Barve, M.; Ou, S.-H.I.; Leal, T.A.; Bekaii-Saab, T.S.; Paweletz, C.P.; Heavey, G.A.; et al. Adagrasib with or without Cetuximab in Colorectal Cancer with Mutated KRAS G12C. N. Engl. J. Med. 2023, 388, 44–54. [Google Scholar] [CrossRef]
- Purkey, H. Discovery of GDC-6036, a Clinical Stage Treatment for KRAS G12C-Positive Cancers. Cancer Res. 2022, 82 (Suppl. S12), ND11. [Google Scholar] [CrossRef]
- Sacher, A.; LoRusso, P.; Patel, M.R.; Miller, W.H.; Garralda, E.; Forster, M.D.; Santoro, A.; Falcon, A.; Kim, T.W.; Paz-Ares, L.; et al. Single-Agent Divarasib (GDC-6036) in Solid Tumors with a KRAS G12C Mutation. N. Engl. J. Med. 2023, 389, 710–721. [Google Scholar] [CrossRef]
- Yuan, Y.; Deng, Y.; Jin, Y.; Pan, Y.; Wang, C.; Wang, Z.; Zhang, Z.; Meng, X.; Hu, Y.; Zhao, M.; et al. Efficacy and Safety of IBI351 (GFH925) Monotherapy in Metastatic Colorectal Cancer Harboring KRASG12C Mutation: Preliminary Results from a Pooled Analysis of Two Phase I Studies. J. Clin. Oncol. 2023, 41, 3586. [Google Scholar] [CrossRef]
- Xu, R.-H.; Xu, Y.; Yan, D.; Munster, P.; Ruan, D.; Deng, Y.; Pan, H.; Underhill, C.R.; Richardson, G.; Nordman, I.; et al. 550O Safety and Efficacy of D-1553 in Combination with Cetuximab in KRAS G12C Mutated Colorectal Cancer (CRC): A Phase II Study. Ann. Oncol. 2023, 34, S410–S411. [Google Scholar] [CrossRef]
- Amodio, V.; Yaeger, R.; Arcella, P.; Cancelliere, C.; Lamba, S.; Lorenzato, A.; Arena, S.; Montone, M.; Mussolin, B.; Bian, Y.; et al. EGFR Blockade Reverts Resistance to KRASG12C Inhibition in Colorectal Cancer. Cancer Discov. 2020, 10, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.B.; Coker, O.; Sorokin, A.; Fella, K.; Barnes, H.; Wong, E.; Kanikarla, P.; Gao, F.; Zhang, Y.; Zhou, L.; et al. KRASG12C-Independent Feedback Activation of Wild-Type RAS Constrains KRASG12C Inhibitor Efficacy. Cell Rep. 2022, 39, 110993. [Google Scholar] [CrossRef]
- Nusrat, M.; Yaeger, R. KRAS Inhibition in Metastatic Colorectal Cancer: An Update. Curr. Opin. Pharmacol. 2023, 68, 102343. [Google Scholar] [CrossRef]
- Kuboki, Y.; Fakih, M.; Strickler, J.; Yaeger, R.; Masuishi, T.; Kim, E.J.; Bestvina, C.M.; Kopetz, S.; Falchook, G.S.; Langer, C.; et al. Sotorasib with Panitumumab in Chemotherapy-Refractory KRASG12C-Mutated Colorectal Cancer: A Phase 1b Trial. Nat. Med. 2024, 30, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.G.; Salvatore, L.; Esaki, T.; Modest, D.P.; Lopez-Bravo, D.P.; Taieb, J.; Karamouzis, M.V.; Ruiz-Garcia, E.; Kim, T.-W.; Kuboki, Y.; et al. Sotorasib plus Panitumumab in Refractory Colorectal Cancer with Mutated KRAS G12C. N. Engl. J. Med. 2023, 389, 2125–2139. [Google Scholar] [CrossRef]
- Hong, D.S.; Kuboki, Y.; Strickler, J.H.; Fakih, M.; Houssiau, H.; Price, T.J.; Elez, E.; Siena, S.; Chan, E.; Nolte-Hippenmeyer, J.; et al. Sotorasib (Soto) plus Panitumumab (Pmab) and FOLFIRI for Previously Treated KRAS G12C-Mutated Metastatic Colorectal Cancer (MCRC): CodeBreaK 101 Phase 1b Safety and Efficacy. J. Clin. Oncol. 2023, 41, 3513. [Google Scholar] [CrossRef]
- Desai, J.; Alonso, G.; Kim, S.H.; Cervantes, A.; Karasic, T.; Medina, L.; Shacham-Shmueli, E.; Cosman, R.; Falcon, A.; Gort, E.; et al. Divarasib plus Cetuximab in KRAS G12C-Positive Colorectal Cancer: A Phase 1b Trial. Nat. Med. 2024, 30, 271. [Google Scholar] [CrossRef]
- Hillig, R.C.; Sautier, B.; Schroeder, J.; Moosmayer, D.; Hilpmann, A.; Stegmann, C.M.; Werbeck, N.D.; Briem, H.; Boemer, U.; Weiske, J.; et al. Discovery of Potent SOS1 Inhibitors That Block RAS Activation via Disruption of the RAS–SOS1 Interaction. Proc. Natl. Acad. Sci. USA 2019, 116, 2551–2560. [Google Scholar] [CrossRef]
- Negrao, M.V.; Cassier, P.A.; Solomon, B.; Schuler, M.; Rohrberg, K.; Cresta, S.; Dooms, C.; Tan, D.S.W.; Loong, H.H.; Amatu, A.; et al. MA06.03 KontRASt-01: Preliminary Safety and Efficacy of JDQ443 + TNO155 in Patients with Advanced, KRAS G12C-Mutated Solid Tumors. J. Thorac. Oncol. 2023, 18, S117–S118. [Google Scholar] [CrossRef]
- Ryan, M.B.; de la Cruz, F.F.; Phat, S.; Myers, D.T.; Wong, E.; Shahzade, H.A.; Hong, C.B.; Corcoran, R.B. Vertical Pathway Inhibition Overcomes Adaptive Feedback Resistance to KrasG12C Inhibition. Clin. Cancer Res. 2020, 26, 1617–1643. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, H.; Miyoshi, H.; Kakizaki, F.; Morimoto, T.; Kawada, K.; Yamamoto, T.; Obama, K.; Sakai, Y.; Taketo, M.M. Efficacious Combination Drug Treatment for Colorectal Cancer That Overcomes Resistance to KRAS G12C Inhibitors. Mol. Cancer Ther. 2019, 22, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Sommerhalder, D.; Thevathasan, J.; Ianopulos, X.; Volinn, W.; Hong, D. Abstract CT242: KRYSTAL-16: A Phase I/Ib Trial of Adagrasib (MRTX849) in Combination with Palbociclib in Patients with Advanced Solid Tumors with KRASG12C Mutation. Cancer Res. 2022, 82, CT242. [Google Scholar] [CrossRef]
- Hallin, J.; Engstrom, L.D.; Hargi, L.; Calinisan, A.; Aranda, R.; Briere, D.M.; Sudhakar, N.; Bowcut, V.; Baer, B.R.; Ballard, J.A.; et al. The KRASG12C Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer Discov. 2020, 10, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Zhang, J.; Liu, P.; Jiao, B.; Wang, Z.; Ren, R. Focal Adhesion Kinase (FAK) Inhibition Synergizes with KRAS G12C Inhibitors in Treating Cancer through the Regulation of the FAK–YAP Signaling. Adv. Sci. 2021, 8, 2100250. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Martin-Romano, P.; Cassier, P.; Johnson, M.; Haura, E.; Lenox, L.; Guo, Y.; Bandyopadhyay, N.; Russell, M.; Shearin, E.; et al. Phase I Study of JNJ-74699157 in Patients with Advanced Solid Tumors Harboring the KRAS G12C Mutation. Oncologist 2022, 27, 536–553. [Google Scholar] [CrossRef]
- Weiss, A.; Lorthiois, E.; Barys, L.; Beyer, K.S.; Bomio-Confaglia, C.; Burks, H.; Chen, X.; Cui, X.; De Kanter, R.; Dharmarajan, L.; et al. Discovery, Preclinical Characterization, and Early Clinical Activity of JDQ443, a Structurally Novel, Potent, and Selective Covalent Oral Inhibitor of KRAS G12C. Cancer Discov. 2022, 12, 1500–1517. [Google Scholar] [CrossRef]
- Awad, M.M.; Liu, S.; Rybkin, I.I.; Arbour, K.C.; Dilly, J.; Zhu, V.W.; Johnson, M.L.; Heist, R.S.; Patil, T.; Riely, G.J.; et al. Acquired Resistance to KRAS G12C Inhibition in Cancer. N. Engl. J. Med. 2021, 384, 2382–2393. [Google Scholar] [CrossRef]
- Cregg, J.; Nichols, R.J.; Yang, Y.C.; Schulze, C.J.; Wang, Z.; Dua, R.; Jiang, J.; Nasholm, N.; Knox, J.E.; Seamon, K.; et al. Abstract ND07: Discovery of RMC-6291, a Tri-Complex KRASG12C(ON) Inhibitor. Cancer Res. 2023, 83, ND07. [Google Scholar] [CrossRef]
- Pelster, M.S.; Yaeger, R.; Klempner, S.J.; Ou, S.-H.I.; Spira, A.I.; Jänne, P.A.; Uboha, N.V.; Gaffar, Y.A.; Newman, G.; Paweletz, C.P.; et al. 549O Adagrasib with or without Cetuximab in Patients with KRASG12C-Mutated Colorectal Cancer (CRC): Analysis of Tumor Biomarkers and Genomic Alterations. Ann. Oncol. 2023, 34, S410. [Google Scholar] [CrossRef]
- Prenen, H.; Fakih, M.; Falchook, G.; Strickler, J.; Hindoyan, A.; Anderson, A.; Ang, A.; Kurata, T.; Price, T. SO-39 Evaluation of Acquired Resistance to Sotorasib in KRAS p.G12C-Mutated Colorectal Cancer: Exploratory Plasma Biomarker Analysis of CodeBreaK 100. Ann. Oncol. 2022, 33, S373. [Google Scholar] [CrossRef]
- Yaeger, R.; Mezzadra, R.; Sinopoli, J.; Bian, Y.; Marasco, M.; Kaplun, E.; Gao, Y.; Zhao, H.; Paula, A.D.C.; Zhu, Y.; et al. Molecular Characterization of Acquired Resistance to KRASG12C–EGFR Inhibition in Colorectal Cancer. Cancer Discov. 2023, 13, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Lin, J.J.; Li, C.; Ryan, M.B.; Zhang, J.; Kiedrowski, L.A.; Michel, A.G.; Syed, M.U.; Fella, K.A.; Sakhi, M.; et al. Clinical Acquired Resistance to KRASG12C Inhibition through a Novel KRAS Switch-II Pocket Mutation and Polyclonal Alterations Converging on RAS–MAPK Reactivation. Cancer Discov. 2021, 11, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Patel, A.S.; Kim, E.; Li, H.; Chen, Y.; Li, S.; Liu, S.; Dilly, J.; Kapner, K.S.; Zhang, N.; et al. Adeno-to-Squamous Transition Drives Resistance to KRAS Inhibition in LKB1 Mutant Lung Cancer. Cancer Cell 2024, 42, 413–428.e7. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, R.B.; Ebi, H.; Turke, A.B.; Coffee, E.M.; Nishino, M.; Cogdill, A.P.; Brown, R.D.; Pelle, P.D.; Dias-Santagata, D.; Hung, K.E.; et al. EGFR-Mediated Reactivation of MAPK Signaling Contributes to Insensitivity of BRAF-Mutant Colorectal Cancers to RAF Inhibition with Vemurafenib. Cancer Discov. 2012, 2, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Prahallad, A.; Sun, C.; Huang, S.; Di Nicolantonio, F.; Salazar, R.; Zecchin, D.; Beijersbergen, R.L.; Bardelli, A.; Bernards, R. Unresponsiveness of Colon Cancer to BRAF(V600E) Inhibition through Feedback Activation of EGFR. Nature 2012, 483, 100–103. [Google Scholar] [CrossRef]
- Tie, J.; Wang, Y.; Tomasetti, C.; Li, L.; Springer, S.; Kinde, I.; Silliman, N.; Tacey, M.; Wong, H.L.; Christie, M.; et al. Circulating Tumor DNA Analysis Detects Minimal Residual Disease and Predicts Recurrence in Patients with Stage II Colon Cancer. Sci. Transl. Med. 2016, 8, 346ra92. [Google Scholar] [CrossRef]
- Montagut, C.; Dalmases, A.; Bellosillo, B.; Crespo, M.; Pairet, S.; Iglesias, M.; Salido, M.; Gallen, M.; Marsters, S.; Tsai, S.P.; et al. Identification of a Mutation in the Extracellular Domain of the Epidermal Growth Factor Receptor Conferring Cetuximab Resistance in Colorectal Cancer. Nat. Med. 2012, 18, 221–223. [Google Scholar] [CrossRef]
- Montagut, C.; Argilés, G.; Ciardiello, F.; Poulsen, T.T.; Dienstmann, R.; Kragh, M.; Kopetz, S.; Lindsted, T.; Ding, C.; Vidal, J.; et al. Efficacy of Sym004 in Patients With Metastatic Colorectal Cancer With Acquired Resistance to Anti-EGFR Therapy and Molecularly Selected by Circulating Tumor DNA Analyses: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018, 4, e175245. [Google Scholar] [CrossRef]
- Ros, J.; Matito, J.; Villacampa, G.; Comas, R.; Garcia, A.; Martini, G.; Baraibar, I.; Saoudi, N.; Salvà, F.; Martin, A.; et al. Plasmatic BRAF-V600E Allele Fraction as a Prognostic Factor in Metastatic Colorectal Cancer Treated with BRAF Combinatorial Treatments. Ann. Oncol. 2023, 34, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Parseghian, C.M.; Loree, J.M.; Morris, V.K.; Liu, X.; Clifton, K.K.; Napolitano, S.; Henry, J.T.; Pereira, A.A.; Vilar, E.; Johnson, B.; et al. Anti-EGFR-Resistant Clones Decay Exponentially after Progression: Implications for Anti-EGFR Re-Challenge. Ann. Oncol. 2019, 30, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Reinert, T.; Henriksen, T.V.; Christensen, E.; Sharma, S.; Salari, R.; Sethi, H.; Knudsen, M.; Nordentoft, I.; Wu, H.T.; Tin, A.S.; et al. Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients With Stages I to III Colorectal Cancer. JAMA Oncol. 2019, 5, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Pietrantonio, F.; Lonardi, S.; Mussolin, B.; Rua, F.; Crisafulli, G.; Bartolini, A.; Fenocchio, E.; Amatu, A.; Manca, P.; et al. Circulating Tumor DNA to Guide Rechallenge with Panitumumab in Metastatic Colorectal Cancer: The Phase 2 CHRONOS Trial. Nat. Med. 2022, 28, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Wang, C.; Fakih, M. Rechallenge With BRAF and Anti-EGFR Inhibitors in Patients With Metastatic Colorectal Cancer Harboring BRAFV600E Mutation Who Progressed on Cetuximab and Encorafenib With or Without Binimetinib: A Case Series. Clin. Color. Cancer 2022, 21, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Ros, J.; Vivancos, A.; Tabernero, J.; Élez, E. Circulating Tumor DNA, and Clinical Features to Guide Rechallenge with BRAF Inhibitors in BRAF-V600E Mutated Metastatic Colorectal Cancer. Ann. Oncol. 2023, 35, 240–241. [Google Scholar] [CrossRef] [PubMed]
- Akhoundova, D.; Pietge, H.; Hussung, S.; Kiessling, M.; Britschgi, C.; Zoche, M.; Rechsteiner, M.; Weber, A.; Fritsch, R.M. Targeting Secondary and Tertiary Resistance to BRAF Inhibition in BRAF V600E–Mutated Metastatic Colorectal Cancer. JCO Precis. Oncol. 2021, 5, 1082–1087. [Google Scholar] [CrossRef]
- Wang, N.; Fang, J.Y. Fusobacterium Nucleatum, a Key Pathogenic Factor and Microbial Biomarker for Colorectal Cancer. Trends Microbiol. 2023, 31, 159–172. [Google Scholar] [CrossRef]
- Marmorino, F.; Piccinno, G.; Rossini, D.; Ghelardi, F.; Murgioni, S.; Salvatore, L.; Nasca, V.; Antoniotti, C.; Daniel, F.; Schietroma, F.; et al. Gut Microbiome Composition as Predictor of the Efficacy of Adding Atezolizumab to First-Line FOLFOXIRI plus Bevacizumab in Metastatic Colorectal Cancer: A Translational Analysis of the AtezoTRIBE Study. J. Clin. Oncol. 2023, 41, 3534. [Google Scholar] [CrossRef]
- Zhu, H.; Li, M.; Bi, D.; Yang, H.; Gao, Y.; Song, F.; Zheng, J.; Xie, R.; Zhang, Y.; Liu, H.; et al. Fusobacterium Nucleatum Promotes Tumor Progression in KRAS p.G12D-Mutant Colorectal Cancer by Binding to DHX15. Nat. Commun. 2024, 15, 1688. [Google Scholar] [CrossRef]

| Clinical Trials Targeting KRAS G12C in mCRC, Completed or with Already Published Data | ||||
|---|---|---|---|---|
| Study Name/ID | Population (n. of Patients) | Treatment Regimen (n. of Patients Treated) | Results | Grade 3 or Higher TRAEs |
| Phase | ||||
| CodeBreaK 100/NCT03600883 | advanced KRAS G12C mutant solid tumors (124, including 42 mCRC) | Sotorasib (AMG510) | limited to mCRC treated with any dose | 52.7%, in the overall population |
| Phase I | ORR 7.1% (3/42) | |||
| DCR 73.8% (31/42) | ||||
| mPFS 4 mo | ||||
| CodeBreaK 100 (CRC expansion cohort)/NCT03600883 | advanced KRAS G12C mutant mCRC (62) | Sotorasib (AMG510) 960 mg qd | ORR 9.7% (6/62) | 10% (6/62) |
| DCR 82.3% (51/62) | ||||
| Phase II | mPFS 4 mo | |||
| KRYSTAL-1/NCT03785249 | advanced KRAS G12C mutant solid tumors (25, including 4 mCRC) | Adagrasib (MRTX849) | limited to evaluable mCRC treated with 600 mg bid | 36% (9/25) |
| Phase I/Ib | ORR 50% (1/2) | |||
| DOR 4.2 mo | ||||
| KRYSTAL-1 (monotherapy arm)/NCT03785249 | advanced KRAS G12C mutant mCRC (44) | Adagrasib (MRTX849) 600 mg bid | ORR 19% (8/43) | 34% (15/43) |
| Phase I/II | DCR 86% (37/43) | |||
| mPFS 5.6 mo | ||||
| NCT04449874 | advanced KRAS G12C mutant solid tumors (137, including 55 mCRC) | Divarasib (GDC-6036) | limited to mCRC population | 7% (4/55) |
| Phase Ib | ORR 29.1% (20/55) | |||
| mPFS 5.6 mo | ||||
| limited to mCRC treated with 400 mg qd | ||||
| ORR 35.9% (14/39) | ||||
| mPFS 6.9 mo | ||||
| pooled analysis of NCT05005234 and NCT05497336 | advanced KRAS G12C mutant solid tumors, including 45 mCRC | Fulzerasib (IBI531) | limited to mCRC patients treated with 600 mg bid | 20% (9/32) |
| Phase I | ORR 43.8% (14/32) | |||
| DCR 87.5% (28/32) | ||||
| Clinical trials targeting EGFR-KRAS G12C in mCRC, completed or with already published data | ||||
| CodeBreaK 101/NCT04185883 | advanced KRAS G12C mutant mCRC (48) | Sotorasib (AMG510) + panitumumab | limited to patients treated with 960 mg qd | 27% (13/48) |
| Phase Ib | ORR 30% (12/40) | |||
| DCR 92.5% (37/40) | ||||
| mPFS 5.7 mo | ||||
| CodeBreaK 101 (subprotocol H)/NCT04185883 | advanced KRAS G12C mCRC previously treated ≥1 prior treatment (33) | Sotorasib (AMG510) 960 mg qd + panitumumab + FOLFIRI (54) | ORR 58.1% | 45.5% (15/33) |
| DCR 93.5% | ||||
| Phase Ib | mPFS 5.7 mo | |||
| CodeBreaK 300/NCT05198934 | advanced KRAS G12C mutant mCRC (160) | Sotorasib (AMG510) 960 qd mg + panitumumab (53) | ORR 26.4% DCR 71.7% | 35.8% (19/53) |
| Phase III | mPFS 5.6 mo | |||
| Sotorasib (AMG510) 240 qd mg + panitumumab (53) | ORR 5.7% DCR 67.9% | 30.2% (16/53) | ||
| mPFS 3.9 | ||||
| SOC (54) | ORR 0% DCR 46.3% | 43.1% (23/54) | ||
| mPFS 2.2 mo | ||||
| KRYSTAL-1 (combination arm)/NCT03785249 | advanced KRAS G12C mutant mCRC (32) | Adagrasib (MRTX849) 600 mg bid + cetuximab | ORR 46% (13/28) | 16% (5/32) |
| Phase I/II | DCR 100% (28/28) | |||
| mPFS 6.9 mo | ||||
| mOS 13.4 mo | ||||
| NCT04449874 (arm C) | advanced KRAS G12C mutant mCRC (29) | Divarasib (GDC-6036) at 400 mg qd (26) + cetuximab | limited to KRAS G12C inhibitor naive population | 37.9% (11/29) |
| Phase Ib | ORR 62.5% (14/24) | |||
| mPFS 8 mo | ||||
| NCT04585035 | advanced KRAS G12C mutant solid tumors, including 29 mCRC | Garsorasib (D-1553) 600 mg bid (29) + cetuximab | ORR 51.7% (15/29) | 10.3% (3/29) |
| Phase I/II | DCR 93.1% (27/29) | |||
| mPFS 7.56 mo | ||||
| Study ID/Name | Treatment Regimen | Population |
|---|---|---|
| Phase | ||
| Ongoing clinical trials evaluating well established anti-KRAS G12C in combination with other compounds | ||
| NCT04975256/KRYSTAL 14 | Adagrasib (MRTX849) + BI 1701963 (inhibitor of KRAS and SOS1 interaction) | advanced KRAS G12C mutant solid tumors |
| Phase I/Ib | ||
| NCT05578092 | Adagrasib (MRTX849) + MRTX0902 (SOS1 inhibitor) | advanced solid tumors KRAS G12C mutant or harboring any mutations in MAPK pathway effectors |
| Phase I/II | ||
| NCT05178888/KRYSTAL-16 | Adagrasib (MRTX849) + palbociclib | advanced KRAS G12C mutant solid tumors |
| Phase I/Ib | ||
| NCT04330664/KRYSTAL-2 | Adagrasib (MRTX849) + TNO155 (SHP2 inhibitor) | advanced KRAS G12C mutant solid tumors |
| Phase I/II | ||
| NCT04793958/KRYSTAL-10 | Adagrasib (MRTX849) + cetuximab vs chemotherapy | advanced KRAS G12C mutant mCRC |
| Phase III | ||
| NCT05722327 | Adagrasib (MRTX849) + cetuximab and irinotecan | advanced KRAS G12C mutant mCRC |
| Phase I | ||
| NCT06024174 | Adagrasib (MRTX849) + BMS-986466 (SHP2 Inhibitor) +/− cetuximab | advanced KRAS G12C mutant NSCLC, PDCA, BTC and CRC |
| Phase 1/2 | ||
| NCT04418661 | Adagrasib (MRTX849) + RMC-4630 (SHP2 inhibitor) | advanced KRAS G12C mutant solid tumors |
| Phase I | ||
| NCT04892017 | DCC-3116 (ULK inhibitor) +/− trametinib, binimetinib, or sotorasib (AMG510) | advanced solid tumors harboring any mutation in RAS/MAPK pathway |
| Phase I/II | ||
| NCT05480865/Argonaut | Sotorasib (AMG510) + BBP-398 (SHP2 inhibitor) | advanced KRAS G12C mutant solid tumors |
| Phase I | ||
| NCT04929223/INTRINSIC | Divarasib (GDC-6036) + Cetuximab +/− FOLFOX or FOLFIRI (in KRAS G12C) | advanced mutant mCRC |
| Phase I/Ib | ||
| NCT05497336 | Fulzerasib (IBI351) + cetuximab (phase Ib) and versus SOC (phase III in mCRC) | advanced KRAS G12C mutant solid tumors (phase Ib) |
| Phase Ib/III | pretreated KRAS G12C mutant mCRC (phase III) | |
| NCT06166836 | Garsorasib (D-1553) + ifebemtinib (IN10018) (FAK inhibitor) | advanced KRAS G12C mutant solid tumors |
| Phase Ib/II | ||
| Ongoing clinical trials evaluating other anti-KRAS G12C inhibitors | ||
| NCT04165031 | LY3499446 +/− several compounds, based on histology (cetuximab in mCRC) | advanced KRAS G12C mutant solid tumors |
| Phase I/II | ||
| NCT 04956640/LOXO-RAS-2000 | LY3537982 +/− several compounds, based on histology (cetuximab in mCRC) | advanced KRAS G12C mutant solid tumors |
| Phase I/II | ||
| NCT04699188/KontRASt-01 | Opnurasib (JDQ443) +/− TNO155 (SHP2 inhibitor) + tislelizumab | advanced KRAS G12C mutant solid tumors |
| Phase Ib/II | ||
| NCT05358249/KontRASt-03 | Opnurasib (JDQ443) + cetuximab (in mCRC) | advanced KRAS G12C mutant solid tumors |
| Phase Ib/II | ||
| NCT05002270 | Glecirasib (JAB-21822) | advanced KRAS G12C mutant solid tumors |
| Phase I/II | ||
| NCT05194995 | Glecirasib (JAB-21822) + cetuximab | advanced KRAS G12C CRC, small intestine cancer and appendiceal cancer |
| Phase Ib/II | ||
| NCT05288205 | Glecirasib (JAB-21822) + JAB-3312 (SHP2 inhibitor) | advanced KRAS G12C mutant solid tumors |
| Phase I/IIa | ||
| NCT04006301 | JNJ-74699157 | advanced KRAS G12C mutant solid tumors |
| Phase I | ||
| NCT05462717 | RMC-6291 | advanced KRAS G12C mutant solid tumors |
| Phase I | ||
| NCT06128551 | RMC-6291 + RMC-6236 (pan-RAS inhibitor) | advanced KRAS G12C mutant solid tumors |
| Phase Ib | ||
| NCT06117371 | BEBT-607 | advanced KRAS G12C mutant solid tumors |
| Phase I/Ib | ||
| NCT06006793 | SY-5933 | advanced KRAS G12C mutant solid tumors |
| Phase I | ||
| NCT06006793 | BPI-421286 | advanced KRAS G12C mutant solid tumors |
| Phase I | ||
| NCT04973163 | BI 1823911 | advanced KRAS G12C mutant solid tumors |
| Phase Ia/Ib | ||
| NCT05410145 | D3S-001 | advanced KRAS G12C mutant solid tumors |
| Phase I | ||
| NCT05768321 | GEC255 | advanced KRAS G12C mutant solid tumors |
| Phase I | ||
| NCT05485974 | HBI-2438 | advanced KRAS G12C mutant solid tumors |
| Phase I | ||
| Main Pharmacokinetics Characteristic of KRAS G12C Inhibitors | |||
|---|---|---|---|
| Name | Sotorasib (AMG510) | Adagrasib (MRTX849) | Divarasib (GDC-6036) |
| Mechanism of action | covalent inhibitor of KRAS G12C | covalent inhibitor of KRAS G12C | covalent inhibitor of KRAS G12C |
| Half-life (hours) | 5.5 ± 1.8 | 24 | 17.6 ± 2.7 |
| Dose | 960 mg qd | 600 mg bid | 400 mg qd |
| Median time to maximum concentration (hours) | 1 | 6 | 2 |
| Other features | CNS penetration | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ros, J.; Vaghi, C.; Baraibar, I.; Saoudi González, N.; Rodríguez-Castells, M.; García, A.; Alcaraz, A.; Salva, F.; Tabernero, J.; Elez, E. Targeting KRAS G12C Mutation in Colorectal Cancer, A Review: New Arrows in the Quiver. Int. J. Mol. Sci. 2024, 25, 3304. https://doi.org/10.3390/ijms25063304
Ros J, Vaghi C, Baraibar I, Saoudi González N, Rodríguez-Castells M, García A, Alcaraz A, Salva F, Tabernero J, Elez E. Targeting KRAS G12C Mutation in Colorectal Cancer, A Review: New Arrows in the Quiver. International Journal of Molecular Sciences. 2024; 25(6):3304. https://doi.org/10.3390/ijms25063304
Chicago/Turabian StyleRos, Javier, Caterina Vaghi, Iosune Baraibar, Nadia Saoudi González, Marta Rodríguez-Castells, Ariadna García, Adriana Alcaraz, Francesc Salva, Josep Tabernero, and Elena Elez. 2024. "Targeting KRAS G12C Mutation in Colorectal Cancer, A Review: New Arrows in the Quiver" International Journal of Molecular Sciences 25, no. 6: 3304. https://doi.org/10.3390/ijms25063304
APA StyleRos, J., Vaghi, C., Baraibar, I., Saoudi González, N., Rodríguez-Castells, M., García, A., Alcaraz, A., Salva, F., Tabernero, J., & Elez, E. (2024). Targeting KRAS G12C Mutation in Colorectal Cancer, A Review: New Arrows in the Quiver. International Journal of Molecular Sciences, 25(6), 3304. https://doi.org/10.3390/ijms25063304





