Abstract
The long-term effects of environmental pollution have been of concern as several pollutants are carcinogenic, potentially inducing a variety of cancers, including childhood cancer, which is a leading cause of death around the world and, thus, is a public health issue. The present scoping review aimed to update and summarize the available literature to detect specific environmental pollutants and their association with certain types of childhood cancer. Studies published from 2013 to 2023 regarding environmental pollution and childhood cancer were retrieved from the PubMed database. A total of 174 studies were eligible for this review and were analyzed. Our search strategy brought up most of the articles that evaluated air pollution (29%) and pesticides (28%). Indoor exposure to chemicals (11%), alcohol and tobacco use during pregnancy (16%), electromagnetic fields (12%), and radon (4%) were the subjects of less research. We found a particularly high percentage of positive associations between prenatal and postnatal exposure to indoor (84%) and outdoor (79%) air pollution, as well as to pesticides (82%), and childhood cancer. Positive associations were found between leukemia and pesticides and air pollution (33% and 27%); CNS tumors and neuroblastoma and pesticides (53% and 43%); and Wilms tumor and other rare cancers were found in association with air pollution (50%). Indoor air pollution was mostly reported in studies assessing several types of cancer (26%). Further studies are needed to investigate the mechanisms underlying the potential associations between indoor/outdoor air pollution and pesticide exposure with childhood cancer risk as more preventable measures could be taken.
1. Introduction
Global environmental pollution is an international public health issue with several health effects. Pollutants are harmful solids, liquids, or gases produced in unusually high concentrations that reduce the quality of the environment. Human activities have adverse effects on the environment by polluting the water, the air, and soil [1]. Large-scale human activities, such as the use of industrial machinery, power-producing stations, combustion engines, and cars, as well as field cultivation techniques, gas stations, fuel tank heaters, and cleaning procedures, influence the environment, emitting most environmental pollutants. Anthropogenic air pollution is one of the biggest health hazards worldwide, accounting for approximately 9 million deaths per year. In addition, air pollution leads to adverse effects on vegetation near factories due to exposure to heavy metals. The long-term effects of environmental pollution have been of concern as several pollutants are carcinogenic, potentially inducing a variety of cancers, including childhood cancer [1,2,3].
Childhood cancer is a leading cause of death around the world, and, thus, it is a public health issue. In many countries, this disease is the second cause of death in children over one year, exceeded only by accidents [4,5]. Approximately 280,000 children under 18 years are diagnosed with cancer each year, and it has been estimated that there will be 13.7 million new cases between 2020 and 2050 [4,5]. In children, the most frequent cancer is leukemia, accounting for 28% of the cases, followed by brain and other central nervous system tumors (26%). Other important types of childhood cancer include lymphoma, hepatoblastoma, retinoblastoma, and Wilms tumor [6,7].
Although the overall pediatric cancer incidence rate has remained steady from 2010 to 2019, it has increased since 1975, causing concern. In addition, adolescent cancer has continued to increase [6]. Important progress has been achieved regarding cure rates, and some countries report remission rates of 90–100%. However, this progress has not been achieved worldwide since some low-income countries report lower remission rates. Additionally, cure rates in adolescents have lagged behind those in children [6]. Contrasting to the enormous advances reached in cure rates, the advances in finding the causes of childhood cancer have not shown much improvement.
Even though the origins and causes of childhood cancer have not been fully understood, genetic, epigenetic, and environmental factors are recognized. It is now accepted, for childhood leukemia, that genetic aberrations potentially driving cancer can arise prenatally, and, together with secondary aberrations and epigenetic changes, the disease may develop postnatally. An important participation of infections, and how the immune system responds to them, has also been suggested as part of leukemia etiology [8,9].
Despite the abundance of research, few causes of childhood cancer have been scientifically established [4,6]. Some genetic conditions, high birth weight, and exposure to ionizing radiation are established risk factors. For many other factors, especially environmental-related ones, the scientific evidence is controversial [4]. Several epidemiological studies have suggested that environmental pollution may have a role in childhood cancer etiology. Prenatal and postnatal exposure to environmental contaminants, including air pollution, electromagnetic fields, pesticides, and radon, have been studied worldwide and are proposed as childhood cancer risk factors. However, despite the vast number of studies available, no conclusions have been drawn given the high variability of results, confounding factors, and bias [2,7,8,10,11]. In addition, the etiologies of the different childhood cancers are heterogeneous; thus, the contribution of certain factors to the development of a specific type of cancer varies. Moreover, the time of exposure can also add complexity to the environment–childhood-cancer association, as this disease has prenatal origins. Thus, additional efforts should be taken to fully clarify the relationship between environmental pollution and the risk of childhood cancer. An analysis of the available literature regarding environmental pollution and childhood cancer is helpful in order to guide the study of the potential causes of childhood cancer, which is a health issue worldwide.
The aim of the present scoping review is to analyze the available literature to detect specific environmental pollutants and their association with certain types of childhood cancer. In this study, we update and summarize the epidemiological evidence published in the last 10 years, assessing the association of prenatal and postnatal exposures to environmental factors with childhood cancer risk to push forward the investigations on the causes of childhood cancer as more preventable measures could be taken. Unlike other reviews on this topic, we have included all types of childhood cancer and not only the most frequent types of cancers; in addition, we have included prenatal exposures, adding value to our study because a broader revision was performed.
2. Results
A total of 6172 articles obtained from electronic MEDLINE (PuBMed) databases were retrieved, and an additional 7 were obtained from the lists of citations. Of these, 209 studies were eligible for this review because they fulfilled the inclusion criteria: (1) original or review epidemiological studies assessing the association between environmental pollution and childhood cancer; (2) published between 2013–2023; and (3) with abstracts in English or Spanish. Pollutants were categorized into the following groups: air pollution, pesticides, tobacco and alcohol exposure, indoor chemical exposure, electromagnetic fields, and radon. Studies regarding ionizing radiation from sources different from radon were excluded, as well as studies including populations other than children. Of these, 174 studies were retained after a second manual screening and elimination of studies that did not meet the inclusion criteria or were duplicated (Figure 1). Searching commands and the number of papers found in each search are described in Table 1. Table 2, Table 3, Table 4, Table 5, Table 6 and Table 7 summarize the 174 papers included in this review.
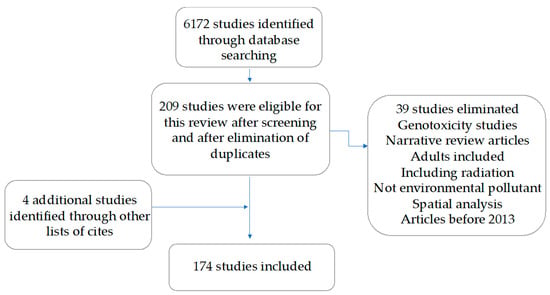
Figure 1.
Study selection process.

Table 1.
Number of articles retrieved by each searching command.

Table 2.
Studies assessing air pollution and childhood cancer risk.

Table 3.
Studies assessing exposure to pesticides and childhood cancer risk.

Table 4.
Studies assessing exposure to tobacco and alcohol and childhood cancer risk.

Table 5.
Studies assessing exposure to indoor chemicals and childhood cancer risk.

Table 6.
Studies assessing exposure to electromagnetic fields and childhood cancer risk.

Table 7.
Studies assessing indoor radon exposure and childhood cancer risk.
Most studies were case–control analyses—we found 69% of papers in this category—and 18% of studies were meta-analyses, systematic reviews, or both (Figure 2). The most investigated type of cancer was leukemia, accounting for 46% of papers reviewed. We found that 19% of the papers assessed three or more types of cancer, and 12% of papers investigated central nervous system cancer; 10% of the articles assessed Wilm’s tumor, retinoblastoma, hepatoblastoma, and other rare tumors (Figure 3). According to our categorization of results (positive association, low association, or no association with environmental pollution), the type of cancer with the most positive associations (78%) was Wilms tumor and other rare tumors, and the type of cancer with the lowest percentage of positive associations was leukemia, in which 60% of studies showed positive associations and 23% of the studies showed negative associations with environmental pollution (Figure 3). We found that 12% of studies analyzed nervous system cancer, and 72% of the studies showed positive associations with environmental pollution.
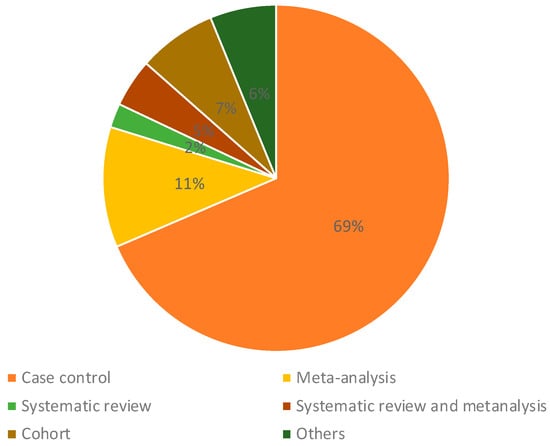
Figure 2.
Types of studies included in this review.
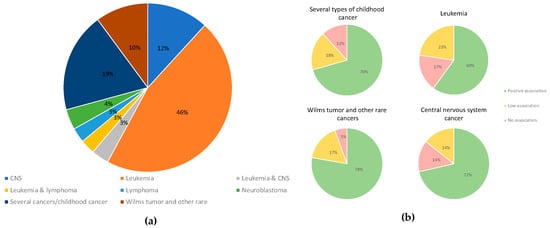
Figure 3.
(a) Types of cancer included in the studies evaluated. (b) The smaller circles show the percentage of papers reporting positive (light green), low (light yellow), or no (light red) associations.
Regarding the type of pollutant, we found that 88% of the investigations assessed chemical environmental factors, and 12% included physical factors such as electromagnetic fields (Figure 4). The most frequent type of environmental factor assessed was air pollution (29%), which included benzene, particulate matter, ultrafine particles, diesel engine exhaust, living in industrially contaminated sites, and living near road traffic. Studies assessing air pollution were followed by studies on pesticides (28%), papers including prenatal or parental exposure to tobacco, alcohol, coffee, and tea (16%), and papers assessing exposure to electromagnetic fields (12%); 11% of the studies assessed indoor chemical exposures (Figure 5).
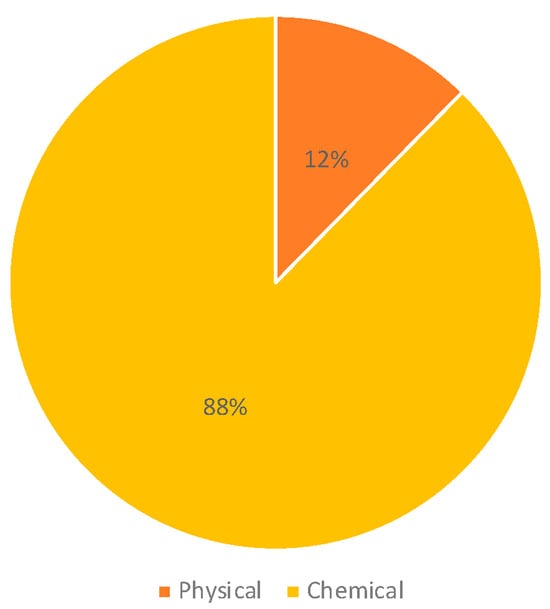
Figure 4.
Type of pollutants included in the studies.
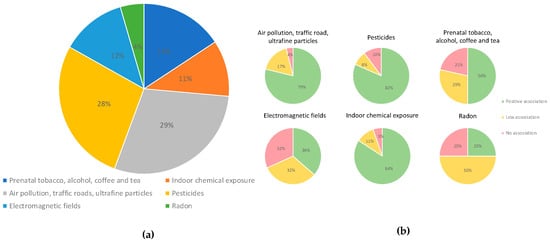
Figure 5.
(a) Types of environmental pollutants analyzed in the studies included. (b) The smaller circles show the percentage of papers demonstrating positive (green), low (blue), or no (red) associations with environmental pollution.
The highest percentage of positive associations (84%) was found in studies assessing indoor chemical exposures, which included volatile organic compounds such as benzene, hydrocarbon solvents, air pollution at the residence (NO2, PM2.5), polychlorinated biphenyls, home painting, home remodeling, and others. A high percentage of positive associations was also seen among studies assessing pesticides (82%) and air pollution (79%). A lower percentage of positive associations was observed among studies assessing prenatal exposure to tobacco and alcohol (50%) and radon (25%) (Figure 5).
As mentioned above, the type of cancer most frequently analyzed was leukemia, ranging from 68% in studies on electromagnetic fields to 40% in studies on air pollution. For all the pollutants, studies assessing more than one type of cancer have been performed, particularly for indoor chemical exposures (37%). An exclusive analysis of childhood CNS cancer has been included, particularly in studies on prenatal tobacco and alcohol exposure and pesticides. Although no studies were found exclusively assessing Wilms tumor and other rare cancers and exposure to electromagnetic fields or radon, these types of cancers could have been included in studies that investigated more than one type of cancer (category “childhood cancer”) (Figure 6).
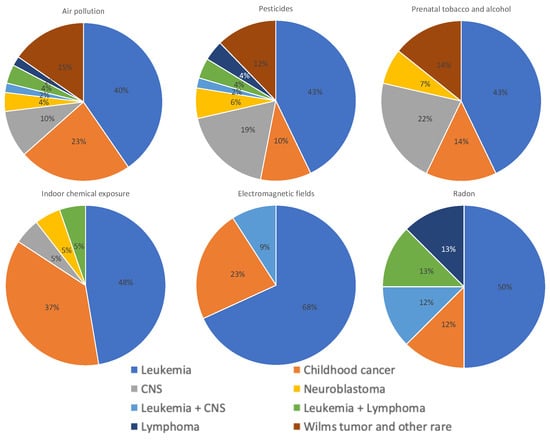
Figure 6.
Distribution of types of cancer assessed in studies on air pollution, pesticides, parental tobacco and alcohol, indoor chemicals, electromagnetic fields, and radon exposures. All studies, those with positive, low, or negative results, were included in this analysis.
An analysis focusing only on studies that found positive associations was performed, and it revealed that leukemia was mostly associated with pesticides and air pollution (33% and 27%, respectively). Studies assessing more than two types of childhood cancer found mostly an association with air pollution and indoor chemical exposure (43% and 26%), whereas studies on CNS tumors and neuroblastoma found associations with pesticides (53% and 43%). Studies assessing Wilms tumor and other rare cancers found an association mostly with air pollution (50%) (Figure 7). In addition, the analysis of only positive associations focusing on the type of pollutant showed that air pollution was associated with leukemia (34%), several types of childhood cancer (24%), and Wilms tumor and other rare cancers (17%); pesticides were associated with leukemia (42%), CNS tumors (20%), and Wilms tumor and other rare cancers (13%); prenatal tobacco and alcohol exposure was associated mostly with leukemia (50%); and indoor air pollution was associated mostly with leukemia (50%) and several types of cancer (38%) (Figure 8).
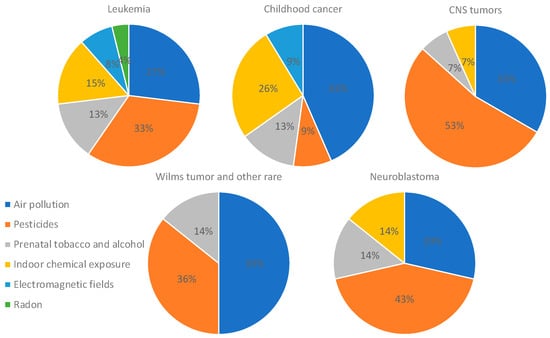
Figure 7.
Distribution of studies showing positive associations between each type of cancer and pollutant. Only studies that found positive associations were included in this analysis.
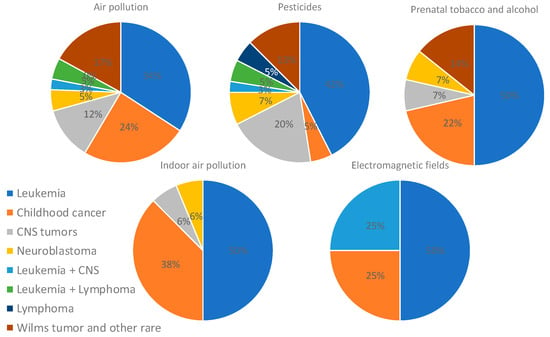
Figure 8.
Distribution of types of cancer assessed in studies on air pollution, pesticides, parental tobacco and alcohol, indoor chemicals, and electromagnetic fields exposures. Only studies showing positive associations were included for this analysis.
Regarding the type of exposure, most studies included postnatal exposure (53%), particularly studies assessing air pollution and pesticides (Figure 9); 23% of the studies analyzed maternal exposure to contaminants during pregnancy, particularly to air pollution, pesticides, and tobacco and alcohol exposures. Studies investigating parental exposures mostly included studies on pesticides and tobacco and alcohol consumption. Indoor chemical exposures were mostly assessed postnatally (Figure 10).
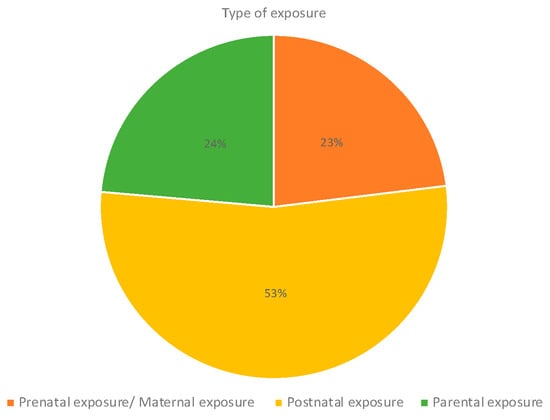
Figure 9.
Type of exposure.
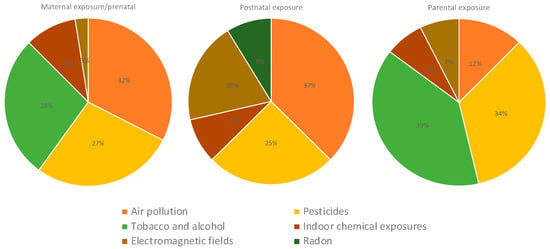
Figure 10.
Type of exposure in studies assessing air pollution, pesticides, tobacco and alcohol, indoor chemical, electromagnetic fields, and radon exposures.
3. Discussion
Environmental pollution is a global public health issue, particularly for susceptible groups such as children, who are vulnerable during their development [1]. Among the multiple childhood diseases associated with environmental pollution, cancer is of concern because it is a leading cause of death for children and adolescents, and the causes have not been fully understood [8]. The analysis of the potential environmental risk factors associated with childhood leukemia is important because preventable measures could be established. In the present review, we update and summarize the epidemiological evidence published in the last 10 years, assessing the association of prenatal and postnatal exposures to environmental factors with childhood cancer risk. Although relatively recent reviews on the topic are available in the literature, in the present study, we have updated the information to 2023 and also have included prenatal studies, which have not been included in recent reviews [2]. Moreover, compared to other recent reviews, we have focused not only on childhood leukemia, but have also included all types of childhood cancer [188]. The inclusion of all types of childhood cancer, as well as the inclusion of prenatal exposures, are the strengths of this review. Overlap of original studies among systematic reviews may exist, which is a limitation of this review. Other limitations of this study are the exclusive use research in PubMed and the exclusion of papers published before 2013. Thus, the conclusions should be taken with caution. The following paragraphs discuss our main findings on each of the groups of contaminants detected.
3.1. Air Pollution
More than one quarter of the literature (29%) retrieved included air pollution. This contaminant was mostly positively associated with leukemia, and it was frequently reported in studies that evaluated several types of childhood cancer (Figure 8). In addition, we observed that studies assessing Wilms tumor and other rare cancers found an association with air pollution (Figure 7). This is the second leading cause of non-communicable diseases globally, and has been classified by IARC as a human carcinogen regarding lung cancer. In addition, particulate matter and several other components of air pollution have also been classified as carcinogenic to humans. Moreover, air pollution is the most widespread environmental carcinogen. The predominant sources of outdoor air pollution are transportation, stationary power generation, industrial and agricultural emissions, and residential heating and cooking [189,190]. In the present study we have separated the analysis of outdoor air pollution from the studies on indoor air pollution. A wide number of studies evaluated air pollution and childhood cancer risk by assessing whether living nearby heavy-traffic roads or proximity to industrial and urban sites is associated with an increased cancer risk. Numerous studies have found positive associations not only with leukemia, but also with CNS cancer, neuroblastoma, Wilms tumor, and bone cancer, among other cancers (Table 2) [13,14,16,24,29,30,31,32,34,37,43,46,54,61]. However, other studies have found mild or negative associations [12,16,21,25,39,45,60,185]. Benzene is one of the major components of air pollution and is carcinogenic to the bone marrow, causing leukemia and myelodysplastic syndromes; it is suggested that it also affects the lymphatic system, causing lymphoma. It has been reported that benzene induces genotoxic and non-genotoxic events in utero, that could potentially lead to childhood leukemia [191]. In murine models bearing preleukemic cells, benzene induced fast leukemic transformation. Benzene metabolites can induce oxidative stress, genotoxicity, epigenetic modifications, aryl hydrocarbon receptor dysregulation, gene expression alterations, and apoptosis induction; these events can lead to the dysregulation of immune response and hematotoxicity, potentially contributing to leukemogenesis (Figure 11) [192]. Although these investigations on benzene could represent a plausible mechanism underlying the association of air pollution exposure and childhood leukemia, for other types of childhood cancer with a different etiology, the mechanisms must be further explored. In countries such as Mexico, where the incidence of childhood leukemia is high, the levels of benzene have been detected above the reference concentration in the urine of children living near shoe workshops [193]. Regulations on the air concentration of benzene and other established carcinogens must be applied as the evidence clearly shows their harmful potential.
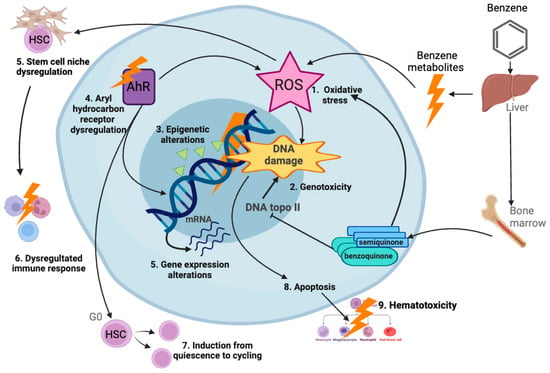
Figure 11.
Mechanisms of benzene-induced leukemia. Benzene is metabolized in liver, lung, and bone marrow, producing metabolites which exert different biological effects, including oxidative stress inducing reactive oxygen species (ROS); genotoxicity, including mutations, chromosome breaks, and aneuploidy; and epigenetic changes, dysregulation of aryl hydrocarbon receptor (AhR), alteration of gene expression, and apoptosis. Oxidative stress can dysregulate hematopoietic stem cell (HSC) niche and lead to a dysregulated immune response. Moreover, Ahr disruption can induce HSCs from quiescence (G0) to cycling, leading to hematotoxicity. In combination, these events can lead to leukemogenesis [192]. Created with BioRender.com.
3.2. Pesticides
The literature on pesticides (49 articles), together with air pollution (52 articles), represents more than one-half of the studies reviewed. Positive associations were found between pesticides and leukemia, as well as CNS tumors (Figure 8). Pesticides are used worldwide in several human activities, and tons of these chemicals are produced globally. However, the health effects of these products have been of concern because they are associated with diseases such as cancer [11]. To reduce environmental pollution and toxicity, research on bio-based pesticides is crucial, in order to have alternatives to replace chemical pesticides [194]. Although their association with childhood cancer has not been fully demonstrated, we found that more than 80% of the epidemiological studies show positive associations with this disease (Table 3, Figure 5). A recent study that evaluated pesticides in the urine samples of parents and children from five European countries reported that 84% of samples showed at least two pesticides, highlighting the global exposure to these chemicals [195]. Unfortunately, most of these studies lack the characterization and quantification of pesticides in the human body, and generally rely only on self-reported uses. In addition, humans are not exposed to a single type of pesticide but to a mixture of them, adding complexity to studies on the relationship between these pollutants and childhood cancer. Worldwide, studies have been performed and have shown that prenatal and postnatal exposure to pesticides is associated with higher risks, not only of leukemia, but also of lymphoma, retinoblastoma, neuroblastoma, CNS cancer, and Wilms tumor (Table 3, Figure 8).
Different classes of pesticides have been associated with childhood cancer, including organochlorides, organophosphates, and pyrethroids (Table 3). Regulatory agencies have considered several pesticides as carcinogens, such as DDT, but, for many others, such as pyrethroids, the evidence has not been enough to be catalogued as carcinogens [196]. The mechanisms underlying the associations of pesticides with childhood cancer may be different according to the type of pesticides. For pyrethroids, which are one of the most common classes of insecticides, evidence has shown that they can induce multiple biological effects potentially linked to cancer. Pyrethroids can induce genotoxic and non-genotoxic effects, and are immunotoxins, neurotoxins, oxidative stress producers, and endocrine disruptors; all these effects could promote the initiation or development of cancer (Figure 12) [197]. Importantly, our results revealed a high percentage of studies showing associations between CNS tumors, neuroblastoma, and pesticides (Figure 7). The mechanisms assessing the potential relationship of pesticides with these types of cancer should be explored. Although the genotoxicity of pesticides has been evaluated, their epigenetic effects need to be fully investigated because their contribution to childhood cancer etiology could be at this level [198]. In addition, although the risks of pesticide exposure have been vastly evaluated, the transgenerational effects of these exposures need more attention. It has been shown that the environmentally induced disease risk can be transmitted to the offspring, via epigenetic mechanisms through female and male germ lines [199].
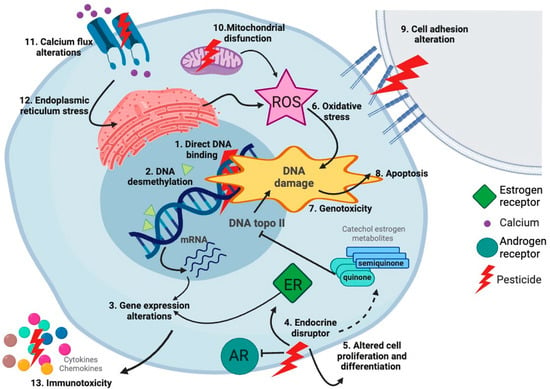
Figure 12.
Mechanisms of pesticide-induced cancer. Pesticides such as pyrethroids exert different biological events that can potentially promote cancer. Pyrethroids can bind DNA, induce epigenetic changes, alter gene expression, be endocrine disruptors, alter cell proliferation and differentiation, induce oxidative stress increasing reactive oxygen species (ROS), be genotoxic, induce apoptosis, alter cell adhesion, disrupt mitochondrial function, alter calcium flux, and induce endoplasmic reticulum stress. All these events in combination could drive leukemogenesis [197]. Created with BioRender.com.
3.3. Tobacco and Alcohol
We found that 16% (28 articles) of the studies are destined to investigate the association of tobacco and alcohol exposure with childhood cancer. However, other studies could have been missed by our searching strategy, given that we did not search “tobacco” or “alcohol” intentionally. The 28 articles presented here were found with the searching strategy described in the following sections. Contrasting with pesticides and air pollution, studies on tobacco and alcohol showed a lower percentage of positive associations. Only 50% of the studies showed a clear association with childhood cancer (Table 4, Figure 5), showing that the association of these pollutants with childhood cancer is even more contradictory. Studies suggest an association of tobacco and alcohol exposure with leukemia, CNS cancer, retinoblastoma, neuroblastoma, and Wilms tumor, particularly during the prenatal period. Although tobacco use accounts for 25% of all cancer deaths globally, and is the primary cause of lung cancer in adults, its role in childhood cancer is less clear [200]. Efforts to reveal whether tobacco and alcohol exposures are related to childhood cancer are needed because these exposures are completely preventable. It is known that cigarettes contain dozens of carcinogens, including benzene, whose leukemogenic potential has already been discussed [201].
3.4. Indoor Chemical Exposure
Studies on chemical exposure at home accounted for 11% of the articles included. For the purposes of this review, indoor exposure was separated from outdoor exposure. However, several of the pollutants are shared between indoor and outdoor exposures, including NO2, benzene, and VOCs; in addition, chemical exposures at home may include paints and other solvents used to clean or carry out home remodeling (Table 5). As expected, as seen with air pollution analysis, a high number of studies showing positive associations between indoor chemical exposures and childhood cancer were observed (Figure 5 and Figure 8). The mechanisms underlying the role of indoor chemical exposure and childhood cancer are expected to be like the mechanisms supporting the associations with outdoor air pollution exposures. We have to consider that the concentrations of chemicals indoors and outdoors might be different. Efforts to inform people of the risks of using certain chemicals at home, such as paints, as well as measures helping to improve indoor air quality, such as correct ventilation or optimal cooking practices, should be improved, particularly in homes with pregnant women and children [7].
3.5. Electromagnetic Fields
Our search strategy retrieved 22 articles that investigated electromagnetic fields and childhood cancer, which represented 12% of the studies included. Given the global expansion in wireless networks, concern has been raised regarding the possible health effects of low-to-mid-frequency electromagnetic fields (LM-EMFs). LM-EFs include extremely low-frequency EMFs (EL-EMFs) which are produced by power lines, electrical wiring, and electrical appliances; and radiofrequency EMFs, which mostly come from wireless telecommunication devices, including cell phones, tablets, and laptop computers [202]. Contrasting to higher-frequency EMFs which includes X-rays and gamma rays and are in the ionizing radiation part of the electromagnetic spectrum, LM-EFs are not known to damage DNA or cells directly. However, other potential cellular effects of LM-EFs have been of concern [203]. The studies retrieved by our search showed a low number of positive associations between EMFs and childhood cancer (36%) (Figure 5), showing a higher controversy of results compared to other environmental risk factors. The studies assessing EMFs mostly included childhood leukemia (Figure 6), showing that further studies are needed to evaluate other specific types of cancer. Although some studies have shown that prenatal and postnatal exposure to EMFs can increase the risk of childhood leukemia (Table 6), the most recent studies have shown mild to no associations [158,159,160].
3.6. Radon
Few studies regarding radon exposure and childhood cancer were retrieved by our search strategy (8 studies). Importantly, we did not include “radon” in the search strategy. Possibly, other studies, including radon and childhood cancer, were missed by our strategy. Ionizing radiation exposure at therapeutic doses was beyond the scope of this review as it is a well-established childhood cancer risk factor. Natural radiation comes from radon exposure, and its relationship with childhood cancer is unclear. Although studies have suggested that domestic radon exposure is associated with higher childhood leukemia risk [180,181], others have shown mild to no association, neither with childhood cancer nor with other cancers such as lymphoma and CNS cancer [102,183,185,186,187]. Thus, the possible role of radon in childhood cancer remains controversial and needs further investigation.
Compared to previous recent reviews, some coincidences have been detected in our analysis. Regarding childhood leukemia, an umbrella review in 2021 found convincing evidence of an association between general prenatal pesticide exposure and the risk of this type of cancer; this result was also retrieved by our study. In the same study, the authors found some level of evidence of an association of EMFs, benzene, indoor air pollution, and prenatal tobacco exposure with childhood leukemia. Our results coincide with this previous study except for EMF exposure, because our study retrieved mostly low or negative results for EMFs. However, this needs to be taken with caution as few articles regarding EMFs were included in this study [188]. A more recent review of the same author confirmed the convincing evidence of the pesticide exposure association with acute lymphoblastic leukemia risk, and found little evidence for radon association with this cancer [204]. In addition, a 2021 scoping review of environmental risk factors and all types of childhood cancer mostly found articles regarding air pollution, chemical exposures, radiation, and residential locations. Our study agrees with finding a great number of articles regarding air pollution and chemical exposures. However, the previously reported scoping review did not include prenatal exposures, and pesticide exposures were included in the category of chemical exposures together with other types of chemicals [2].
Our results show that several studies have found a positive association between certain types of cancer and specific pollutants. Given its highest frequency, most pollutants were expected to be associated with childhood leukemia. We observed that, together, indoor and outdoor air pollution add up to 42% of the studies assessing causes of childhood leukemia and showing positive results, and pesticides represented 33%. Regarding CNS tumors and neuroblastoma, 53% and 43% of the studies, respectively, presented positive associations with pesticides. Studies that analyzed more than two types of childhood cancer found associations with indoor and outdoor air pollution (69%). Although epidemiological data strongly suggest associations of air pollution and pesticides with childhood cancer, particularly leukemia and SNC tumors, more mechanistic evidence is needed. Studies are required to demonstrate that prenatal or postnatal exposure to these chemicals can promote biological events associated with leukemia and brain tumor etiology, such as specific gene mutations, epigenetic changes, the modification of signaling pathways, increased proliferation, and escape from apoptosis. In utero studies with animal models are particularly valuable, given the prenatal origin of childhood cancer. Additionally, analyses of exposomes from cancer patients and controls are valuable for detecting specific levels of chemicals included in the global groups of “air pollution” and “pesticides”. Research on the effects of individual chemicals is important, but it is also needed for evaluating how all these molecules interact with each other and collaborate in promoting childhood cancer, which could be achieved through AI models. On the other hand, the epidemiological evidence regarding electromagnetic fields is still controversial, and mostly negative results were found in our review. Thus, more epidemiological studies are needed regarding electromagnetic field exposure and childhood cancer risk.
This review shows that, in particular, outdoor and indoor air pollution and pesticides are associated with childhood cancer in numerous studies, which could help guide the research into the causes of childhood cancer. In addition, regulatory measures could be considered based on these results. For example, the re-evaluation of specific contaminants is necessary in order to update their classifications as carcinogens. Additionally, more regulatory measures are needed to improve the air quality in countries like Mexico City, which has a history of high environmental pollution. Furthermore, disseminating information and education to the global population on the safe use of chemicals at home is important in order to avoid high exposure indoors. Regulatory measures are urgently needed to prevent childhood cancer, which is a global health issue with a worryingly increasing incidence. It is important to highlight that this review shows limitations such as the overlap of original studies and systematic reviews; therefore, the conclusions should be taken with caution. The strengths of this review are the inclusion of all types of childhood cancer because most reviews focus only on specific types of cancer, and the inclusion of prenatal exposures which has also been eliminated in some review studies. Prenatal exposures are important given the intrauterine origin of childhood cancer. It is hypothesized that genetic alterations may arise in utero, and, later in life, this could drive carcinogenesis, if secondary mutations arise along with additional epigenetic events. Detecting environmental exposures that could induce prenatal genetic alterations is important for the prevention of childhood cancer.
4. Materials and Methods
4.1. Data Source and Search Strategy
An extensive peer-reviewed original epidemiological search of MEDLINE (PuBMed) database was conducted in January and June 2023 to identify studies regarding childhood cancer and environmental factors. Three researchers participated independently in the search. Studies included were limited to studies on humans. The following search strategy was applied: “child” OR “childhood” AND “cancer” AND “pollution”, “pesticides”, “magnetic fields”, “benzene”, “smoke”, “water pollution”, and “air pollution”. Studies regarding radiation and childhood cancer were not included in this study as radiation is a well-established cause of childhood cancer. However, exposure to indoor radon was included as controversy still exists. Searching was performed through titles and abstract screening, and, when needed, additional information was obtained from the main article. Reference lists of articles were examined for additional relevant literature.
4.2. Study Selection
Criteria for inclusion and exclusion were defined previously. We included articles published between 2013 and 2023. No geographical or language restrictions were applied. However, when the information of interest was not available in abstracts, studies in languages other than English or Spanish were excluded. Studies selected included case–control studies, cohort studies, ecological studies, systematic reviews, and meta-analyses assessing environmental exposure and childhood cancer. Exposure times included prenatal and childhood time windows. Studies assessing parental exposures were also included. Risk assessment studies, in vitro and in vivo assays, and narrative, scoping, or umbrella reviews were not included but were evaluated for additional literature. Duplicates were eliminated using Zotero 6.0.32.
4.3. Data Extraction
For each included article, we recorded the first author’s name and year of publication, type of childhood cancer included, type of pollutant investigated, country, study design, main outcomes and results, and number of participants. Data were summarized in a table.
4.4. Data Analysis
A second review was performed to eliminate articles that did not meet all the criteria described in Section 4.2. Data were organized on graphics containing the type of environmental factor, type of cancer analyzed, type of study, type of exposure, and type of association. Each outcome was evaluated and categorized as “positive association”, when a clear and significant association between the risk factor and childhood cancer was reported; “low association” when results did show some association but were taken with caution because confounding factors and bias were not excluded; and “no association” when results did not show an association between the investigated risk factor and childhood cancer.
5. Conclusions
Environmental pollution and childhood cancer are both worldwide health issues that deserve considerable attention. A vast amount of literature shows that childhood cancer risk may be related to environmental prenatal and early childhood exposures. According to the findings of this scoping review, prenatal and postnatal exposures to indoor and outdoor air pollution and to pesticides seem to be positively associated with childhood cancer risks, including leukemia, CNS cancer, Wilms tumor, and other rare childhood cancers, given the high percentage of studies showing positive associations. There is some evidence linking radon and electromagnetic field exposure to pediatric cancer; however, the correlation is not strong, given the large amount of research demonstrating weak or negative relationships. However, some limitations of this review must be considered, including the overlap of original studies across systematic reviews.
Further studies are needed to investigate the mechanisms underlying the potential associations between air pollution, pesticide exposure, and childhood cancer risk. Determining the specific air pollution components, as well as the group of pesticides, related to childhood cancer etiology and their mechanisms could help to establish better regulatory preventive measures, and to detect vulnerable populations. Besides genotoxicity and carcinogenicity studies, investigations assessing other potential biological effects, including epigenetic modifications, are necessary. Additionally, studies assessing the potential transgenerational effects of these pollutants regarding childhood cancer are needed.
Author Contributions
Conceptualization, data search, data analysis, data summary, data curation, and writing—original draft preparation, M.d.P.N.-M.; data search, data analysis, data summary, and data curation, C.S.-L.; data analysis, data summary, and data curation, F.G.-C.; and data analysis, data summary, data curation, and manuscript supervision, P.P.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Instituto Nacional de Pediatria Recursos Fiscales 2024.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Buser, J.M.; Lake, K.; Ginier, E. Environmental Risk Factors for Childhood Cancer in an Era of Global Climate Change: A Scoping Review. J. Pediatr. Health Care Off. Publ. Natl. Assoc. Pediatr. Nurse Assoc. Pract. 2022, 36, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Cazzolla Gatti, R. Why We Will Continue to Lose Our Battle with Cancers If We Do Not Stop Their Triggers from Environmental Pollution. Int. J. Environ. Res. Public Health 2021, 18, 6107. [Google Scholar] [CrossRef] [PubMed]
- Childhood Cancer Inequalities in the WHO European Region. Available online: https://www.who.int/europe/publications/i/item/9789289057615 (accessed on 30 October 2023).
- PAHO WHO|Pan American Health Organization. Childhood and Adolescence Cancer. Available online: https://www.paho.org/en/topics/childhood-and-adolescence-cancer (accessed on 30 October 2023).
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Fucic, A.; Guszak, V.; Mantovani, A. Transplacental exposure to environmental carcinogens: Association with childhood cancer risks and the role of modulating factors. Reprod. Toxicol. 2017, 72, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.; Hornhardt, S.; Erdmann, F.; Sánchez-García, I.; Fischer, U.; Schüz, J.; Ziegelberger, G. Risk Factors for Childhood Leukemia: Radiation and Beyond. Front. Public Health 2021, 9, 805757. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat. Rev. Cancer 2018, 18, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Risk Factors and Causes of Childhood Cancer. Available online: https://www.cancer.org/cancer/cancer-in-children/risk-factors-and-causes.html (accessed on 30 October 2023).
- Iqbal, S.; Ali, S.; Ali, I. Maternal pesticide exposure and its relation to childhood cancer: An umbrella review of meta-analyses. Int. J. Environ. Health Res. 2022, 32, 1609–1627. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, R.; Morimoto, L.M.; Longcore, T.; Franklin, M.; Rogne, T.; Metayer, C.; Wiemels, J.L.; Ma, X. Outdoor artificial light at night, air pollution, and risk of childhood acute lymphoblastic leukemia in the California Linkage Study of Early-Onset Cancers. Sci. Rep. 2023, 13, 583. [Google Scholar] [CrossRef]
- Malavolti, M.; Malagoli, C.; Filippini, T.; Wise, L.A.; Bellelli, A.; Palazzi, G.; Cellini, M.; Costanzini, S.; Teggi, S.; Vinceti, M. Residential proximity to petrol stations and risk of childhood leukemia. Eur. J. Epidemiol. 2023, 38, 771–782. [Google Scholar] [CrossRef]
- Kreis, C.; Héritier, H.; Scheinemann, K.; Hengartner, H.; de Hoogh, K.; Röösli, M.; Spycher, B.D. Childhood cancer and traffic-related air pollution in Switzerland: A nationwide census-based cohort study. Environ. Int. 2022, 166, 107380. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Lee, T.H.; Kim, S.; Song, M.; Bae, S. Association between long-term exposure to particulate matter and childhood cancer: A retrospective cohort study. Environ. Res. 2022, 205, 112418. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, A.; Konstantinoudis, G.; Kreis, C.; Diezi, M.; Ammann, R.A.; Zwahlen, M.; Kühni, C.; Spycher, B.D. Childhood cancer and residential proximity to petrol stations: A nationwide registry-based case-control study in Switzerland and an updated meta-analysis. Int. Arch. Occup. Environ. Health 2022, 95, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Asenjo, S.; Nuñez, O.; Segú-Tell, J.; Pardo Romaguera, E.; Cañete Nieto, A.; Martín-Méndez, I.; Bel-Lan, A.; García-Pérez, J.; Cárceles-Álvarez, A.; Ortega-García, J.A.; et al. Cadmium [Cd] and Lead [Pb] topsoil levels and incidence of childhood leukemias. Environ. Geochem. Health 2022, 44, 2341–2354. [Google Scholar] [CrossRef] [PubMed]
- Onyije, F.M.; Hosseini, B.; Togawa, K.; Schüz, J.; Olsson, A. Cancer Incidence and Mortality among Petroleum Industry Workers and Residents Living in Oil Producing Communities: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 4343. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.G.; Vermeulen, R.; Cardoso, M.R.A.; de Oliveira Latorre, M.d.R.D.; Hystad, P.; Downward, G.S.; Nardocci, A.C. Residential traffic exposure and lymphohematopoietic malignancies among children in the city of São Paulo, Brazil: An ecological study. Cancer Epidemiol. 2021, 70, 101859. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, E.; Lima, I.; Hatzopoulou, M.; Van Ryswyk, K.; van Donkelaar, A.; Martin, R.V.; Chen, H.; Stieb, D.M.; Crighton, E.; Burnett, R.T.; et al. Ambient ultrafine particle concentrations and incidence of childhood cancers. Environ. Int. 2020, 145, 106135. [Google Scholar] [CrossRef]
- Hvidtfeldt, U.A.; Erdmann, F.; Urhoj, S.K.; Brandt, J.; Geels, C.; Ketzel, M.; Frohn, L.M.; Christensen, J.H.; Sørensen, M.; Raaschou-Nielsen, O. Residential Exposure to PM2.5 Components and Risk of Childhood Non-Hodgkin Lymphoma in Denmark: A Nationwide Register-Based Case-Control Study. Int. J. Environ. Res. Public Health 2020, 17, 8949. [Google Scholar] [CrossRef]
- Volk, J.; Heck, J.E.; Schmiegelow, K.; Hansen, J. Parental occupational exposure to diesel engine exhaust in relation to childhood leukaemia and central nervous system cancers: A register-based nested case-control study in Denmark 1968–2016. Occup. Environ. Med. 2019, 76, 809–817. [Google Scholar] [CrossRef]
- Peckham-Gregory, E.C.; Ton, M.; Rabin, K.R.; Danysh, H.E.; Scheurer, M.E.; Lupo, P.J. Maternal Residential Proximity to Major Roadways and the Risk of Childhood Acute Leukemia: A Population-Based Case-Control Study in Texas, 1995–2011. Int. J. Environ. Res. Public Health 2019, 16, 2029. [Google Scholar] [CrossRef]
- García-Pérez, J.; Gómez-Barroso, D.; Tamayo-Uria, I.; Ramis, R. Methodological approaches to the study of cancer risk in the vicinity of pollution sources: The experience of a population-based case-control study of childhood cancer. Int. J. Health Geogr. 2019, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.H.; Li, J.; Wang, X.Y.; Yu, Y.; Ren, M.M.; Zhou, J. A Meta-analysis of Traffic-related Air Pollution and Risk of Childhood Leukemia. J. Pediatr. Hematol. Oncol. 2019, 41, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Hatch, E.E.; Rothman, K.J.; Heck, J.E.; Park, A.S.; Crippa, A.; Orsini, N.; Vinceti, M. Association between Outdoor Air Pollution and Childhood Leukemia: A Systematic Review and Dose-Response Meta-Analysis. Environ. Health Perspect. 2019, 127, 46002. [Google Scholar] [CrossRef] [PubMed]
- Seifi, M.; Niazi, S.; Johnson, G.; Nodehi, V.; Yunesian, M. Exposure to ambient air pollution and risk of childhood cancers: A population-based study in Tehran, Iran. Sci. Total Environ. 2019, 646, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Heck, J.E.; Ritz, B.; Cockburn, M.; Escobedo, L.A.; von Ehrenstein, O.S. Prenatal Exposure to Air Toxics and Malignant Germ Cell Tumors in Young Children. J. Occup. Environ. Med. 2019, 61, 529–534. [Google Scholar] [CrossRef]
- Iavarone, I.; Buzzoni, C.; Stoppa, G.; Steliarova-Foucher, E.; SENTIERI-AIRTUM Working Group. Cancer incidence in children and young adults living in industrially contaminated sites: From the Italian experience to the development of an international surveillance system. Epidemiol. Prev. 2018, 42, 76–85. [Google Scholar] [PubMed]
- Kirkeleit, J.; Riise, T.; Bjørge, T.; Christiani, D.C.; Bråtveit, M.; Baccarelli, A.; Mattioli, S.; Hollund, B.E.; Gjertsen, B.T. Maternal exposure to gasoline and exhaust increases the risk of childhood leukaemia in offspring—A prospective study in the Norwegian Mother and Child Cohort Study. Br. J. Cancer 2018, 119, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.V.; Lupo, P.J.; Pompeii, L.A.; Danysh, H.E. Maternal Residential Proximity to Major Roadways and Pediatric Embryonal Tumors in Offspring. Int. J. Environ. Res. Public Health 2018, 15, 505. [Google Scholar] [CrossRef]
- Ortega-García, J.A.; López-Hernández, F.A.; Cárceles-Álvarez, A.; Fuster-Soler, J.L.; Sotomayor, D.I.; Ramis, R. Childhood cancer in small geographical areas and proximity to air-polluting industries. Environ. Res. 2017, 156, 63–73. [Google Scholar] [CrossRef]
- Janitz, A.E.; Campbell, J.E.; Magzamen, S.; Pate, A.; Stoner, J.A.; Peck, J.D. Benzene and childhood acute leukemia in Oklahoma. Environ. Res. 2017, 158, 167–173. [Google Scholar] [CrossRef]
- Ramis, R.; Tamayo-Uria, I.; Gómez-Barroso, D.; López-Abente, G.; Morales-Piga, A.; Pardo Romaguera, E.; Aragones, N.; García-Pérez, J. Risk factors for central nervous system tumors in children: New findings from a case-control study. PLoS ONE 2017, 12, e0171881. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, É.; Bélair, M.A.; Do, M.T.; Stieb, D.M.; Hystad, P.; van Donkelaar, A.; Martin, R.V.; Crouse, D.L.; Crighton, E.; Chen, H.; et al. Maternal exposure to ambient air pollution and risk of early childhood cancers: A population-based study in Ontario, Canada. Environ. Int. 2017, 100, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Spycher, B.D.; Lupatsch, J.E.; Huss, A.; Rischewski, J.; Schindera, C.; Spoerri, A.; Vermeulen, R.; Kuehni, C.E.; Swiss Paediatric Oncology Group; Swiss National Cohort Study Group. Parental occupational exposure to benzene and the risk of childhood cancer: A census-based cohort study. Environ. Int. 2017, 108, 84–91. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, J.; Morales-Piga, A.; Gómez-Barroso, D.; Tamayo-Uria, I.; Pardo Romaguera, E.; López-Abente, G.; Ramis, R. Risk of bone tumors in children and residential proximity to industrial and urban areas: New findings from a case-control study. Sci. Total Environ. 2017, 579, 1333–1342. [Google Scholar] [CrossRef]
- Janitz, A.E.; Ramachandran, G.; Tomlinson, G.E.; Krailo, M.; Richardson, M.; Spector, L. Maternal and paternal occupational exposures and hepatoblastoma: Results from the HOPE study through the Children’s Oncology Group. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Janitz, A.E.; Campbell, J.E.; Magzamen, S.; Pate, A.; Stoner, J.A.; Peck, J.D. Traffic-related air pollution and childhood acute leukemia in Oklahoma. Environ. Res. 2016, 148, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Danysh, H.E.; Zhang, K.; Mitchell, L.E.; Scheurer, M.E.; Lupo, P.J. Maternal residential proximity to major roadways at delivery and childhood central nervous system tumors. Environ. Res. 2016, 146, 315–322. [Google Scholar] [CrossRef]
- von Ehrenstein, O.S.; Heck, J.E.; Park, A.S.; Cockburn, M.; Escobedo, L.; Ritz, B. In Utero and Early-Life Exposure to Ambient Air Toxics and Childhood Brain Tumors: A Population-Based Case-Control Study in California, USA. Environ. Health Perspect. 2016, 124, 1093–1099. [Google Scholar] [CrossRef]
- Symanski, E.; Tee Lewis, P.G.; Chen, T.Y.; Chan, W.; Lai, D.; Ma, X. Air toxics and early childhood acute lymphocytic leukemia in Texas, a population based case control study. Environ. Health Glob. Access Sci. Source 2016, 15, 70. [Google Scholar] [CrossRef]
- Magnani, C.; Ranucci, A.; Badaloni, C.; Cesaroni, G.; Ferrante, D.; Miligi, L.; Mattioli, S.; Rondelli, R.; Bisanti, L.; Zambon, P.; et al. Road Traffic Pollution and Childhood Leukemia: A Nationwide Case-control Study in Italy. Arch. Med. Res. 2016, 47, 694–705. [Google Scholar] [CrossRef]
- García-Pérez, J.; Morales-Piga, A.; Gómez, J.; Gómez-Barroso, D.; Tamayo-Uria, I.; Romaguera, E.P.; Fernández-Navarro, P.; López-Abente, G.; Ramis, R. Association between residential proximity to environmental pollution sources and childhood renal tumors. Environ. Res. 2016, 147, 405–414. [Google Scholar] [CrossRef]
- García-Pérez, J.; Morales-Piga, A.; Gómez-Barroso, D.; Tamayo-Uria, I.; Pardo Romaguera, E.; López-Abente, G.; Ramis, R. Residential proximity to environmental pollution sources and risk of rare tumors in children. Environ. Res. 2016, 151, 265–274. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, J.; Morales-Piga, A.; Gómez-Barroso, D.; Tamayo-Uria, I.; Pardo Romaguera, E.; Fernández-Navarro, P.; Lopez-Abente, G.; Ramis, R. Risk of neuroblastoma and residential proximity to industrial and urban sites: A case-control study. Environ. Int. 2016, 92–93, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Carlos-Wallace, F.M.; Zhang, L.; Smith, M.T.; Rader, G.; Steinmaus, C. Parental, In Utero, and Early-Life Exposure to Benzene and the Risk of Childhood Leukemia: A Meta-Analysis. Am. J. Epidemiol. 2016, 183, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Heck, J.E.; Malagoli, C.; Del Giovane, C.; Vinceti, M. A review and meta-analysis of outdoor air pollution and risk of childhood leukemia. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2015, 33, 36–66. [Google Scholar] [CrossRef] [PubMed]
- Spycher, B.D.; Feller, M.; Röösli, M.; Ammann, R.A.; Diezi, M.; Egger, M.; Kuehni, C.E. Childhood cancer and residential exposure to highways: A nationwide cohort study. Eur. J. Epidemiol. 2015, 30, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Malagoli, C.; Malavolti, M.; Costanzini, S.; Fabbri, S.; Tezzi, S.; Palazzi, G.; Arcolin, E.; Vinceti, M. Increased incidence of childhood leukemia in urban areas: A population-based case-control study. Epidemiol. Prev. 2015, 39 (Suppl. S1), 102–107. [Google Scholar]
- Heck, J.E.; Park, A.S.; Qiu, J.; Cockburn, M.; Ritz, B. Retinoblastoma and ambient exposure to air toxics in the perinatal period. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 182–186. [Google Scholar] [CrossRef]
- Houot, J.; Marquant, F.; Goujon, S.; Faure, L.; Honoré, C.; Roth, M.H.; Hémon, D.; Clavel, J. Residential Proximity to Heavy-Traffic Roads, Benzene Exposure, and Childhood Leukemia-The GEOCAP Study, 2002–2007. Am. J. Epidemiol. 2015, 182, 685–693. [Google Scholar] [CrossRef]
- Greenop, K.R.; Hinwood, A.L.; Fritschi, L.; Scott, R.J.; Attia, J.; Ashton, L.J.; Heath, J.A.; Armstrong, B.K.; Milne, E. Vehicle refuelling, use of domestic wood heaters and the risk of childhood brain tumours: Results from an Australian case-control study. Pediatr. Blood Cancer 2015, 62, 229–234. [Google Scholar] [CrossRef]
- García-Pérez, J.; López-Abente, G.; Gómez-Barroso, D.; Morales-Piga, A.; Romaguera, E.P.; Tamayo, I.; Fernández-Navarro, P.; Ramis, R. Childhood leukemia and residential proximity to industrial and urban sites. Environ. Res. 2015, 140, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, S.; Li, Z.; Zhu, J.; Bi, Y.; Bai, Y.; Wang, H. Maternal benzene exposure during pregnancy and risk of childhood acute lymphoblastic leukemia: A meta-analysis of epidemiologic studies. PLoS ONE 2014, 9, e110466. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Ritz, B.; Wilhelm, M.; Qiu, J.; Cockburn, M.; Heck, J.E. Prenatal exposure to air toxics and risk of Wilms tumor in 0- to 5-year-old children. J. Occup. Environ. Med. 2014, 56, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Boothe, V.L.; Boehmer, T.K.; Wendel, A.M.; Yip, F.Y. Residential traffic exposure and childhood leukemia: A systematic review and meta-analysis. Am. J. Prev. Med. 2014, 46, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Heck, J.E.; Park, A.S.; Qiu, J.; Cockburn, M.; Ritz, B. Risk of leukemia in relation to exposure to ambient air toxics in pregnancy and early childhood. Int. J. Hyg. Environ. Health 2014, 217, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Badaloni, C.; Ranucci, A.; Cesaroni, G.; Zanini, G.; Vienneau, D.; Al-Aidrous, F.; De Hoogh, K.; Magnani, C.; Forastiere, F.; SETIL Study Group. Air pollution and childhood leukaemia: A nationwide case-control study in Italy. Occup. Environ. Med. 2013, 70, 876–883. [Google Scholar] [CrossRef]
- Heck, J.E.; Wu, J.; Lombardi, C.; Qiu, J.; Meyers, T.J.; Wilhelm, M.; Cockburn, M.; Ritz, B. Childhood cancer and traffic-related air pollution exposure in pregnancy and early life. Environ. Health Perspect. 2013, 121, 1385–1391. [Google Scholar] [CrossRef]
- Ghosh, J.K.C.; Heck, J.E.; Cockburn, M.; Su, J.; Jerrett, M.; Ritz, B. Prenatal exposure to traffic-related air pollution and risk of early childhood cancers. Am. J. Epidemiol. 2013, 178, 1233–1239. [Google Scholar] [CrossRef]
- Peters, S.; Glass, D.C.; Reid, A.; de Klerk, N.; Armstrong, B.K.; Kellie, S.; Ashton, L.J.; Milne, E.; Fritschi, L. Parental occupational exposure to engine exhausts and childhood brain tumors. Int. J. Cancer 2013, 132, 2975–2979. [Google Scholar] [CrossRef]
- Heck, J.E.; Park, A.S.; Qiu, J.; Cockburn, M.; Ritz, B. An exploratory study of ambient air toxics exposure in pregnancy and the risk of neuroblastoma in offspring. Environ. Res. 2013, 127, 1–6. [Google Scholar] [CrossRef]
- Ward, M.H.; Madrigal, J.M.; Jones, R.R.; Friesen, M.C.; Falk, R.T.; Koebel, D.; Metayer, C. Glyphosate in house dust and risk of childhood acute lymphoblastic leukemia in California. Environ. Int. 2023, 172, 107777. [Google Scholar] [CrossRef] [PubMed]
- Rafeeinia, A.; Asadikaram, G.; Moazed, V.; Darabi, M.K. Organochlorine pesticides may induce leukemia by methylation of CDKN2B and MGMT promoters and histone modifications. Gene 2023, 851, 146976. [Google Scholar] [CrossRef] [PubMed]
- Rossides, M.; Kampitsi, C.E.; Talbäck, M.; Mogensen, H.; Wiebert, P.; Tettamanti, G.; Feychting, M. Occupational exposure to pesticides in mothers and fathers and risk of cancer in the offspring: A register-based case-control study from Sweden [1960–2015]. Environ. Res. 2022, 214 Pt 1, 113820. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; Ritz, B.; Cockburn, M.; Heck, J.E. Prenatal ambient pesticide exposure and childhood retinoblastoma. Int. J. Hyg. Environ. Health 2022, 245, 114025. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Feulefack, J.; Sergi, C.M. Pre-conceptional and prenatal exposure to pesticides and pediatric neuroblastoma. A meta-analysis of nine studies. Environ. Toxicol. Pharmacol. 2022, 90, 103790. [Google Scholar] [CrossRef]
- El-Helaly, S.; Khashaba, E.; El Domiaty, H.; Darwish, A. Parental occupational and environmental risk factors for childhood bone cancer in Mansoura oncology center: A case control study. Int. J. Environ. Health Res. 2022, 34, 248–256. [Google Scholar] [CrossRef]
- Khan, A.; Feulefack, J.; Sergi, C.M. Exposure to pesticides and pediatric Wilms’ tumor. A meta-analysis on pre-conception and pregnancy parental exposure with an IARC/WHO commentary. Hum. Exp. Toxicol. 2022, 41, 9603271221136211. [Google Scholar] [CrossRef] [PubMed]
- Bamouni, S.; Hémon, D.; Faure, L.; Clavel, J.; Goujon, S. Residential proximity to croplands at birth and childhood leukaemia. Environ. Health Glob. Access Sci. Source 2022, 21, 103. [Google Scholar] [CrossRef]
- Onyije, F.M.; Olsson, A.; Erdmann, F.; Magnani, C.; Petridou, E.; Clavel, J.; Miligi, L.; Bonaventure, A.; Ferrante, D.; Piro, S.; et al. Parental occupational exposure to combustion products, metals, silica and asbestos and risk of childhood leukaemia: Findings from the Childhood Cancer and Leukaemia International Consortium [CLIC]. Environ. Int. 2022, 167, 107409. [Google Scholar] [CrossRef]
- Feulefack, J.; Khan, A.; Forastiere, F.; Sergi, C.M. Parental Pesticide Exposure and Childhood Brain Cancer: A Systematic Review and Meta-Analysis Confirming the IARC/WHO Monographs on Some Organophosphate Insecticides and Herbicides. Children 2021, 8, 1096. [Google Scholar] [CrossRef]
- Nguyen, A.; Crespi, C.M.; Vergara, X.; Kheifets, L. Commercial outdoor plant nurseries as a confounder for electromagnetic fields and childhood leukemia risk. Environ. Res. 2022, 212, 113446. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, J.M.; Jones, R.R.; Gunier, R.B.; Whitehead, T.P.; Reynolds, P.; Metayer, C.; Ward, M.H. Residential exposure to carbamate, organophosphate, and pyrethroid insecticides in house dust and risk of childhood acute lymphoblastic leukemia. Environ. Res. 2021, 201, 111501. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.; Bailey, H.D.; Kartal-Kaess, M.; Renella, R.; Berthet, A.; Spycher, B.D. Parental occupational exposure to pesticides and risk of childhood cancer in Switzerland: A census-based cohort study. BMC Cancer 2020, 20, 819. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.M.; Jones, R.R.; Booth, B.J.; Olsson, A.C.; Kromhout, H.; Straif, K.; Vermeulen, R.; Tikellis, G.; Paltiel, O.; Golding, J.; et al. Parental occupational exposure to pesticides, animals and organic dust and risk of childhood leukemia and central nervous system tumors: Findings from the International Childhood Cancer Cohort Consortium [I4C]. Int. J. Cancer 2020, 146, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Park, A.S.; Ritz, B.; Yu, F.; Cockburn, M.; Heck, J.E. Prenatal pesticide exposure and childhood leukemia—A California statewide case-control study. Int. J. Hyg. Environ. Health 2020, 226, 113486. [Google Scholar] [CrossRef]
- Mavoungou, S.; Rios, P.; Pacquement, H.; Nolla, M.; Rigaud, C.; Simonin, M.; Bertrand, Y.; Lambilliotte, A.; Faure, L.; Orsi, L.; et al. Maternal exposure to pesticides and risk of childhood lymphoma in France: A pooled analysis of the ESCALE and ESTELLE studies [SFCE]. Cancer Epidemiol. 2020, 68, 101797. [Google Scholar] [CrossRef] [PubMed]
- Rios, P.; Bauer, H.; Schleiermacher, G.; Pasqualini, C.; Boulanger, C.; Thebaud, E.; Gandemer, V.; Pellier, I.; Verschuur, A.; Sudour-Bonnange, H.; et al. Environmental exposures related to parental habits in the perinatal period and the risk of Wilms’ tumor in children. Cancer Epidemiol. 2020, 66, 101706. [Google Scholar] [CrossRef]
- Coste, A.; Goujon, S.; Faure, L.; Hémon, D.; Clavel, J. Agricultural crop density in the municipalities of France and incidence of childhood leukemia: An ecological study. Environ. Res. 2020, 187, 109517. [Google Scholar] [CrossRef]
- Patel, D.M.; Gyldenkærne, S.; Jones, R.R.; Olsen, S.F.; Tikellis, G.; Granström, C.; Dwyer, T.; Stayner, L.T.; Ward, M.H. Residential proximity to agriculture and risk of childhood leukemia and central nervous system tumors in the Danish national birth cohort. Environ. Int. 2020, 143, 105955. [Google Scholar] [CrossRef]
- Van Maele-Fabry, G.; Gamet-Payrastre, L.; Lison, D. Household exposure to pesticides and risk of leukemia in children and adolescents: Updated systematic review and meta-analysis. Int. J. Hyg. Environ. Health 2019, 222, 49–67. [Google Scholar] [CrossRef]
- Bunch, K.J.; Kendall, G.M.; Stiller, C.A.; Vincent, T.J.; Murphy, M.F.G. Case-control study of paternal occupational exposures and childhood lymphoma in Great Britain, 1962–2010. Br. J. Cancer 2019, 120, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, M.K.; Dessypris, N.; Papadakis, V.; Tragiannidis, A.; Bouka, E.; Hatzipantelis, E.; Moschovi, M.; Papakonstantinou, E.; Polychronopoulou, S.; Sgouros, S.; et al. Perinatal and early life risk factors for childhood brain tumors: Is instrument-assisted delivery associated with higher risk? Cancer Epidemiol. 2019, 59, 178–184. [Google Scholar] [CrossRef]
- Hyland, C.; Gunier, R.B.; Metayer, C.; Bates, M.N.; Wesseling, C.; Mora, A.M. Maternal residential pesticide use and risk of childhood leukemia in Costa Rica. Int. J. Cancer 2018, 143, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Ferri, G.M.; Guastadisegno, C.M.; Intranuovo, G.; Cavone, D.; Birtolo, F.; Cecinati, V.; Pappalardi, B.; Corsi, P.; Vimercati, L.; Santoro, N. Maternal Exposure to Pesticides, Paternal Occupation in the Army/Police Force, and CYP2D6*4 Polymorphism in the Etiology of Childhood Acute Leukemia. J. Pediatr. Hematol. Oncol. 2018, 40, e207–e214. [Google Scholar] [CrossRef] [PubMed]
- Vidart d’Egurbide Bagazgoïtia, N.; Bailey, H.D.; Orsi, L.; Lacour, B.; Guerrini-Rousseau, L.; Bertozzi, A.I.; Leblond, P.; Faure-Conter, C.; Pellier, I.; Freycon, C.; et al. Maternal residential pesticide use during pregnancy and risk of malignant childhood brain tumors: A pooled analysis of the ESCALE and ESTELLE studies [SFCE]. Int. J. Cancer 2018, 142, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Desai, V. Exposure to permethrin and cancer risk: A systematic review. Crit. Rev. Toxicol. 2018, 48, 433–442. [Google Scholar] [CrossRef]
- Van Maele-Fabry, G.; Gamet-Payrastre, L.; Lison, D. Residential exposure to pesticides as risk factor for childhood and young adult brain tumors: A systematic review and meta-analysis. Environ. Int. 2017, 106, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Gunier, R.B.; Kang, A.; Hammond, S.K.; Reinier, K.; Lea, C.S.; Chang, J.S.; Does, M.; Scelo, G.; Kirsch, J.; Crouse, V.; et al. A task-based assessment of parental occupational exposure to pesticides and childhood acute lymphoblastic leukemia. Environ. Res. 2017, 156, 57–62. [Google Scholar] [CrossRef]
- Eerjaee, A.; Niknam, M.; Sadeghi, A.; Dehghani, M.; Safaei, Z.; Teshnizi, S.H.; Karimi, M. A Significant Breakthrough in the Incidence of Childhood Cancers and Evaluation of its Risk Factors in Southern Iran. Indian J. Med. Paediatr. Oncol. Off. J. Indian Soc. Med. Paediatr. Oncol. 2017, 38, 158–164. [Google Scholar]
- Rios, P.; Bailey, H.D.; Lacour, B.; Valteau-Couanet, D.; Michon, J.; Bergeron, C.; Boutroux, H.; Defachelles, A.S.; Gambart, M.; Sirvent, N.; et al. Maternal use of household pesticides during pregnancy and risk of neuroblastoma in offspring. A pooled analysis of the ESTELLE and ESCALE French studies [SFCE]. Cancer Causes Control CCC. 2017, 28, 1125–1132. [Google Scholar] [CrossRef]
- Omidakhsh, N.; Ganguly, A.; Bunin, G.R.; von Ehrenstein, O.S.; Ritz, B.; Heck, J.E. Residential Pesticide Exposures in Pregnancy and the Risk of Sporadic Retinoblastoma: A Report From the Children’s Oncology Group. Am. J. Ophthalmol. 2017, 176, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Febvey, O.; Schüz, J.; Bailey, H.D.; Clavel, J.; Lacour, B.; Orsi, L.; Lightfoot, T.; Roman, E.; Vermeulen, R.; Kromhout, H.; et al. Risk of Central Nervous System Tumors in Children Related to Parental Occupational Pesticide Exposures in three European Case-Control Studies. J. Occup. Environ. Med. 2016, 58, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Barroso, D.; García-Pérez, J.; López-Abente, G.; Tamayo-Uria, I.; Morales-Piga, A.; Pardo Romaguera, E.; Ramis, R. Agricultural crop exposure and risk of childhood cancer: New findings from a case-control study in Spain. Int. J. Health Geogr. 2016, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Malagoli, C.; Costanzini, S.; Heck, J.E.; Malavolti, M.; De Girolamo, G.; Oleari, P.; Palazzi, G.; Teggi, S.; Vinceti, M. Passive exposure to agricultural pesticides and risk of childhood leukemia in an Italian community. Int. J. Hyg. Environ. Health 2016, 219, 742–748. [Google Scholar] [CrossRef]
- Chen, S.; Gu, S.; Wang, Y.; Yao, Y.; Wang, G.; Jin, Y.; Wu, Y. Exposure to pyrethroid pesticides and the risk of childhood brain tumors in East China. Environ. Pollut. 2016, 218, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chang, C.H.; Tao, L.; Lu, C. Residential Exposure to Pesticide During Childhood and Childhood Cancers: A Meta-Analysis. Pediatrics 2015, 136, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Maryam, Z.; Sajad, A.; Maral, N.; Zahra, L.; Sima, P.; Zeinab, A.; Zahra, M.; Fariba, E.; Sezaneh, H.; Davood, M. Relationship between exposure to pesticides and occurrence of acute leukemia in Iran. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 239–244. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.; Shi, R.; Chen, D.; Wang, X.; Kamijima, M.; Sakai, K.; Nakajima, T.; Khalequzzaman, M.; Zhou, Y.; et al. Household pesticide exposure and the risk of childhood acute leukemia in Shanghai, China. Environ. Sci. Pollut. Res. Int. 2015, 22, 11755–11763. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Tian, Y.; Shi, R.; Wang, X.; Hu, Y.; Ji, X.; Han, K.; Hu, S.; Mao, S.; et al. Relationship between risk of childhood acute leukemia and children’s and parents’ lifestyles and household environment exposure. Chin. J. Prev. Med. 2015, 49, 792–799. [Google Scholar]
- Bailey, H.D.; Infante-Rivard, C.; Metayer, C.; Clavel, J.; Lightfoot, T.; Kaatsch, P.; Roman, E.; Magnani, C.; Spector, L.G.; Th Petridou, E.T.; et al. Home pesticide exposures and risk of childhood leukemia: Findings from the childhood leukemia international consortium. Int. J. Cancer 2015, 137, 2644–2663. [Google Scholar] [CrossRef]
- Zheng, R.; Zhang, Q.; Zhang, Q.; Yang, L.; Zhang, Z.; Huang, F. Occupational exposure to pentachlorophenol causing lymphoma and hematopoietic malignancy for two generations. Toxicol. Ind. Health 2015, 31, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Kunkle, B.; Bae, S.; Singh, K.P.; Roy, D. Increased risk of childhood brain tumors among children whose parents had farm-related pesticide exposures during pregnancy. JP J. Biostat. 2014, 11, 89–101. [Google Scholar] [PubMed]
- Kumar, A.; Vashist, M.; Rathee, R. Maternal factors and risk of childhood leukemia. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 781–784. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bailey, H.D.; Fritschi, L.; Infante-Rivard, C.; Glass, D.C.; Miligi, L.; Dockerty, J.D.; Lightfoot, T.; Clavel, J.; Roman, E.; Spector, L.G.; et al. Parental occupational pesticide exposure and the risk of childhood leukemia in the offspring: Findings from the childhood leukemia international consortium. Int. J. Cancer 2014, 135, 2157–2172. [Google Scholar] [CrossRef] [PubMed]
- Van Maele-Fabry, G.; Hoet, P.; Lison, D. Parental occupational exposure to pesticides as risk factor for brain tumors in children and young adults: A systematic review and meta-analysis. Environ. Int. 2013, 56, 19–31. [Google Scholar] [CrossRef]
- Ferreira, J.D.; Couto, A.C.; Pombo-de-Oliveira, M.S.; Koifman, S.; Brazilian Collaborative Study Group of Infant Acute Leukemia. In utero pesticide exposure and leukemia in Brazilian children < 2 years of age. Environ. Health Perspect. 2013, 121, 269–275. [Google Scholar] [PubMed]
- Greenop, K.R.; Peters, S.; Bailey, H.D.; Fritschi, L.; Attia, J.; Scott, R.J.; Glass, D.C.; De Klerk, N.H.; Alvaro, F.; Armstrong, B.K.; et al. Exposure to pesticides and the risk of childhood brain tumors. Cancer Causes Control CCC 2013, 24, 1269–1278. [Google Scholar] [CrossRef]
- Metayer, C.; Colt, J.S.; Buffler, P.A.; Reed, H.D.; Selvin, S.; Crouse, V.; Ward, M.H. Exposure to herbicides in house dust and risk of childhood acute lymphoblastic leukemia. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 363–370. [Google Scholar] [CrossRef]
- Abdolahi, A.; van Wijngaarden, E.; McClean, M.D.; Herrick, R.F.; Allen, J.G.; Ganguly, A.; Bunin, G.R. A case-control study of paternal occupational exposures and the risk of childhood sporadic bilateral retinoblastoma. Occup. Environ. Med. 2013, 70, 372–379. [Google Scholar] [CrossRef]
- Wimberly, C.E.; Gulrajani, N.B.; Russ, J.B.; Landi, D.; Wiemels, J.L.; Towry, L.; Wiencke, J.K.; Walsh, K.M. Maternal prenatal use of alcohol, tobacco, and illicit drugs and associations with childhood cancer subtypes. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2024, 33, 347–354. [Google Scholar] [CrossRef]
- Xu, K.; Li, S.; Whitehead, T.P.; Pandey, P.; Kang, A.Y.; Morimoto, L.M.; Kogan, S.C.; Metayer, C.; Wiemels, J.L.; de Smith, A.J. Epigenetic biomarkers of prenatal tobacco smoke exposure are associated with gene deletions in childhood acute lymphoblastic leukemia. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2021, 30, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Alyahya, M.S.; Al-Sheyab, N.A.; Amro, B. Parental Smoking Behavior and Childhood Cancer: A Case-control Study. Am. J. Health Behav. 2020, 44, 572–590. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, L.E.; Erdmann, F.; Wesseling, C.; Winther, J.F.; Mora, A.M. Parental tobacco smoking and risk of childhood leukemia in Costa Rica: A population-based case-control study. Environ. Res. 2020, 180, 108827. [Google Scholar] [CrossRef]
- Doganis, D.; Katsimpris, A.; Panagopoulou, P.; Bouka, P.; Bouka, E.; Moschovi, M.; Polychronopoulou, S.; Papakonstantinou, E.; Tragiannidis, A.; Katzilakis, N.; et al. Maternal lifestyle characteristics and Wilms tumor risk in the offspring: A systematic review and meta-analysis. Cancer Epidemiol. 2020, 67, 101769. [Google Scholar] [CrossRef] [PubMed]
- Medina-Sanson, A.; Núñez-Enríquez, J.C.; Hurtado-Cordova, E.; Pérez-Saldivar, M.L.; Martínez-García, A.; Jiménez-Hernández, E.; Fernández-López, J.C.; Martín-Trejo, J.A.; Pérez-Lorenzana, H.; Flores-Lujano, J.; et al. Genotype-Environment Interaction Analysis of NQO1, CYP2E1, and NAT2 Polymorphisms and the Risk of Childhood Acute Lymphoblastic Leukemia: A Report From the Mexican Interinstitutional Group for the Identification of the Causes of Childhood Leukemia. Front. Oncol. 2020, 10, 571869. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lu, J.; Lu, J. Paternal Smoking Before Conception and During Pregnancy Is Associated With an Increased Risk of Childhood Acute Lymphoblastic Leukemia: A Systematic Review and Meta-Analysis of 17 Case-Control Studies. J. Pediatr. Hematol. Oncol. 2020, 42, 32–40. [Google Scholar] [CrossRef]
- Dong, C.; Wang, M.; Zhang, J.; Zhang, R.; Liu, X.; Zheng, Z.; Yang, L. Tobacco smoke exposure and the risk of childhood acute lymphoblastic leukemia and acute myeloid leukemia: A meta-analysis. Medicine 2019, 98, e16454. [Google Scholar]
- Rios, P.; Bailey, H.D.; Poulalhon, C.; Valteau-Couanet, D.; Schleiermacher, G.; Bergeron, C.; Petit, A.; Defachelles, A.-S.; Marion, G.; Sirvent, N.; et al. Parental smoking, maternal alcohol consumption during pregnancy and the risk of neuroblastoma in children. A pooled analysis of the ESCALE and ESTELLE French studies. Int. J. Cancer 2019, 145, 2907–2916. [Google Scholar] [CrossRef]
- Kessous, R.; Wainstock, T.; Sheiner, E. Smoking during pregnancy as a possible risk factor for pediatric neoplasms in the offspring: A population-based cohort study. Addict. Behav. 2019, 90, 349–353. [Google Scholar] [CrossRef]
- Milne, E.; Greenop, K.R.; Petridou, E.; Bailey, H.D.; Orsi, L.; Kang, A.Y.; Baka, M.; Bonaventure, A.; Kourti, M.; Metayer, C.; et al. Maternal consumption of coffee and tea during pregnancy and risk of childhood ALL: A pooled analysis from the childhood Leukemia International Consortium. Cancer Causes Control CCC 2018, 29, 539–550. [Google Scholar] [CrossRef]
- de Smith, A.J.; Kaur, M.; Gonseth, S.; Endicott, A.; Selvin, S.; Zhang, L.; Roy, R.; Shao, X.; Hansen, H.M.; Kang, A.Y.; et al. Correlates of Prenatal and Early-Life Tobacco Smoke Exposure and Frequency of Common Gene Deletions in Childhood Acute Lymphoblastic Leukemia. Cancer Res. 2017, 77, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Tettamanti, G.; Ljung, R.; Mathiesen, T.; Schwartzbaum, J.; Feychting, M. Maternal smoking during pregnancy and the risk of childhood brain tumors: Results from a Swedish cohort study. Cancer Epidemiol. 2016, 40, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Vienneau, D.; Infanger, D.; Feychting, M.; Schüz, J.; Schmidt, L.S.; Poulsen, A.H.; Tettamanti, G.; Klæboe, L.; Kuehni, C.E.; Tynes, T.; et al. A multinational case-control study on childhood brain tumours, anthropogenic factors, birth characteristics and prenatal exposures: A validation of interview data. Cancer Epidemiol. 2016, 40, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Azary, S.; Ganguly, A.; Bunin, G.R.; Lombardi, C.; Park, A.S.; Ritz, B.; Heck, J.E. Sporadic Retinoblastoma and Parental Smoking and Alcohol Consumption before and after Conception: A Report from the Children’s Oncology Group. PLoS ONE 2016, 11, e0151728. [Google Scholar] [CrossRef] [PubMed]
- Metayer, C.; Petridou, E.; Aranguré, J.M.M.; Roman, E.; Schüz, J.; Magnani, C.; Mora, A.M.; Mueller, B.A.; de Oliveira, M.S.P.; Dockerty, J.D.; et al. Parental Tobacco Smoking and Acute Myeloid Leukemia: The Childhood Leukemia International Consortium. Am. J. Epidemiol. 2016, 184, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.; Wang, H.; Han, S.; Jin, Y.; Lu, J.; Han, W.; Shi, J.; Guo, Y.; Ni, X. Maternal smoking during pregnancy and risk of childhood neuroblastoma: Systematic review and meta-analysis. J. Cancer Res. Ther. 2016, 12, 999–1005. [Google Scholar] [PubMed]
- Orsi, L.; Rudant, J.; Ajrouche, R.; Leverger, G.; Baruchel, A.; Nelken, B.; Pasquet, M.; Michel, G.; Bertrand, Y.; Ducassou, S.; et al. Parental smoking, maternal alcohol, coffee and tea consumption during pregnancy, and childhood acute leukemia: The ESTELLE study. Cancer Causes Control CCC 2015, 26, 1003–1017. [Google Scholar] [CrossRef]
- Momen, N.C.; Olsen, J.; Gissler, M.; Li, J. Exposure to maternal smoking during pregnancy and risk of childhood cancer: A study using the Danish national registers. Cancer Causes Control CCC 2016, 27, 341–349. [Google Scholar] [CrossRef]
- Mattioli, S.; Farioli, A.; Legittimo, P.; Miligi, L.; Benvenuti, A.; Ranucci, A.; Salvan, A.; Rondelli, R.; Magnani, C.; on behalf of the SETIL Study Group. Tobacco smoke and risk of childhood acute non-lymphocytic leukemia: Findings from the SETIL study. PLoS ONE 2014, 9, e111028. [Google Scholar] [CrossRef][Green Version]
- Grufferman, S.; Lupo, P.J.; Vogel, R.I.; Danysh, H.E.; Erhardt, E.B.; Ognjanovic, S. Parental military service, agent orange exposure, and the risk of rhabdomyosarcoma in offspring. J. Pediatr. 2014, 165, 1216–1221. [Google Scholar] [CrossRef]
- Farioli, A.; Legittimo, P.; Mattioli, S.; Miligi, L.; Benvenuti, A.; Ranucci, A.; Salvan, A.; Rondelli, R.; Conter, V.; Magnani, C. Tobacco smoke and risk of childhood acute lymphoblastic leukemia: Findings from the SETIL case-control study. Cancer Causes Control CCC 2014, 25, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Greenop, K.R.; Miller, M.; Attia, J.; Ashton, L.J.; Cohn, R.; Armstrong, B.K.; Milne, E. Maternal consumption of coffee and tea during pregnancy and risk of childhood brain tumors: Results from an Australian case-control study. Cancer Causes Control CCC 2014, 25, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, J.; Lan, H.; Zhao, G.; Huang, C. A meta-analysis of parental smoking and the risk of childhood brain tumors. PLoS ONE 2014, 9, e102910. [Google Scholar] [CrossRef] [PubMed]
- Barrington-Trimis, J.L.; Searles Nielsen, S.; Preston-Martin, S.; Gauderman, W.J.; Holly, E.A.; Farin, F.M.; Mueller, B.A.; McKean-Cowdin, R. Parental smoking and risk of childhood brain tumors by functional polymorphisms in polycyclic aromatic hydrocarbon metabolism genes. PLoS ONE 2013, 8, e79110. [Google Scholar] [CrossRef] [PubMed]
- Metayer, C.; Zhang, L.; Wiemels, J.L.; Bartley, K.; Schiffman, J.; Ma, X.; Aldrich, M.C.; Chang, J.S.; Selvin, S.; Fu, C.H.; et al. Tobacco smoke exposure and the risk of childhood acute lymphoblastic and myeloid leukemias by cytogenetic subtype. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2013, 22, 1600–1611. [Google Scholar] [CrossRef] [PubMed]
- Milne, E.; Greenop, K.R.; Scott, R.J.; Ashton, L.J.; Cohn, R.J.; de Klerk, N.H.; Lyon, J.L.; Swanson, G.M.; Weiss, N.S.; West, D.; et al. Parental smoking and risk of childhood brain tumors. Int. J. Cancer 2013, 133, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Rossides, M.; Kampitsi, C.E.; Talbäck, M.; Mogensen, H.; Wiebert, P.; Feychting, M.; Tettamanti, G. Risk of Cancer in Children of Parents Occupationally Exposed to Hydrocarbon Solvents and Engine Exhaust Fumes: A Register-Based Nested Case-Control Study from Sweden [1960–2015]. Environ. Health Perspect. 2022, 130, 77002. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, D.; Shi, R.; Kamijima, M.; Sakai, K.; Tian, Y.; Gao, Y. Indoor volatile organic compounds exposures and risk of childhood acute leukemia: A case-control study in shanghai. J. Environ. Sci. Health Part A Tox Hazard. Subst. Environ. Eng. 2021, 56, 190–198. [Google Scholar] [CrossRef]
- Stayner, L.T.; Schullehner, J.; Semark, B.D.; Jensen, A.S.; Trabjerg, B.B.; Pedersen, M.; Olsen, J.; Hansen, B.; Ward, M.H.; Jones, R.R.; et al. Exposure to nitrate from drinking water and the risk of childhood cancer in Denmark. Environ. Int. 2021, 155, 106613. [Google Scholar] [CrossRef]
- Hvidtfeldt, U.A.; Erdmann, F.; Urhøj, S.K.; Brandt, J.; Geels, C.; Ketzel, M.; Frohn, L.M.; Christensen, J.H.; Sørensen, M.; Raaschou-Nielsen, O. Air pollution exposure at the residence and risk of childhood cancers in Denmark: A nationwide register-based case-control study. eClinicalMedicine 2020, 28, 100569. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, P.; Liang, G.; Zhang, N.; Wang, C.; Wang, Y.; Nie, L.; Lv, X.; Li, W.; Guo, Q.; et al. Maternal prenatal exposure to environmental factors and risk of childhood acute lymphocytic leukemia: A hospital-based case-control study in China. Cancer Epidemiol. 2019, 58, 146–152. [Google Scholar] [CrossRef]
- Raaschou-Nielsen, O.; Hvidtfeldt, U.A.; Roswall, N.; Hertel, O.; Poulsen, A.H.; Sørensen, M. Ambient benzene at the residence and risk for subtypes of childhood leukemia, lymphoma and CNS tumor. Int. J. Cancer 2018, 143, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Loffredo, C.A.; Mitra, P.S.; Trnovec, T.; Palkovicova Murinova, L.; Sovcikova, E.; Hoffman, E.P.; Makambi, K.H.; Dutta, S.K. PCB exposure and potential future cancer incidence in Slovak children: An assessment from molecular finger printing by Ingenuity Pathway Analysis [IPA®] derived from experimental and epidemiological investigations. Environ. Sci. Pollut. Res. Int. 2018, 25, 16493–16507. [Google Scholar] [CrossRef] [PubMed]
- Park, A.S.; Ritz, B.; Ling, C.; Cockburn, M.; Heck, J.E. Exposure to ambient dichloromethane in pregnancy and infancy from industrial sources and childhood cancers in California. Int. J. Hyg. Environ. Health 2017, 220, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, T.P.; Adhatamsoontra, P.; Wang, Y.; Arcolin, E.; Sender, L.; Selvin, S.; Metayer, C. Home remodeling and risk of childhood leukemia. Ann. Epidemiol. 2017, 27, 140–144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Metayer, C.; Scelo, G.; Kang, A.Y.; Gunier, R.B.; Reinier, K.; Lea, S.; Chang, J.S.; Selvin, S.; Kirsch, J.; Crouse, V.; et al. A task-based assessment of parental occupational exposure to organic solvents and other compounds and the risk of childhood leukemia in California. Environ. Res. 2016, 151, 174–183. [Google Scholar] [CrossRef]
- Jiang, W.C.; Wu, S.Y.; Ke, Y.B. Association of exposure to environmental chemicals with risk of childhood acute lymphocytic leukemia. Chin. J. Prev. Med. 2016, 50, 893–899. [Google Scholar]
- Bailey, H.D.; Metayer, C.; Milne, E.; Petridou, E.T.; Infante-Rivard, C.; Spector, L.G.; Clavel, J.; Dockerty, J.D.; Zhang, L.; Armstrong, B.K.; et al. Home paint exposures and risk of childhood acute lymphoblastic leukemia: Findings from the Childhood Leukemia International Consortium. Cancer Causes Control CCC 2015, 26, 1257–1270. [Google Scholar] [CrossRef]
- Ward, M.H.; Colt, J.S.; Deziel, N.C.; Whitehead, T.P.; Reynolds, P.; Gunier, R.B.; Nishioka, M.; Dahl, G.V.; Rappaport, S.M.; Buffler, P.A.; et al. Residential levels of polybrominated diphenyl ethers and risk of childhood acute lymphoblastic leukemia in California. Environ. Health Perspect. 2014, 122, 1110–1116. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Kamijima, M.; Sakai, K.; Khalequzzaman, M.; Nakajima, T.; Shi, R.; Wang, X.; Chen, D.; Ji, X.; et al. Quantitative assessments of indoor air pollution and the risk of childhood acute leukemia in Shanghai. Environ. Pollut. Barking Essex 2014, 187, 81–89. [Google Scholar] [CrossRef]
- Parodi, S.; Merlo, D.F.; Ranucci, A.; Miligi, L.; Benvenuti, A.; Rondelli, R.; Magnani, C.; Haupt, R.; SETIL Working Group. Risk of neuroblastoma, maternal characteristics and perinatal exposures: The SETIL study. Cancer Epidemiol. 2014, 38, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Glass, D.C.; Greenop, K.R.; Armstrong, B.K.; Kirby, M.; Milne, E.; Fritschi, L. Childhood brain tumours: Associations with parental occupational exposure to solvents. Br. J. Cancer 2014, 111, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Ruckart, P.Z.; Bove, F.J.; Maslia, M. Evaluation of exposure to contaminated drinking water and specific birth defects and childhood cancers at Marine Corps Base Camp Lejeune, North Carolina: A case-control study. Environ. Health Glob. Access Sci. Source 2013, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Gao, Y.; Zhang, Y.; Gao, Y.J.; Zhu, S.; Wang, X.J.; Jin, P.; Tian, Y. Relationship between parental exposure to chemicals and risk of childhood acute leukemia. Chin. J. Ind. Hyg. Occup. Dis. 2013, 31, 413–417. [Google Scholar]
- Brabant, C.; Geerinck, A.; Beaudart, C.; Tirelli, E.; Geuzaine, C.; Bruyère, O. Exposure to magnetic fields and childhood leukemia: A systematic review and meta-analysis of case-control and cohort studies. Rev. Environ. Health 2022, 38, 229–253. [Google Scholar] [CrossRef] [PubMed]
- Amoon, A.T.; Swanson, J.; Magnani, C.; Johansen, C.; Kheifets, L. Pooled analysis of recent studies of magnetic fields and childhood leukemia. Environ. Res. 2022, 204, 111993. [Google Scholar] [CrossRef] [PubMed]
- Seomun, G.; Lee, J.; Park, J. Exposure to extremely low-frequency magnetic fields and childhood cancer: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251628. [Google Scholar] [CrossRef]
- Núñez-Enríquez, J.C.; Correa-Correa, V.; Flores-Lujano, J.; Pérez-Saldivar, M.L.; Jiménez-Hernández, E.; Martín-Trejo, J.A.; Espinoza-Hernández, L.E.; Medina-Sanson, A.; Cárdenas-Cardos, R.; Flores-Villegas, L.V.; et al. Extremely Low-Frequency Magnetic Fields and the Risk of Childhood B-Lineage Acute Lymphoblastic Leukemia in a City With High Incidence of Leukemia and Elevated Exposure to ELF Magnetic Fields. Bioelectromagnetics 2020, 41, 581–597. [Google Scholar] [CrossRef]
- Crespi, C.M.; Swanson, J.; Vergara, X.P.; Kheifets, L. Childhood leukemia risk in the California Power Line Study: Magnetic fields versus distance from power lines. Environ. Res. 2019, 171, 530–535. [Google Scholar] [CrossRef]
- Auger, N.; Bilodeau-Bertrand, M.; Marcoux, S.; Kosatsky, T. Residential exposure to electromagnetic fields during pregnancy and risk of child cancer: A longitudinal cohort study. Environ. Res. 2019, 176, 108524. [Google Scholar] [CrossRef]
- Talibov, M.; Olsson, A.; Bailey, H.; Erdmann, F.; Metayer, C.; Magnani, C.; Petridou, E.; Auvinen, A.; Spector, L.; Clavel, J.; et al. Parental occupational exposure to low-frequency magnetic fields and risk of leukaemia in the offspring: Findings from the Childhood Leukaemia International Consortium [CLIC]. Occup. Environ. Med. 2019, 76, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Amoon, A.T.; Crespi, C.M.; Ahlbom, A.; Bhatnagar, M.; Bray, I.; Bunch, K.J.; Clavel, J.; Feychting, M.; Hémon, D.; Johansen, C.; et al. Proximity to overhead power lines and childhood leukaemia: An international pooled analysis. Br. J. Cancer 2018, 119, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zhao, C.; Jin, Y.; Lei, Y.; Lu, L.; Chen, G. Association between parental occupational exposure to extremely low frequency magnetic fields and childhood nervous system tumors risk: A meta-analysis. Sci. Total Environ. 2018, 642, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Kheifets, L.; Crespi, C.M.; Hooper, C.; Cockburn, M.; Amoon, A.T.; Vergara, X.P. Residential magnetic fields exposure and childhood leukemia: A population-based case-control study in California. Cancer Causes Control CCC 2017, 28, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Fei, Y.; Wei, X.; Guo, J.; Jiang, X.; Lu, L.; Chen, G. Associations of parental occupational exposure to extremely low-frequency magnetic fields with childhood leukemia risk. Leuk. Lymphoma 2016, 57, 2855–2862. [Google Scholar] [CrossRef] [PubMed]
- Crespi, C.M.; Vergara, X.P.; Hooper, C.; Oksuzyan, S.; Wu, S.; Cockburn, M.; Kheifets, L. Childhood leukaemia and distance from power lines in California: A population-based case-control study. Br. J. Cancer 2016, 115, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Bunch, K.J.; Swanson, J.; Vincent, T.J.; Murphy, M.F.G. Epidemiological study of power lines and childhood cancer in the UK: Further analyses. J. Radiol. Prot. Off. J. Soc. Radiol. Prot. 2016, 36, 437–455. [Google Scholar] [CrossRef]
- Dechent, D.; Driessen, S. Re: Role of Electromagnetic Field Exposure in Childhood Acute Lymphoblastic Leukemia and No Impact of Urinary Alpha- Amylase—A Case Control Study in Tehran, Iran. Asian Pac. J. Cancer Prev. APJCP 2016, 17, 877–878. [Google Scholar] [CrossRef][Green Version]
- Tabrizi, M.M.; Bidgoli, S.A. Increased risk of childhood acute lymphoblastic leukemia [ALL] by prenatal and postnatal exposure to high voltage power lines: A case control study in Isfahan, Iran. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 2347–2350. [Google Scholar] [CrossRef]
- Bunch, K.J.; Swanson, J.; Vincent, T.J.; Murphy, M.F.G. Magnetic fields and childhood cancer: An epidemiological investigation of the effects of high-voltage underground cables. J. Radiol. Prot. Off. J. Soc. Radiol. Prot. 2015, 35, 695–705. [Google Scholar] [CrossRef]
- Pedersen, C.; Johansen, C.; Schüz, J.; Olsen, J.H.; Raaschou-Nielsen, O. Residential exposure to extremely low-frequency magnetic fields and risk of childhood leukaemia, CNS tumour and lymphoma in Denmark. Br. J. Cancer 2015, 113, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Salvan, A.; Ranucci, A.; Lagorio, S.; Magnani, C.; SETIL Research Group. Childhood leukemia and 50 Hz magnetic fields: Findings from the Italian SETIL case-control study. Int. J. Environ. Res. Public Health 2015, 12, 2184–2204. [Google Scholar] [CrossRef] [PubMed]
- Bunch, K.J.; Keegan, T.J.; Swanson, J.; Vincent, T.J.; Murphy, M.F.G. Residential distance at birth from overhead high-voltage powerlines: Childhood cancer risk in Britain 1962–2008. Br. J. Cancer 2014, 110, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, X.; Wang, C.; Yan, K.; Lin, X.; Li, S.; Bao, H.; Liu, X. Magnetic fields exposure and childhood leukemia risk: A meta-analysis based on 11,699 cases and 13,194 controls. Leuk. Res. 2014, 38, 269–274. [Google Scholar] [CrossRef]
- Pedersen, C.; Bräuner, E.V.; Rod, N.H.; Albieri, V.; Andersen, C.E.; Ulbak, K.; Hertel, O.; Johansen, C.; Schüz, J.; Raaschou-Nielsen, O. Distance to high-voltage power lines and risk of childhood leukemia—An analysis of confounding by and interaction with other potential risk factors. PLoS ONE 2014, 9, e107096. [Google Scholar] [CrossRef] [PubMed]
- Sermage-Faure, C.; Demoury, C.; Rudant, J.; Goujon-Bellec, S.; Guyot-Goubin, A.; Deschamps, F.; Hemon, D.; Clavel, J. Childhood leukaemia close to high-voltage power lines—The Geocap study, 2002–2007. Br. J. Cancer 2013, 108, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, L.T.N.; Park, D.; Lee, Y.C. Human Health Impacts of Residential Radon Exposure: Updated Systematic Review and Meta-Analysis of Case-Control Studies. Int. J. Environ. Res. Public Health 2022, 20, 97. [Google Scholar] [CrossRef]
- Moon, J.; Yoo, H. Residential radon exposure and leukemia: A meta-analysis and dose-response meta-analyses for ecological, case-control, and cohort studies. Environ. Res. 2021, 202, 111714. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, L.; Chen, Q.; Wei, J.; Cao, G.; Zhang, J. Domestic radon exposure and risk of childhood leukemia: A meta-analysis. J. BUON Off. J. Balk. Union. Oncol. 2020, 25, 1035–1041. [Google Scholar]
- Nikkilä, A.; Arvela, H.; Mehtonen, J.; Raitanen, J.; Heinäniemi, M.; Lohi, O.; Auvinen, A. Predicting residential radon concentrations in Finland: Model development, validation, and application to childhood leukemia. Scand. J. Work Environ. Health 2020, 46, 278–292. [Google Scholar] [CrossRef]
- Chen, J.; Xie, L. Domestic radon exposure and childhood leukaemia and lymphoma: A population-based study in canada. Radiat. Prot. Dosimetry 2019, 184, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Peckham, E.C.; Scheurer, M.E.; Danysh, H.E.; Lubega, J.; Langlois, P.H.; Lupo, P.J. Residential Radon Exposure and Incidence of Childhood Lymphoma in Texas, 1995–2011. Int. J. Environ. Res. Public Health 2015, 12, 12110–12126. [Google Scholar] [CrossRef] [PubMed]
- Del Risco Kollerud, R.; Blaasaas, K.G.; Claussen, B. Risk of leukaemia or cancer in the central nervous system among children living in an area with high indoor radon concentrations: Results from a cohort study in Norway. Br. J. Cancer 2014, 111, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Hauri, D.; Spycher, B.; Huss, A.; Zimmermann, F.; Grotzer, M.; von der Weid, N.; Weber, D.; Spoerri, A.; Kuehni, C.E.; Röösli, M.; et al. Domestic radon exposure and risk of childhood cancer: A prospective census-based cohort study. Environ. Health Perspect. 2013, 121, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Onyije, F.M.; Olsson, A.; Baaken, D.; Erdmann, F.; Stanulla, M.; Wollschläger, D.; Schüz, J. Environmental Risk Factors for Childhood Acute Lymphoblastic Leukemia: An Umbrella Review. Cancers 2022, 14, 382. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.iarc.who.int/wp-content/uploads/2018/07/pr221_E.pdf (accessed on 10 January 2024).
- Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono109-F07.pdf (accessed on 10 January 2024).
- Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono100F-24.pdf (accessed on 10 January 2024).
- McHale, C.M.; Zhang, L.; Smith, M.T. Current understanding of the mechanism of benzene-induced leukemia in humans: Implications for risk assessment. Carcinogenesis 2012, 33, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Pech, K.; Pérez-Herrera, N.; Vértiz-Hernández, Á.A.; Lajous, M.; Farías, P. Health Risk Assessment in Children Occupationally and Para-Occupationally Exposed to Benzene Using a Reverse-Translation PBPK Model. Int. J. Environ. Res. Public Health 2023, 20, 2275. [Google Scholar] [CrossRef] [PubMed]
- Gruľová, D.; Caputo, L.; Elshafie, H.S.; Baranová, B.; De Martino, L.; Sedlák, V.; Gogaľová, Z.; Poráčová, J.; Camele, I.; De Feo, V. Thymol Chemotype Origanum vulgare L. Essential Oil as a Potential Selective Bio-Based Herbicide on Monocot Plant Species. Molecules 2020, 25, 595. [Google Scholar] [CrossRef]
- Ottenbros, I.; Lebret, E.; Huber, C.; Lommen, A.; Antignac, J.P.; Čupr, P.; Šulc, L.; Mikeš, O.; Szigeti, T.; Középesy, S.; et al. Assessment of exposure to pesticide mixtures in five European countries by a harmonized urinary suspect screening approach. Int. J. Hyg. Environ. Health 2023, 248, 114105. [Google Scholar] [CrossRef]
- Available online: https://www.iarc.who.int/wp-content/uploads/2018/07/pr236_E.pdf (accessed on 10 January 2024).
- Navarrete-Meneses, M.d.P.; Pérez-Vera, P. Pyrethroid pesticide exposure and hematological cancer: Epidemiological, biological and molecular evidence. Rev. Environ. Health 2019, 34, 197–210. [Google Scholar] [CrossRef]
- Navarrete-Meneses, M.d.P.; Salas-Labadía, C.; Juárez-Velázquez, M.d.R.; Moreno-Lorenzana, D.; Gómez-Chávez, F.; Olaya-Vargas, A.; Pérez-Vera, P. Exposure to Insecticides Modifies Gene Expression and DNA Methylation in Hematopoietic Tissues In Vitro. Int. J. Mol. Sci. 2023, 24, 6259. [Google Scholar] [CrossRef]
- Nicolella, H.D.; de Assis, S. Epigenetic Inheritance: Intergenerational Effects of Pesticides and Other Endocrine Disruptors on Cancer Development. Int. J. Mol. Sci. 2022, 23, 4671. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/europe/news/item/03-02-2021-world-cancer-day-know-the-facts-tobacco-and-alcohol-both-cause-cancer#:~:text=People%20who%20use%20both%20alcohol,up%20to%2030%20times%20higher (accessed on 10 January 2024).
- Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono100E-6.pdf (accessed on 10 January 2024).
- NCI. Electromagnetic Fields and Cancer. 2022. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/radiation/electromagnetic-fields-fact-sheet (accessed on 10 January 2024).
- Schüz, J.; Erdmann, F. Environmental Exposure and Risk of Childhood Leukemia: An Overview. Arch. Med. Res. 2016, 47, 607–614. [Google Scholar] [CrossRef]
- Onyije, F.M.; Olsson, A.; Bouaoun, L.; Schüz, J. Synthesized evidence for childhood acute lymphoblastic leukemia. Front. Pediatr. 2023, 11, 1209330. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).