Treatment of Thoracic SMARCA4-Deficient Undifferentiated Tumors: Where We Are and Where We Will Go
Abstract
1. Introduction
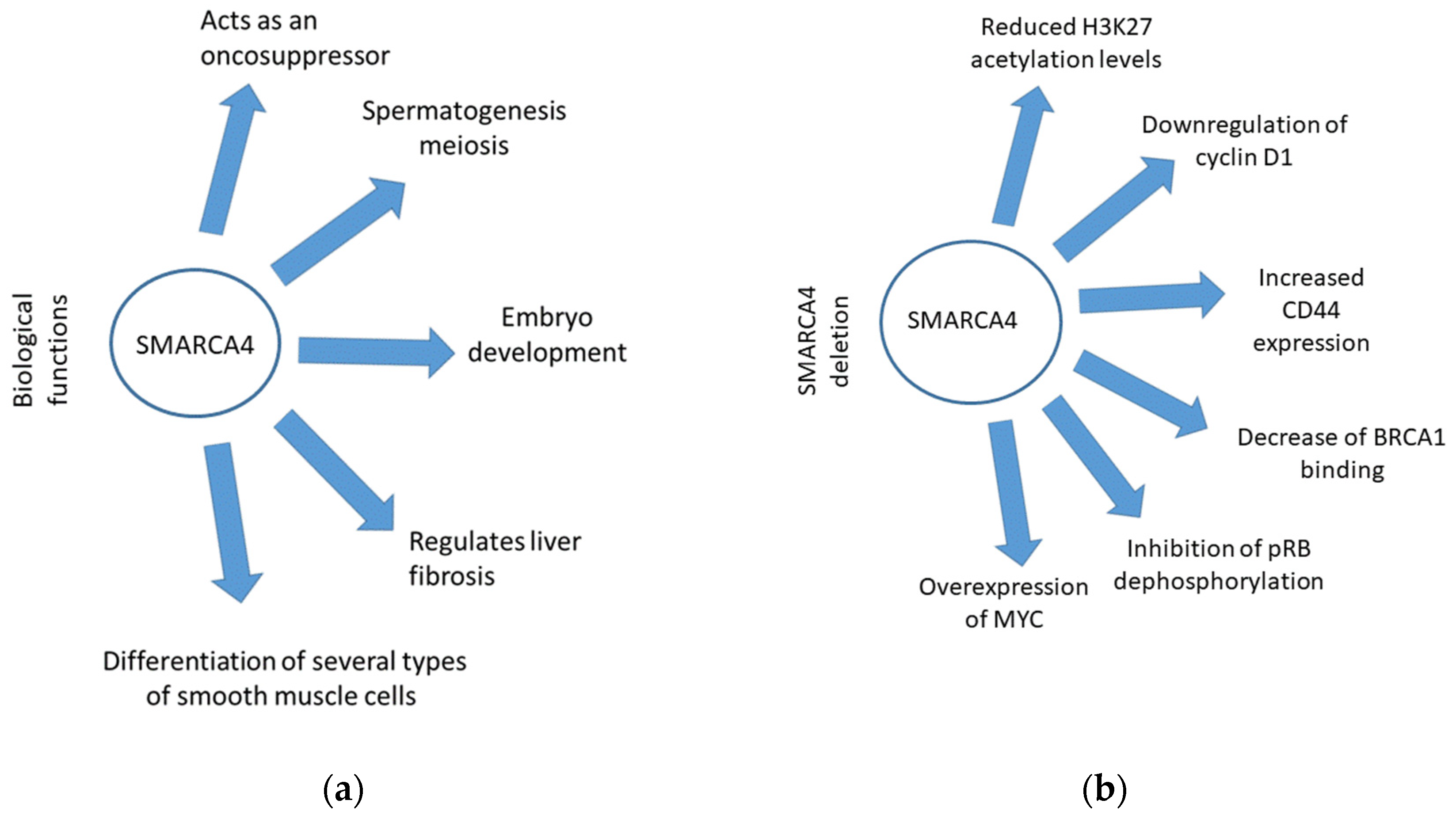
2. Role of SMARCA4 in Cancer Development
3. Clinical Pathological Features
| SMARCA4-UT Features | Description | References |
|---|---|---|
| Patient population | Young to middle-aged adults; predominantly male with smoking history. | [2] |
| Imaging | CT scan shows a large mass involving the mediastinum and lung parenchyma; strong 18F-fluorodeoxyglucose affinity. | [3] |
| Histopathology | Rhabdoid and/or epithelioid tumor cells organized in non-cohesive clusters without epithelial architecture and with extensive necrosis. High Ki-67 rate of around 70%. | [57,58,59,60,61] |
Treatment:
| High rate of recurrence; useful only for stage I. Weak response. Resistance. Promising efficacy | [4,5,6,7,8,9,56,60,62,63] |
| Methods | Markers | Expression | References |
|---|---|---|---|
| IHC | CK7 CAM5.2 AE1/3 EMA | Only in some cases in a focal/weak manner | [58] |
| P40 P63 TTF1 Desmin NUT S100 WT-1 | Usually negative | [58,60] | |
| Vimentin Synaptophysin | Positive in some cases | [38] | |
| SOX2 SALL4 CD34 | Positive | [60] | |
| Claudin-4 | Negative | [60] | |
| CD138 CD99 CD30 | Focal and weak expression | [61] | |
| SMARCA2 | Negative (different from SMARCA4-deficient NSCLC) | [58,61] | |
| SMARCAB-1 | Overexpression | ||
| NGS | P53 | 81–56% | [64,65] |
| KEAP1 | 41% | ||
| STK11 | 39–15% | ||
| Kras | 36–15% | ||
| NF1 | 15% | ||
| PTEN | 11% | ||
| EGFR | Negative | [66] | |
| NGS/IHC/FISH | ROS1 | Negative | |
| ALK | Some cases reported in the literature | [4,66] |
4. Differential Diagnosis from Other Malignancies
5. Treatment: Chemotherapy versus Immunotherapy
6. Treatment: Novel Targets
7. SMARCA4-UT and Co-Occurring Mutations
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lin, Y.; Yu, B.; Sun, H.; Zhang, H.; Hu, Z.; Zhang, Y.; Wu, Z.; Sun, S.; Zhao, X.; Yu, H.; et al. Promising efficacy of immune checkpoint inhibitor plus chemotherapy for thoracic SMARCA4-deficient undifferentiated tumor. J. Cancer Res. Clin. Oncol. 2023, 149, 8663–8671. [Google Scholar] [CrossRef]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef]
- Crombé, A.; Alberti, N.; Villard, N.; Pilleul, F.; Buy, X.; Le Loarer, F.; Kind, M. Imaging features of SMARCA4-deficient thoracic sarcomas: A multi-centric study of 21 patients. Eur. Radiol. 2019, 29, 4730–4741. [Google Scholar] [CrossRef]
- Luo, J.; Ding, B.; Campisi, A.; Chen, T.; Teng, H.; Ji, C. Molecular, clinicopathological characteristics and surgical results of resectable SMARCA4-deficient thoracic tumors. J. Cancer Res. Clin. Oncol. 2023, 149, 4455–4463. [Google Scholar] [CrossRef]
- Yang, P.; Xiong, F.; Lin, Y.; Liang, P.; Tang, C. Effectiveness of tislelizumab when combined with etoposide and carboplatin in patients with SMARCA4-deficient undifferentiated thoracic tumor: A case report. Transl. Cancer Res. 2023, 12, 1041–1048. [Google Scholar] [CrossRef]
- Henon, C.; Blay, J.Y.; Massard, C.; Mir, O.; Bahleda, R.; Dumont, S.; Postel-Vinay, S.; Adam, J.; Soria, J.C.; Le Cesne, A. Long lasting major response to pembrolizumab in a thoracic malignant rhabdoid-like SMARCA4-deficient tumor. Ann. Oncol. 2019, 30, 1401–1403. [Google Scholar] [CrossRef]
- Shi, L.; Lin, L.; Ding, Y.; Zeng, Y.; Chen, X. Case report: A rapid response to immunotherapy in a thoracic SMARCA4-deficient undifferentiated tumor with respiratory failure. Front. Oncol. 2022, 12, 1020875. [Google Scholar] [CrossRef]
- Naito, T.; Umemura, S.; Nakamura, H.; Zenke, Y.; Udagawa, H.; Kirita, K.; Matsumoto, S.; Yoh, K.; Niho, S.; Motoi, N.; et al. Successful treatment with nivolumab for SMARCA4-deficient non-small cell lung carcinoma with a high tumor mutation burden: A case report. Thorac. Cancer 2019, 10, 1285–1288. [Google Scholar] [CrossRef]
- Utsumi, T.; Taniguchi, Y.; Noda, Y.; Fukai, M.; Kibata, K.; Murakawa, T. SMARCA4-deficient undifferentiated tumor that responded to chemotherapy in combination with immune checkpoint inhibitors: A case report. Thorac. Cancer 2022, 13, 2264–2266. [Google Scholar] [CrossRef]
- Lee, E.K.; Esselen, K.M.; Kolin, D.L.; Lee, L.J.; Matulonis, U.A.; Konstantinopoulos, P.A. Combined CDK4/6 and PD-1 Inhibition in Refractory SMARCA4-Deficient Small-Cell Carcinoma of the Ovary, Hypercalcemic Type. JCO Precis. Oncol. 2020, 4, 736–742. [Google Scholar] [CrossRef]
- Tagal, V.; Wei, S.; Zhang, W.; Brekken, R.A.; Posner, B.A.; Peyton, M.; Girard, L.; Hwang, T.; Wheeler, D.A.; Minna, J.D. SMARCA4-inactivating mutations increase sensitivity to Aurora kinase A inhibitor VX-680 in non-small cell lung cancers. Nat. Commun. 2017, 8, 14098. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, Y.H.; Li, L.Y.; Lang, J.; Yeh, S.P.; Shi, B.; Yang, C.C.; Yang, J.Y.; Lin, C.Y.; Lai, C.C.; et al. CDK1-dependent phosphorylation of EZH2 suppresses methylation of H3K27 and promotes osteogenic differentiation of human mesenchymal stem cells. Nat. Cell Biol. 2011, 13, 87–94. [Google Scholar] [CrossRef]
- Januario, T.; Ye, X.; Bainer, R.; Alicke, B.; Smith, T.; Haley, B.; Modrusan, Z.; Gould, S.; Yauch, R.L. PRC2-mediated repression of SMARCA2 predicts EZH2 inhibitor activity in SWI/SNF mutant tumors. Proc. Natl. Acad. Sci. USA 2017, 114, 12249–12254. [Google Scholar] [CrossRef]
- Julia, E.; Salles, G. EZH2 inhibition by tazemetostat: Mechanisms of action, safety and efficacy in relapsed/refractory follicular lymphoma. Future Oncol. 2021, 17, 2127–2140. [Google Scholar] [CrossRef]
- Wilson, B.G.; Wang, X.; Shen, X.; McKenna, E.S.; Lemieux, M.E.; Cho, Y.J.; Koellhoffer, E.C.; Pomeroy, S.L.; Orkin, S.H.; Roberts, C.W. Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation. Cancer Cell 2010, 18, 316–328, Erratum in Cancer Cell 2011, 19, 153. [Google Scholar] [CrossRef]
- Yuan, H.; Nishikori, M.; Otsuka, Y.; Arima, H.; Kitawaki, T.; Takaori-Kondo, A. The EZH2 inhibitor tazemetostat upregulates the expression of CCL17/TARC in B-cell lymphoma and enhances T-cell recruitment. Cancer Sci. 2021, 112, 4604–4616. [Google Scholar] [CrossRef]
- Simeone, N.; Frezza, A.M.; Zaffaroni, N.; Stacchiotti, S. Tazemetostat for advanced epithelioid sarcoma: Current status and future perspectives. Future Oncol. 2021, 17, 1253–1263. [Google Scholar] [CrossRef]
- Italiano, A.; Soria, J.C.; Toulmonde, M.; Michot, J.M.; Lucchesi, C.; Varga, A.; Coindre, J.M.; Blakemore, S.J.; Clawson, A.; Suttle, B.; et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: A first-in-human, open-label, phase 1 study. Lancet Oncol. 2018, 19, 649–659. [Google Scholar] [CrossRef]
- Chi, S.N.; Yi, J.S.; Williams, P.M.; Roy-Chowdhuri, S.; Patton, D.R.; Coffey, B.D.; Reid, J.M.; Piao, J.; Saguilig, L.; Alonzo, T.A.; et al. Tazemetostat for tumors harboring SMARCB1/SMARCA4 or EZH2 alterations: Results from NCI-COG pediatric MATCH APEC1621C. J. Natl. Cancer Inst. 2023, 115, 1355–1363. [Google Scholar] [CrossRef]
- Available online: https://www.centerwatch.com/clinical-trials/listings/NCT05407441/tazemetostatnivo-ipi-in-ini1-negsmarca4-def-tumors (accessed on 15 January 2024).
- Tischkowitz, M.; Huang, S.; Banerjee, S.; Hague, J.; Hendricks, W.P.; Huntsman, D.G.; Lang, J.D.; Orlando, K.A.; Oza, A.M.; Pautier, P.; et al. Small-cell carcinoma of the ovary, hypercalcemic type–genetics, new treatment targets, and current management guidelines. Clin. Cancer Res. 2020, 26, 3908–3917. [Google Scholar] [CrossRef]
- Zou, L.; Elledge, S.J. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 2003, 300, 1542–1548. [Google Scholar] [CrossRef]
- Qi, L.; Wenyuan, Q.; Yang, Z.; Lihong, H.; Shuhui, C.; Yuanfeng, X. A new wave of innovations within the DNA damage response. Signal Transduct. Target. Ther. 2023, 8, 338. [Google Scholar] [CrossRef]
- Gupta, M.; Concepcion, C.P.; Fahey, C.G.; Keshishian, H.; Bhutkar, A.; Brainson, C.F.; Sanchez-Rivera, F.J.; Pessina, P.; Kim, J.Y.; Simoneau, A.; et al. BRG1 Loss Predisposes Lung Cancers to Replicative Stress and ATR Dependency. Cancer Res. 2020, 80, 3841–3854. [Google Scholar] [CrossRef]
- Roberts, C.W.; Orkin, S.H. The SWI/SNF complex--chromatin and cancer. Nat. Rev. Cancer 2004, 4, 133–142. [Google Scholar] [CrossRef]
- Wang, X.; Haswell, J.R.; Roberts, C.W. Molecular pathways: SWI/SNF (BAF) complexes are frequently mutated in cancer—Mechanisms and potential therapeutic insights. Clin. Cancer Res. 2014, 20, 21–27. [Google Scholar] [CrossRef]
- Clapier, C.R.; Cairns, B.R. The biology of chromatin remodeling complexes. Annu. Rev. Biochem. 2009, 78, 273–304. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, M.; Fang, H.; El-Mounayri, O.; Rodenberg, J.M.; Imbalzano, A.N.; Herring, B.P. The SWI/SNF chromatin remodeling complex regulates myocardin-induced smooth muscle-specific gene expression. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 921–928. [Google Scholar] [CrossRef]
- Magnani, L.; Cabot, R.A. Manipulation of SMARCA2 and SMARCA4 transcript levels in porcine embryos differentially alters development and expression of SMARCA1, SOX2, NANOG, and EIF1. Reproduction 2009, 137, 23–33. [Google Scholar] [CrossRef]
- Kim, Y.; Fedoriw, A.M.; Magnuson, T. An essential role for a mammalian SWI/SNF chromatin-remodeling complex during male meiosis. Development 2012, 139, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Kang, A.; Kuai, Y.; Guo, Y.; Miao, X.; Zhu, L.; Kong, M.; Li, N. The chromatin remodeling protein BRG1 regulates HSC-myofibroblast differentiation and liver fibrosis. Cell Death Dis. 2023, 14, 826. [Google Scholar] [CrossRef]
- Errico, A. Genetics: SMARCA4 mutated in SCCOHT. Nat. Rev. Clin. Oncol. 2014, 11, 302. [Google Scholar] [CrossRef]
- Le Loarer, F.; Watson, S.; Pierron, G.; de Montpreville, V.T.; Ballet, S.; Firmin, N.; Auguste, A.; Pissaloux, D.; Boyault, S.; Paindavoine, S.; et al. SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF-deficient sarcomas. Nat. Genet. 2015, 47, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Kolin, D.L.; Quick, C.M.; Dong, F.; Fletcher, C.D.M.; Stewart, C.J.R.; Soma, A.; Hornick, J.L.; Nucci, M.R.; Howitt, B.E. SMARCA4-deficient Uterine Sarcoma and Undifferentiated Endometrial Carcinoma Are Distinct Clinicopathologic Entities. Am. J. Surg. Pathol. 2020, 44, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Nakra, T.; Kakkar, A.; Mathur, S.R.; Jain, D.; Kumar, S. SMARCA4-deficient Undifferentiated Uterine Sarcoma: Clinicopathological Features of an Emerging Entity. Int. J. Surg. Pathol. 2023, 31, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Ahadi, M.S.; Fuchs, T.L.; Clarkson, A.; Sheen, A.; Sioson, L.; Chou, A.; Gill, A.J. Switch/sucrose-non-fermentable (SWI/SNF) complex (SMARCA4, SMARCA2, INI1/SMARCB1)-deficient colorectal carcinomas are strongly associated with microsatellite instability: An incidence study in 4508 colorectal carcinomas. Histopathology 2022, 80, 906–921. [Google Scholar] [CrossRef] [PubMed]
- Reisman, D.N.; Sciarrotta, J.; Wang, W.; Funkhouser, W.K.; Weissman, B.E. Loss of BRG1/BRM in human lung cancer cell lines and primary lung cancers: Correlation with poor prognosis. Cancer Res. 2003, 63, 560–566. [Google Scholar]
- Yoshida, A. NUT carcinoma and thoracic SMARCA4-deficient undifferentiated tumour: Facts and controversies. Histopathology 2023, 84, 86–101. [Google Scholar] [CrossRef]
- Bhat-Nakshatri, P.; Khatpe, A.S.; Chen, D.; Batic, K.; Mang, H.; Herodotou, C.; McGuire, P.C.; Xuei, X.; Erdogan, C.; Gao, H.; et al. Signaling Pathway Alterations Driven by BRCA1 and BRCA2 Germline Mutations are Sufficient to Initiate Breast Tumorigenesis by the PIK3CAH1047R Oncogene. Cancer Res. Commun. 2023, 4, 38–54. [Google Scholar] [CrossRef]
- Bochar, D.A.; Wang, L.; Beniya, H.; Kinev, A.; Xue, Y.; Lane, W.S.; Wang, W.; Kashanchi, F.; Shiekhattar, R. BRCA1 is associated with a human SWI/SNF-related complex: Linking chromatin remodeling to breast cancer. Cell 2000, 102, 257–265. [Google Scholar] [CrossRef]
- Orvis, T.; Hepperla, A.; Walter, V.; Song, S.; Simon, J.; Parker, J.; Wilkerson, M.D.; Desai, N.; Major, M.B.; Hayes, D.N.; et al. BRG1/SMARCA4 inactivation promotes non-small cell lung cancer aggressiveness by altering chromatin organization. Cancer Res. 2014, 74, 6486–6498. [Google Scholar] [CrossRef] [PubMed]
- Romero, O.A.; Setien, F.; John, S.; Gimenez-Xavier, P.; Gómez-López, G.; Pisano, D.; Condom, E.; Villanueva, A.; Hager, G.L.; Sanchez-Cespedes, M. The tumour suppressor and chromatin-remodelling factor BRG1 antagonizes Myc activity and promotes cell differentiation in human cancer. EMBO Mol. Med. 2012, 4, 603–616. [Google Scholar] [CrossRef]
- Kang, Q.; Peng, Z.; Ming, N.; Qin, F.; Jishi, W. BRG1 Regulates Adult B-Cell Acute Lymphoblastic Leukemia Proliferation and Apoptosis By Interacting with c-MYC and Activating PI3K/AKT Pathway. Blood 2023, 142 (Suppl. S1), 5725. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Li, W.; Ma, L.; Zheng, D.; Li, L.; Yang, W.; Chu, M.; Chen, W.; Mailman, R.B.; et al. Transcriptional repression by the BRG1-SWI/SNF complex affects the pluripotency of human embryonic stem cells. Stem Cell Rep. 2014, 3, 460–474. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, Q.; Gu, J.; Xuan, Z.; Wu, J.I. SMARCA4/Brg1 coordinates genetic and epigenetic networks underlying Shh-type medulloblastoma development. Oncogene 2016, 35, 5746–5758. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, C.P.; Ma, S.; LaFave, L.M.; Bhutkar, A.; Liu, M.; DeAngelo, L.P.; Kim, J.Y.; Del Priore, I.; Schoenfeld, A.J.; Miller, M.; et al. Buenrostro, Tyler Jacks; Smarca4 Inactivation Promotes Lineage-Specific Transformation and Early Metastatic Features in the Lung. Cancer Discov. 2022, 12, 562–585. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Cui, K.; Zhao, K. BRG1 controls the activity of the retinoblastoma protein via regulation of p21CIP1/WAF1/SDI. Mol. Cell Biol. 2004, 24, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Sun, L.; Xu, F.; Liu, L.; Hu, F.; Song, D.; Hou, Z.; Wu, W.; Luo, X.; Wang, J.; et al. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res. 2019, 79, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Chen, J.; Zheng, H.; Lin, A.; Li, J.; Wu, W.; Jie, Q. MiR-199a-5p-containing macrophage-derived extracellular vesicles inhibit SMARCA4 and alleviate atherosclerosis by reducing endothelial cell pyroptosis. Cell Biol. Toxicol. 2022, 39, 591–605. [Google Scholar] [CrossRef]
- Rosson, G.B.; Bartlett, C.; Reed, W.; Weissman, B.E. BRG1 loss in MiaPaCa2 cells induces an altered cellular morphology and disruption in the organization of the actin cytoskeleton. J. Cell. Physiol. 2005, 205, 286–294. [Google Scholar] [CrossRef]
- Navickas, S.M.; Giles, K.; Brettingham-Moore, K.H. The role of chromatin remodeler SMARCA4/BRG1 in brain cancers: A potential therapeutic target. Oncogene 2023, 42, 2363–2373. [Google Scholar] [CrossRef]
- Chatzopoulos, K.; Boland, J.M. Update on genetically defined lung neoplasms: NUT carcinoma and thoracic SMARCA4-deficient undifferentiated tumors. Virchows Arch. 2021, 478, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Sun, L.; Salyana, M.; Eric, M.; Gotlieb, V.; Wang, J.C. SMARCA4-Deficient Undifferentiated Tumor of Lung Mass-A Rare Tumor With the Rarer Occurrence of Brain Metastasis: A Case Report and Review of the Literature. J. Investig. Med. High Impact Case Rep. 2022, 10, 23247096221074864. [Google Scholar] [CrossRef] [PubMed]
- Sauter, J.L.; Graham, R.P.; Larsen, B.T.; Jenkins, S.M.; Roden, A.C.; Boland, J.M. SMARCA4-deficient thoracic sarcoma: A distinctive clinicopathological entity with undifferentiated rhabdoid morphology and aggressive behavior. Mod. Pathol. 2017, 30, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Nambirajan, A.; Jain, D. Recent updates in thoracic SMARCA4-deficient undifferentiated tumor. Semin. Diagn. Pathol. 2021, 38, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Yang, Z.; Li, N. 68 Ga-DOTA-FAPI-04 PET/CT in the Detection of Thoracic SMARCA4-Deficient Undifferentiated Tumor. Clin. Nucl. Med. 2023, 48, 1102–1104. [Google Scholar] [CrossRef]
- Yoshida, A.; Kobayashi, E.; Kubo, T.; Kodaira, M.; Motoi, T.; Motoi, N.; Yonemori, K.; Ohe, Y.; Watanabe, S.I.; Kawai, A.; et al. Clinicopathological and molecular characterization of SMARCA4-deficient thoracic sarcomas with comparison to potentially related entities. Mod. Pathol. 2017, 30, 797–809. [Google Scholar] [CrossRef]
- Rekhtman, N.; Montecalvo, J.; Chang, J.C.; Alex, D.; Ptashkin, R.N.; Ai, N.; Sauter, J.L.; Kezlarian, B.; Jungbluth, A.; Desmeules, P.; et al. SMARCA4-Deficient Thoracic Sarcomatoid Tumors Represent Primarily Smoking-Related Undifferentiated Carcinomas Rather Than Primary Thoracic Sarcomas. J. Thorac. Oncol. 2020, 15, 231–247. [Google Scholar] [CrossRef]
- Agaimy, A.; Fuchs, F.; Moskalev, E.A.; Sirbu, H.; Hartmann, A.; Haller, F. SMARCA4-deficient pulmonary adenocarcinoma: Clinicopathological, immunohistochemical, and molecular characteristics of a novel aggressive neoplasm with a consistent TTF1neg/CK7pos/HepPar-1pos immunophenotype. Virchows Arch. 2017, 471, 599–609. [Google Scholar] [CrossRef]
- Kunimasa, K.; Nakamura, H.; Sakai, K.; Tamiya, M.; Kimura, M.; Inoue, T.; Nishino, K.; Kuhara, H.; Nakatsuka, S.I.; Nishio, K.; et al. Patients with SMARCA4-deficient thoracic sarcoma and severe skeletal-related events. Lung Cancer 2019, 132, 59–64. [Google Scholar] [CrossRef]
- Pokhrel, A.; Yadav, R.; Manvar, K.K.; Wu, R.; Jaswani, V.; Wasserman, C.B.; Wang, J.C. Chemotherapy and Immune Checkpoint Inhibitors in a Case of SMARCA4-dUT: A Case Report and Review of Literature. J. Investig. Med. High Impact Case Rep. 2023, 11, 23247096231176220. [Google Scholar] [CrossRef]
- Maartens, D.J.; Moolla, M.S.; Ndaba, S.; Vlok, S.S.C.; Hendricks, F.; Koegelenberg, C.F.N.; van Wyk, A.C. Thoracic SMARCA4-deficient undifferentiated tumour: Diagnostic challenges and potential for misdiagnosis in small tissue samples. Respirol. Case Rep. 2023, 11, e01238. [Google Scholar] [CrossRef]
- Marshall, M.; Khader, S.; Beasley, S.; Lajara, S. Thoracic SMARCA4-deficient undifferentiated tumor with associated granulomatous reaction and response to pembrolizumab. Diagn. Cytopathol. 2023, 51, E287–E293. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, A.J.; Bandlamudi, C.; Lavery, J.A.; Montecalvo, J.; Namakydoust, A.; Rizvi, H.; Egger, J.; Concepcion, C.P.; Paul, S.; Arcila, M.E.; et al. The Genomic Landscape of SMARCA4 Alterations and Associations with Outcomes in Patients with Lung Cancer. Clin. Cancer Res. 2020, 26, 5701–5708. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Jin, Y.; Cao, Z.; Lu, L.; Li, Z. Clinicopathological characteristics and treatment outcomes of advanced SMARCA4-deficient thoracic tumors. Cancer Med. 2023, 13, e6809. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Han, W.; Pan, H. Thoracic SMARCA4-Deficient Undifferentiated Tumor With ALK Fusion Treated With Alectinib Achieved Remarkable Tumor Regression: Case Report. JTO Clin. Res. Rep. 2023, 4, 100476. [Google Scholar] [CrossRef] [PubMed]
- Asou, C.; Maeda, T.; Yamaguchi, O.; Okamura, D.; Ishikawa, M.; Kohri, M.; Tsukasaki, K.; Matsuda, A.; Asou, N.; Satoh, T.; et al. Thoracic SMARCA4-deficient undifferentiated tumor resembling malignant lymphoma. Rinsho Ketsueki 2023, 64, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Helmink, A.J.; Alshomrani, A.; Lauer, S.R.; Yuil-Valdes, A. Thoracic SMARCA4-Deficient Undifferentiated Tumors with Unusual Presentations: A Case Series. Int. J. Surg. Pathol. 2023, 10668969231206350. [Google Scholar] [CrossRef] [PubMed]
- Kolin, D.L.; Dong, F.; Baltay, M.; Lindeman, N.; MacConaill, L.; Nucci, M.R.; Crum, C.P.; Howitt, B.E. SMARCA4-deficient undifferentiated uterine sarcoma (malignant rhabdoid tumor of the uterus): A clinicopathologic entity distinct from undifferentiated carcinoma. Mod. Pathol. 2018, 31, 1442–1456. [Google Scholar] [CrossRef]
- Khanchel, F.; Hedhili, R.; Zenaidi, H.; Helal, I.; Yahmadi, A.; Ben, N.H.; Ksontini, F.; Ben Brahim, E.; Jouini, R.; Chadli, A. SMARCA4-deficient thoracic sarcoma revealed by metastasis to the small intestine: A diagnostic dilemma. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 1155–1158. [Google Scholar] [CrossRef]
- Ramos, P.; Karnezis, A.N.; Craig, D.W.; Sekulic, A.; Russell, M.L.; Hendricks, W.P.; Corneveaux, J.J.; Barrett, M.T.; Shumansky, K.; Yang, Y.; et al. Small cell carcinoma of the ovary, hypercalcemic type, displays frequent inactivating germline and somatic mutations in SMARCA4. Nat. Genet. 2014, 46, 427–429. [Google Scholar] [CrossRef]
- Kuwamoto, S.; Matsushita, M.; Takeda, K.; Tanaka, N.; Endo, Y.; Yamasaki, A.; Kohashi, K.; Oda, Y.; Horie, Y. SMARCA4-deficient thoracic sarcoma: Report of a case and insights into how to reach the diagnosis using limited samples and resources. Hum. Pathol. 2017, 70, 92–97. [Google Scholar] [CrossRef]
- Kothandapani, A.; Gopalakrishnan, K.; Kahali, B.; Reisman, D.; Patrick, S.M. Downregulation of SWI/SNF chromatin remodeling factor subunits modulates cisplatin cytotoxicity. Exp. Cell Res. 2012, 318, 1973–1986. [Google Scholar] [CrossRef]
- Xue, Y.; Morris, J.L.; Yang, K.; Fu, Z.; Zhu, X.; Johnson, F.; Meehan, B.; Witkowski, L.; Yasmeen, A.; Golenar, T.; et al. SMARCA4/2 loss inhibits chemotherapy-induced apoptosis by restricting IP3R3-mediated Ca2+ flux to mitochondria. Nat. Commun. 2021, 12, 5404, Erratum in Nat. Commun. 2023, 14, 1552. [Google Scholar] [CrossRef]
- Bell, E.H.; Chakraborty, A.R.; Mo, X.; Liu, Z.; Shilo, K.; Kirste, S.; Stegmaier, P.; McNulty, M.; Karachalion, N.; Rosell, R.; et al. SMARCA4/BRG1 Is a Novel Prognostic Biomarker Predictive of Cisplatin-Based Chemotherapy Outcomes in Resected Non-Small Cell Lung Cancer. Clin. Cancer Res. 2016, 22, 2396–2404. [Google Scholar] [CrossRef]
- Kunimasa, K.; Okami, J.; Takenaka, S.; Honma, K.; Kukita, Y.; Nagata, S.; Kawamura, T.; Inoue, T.; Tamiya, M.; Kuhara, H.; et al. Conversion Surgery for Advanced Thoracic SMARCA4-Deficient Undifferentiated Tumor With Atezolizumab in Combination With Bevacizumab, Paclitaxel, and Carboplatin Treatment: A Case Report. JTO Clin. Res. Rep. 2021, 2, 100235. [Google Scholar] [CrossRef]
- Soto-Castillo, J.J.; Lavata-Marti, L.; Fort-Culillas, R.; Andreu-Cobo, P.; Moreno, R.; Codony, C.; García Del Muro, X.; Alemany, R.; Piulats, J.M.; Martin-Liberal, J. SWI/SNF Complex Alterations in Tumors with Rhabdoid Features: Novel Therapeutic Approaches and Opportunities for Adoptive Cell Therapy. Int. J. Mol. Sci. 2023, 24, 11143. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Kobayashi, A.; Jiang, P.; Ferrari de Andrade, L.; Tay, R.E.; Luoma, A.M.; Tsoucas, D.; Qiu, X.; Lim, K.; Rao, P.; et al. A major chromatin regulator determines resistance of tumor cells to T cell-mediated killing. Science 2018, 359, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Gantzer, J.; Davidson, G.; Vokshi, B.; Weingertner, N.; Bougoüin, A.; Moreira, M.; Lindner, V.; Lacroix, G.; Mascaux, C.; Chenard, M.P.; et al. Immune-Desert Tumor Microenvironment in Thoracic SMARCA4-Deficient Undifferentiated Tumors with Limited Efficacy of Immune Checkpoint Inhibitors. Oncologist 2022, 27, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, C.; Iizuka, A.; Ikeya, T.; Miyata, H.; Maeda, C.; Ashizawa, T.; Nagashima, T.; Urakami, K.; Shimoda, Y.; Ohshima, K.; et al. Impact of Mutations in Subunit Genes of the Mammalian SWI/SNF Complex on Immunological Tumor Microenvironment. Cancer Genom. Proteom. 2024, 21, 88–101. [Google Scholar] [CrossRef]
- Bever, K.M.; Le, D.T. DNA repair defects and implications for immunotherapy. J. Clin. Investig. 2018, 128, 4236–4242. [Google Scholar] [CrossRef] [PubMed]
- Shinno, Y.; Yoshida, A.; Masuda, K.; Matsumoto, Y.; Okuma, Y.; Yoshida, T.; Goto, Y.; Horinouchi, H.; Yamamoto, N.; Yatabe, Y.; et al. Efficacy of Immune Checkpoint Inhibitors in SMARCA4-Deficient Thoracic Tumor. Clin. Lung Cancer 2022, 23, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhang, B.; Nie, L.G.; Wu, S.K.; Zhao, H.; Li, D.; Di, J.T. Thoracic SMARCA4-deficient undifferentiated tumor-pathological diagnosis and combined immune checkpoint inhibitor treatment. Beijing Da Xue Xue Bao Yi Xue Ban 2023, 55, 351–356. [Google Scholar] [CrossRef]
- Wang, J.; Elghawy, O.; Kurpiel, B.; Kaur, V. Diagnosis and management of gastrointestinal SMARCA4-deficient undifferentiated tumors. Clin. J. Gastroenterol. 2023, 16, 807–814. [Google Scholar] [CrossRef]
- Xue, Y.; Meehan, B.; Macdonald, E.; Venneti, S.; Wang, X.Q.D.; Witkowski, L.; Jelinic, P.; Kong, T.; Martinez, D.; Morin, G.; et al. CDK4/6 inhibitors target SMARCA4-determined cyclin D1 deficiency in hypercalcemic small cell carcinoma of the ovary. Nat. Commun. 2019, 10, 558. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Meehan, B.; Fu, Z.; Wang, X.Q.D.; Fiset, P.O.; Rieker, R.; Levins, C.; Kong, T.; Zhu, X.; Morin, G.; et al. SMARCA4 loss is synthetic lethal with CDK4/6 inhibition in non-small cell lung cancer. Nat. Commun. 2019, 10, 557. [Google Scholar] [CrossRef] [PubMed]
- Zeverijn, L.J.; Looze, E.J.; Thavaneswaran, S.; van Berge Henegouwen, J.M.; Simes, R.J.; Hoes, L.R.; Sjoquist, K.M.; van der Wijngaart, H.; Sebastian, L.; Geurts, B.S.; et al. Limited clinical activity of palbociclib and ribociclib monotherapy in advanced cancers with cyclin D-CDK4/6 pathway alterations in the Dutch DRUP and Australian MoST trials. Int. J. Cancer 2023, 153, 1413–1422. [Google Scholar] [CrossRef]
- Deribe, Y.L.; Sun, Y.; Terranova, C.; Khan, F.; Martinez-Ledesma, J.; Gay, J.; Gao, G.; Mullinax, R.A.; Khor, T.; Feng, N.; et al. Author Correction: Mutations in the SWI/SNF complex induce a targetable dependence on oxidative phosphorylation in lung cancer. Nat. Med. 2018, 24, 1627, Erratum in Nat. Med. 2018, 24, 1047–1057. [Google Scholar] [CrossRef]
- Yap, T.A.; Daver, N.; Mahendra, M.; Zhang, J.; Kamiya-Matsuoka, C.; Meric-Bernstam, F.; Kantarjian, H.M.; Ravandi, F.; Collins, M.E.; Francesco, M.E.; et al. Complex I inhibitor of oxidative phosphorylation in advanced solid tumors and acute myeloid leukemia: Phase I trials. Nat. Med. 2023, 29, 115–126. [Google Scholar] [CrossRef]
- Pilié, P.G.; Gay, C.M.; Byers, L.A.; O’Connor, M.J.; Yap, T.A. PARP Inhibitors: Extending Benefit Beyond BRCA-Mutant Cancers. Clin. Cancer Res. 2019, 25, 3759–3771. [Google Scholar] [CrossRef]
- Alessi, J.V.; Elkrief, A.; Ricciuti, B.; Wang, X.; Cortellini, A.; Vaz, V.R.; Lamberti, G.; Frias, R.L.; Venkatraman, D.; Fulgenzi, C.A.M.; et al. Clinicopathologic and Genomic Factors Impacting Efficacy of First-Line Chemoimmunotherapy in Advanced NSCLC. J. Thorac. Oncol. 2023, 18, 731–743. [Google Scholar] [CrossRef]
- Alessi, J.V.; Ricciuti, B.; Spurr, L.F.; Gupta, H.; Li, Y.Y.; Glass, C.; Nishino, M.; Cherniack, A.D.; Lindsay, J.; Sharma, B.; et al. SMARCA4 and Other SWItch/Sucrose NonFermentable Family Genomic Alterations in NSCLC: Clinicopathologic Characteristics and Outcomes to Immune Checkpoint Inhibition. J. Thorac. Oncol. 2021, 16, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ahmed, T.; Petty, W.J.; Grant, S.; Ruiz, J.; Lycan, T.W.; Topaloglu, U.; Chou, P.C.; Miller, L.D.; Hawkins, G.A.; et al. SMARCA4 mutations in KRAS-mutant lung adenocarcinoma: A multi-cohort analysis. Mol. Oncol. 2021, 15, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Negrao, M.V.; Araujo, H.A.; Lamberti, G.; Cooper, A.J.; Akhave, N.S.; Zhou, T.; Delasos, L.; Hicks, J.K.; Aldea, M.; Minuti, G.; et al. Comutations and KRASG12C Inhibitor Efficacy in Advanced NSCLC. Cancer Discov. 2023, 13, 1556–1571. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, V.; Catino, A.; Montrone, M.; Montagna, E.S.; Pesola, F.; Marech, I.; Pizzutilo, P.; Nardone, A.; Perrone, A.; Gesualdo, M.; et al. Treatment of Thoracic SMARCA4-Deficient Undifferentiated Tumors: Where We Are and Where We Will Go. Int. J. Mol. Sci. 2024, 25, 3237. https://doi.org/10.3390/ijms25063237
Longo V, Catino A, Montrone M, Montagna ES, Pesola F, Marech I, Pizzutilo P, Nardone A, Perrone A, Gesualdo M, et al. Treatment of Thoracic SMARCA4-Deficient Undifferentiated Tumors: Where We Are and Where We Will Go. International Journal of Molecular Sciences. 2024; 25(6):3237. https://doi.org/10.3390/ijms25063237
Chicago/Turabian StyleLongo, Vito, Annamaria Catino, Michele Montrone, Elisabetta Sara Montagna, Francesco Pesola, Ilaria Marech, Pamela Pizzutilo, Annalisa Nardone, Antonella Perrone, Monica Gesualdo, and et al. 2024. "Treatment of Thoracic SMARCA4-Deficient Undifferentiated Tumors: Where We Are and Where We Will Go" International Journal of Molecular Sciences 25, no. 6: 3237. https://doi.org/10.3390/ijms25063237
APA StyleLongo, V., Catino, A., Montrone, M., Montagna, E. S., Pesola, F., Marech, I., Pizzutilo, P., Nardone, A., Perrone, A., Gesualdo, M., & Galetta, D. (2024). Treatment of Thoracic SMARCA4-Deficient Undifferentiated Tumors: Where We Are and Where We Will Go. International Journal of Molecular Sciences, 25(6), 3237. https://doi.org/10.3390/ijms25063237






