Neutrophil Gelatinase-Associated Lipocalin for the Differentiation of Mucinous Pancreatic Cystic Lesions
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Biomarkers Analysis
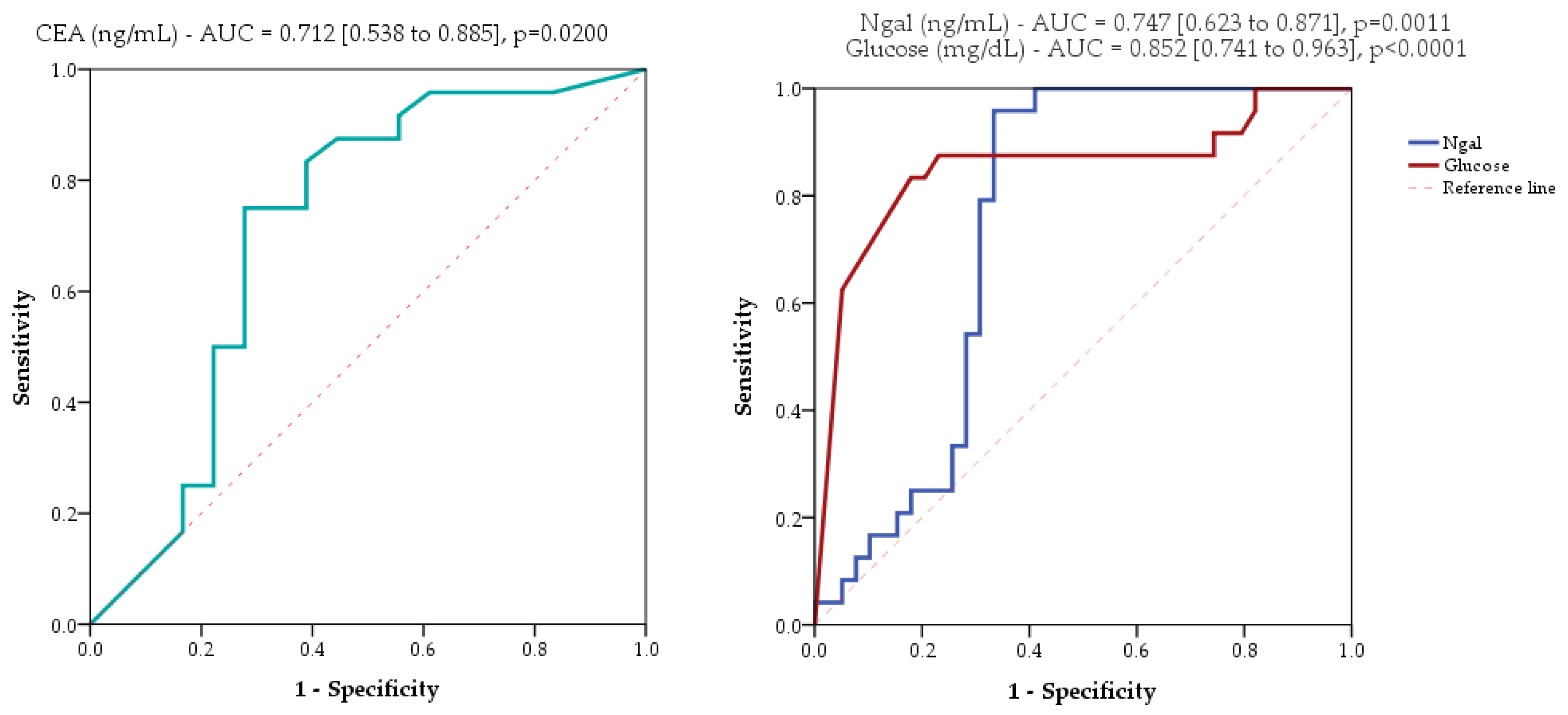
2.3. Evaluated Markers as Diagnostic Tools
3. Discussion
4. Materials and Methods
4.1. Study Design
4.1.1. Inclusion Criteria
4.1.2. Exclusion Criteria
4.2. Procedure
4.3. Preparation of Samples
4.4. Sandwich Enzyme-Linked Immunosorbent Assays
4.5. Glucose Level Measurements
4.6. Definitions
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zerboni, G.; Signoretti, M.; Crippa, S.; Falconi, M.; Arcidiacono, P.G.; Capurso, G. Systematic review and meta-analysis: Prevalence of incidentally detected pancreatic cystic lesions in asymptomatic individuals. Pancreatology 2019, 19, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Kromrey, M.L.; Bülow, R.; Hübner, J.; Paperlein, C.; Lerch, M.M.; Ittermann, T.; Völzke, H.; Mayerle, J.; Kühn, J.P. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut 2018, 67, 138–145. [Google Scholar] [CrossRef]
- Tan, J.H.; Chin, W.; Shaikh, A.L.; Zheng, S. Pancreatic pseudocyst: Dilemma of its recent management (Review). Exp. Ther. Med. 2021, 21, 159. [Google Scholar] [CrossRef] [PubMed]
- Pezzilli, R.; Buscarini, E.; Pollini, T.; Bonamini, D.; Marchegiani, G.; Crippa, S.; Belfiori, G.; Sperti, C.; Moletta, L.; Pozza, G.; et al. Epidemiology, clinical features and diagnostic work-up of cystic neoplasms of the pancreas: Interim analysis of the prospective PANCY survey. Dig. Liver Dis. 2020, 52, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Del Chiaro, M.; Segersvärd, R.; Pozzi Mucelli, R.; Rangelova, E.; Kartalis, N.; Ansorge, C.; Arnelo, U.; Blomberg, J.; Löhr, M.; Verbeke, C. Comparison of preoperative conference-based diagnosis with histology of cystic tumors of the pancreas. Ann. Surg. Oncol. 2014, 21, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Wang, Z.J.; Pan, C.Y.; Wu, C.; Li, Z.S.; Jin, Z.D.; Wang, K.X. Comparative Performance of Endoscopic Ultrasound-Based Techniques in Patients with Pancreatic Cystic Lesions: A Network Meta-Analysis. Am. J. Gastroenterol. 2023, 118, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Gorris, M.; Janssen, Q.P.; Besselink, M.G.; van den Broek, B.L.J.; van Eijck, C.H.J.; van Gils, M.J.; Koerkamp, B.G.; Struik, F.; van Driel, L.M.; van Hooft, J.E. Sensitivity of CT, MRI, and EUS-FNA/B in the preoperative workup of histologically proven left-sided pancreatic lesions. Pancreatology 2022, 22, 136–141. [Google Scholar] [CrossRef]
- Gorris, M.; Dijk, F.; Farina, A.; Halfwerk, J.B.; Hooijer, G.K.; Lekkerkerker, S.J.; Voermans, R.P.; Wielenga, M.C.; Besselink, M.G.; van Hooft, J.E. Validation of combined carcinoembryonic antigen and glucose testing in pancreatic cyst fluid to differentiate mucinous from non-mucinous cysts. Surg. Endosc. 2023, 37, 3739–3746. [Google Scholar] [CrossRef]
- Smith, Z.L.; Satyavada, S.; Simons-Linares, R.; Mok, S.R.S.; Martinez Moreno, B.; Aparicio, J.R.; Chahal, P. Intracystic Glucose and Carcinoembryonic Antigen in Differentiating Histologically Confirmed Pancreatic Mucinous Neoplastic Cysts. Am. J. Gastroenterol. 2022, 117, 478–485. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kaur, S.; Guha, S.; Batra, S.K. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim. Biophys. Acta 2012, 1826, 129–169. [Google Scholar] [CrossRef]
- Clerico, A.; Galli, C.; Fortunato, A.; Ronco, C. Neutrophil gelatinase-associated lipocalin (NGAL) as biomarker of acute kidney injury: A review of the laboratory characteristics and clinical evidences. Clin. Chem. Lab. Med. 2012, 50, 1505–1517. [Google Scholar] [CrossRef]
- Devarajan, P. Neutrophil gelatinase-associated lipocalin--an emerging troponin for kidney injury. Nephrol. Dial. Transplant. 2008, 23, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Donato, V.; Lacquaniti, A.; Fazio, M.R.; Bono, C.; Coppolino, G.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL) in human neoplasias: A new protein enters the scene. Cancer Lett. 2010, 288, 10–16. [Google Scholar] [CrossRef]
- Roli, L.; Pecoraro, V.; Trenti, T. Can NGAL be employed as prognostic and diagnostic biomarker in human cancers? A systematic review of current evidence. Int. J. Biol. Markers 2017, 32, e53–e61. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, M.; Rydzewska-Rosolowska, A.; Rydzewski, A.; Rydzewska, G. Urinary neutrophil gelatinase-associated lipocalin as an early predictor of disease severity and mortality in acute pancreatitis. Pancreas 2015, 44, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, F.C.; Levy, M.J.; Jackson, R.A.; Murphy, S.J.; Halling, K.C.; Kipp, B.R.; Graham, R.P.; Zhang, L. Lipocalin-2 Expression in Pancreas Adenocarcinoma Tumor Microenvironment via Endoscopic Ultrasound Fine Needle Biopsy Is Feasible and May Reveal a Therapeutic Target. Pancreas 2020, 49, e98–e99. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Baine, M.J.; Guha, S.; Ochi, N.; Chakraborty, S.; Mallya, K.; Thomas, C.; Crook, J.; Wallace, M.B.; Woodward, T.A.; et al. Neutrophil gelatinase-associated lipocalin, macrophage inhibitory cytokine 1, and carbohydrate antigen 19-9 in pancreatic juice: Pathobiologic implications in diagnosing benign and malignant disease of the pancreas. Pancreas 2013, 42, 494–501. [Google Scholar] [CrossRef]
- Levink, I.J.M.; Visser, I.J.; Koopmann, B.D.M.; van Driel, L.M.J.W.; Poley, J.W.; Cahen, D.L.; Bruno, M.J.; Fuhler, G.M. Protein biomarkers in pancreatic juice and serum for identification of pancreatic cancer. Gastrointest. Endosc. 2022, 96, 801–813.e2. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, M.; Degowska, M.; Rydzewska, G. Cystic fluid neutrophil gelatinase-associated lipocalin (NGAL) concentration in differential diagnosis of pancreatic cystic lesions: A new factor enters the scene? Prz. Gastroenterol. 2018, 13, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta-A Friend or Foe in Malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Kim, D.B.; Ko, S.H.; Jo, Y.H.; Kim, M.J. Induction mechanism of lipocalin-2 expression by co-stimulation with interleukin-1β and interferon-γ in RINm5F beta-cells. Biochem. Biophys. Res. Commun. 2013, 434, 577–583. [Google Scholar] [CrossRef]
- Khan, I.; Baig, M.; Bandepalle, T.; Puli, S.R. Utility of Cyst Fluid Carcinoembryonic Antigen in Differentiating Mucinous and Non-mucinous Pancreatic Cysts: An Updated Meta-Analysis. Dig. Dis. Sci. 2022, 67, 4541–4548. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Calderón, E.; Md, B.M.; Casellas, J.A.; Aparicio, J.R. Intracystic Glucose Levels Appear Useful for Diagnosis of Pancreatic Cystic Lesions: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2022, 67, 2562–2570. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.R.; Garg, R.; Rustagi, T. Pancreatic cyst fluid glucose in differentiating mucinous from nonmucinous pancreatic cysts: A systematic review and meta-analysis. Gastrointest. Endosc. 2021, 94, 698–712.e6. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.A.; Yip-Schneider, M.T.; Simpson, R.E.; Dolejs, S.; Schneider, J.G.; Wu, H.; Ceppa, E.P.; Park, W.; Schmidt, C.M. Pancreatic cyst fluid glucose: Rapid, inexpensive, and accurate diagnosis of mucinous pancreatic cysts. Surgery 2018, 163, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Barutcuoglu, B.; Oruc, N.; Ak, G.; Kucukokudan, S.; Aydın, A.; Nart, D.; Harman, M. Co-analysis of pancreatic cyst fluid carcinoembryonic antigen and glucose with novel cut-off levels better distinguishes between mucinous and non-mucinous neoplastic pancreatic cystic lesions. Ann. Clin. Biochem. 2022, 59, 125–133. [Google Scholar] [CrossRef]
- Abdellah, T.A.; Shalaby, S.M.; Salem, A.A.; Yousef, T.M.; Saleh, S.A.; Saleh, H.H.; Zaghloul, M.S.; El-Nady, M.A. Pancreatic cyst fluid interleukin-1 beta (IL-1β) level in predicting the risk of malignancy in pancreatic cysts. Egypt. J. Immunol. 2022, 29, 75–83. [Google Scholar] [CrossRef]
- Kaddah, M.; Okasha, H.H.; Hasan, E.M.; Elbaz, T.; El Ansary, M.; Khattab, H.; Yosry, A. The Role of Interleukin 1 Beta in Differentiating Malignant from Benign Pancreatic Cysts. J. Interferon Cytokine Res. 2022, 42, 118–126. [Google Scholar] [CrossRef]
- Gomez-Chou, S.B.; Swidnicka-Siergiejko, A.K.; Badi, N.; Chavez-Tomar, M.; Lesinski, G.B.; Bekaii-Saab, T.; Farren, M.R.; Mace, T.A.; Schmidt, C.; Liu, Y.; et al. Lipocalin-2 Promotes Pancreatic Ductal Adenocarcinoma by Regulating Inflammation in the Tumor Microenvironment. Cancer Res. 2017, 77, 2647–2660. [Google Scholar] [CrossRef]
- Kuhlmann, L.; Nadler, W.M.; Kerner, A.; Hanke, S.A.; Noll, E.M.; Eisen, C.; Espinet, E.; Vogel, V.; Trumpp, A.; Sprick, M.R.; et al. Identification and Validation of Novel Subtype-Specific Protein Biomarkers in Pancreatic Ductal Adenocarcinoma. Pancreas 2017, 46, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Rehwald, C.; Schnetz, M.; Urbschat, A.; Mertens, C.; Meier, J.K.; Bauer, R.; Baer, P.; Winslow, S.; Roos, F.C.; Zwicker, K.; et al. The iron load of lipocalin-2 (LCN-2) defines its pro-tumour function in clear-cell renal cell carcinoma. Br. J. Cancer 2020, 122, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Sánchez, G.S.; Pita-Grisanti, V.; Quiñones-Díaz, B.; Gumpper, K.; Cruz-Monserrate, Z.; Vivas-Mejía, P.E. Biological Functions and Therapeutic Potential of Lipocalin 2 in Cancer. Int. J. Mol. Sci. 2020, 21, 4365. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Kaur, S.; Muddana, V.; Sharma, N.; Wittel, U.A.; Papachristou, G.I.; Whitcomb, D.; Brand, R.E.; Batra, S.K. Elevated serum neutrophil gelatinase-associated lipocalin is an early predictor of severity and outcome in acute pancreatitis. Am. J. Gastroenterol. 2010, 105, 2050–2059. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.; Muniyan, S.; Thompson, C.M.; Kaur, S.; Jain, M.; Singh, R.K.; Dhaliwal, A.; Cox, J.L.; Akira, S.; Singh, S.; et al. Neutrophil Gelatinase-Associated Lipocalin Protects Acinar Cells from Cerulein-Induced Damage during Acute Pancreatitis. Pancreas 2020, 49, 1297–1306. [Google Scholar] [CrossRef]
- Oh, C.H.; Lee, J.K.; Song, T.J.; Park, J.S.; Lee, J.M.; Son, J.H.; Jang, D.K.; Choi, M.; Byeon, J.S.; Lee, I.S.; et al. Clinical Practice Guidelines for the Endoscopic Management of Peripancreatic Fluid Collections. Clin. Endosc. 2021, 54, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Faias, S.; Pereira, L.; Roque, R.; Chaves, P.; Torres, J.; Cravo, M.; Pereira, A.D. Excellent Accuracy of Glucose Level in Cystic Fluid for Diagnosis of Pancreatic Mucinous Cysts. Dig. Dis. Sci. 2020, 65, 2071–2078. [Google Scholar] [CrossRef]
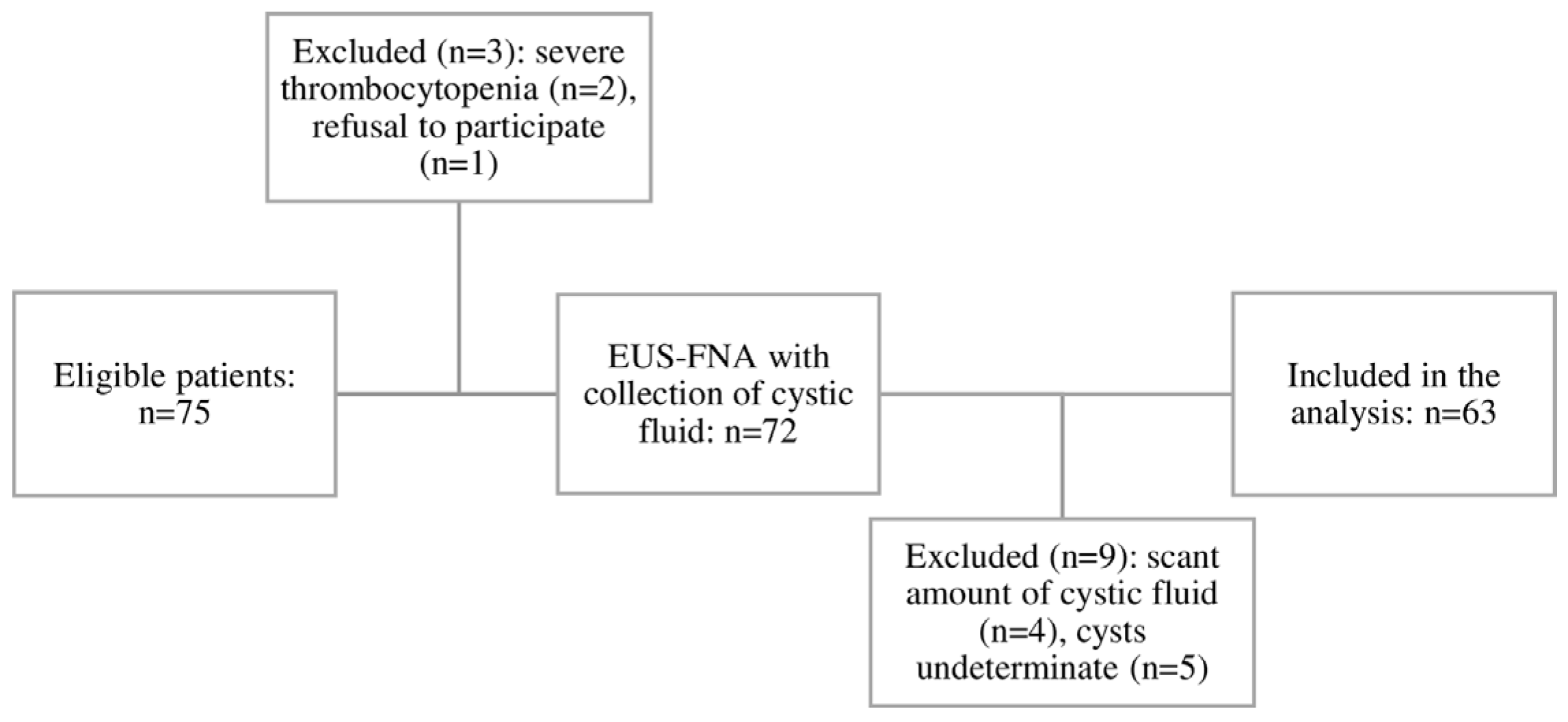

| Characteristic | All, n = 63 | Pseudocysts, n = 33 | Non-Inflammatory PCLs, n = 30 | Stat. (p-Value) |
|---|---|---|---|---|
| Age, years a | 56 (14) | 53.7 (13) | 58.6 (14.8) | −1.4 (0.1673) |
| Sex male b | 33 (52.4) | 24 (72.7) | 9 (30) | 11.5 (0.0007) |
| Alcohol b | 17 (27) | 13 (39.4) | 4 (13.3) | 5.4 (0.0199) |
| Smoker b | 22 (34.9) | 13 (39.4) | 9 (30) | 0.6 (0.4347) |
| Localization b | 0.06 (0.9718) | |||
| Head | 30 (47.6) | 16 (48.5) | 14 (46.7) | |
| Body | 18 (28.6) | 9 (27.3) | 9 (30) | |
| Tail | 15 (23.8) | 8 (24.2) | 7 (23.3) | |
| Cyst size, mm c | 38 [25 to 57.5] | 50 [40 to 60] | 27 [20 to 35] | 4.1 (<0.0001) |
| Final diagnosis b | n.a. | |||
| Cystic ductal adk | 2 (3.2) | 2 (6.7) | ||
| MCN | 3 (4.8) | 3 (10) | ||
| BD-IPMN | 19 (30.2) | 19 (63.3) | ||
| SCN | 6 (9.5) | 6 (20) | ||
| PC | 33 (52.4) | 33 (100) |
| Marker | Pseudocyst, n = 33 | Non-Inflammatory PCLs, n = 30 | Stat. (p-Value) | Mucinous, n = 24 | Non-Mucious, n = 39 | Stat. (p-Value) |
|---|---|---|---|---|---|---|
| Biomarkers in cystic fluid | ||||||
| NGAL, ng/mL | 1199.4 [828.7 to 1510.4] | 669.9 [417.2 to 762.7] | 5.1 (<0.0001) | 728.7 [521.5 to 768.671] | 960.9 [538 to 1475.362] | −3.3 (0.0011) |
| IL-1β, ng/dL | 63.8 [58.6 to 74.1] | 64.9 [55.9 to 136.4] | −0.2 (0.8688) | 62.3 [57.3 to 140.518] | 63.8 [58.6 to 79.273] | −0.03 (0.9774) |
| Glucose, mg/dL | 88 [27 to 132] | 19 [19 to 23.8] | 4.2 (<0.0001) | 19 [19 to 20] | 88 [26.5 to 138] | −4.7 (<0.0001) |
| CEA *, n (%) | 2 (6.1) | 18 (60) | 21.1 (<0.001) | 18 (75) | 2 (5.1) | 33.5 (<0.0001) |
| Biomarkers in serum | ||||||
| NGAL, ng/mL | 648.8 [603.8 to 665.6] | 643 [569.7 to 673.1] | 0.3 (0.7779) | 638.9 [555.3 to 675.4] | 651 [608.9 to 666.3] | −0.6 (0.5475) |
| IL1β, ng/dL | 153.1 [143.1 to 160.4] | 143.5 [128.5 to 156.3] | −1.35 (0.1774) | 142.6 [123.8 to 156.1] | 153.1 [143.1 to 160.4] | 1.47 (0.141) |
| Criterion | Ngal 500–800 ng/mL | Glucose ≤50 mg/dL | CEA ≥192 ng/mL | Ngal 500–800 ng/mL | Glucose ≤50 mg/dL | CEA ≥192 ng/mL | |
|---|---|---|---|---|---|---|---|
| Characteristic | |||||||
| No. of pts | All cysts (n = 63) | Cysts without infected pseudocysts * (n = 55) | |||||
| Contingency | |||||||
| True positive | 17 | 21 | 21 | 17 | 21 | 18 | |
| False positive | 3 | 12 | 5 | 2 | 4 | 1 | |
| False negative | 7 | 3 | 3 | 7 | 3 | 6 | |
| True negative | 36 | 27 | 34 | 29 | 27 | 30 | |
| χ2 (p-value) | 27.3 (<0.0001) | 19.2 (<0.0001) | 34.2 (<0.0001) | 24.8 (<0.0001) | 30.4 (<0.0001) | 30.8 (<0.0001) | |
| Sensitivity, % | 70.8 [52.6 to 89.0] | 87.5 [74.3 to 100] | 87.5 [74.3 to 100] | 70.8 [52.6 to 89.0] | 87.5 [74.3 to 100] | 75.0 [57.7 to 92.3] | |
| Specificity, % | 92.3 [83.9 to 100] | 69.2 [54.7 to 83.7] | 87.2 [76.7 to 97.7] | 93.5 [84.9 to 100] | 87.1 [75.3 to 98.9] | 96.8 [90.6 to 100] | |
| +LR | 9.21 [3.01 to 28.14] | 2.84 [1.73 to 4.66] | 6.83 [2.97 to 15.7] | 10.98 [2.80 to 42.98] | 6.78 [2.68 to 14.14] | 23.25 [3.3 to 162.1] | |
| −LR | 0.32 [0.17 to 0.59] | 0.18 [0.06 to 0.53] | 0.14 [0.05 to 0.42] | 0.31 [0.17 to 0.59] | 0.14 [0.05 to 0.42] | 0.26 [0.13 to 0.52] | |
| Accuracy, % | 84.1 [75.1 to 93.2] | 76.2 [65.7 to 86.7] | 87.3 [79.1 to 95.5] | 83.6 [73.9 to 93.4] | 87.3 [78.5 to 96.1] | 87.3 [78.5 to 96.1] | |
| +CUI | 0.602 [0.390–0.814] | 0.557 [0.364 to 0.750] | 0.707 [0.531 to 0.882] | 0.634 [0.427 to 0.840] | 0.735 [0.566 to 0.905] | 0.711 [0.525 to 0.896] | |
| −CUI | 0.773 [0.688 to 0.858] | 0.623 [0.503 to 0.743] | 0.801 [0.716 to 0.886] | 0.754 [0.657 to 0.850] | 0.786 [0.686 to 0.882] | 0.806 [0.720 to 0.893] | |
| For case-finding | Fair | Fair | Good | Fair | Good | Good | |
| For screening | Good | Fair | Good | Good | Good | Good | |
| Histopathology, n = 8 | Cytology, n = 61 | |
|---|---|---|
| True positive | 6 | 16 |
| False positive | 0 | 3 |
| False negative | 2 | 6 |
| True negative | 0 | 36 |
| χ2 (p-value) | n.a. | 59.4 (<0.0001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olar, M.P.; Iacobescu, M.; Bolboacă, S.D.; Pojoga, C.; Moșteanu, O.; Seicean, R.; Rusu, I.; Banc, O.; Iuga, C.A.; Seicean, A. Neutrophil Gelatinase-Associated Lipocalin for the Differentiation of Mucinous Pancreatic Cystic Lesions. Int. J. Mol. Sci. 2024, 25, 3224. https://doi.org/10.3390/ijms25063224
Olar MP, Iacobescu M, Bolboacă SD, Pojoga C, Moșteanu O, Seicean R, Rusu I, Banc O, Iuga CA, Seicean A. Neutrophil Gelatinase-Associated Lipocalin for the Differentiation of Mucinous Pancreatic Cystic Lesions. International Journal of Molecular Sciences. 2024; 25(6):3224. https://doi.org/10.3390/ijms25063224
Chicago/Turabian StyleOlar, Miruna Patricia, Maria Iacobescu, Sorana D. Bolboacă, Cristina Pojoga, Ofelia Moșteanu, Radu Seicean, Ioana Rusu, Oana Banc, Cristina Adela Iuga, and Andrada Seicean. 2024. "Neutrophil Gelatinase-Associated Lipocalin for the Differentiation of Mucinous Pancreatic Cystic Lesions" International Journal of Molecular Sciences 25, no. 6: 3224. https://doi.org/10.3390/ijms25063224
APA StyleOlar, M. P., Iacobescu, M., Bolboacă, S. D., Pojoga, C., Moșteanu, O., Seicean, R., Rusu, I., Banc, O., Iuga, C. A., & Seicean, A. (2024). Neutrophil Gelatinase-Associated Lipocalin for the Differentiation of Mucinous Pancreatic Cystic Lesions. International Journal of Molecular Sciences, 25(6), 3224. https://doi.org/10.3390/ijms25063224







