Emerging Roles of B56 Phosphorylation and Binding Motif in PP2A-B56 Holoenzyme Biological Function
Abstract
1. Introduction
2. The Important Role of B56 Phosphorylation in PP2A-B56 Function and Its Mechanism
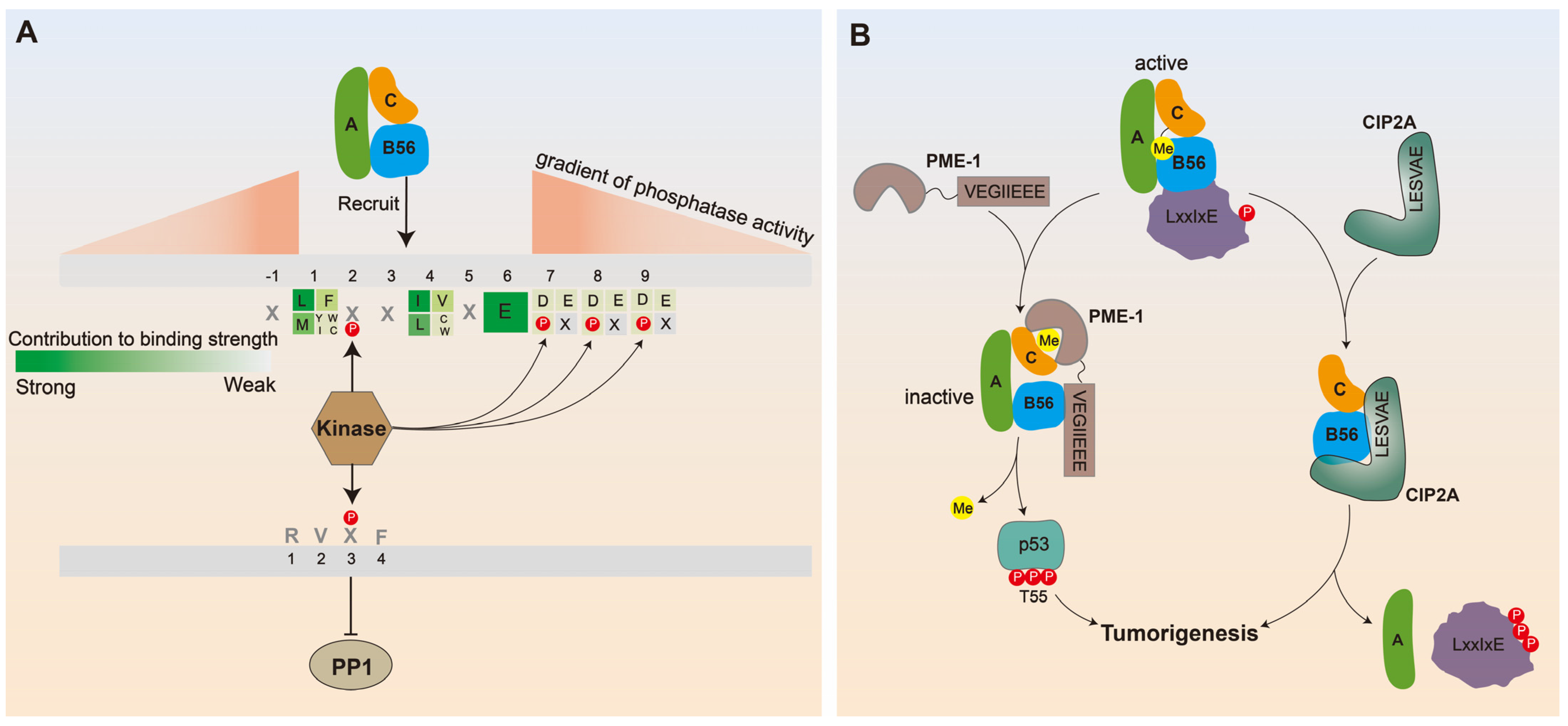
2.1. Biological Function of B56 Phosphorylation
2.2. The Mechanism of B56 Phosphorylation Exerting Its Biological Effect
3. The Contribution of LxxIxE Motif to Decipher the Related Biological Functions of PP2A-B56
3.1. Overview of LxxIxE Motifs on the B56 Subunit Binding Protein
3.2. Deciphering the Biological Function of PP2A-B56 through LxxIxE Motif
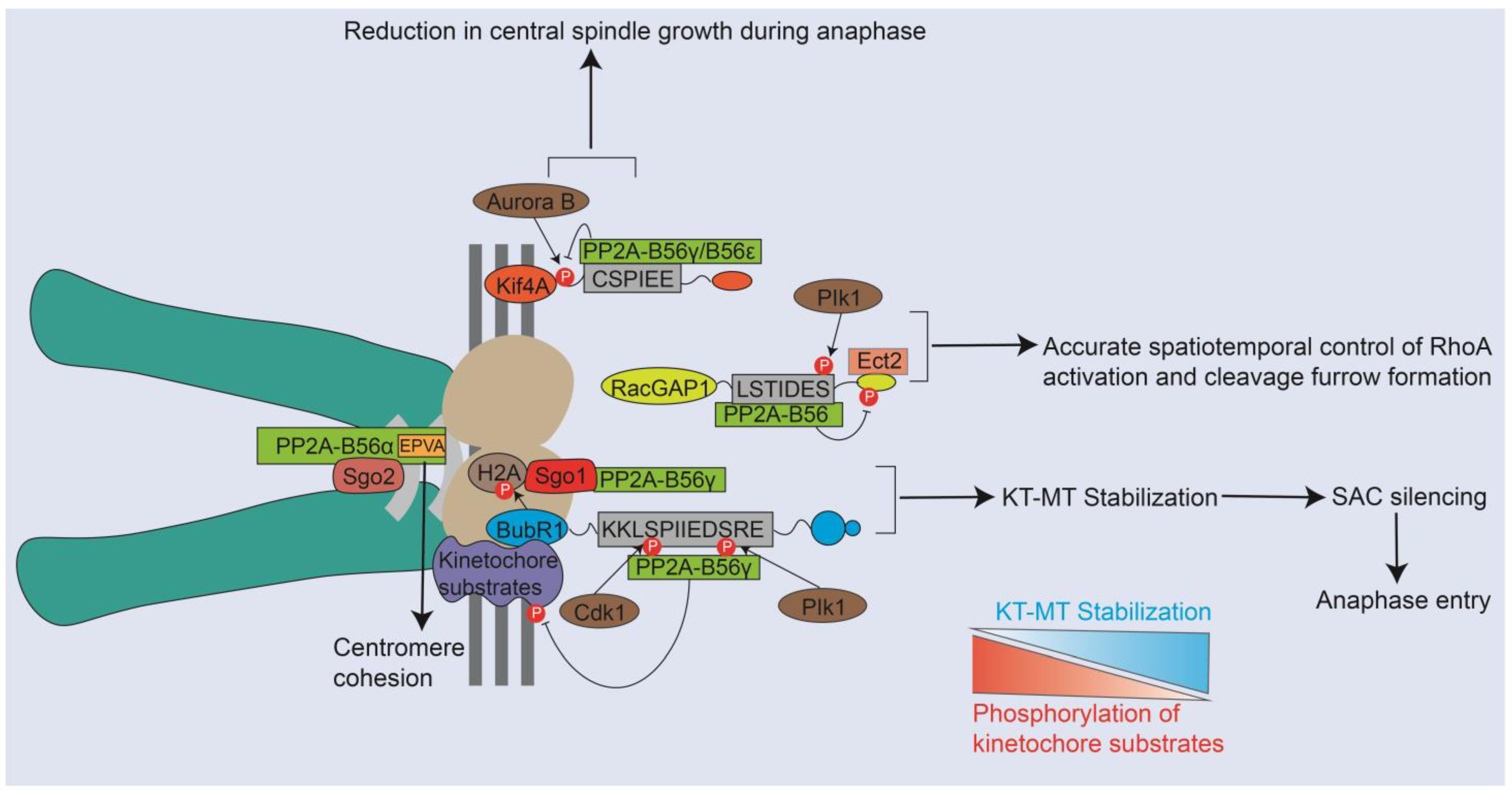
4. Emerging Role of PP2A-B56 Complexes during Mitosis via the LxxIxE-Mediated B56 Binding
4.1. PP2A-B56 Is Recruited to the Strategic Place by Mitotic Regulators Containing Motifs to Achieve Mitotic Regulation
4.2. The Participation of Different PP2A-B56 Complexes in Distinct Mitotic Events Was Decrypted from the Perspective of Motif
5. Motif-Based Understanding on Manipulation of PP2A-B56 by Diverse Viral Families
5.1. PP2A-B56 Is the Main PP2A Subfamily Attacked by Viruses
5.2. The Discovery of Binding Motifs in Various Clinically-Relevant Viruses Revealed Why PP2A-B56 Was Hijacked by the Virus
5.3. B56γ Phosphorylation at Ser510 Is Predicted to be an Effective Intervention against HBV-Induced HCC
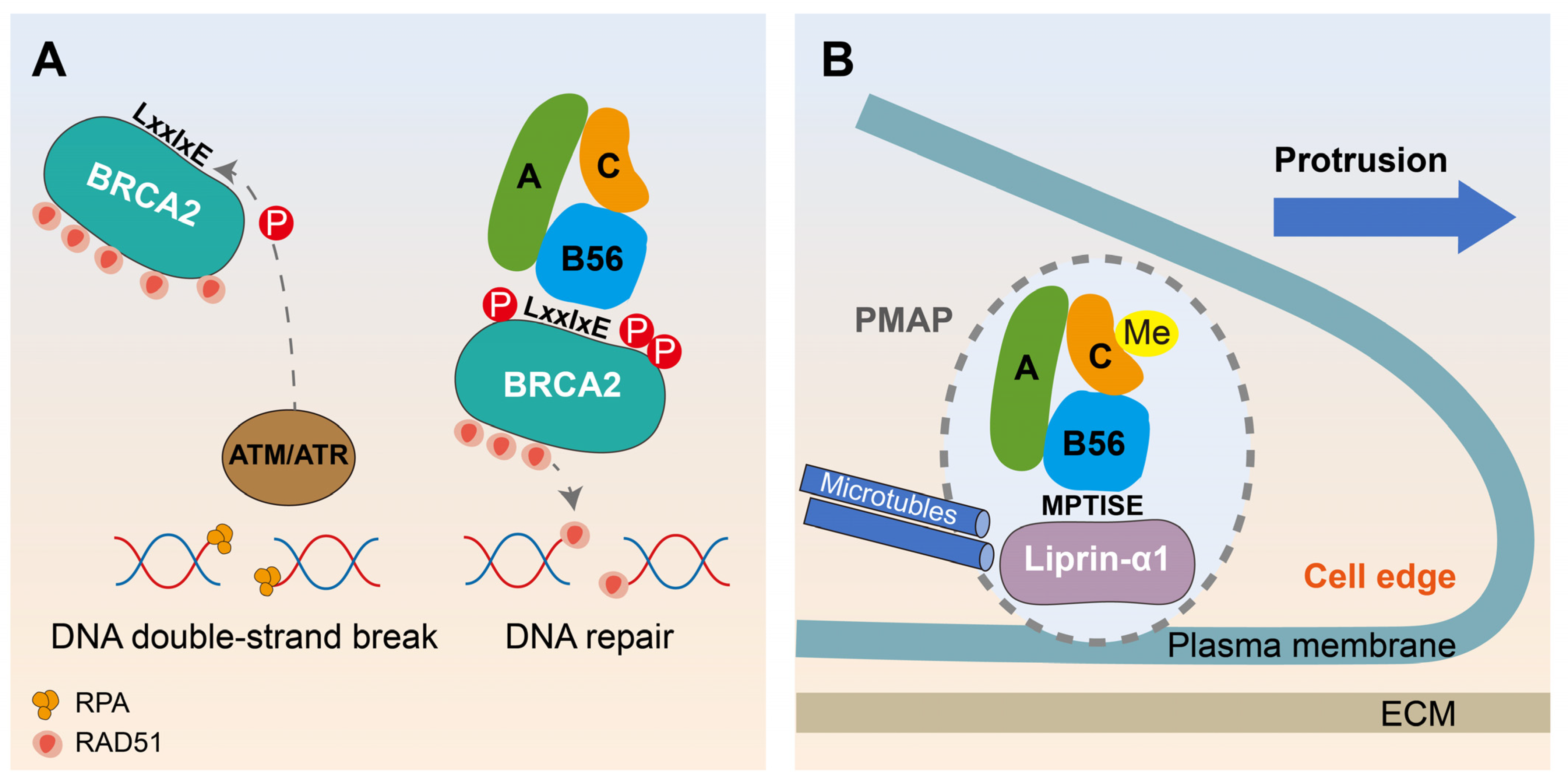
6. The Contribution of PP2A-B56 to Tumor Suppression and its Dual Role in Breast Cancer through LxxIxE Motif Mimicry
6.1. The PP2A-B56 Subfamily Plays an Important Role in Various Tumor Inhibition
6.2. Motif-Based Decryption of PP2A-B56 Promoting DNA Damage Repair and Migration in Breast Cancer
7. Conclusions
- B56 phosphorylation plays an important role in a variety of biological processes by regulating PP2A activity and its ability to bind substrates.
- The discovery of a LxxIxE motif that achieves PP2A-B56 specific binding and deciphers the substrate and phosphorylation site preference regulated by PP2A-B56, and the interaction mechanism between phosphatases and kinases.
- The discovery of B56-binding motifs in mitotic regulators not only decrypts the role of PP2A-B56 in mitosis, but more importantly, sheds light on the reasons why different PP2A-B56 complexes participate in different mitotic events.
- LxxIxE-mediated B56 binding in virus infection makes PP2A-B56 a key target for virus manipulation, and the elevation of B56γ phosphorylation at S510 is predicted to be an effective treatment for HBV-induced HCC.
- LxxIxE motif-based decryption of PP2A-B56 promoting DNA damage repair and migration expands the understanding of the PP2A-B56 function on cancer.
8. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Peris, I.; Romero-Murillo, S.; Vicente, C.; Narla, G.; Odero, M.D. Regulation and role of the PP2A-B56 holoenzyme family in cancer. Biochim. Et Biophys. Acta (BBA)—Rev. Cancer 2023, 1878, 188953. [Google Scholar] [CrossRef]
- Haanen, T.J.; O’Connor, C.M.; Narla, G. Biased holoenzyme assembly of protein phosphatase 2A (PP2A): From cancer to small molecules. J. Biol. Chem. 2022, 298, 102656. [Google Scholar] [CrossRef]
- Reynhout, S.; Janssens, V. Physiologic functions of PP2A: Lessons from genetically modified mice. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Res. 2019, 1866, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Fowle, H.; Zhao, Z.; Graña, X. PP2A holoenzymes, substrate specificity driving cellular functions and deregulation in cancer. Adv. Cancer Res. 2019, 144, 55–93. [Google Scholar]
- Sandal, P.; Jong, C.J.; Merrill, R.A.; Song, J.; Strack, S. Protein phosphatase 2A—Structure, function and role in neurodevelopmental disorders. J. Cell Sci. 2021, 134, jcs248187. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, I.; Kirito, K. Metformin inhibits JAK2V617F activity in MPN cells by activating AMPK and PP2A complexes containing the B56α subunit. Exp. Hematol. 2016, 44, 1156–1165.e4. [Google Scholar] [CrossRef] [PubMed]
- Zolnierowicz, S. Type 2A protein phosphatase, the complex regulator of numerous signaling pathways. Biochem. Pharmacol. 2000, 60, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Ditsworth, D.; Lindsten, T.; Thompson, C.B. Alpha4 is an essential regulator of PP2A phosphatase activity. Mol. Cell 2009, 36, 51–60. [Google Scholar] [CrossRef]
- Stanevich, V.; Jiang, L.; Satyshur, K.A.; Li, Y.; Jeffrey, P.D.; Li, Z.; Menden, P.; Semmelhack, M.F.; Xing, Y. The structural basis for tight control of PP2A methylation and function by LCMT-1. Mol. Cell 2011, 41, 331–342. [Google Scholar] [CrossRef]
- Guo, F.; Stanevich, V.; Wlodarchak, N.; Sengupta, R.; Jiang, L.; Satyshur, K.A.; Xing, Y. Structural basis of PP2A activation by PTPA, an ATP-dependent activation chaperone. Cell Res. 2014, 24, 190–203. [Google Scholar] [CrossRef]
- Longin, S.; Zwaenepoel, K.; Louis, J.V.; Dilworth, S.; Goris, J.; Janssens, V. Selection of protein phosphatase 2A regulatory subunits is mediated by the C terminus of the catalytic Subunit. J. Biol. Chem. 2007, 282, 26971–26980. [Google Scholar] [CrossRef]
- Mazhar, S.; Leonard, D.; Sosa, A.; Schlatzer, D.; Thomas, D.; Narla, G. Challenges and Reinterpretation of Antibody-Based Research on Phosphorylation of Tyr(307) on PP2Ac. Cell Rep. 2020, 30, 3164–3170.e3. [Google Scholar] [CrossRef] [PubMed]
- Shouse, G.P.; Nobumori, Y.; Panowicz, M.J.; Liu, X. ATM-mediated phosphorylation activates the tumor-suppressive function of B56γ–PP2A. Oncogene 2011, 30, 3755–3765. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Liu, C.; Lin, X.; Sun, B.; Su, C. PPP2R5A: A multirole protein phosphatase subunit in regulating cancer development. Cancer Lett. 2018, 414, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.-H.; McAvoy, T.; Rakhilin, S.V.; Nishi, A.; Greengard, P.; Nairn, A.C. Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56δ subunit. Proc. Natl. Acad. Sci. USA 2007, 104, 2979–2984. [Google Scholar] [CrossRef] [PubMed]
- Kirchhefer, U.; Heinick, A.; König, S.; Kristensen, T.; Müller, F.U.; Seidl, M.D.; Boknik, P. Protein Phosphatase 2A Is Regulated by Protein Kinase Cα (PKCα)-dependent Phosphorylation of Its Targeting Subunit B56α at Ser41. J. Biol. Chem. 2014, 289, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Sacco, F.; Silvestri, A.; Posca, D.; Pirrò, S.; Gherardini Pier, F.; Castagnoli, L.; Mann, M.; Cesareni, G. Deep Proteomics of Breast Cancer Cells Reveals that Metformin Rewires Signaling Networks Away from a Pro-growth State. Cell Syst. 2016, 2, 159–171. [Google Scholar] [CrossRef]
- Ranieri, A.; Kemp, E.; Burgoyne, J.R.; Avkiran, M. β-Adrenergic regulation of cardiac type 2A protein phosphatase through phosphorylation of regulatory subunit B56δ at S573. J. Mol. Cell. Cardiol. 2018, 115, 20–31. [Google Scholar] [CrossRef]
- Sontag, J.-M.; Sontag, E. Protein phosphatase 2A dysfunction in Alzheimer’s disease. Front. Mol. Neurosci. 2014, 7, 16. [Google Scholar] [CrossRef]
- Hertz Emil Peter, T.; Kruse, T.; Davey Norman, E.; López-Méndez, B.; Sigurðsson Jón, O.; Montoya, G.; Olsen Jesper, V.; Nilsson, J. A Conserved Motif Provides Binding Specificity to the PP2A-B56 Phosphatase. Mol. Cell 2016, 63, 686–695. [Google Scholar] [CrossRef]
- Kruse, T.; Gnosa, S.P.; Nasa, I.; Garvanska, D.H.; Hein, J.B.; Nguyen, H.; Samsøe-Petersen, J.; Lopez-Mendez, B.; Hertz, E.P.T.; Schwarz, J.; et al. Mechanisms of site-specific dephosphorylation and kinase opposition imposed by PP2A regulatory subunits. EMBO J. 2020, 39, e103695. [Google Scholar] [CrossRef]
- Wang, X.; Garvanska, D.H.; Nasa, I.; Ueki, Y.; Zhang, G.; Kettenbach, A.N.; Peti, W.; Nilsson, J.; Page, R. A dynamic charge-charge interaction modulates PP2A:B56 substrate recruitment. eLife 2020, 9, e55966. [Google Scholar] [CrossRef] [PubMed]
- Letourneux, C.; Rocher, G.; Porteu, F. B56-containing PP2A dephosphorylate ERK and their activity is controlled by the early gene IEX-1 and ERK. EMBO J. 2006, 25, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, V.R.; Kurinna, S.M.; Karanjeet, K.B.; Schuster, T.F.; Martelli, A.M.; McCubrey, J.A.; Ruvolo, P.P. PKR regulates B56(alpha)-mediated BCL2 phosphatase activity in acute lymphoblastic leukemia-derived REH cells. J. Biol. Chem. 2008, 283, 35474–35485. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Wu, C.-G.; Jia, W.; Xing, Y.; Tibbetts, R.S. Roles of constitutive and signal-dependent protein phosphatase 2A docking motifs in burst attenuation of the cyclic AMP response element-binding protein. J. Biol. Chem. 2021, 297, 100908. [Google Scholar] [CrossRef] [PubMed]
- Kruse, T.; Biedenkopf, N.; Hertz, E.P.T.; Dietzel, E.; Stalmann, G.; López-Méndez, B.; Davey, N.E.; Nilsson, J.; Becker, S. The Ebola Virus Nucleoprotein Recruits the Host PP2A-B56 Phosphatase to Activate Transcriptional Support Activity of VP30. Mol. Cell 2018, 69, 136–145.e6. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bajaj, R.; Bollen, M.; Peti, W.; Page, R. Expanding the PP2A Interactome by Defining a B56-Specific SLiM. Structure 2016, 24, 2174–2181. [Google Scholar] [CrossRef]
- Wu, C.-G.; Chen, H.; Guo, F.; Yadav, V.K.; McIlwain, S.J.; Rowse, M.; Choudhary, A.; Lin, Z.; Li, Y.; Gu, T.; et al. PP2A-B′ holoenzyme substrate recognition, regulation and role in cytokinesis. Cell Discov. 2017, 3, 17027. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.; Cordeiro, M.H.; Davey, N.E.; Vallardi, G.; Ciliberto, A.; Gross, F.; Saurin, A.T. PP1 and PP2A Use Opposite Phospho-dependencies to Control Distinct Processes at the Kinetochore. Cell Rep. 2019, 28, 2206–2219.e8. [Google Scholar] [CrossRef]
- Wang, J.; Okkeri, J.; Pavic, K.; Wang, Z.Z.; Kauko, O.; Halonen, T.; Sarek, G.; Ojala, P.M.; Rao, Z.H.; Xu, W.Q.; et al. Oncoprotein CIP2A is stabilized via interaction with tumor suppressor PP2A/B56. EMBO Rep. 2017, 18, 437–450. [Google Scholar] [CrossRef]
- Pavic, K.; Gupta, N.; Omella, J.D.; Derua, R.; Aakula, A.; Huhtaniemi, R.; Määttä, J.A.; Höfflin, N.; Okkeri, J.; Wang, Z.; et al. Structural mechanism for inhibition of PP2A-B56α and oncogenicity by CIP2A. Nat. Commun. 2023, 14, 1143. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.R.; Puustinen, P.; Niemelä, M.; Ahola, R.; Arnold, H.; Böttzauw, T.; Ala-Aho, R.; Nielsen, C.; Ivaska, J.; Taya, Y.; et al. CIP2A inhibits PP2A in human malignancies. Cell 2007, 130, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.F.; Liu, C.Y.; Lin, Y.C.; Yu, H.C.; Liu, T.H.; Hou, D.R.; Chen, P.J.; Cheng, A.L. CIP2A mediates effects of bortezomib on phospho-Akt and apoptosis in hepatocellular carcinoma cells. Oncogene 2010, 29, 6257–6266. [Google Scholar] [CrossRef]
- Li, Y.; Balakrishnan, V.K.; Rowse, M.; Wu, C.-G.; Bravos, A.P.; Yadav, V.K.; Ivarsson, Y.; Strack, S.; Novikova, I.V.; Xing, Y. Coupling to short linear motifs creates versatile PME-1 activities in PP2A holoenzyme demethylation and inhibition. eLife 2022, 11, e79736. [Google Scholar] [CrossRef] [PubMed]
- Lambrus, B.G.; Holland, A.J. A New Mode of Mitotic Surveillance. Trends Cell Biol. 2017, 27, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Pachis, S.T.; Kops, G.J.P.L. Leader of the SAC: Molecular mechanisms of Mps1/TTK regulation in mitosis. Open Biol. 2018, 8, 180109. [Google Scholar] [CrossRef] [PubMed]
- Gama Braga, L.; Cisneros, A.F.; Mathieu, M.M.; Clerc, M.; Garcia, P.; Lottin, B.; Garand, C.; Thebault, P.; Landry, C.R.; Elowe, S. BUBR1 Pseudokinase Domain Promotes Kinetochore PP2A-B56 Recruitment, Spindle Checkpoint Silencing, and Chromosome Alignment. Cell Rep. 2020, 33, 108397. [Google Scholar] [CrossRef]
- Musacchio, A. The Molecular Biology of Spindle Assembly Checkpoint Signaling Dynamics. Curr. Biol. 2015, 25, R1002–R1018. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Yu, T.; Yang, H.; Virshup, D.M.; Kops, G.J.P.L.; Lee, S.H.; Zhou, W.; Li, X.; Xu, W.; et al. Crystal structure of a PP2A B56-BubR1 complex and its implications for PP2A substrate recruitment and localization. Protein Cell 2016, 7, 516–526. [Google Scholar] [CrossRef]
- Kruse, T.; Zhang, G.; Larsen MS, Y.; Lischetti, T.; Streicher, W.; Kragh Nielsen, T.; Bjørn, S.P.; Nilsson, J. Direct binding between BubR1 and B56–PP2A phosphatase complexes regulate mitotic progression. J. Cell Sci. 2013, 126, 1086–1092. [Google Scholar] [CrossRef]
- Funabiki, H.; Wynne, D.J. Making an effective switch at the kinetochore by phosphorylation and dephosphorylation. Chromosoma 2013, 122, 135–158. [Google Scholar] [CrossRef] [PubMed]
- Ueki, Y.; Hadders, M.A.; Weisser, M.B.; Nasa, I.; Sotelo-Parrilla, P.; Cressey, L.E.; Gupta, T.; Hertz, E.P.T.; Kruse, T.; Montoya, G.; et al. A highly conserved pocket on PP2A-B56 is required for hSgo1 binding and cohesion protection during mitosis. EMBO Rep. 2021, 22, e52295. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.N.; Cundell, M.J.; Barr, F.A. KIF4A and PP2A–B56 form a spatially restricted feedback loop opposing Aurora B at the anaphase central spindle. J. Cell Biol. 2014, 207, 683–693. [Google Scholar] [CrossRef]
- Foley, E.A.; Maldonado, M.; Kapoor, T.M. Formation of stable attachments between kinetochores and microtubules depends on the B56-PP2A phosphatase. Nat. Cell Biol. 2011, 13, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Vallardi, G.; Allan, L.A.; Crozier, L.; Saurin, A.T. Division of labour between PP2A-B56 isoforms at the centromere and kinetochore. eLife 2019, 8, e42619. [Google Scholar] [CrossRef] [PubMed]
- Barski, M.S.; Minnell, J.J.; Maertens, G.N. PP2A Phosphatase as an Emerging Viral Host Factor. Front. Cell. Infect. Microbiol. 2021, 11, 721. [Google Scholar] [CrossRef] [PubMed]
- Marelli, S.; Williamson, J.C.; Protasio, A.V.; Naamati, A.; Greenwood, E.J.D.; Deane, J.E.; Lehner, P.J.; Matheson, N.J. Antagonism of PP2A is an independent and conserved function of HIV-1 Vif and causes cell cycle arrest. eLife 2020, 9, e53036. [Google Scholar] [CrossRef]
- Bhatt, V.; Shi, K.; Salamango, D.J.; Moeller, N.H.; Pandey, K.K.; Bera, S.; Bohl, H.O.; Kurniawan, F.; Orellana, K.; Zhang, W.; et al. Structural basis of host protein hijacking in human T-cell leukemia virus integration. Nat. Commun. 2020, 11, 3121. [Google Scholar] [CrossRef]
- Sugden, B.; Cho, U.S.; Morrone, S.; Sablina, A.A.; Arroyo, J.D.; Hahn, W.C.; Xu, W. Structural Basis of PP2A Inhibition by Small t Antigen. PLoS Biol. 2007, 5, e202. [Google Scholar]
- Anwar, M.I.; Li, N.; Zhou, Q.; Chen, M.; Hu, C.; Wu, T.; Chen, H.; Li, Y.P.; Zhou, Y. PPP2R5D promotes hepatitis C virus infection by binding to viral NS5B and enhancing viral RNA replication. Virol. J. 2022, 19, 118. [Google Scholar] [CrossRef]
- Li, T.-Y.; Yang, Y.; Zhou, G.; Tu, Z.-K. Immune suppression in chronic hepatitis B infection associated liver disease: A review. World J. Gastroenterol. 2019, 25, 3527–3537. [Google Scholar] [CrossRef]
- Li, Y.; He, M.; Wang, Z.; Duan, Z.; Guo, Z.; Wang, Z.; Gong, R.; Chu, T.; Cai, J.; Gao, B. STING signaling activation inhibits HBV replication and attenuates the severity of liver injury and HBV-induced fibrosis. Cell. Mol. Immunol. 2021, 19, 92–107. [Google Scholar] [CrossRef]
- Kanda, T.; Goto, T.; Hirotsu, Y.; Moriyama, M.; Omata, M. Molecular Mechanisms Driving Progression of Liver Cirrhosis towards Hepatocellular Carcinoma in Chronic Hepatitis B and C Infections: A Review. Int. J. Mol. Sci. 2019, 20, 1358. [Google Scholar] [CrossRef]
- Bybee, G.; Moeun, Y.; Wang, W.; Kharbanda, K.K.; Poluektova, L.Y.; Kidambi, S.; Osna, N.A.; Ganesan, M. Increased liver stiffness promotes hepatitis B progression by impairing innate immunity in CCl4-induced fibrotic HBV+ transgenic mice. Front. Immunol. 2023, 14, 1166171. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.; Xi, J.; Luckenbaugh, L.; Hu, J. Multiple roles of PP2A binding motif in hepatitis B virus core linker and PP2A in regulating core phosphorylation state and viral replication. PLoS Pathog. 2021, 17, e1009230. [Google Scholar]
- Maksimova, V.; Panfil, A.R. Human T-Cell Leukemia Virus Type 1 Envelope Protein: Post-Entry Roles in Viral Pathogenesis. Viruses 2022, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Barski, M.S.; Minnell, J.J.; Hodakova, Z.; Pye, V.E.; Nans, A.; Cherepanov, P.; Maertens, G.N. Cryo-EM structure of the deltaretroviral intasome in complex with the PP2A regulatory subunit B56γ. Nat. Commun. 2020, 11, 5043. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, E.J.D.; Matheson, N.J.; Wals, K.; van den Boomen, D.J.H.; Antrobus, R.; Williamson, J.C.; Lehner, P.J. Temporal proteomic analysis of HIV infection reveals remodelling of the host phosphoproteome by lentiviral Vif variants. eLife 2016, 5, e18296. [Google Scholar] [CrossRef] [PubMed]
- Naamati, A.; Williamson, J.C.; Greenwood, E.J.D.; Marelli, S.; Lehner, P.J.; Matheson, N.J. Functional proteomic atlas of HIV infection in primary human CD4+ T cells. eLife 2019, 8, e41431. [Google Scholar] [CrossRef] [PubMed]
- Salamango, D.J.; McCann, J.L.; Demir, Ö.; Becker, J.T.; Wang, J.; Lingappa, J.R.; Temiz, N.A.; Brown, W.L.; Amaro, R.E.; Harris, R.S.; et al. Functional and Structural Insights into a Vif/PPP2R5 Complex Elucidated Using Patient HIV-1 Isolates and Computational Modeling. J. Virol. 2020, 94, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Shindo, K.; Matsui, Y.; Shirakawa, K.; Takaori-Kondo, A. Critical role of PP2A-B56 family protein degradation in HIV-1 Vif mediated G2 cell cycle arrest. Biochem. Biophys. Res. Commun. 2020, 527, 257–263. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- He, C.; Qiu, Y.; Han, P.; Chen, Y.; Zhang, L.; Yuan, Q.; Zhang, T.; Cheng, T.; Yuan, L.; Huang, C.; et al. ER stress regulating protein phosphatase 2A-B56γ, targeted by hepatitis B virus X protein, induces cell cycle arrest and apoptosis of hepatocytes. Cell Death Dis. 2018, 9, 762. [Google Scholar] [CrossRef]
- Che, L.; Du, Z.B.; Wang, W.H.; Wu, J.S.; Han, T.; Chen, Y.Y.; Han, P.Y.; Lei, Z.; Chen, X.X.; He, Y.; et al. Intracellular antibody targeting HBx suppresses invasion and metastasis in hepatitis B virus-related hepatocarcinogenesis via protein phosphatase 2A-B56γ-mediated dephosphorylation of protein kinase B. Cell Prolif. 2022, 55, e13304. [Google Scholar] [CrossRef]
- Sangodkar, J.; Farrington, C.C.; McClinch, K.; Galsky, M.D.; Kastrinsky, D.B.; Narla, G. All roads lead to PP2A: Exploiting the therapeutic potential of this phosphatase. FEBS J. 2015, 283, 1004–1024. [Google Scholar] [CrossRef]
- Ramaswamy, K.; Spitzer, B.; Kentsis, A. Therapeutic Re-Activation of Protein Phosphatase 2A in Acute Myeloid Leukemia. Front. Oncol. 2015, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Allen-Petersen, B.L.; Risom, T.; Feng, Z.; Wang, Z.; Jenny, Z.P.; Thoma, M.C.; Pelz, K.R.; Morton, J.P.; Sansom, O.J.; Lopez, C.D.; et al. Activation of PP2A and Inhibition of mTOR Synergistically Reduce MYC Signaling and Decrease Tumor Growth in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2019, 79, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, C.; Libbrecht, L.; Sagaert, X.; Pauwels, P.; Hoorne, Y.; Crowther, J.; Louis, J.V.; Sents, W.; Sablina, A.; Janssens, V. Loss of protein phosphatase 2A regulatory subunit B56δ promotes spontaneous tumorigenesis in vivo. Oncogene 2017, 37, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Shouse, G.P.; Nobumori, Y.; Liu, X. A B56γ mutation in lung cancer disrupts the p53-dependent tumor-suppressor function of protein phosphatase 2A. Oncogene 2010, 29, 3933–3941. [Google Scholar] [CrossRef][Green Version]
- Mannava, S.; Omilian, A.R.; Wawrzyniak, J.A.; Fink, E.E.; Zhuang, D.; Miecznikowski, J.C.; Marshall, J.R.; Soengas, M.S.; Sears, R.C.; Morrison, C.D.; et al. PP2A-B56α controls oncogene-induced senescence in normal and tumor human melanocytic cells. Oncogene 2012, 31, 1484–1492. [Google Scholar] [CrossRef][Green Version]
- Ito, A.; Kataoka, T.R.; Watanabe, M.; Nishiyama, K.; Mazaki, Y.; Sabe, H.; Kitamura, Y.; Nojima, H. A truncated isoform of the PP2A B56 subunit promotes cell motility through paxillin phosphorylation. EMBO J. 2000, 19, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Rasool, R.U.; O’Connor, C.M.; Das, C.K.; Alhusayan, M.; Verma, B.K.; Islam, S.; Frohner, I.E.; Deng, Q.; Mitchell-Velasquez, E.; Sangodkar, J.; et al. Loss of LCMT1 and biased protein phosphatase 2A heterotrimerization drive prostate cancer progression and therapy resistance. Nat. Commun. 2023, 14, 5253. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Possemato, R.; Campbell, K.T.; Plattner, C.A.; Pallas, D.C.; Hahn, W.C. Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell 2004, 5, 127–136. [Google Scholar] [CrossRef] [PubMed]
- De Palma, R.M.; Parnham, S.R.; Li, Y.; Oaks, J.J.; Peterson, Y.K.; Szulc, Z.M.; Roth, B.M.; Xing, Y.; Ogretmen, B. The NMR-based characterization of the FTY720-SET complex reveals an alternative mechanism for the attenuation of the inhibitory SET-PP2A interaction. FASEB J. 2019, 33, 7647–7666. [Google Scholar] [CrossRef] [PubMed]
- Irie, A.; Harada, K.; Araki, N.; Nishimura, Y. Phosphorylation of SET protein at Ser171 by protein kinase D2 diminishes its inhibitory effect on protein phosphatase 2A. PLoS ONE 2012, 7, e51242. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Pimanda, J.E. Clinical significance of cancerous inhibitor of protein phosphatase 2A in human cancers. Int. J. Cancer 2016, 138, 525–532. [Google Scholar] [CrossRef]
- Guo, B.; Wu, S.; Zhu, X.; Zhang, L.; Deng, J.; Li, F.; Wang, Y.; Zhang, S.; Wu, R.; Lu, J.; et al. Micropeptide CIP2A-BP encoded by LINC00665 inhibits triple-negative breast cancer progression. EMBO J. 2020, 39, e102190. [Google Scholar] [CrossRef]
- Enjoji, S.; Yabe, R.; Tsuji, S.; Yoshimura, K.; Kawasaki, H.; Sakurai, M.; Sakai, Y.; Takenouchi, H.; Yoshino, S.; Hazama, S.; et al. Stemness Is Enhanced in Gastric Cancer by a SET/PP2A/E2F1 Axis. Mol. Cancer Res. 2018, 16, 554–563. [Google Scholar] [CrossRef]
- Kwun, H.J.; Shuda, M.; Camacho, C.J.; Gamper, A.M.; Thant, M.; Chang, Y.; Moore, P.S. Restricted protein phosphatase 2A targeting by Merkel cell polyomavirus small T antigen. J. Virol. 2015, 89, 4191–4200. [Google Scholar] [CrossRef]
- Ambjørn, S.M.; Duxin, J.P.; Hertz, E.P.T.; Nasa, I.; Duro, J.; Kruse, T.; Lopez-Mendez, B.; Rymarczyk, B.; Cressey, L.E.; van Overeem Hansen, T.; et al. A complex of BRCA2 and PP2A-B56 is required for DNA repair by homologous recombination. Nat. Commun. 2021, 12, 5748. [Google Scholar] [CrossRef]
- de Curtis, I. Biomolecular Condensates at the Front: Cell Migration Meets Phase Separation. Trends Cell Biol. 2021, 31, 145–148. [Google Scholar] [CrossRef]
- Ripamonti, M.; Lamarca, A.; Davey, N.E.; Tonoli, D.; Surini, S.; de Curtis, I. A functional interaction between liprin-α1 and B56γ regulatory subunit of protein phosphatase 2A supports tumor cell motility. Commun. Biol. 2022, 5, 1025. [Google Scholar] [CrossRef]
- Shah, V.M.; English, I.A.; Sears, R.C. Select Stabilization of a Tumor-Suppressive PP2A Heterotrimer. Trends Pharmacol. Sci. 2020, 41, 595–597. [Google Scholar] [CrossRef]
- Leonard, D.; Huang, W.; Izadmehr, S.; O’Connor, C.M.; Wiredja, D.D.; Wang, Z.; Zaware, N.; Chen, Y.; Schlatzer, D.M.; Kiselar, J.; et al. Selective PP2A Enhancement through Biased Heterotrimer Stabilization. Cell 2020, 181, 688–701.e16. [Google Scholar] [CrossRef] [PubMed]
- Vicente, C.; Arriazu, E.; Martínez-Balsalobre, E.; Peris, I.; Marcotegui, N.; García-Ramírez, P.; Pippa, R.; Rabal, O.; Oyarzábal, J.; Guruceaga, E.; et al. A novel FTY720 analogue targets SET-PP2A interaction and inhibits growth of acute myeloid leukemia cells without inducing cardiac toxicity. Cancer Lett. 2020, 468, 1–13. [Google Scholar] [CrossRef]
- McClinch, K.; Avelar, R.A.; Callejas, D.; Izadmehr, S.; Wiredja, D.; Perl, A.; Sangodkar, J.; Kastrinsky, D.B.; Schlatzer, D.; Cooper, M.; et al. Small-Molecule Activators of Protein Phosphatase 2A for the Treatment of Castration-Resistant Prostate Cancer. Cancer Res. 2018, 78, 2065–2080. [Google Scholar] [CrossRef] [PubMed]
- Sangodkar, J.; Perl, A.; Tohme, R.; Kiselar, J.; Kastrinsky, D.B.; Zaware, N.; Izadmehr, S.; Mazhar, S.; Wiredja, D.D.; O’Connor, C.M.; et al. Activation of tumor suppressor protein PP2A inhibits KRAS-driven tumor growth. J. Clin. Investig. 2017, 127, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Peris, I.; Romero-Murillo, S.; Martínez-Balsalobre, E.; Farrington, C.C.; Arriazu, E.; Marcotegui, N.; Jiménez-Muñoz, M.; Alburquerque-Prieto, C.; Torres-López, A.; Fresquet, V.; et al. Activation of the PP2A-B56α heterocomplex synergizes with venetoclax therapies in AML through BCL2 and MCL1 modulation. Blood 2023, 141, 1047–1059. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Jiang, H.; Yin, H.; Zhao, X.; Zhang, Y. Emerging Roles of B56 Phosphorylation and Binding Motif in PP2A-B56 Holoenzyme Biological Function. Int. J. Mol. Sci. 2024, 25, 3185. https://doi.org/10.3390/ijms25063185
Zhang Y, Jiang H, Yin H, Zhao X, Zhang Y. Emerging Roles of B56 Phosphorylation and Binding Motif in PP2A-B56 Holoenzyme Biological Function. International Journal of Molecular Sciences. 2024; 25(6):3185. https://doi.org/10.3390/ijms25063185
Chicago/Turabian StyleZhang, Yanqiao, Haonan Jiang, Haimeng Yin, Xinyuan Zhao, and Yali Zhang. 2024. "Emerging Roles of B56 Phosphorylation and Binding Motif in PP2A-B56 Holoenzyme Biological Function" International Journal of Molecular Sciences 25, no. 6: 3185. https://doi.org/10.3390/ijms25063185
APA StyleZhang, Y., Jiang, H., Yin, H., Zhao, X., & Zhang, Y. (2024). Emerging Roles of B56 Phosphorylation and Binding Motif in PP2A-B56 Holoenzyme Biological Function. International Journal of Molecular Sciences, 25(6), 3185. https://doi.org/10.3390/ijms25063185






