The Potential of Twendee X® as a Safe Antioxidant Treatment for Systemic Sclerosis
Abstract
1. Introduction
2. Results and Discussion
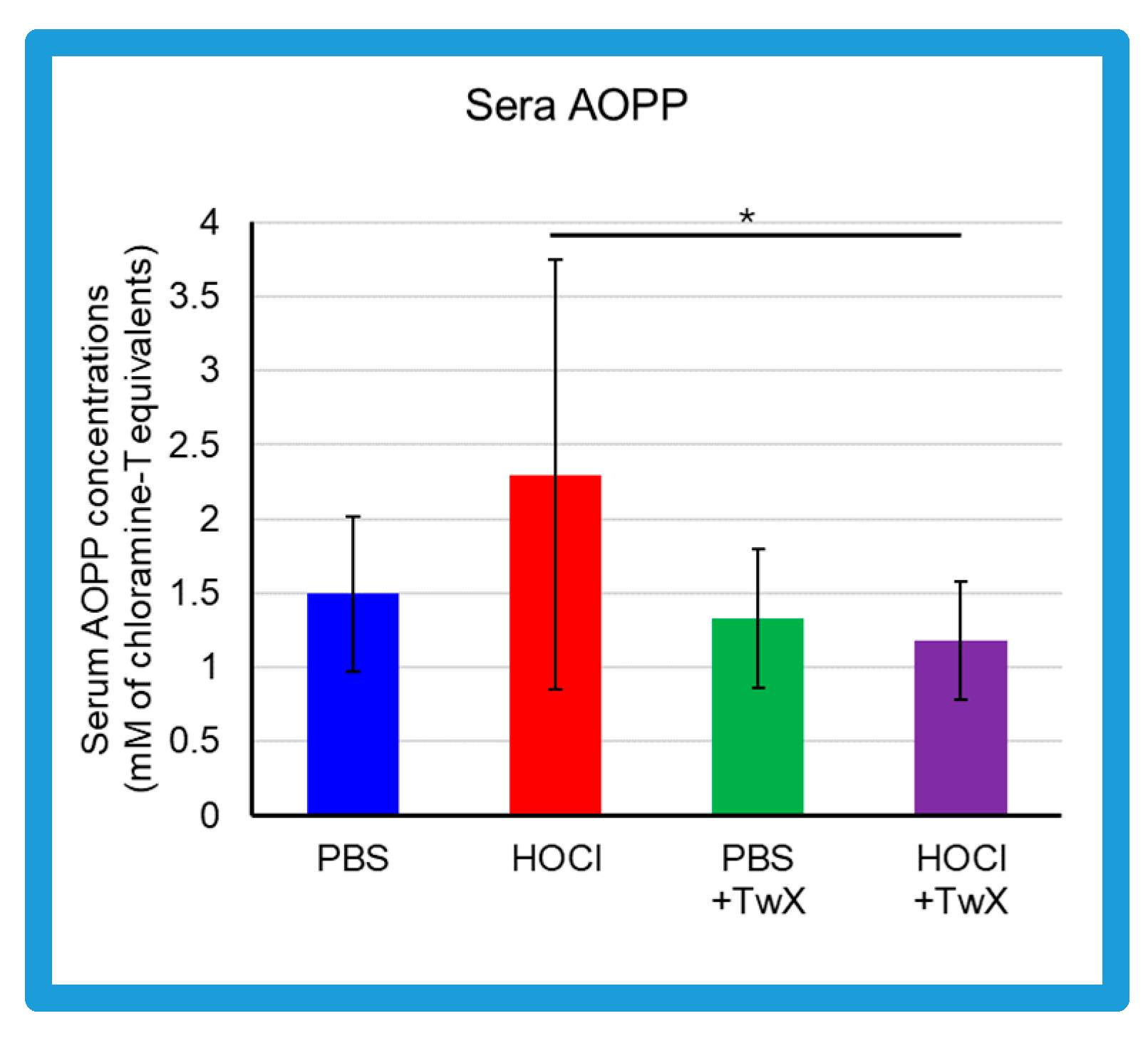
2.1. Reduction of OS in HOCl-Induced SSc by TwX
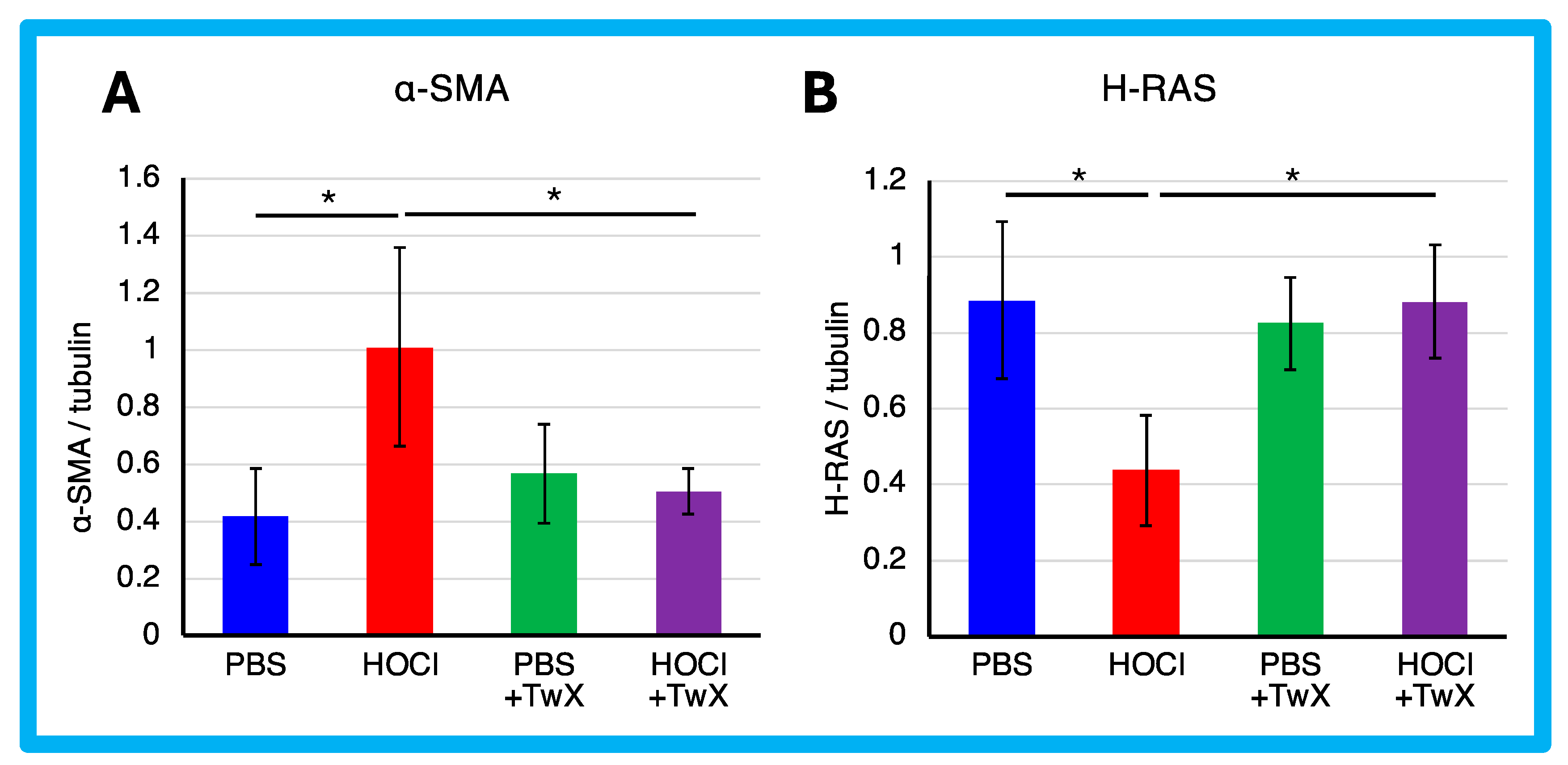
2.2. Inhibition of HOCl-Induced Skin and Lung Fibrosis by TwX
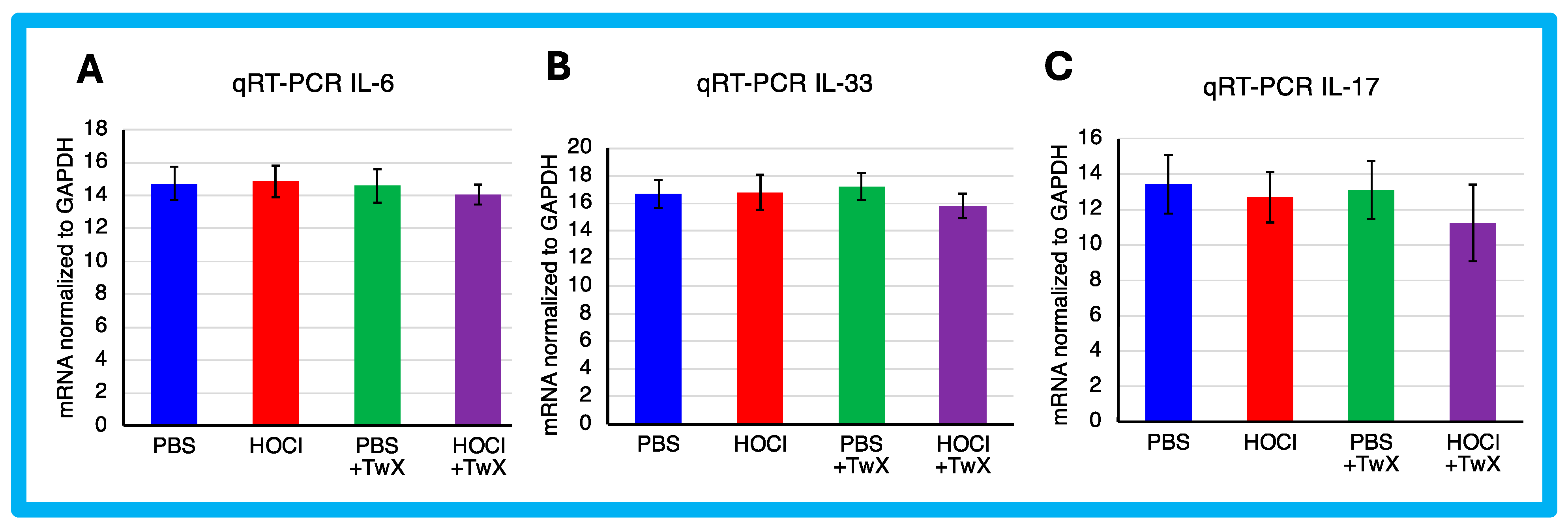
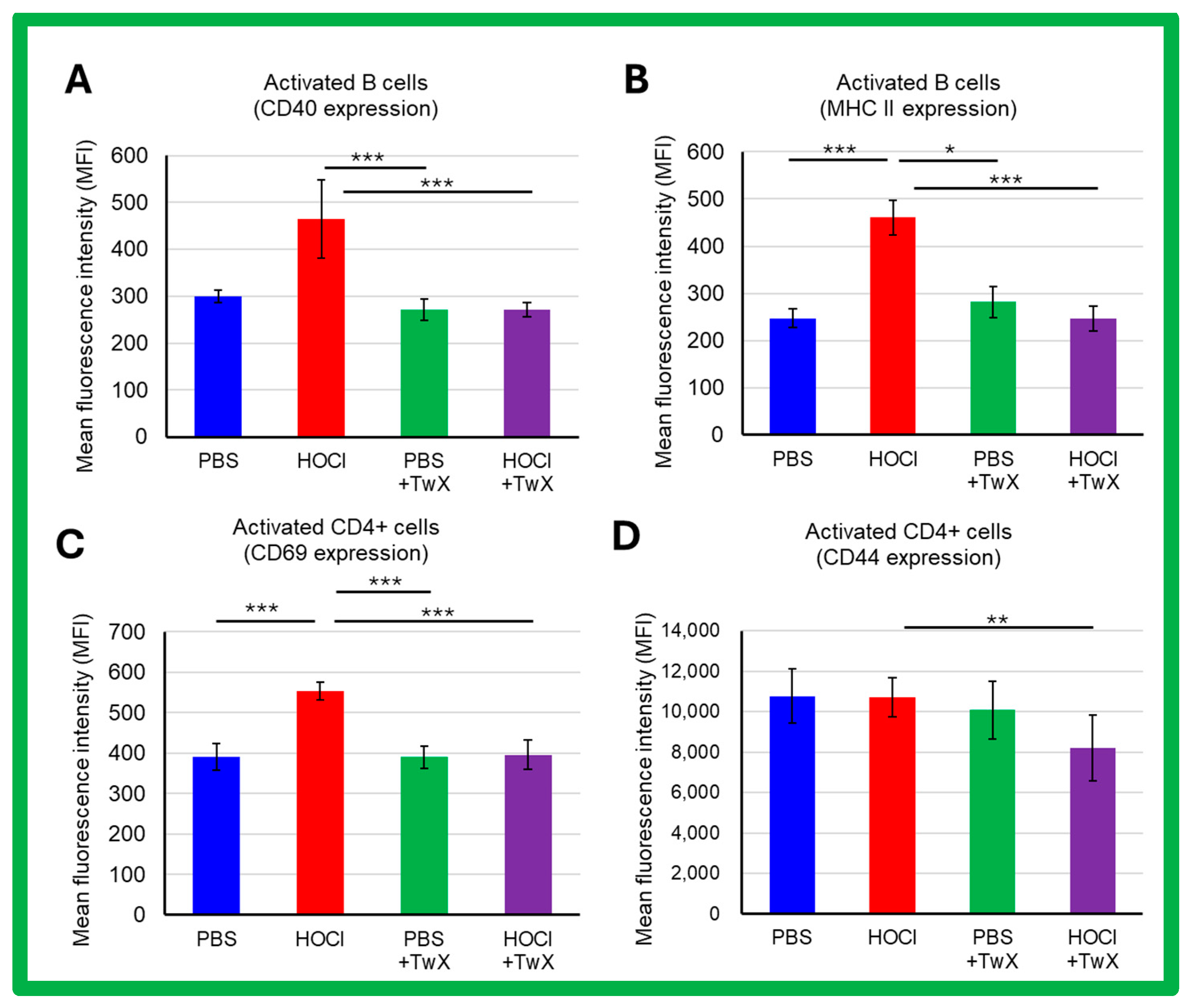
2.3. Effects on Inflammation and Immunity in SSc
3. Materials and Methods
3.1. Materials
3.2. Chemically Inducing SSc in Mice In Vivo
3.3. Evaluating Fibrosis
3.4. Serum AOPP Measurement
3.5. Collagen Content Measurement
3.6. Histopathologic Analysis
3.7. Evaluation of Fibroblasts
3.8. Evaluation of mRNA Expressions
3.9. Evaluating Immune Status
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramos-Casals, M.; Fonollosa-Pla, V.; Brito-Zerón, P.; Sisó-Almirall, A. Targeted therapy for systemic sclerosis: How close are we? Nat. Rev. Rheumatol. 2010, 6, 269–278. [Google Scholar] [CrossRef]
- Zuo, X.; Zhang, L.; Luo, H.; Li, Y.; Zhu, H. Systematic approach to understanding the pathogenesis of systemic sclerosis. Clin. Genet. 2017, 92, 365–371. [Google Scholar] [CrossRef]
- Yang, H.; Cheong, S.; He, Y.; Lu, F. Mesenchymal stem cell-based therapy for autoimmune-related fibrotic skin diseases-systemic sclerosis and sclerodermatous graft-versus-host disease. Stem Cell Res. Ther. 2023, 14, 372. [Google Scholar] [CrossRef]
- LeRoy, E.C.; Medsger, T.A., Jr. Criteria for the classification of early systemic sclerosis. J. Rheumatol. 2001, 28, 1573–1576. [Google Scholar]
- Varga, J.; Abraham, D.J. Systemic sclerosis: A prototypic multisystem fibrotic disorder. J. Clin. Investig. 2007, 117, 557–567. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Andréasson, K.; Smith, V. Systemic sclerosis. Lancet 2023, 401, 304–318. [Google Scholar] [CrossRef]
- Tamby, M.C.; Chanseaud, Y.; Guillevin, L.; Mouthon, L. New insights into the pathogenesis of systemic sclerosis. Autoimmunity Rev. 2003, 2, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Wei, J.; Varga, J. Understanding fibrosis in systemic sclerosis: Shifting paradigms, emerging opportunities. Nat. Rev. Rheumatol. 2011, 8, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zuo, X.X.; Li, Y.S.; Gao, S.M.; Dai, X.D.; Zhu, H.L.; Luo, H. Integration of microRNA and mRNA expression profiles in the skin of systemic sclerosis patients. Sci. Rep. 2017, 7, 42899. [Google Scholar] [CrossRef] [PubMed]
- Bieber, K.; Hundt, J.E.; Yu, X.; Ehlers, M.; Petersen, F.; Karsten, C.M.; Köhl, J.; Kridin, K.; Kalies, K.; Kasprick, A.; et al. Autoimmune pre-disease. Autoimmun. Rev. 2023, 22, 103236. [Google Scholar] [CrossRef] [PubMed]
- Committee for the Assessment of NIH Research on Autoimmune Diseases; Board on Population Health and Public Health Practice; Health and Medicine Division; National Academies of Sciences, Engineering, and Medicine. Enhancing NIH Research on Autoimmune Disease; National Academies Press (US): Washington, DC, USA, 2022. [Google Scholar]
- Gabrielli, A.; Avvedimento, E.V.; Krieg, T. Scleroderma. N. Engl. J. Med. 2009, 360, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Servettaz, A.; Guilpain, P.; Goulvestre, C.; Chéreau, C.; Hercend, C.; Nicco, C.; Guillevin, L.; Weill, B.; Mouthon, L.; Batteux, F. Radical oxygen species production induced by advanced oxidation protein products predicts clinical evolution and response to treatment in systemic sclerosis. Ann. Rheum. Dis. 2007, 66, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Simonini, G.; Cerinic, M.M.; Generini, S.; Zoppi, M.; Anichini, M.; Cesaretti, C.; Pignone, A.; Falcini, F.; Lotti, T.; Cagnoni, M. Oxidative stress in Systemic Sclerosis. Mol. Cell Biochem. 1999, 196, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Sambo, P.; Baroni, S.S.; Luchetti, M.; Paroncini, P.; Dusi, S.; Orlandini, G.; Gabrielli, A. Oxidative stress in scleroderma: Maintenance of scleroderma fibroblast phenotype by the constitutive up-regulation of reactive oxygen species generation through the NADPH oxidase complex pathway. Arthritis Rheum. 2001, 44, 2653–2664. [Google Scholar] [CrossRef]
- Ogawa, F.; Shimizu, K.; Muroi, E.; Hara, T.; Hasegawa, M.; Takehara, K.; Sato, S. Serum levels of 8-isoprostane, a marker of oxidative stress, are elevated in patients with systemic sclerosis. Rheumatology 2006, 45, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Bourji, K.; Meyer, A.; Chatelus, E.; Pincemail, J.; Pigatto, E.; Defraigne, J.O.; Singh, F.; Charlier, C.; Geny, B.; Gottenberg, J.E.; et al. High reactive oxygen species in fibrotic and nonfibrotic skin of patients with diffuse cutaneous systemic sclerosis. Free Radic. Biol. Med. 2015, 87, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Abdulle, A.E.; Diercks, G.F.H.; Feelisch, M.; Mulder, D.J.; van Goor, H. The Role of Oxidative Stress in the Development of Systemic Sclerosis Related Vasculopathy. Front. Physiol. 2018, 9, 1177. [Google Scholar] [CrossRef]
- Gabrielli, A.; Svegliati, S.; Moroncini, G.; Amico, D. New insights into the role of oxidative stress in scleroderma fibrosis. Open Rheumatol. J. 2012, 6, 87–95. [Google Scholar] [CrossRef]
- Gabrielli, A.; Svegliati, S.; Moroncini, G.; Pomponio, G.; Santillo, M.; Avvedimento, E.V. Oxidative stress and the pathogenesis of scleroderma: The Murrell’s hypothesis revisited. Semin. Immunopathol. 2008, 30, 329–337. [Google Scholar] [CrossRef]
- Almeida, C.; Almeida, I.; Vasconcelos, C. Quality of life in systemic sclerosis. Autoimmune Rev. 2015, 14, 1087–1096. [Google Scholar] [CrossRef]
- Abouyahya, I.; Liem, S.I.E.; Amoura, Z.; Fonseca, J.E.; Chaigne, B.; Cutolo, M.; Doria, A.; Fischer-Betz, R.; Guimaraes, V.; Huizinga, T.W.J.; et al. Health-related quality of life in patients with mixed connective tissue disease: A comparison with matched systemic sclerosis patients. Clin. Exp. Rheumatol. 2022, 40 (Suppl. 134), 66–70. [Google Scholar] [CrossRef]
- Herrick, A.L. Pathogenesis of raynaud’s phenomenon. Rheumatology 2005, 44, 587–596. [Google Scholar] [CrossRef]
- McCord, J.M. Oxygen-derived free radicals in postischemic tissue injury. N. Engl. J. Med. 1985, 312, 159–163. [Google Scholar] [CrossRef]
- Sinning, C.; Westermann, D.; Clemmensen, P. Oxidative stress in ischemia and reperfusion: Current concepts, novel ideas and future perspectives. Biomark. Med. 2017, 11, 11031–11040. [Google Scholar] [CrossRef]
- Simonini, G.; Pignone, A.; Generini, S.; Falcini, F.; Cerinic, M.M. Emerging potentials for an antioxidant therapy as a new approach to the treatment of systemic sclerosis. Toxicology 2000, 155, 1–15. [Google Scholar] [CrossRef]
- Sambo, P.; Jannino, L.; Candela, M.; Salvi, A.; Donini, M.; Dusi, S.; Luchetti, M.M.; Gabrielli, A. Monocytes of patients with systemic sclerosis (scleroderma) spontaneously release in vitro increased amounts of superoxide anion. J. Investig. Dermatol. 1999, 112, 78–84. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Svegliati, S.; Cancello, R.; Sambo, P.; Luchetti, M.; Paroncini, P.; Orlandini, G.; Discepoli, G.; Paterno, R.; Santillo, M.; Cuozzo, C.; et al. Platelet-derived growth factor and reactive oxygen species regulate Ras protein levels in primary human fibroblasts via ERK1/2: Amplification of ROS and Ras in systemic sclerosis fibro-blasts. J. Biol. Chem. 2005, 280, 36474–36482. [Google Scholar] [CrossRef]
- Inufusa, H. Composition for Protection against Cytotoxic Effects. TIMA Foundation. Patent No. 5777821, 9 September 2015. [Google Scholar]
- You, F.; Harakawa, Y.; Yoshikawa, T.; Inufusa, H. Why Does the Antioxidant Complex Twendee X® Prevent Dementia? Int. J. Mol. Sci. 2023, 24, 13018. [Google Scholar] [CrossRef]
- Tadokoro, K.; Morihara, R.; Ohta, Y.; Hishikawa, N.; Kawano, S.; Sasaki, R.; Matsumoto, N.; Nomura, E.; Nakano, Y.; Takahashi, Y.; et al. Clinical Benefits of Antioxidative Supplement Twendee X for Mild Cognitive Impairment: A Multicenter, Randomized, Double-Blind, and Placebo-Controlled Prospective Interventional Study. J. Alzheimers Dis. 2019, 71, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yamashita, T.; Shang, J.; Shi, X.; Morihara, R.; Huang, Y.; Sato, K.; Takemoto, M.; Hishikawa, N.; Ohta, Y.; et al. Clinical and Pathological Benefit of Twendee X in Alzheimer’s Disease Transgenic Mice with Chronic Cerebral Hypoperfusion. J. Stroke Cerebrovasc. Dis. 2019, 28, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yamashita, T.; Shang, J.; Shi, X.; Morihara, R.; Huang, Y.; Sato, K.; Takemoto, M.; Hishikawa, N.; Ohta, Y.; et al. Twendee X Ameliorates Phosphorylated Tau, α-Synuclein and Neurovascular Dysfunction in Alzheimer’s Disease Transgenic Mice with Chronic Cerebral Hypoperfusion. J. Stroke Cerebrovasc. Dis. 2019, 28, 104310. [Google Scholar] [CrossRef]
- Kusaki, M.; Ohta, Y.; Inufusa, H.; Yamashita, T.; Morihara, R.; Nakano, Y.; Liu, X.; Shang, J.; Tian, F.; Fukui, Y.; et al. Neuroprotective Effects of a Novel Antioxidant Mixture Twendee X in Mouse Stroke Model. J. Stroke Cerebrovasc. Dis. 2017, 26, 1191–1196. [Google Scholar] [CrossRef]
- You, F.; Harakawa, Y.; Yoshikawa, T.; Inufusa, H. Controlling Gut Microbiota by Twendee X® May Contribute to Dementia Prevention. Int. J. Mol. Sci. 2023, 24, 16642. [Google Scholar] [CrossRef] [PubMed]
- Morozan, A.; Joy, S.; Fujii, U.; Fraser, R.; Watters, K.; Martin, J.G.; Colmegna, I. Superiority of systemic bleomycin to intradermal HOCl for the study of interstitial lung disease. Sci. Rep. 2023, 13, 20577. [Google Scholar] [CrossRef] [PubMed]
- Maria, A.T.J.; Toupet, K.; Maumus, M.; Rozier, P.; Vozenin, M.C.; Le Quellec, A.; Jorgensen, C.; Noël, D.; Guilpain, P. Fibrosis Development in HOCl-Induced Systemic Sclerosis: A Multistage Process Hampered by Mesenchymal Stem Cells. Front. Immunol. 2018, 9, 2571. [Google Scholar] [CrossRef] [PubMed]
- Servettaz, A.; Goulvestre, C.; Kavian, N.; Nicco, C.; Guilpain, P.; Chéreau, C.; Vuiblet, V.; Guillevin, L.; Mouthon, L.; Weill, B.; et al. Selective oxidation of DNA topoisomerase 1 induces systemic sclerosis in the mouse. J. Immunol. 2009, 182, 5855–5864. [Google Scholar] [CrossRef] [PubMed]
- Batteux, F.; Kavian, N.; Servettaz, A. New insights on chemically induced animal models of systemic sclerosis. Curr. Opin. Rheumatol. 2011, 23, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, G.L.; Irrera, N.; Pizzino, G.; Santoro, D.; Roberts, W.N.; Bagnato, G.; Pallio, G.; Vaccaro, M.; Squadrito, F.; Saitta, A.; et al. Dual αvβ3 and αvβ5 blockade attenuates fibrotic and vascular alterations in a murine model of systemic sclerosis. Clin. Sci. 2018, 132, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, G.; Bitto, A.; Pizzino, G.; Irrera, N.; Sangari, D.; Cinquegrani, M.; Roberts, W.N.; Matucci Cerinic, M.; Squadrito, F.; Altavilla, D.; et al. Simvastatin attenuates the development of pulmonary and cutaneous fibrosis in a murine model of systemic sclerosis. Rheumatology 2013, 52, 1377–1386. [Google Scholar] [CrossRef]
- Bagnato, G.; Bitto, A.; Pizzino, G.; Roberts, W.N.; Squadrito, F.; Altavilla, D.; Bagnato, G.; Saitta, A. Propylthiouracil modulates aortic vasculopathy in the oxidative stress model of systemic sclerosis. Vasc. Pharmacol. 2015, 71, 79–83. [Google Scholar] [CrossRef]
- Allanore, Y.; Borderie, D.; Lemaréchal, H.; Ekindjian, O.G.; Kahan, A. Acute and sustained effects of dihydropyridine-type calcium channel antagonists on oxidative stress in systemic sclerosis. Am. J. Med. 2004, 116, 595–600. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Al-Adwi, Y.; Atzeni, I.M.; Doornbos-van der Meer, B.; Abdulle, A.E.; van Roon, A.M.; Stel, A.; van Goor, H.; Smit, A.J.; Westra, J.; Mulder, D.J. Release of High-Mobility Group Box-1 after a Raynaud’s Attack Leads to Fibroblast Activation and Interferon-γ Induced Protein-10 Production: Role in Systemic Sclerosis Pathogenesis. Antioxidants 2023, 12, 794. [Google Scholar] [CrossRef]
- Murrell, D.F. A radical proposal for the pathogenesis of scleroderma. J. Am. Acad. Dermatol. 1993, 28, 78–85. [Google Scholar] [CrossRef]
- Amstad, P.; Peskin, A.; Shah, G.; Mirault, M.E.; Moret, R.; Zbinden, I.; Cerutti, P. The balance between Cu,Zn-superoxide dismutase and catalase affects the sensitivity of mouse epidermal cells to oxidative stress. Biochemistry 1991, 30, 9305–9313. [Google Scholar] [CrossRef]
- Marut, W.K.; Kavian, N.; Servettaz, A.; Nicco, C.; Ba, L.A.; Doering, M.; Chéreau, C.; Jacob, C.; Weill, B.; Batteux, F. The organotelluride catalyst (PHTE)₂NQ prevents HOCl-induced systemic sclerosis in mouse. J. Investig. Dermatol. 2012, 132, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chu, H.; Jiang, S.; Liu, Q.; Liu, L.; Xue, Y.; Zheng, S.; Wan, W.; Qiu, J.; Wang, J.; et al. Sirt1 ameliorates systemic sclerosis by targeting the mTOR pathway. J. Dermatol. Sci. 2017, 87, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, A.; Yanaba, K.; Ogawa, A.; Iwata, Y.; Ogawa, F.; Takenaka, M.; Shimizu, K.; Asano, Y.; Kadono, T.; Sato, S. The specific free radical scavenger edaravone suppresses fibrosis in the bleomycin- induced and tight skin mouse models of systemic sclerosis. Arthritis Rheum. 2011, 63, 3086–3097. [Google Scholar] [CrossRef] [PubMed]
- Baral, H.; Sekiguchi, A.; Uchiyama, A.; Nisaa Amalia, S.; Yamazaki, S.; Inoue, Y.; Yokoyama, Y.; Ogino, S.; Torii, R.; Hosoi, M.; et al. Inhibition of skin fibrosis in systemic sclerosis by botulinum toxin B via the suppression of oxidative stress. J. Dermatol. 2021, 48, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Wu, Q.; Xu, X.; Xing, Y.; Liang, J.; Lin, Q.; Huang, M.; Chen, Y.; Lin, B.; Chen, W. Resveratrol Ameliorates Systemic Sclerosis via Suppression of Fibrosis and Inflammation Through Activation of SIRT1/mTOR Signaling. Drug Des. Devel Ther. 2020, 14, 5337–5348. [Google Scholar] [CrossRef] [PubMed]
- Edaravone Acute Infarction Study Group. Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction: Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc. Dis. 2003, 15, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Yamashita, T.; Tsunoda, K.; Matsumoto, N.; Tadokoro, K.; Sasaki, R.; Abe, K. In Vitro Free Radical Scavenging Activities of Dietary Supplements by Electron Spin Resonance. Brain Suppl. 2020, 2, 1–12. [Google Scholar]
- Spadoni, T.; Svegliati Baroni, S.; Amico, D.; Albani, L.; Moroncini, G.; Avvedimento, E.V.; Gabrielli, A. A reactive oxygen species-mediated loop maintains increased expression of NADPH oxidases 2 and 4 in skin fibroblasts from patients with systemic sclerosis. Arthritis Rheumatol. 2015, 67, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Grande, M.T.; Fuentes-Calvo, I.; Arévalo, M.; Heredia, F.; Santos, E.; Martínez-Salgado, C.; Rodríguez-Puyol, D.; Nieto, M.A.; López-Novoa, J.M. Deletion of H-Ras decreases renal fibrosis and myofibroblast activation following ureteral obstruction in mice. Kidney Int. 2010, 77, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Tan, J.; Chen, W.; Du, Q.; Xie, B.; Wang, N.; Zhu, H.; Wang, K. The Fibrosis and Immunological Features of Hypochlorous Acid Induced Mouse Model of Systemic Sclerosis. Front. Immunol. 2019, 10, 1861. [Google Scholar] [CrossRef] [PubMed]
- Amico, D.; Spadoni, T.; Rovinelli, M.; Serafini, M.; D’Amico, G.; Campelli, N.; Svegliati Baroni, S.; Gabrielli, A. Intracellular free radical production by peripheral blood T lymphocytes from patients with systemic sclerosis: Role of NADPH oxidase and ERK1/2. Arthritis Res Ther. 2015, 17, 68. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, M.; Yang, H.; Qu, R.; Qiu, Y.; Hao, J.; Bi, H.; Guo, D. Regulatory Mechanism of M1/M2 Macrophage Polarization in the Development of Autoimmune Diseases. Mediat. Inflamm. 2023, 2023, 8821610. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Chung, E.J.; Kwon, S.; Shankavaram, U.; White, A.O.; Das, S.; Citrin, D.E. Natural variation in macrophage polarization and function impact pneumocyte senescence and susceptibility to fibrosis. Aging 2022, 14, 7692–7717. [Google Scholar] [CrossRef] [PubMed]
- Jeljeli, M.; Riccio, L.G.C.; Doridot, L.; Chêne, C.; Nicco, C.; Chouzenoux, S.; Deletang, Q.; Allanore, Y.; Kavian, N.; Batteux, F. Trained immunity modulates inflammation-induced fibrosis. Nat. Commun. 2019, 10, 5670. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Karim, M.R.; Izawa, T.; Kuwamura, M.; Yamate, J. Immunophenotypical characterization of M1/M2 macrophages and lymphocytes in cisplatin-induced rat progressive renal fibrosis. Cells 2021, 10, 257. [Google Scholar] [CrossRef]
- Chêne, C.; Rongvaux-Gaïda, D.; Thomas, M.; Rieger, F.; Nicco, C.; Batteux, F. Optimal combination of arsenic trioxide and copper ions to prevent autoimmunity in a murine HOCl-induced model of systemic sclerosis. Front. Immunol. 2023, 14, 1149869. [Google Scholar] [CrossRef]
- Duan, J.; Liu, X.; Wang, H.; Guo, S.W. The M2a macrophage subset may be critically involved in the fibrogenesis of endometriosis in mice. Reprod. Biomed. Online 2018, 37, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Woessner, J.F., Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch. Biochem. Biophys. 1961, 93, 440–447. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, F.; Nicco, C.; Harakawa, Y.; Yoshikawa, T.; Inufusa, H. The Potential of Twendee X® as a Safe Antioxidant Treatment for Systemic Sclerosis. Int. J. Mol. Sci. 2024, 25, 3064. https://doi.org/10.3390/ijms25053064
You F, Nicco C, Harakawa Y, Yoshikawa T, Inufusa H. The Potential of Twendee X® as a Safe Antioxidant Treatment for Systemic Sclerosis. International Journal of Molecular Sciences. 2024; 25(5):3064. https://doi.org/10.3390/ijms25053064
Chicago/Turabian StyleYou, Fukka, Carole Nicco, Yoshiaki Harakawa, Toshikazu Yoshikawa, and Haruhiko Inufusa. 2024. "The Potential of Twendee X® as a Safe Antioxidant Treatment for Systemic Sclerosis" International Journal of Molecular Sciences 25, no. 5: 3064. https://doi.org/10.3390/ijms25053064
APA StyleYou, F., Nicco, C., Harakawa, Y., Yoshikawa, T., & Inufusa, H. (2024). The Potential of Twendee X® as a Safe Antioxidant Treatment for Systemic Sclerosis. International Journal of Molecular Sciences, 25(5), 3064. https://doi.org/10.3390/ijms25053064





