Oxy-Inflammation in Humans during Underwater Activities
Abstract
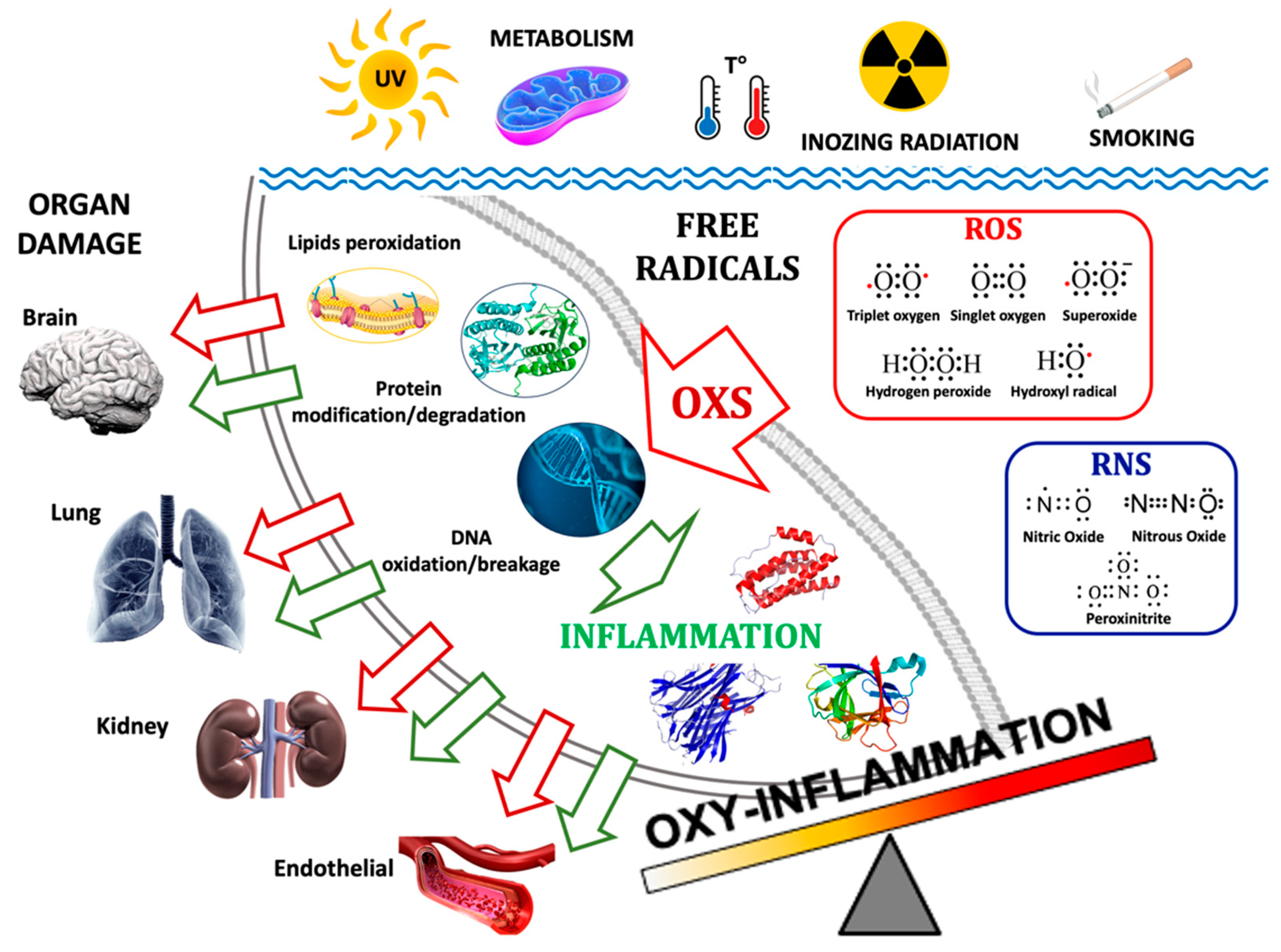
1. Introduction
2. Oxy-Inflammation-Related Mechanisms and Outcomes
2.1. Oxidative Stress in Hypoxia and Hyperoxia
2.2. Inflammation in Hypoxia and Hyperoxia
2.3. Endothelial Dysfunction
2.4. Hypoxia, Reoxygenation, and Hyperoxia
2.5. Oxy-Inflammation Biomarkers
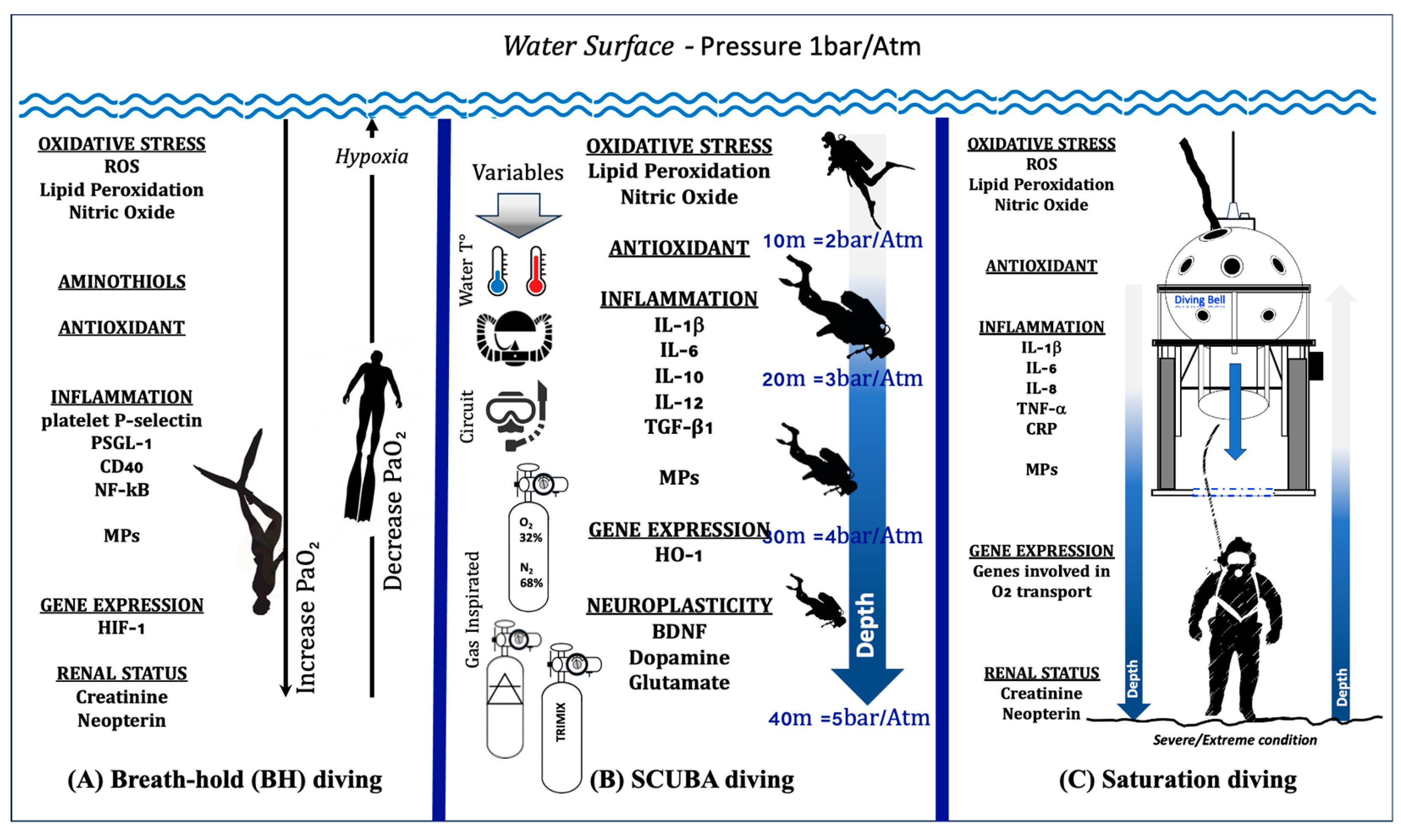
3. The Oxy-Inflammation Mechanism in Underwater Activity
3.1. Breath-Hold (BH) Diving
3.2. Self-Contained Underwater Breathing Apparatus (SCUBA) and Closed-Circuit Rebreather (CCR) Diving
3.3. Saturation Diving
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Amino acid |
| ABG | Arterial blood gas |
| BH | Breath-hold diving |
| CAT | Catalase |
| CCR | Closed-Circuit Rebreather |
| CDM | Compartmental decompression model |
| CRP | Protein C-reactive |
| DCS | Decompression sickness |
| EGF | Epithelial growth factor |
| HIF | Hypoxia-inducible factor |
| IL- | Interleukin |
| IRI | Ischemia–reperfusion |
| NO | Nitric oxide |
| NK | Natural Killer |
| O2 | Oxygen |
| O22− | Superoxide anion |
| ONOO | Peroxinitrite |
| OxS | Oxidative stress |
| PaCO2 | Arterial partial CO2 pressure |
| pO2 | Partial O2 pressure |
| pGSN | Plasma gelsolin |
| RDS | Ratio decompression strategy |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| SCUBA | Self-Contained Underwater Breathing Apparatus |
| SOD | Superoxide dismutase |
| TAC | Total Antioxidant Capacity |
| 8-OH-dG | 8-OH-2-deoxyguanosine |
| 8-iso-PGF2α | 8-isoprostane |
References
- Wilmshurst, P. ABC of Oxygen. Diving and Oxygen. BMJ 1998, 317, 996–999. [Google Scholar] [CrossRef]
- Lee, Y.I.; Ye, B.J. Underwater and Hyperbaric Medicine as a Branch of Occupational and Environmental Medicine. Ann. Occup. Environ. Med. 2013, 25, 39. [Google Scholar] [CrossRef]
- Balestra, C.; Mrakic-Sposta, S.; Virgili, F. Oxygen Variations—Insights into Hypoxia, Hyperoxia and Hyperbaric Hyperoxia—Is the Dose the Clue? Int. J. Mol. Sci. 2023, 24, 13472. [Google Scholar] [CrossRef]
- Kramer, M.R.; Godfrey, S. Dead Sea: Natural oxygen enrichment at low altitude. Isr. J. Med. Sci. 1996, 32, S20–S23. [Google Scholar]
- Gouin, E.; Balestra, C.; Orsat, J.; Dugrenot, E.; L’Her, E. Pulmonary Effects of One Week of Repeated Recreational Closed-Circuit Rebreather Dives in Cold Water. Medicina 2023, 59, 81. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.R., III; Murphy-Lavoie, H.M. Diving Rebreathers. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024; Bookshelf ID: NBK430743. [Google Scholar]
- Balestra, C.; Guerrero, F.; Theunissen, S.; Germonpre, P.; Lafere, P. Physiology of repeated mixed gas 100-m wreck dives using a closed-circuit rebreather: A field bubble study. Eur. J. Appl. Physiol. 2022, 122, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Tsur, N.; Bar, R.; Hilly, O.; Handzel, O. Balloon Eustachian tuboplasty in a professional Navy SEAL diver: Case report. Undersea Hyperb. Med. 2020, 47, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.J.; Doolette, D.J. Recreational technical diving part 1: An introduction to technical diving methods and activities. Diving Hyperb. Med. 2013, 43, 86–93. [Google Scholar] [PubMed]
- Imbert, J.P.; Egi, S.M.; Balestra, C. Vascular Function Recovery Following Saturation Diving. Medicina 2022, 58, 1476. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, R.; Gray, H.B. Preface on making Oxygen. Inorg. Chem. 2008, 47, 1697–1699. [Google Scholar] [CrossRef] [PubMed]
- Kamata, H.; Hirata, H. Redox regulation of cellular signaling. Cell. Signal. 1999, 11, 1–14. [Google Scholar] [CrossRef]
- Rimessi, A.; Previati, M.; Nigro, F.; Wieckowski, M.R.; Pinton, P. Mitochondrial reactive oxygen species and inflammation: Molecular mechanisms, diseases and promising therapies. Int. J. Biochem. Cell Biol. 2016, 81, 281–293. [Google Scholar] [CrossRef]
- Bergendi, L.; Benes, L.; Durackova, Z.; Ferencik, M. Chemistry, physiology and pathology of free radicals. Life Sci. 1999, 65, 1865–1874. [Google Scholar] [CrossRef]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M.; Forman, H.J. Redox homeostasis: The Golden Mean of healthy living. Redox Biol. 2016, 8, 205–215. [Google Scholar] [CrossRef]
- Ryter, S.W.; Nakahira, K.; Haspel, J.A.; Choi, A.M. Autophagy in pulmonary diseases. Annu. Rev. Physiol. 2012, 74, 377–401. [Google Scholar] [CrossRef] [PubMed]
- Sunil, V.R.; Shen, J.; Patel-Vayas, K.; Gow, A.J.; Laskin, J.D.; Laskin, D.L. Role of reactive nitrogen species generated via inducible nitric oxide synthase in vesicant-induced lung injury, inflammation and altered lung functioning. Toxicol. Appl. Pharmacol. 2012, 261, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zou, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Debevec, T.; Millet, G.P.; Pialoux, V. Hypoxia-Induced Oxidative Stress Modulation with Physical Activity. Front. Physiol. 2017, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- McGarry, T.; Biniecka, M.; Veale, D.J.; Fearon, U. Hypoxia, oxidative stress and inflammation. Free Radic. Biol. Med. 2018, 125, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.S.; Phuyal, P.; Shah, N. Oxygen Toxicity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Smit, B.; Smulders, Y.M.; van der Wouden, J.C.; Straaten, H.M.O.-V.; Man, A.M.E.S.-D. Hemodynamic effects of acute hyperoxia: Systematic review and meta-analysis. Crit. Care 2018, 22, 45. [Google Scholar] [CrossRef]
- Hackett, P.H.; Roach, R.C. High-altitude illness. N. Engl. J. Med. 2001, 345, 107–114. [Google Scholar] [CrossRef]
- Hartmann, G.; Tschop, M.; Fischer, R.; Bidlingmaier, C.; Riepl, R.; Tschop, K.; Hautmann, H.; Endres, S.; Toepfer, M. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine 2000, 12, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Nejfelt, M.K.; Chi, S.M.; Antonarakis, S.E. Hypoxia-inducible nuclear factors bind to an enhancer ele-ment located 3′ to the human erythropoietin gene. Proc. Natl. Acad. Sci. USA 1991, 88, 5680–5684. [Google Scholar] [CrossRef]
- Salvagno, M.; Coppalini, G.; Taccone, F.S.; Strapazzon, G.; Mrakic-Sposta, S.; Rocco, M.; Khalife, M.; Balestra, C. The Normobaric Oxygen Paradox-Hyperoxic Hypoxic Paradox: A Novel Expedient Strategy in Hematopoiesis Clinical Issues. Int. J. Mol. Sci. 2022, 24, 82. [Google Scholar] [CrossRef]
- Kiboub, F.Z.; Balestra, C.; Loennechen, O.; Eftedal, I. Hemoglobin and Erythropoietin After Commercial Saturation Diving. Front. Physiol. 2018, 9, 1176. [Google Scholar] [CrossRef]
- Koch, A.; Kähler, W.; Klapa, S.; Grams, B.; van Ooij, P.J.A.M. The conundrum of using hyperoxia in COVID-19 treatment strategies: May intermittent therapeutic hyperoxia play a helpful role in the expression of the surface receptors ACE2 and Furin in lung tissue via triggering of HIF-1α? Intensiv. Care Med. Exp. 2020, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Oxygen sensing, homeostasis, and disease. N. Engl. J. Med. 2011, 365, 537–547. [Google Scholar] [CrossRef]
- Fong, G.H.; Takeda, K. Role and regulation of prolyl hydroxylase domain proteins. Cell Death Differ. 2008, 15, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Naugler, W.E.; Karin, M. NF-kappaB, and cancer-identifying targets and mechanisms. Curr. Opin. Genet. Dev. 2008, 18, 19–26. [Google Scholar] [CrossRef]
- Taylor, C.T. Interdependent roles for Hypoxia-inducible factor and nuclear factor-kappaB in hypoxic inflammation. J. Physiol. 2008, 586, 4055–4059. [Google Scholar] [CrossRef] [PubMed]
- Leveque, C.; Mrakic Sposta, S.; Theunissen, S.; Germonpré, P.; Lambrechts, K.; Vezzoli, A.; Gussoni, M.; Levenez, M.; Lafère, P.; Guerrero, F.; et al. Oxidative Stress Response Kinetics after 60 Minutes at Different Levels (10% or 15%) of Normobaric Hypoxia Exposure. Int. J. Mol. Sci. 2023, 24, 10188. [Google Scholar] [CrossRef]
- Cummins, E.P.; Berra, E.; Comerford, K.M.; Ginouves, A.; Fitzgerald, K.T.; Seeballuck, F.; Godson, C.; Nielsen, J.E.; Moynagh, P.; Pouyssegur, J.; et al. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving in-sight into hypoxia-induced NFkappaB activity. Proc. Natl. Acad. Sci. USA 2006, 103, 18154–18159. [Google Scholar] [CrossRef] [PubMed]
- Rius, J.; Guma, M.; Schachtrup, C.; Akassoglou, K.; Zinkernagel, A.S.; Nizet, V.; Johnson, R.S.; Haddad, G.G.; Karin, M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 2008, 453, 807–811. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.P.; Bald, A.; Cassatella, M.A. Activation of the NF-κB Pathway by Inflammatory Stimuli in Human Neutrophils. Blood 1997, 89, 3421–3433. [Google Scholar] [CrossRef]
- Ben-Shoshan, J.; Afek, A.; Maysel-Auslender, S.; Barzelay, A.; Rubinstein, A.; Keren, G.; George, J. HIF-1alpha overex-pression and experimental murine atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 665–670. [Google Scholar] [CrossRef]
- Alonso, D.; Serrano, E.; Bermejo, F.J.; Corral, R.S. HIF-1alpha-regulated MIF activation and Nox2-dependent ROS generation promote Leishmania amazonensis killing by macrophages under Hypoxia. Cell Immunol. 2019, 335, 15–21. [Google Scholar] [CrossRef]
- Damiani, E.; Donati, A.; Girardis, M. Oxygen in the critically ill: Friend or foe? Curr. Opin. Anaesthesiol. 2018, 31, 129–135. [Google Scholar] [CrossRef]
- Jamieson, D.; Chance, B.; Cadenas, E.; Boveris, A. The relation of free radical production to hyperoxia. Annu. Rev. Physiol. 1986, 48, 703–719. [Google Scholar] [CrossRef]
- Jackson, R.M. Pulmonary oxygen toxicity. Chest 1985, 88, 900–905. [Google Scholar] [CrossRef]
- Bhandari, V. Molecular mechanisms of hyperoxia-induced acute lung injury. Front. Biosci. 2008, 13, 6653–6661. [Google Scholar] [CrossRef]
- Li, L.F.; Yang, C.T.; Huang, C.C.; Liu, Y.Y.; Kao, K.C.; Lin, H.C. Low-molecular-weight heparin reduces hyperoxia-augmented ventilator-induced lung injury via serine/threonine kinase-protein kinase B. Respir. Res. 2011, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Qadan, M.; Battista, C.; Gardner, S.A.; Anderson, G.; Akca, O.; Polk, H.C., Jr. Oxygen and surgical site infection: A study of underlying immunologic mechanisms. Anesthesiology 2010, 113, 369–377. [Google Scholar] [CrossRef]
- McInturff, A.M.; Cody, M.J.; Elliott, E.A.; Glenn, J.W.; Rowley, J.W.; Rondina, M.T.; Yost, C.C. Mammalian target of rapamycin regulates neutrophil extracellular trap formation via induction of hypoxia-inducible factor 1 alpha. Blood 2012, 120, 3118–3125. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, P.J.; Hickman-Davis, J.M.; Davis, I.C.; Matalon, S. Hyperoxia impairs antibacterial function of macrophages through effects on actin. Am. J. Respir. Cell Mol. Biol. 2003, 28, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Xie, K.; Li, N.; Qin, M.; Lu, Y.; Ma, S.; Ji, G.; Xiong, L. 100% oxygen inhalation protects against zymosan-induced sterile sepsis in mice: The roles of inflammatory cytokines and antioxidant enzymes. Shock 2009, 32, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bai, X.; Du, K.; Huang, Y.; Wang, W.; Zhao, Y.; Pei, Y.; Mu, J.; Han, H.; Hu, S.; et al. Activation of the cholinergic anti-inflammatory pathway contributes to the protective effects of 100% oxygen inhalation on zymosan-induced generalized inflammation in mice. J. Surg. Res. 2012, 174, e75–e83. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Ferrer, M.D.; Batle, J.M.; Tauler, P.; Tur, J.A.; Pons, A. Scuba diving increases erythrocyte and plasma antioxidant defenses and spares NO without oxidative damage. Med. Sci. Sport. Exerc. 2009, 41, 1271–1276. [Google Scholar] [CrossRef]
- Forstermann, U. Nitric oxide and oxidative stress in vascular disease. Pflug. Arch. 2010, 459, 923–939. [Google Scholar] [CrossRef]
- Landmesser, U.; Dikalov, S.; Price, S.R.; McCann, L.; Fukai, T.; Holland, S.M.; Mitch, W.E.; Harrison, D.G. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Investig. 2003, 111, 1201–1209. [Google Scholar] [CrossRef]
- Solich-Talanda, M.; Żebrowska, A.; Mikołajczyk, R.; Kostorz-Nosal, S.; Ziora, D.; Jastrzębski, D.; Siermontowski, P. Effect of Apnea-Induced Hypoxia on Cardiovascular Adaptation and Circulating Biomarkers of Oxidative Stress in Elite Breath-Hold Divers. Front. Physiol. 2021, 12, 726434. [Google Scholar] [CrossRef]
- Theunissen, S.; Guerrero, F.; Sponsiello, N.; Cialoni, D.; Pieri, M.; Germonpre, P.; Obeid, G.; Tillmans, F.; Papadopoulou, V.; Hemelryck, W.; et al. Nitric oxide-related endothelial changes in breath-hold and scuba divers. Undersea Hyperb. Med. 2013, 40, 135–144. [Google Scholar]
- Arya, A.K.; Balestra, C.; Bhopale, V.M.; Tuominen, L.J.; Raisanen-Sokolowski, A.; Dugrenot, E.; L’Her, E.; Bhat, A.R.; Thom, S.R. Elevations of Extracellular Vesicles and Inflammatory Biomarkers in Closed Circuit SCUBA Divers. Int. J. Mol. Sci. 2023, 24, 5969. [Google Scholar] [CrossRef]
- Sharma, R.I.; Marcinkowska, A.B.; Mankowska, N.D.; Waśkow, M.; Kot, J.; Winklewski, P.J. Cognitive Functions in Scuba, Technical and Saturation Diving. Biology 2023, 12, 229. [Google Scholar] [CrossRef]
- Nossum, V.; Hjelde, A.; Brubakk, A.O. Small amounts of venous gas embolism cause delayed impairment of endothelial function and increase polymorphonuclear neutrophil infiltration. Eur. J. Appl. Physiol. 2002, 86, 209–214. [Google Scholar] [CrossRef]
- Klinger, A.L.; Pichette, B.; Sobolewski, P.; Eckmann, D.M. Mechanotransductional basis of endothelial cell response to intravascular bubbles. Integr. Biol. 2011, 3, 1033–1042. [Google Scholar] [CrossRef]
- Pontier, J.M.; Gempp, E.; Ignatescu, M. Blood platelet-derived microparticles release and bubble formation after an open-sea air dive. Appl. Physiol. Nutr. Metab. 2012, 37, 888–892. [Google Scholar] [CrossRef]
- Brett, K.D.; Nugent, N.Z.; Fraser, N.K.; Bhopale, V.M.; Yang, M.; Thom, S.R. Microparticle and interleukin-1β production with human simulated compressed air diving. Sci. Rep. 2019, 9, 13320. [Google Scholar] [CrossRef] [PubMed]
- Barak, O.F.; Janjic, N.; Drvis, I.; Mijacika, T.; Mudnic, I.; Coombs, G.B.; Thom, S.R.; Madic, D.; Dujic, Z. Vascular dysfunction following breath-hold diving. Can. J. Physiol. Pharmacol. 2020, 98, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, J.; Ascensao, A.; Viscor, G.; Soares, J.; Oliveira, J.; Marques, F.; Duarte, J. Oxidative stress in humans during and after 4 hours of hypoxia at a simulated altitude of 5500 m. Aviat. Space Environ. Med. 2004, 75, 16–22. [Google Scholar] [PubMed]
- Irarrázaval, S.; Allard, C.; Campodónico, J.; Pérez, D.; Strobel, P.; Vásquez, L.; Urquiaga, I.; Echeverría, G.; Leighton, F. Oxidative Stress in Acute Hypobaric Hypoxia. High Alt. Med. Biol. 2017, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Mrakic-Sposta, S.; Gussoni, M.; Dellanoce, C.; Marzorati, M.; Montorsi, M.; Rasica, L.; Pratali, L.; D’Angelo, G.; Martinelli, M.; Bastiani, L.; et al. Effects of acute and sub-acute hypobaric hypoxia on oxidative stress: A field study in the Alps. Eur. J. Appl. Physiol. 2021, 121, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Liu, G.; Wu, G.; Wang, R.; Zhang, J. High altitude hypoxia and oxidative stress: The new hope brought by free radical scavengers. Life Sci. 2023, 336, 122319. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Mallet, R.T.; Pialoux, V.; Millet, G.P.; Burtscher, M. Adaptive Responses to Hypoxia and/or Hyperoxia in Humans. Antioxid. Redox Signal. 2022, 37, 887–912. [Google Scholar] [CrossRef] [PubMed]
- Mrakic Sposta, S.; Montorsi, M.; Porcelli, S.; Marzorati, M.; Healey, B.; Dellanoce, C.; Vezzoli, A. Effects of Prolonged Exposure to Hypobaric Hypoxia on Oxidative Stress: Overwintering in Antarctic Concordia Station. Oxid. Med. Cell Longev. 2022, 2022, 4430032. [Google Scholar] [CrossRef]
- Rathor, R.; Suryakumar, G.; Singh, S.N. Diet and redox state in maintaining skeletal muscle health and performance at high altitude. Free. Radic. Biol. Med. 2021, 174, 305–320. [Google Scholar] [CrossRef]
- Verratti, V.; Bondi, D.; Jandova, T.; Camporesi, E.; Paoli, A.; Bosco, G. Sex Hormones Response to Physical Hyperoxic and Hyperbaric Stress in Male Scuba Divers: A Pilot Study. Adv. Exp. Med. Biol. 2019, 1176, 53–62. [Google Scholar] [CrossRef]
- Balestra, C.; Lambrechts, K.; Mrakic-Sposta, S.; Vezzoli, A.; Levenez, M.; Germonpré, P.; Virgili, F.; Bosco, G.; Lafère, P. Hypoxic and Hyperoxic Breathing as a Complement to Low-Intensity Physical Exercise Programs: A Proof-of-Principle Study. Int. J. Mol. Sci. 2021, 22, 9600. [Google Scholar] [CrossRef]
- Leveque, C.; Mrakic-Sposta, S.; Theunissen, S.; Germonpr, P.; Lambrechts, K.; Vezzoli, A.; Bosco, G.; Lévenéz, M.; Lafèvre, P.; Guerrero, F.; et al. Oxidative Stress Response Kinetics after 60 Minutes at Different (1.4 ATA and 2.5 ATA) Hyperbaric Hyperoxia Exposures. Int. J. Mol. Sci. 2023, 24, 12361. [Google Scholar] [CrossRef] [PubMed]
- Brugniaux, J.V.; Coombs, G.B.; Barak, O.F.; Dujic, Z.; Sekhon, M.S.; Ainslie, P.N. Highs and lows of hyperoxia: Physiological, performance, and clinical aspects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R1–R27. [Google Scholar] [CrossRef] [PubMed]
- Modun, D.; Krnic, M.; Vukovic, J.; Kokic, V.; Kukoc-Modun, L.; Tsikas, D.; Dujic, Z. Plasma nitrite concentration decreases after hyperoxia-induced oxidative stress in healthy humans. Clin. Physiol. Funct. Imaging 2012, 32, 404–408. [Google Scholar] [CrossRef]
- Thom, S.R.; Bhopale, V.; Fisher, D.; Manevich, Y.; Huang, P.L.; Buerk, D.G. Stimulation of nitric oxide synthase in cerebral cortex due to elevated partial pressures of oxygen: An oxidative stress response. J. Neurobiol. 2002, 51, 85–100. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Porcelli, S.; Pugliese, L.; Pavei, G.; Bellistri, G.; Montorsi, M.; Tacchini, P.; Vezzoli, A. Training effects on ROS production determined by electron paramagnetic resonance in master swimmers. Oxid. Med. Cell. Longev. 2015, 2015, 804794. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, A.; Pinho, R.A.; Ugbolue, U.C.; He, Y.; Meng, Y.; Gu, Y. Effect of Running Exercise on Oxidative Stress Biomarkers: A Systematic Review. Front. Physiol. 2021, 11, 610112. [Google Scholar] [CrossRef]
- Brizzolari, A.; Bosco, G.; Vezzoli, A.; Dellanoce, C.; Barassi, A.; Paganini, M.; Cialoni, D.; Mrakic-Sposta, S. Seasonal Oxy-Inflammation and Hydration Status in Non-Elite Freeskiing Racer: A Pilot Study by Non-Invasive Analytic Method. Int. J. Environ. Res. Public Health 2023, 20, 3157. [Google Scholar] [CrossRef]
- Goto, C.; Higashi, Y.; Kimura, M.; Noma, K.; Hara, K.; Nakagawa, K.; Kawamura, M.; Chayama, K.; Yoshizumi, M.; Nara, I. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: Role of endothelium-dependent nitric oxide and oxidative stress. Circulation 2003, 108, 530–535. [Google Scholar] [CrossRef]
- Vezzoli, A.; Dellanoce, C.; Mrakic-Sposta, S.; Montorsi, M.; Moretti, S.; Tonini, A.; Pratali, L.; Accinni, R. Oxidative Stress Assessment in Response to Ultraendurance Exercise: Thiols Redox Status and ROS Production according to Duration of a Competitive Race. Oxid. Med. Cell. Longev. 2016, 2016, 6439037. [Google Scholar] [CrossRef]
- Granger, D.N.; Kvietysb, P.R. Reperfusion injury, and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Nunez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Mills, K.H. TLR-dependent T cell activation in autoimmunity. Nat. Rev. Immunol. 2011, 11, 807–822. [Google Scholar] [CrossRef] [PubMed]
- Kruger, B.; Krick, S.; Dhillon, N.; Lerner, S.M.; Ames, S.; Bromberg, J.S.; Lin, M.; Walsh, L.; Vella, J.; Fischereder, M.; et al. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc. Natl. Acad. Sci. USA 2009, 106, 3390–3395. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yin, H.; Han, J.; Huang, B.; Xu, J.; Zheng, F.; Tan, Z.; Fang, M.; Rui, L.; Chen, D.; et al. Extracellular hmgb1 functions as an innate immune mediator implicated in murine cardiac allograft acute rejection. Am. J. Transplant. 2007, 7, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Moser, B.; Szabolcs, M.J.; Ankersmit, H.J.; Lu, Y.; Qu, W.; Weinberg, A.; Herold, K.C.; Schmidt, A.M. Blockade of RAGE suppresses alloimmune reactions in vitro and delays allograft rejection in murine heart transplantation. Am. J. Transplant. 2007, 7, 293–302. [Google Scholar] [CrossRef]
- Rybnikova, E.A.; Nalivaeva, N.N.; Zenko, M.Y.; Baranova, K.A. Intermittent Hypoxic Training as an Effective Tool for Increasing the Adaptive Potential, Endurance and Working Capacity of the Brain. Front. Neurosci. 2022, 16, 941740. [Google Scholar] [CrossRef] [PubMed]
- Lakka, T.A.; Venalainen, J.M.; Rauramaa, R.; Salonen, R.; Tuomilehto, J.; Salonen, J.T. Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction. N. Engl. J. Med. 1994, 330, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Briones, A.M.; Touyz, R.M. Moderate exercise decreases inflammation and oxidative stress in hypertension: But what are the mechanisms? Hypertension 2009, 54, 1206–1208. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moller, P.; Loft, S.; Lundby, C.; Olsen, N.V. Acute hypoxia and hypoxic exercise induce DNA strand breaks and oxidative DNA damage in humans. FASEB J. 2001, 15, 1181–1186. [Google Scholar] [CrossRef]
- Wozniak, A.; Drewa, G.; Chesy, G.; Rakowski, A.; Rozwodowska, M.; Olszewska, D. Effect of altitude training on sportsmen’s peroxidation and antioxidant enzymes. Med. Sci. Sport. Exerc. 2001, 33, 1109–1113. [Google Scholar] [CrossRef]
- Ridker, P.M.; Cushman, M.; Stampfer, M.J.; Tracy, R.P.; Hennekens, C.H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med. 1997, 336, 973–979. [Google Scholar] [CrossRef]
- Verma, S.; Buchanan, M.R.; Anderson, T.J. Endothelial function testing as a biomarker of vascular disease. Circulation 2003, 108, 2054–2059. [Google Scholar] [CrossRef]
- Watson, C.; Whittaker, S.; Smith, N.; Vora, A.J.; Dumonde, D.C.; Brown, K.A. IL-6 acts on endothelial cells to preferentially increase their lymphocyte adherence. Clin. Exp. Immunol. 1996, 105, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Virgili, F.; Cervellati, C.; Pecorelli, A. OxInflammation: From Subclinical Condition to Pathological Biomarker. Front. Physiol. 2018, 9, 858. [Google Scholar] [CrossRef] [PubMed]
- Heusser, K.; Dzamonja, G.; Tank, J.; Palada, I.; Valic, Z.; Bakovic, D.; Obad, A.; Ivancev, V.; Breskovic, T.; Diedrich, A.; et al. Cardiovascular regulation during apnea in elite divers. Hypertension 2009, 53, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Bosco, G.; Rizzato, A.; Moon, R.E.; Camporesi, E.M. Environmental Physiology and Diving Medicine. Front. Psychol. 2018, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G. Extreme human breath-hold diving. Eur. J. Appl. Physiol. 2001, 84, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Muth, C.M.; Radermacher, P.; Pittner, A.; Steinacker, J.; Schabana, R.; Hamich, S.; Paulat, K.; Calzia, E. Arterial blood gases during diving in elite apnea divers. Int. J. Sport. Med. 2003, 24, 104–107. [Google Scholar] [CrossRef]
- Garbella, E.; Piarulli, A.; Fornai, E.; Pingitore, A.; Prediletto, R. Preliminary observations on the effect of hypoxic and hyperbaric stress on pulmonary gas exchange in breath-hold divers. Diving Hyperb. Med. 2011, 41, 97–100. [Google Scholar]
- Bosco, G.; Rizzato, A.; Martani, L.; Schiavo, S.; Talamonti, E.; Garetto, G.; Paganini, M.; Camporesi, E.M.; Moon, R.E. Arterial Blood Gas Analysis in Breath-Hold Divers at Depth. Front. Physiol. 2018, 9, 1558. [Google Scholar] [CrossRef]
- Paganini, M.; Moon, R.E.; Giacon, T.A.; Cialoni, D.; Martani, L.; Zucchi, L.; Garetto, G.; Talamonti, E.; Camporesi, E.M.; Bosco, G. Relative hypoxemia at depth during breath-hold diving investigated through arterial blood gas analysis and lung ultrasound. J. Appl. Physiol. 2023, 135, 863–871. [Google Scholar] [CrossRef]
- Barković, I.; Jurilj, Z.; Marinelli, F.; Maričić, V.; Pavlović, M.; Wensveen, T.T.; Peršić, V. Arterial blood gases’ analysis in elite breath-hold divers at extreme depths. Eur. J. Appl Physiol. 2023, 123, 857–865. [Google Scholar] [CrossRef]
- Scott, T.; van Waart, H.; Vrijdag, X.C.E.; Mullins, D.; Mesley, P.; Mitchell, S.J. Arterial blood gas measurements during deep open-water breath-hold dives. J. Appl. Physiol. 2021, 130, 1490–1495. [Google Scholar] [CrossRef]
- Fitz-Clarke, J.R. Mechanics of airway and alveolar collapse in human breath-hold diving. Respir. Physiol. Neurobiol. 2007, 159, 202–210. [Google Scholar] [CrossRef]
- Bosco, G.; Paganini, M.; Rizzato, A.; Martani, L.; Garetto, G.; Lion, J.; Camporesi, E.M.; Moon, R.E. Arterial blood gases in divers at the surface after prolonged breath-hold. Eur. J. Appl. Physiol. 2020, 120, 505–512. [Google Scholar] [CrossRef]
- Patrician, A.; Dujić, Z.; Spajić, B.; Drviš, I.; Ainslie, P.N. Breath-Hold Diving—The Physiology of Diving Deep and Returning. Front. Physiol. 2021, 12, 639377. [Google Scholar] [CrossRef]
- Barlow, M.J.; Elia, A.; Shannon, O.M.; Zacharogianni, A.; Lodin-Sundstrom, A. The Effect of a Dietary Nitrate Supplementation in the Form of a Single Shot of Beetroot Juice on Static and Dynamic Apnea Performance. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, S.; Schumacker, J.; Guerrero, F.; Tillmans, F.; Boutros, A.; Lambrechts, K.; Mazur, A.; Pieri, M.; Germonpre, P.; Balestra, C. Dark chocolate reduces endothelial dysfunction after successive breath-hold dives in cool water. Eur. J. Appl. Physiol. 2013, 113, 2967–2975. [Google Scholar] [CrossRef] [PubMed]
- Mrakic-Sposta, S.; Vezzoli, A.; Rizzato, A.; Della Noce, C.; Malacrida, S.; Montorsi, M.; Paganini, M.; Cancellara, P.; Bosco, G. Oxidative stress assessment in breath-hold diving. Eur. J. Appl. Physiol. 2019, 119, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Dolscheid-Pommerich, R.C.; Weikert, J.; Reinicke, M.; Fimmers, R.; Stoffel-Wagner, B.; Erdfelder, F.; Ceglarek, U.; Eichhorn, L. Changes in PUFA and eicosanoid metabolism during/after apnea diving: A prospective single-center study. Undersea Hyperb. Med. 2020, 47, 539–549. [Google Scholar] [CrossRef]
- Data, P.G.; Di Tano, G.; Arborelius, M., Jr.; Polidoro, G.; Arduini, A. Change in plasma amino acid concentrations during breath-hold diving at high altitude. Clin. Physiol. Biochem. 1988, 6, 327–333. [Google Scholar]
- Cialoni, D.; Brizzolari, A.; Sponsiello, N.; Lancellotti, V.; Bosco, G.; Marroni, A.; Barassi, A. Serum Amino Acid Profile Changes After Repetitive Breath-Hold Dives: A Preliminary Study. Sport. Med. Open 2022, 8, 80. [Google Scholar] [CrossRef]
- Zaccaria, M.; Borea, P.A.; Opocher, G.; Ponchia, A.; Varani, K.; Fraccarollo, D.; Scandellari, C. Effects of high-altitude chronic Hypoxia on platelet alpha 2-receptors in man. Eur. J. Clin. Investig. 1997, 27, 316–321. [Google Scholar] [CrossRef]
- Bosco, G.; Yang, Z.J.; Savini, F.; Nubile, G.; Data, P.G.; Wang, J.P.; Camporesi, E.M. Environmental stress on diving-induced platelet activation. Undersea Hyperb. Med. 2001, 28, 207–211. [Google Scholar]
- Goebel, M.U.; Mills, P.J.; Irwin, M.R.; Ziegler, M.G. Interleukin-6 and tumor necrosis factor-alpha production after acute psychological stress, exercise, and infused isoproterenol: Differential effects and pathways. Psychosom. Med. 2000, 62, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Kurose, I.; Anderson, D.C.; Miyasaka, M.; Tamatani, T.; Paulson, J.C.; Todd, R.F.; Rusche, J.R.; Granger, D.N. Molecular determinants of reperfusion-induced leukocyte adhesion and vascular protein leakage. Circ. Res. 1994, 74, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Lievens, D.; Zernecke, A.; Seijkens, T.; Soehnlein, O.; Beckers, L.; Munnix, I.C.; Wijnands, E.; Goossens, P.; van Kruchten, R.; Thevissen, L.; et al. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood 2010, 116, 4317–4327. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Oku, T.; Tsuji, T. Platelets attenuate production of cytokines and nitric oxide by macrophages in response to bacterial endotoxin. Platelets 2016, 27, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Gudbrandsdottir, S.; Hasselbalch, H.C.; Nielsen, C.H. Activated platelets enhance IL-10 secretion and reduce TNF-alpha secretion by monocytes. J. Immunol. 2013, 191, 4059–4067. [Google Scholar] [CrossRef] [PubMed]
- Eftedal, I.; Flatberg, A.; Davis, I.; Dujic, Z. Immune and inflammatory responses to freediving calculated from leukocyte gene expression profiles. Physiol. Genom. 2016, 48, 795–802. [Google Scholar] [CrossRef]
- Wang, Z.L.; Liu, J.Y.; Zhou, C.J.; Wang, M.B.; Wang, H.Y.; Xu, Y. Risk factors and impacts on prognosis of ultrasound lung comets in patients undergoing hemodialysis. Zhonghua Yi Xue Za Zhi 2017, 97, 3796–3801. [Google Scholar] [CrossRef]
- Boussuges, A.; Coulange, M.; Bessereau, J.; Gargne, O.; Ayme, K.; Gavarry, O.; Fontanari, P.; Joulia, F. Ultrasound lung comets induced by repeated breath-hold diving, a study in underwater fishermen. Scand. J. Med. Sci. Sports 2011, 21, e384–e392. [Google Scholar] [CrossRef] [PubMed]
- Tojo, K.; Nagamine, Y.; Yazawa, T.; Mihara, T.; Baba, Y.; Ota, S.; Goto, T.; Kurahashi, K. Atelectasis causes alveolar hypoxia-induced inflammation during uneven mechanical ventilation in rats. Intensive Care Med. Exp. 2015, 3, 56. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Ramos, C.A.; Ramírez-Jirano, L.J.; Bitzer-Quintero, O.K.; Vázquez-Medina, J.P.; Gaxiola-Robles, R.; Zenteno-Savín, T. Dolphin leukocytes exhibit an attenuated cytokine response and increase heme oxygenase activity upon exposure to lipopolysaccharides. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2023, 281, 111438. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, L.; Dolscheid-Pommerich, R.; Erdfelder, F.; Ayub, M.A.; Schmitz, T.; Werner, N.; Jansen, F. Sustained apnea induces endothelial activation. Clin. Cardiol. 2017, 40, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Lichtenauer, M.; Goebel, B.; Fritzenwanger, M.; Foster, M.; Betge, S.; Laute, A.; Figulla, H.-R.; Jung, C. Simulated temporary hypoxia triggers the release of CD31+/Annexin+ endothelial microparticles: A prospective pilot study in humans. Clin. Hemorheol. Microcirc. 2015, 61, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Brubakk, A.O.; Duplancic, D.; Valic, Z.; Palada, I.; Obad, A.; Bakovic, D.; Wisloff, U.; Dujic, Z. A single air dive reduces arterial endothelial function in man. J. Physiol. 2005, 566, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Obad, A.; Marinovic, J.; Ljubkovic, M.; Breskovic, T.; Modun, D.; Boban, M.; Dujic, Z. Successive deep dives impair endothelial function and enhance oxidative stress in man. Clin. Physiol. Funct. Imaging 2010, 30, 432–438. [Google Scholar] [CrossRef]
- Perovic, A.; Nikolac, N.; Braticevic, M.N.; Milcic, A.; Sobocanec, S.; Balog, T.; Dabelic, S.; Dumic, J. Does recreational scuba diving have clinically significant effect on routine haematological parameters? Biochem. Med. 2017, 27, 325–331. [Google Scholar] [CrossRef]
- Sureda, A.; Batle, J.M.; Ferrer, M.D.; Cases, N.; Aguiló, A.; Pons, A. Neutrophil tolerance to oxidative stress induced by hypoxia/reoxygenation. Free Radic. Res. 2004, 38, 1003–1009. [Google Scholar] [CrossRef]
- Bosco, G.; Yang, Z.J.; Di Tano, G.; Camporesi, E.M.; Faralli, F.; Savini, F.; Landolfi, A.; Doria, C.; Fano, G. Effect of in-water Oxygen prebreathing at different depths on decompression-induced bubble formation and platelet activation. J. Appl. Physiol. 2010, 108, 1077–1083. [Google Scholar] [CrossRef]
- Ferrer, M.D.; Sureda, A.; Batle, J.M.; Tauler, P.; Tur, J.A.; Pons, A. Scuba diving enhances endogenous antioxidant defenses in lymphocytes and neutrophils. Free Radic. Res. 2007, 41, 274–281. [Google Scholar] [CrossRef]
- Perovic, A.; Sobocanec, S.; Dabelic, S.; Balog, T.; Dumic, J. Effect of scuba diving on the oxidant/antioxidant status, SIRT1 and SIRT3 expression in recreational divers after a winter nondive period. Free Radic. Res. 2018, 52, 188–197. [Google Scholar] [CrossRef]
- Faraci, F.M.; Didion, S.P. Vascular protection: Superoxide dismutase isoforms in the vessel wall. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Batle, J.M.; Ferrer, M.D.; Mestre-Alfaro, A.; Tur, J.A.; Pons, A. Scuba diving activates vascular antioxidant system. Int. J. Sport. Med. 2012, 33, 531–536. [Google Scholar] [CrossRef]
- Bosco, G.; Giacon, T.A.; Paolocci, N.; Vezzoli, A.; Noce, C.D.; Paganini, M.; Agrimi, J.; Garetto, G.; Cialoni, D.; D’Alessandro, N.; et al. Dopamine/BDNF loss underscores narcosis cognitive impairment in divers: A proof of concept in a dry condition. Eur. J. Appl. Physiol. 2022, 123, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.; Kang, S.; Kim, S.H.; Kim, J.C.; Yang, M.; Moon, C. Brain-derived neurotropic factor and GABAergic transmission in neurodegeneration and neuroregeneration. Neural. Regen. Res. 2017, 12, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Lavoute, C.; Weiss, M.; Rostain, J.C. Alterations in nigral NMDA and GABAA receptor control of the striatal dopamine level after repetitive exposures to nitrogen narcosis. Exp. Neurol. 2008, 212, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, F.; Costalat, G.; Allinger, J.; Balestra, C. Possible causes of narcosis-like symptoms in freedivers. Undersea Hyperb. Med. 2023, 50, 85–93. [Google Scholar] [CrossRef]
- Di Pumpo, F.; Gualtiero, M.; Paganini, M.; Cialoni, D.; Giacomo, G.; Alessandro Cipriano, A.; Giacon, T.A.; Martani, L.; Camporesi, E.; Bosco, G. Comparison between Arterial Blood Gases and Oxygen Reserve Index™ in a SCUBA Diver: A Case Report. Healthcare 2023, 11, 1102. [Google Scholar] [CrossRef]
- Rocco, M.; Pelaia, P.; Di Benedetto, P.; Conte, G.; Maggi, L.; Fiorelli, S.; Mercieri, M.; Balestra, C.; De Blasi, R.A.; on behalf of ROAD Project Investigators. Inert gas narcosis in scuba diving, different gases different reactions. Eur. J. Appl. Physiol. 2019, 119, 247–255. [Google Scholar] [CrossRef]
- Lafere, P.; Balestra, C.; Hemelryck, W.; Guerrero, F.; Germonpre, P. Do Environmental Conditions Contribute to Narcosis Onset and Symptom Severity? Int. J. Sport. Med. 2016, 37, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Ersoz, G.; Ocakcioglu, B.; Bastug, M.; Ficicilar, H.; Yavuzer, S. Platelet aggregation and release function in hyperbaric oxygenation. Undersea Hyperb. Med. 1998, 25, 229–232. [Google Scholar] [PubMed]
- Hjelde, A.; Bergh, K.; Brubakk, A.O.; Iversen, O.J. Complement activation in divers after repeated air/heliox dives and its possible relevance to DCS. J. Appl. Physiol. 1995, 78, 1140–1144. [Google Scholar] [CrossRef]
- Morabito, C.; Bosco, G.; Pilla, R.; Corona, C.; Mancinelli, R.; Yang, Z.; Camporesi, E.M.; Fano, G.; Mariggio, M.A. Effect of pre-breathing Oxygen at different depth on oxidative status and calcium concentration in lymphocytes of scuba divers. Acta Physiol. 2011, 202, 69–78. [Google Scholar] [CrossRef]
- Dumić, J.; Cvetko, A.; Abramović, I.; Goreta, S.S.; Perović, A.; Bratičević, M.N.; Kifer, D.; Sinčić, N.; Gornik, O.; Žarak, M. Changes in Specific Biomarkers Indicate Cardiac Adaptive and Anti-inflammatory Response of Repeated Recreational SCUBA Diving. Front. Cardiovasc. Med. 2022, 9, 855682. [Google Scholar] [CrossRef]
- Tillmans, F.; Sharghi, R.; Noy, T.; Kähler, W.; Klapa, S.; Sartisohn, S.; Sebensm, S.; Kochm, A. Effect of Hyperoxia on the immune status of oxygen divers and endurance athletes. Free Radic. Res. 2019, 53, 522–534. [Google Scholar] [CrossRef]
- Eckmann, D.M.; Armstead, S.C. Influence of endothelial glycocalyx degradation and surfactants on air embolism adhesion. Anesthesiology 2006, 105, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Philp, R.B.; Inwood, M.J.; Warren, B.A. Interactions between gas bubbles and components of the blood: Implications in decompression sickness. Aerosp. Med. 1972, 43, 946–953. [Google Scholar]
- Philp, R.B.; Schacham, P.; Gowdey, C.W. Involvement of platelets and microthrombi in experimental decompression sickness: Similarities with disseminated intravascular coagulation. Aerosp. Med. 1971, 42, 494–502. [Google Scholar]
- Kuroiwa, K. The functional and biochemical changes of platelets in experimental decompression sickness of rabbits. Bull. Tokyo Med. Dent. Univ. 1984, 31, 73–84. [Google Scholar]
- Bennett, M.; Mitchell, S.; Dominguez, A. Adjunctive treatment of decompression illness with a non-steroidal anti-inflammatory drug (tenoxicam) reduces compression requirement. Undersea Hyperb. Med. 2003, 30, 195–205. [Google Scholar] [PubMed]
- Ersson, A.; Walles, M.; Ohlsson, K.; Ekholm, A. Chronic hyperbaric exposure activates proinflammatory mediators in humans. J. Appl. Physiol. 2002, 92, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.R.; Palombo, L.J.; Jensen, A.E.; Bernards, J.R. Efficacy of closed cell wet-suit at various depths and gas mixtures for thermoprotection during military training dives. Front. Physiol. 2023, 14, 1165196. [Google Scholar] [CrossRef]
- Li, Y.; Liu, K.; Kang, Z.M.; Sun, X.J.; Liu, W.W.; Mao, Y.F. Helium preconditioning protects against neonatal hypoxia-ischemia via nitric oxide-mediated up-regulation of antioxidants in a rat model. Behav. Brain Res. 2016, 300, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Bhopale, V.M.; Ruhela, D.; Brett, K.D.; Nugent, N.Z.; Fraser, N.K.; Levinson, S.L.; DiNubile, M.J.; Thom, S.R. Plasma gelsolin modulates the production and fate of IL-1β-containing microparticles following high-pressure exposure and decompression. J. Appl. Physiol. 2021, 130, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Balestra, C.; Arya, A.K.; Leveque, C.; Virgili, F.; Germonpre, P.; Lambrechts, K.; Lafere, P.; Thom, S.R. Varying Oxygen Partial Pressure Elicits Blood-Borne Microparticles Expressing Different Cell-Specific Proteins-Toward a Targeted Use of Oxygen? Int. J. Mol. Sci. 2022, 23, 7888. [Google Scholar] [CrossRef]
- Rocco, M.; Maggi, L.; Loffredo, C.; Pelli, M.; Di Benedetto, P.; Fiorelli, S.; Simmaco, M.; De Blasi, R.A. The impact of different gas mixtures on inflammatory responses in advanced recreational divers. Diving Hyperb. Med. 2021, 51, 140–146. [Google Scholar] [CrossRef]
- Yang, M.; Milovanova, T.N.; Bogush, M.; Uzun, G.; Bhopale, V.M.; Thom, S.R. Microparticle enlargement and altered surface proteins after air decompression are associated with inflammatory vascular injuries. J. Appl. Physiol. 2012, 112, 204–211. [Google Scholar] [CrossRef]
- Landolfi, A.; Yang, Z.J.; Savini, F.; Camporesi, E.M.; Faralli, F.; Bosco, G. Pretreatment with hyperbaric oxygenation reduces bubble formation and platelet activation. Sport Sci. Health 2006, 1, 122–128. [Google Scholar] [CrossRef]
- Camporesi, E.M.; Bosco, G. Hyperbaric oxygen pretreatment and preconditioning. Undersea Hyperb. Med. 2014, 41, 259–263. [Google Scholar]
- Dreyer, S.; Schneppendahl, J.; Moeller, F.; Koch, A.; Muth, T.; Schipke, J.D. An Updated Narrative Review on Er-gometric Systems Applied to Date in Assessing Divers’ Fitness. Healthcare 2021, 9, 1044. [Google Scholar] [CrossRef]
- Baj, Z.; Olszanski, R.; Majewska, E.; Konarski, M. The effect of air and nitrox divings on platelet activation tested by flow cytometry. Aviat. Space Environ. Med. 2000, 71, 925–928. [Google Scholar]
- Bosco, G.; Rizzato, A.; Quartesan, S.; Camporesi, E.; Mangar, D.; Paganini, M.; Cenci, L.; Malacrida, S.; Mrakic-Sposta, S.; Moretti, S.; et al. Effects of the Ketogenic diet in overweight divers breathing Enriched Air Nitrox. Sci. Rep. 2018, 8, 2655. [Google Scholar] [CrossRef]
- Gronow, G.; Kahler, W.; Koch, A.; Klause, N. Benzoate hydroxylation: A measure of oxidative stress in divers. Adv. Exp. Med. Biol. 2005, 566, 223–229. [Google Scholar] [CrossRef]
- Doubt, T.J. Cardiovascular and thermal responses to SCUBA diving. Med. Sci. Sport. Exerc. 1996, 28, 581–586. [Google Scholar] [CrossRef]
- Madden, D.; Thom, S.R.; Milovanova, T.N.; Yang, M.; Bhopale, V.M.; Ljubkovic, M.; Dujic, Z. Exercise before SCUBA diving ameliorates decompression-induced neutrophil activation. Med. Sci. Sports Exerc. 2014, 46, 1928–1935. [Google Scholar] [CrossRef] [PubMed]
- Spisni, E.; Marabotti, C.; De Fazio, L.; Valerii, M.C.; Cavazza, E.; Brambilla, S.; Hoxha, K.; L’Abbate, A.; Longobardi, P. A comparative evaluation of two decompression procedures for technical diving using inflammatory responses: Compartmental versus ratio deco. Diving Hyperb. Med. 2017, 47, 9–16. [Google Scholar] [CrossRef]
- Romsbotn, S.; Eftedal, I.; Vaag, J.R. A Work Environment Under Pressure: Psychosocial Job Demands and Resources Among Saturation Divers. Front. Public Health 2022, 10, 765197. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, S.; Deussen, A.; Berndt, D.; Schipke, J.D. How to Survive 33 min after the Umbilical of a Saturation Diver Severed at a Depth of 90 msw? Healthcare 2022, 10, 453. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.C. Rescue of a saturation diver, unconscious due to an explosion underwater. Int. Marit. Health 2021, 72, 46–48. [Google Scholar] [CrossRef]
- Gjerde, K.Ø.; Ryggvik, H. On the Edge, Under Water: Offshore Diving in Norway, 2nd ed.; Wigestrand Forlag: Stavanger, Norway, 2014. [Google Scholar]
- Ramnefjell, M.P.; Morild, I.; Mørk, S.J.; Lilleng, P.K. Fatal diving accidents in western Norway 1983–2007. Forensic Sci. Int. 2012, 223, e22–e26. [Google Scholar] [CrossRef]
- Keatinge, W.R.; Hayward, M.G.; McIver, N.K. Hypothermia during saturation diving in the North Sea. Br. Med. J. 1980, 280, 291. [Google Scholar] [CrossRef]
- Beyerstein, G.; Lang, M.A.; Smith, N.E. Commercial Diving: Surface-Mixed Gas, Sur-D-O2, Bell Bounce, Saturation. In Proceedings of the Advanced Scientific Diving Workshop, Washington, DC, USA, 23–24 February 2006. [Google Scholar]
- Brubakk, A.O.; Ross, J.A.; Thom, S.R. Saturation diving; physiology and pathophysiology. Compr. Physiol. 2014, 4, 1229–1272. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; D’Alessandro, F.; Paganini, M.; Dellanoce, C.; Cialoni, D.; Bosco, G. Change in Oxidative Stress Biomarkers During 30 Days in Saturation Dive: A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 7118. [Google Scholar] [CrossRef] [PubMed]
- Zwart, S.R.; Jessup, J.M.; Ji, J.; Smith, S.M. Saturation Diving Alters Folate Status and Biomarkers of DNA Damage and Repair. PLoS ONE 2012, 7, e31058. [Google Scholar] [CrossRef]
- Imbert, J.P.; Balestra, C.; Kiboub, F.Z.; Loennechen, Ø.; Eftedal, I. Commercial Divers’ Subjective Evaluation of Saturation. Front. Psychol. 2019, 9, 2774. [Google Scholar] [CrossRef]
- Monnoyer, R.; Eftedal, I.; Hjelde, A.; Deb, S.; Haugum, K.; Lautridou, J. Functional Profiling Reveals Altered Metabolic Activity in Divers’ Oral Microbiota During Commercial Heliox Saturation Diving. Front. Physiol. 2021, 12, 702634. [Google Scholar] [CrossRef]
- Sureda, A.; Batle, J.M.; Capo, X.; Martorell, M.; Cordova, A.; Tur, J.A.; Pons, A. Scuba diving induces nitric oxide synthesis and the expression of inflammatory and regulatory genes of the immune response in neutrophils. Physiol. Genom. 2014, 46, 647–654. [Google Scholar] [CrossRef]
- Krog, J.; Tønnesen, E.K.; Jepsen, C.F.; Parner, E.; Segadal, K.; Hope, A.; Ulvik, R.J.; Holland, M.E. Nural killer cells as biomarkers of hyperbaric stress during a dry heliox saturation dive. Aviat. Space Environ. Med. 2010, 81, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Domoto, H.; Iwaya, K.; Ikomi, F.; Matsuo, H.; Tadano, Y.; Fujii, S.; Tachi, K.; Itoh, Y.; Sato, M.; Inoue, K.; et al. Upregulation of antioxidant proteins in the plasma proteome during saturation diving: Unique coincidence under hypobaric Hypoxia. PLoS ONE 2016, 11, e0163804. [Google Scholar] [CrossRef] [PubMed]
- Djurhuus, R.; Segadal, K.; Svardal, A.M. Glutathione in blood cells decreases without DNA breaks after a simulated saturation dive to 250 msw. Aviat. Space Environ. Med. 2006, 77, 597–604. [Google Scholar]
- Kiboub, F.Z.; Møllerløkken, A.; Hjelde, A.; Flatberg, A.; Loennechen, Ø.; Eftedal, I. Blood Gene Expression and Vascular Function Biomarkers in Professional Saturation Diving. Front. Physiol. 2018, 9, 937. [Google Scholar] [CrossRef]
- Smith, S.M.; Davis-Street, J.E.; Fesperman, J.V.; Smith, M.D.; Rice, B.L.; Zwart, S.R. Nutritional assessment during a 14-d saturation dive: The NASA Extreme Environment Mission Operations V Project. J. Nutr. 2004, 134, 1765–1771. [Google Scholar] [CrossRef]
- Ikeda, M.; Nakabayashi, K.; Shinkai, M.; Hara, Y.; Kizaki, T.; Oh-Ishi, S.; Ohno, H. Supplementation of antioxidants prevents oxidative stress during a deep saturation dive. Tohoku J. Exp. Med. 2004, 203, 353–357. [Google Scholar] [CrossRef]
- Deb, S.K.; Swinton, P.A.; Dolan, E. Nutritional considerations during prolonged exposure to a confined, hyperbaric, hyperoxic environment: Recommendations for saturation divers. Extrem. Physiol. Med. 2016, 5, 1. [Google Scholar] [CrossRef]
- Deb, S.K.; Dolan, E.; Hambly, C.; Speakman, J.R.; Eftedal, O.; Zariwala, M.G.; Eftedal, I. The Assessment of Daily Energy Expenditure of Commercial Saturation Divers Using Doubly Labelled Water. Front. Physiol. 2021, 12, 687605. [Google Scholar] [CrossRef]
- Monnoyer, R.; Lautridou, J.; Deb, S.; Hjelde, A.; Eftedal, I. Using Salivary Biomarkers for Stress Assessment in Offshore Saturation Diving: A Pilot Study. Front. Physiol. 2021, 12, 791525. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Alhayaza, R.; Haque, E.; Karbasiafshar, C.; Sellke, F.W.; Abid, M.R. The Relationship Between Reactive Oxygen Species and Endothelial Cell Metabolism. Front. Chem. 2020, 8, 592688. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, E.; Ananiev, J.; Yovchev, Y.; Arabadzhiev, G.; Abrashev, H.; Abrasheva, D.; Atanasov, V.; Kostandieva, R.; Mitev, M.; Petkova-Parlapanska, K.; et al. COVID-19 Complications: Oxidative Stress, Inflammation, and Mitochondrial and Endothelial Dysfunction. Int. J. Mol. Sci. 2023, 24, 14876. [Google Scholar] [CrossRef]
- Comhair, S.A.; Erzurum, S.C. Antioxidant responses to oxidant-mediated lung diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, 246–255. [Google Scholar] [CrossRef]
- Gempp, E.; Blatteau, J.E. Preconditioning Methods and Mechanisms for Preventing the Risk of Decompression Sickness in Scuba Divers: A Review. Res. Sports Med. 2010, 18, 205–218. [Google Scholar] [CrossRef]
- Winkler, B.; Muth, C.M.; Piepho, T. Hyperbare Therapie und Tauchmedizin—Tauchmedizin: Status quo und Ausblick. Anästhesiol Intensivmed Notfallmed Schmerzther 2015, 50, 638–646. [Google Scholar] [CrossRef]
- Madden, D.; Thom, S.R.; Dujic, Z. Exercise before and after SCUBA diving and the role of cellular microparticles in decompression stress. Med. Hypotheses 2016, 86, 80–84. [Google Scholar] [CrossRef]
- Held, H.E.; Pendergast, D.R. The effects of respiratory muscle training on respiratory mechanics and energy cost. Respir. Physiol. Neurobiol. 2014, 200, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Germonpré, P.; Balestra, C. Preconditioning to Reduce Decompression Stress in Scuba Divers. Aerosp. Med. Hum. Perform. 2017, 88, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Šegrt Ribičić, I.; Valić, M.; Lušić Kalcina, L.; Božić, J.; Obad, A.; Glavaš, D.; Glavičić, I.; Valić, Z. Effects of Oxygen Prebreathing on Bubble Formation, Flow-Mediated Dilatation, and Psychomotor Performance during Trimix Dives. Sports 2024, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Barak, O.F.; Caljkusic, K.; Hoiland, R.L.; Ainslie, P.N.; Thom, S.R.; Yang, M.; Jovanov, P.; Dujic, Z. Differential influence of vitamin C on the peripheral and cerebral circulation after diving and exposure to hyperoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R759–R767. [Google Scholar] [CrossRef]
- Thiele, R.H. Subcellular Energetics and Metabolism: Potential Therapeutic Applications. Obstet. Anesthesia Dig. 2017, 124, 1872–1885. [Google Scholar] [CrossRef]
- Arieli, R. Nanobubbles Form at Active Hydrophobic Spots on the Luminal Aspect of Blood Vessels: Consequences for Decompression Illness in Diving and Possible Implications for Autoimmune Disease—An Overview. Front. Physiol. 2017, 8, 591. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Barak, O.F.; Dujic, Z.; Madden, D.; Bhopale, V.M.; Bhullar, J.; Thom, S.R. Ascorbic acid supplementation diminishes microparticle elevations and neutrophil activation following SCUBA diving. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R338–R344. [Google Scholar] [CrossRef] [PubMed]
- Obad, A.; Valic, Z.; Palada, I.; Brubakk, A.O.; Modun, D.; Dujić, Z. Antioxidant Pretreatment and Reduced Arterial Endothelial Dysfunction After Diving. Aviat. Space Environ. Med. 2007, 78, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vezzoli, A.; Mrakic-Sposta, S.; Brizzolari, A.; Balestra, C.; Camporesi, E.M.; Bosco, G. Oxy-Inflammation in Humans during Underwater Activities. Int. J. Mol. Sci. 2024, 25, 3060. https://doi.org/10.3390/ijms25053060
Vezzoli A, Mrakic-Sposta S, Brizzolari A, Balestra C, Camporesi EM, Bosco G. Oxy-Inflammation in Humans during Underwater Activities. International Journal of Molecular Sciences. 2024; 25(5):3060. https://doi.org/10.3390/ijms25053060
Chicago/Turabian StyleVezzoli, Alessandra, Simona Mrakic-Sposta, Andrea Brizzolari, Costantino Balestra, Enrico Maria Camporesi, and Gerardo Bosco. 2024. "Oxy-Inflammation in Humans during Underwater Activities" International Journal of Molecular Sciences 25, no. 5: 3060. https://doi.org/10.3390/ijms25053060
APA StyleVezzoli, A., Mrakic-Sposta, S., Brizzolari, A., Balestra, C., Camporesi, E. M., & Bosco, G. (2024). Oxy-Inflammation in Humans during Underwater Activities. International Journal of Molecular Sciences, 25(5), 3060. https://doi.org/10.3390/ijms25053060









