Exploring the Potential of Exosomes as Biomarkers in Tuberculosis and Other Diseases
Abstract
1. Introduction
1.1. Biomarkers
1.2. Functions of Exosomal Cargo
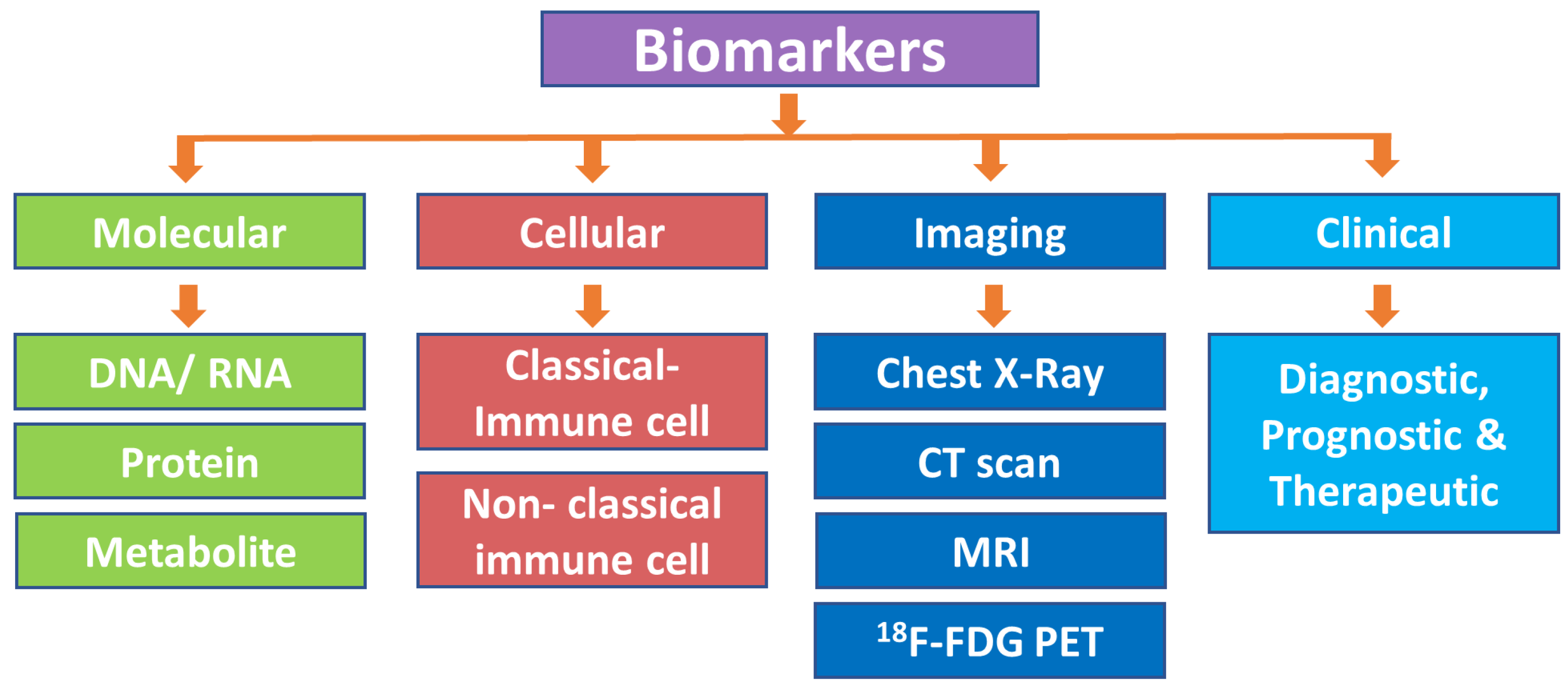
2. Classification of Biomarkers
2.1. Molecular Biomarkers
2.1.1. DNA/RNA-Based Biomarkers
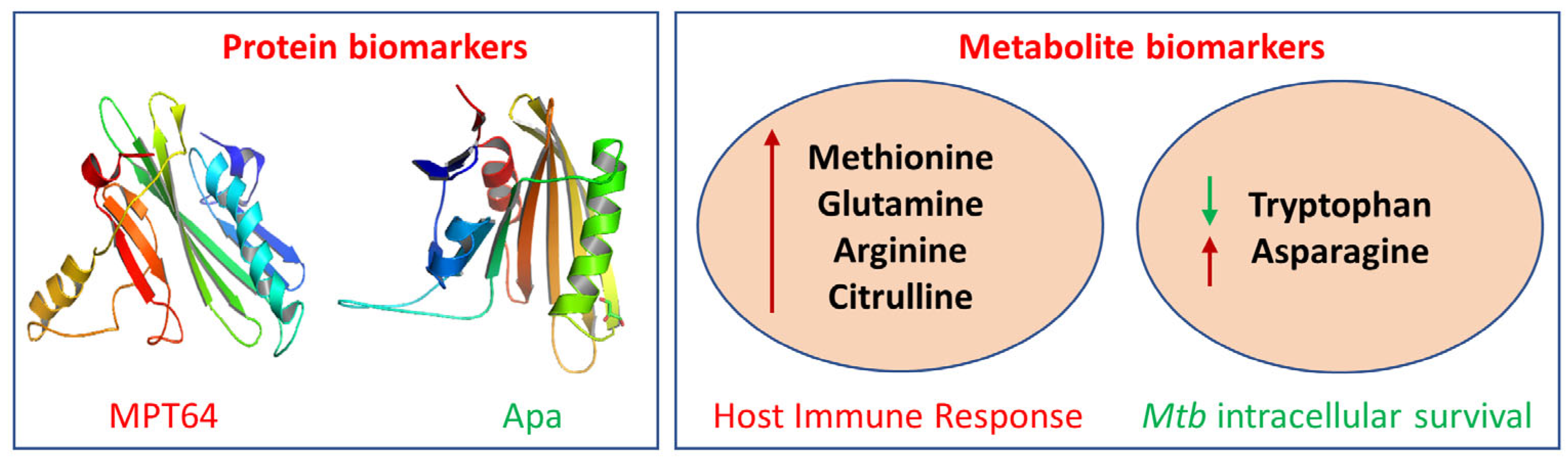
2.1.2. Protein-Based Biomarkers
2.1.3. Metabolite-Based Biomarkers
2.2. Cell-Based Biomarkers
2.2.1. Classical Immune Cell-Based Biomarkers
2.2.2. Nonclassical Immune Cell-Based Biomarkers
2.3. Imaging-Based Biomarkers
2.4. Clinical Application-Based Prognostic, Diagnostic, and Therapeutic Biomarkers
3. Methods of Isolating Exosomes from Biofluids
4. Development of Biomarkers: Discovery and Validation Process
5. Future Perspectives and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Frahm, M.; Goswami, N.D.; Owzar, K.; Hecker, E.; Mosher, A.; Cadogan, E.; Nahid, P.; Ferrari, G.; Stout, J.E. Discriminating between Latent and Active Tuberculosis with Multiple Biomarker Responses. Tuberculosis 2011, 91, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Bodaghi, A.; Fattahi, N.; Ramazani, A. Biomarkers: Promising and Valuable Tools towards Diagnosis, Prognosis and Treatment of COVID-19 and Other Diseases. Heliyon 2023, 9, e13323. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.; Bartok, B.; Oler, E.; Liang, K.; Budinski, Z.; Berjanskii, M.; Guo, A.; Cao, X.; Wilson, M. MarkerDB: An Online Database of Molecular Biomarkers. Nucleic Acids Res. 2021, 49, D1259–D1267. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yao, Y.; Qian, Y.; Qiu, D.; Cao, H.; Xiang, H.; Wang, J. Cargoes of Exosomes Function as Potential Biomarkers for Mycobacterium Tuberculosis Infection. Front. Immunol. 2023, 14, 1254347. [Google Scholar] [CrossRef]
- Cannas, A.; Goletti, D.; Girardi, E.; Chiacchio, T.; Calvo, L.; Cuzzi, G.; Piacentini, M.; Melkonyan, H.; Umansky, S.R.; Lauria, F.N. Mycobacterium Tuberculosis DNA Detection in Soluble Fraction of Urine from Pulmonary Tuberculosis Patients. Int. J. Tuberc. Lung Dis. 2008, 12, 146–151. [Google Scholar]
- Minion, J.; Leung, E.; Talbot, E.; Dheda, K.; Pai, M.; Menzies, D. Diagnosing Tuberculosis with Urine Lipoarabinomannan: Systematic Review and Meta-Analysis. Eur. Respir. J. 2011, 38, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Walzl, G.; Ronacher, K.; Hanekom, W.; Scriba, T.J.; Zumla, A. Immunological Biomarkers of Tuberculosis. Nat. Rev. Immunol. 2011, 11, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Wanchu, A.; Dong, Y.; Sethi, S.; Myneedu, V.P.; Nadas, A.; Liu, Z.; Belisle, J.; Laal, S. Biomarkers for Clinical and Incipient Tuberculosis: Performance in a TB-Endemic Country. PLoS ONE 2008, 3, e2071. [Google Scholar] [CrossRef]
- Xu, F.; Ni, M.; Qu, S.; Duan, Y.; Zhang, H.; Qin, Z. Molecular Markers of Tuberculosis and Their Clinical Relevance: A Systematic Review and Meta-Analysis. Ann. Palliat. Med. 2022, 11, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Bjerrum, S.; Schiller, I.; Dendukuri, N.; Kohli, M.; Nathavitharana, R.R.; Zwerling, A.A.; Denkinger, C.M.; Steingart, K.R.; Shah, M. Lateral Flow Urine Lipoarabinomannan Assay for Detecting Active Tuberculosis in People Living with HIV. Cochrane Database Syst. Rev. 2019, 2019, CD011420. [Google Scholar] [CrossRef] [PubMed]
- Villageliu, D.N.; Samuelson, D.R. The Role of Bacterial Membrane Vesicles in Human Health and Disease. Front. Microbiol. 2022, 13, 828704. [Google Scholar] [CrossRef]
- Gan, Y.; Zhao, G.; Wang, Z.; Zhang, X.; Wu, M.X.; Lu, M. Bacterial Membrane Vesicles: Physiological Roles, Infection Immunology, and Applications. Adv. Sci. 2023, 10, 2301357. [Google Scholar] [CrossRef] [PubMed]
- Schorey, J.S.; Cheng, Y.; Singh, P.P.; Smith, V.L. Exosomes and Other Extracellular Vesicles in Host–Pathogen Interactions. EMBO Rep. 2015, 16, 24–43. [Google Scholar] [CrossRef]
- Arteaga-Blanco, L.A.; Bou-Habib, D.C. The Role of Extracellular Vesicles from Human Macrophages on Host-Pathogen Interaction. Int. J. Mol. Sci. 2021, 22, 10262. [Google Scholar] [CrossRef]
- Giri, P.K.; Schorey, J.S. Exosomes Derived from M. Bovis BCG Infected Macrophages Activate Antigen-Specific CD4+ and CD8+ T Cells In Vitro and In Vivo. PLoS ONE 2008, 3, e2461. [Google Scholar] [CrossRef] [PubMed]
- Schorey, J.S.; Bhatnagar, S. Exosome Function: From Tumor Immunology to Pathogen Biology. Traffic 2008, 9, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Kruh-Garcia, N.; Wolfe, L.; Dobos, K. Deciphering the Role of Exosomes in Tuberculosis. Tuberculosis 2015, 95, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Kruh-Garcia, N.A.; Wolfe, L.M.; Chaisson, L.H.; Worodria, W.O.; Nahid, P.; Schorey, J.S.; Davis, J.L.; Dobos, K.M. Detection of Mycobacterium Tuberculosis Peptides in the Exosomes of Patients with Active and Latent M. Tuberculosis Infection Using MRM-MS. PLoS ONE 2014, 9, e103811. [Google Scholar] [CrossRef] [PubMed]
- Holme, P.A.; Solum, N.O.; Brosstad, F.; Røger, M.; Abdelnoor, M. Demonstration of Platelet-Derived Microvesicles in Blood from Patients with Activated Coagulation and Fibrinolysis Using a Filtration Technique and Western Blotting. Thromb. Haemost. 1994, 72, 666–671. [Google Scholar] [PubMed]
- Hess, C.; Sadallah, S.; Hefti, A.; Landmann, R.; Schifferli, J.A. Ectosomes Released by Human Neutrophils Are Specialized Functional Units. J. Immunol. 1999, 163, 4564–4573. [Google Scholar] [CrossRef]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding Microvesicles: Artefacts No More. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar] [CrossRef]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, É.; Pap, E.; Kittel, Á.; et al. Membrane Vesicles, Current State-of-the-Art: Emerging Role of Extracellular Vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Biadglegne, F.; König, B.; Rodloff, A.C.; Dorhoi, A.; Sack, U. Composition and Clinical Significance of Exosomes in Tuberculosis: A Systematic Literature Review. J. Clin. Med. 2021, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-F.; Pi, J.; Xu, J.-F. Emerging Role of Exosomes in Tuberculosis: From Immunity Regulations to Vaccine and Immunotherapy. Front. Immunol. 2021, 12, 628973. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.P.; LeMaire, C.; Tan, J.C.; Zeng, E.; Schorey, J.S. Exosomes Released from M.Tuberculosis Infected Cells Can Suppress IFN-γ Mediated Activation of Naïve Macrophages. PLoS ONE 2011, 6, e18564. [Google Scholar] [CrossRef]
- Biadglegne, F.; Schmidt, J.R.; Engel, K.M.; Lehmann, J.; Lehmann, R.T.; Reinert, A.; König, B.; Schiller, J.; Kalkhof, S.; Sack, U. Mycobacterium Tuberculosis Affects Protein and Lipid Content of Circulating Exosomes in Infected Patients Depending on Tuberculosis Disease State. Biomedicines 2022, 10, 783. [Google Scholar] [CrossRef]
- Jan, A.; Rahman, S.; Khan, S.; Tasduq, S.; Choi, I. Biology, Pathophysiological Role, and Clinical Implications of Exosomes: A Critical Appraisal. Cells 2019, 8, 99. [Google Scholar] [CrossRef]
- Buzas, E.; György, B.; Nagy, G.; Falus, A.; Gay, S. Emerging Role of Extracellular Vesicles in Inflammatory Diseases. Nat. Rev. Rheumatol. 2014, 10, 356–364. [Google Scholar] [CrossRef]
- Simpson, R.J.; Lim, J.W.E.; Moritz, R.L.; Mathivanan, S. Exosomes: Proteomic Insights and Diagnostic Potential. Expert. Rev. Proteom. 2009, 6, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Cocucci, E.; Meldolesi, J. Ectosomes. Curr. Biol. 2011, 21, R940–R941. [Google Scholar] [CrossRef]
- Battistelli, M.; Falcieri, E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Sivanantham, A.; Life, Y.J. Impact of Storage Conditions on EV Integrity/Surface Markers and Cargos. Life 2022, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Mehaffy, C.; Kruh-Garcia, N.A.; Graham, B.; Jarlsberg, L.G.; Willyerd, C.E.; Borisov, A.; Sterling, T.R.; Nahid, P.; Dobos, K.M. Identification of Mycobacterium Tuberculosis Peptides in Serum Extracellular Vesicles from Persons with Latent Tuberculosis Infection. J. Clin. Microbiol. 2020, 58, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Jensen, S.S.; Lim, J.W.E. Proteomic Profiling of Exosomes: Current Perspectives. Proteomics 2008, 8, 4083–4099. [Google Scholar] [CrossRef]
- Maguire, G. Exosomes: Smart Nanospheres for Drug Delivery Naturally Produced by Stem Cells. In Fabrication and Self-Assembly of Nanobiomaterials; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 179–209. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.; Lötvall, J. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Thakur, B.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-Stranded DNA in Exosomes: A Novel Biomarker in Cancer Detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef]
- Li, X.B.; Zhang, Z.R.; Schluesener, H.J.; Xu, S.Q. Role of Exosomes in Immune Regulation. J. Cell Mol. Med. 2006, 10, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Grizzle, W. Exosomes: A Novel Pathway of Local and Distant Intercellular Communication That Facilitates the Growth and Metastasis of Neoplastic Lesions. Am. J. Pathol. 2014, 184, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Boussac, M.; Véron, P.; Ricciardi-Castagnoli, P.; Raposo, G.; Garin, J.; Amigorena, S. Proteomic Analysis of Dendritic Cell-Derived Exosomes: A Secreted Subcellular Compartment Distinct from Apoptotic Vesicles. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.M.; Carneiro, F.; Machado, J.C.; Melo, S.A. Exosomes and Immune Response in Cancer: Friends or Foes? Front. Immunol. 2018, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular Vesicles as Tools and Targets in Therapy for Diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Bertolone, L.; Castagna, A.; Manfredi, M.; De Santis, D.; Ambrosani, F.; Antinori, E.; Mulatero, P.; Danese, E.; Marengo, E.; Barberis, E.; et al. Proteomic Analysis of Urinary Extracellular Vesicles Highlights Specific Signatures for Patients with Primary Aldosteronism. Front. Endocrinol. 2023, 14, 1096441. [Google Scholar] [CrossRef]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and Proteomic Profiling of Exosomes in Human Urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hill, S.; Luther, J.M.; Hachey, D.L.; Schey, K.L. Proteomic Analysis of Urine Exosomes by Multidimensional Protein Identification Technology (MudPIT). Proteomics 2012, 12, 329–338. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-Mediated Isothermal Amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- O’Grady, J.; Bates, M.; Chilukutu, L.; Mzyece, J.; Cheelo, B.; Chilufya, M.; Mukonda, L.; Mumba, M.; Tembo, J.; Chomba, M.; et al. Evaluation of the Xpert MTB/RIF Assay at a Tertiary Care Referral Hospital in a Setting Where Tuberculosis and HIV Infection Are Highly Endemic. Clin. Infect. Dis. 2012, 55, 1171–1178. [Google Scholar] [CrossRef]
- Kraus, G.; Cleary, T.; Miller, N.; Seivright, R.; Young, A.; Spruill, G.; Hnatyszyn, H. Rapid and Specific Detection of the Mycobacterium Tuberculosis Complex Using Fluorogenic Probes Andreal-Time PCR. Mol. Cell Probes 2001, 15, 375–383. [Google Scholar] [CrossRef]
- Cho, S.M.; Shin, S.; Kim, Y.; Song, W.; Hong, S.G.; Jeong, S.H.; Kang, M.S.; Lee, K.A. A Novel Approach for Tuberculosis Diagnosis Using Exosomal DNA and Droplet Digital PCR. Clin. Microbiol. Infect. 2020, 26, e1–e942. [Google Scholar] [CrossRef]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of MicroRNAs in Serum: A Novel Class of Biomarkers for Diagnosis of Cancer and Other Diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef]
- Alipoor, S.D.; Mortaz, E.; Tabarsi, P.; Farnia, P.; Mirsaeidi, M.; Garssen, J.; Movassaghi, M.; Adcock, I.M. Bovis Bacillus Calmette–Guerin (BCG) Infection Induces Exosomal MiRNA Release by Human Macrophages. J. Transl. Med. 2017, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.P.; Li, L.; Schorey, J.S. Exosomal RNA from Mycobacterium Tuberculosis-infected Cells Is Functional in Recipient Macrophages. Traffic 2015, 16, 555–571. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Zhang, X.; Li, C.; Yang, T.; Wang, J.; Pan, L.; Jia, H.; Li, Z.; Sun, Q.; Yue, L.; et al. Small RNA Profiles of Serum Exosomes Derived from Individuals with Latent and Active Tuberculosis. Front. Microbiol. 2019, 10, 1174. [Google Scholar] [CrossRef]
- Alipoor, S.D.; Tabarsi, P.; Varahram, M.; Movassaghi, M.; Dizaji, M.K.; Folkerts, G.; Garssen, J.; Adcock, I.M.; Mortaz, E. Serum Exosomal MiRNAs Are Associated with Active Pulmonary Tuberculosis. Dis. Markers 2019, 2019, 1907426. [Google Scholar] [CrossRef] [PubMed]
- Beatty, W.L.; Russell, D.G. Identification of Mycobacterial Surface Proteins Released into Subcellular Compartments of Infected Macrophages. Infect. Immun. 2000, 68, 6997–7002. [Google Scholar] [CrossRef] [PubMed]
- Kruh-Garcia, N.A.; Schorey, J.S.; Dobos, K.M. Exosomes: New Tuberculosis Biomarkers-Prospects from the Bench to the Clinic. In Understanding Tuberculosis: Global Experiences and Innovative Approaches to the Diagnosis; In Tech: Rijeka, Croatia, 2012; pp. 395–410. [Google Scholar]
- Giri, P.K.; Kruh, N.A.; Dobos, K.M.; Schorey, J.S. Proteomic Analysis Identifies Highly Antigenic Proteins in Exosomes from M. Tuberculosis-infected and Culture Filtrate Protein-treated Macrophages. Proteomics 2010, 10, 3190–3202. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.-J.; Li, Y.-P.; Wang, J.-Y.; Zhou, J.; Guo, X.-G. MPT64 Assays for the Rapid Detection of Mycobacterium Tuberculosis. BMC Infect. Dis. 2021, 21, 336. [Google Scholar] [CrossRef]
- Satchidanandam, V.; Kumar, N.; Biswas, S.; Jumani, R.S.; Jain, C.; Rani, R.; Aggarwal, B.; Singh, J.; Kotnur, M.R.; Sridharan, A. The Secreted Protein Rv1860 of Mycobacterium Tuberculosis Stimulates Human Polyfunctional CD8+ T Cells. Clin. Vaccine Immunol. 2016, 23, 282–293. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Shinagawa, K.; Castellino, F.J.; Schorey, J.S. Exosomes Released from Macrophages Infected with Intracellular Pathogens Stimulate a Proinflammatory Response In Vitro and In Vivo. Blood 2007, 110, 3234–3244. [Google Scholar] [CrossRef]
- Laulagnier, K.; Motta, C.; Hamdi, S.; Roy, S.; Fauvelle, F.; Pageaux, J.-F.; Kobayashi, T.; Salles, J.-P.; Perret, B.; Bonnerot, C. Mast Cell-and Dendritic Cell-Derived Exosomes Display a Specific Lipid Composition and an Unusual Membrane Organization. Biochem. J. 2004, 380, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Bard, M.P.; Hegmans, J.P.; Hemmes, A.; Luider, T.M.; Willemsen, R.; Severijnen, L.-A.A.; van Meerbeeck, J.P.; Burgers, S.A.; Hoogsteden, H.C.; Lambrecht, B.N. Proteomic Analysis of Exosomes Isolated from Human Malignant Pleural Effusions. Am. J. Respir. Cell Mol. Biol. 2004, 31, 114–121. [Google Scholar] [CrossRef]
- Thomas, C.E.; Sexton, W.; Benson, K.; Sutphen, R.; Koomen, J. Urine Collection and Processing for Protein Biomarker Discovery and Quantification. Cancer Epidemiol. Biomark. Prev. 2010, 19, 953–959. [Google Scholar] [CrossRef]
- Mears, R.; Craven, R.A.; Hanrahan, S.; Totty, N.; Upton, C.; Young, S.L.; Patel, P.; Selby, P.J.; Banks, R.E. Proteomic Analysis of Melanoma-derived Exosomes by Two-dimensional Polyacrylamide Gel Electrophoresis and Mass Spectrometry. Proteomics 2004, 4, 4019–4031. [Google Scholar] [CrossRef]
- Koyama, Y.; Ito, T.; Hasegawa, A.; Eriguchi, M.; Inaba, T.; Ushigusa, T.; Sugiura, K. Exosomes Derived from Tumor Cells Genetically Modified to Express Mycobacterium Tuberculosis Antigen: A Novel Vaccine for Cancer Therapy. Biotechnol. Lett. 2016, 38, 1857–1866. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.; Choi, D.; Lee, J.S.; Kim, D.; Go, G.; Park, S.; Kim, S.H.; Shin, J.H.; Chang, C.L.; et al. Proteomic Analysis of Extracellular Vesicles Derived from Mycobacterium Tuberculosis. Proteomics 2015, 15, 3331–3337. [Google Scholar] [CrossRef] [PubMed]
- Diaz, G.; Wolfe, L.M.; Kruh-Garcia, N.A.; Dobos, K.M. Changes in the Membrane-Associated Proteins of Exosomes Released from Human Macrophages after Mycobacterium Tuberculosis Infection. Sci. Rep. 2016, 6, 37975. [Google Scholar] [CrossRef]
- Huang, C.; Pan, L.; Shen, X.; Tian, H.; Guo, L.; Zhang, Z.; Liu, X. Hsp16. 3 of Mycobacterium Tuberculosis in Exosomes as a Biomarker of Tuberculosis. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2427–2430. [Google Scholar] [CrossRef] [PubMed]
- Wallis, R.S.; Perkins, M.; Phillips, M.; Joloba, M.; Demchuk, B.; Namale, A.; Johnson, J.L.; Williams, D.; Wolski, K.; Teixeira, L. Induction of the Antigen 85 Complex of Mycobacterium Tuberculosis in Sputum: A Determinant of Outcome in Pulmonary Tuberculosis Treatment. J. Infect. Dis. 1998, 178, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, R.S.; Rajan, A.N.; Ramteke, S.S.; Agrawal, V.S.; Kelkar, S.S.; Purohit, H.J.; Taori, G.M.; Daginawala, H.F. Diagnosis of Tuberculosis in an Indian Population by an Indirect ELISA Protocol Based on Detection of Antigen 85 Complex: A Prospective Cohort Study. BMC Infect. Dis. 2007, 7, 74. [Google Scholar] [CrossRef]
- Chanteau, S.; Rasolofo, V.; Rasolonavalona, T.; Ramarokoto, H.; Horn, C.; Auregan, G.; Marchal, G. 45/47 Kilodalton (APA) Antigen Capture and Antibody Detection Assays for the Diagnosis of Tuberculosis. Int. J. Tuberc. Lung Dis. 2000, 4, 377–383. [Google Scholar] [PubMed]
- Rajan, A.N.; Kashyap, R.S.; Purohit, H.J.; Taori, G.M.; Daginawala, H.F. Serodiagnosis of Tuberculosis Based on the Analysis of the 65 KD Heat Shock Protein of Mycobacterium Tuberculosis. Int. J. Tuberc. Lung Dis. 2007, 11, 792–797. [Google Scholar]
- Haldar, S.; Sankhyan, N.; Sharma, N.; Bansal, A.; Jain, V.; Gupta, V.K.; Juneja, M.; Mishra, D.; Kapil, A.; Singh, U.B.; et al. Detection of Mycobacterium Tuberculosis GlcB or HspX Antigens or DevR DNA Impacts the Rapid Diagnosis of Tuberculous Meningitis in Children. PLoS ONE 2012, 7, e44630. [Google Scholar] [CrossRef]
- Kashino, S.S.; Pollock, N.; Napolitano, D.R.; Rodrigues, V., Jr.; Campos-Neto, A. Identification and Characterization of Mycobacterium Tuberculosis Antigens in Urine of Patients with Active Pulmonary Tuberculosis: An Innovative and Alternative Approach of Antigen Discovery of Useful Microbial Molecules. Clin. Exp. Immunol. 2008, 153, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, D.R.; Pollock, N.; Kashino, S.S.; Rodrigues, V., Jr.; Campos-Neto, A. Identification of Mycobacterium Tuberculosis Ornithine Carboamyltransferase in Urine as a Possible Molecular Marker of Active Pulmonary Tuberculosis. Clin. Vaccine Immunol. 2008, 15, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Pollock, N.R.; Macovei, L.; Kanunfre, K.; Dhiman, R.; Restrepo, B.I.; Zarate, I.; Pino, P.A.; Mora-Guzman, F.; Fujiwara, R.T.; Michel, G. Validation of Mycobacterium Tuberculosis Rv1681 Protein as a Diagnostic Marker of Active Pulmonary Tuberculosis. J. Clin. Microbiol. 2013, 51, 1367–1373. [Google Scholar] [CrossRef]
- Young, B.L.; Mlamla, Z.; Gqamana, P.P.; Smit, S.; Roberts, T.; Peter, J.; Theron, G.; Govender, U.; Dheda, K.; Blackburn, J. The Identification of Tuberculosis Biomarkers in Human Urine Samples. Eur. Respir. J. 2014, 43, 1719–1729. [Google Scholar] [CrossRef]
- Kashyap, R.S.; Ramteke, S.S.; Morey, S.H.; Purohit, H.J.; Taori, G.M.; Daginawala, H.F. Diagnostic Value of Early Secreted Antigenic Target-6 for the Diagnosis of Tuberculous Meningitis Patients. Infection 2009, 37, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Nayak, A.R.; Husain, A.A.; Panchbhai, M.S.; Chandak, N.; Purohit, H.J.; Taori, G.M.; Daginawala, H.F.; Kashyap, R.S. Mycobacterial Dormancy Regulon Protein Rv2623 as a Novel Biomarker for the Diagnosis of Latent and Active Tuberculous Meningitis. Dis. Markers 2013, 35, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.M.; Siddiqa, A.; Jones, D.P.; Liu, K.; Kempker, R.R.; Nizam, A.; Shah, N.S.; Ismail, N.; Ouma, S.G.; Tukvadze, N. Tryptophan Catabolism Reflects Disease Activity in Human Tuberculosis. JCI Insight 2020, 5, e137131. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, X.-X.; Li, J.-C. Biomarker Discovery for Tuberculosis Using Metabolomics. Front. Mol. Biosci. 2023, 10, 1099654. [Google Scholar] [CrossRef]
- Amalia, F.; Syamsunarno, M.; Triatin, R.; Fatimah, S.; Chaidir, L.; Achmad, T. The Role of Amino Acids in Tuberculosis Infection: A Literature Review. Metabolites 2022, 12, 933. [Google Scholar] [CrossRef]
- Sinclair, L.V.; Howden, A.J.M.; Brenes, A.; Spinelli, L.; Hukelmann, J.L.; Macintyre, A.N.; Liu, X.; Thomson, S.; Taylor, P.M.; Rathmell, J.C.; et al. Antigen Receptor Control of Methionine Metabolism in T Cells. eLife 2019, 8, e44210. [Google Scholar] [CrossRef]
- Cho, Y.; Park, Y.; Sim, B.; Kim, J.; Lee, H.; Cho, S.-N.; Kang, Y.A.; Lee, S.-G. Identification of Serum Biomarkers for Active Pulmonary Tuberculosis Using a Targeted Metabolomics Approach. Sci. Rep. 2020, 10, 3825. [Google Scholar] [CrossRef]
- Chambers, J.W.; Maguire, T.G.; Alwine, J.C. Glutamine Metabolism Is Essential for Human Cytomegalovirus Infection. J. Virol. 2010, 84, 1867–1873. [Google Scholar] [CrossRef]
- Koeken, V.A.C.M.; Lachmandas, E.; Riza, A.; Matzaraki, V.; Li, Y.; Kumar, V.; Oosting, M.; Joosten, L.A.B.; Netea, M.G.; van Crevel, R. Role of Glutamine Metabolism in Host Defense against Mycobacterium Tuberculosis Infection. J. Infect. Dis. 2019, 219, 1662–1670. [Google Scholar] [CrossRef]
- Qualls, J.E.; Murray, P.J. Immunometabolism within the Tuberculosis Granuloma: Amino Acids, Hypoxia, and Cellular Respiration. Semin. Immunopathol. 2016, 38, 139–152. [Google Scholar] [CrossRef]
- Ralph, A.P.; Waramori, G.; Pontororing, G.J.; Kenangalem, E.; Wiguna, A.; Tjitra, E.; Lolong, D.B.; Yeo, T.W.; Chatfield, M.D.; Soemanto, R.K. L-Arginine and Vitamin D Adjunctive Therapies in Pulmonary Tuberculosis: A Randomised, Double-Blind, Placebo-Controlled Trial. PLoS ONE 2013, 8, e70032. [Google Scholar] [CrossRef] [PubMed]
- Lutgens, L.; Lambin, P. Biomarkers for Radiation-Induced Small Bowel Epithelial Damage: An Emerging Role for Plasma Citrulline. World J. Gastroenterol. 2007, 13, 3033–3042. [Google Scholar] [CrossRef] [PubMed]
- Bahri, S.; Zerrouk, N.; Aussel, C.; Moinard, C.; Crenn, P.; Curis, E.; Chaumeil, J.-C.; Cynober, L.; Sfar, S. Citrulline: From Metabolism to Therapeutic Use. Nutrition 2013, 29, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Vrieling, F.; Alisjahbana, B.; Sahiratmadja, E.; van Crevel, R.; Harms, A.C.; Hankemeier, T.; Ottenhoff, T.H.M.; Joosten, S.A. Plasma Metabolomics in Tuberculosis Patients with and without Concurrent Type 2 Diabetes at Diagnosis and during Antibiotic Treatment. Sci. Rep. 2019, 9, 18669. [Google Scholar] [CrossRef] [PubMed]
- Kurpad, A. V The Requirements of Protein & Amino Acid during Acute & Chronic Infections. Indian J. Med. Res. 2006, 124, 129–148. [Google Scholar]
- Suchard, M.S.; Adu-Gyamfi, C.G.; Cumming, B.M.; Savulescu, D.M. Evolutionary Views of Tuberculosis: Indoleamine 2, 3-dioxygenase Catalyzed Nicotinamide Synthesis Reflects Shifts in Macrophage Metabolism: Indoleamine 2, 3-dioxygenase Reflects Altered Macrophage Metabolism during Tuberculosis Pathogenesis. BioEssays 2020, 42, e1900220. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Huff, J.; Janik, K.; Walter, K.; Keller, C.; Ehlers, S.; Bossmann, S.H.; Niederweis, M. Expression of the OmpATb Operon Accelerates Ammonia Secretion and Adaptation of Mycobacterium Tuberculosis to Acidic Environments. Mol. Microbiol. 2011, 80, 900–918. [Google Scholar] [CrossRef] [PubMed]
- Borah, K.; Beyß, M.; Theorell, A.; Wu, H.; Basu, P.; Mendum, T.A.; Nöh, K.; Beste, D.J.V.; McFadden, J. Intracellular Mycobacterium Tuberculosis Exploits Multiple Host Nitrogen Sources during Growth in Human Macrophages. Cell Rep. 2019, 29, 3580–3591. [Google Scholar] [CrossRef] [PubMed]
- Harari, A.; Rozot, V.; Enders, F.B.; Perreau, M.; Stalder, J.M.; Nicod, L.P.; Cavassini, M.; Calandra, T.; Blanchet, C.L.; Jaton, K. Dominant TNF-A+ Mycobacterium Tuberculosis–Specific CD4+ T Cell Responses Discriminate between Latent Infection and Active Disease. Nat. Med. 2011, 17, 372–376. [Google Scholar] [CrossRef]
- Nikolova, M.; Markova, R.; Drenska, R.; Muhtarova, M.; Todorova, Y.; Dimitrov, V.; Taskov, H.; Saltini, C.; Amicosante, M. Antigen-Specific CD4-and CD8-Positive Signatures in Different Phases of Mycobacterium Tuberculosis Infection. Diagn. Microbiol. Infect. Dis. 2013, 75, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zheng, X.; Yi, L.; Wang, J.; Wang, X.; Wei, P.; Jia, H.; Zhou, L.; Zhao, Y.; Zhang, H. CD 137 Is a Useful Marker for Identifying CD 4+ T Cell Responses to Mycobacterium Tuberculosis. Scand. J. Immunol. 2017, 85, 372–380. [Google Scholar] [CrossRef]
- Ahmed, M.I.M.; Ntinginya, N.E.; Kibiki, G.; Mtafya, B.A.; Semvua, H.; Mpagama, S.; Mtabho, C.; Saathoff, E.; Held, K.; Loose, R.; et al. Phenotypic Changes on Mycobacterium Tuberculosis-Specific CD4 T Cells as Surrogate Markers for Tuberculosis Treatment Efficacy. Front. Immunol. 2018, 9, 2247. [Google Scholar] [CrossRef]
- Streitz, M.; Tesfa, L.; Yildirim, V.; Yahyazadeh, A.; Ulrichs, T.; Lenkei, R.; Quassem, A.; Liebetrau, G.; Nomura, L.; Maecker, H. Loss of Receptor on Tuberculin-Reactive T-Cells Marks Active Pulmonary Tuberculosis. PLoS ONE 2007, 2, e735. [Google Scholar] [CrossRef]
- Adekambi, T.; Ibegbu, C.C.; Kalokhe, A.S.; Yu, T.; Ray, S.M.; Rengarajan, J. Distinct Effector Memory CD4+ T Cell Signatures in Latent Mycobacterium Tuberculosis Infection, BCG Vaccination and Clinically Resolved Tuberculosis. PLoS ONE 2012, 7, e36046. [Google Scholar] [CrossRef]
- Acharya, M.P.; Pradeep, S.P.; Murthy, V.S.; Chikkannaiah, P.; Kambar, V.; Narayanashetty, S.; Burugina Nagaraja, S.; Gangadhar, N.; Yoganand, R.; Satchidanandam, V. CD38+ CD27–TNF-A+ on Mtb-Specific CD4+ T Cells Is a Robust Biomarker for Tuberculosis Diagnosis. Clin. Infect. Dis. 2021, 73, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Estévez, O.; Anibarro, L.; Garet, E.; Pallares, Á.; Pena, A.; Villaverde, C.; Del Campo, V.; González-Fernández, Á. Identification of Candidate Host Serum and Saliva Biomarkers for a Better Diagnosis of Active and Latent Tuberculosis Infection. PLoS ONE 2020, 15, e0235859. [Google Scholar] [CrossRef]
- De Libero, G.; Singhal, A.; Lepore, M.; Mori, L. Nonclassical T Cells and Their Antigens in Tuberculosis. Cold Spring Harb. Perspect. Med. 2014, 4, a018473. [Google Scholar] [CrossRef] [PubMed]
- Nouailles, G.; Dorhoi, A.; Koch, M.; Zerrahn, J.; Weiner, J.; Faé, K.C.; Arrey, F.; Kuhlmann, S.; Bandermann, S.; Loewe, D.; et al. CXCL5-Secreting Pulmonary Epithelial Cells Drive Destructive Neutrophilic Inflammation in Tuberculosis. J. Clin. Investig. 2014, 124, 1268–1282. [Google Scholar] [CrossRef]
- Roy, S.; Sharma, S.; Sharma, M.; Aggarwal, R.; Bose, M. Induction of Nitric Oxide Release from the Human Alveolar Epithelial Cell Line A549: An in Vitro Correlate of Innate Immune Response to Mycobacterium Tuberculosis. Immunology 2004, 112, 471–480. [Google Scholar] [CrossRef]
- Petursdottir, D.H.; Chuquimia, O.D.; Freidl, R.; Fernandez, C. Macrophage Control of Phagocytosed Mycobacteria Is Increased by Factors Secreted by Alveolar Epithelial Cells through Nitric Oxide Independent Mechanisms. PLoS ONE 2014, 9, e103411. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.D. Iron Metabolism at the Host Pathogen Interface: Lipocalin 2 and the Pathogen-Associated IroA Gene Cluster. Int. J. Biochem. Cell Biol. 2007, 39, 1776–1780. [Google Scholar] [CrossRef] [PubMed]
- Saiga, H.; Nishimura, J.; Kuwata, H.; Okuyama, M.; Matsumoto, S.; Sato, S.; Matsumoto, M.; Akira, S.; Yoshikai, Y.; Honda, K. Lipocalin 2-Dependent Inhibition of Mycobacterial Growth in Alveolar Epithelium. J. Immunol. 2008, 181, 8521–8527. [Google Scholar] [CrossRef]
- Harriff, M.J.; Cansler, M.E.; Toren, K.G.; Canfield, E.T.; Kwak, S.; Gold, M.C.; Lewinsohn, D.M. Human Lung Epithelial Cells Contain Mycobacterium Tuberculosis in a Late Endosomal Vacuole and Are Efficiently Recognized by CD8+ T Cells. PLoS ONE 2014, 9, e97515. [Google Scholar] [CrossRef]
- Blomgran, R.; Ernst, J.D. Lung Neutrophils Facilitate Activation of Naive Antigen-Specific CD4+ T Cells during Mycobacterium Tuberculosis Infection. J. Immunol. 2011, 186, 7110–7119. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Pando, R.; Jeyanathan, M.; Mengistu, G.; Aguilar, D.; Orozco, H.; Harboe, M.; Rook, G.A.W.; Bjune, G. Persistence of DNA from Mycobacterium Tuberculosis in Superficially Normal Lung Tissue during Latent Infection. Lancet 2000, 356, 2133–2138. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, S.; Sargentini, V.; Pardini, M.; Giannoni, F.; De Spirito, M.; Gagliardi, M.C.; Greco, E.; Teloni, R.; Fraziano, M.; Nisini, R. Mycobacterium Tuberculosis May Escape Helper T Cell Recognition by Infecting Human Fibroblasts. Hum. Immunol. 2013, 74, 722–729. [Google Scholar] [CrossRef]
- O’Kane, C.M.; Boyle, J.J.; Horncastle, D.E.; Elkington, P.T.; Friedland, J.S. Monocyte-Dependent Fibroblast CXCL8 Secretion Occurs in Tuberculosis and Limits Survival of Mycobacteria within Macrophages. J. Immunol. 2007, 178, 3767–3776. [Google Scholar] [CrossRef] [PubMed]
- Khader, S.A.; Guglani, L.; Rangel-Moreno, J.; Gopal, R.; Fallert Junecko, B.A.; Fountain, J.J.; Martino, C.; Pearl, J.E.; Tighe, M.; Lin, Y. IL-23 Is Required for Long-Term Control of Mycobacterium Tuberculosis and B Cell Follicle Formation in the Infected Lung. J. Immunol. 2011, 187, 5402–5407. [Google Scholar] [CrossRef]
- Neyrolles, O.; Hernández-Pando, R.; Pietri-Rouxel, F.; Fornès, P.; Tailleux, L.; Payán, J.A.B.; Pivert, E.; Bordat, Y.; Aguilar, D.; Prévost, M.-C. Is Adipose Tissue a Place for Mycobacterium Tuberculosis Persistence? PLoS ONE 2006, 1, e43. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Ryu, M.-J.; Byun, E.-H.; Kim, W.S.; Whang, J.; Min, K.-N.; Shong, M.; Kim, H.-J.; Shin, S.J. Differential Immune Response of Adipocytes to Virulent and Attenuated Mycobacterium Tuberculosis. Microbes Infect. 2011, 13, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, G.; Suberbielle, E.; Monnet, C.; Duplan, V.; Martin-Blondel, G.; Farrugia, F.; Le Masson, G.; Liblau, R.; Gonzalez-Dunia, D. Neurons Are MHC Class I-Dependent Targets for CD8 T Cells upon Neurotropic Viral Infection. PLoS Pathog. 2011, 7, e1002393. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Systematic Screening for Active Tuberculosis: Principles and Recommendations; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Yeh, J.J.; Yu, J.K.-L.; Teng, W.-B.; Chou, C.-H.; Hsieh, S.-P.; Lee, T.-L.; Wu, M.-T. High-Resolution CT for Identify Patients with Smear-Positive, Active Pulmonary Tuberculosis. Eur. J. Radiol. 2012, 81, 195–201. [Google Scholar] [CrossRef]
- Sharma, A.; Chhabra, H.S.; Mahajan, R.; Chabra, T.; Batra, S. Magnetic Resonance Imaging and GeneXpert: A Rapid and Accurate Diagnostic Tool for the Management of Tuberculosis of the Spine. Asian Spine J. 2016, 10, 850–856. [Google Scholar] [CrossRef][Green Version]
- Kim, I.-J.; Lee, J.S.; Kim, S.-J.; Kim, Y.-K.; Jeong, Y.J.; Jun, S.; Nam, H.Y.; Kim, J.S. Double-Phase 18 F-FDG PET-CT for Determination of Pulmonary Tuberculoma Activity. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 808–814. [Google Scholar] [CrossRef]
- Gautam, U.S.; Foreman, T.W.; Bucsan, A.N.; Veatch, A.V.; Alvarez, X.; Adekambi, T.; Golden, N.A.; Gentry, K.M.; Doyle-Meyers, L.A.; Russell-Lodrigue, K.E.; et al. In Vivo Inhibition of Tryptophan Catabolism Reorganizes the Tuberculoma and Augments Immune-Mediated Control of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2018, 115, E62–E71. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Suda, T.; Asada, K.; Miwa, S.; Suzuki, M.; Fujie, M.; Furuhashi, K.; Nakamura, Y.; Inui, N.; Shirai, T. Serum Indoleamine 2, 3-Dioxygenase Activity Predicts Prognosis of Pulmonary Tuberculosis. Clin. Vaccine Immunol. 2012, 19, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Cakır, G.; Gumus, S.; Ucar, E.; Kaya, H.; Tozkoparan, E.; Akgul, E.O.; Karaman, B.; Deniz, O.; Kurt, I.; Ozkan, M. Serum Chitotriosidase Activity in Pulmonary Tuberculosis: Response to Treatment and Correlations with Clinical Parameters. Ann. Lab. Med. 2012, 32, 184–189. [Google Scholar] [CrossRef]
- Eribo, O.A.; Leqheka, M.S.; Malherbe, S.T.; McAnda, S.; Stanley, K.; van der Spuy, G.D.; Walzl, G.; Chegou, N.N. Host Urine Immunological Biomarkers as Potential Candidates for the Diagnosis of Tuberculosis. Int. J. Infect. Dis. 2020, 99, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Han, Y.-S.; Yi, W.-J.; Huang, H.; Li, Z.-B.; Shi, L.-Y.; Wei, L.-L.; Yu, Y.; Jiang, T.-T.; Li, J.-C. Serum SCD14, PGLYRP2 and FGA as Potential Biomarkers for Multidrug-resistant Tuberculosis Based on Data-independent Acquisition and Targeted Proteomics. J. Cell Mol. Med. 2020, 24, 12537–12549. [Google Scholar] [CrossRef] [PubMed]
- Mutavhatsindi, H.; Calder, B.; McAnda, S.; Malherbe, S.T.; Stanley, K.; Kidd, M.; Walzl, G.; Chegou, N.N. Identification of Novel Salivary Candidate Protein Biomarkers for Tuberculosis Diagnosis: A Preliminary Biomarker Discovery Study. Tuberculosis 2021, 130, 102118. [Google Scholar] [CrossRef] [PubMed]
- Verber, N.S.; Shepheard, S.R.; Sassani, M.; McDonough, H.E.; Moore, S.A.; Alix, J.J.P.; Wilkinson, I.D.; Jenkins, T.M.; Shaw, P.J. Biomarkers in Motor Neuron Disease: A State of the Art Review. Front. Neurol. 2019, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, P.; Zhang, T.; Xu, Z.; Huang, X.; Wang, R.; Du, L. Review on Strategies and Technologies for Exosome Isolation and Purification. Front. Bioeng. Biotechnol. 2022, 9, 811971. [Google Scholar] [CrossRef]
- Li, J.; He, X.; Deng, Y.; Yang, C. An Update on Isolation Methods for Proteomic Studies of Extracellular Vesicles in Biofluids. Molecules 2019, 24, 3516. [Google Scholar] [CrossRef]
- Livshits, M.A.; Khomyakova, E.; Evtushenko, E.G.; Lazarev, V.N.; Kulemin, N.A.; Semina, S.E.; Generozov, E.V.; Govorun, V.M. Isolation of Exosomes by Differential Centrifugation: Theoretical Analysis of a Commonly Used Protocol. Sci. Rep. 2015, 5, 17319. [Google Scholar] [CrossRef]
- Yang, X.-X.; Sun, C.; Wang, L.; Guo, X.-L. New Insight into Isolation, Identification Techniques and Medical Applications of Exosomes. J. Control. Release 2019, 308, 119–129. [Google Scholar] [CrossRef]
- Gupta, S.; Rawat, S.; Arora, V.; Kottarath, S.K.; Dinda, A.K.; Vaishnav, P.K.; Nayak, B.; Mohanty, S. An Improvised One-Step Sucrose Cushion Ultracentrifugation Method for Exosome Isolation from Culture Supernatants of Mesenchymal Stem Cells. Stem Cell Res. Ther. 2018, 9, 180. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.W.; Sturk, A.; Nieuwland, R. Single-step Isolation of Extracellular Vesicles by Size-exclusion Chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef]
- Weng, Y.; Sui, Z.; Shan, Y.; Hu, Y.; Chen, Y.; Zhang, L.; Zhang, Y. Effective Isolation of Exosomes with Polyethylene Glycol from Cell Culture Supernatant for In-Depth Proteome Profiling. Analyst 2016, 141, 4640–4646. [Google Scholar] [CrossRef]
- Vergauwen, G.; Dhondt, B.; Van Deun, J.; De Smedt, E.; Berx, G.; Timmerman, E.; Gevaert, K.; Miinalainen, I.; Cocquyt, V.; Braems, G.; et al. Confounding Factors of Ultrafiltration and Protein Analysis in Extracellular Vesicle Research. Sci. Rep. 2017, 7, 2704. [Google Scholar] [CrossRef]
- Zhou, Y.; Mohamadi, R.M.; Poudineh, M.; Kermanshah, L.; Ahmed, S.; Safaei, T.S.; Stojcic, J.; Nam, R.K.; Sargent, E.H.; Kelley, S.O. Interrogating Circulating Microsomes and Exosomes Using Metal Nanoparticles. Small 2016, 12, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Davey, M.; Chute, I.C.; Griffiths, S.G.; Lewis, S.; Chacko, S.; Barnett, D.; Crapoulet, N.; Fournier, S.; Joy, A.; et al. Rapid Isolation of Extracellular Vesicles from Cell Culture and Biological Fluids Using a Synthetic Peptide with Specific Affinity for Heat Shock Proteins. PLoS ONE 2014, 9, e110443. [Google Scholar] [CrossRef]
- Balaj, L.; Atai, N.A.; Chen, W.; Mu, D.; Tannous, B.A.; Breakefield, X.O.; Skog, J.; Maguire, C.A. Heparin Affinity Purification of Extracellular Vesicles. Sci. Rep. 2015, 5, 10266. [Google Scholar] [CrossRef]
- Zarovni, N.; Corrado, A.; Guazzi, P.; Zocco, D.; Lari, E.; Radano, G.; Muhhina, J.; Fondelli, C.; Gavrilova, J.; Chiesi, A. Integrated Isolation and Quantitative Analysis of Exosome Shuttled Proteins and Nucleic Acids Using Immunocapture Approaches. Methods 2015, 87, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.T.; Witwer, K.W.; van Balkom, B.W.M.; de Beer, J.; Brodie, C.; Corteling, R.L.; Gabrielsson, S.; Gimona, M.; Ibrahim, A.G.; de Kleijn, D.; et al. Concise Review: Developing Best-Practice Models for the Therapeutic Use of Extracellular Vesicles. Stem Cells Transl. Med. 2017, 6, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Helwa, I.; Cai, J.; Drewry, M.D.; Zimmerman, A.; Dinkins, M.B.; Khaled, M.L.; Seremwe, M.; Dismuke, W.M.; Bieberich, E.; Stamer, W.D.; et al. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS ONE 2017, 12, e0170628. [Google Scholar] [CrossRef]
- Chen, C.; Skog, J.; Hsu, C.-H.; Lessard, R.T.; Balaj, L.; Wurdinger, T.; Carter, B.S.; Breakefield, X.O.; Toner, M.; Irimia, D. Microfluidic Isolation and Transcriptome Analysis of Serum Microvesicles. Lab Chip 2010, 10, 505–511. [Google Scholar] [CrossRef]
- Rifai, N.; Gillette, M.A.; Carr, S.A. Protein Biomarker Discovery and Validation: The Long and Uncertain Path to Clinical Utility. Nat. Biotechnol. 2006, 24, 971–983. [Google Scholar] [CrossRef]
- Aebersold, R.; Mann, M. Mass Spectrometry-Based Proteomics. Nature 2003, 422, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Arya, R.; Dabral, D.; Faruquee, H.M.; Mazumdar, H.; Patgiri, S.J.; Deka, T.; Basumatary, R.; Kupa, R.; Semy, C.; Kapfo, W.; et al. Serum Small Extracellular Vesicles Proteome of Tuberculosis Patients Demonstrated Deregulated Immune Response. Proteom. Clin. Appl. 2020, 14, 1900062. [Google Scholar] [CrossRef] [PubMed]
- English, P.A.; Williams, J.A.; Martini, J.-F.; Motzer, R.J.; Valota, O.; Buller, R.E. A Case for the Use of Receiver Operating Characteristic Analysis of Potential Clinical Efficacy Biomarkers in Advanced Renal Cell Carcinoma. Future Oncol. 2016, 12, 175–182. [Google Scholar] [CrossRef]
- Vitzthum, F.; Behrens, F.; Anderson, N.L.; Shaw, J.H. Proteomics: From Basic Research to Diagnostic Application. A Review of Requirements & Needs. J. Proteome Res. 2005, 4, 1086–1097. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.M.; Moher, D.; Rennie, D.; De Vet, H.C.W.; Lijmer, J.G. The STARD Statement for Reporting Studies of Diagnostic Accuracy: Explanation and Elaboration. Ann. Intern. Med. 2003, 138, W1–W12. [Google Scholar] [CrossRef]


| SN | Total/Subproteome | Proteins | Method of Isolation | Instruments Used | Ref. |
|---|---|---|---|---|---|
| 1 | Sputum/Serum | Rv3804c-FbpA | Spectrophotometer | [72,73] | |
| 2 | Sputum/Serum | Rv1860-Apa | Ag/Ab assay | Spectrophotometer | [74] |
| 3 | Serum | Rv0440-GroEL2 | Spectrophotometer | [75] | |
| 4 | CSF/Serum | Rv1837c-GlcB, Rv2031c-HspX, Rv0934-PstS1 | Spectrophotometer | [76] | |
| 5 | Serum | Rv2244-AcpM, Rv3804c-Ag85a, Rv1886c-Ag85b, Rv0129c-Ag85c, Rv1860-Apa, Rv3841-BfrB, Rv1827-Cfp17, Rv0350-DnaK, Rv0363c-Fba, Rv1837c-GlcB, Rv3418c-GroES, Rv2031c-HspX, Rv0066c-Icd2, Rv1908c-KatG, Rv1980c-Mpt64, Rv3248c-SahH, Rv0009-PpiA | ExoQuick | LTQ linear ion trap | [19] |
| 6 | Serum | Rv0129c-Ag85c, Rv1837c-GclB, Rv1860-MPT32, Rv1980c-MPT64, Rv2031c-HspX, Rv2376c-Cfp2, Rv3248c-SahH, Rv3418c-GroES, Rv3841-BfrB, Rv0350-DnaK, Rv1886c-Ag85B, Rv3874-Cfp10, Rv3875-EsxA, Rv2220-GlnA1, Rv3441c-MrsA, Rv0009-PpiA, Rv2244-AcpM, Rv3804c-Ag85A, Rv1827-GarA | ExoQuick | Xevo TQ-S mass spectrometer | [36] |
| 7 | Plasma | Hsp16.3 | Ultracentrifugation | [71] | |
| 8 | Urine | Rv1656-ArgF, Rv3341-MetA, Rv2392-cysH | Ultrafiltration | LCQ-DECA XP | [77,78] |
| 9 | Urine | Rv1681-MoeX | LCQ-DECA XP | [79] | |
| 10 | Urine | Rv0014c-PknB, Rv2748c-FtsK, Rv1664-Pks9, Rv1161-NarG, Rv2490c-PE_PGRS43, Rv0578c-PE_PGRS7 | Ultrafiltration | LTQ-Orbitrap Velos Pro | [80] |
| 11 | H37Rv-infected J774 cells and CFP-treated J774 cells | Rv0129c-Antigen 85C, Rv0211-PckA, Rv0350-DnaK, Rv0462-LpdC, Rv0896-GltA2, Rv0934-PstS1, Rv1448-Tal, Rv1827-Cfp17, Rv1837-GlcB, Rv1860-Apa, Rv1886c-Antigen 85B, Rv1908c-KatG, Rv1926c-Mpt63, Rv1932-Tpx, Rv1980c-Mpt64, Rv2031c-HspX, Rv2220-GlnA1, Rv2244-AcpM, Rv2376c-Cfp2, Rv2467-PepN, Rv2780-Ald, Rv2878c-Mpt53, Rv3248c-SahH, Rv3418 -GroES, Rv3804c-Antigen 85A | Filtration and ultracentrifugation | LCQ DECA XP | [60] |
| 12 | Culture | Rv2244-AcpM, Rv1860-Apa, Rv1793-FadA3, Rv0363c-Fba, Rv3804c-FbpA, Rv1908c-KatG, Rv2945c-LppX, Rv3763-LpqH, Rv0040c-Mtc28, Rv1017c-Prs, Rv0934-PstS1, Rv3846-SodB, Rv1793-EsxN, Rv2220-GlnA1 | Density gradient ultracentrifugation | LTQ-Orbitrap Velos | [69] |
| 13 | Cerebrospinal fluid | Rv3875-ESAT6 | 1D-2D PAGE | Spectrophotometer | [81] |
| 14 | Cerebrospinal fluid | Rv2623-TB31.7 | Spectrophotometer | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arya, R.; Jit, B.P.; Kumar, V.; Kim, J.J. Exploring the Potential of Exosomes as Biomarkers in Tuberculosis and Other Diseases. Int. J. Mol. Sci. 2024, 25, 2885. https://doi.org/10.3390/ijms25052885
Arya R, Jit BP, Kumar V, Kim JJ. Exploring the Potential of Exosomes as Biomarkers in Tuberculosis and Other Diseases. International Journal of Molecular Sciences. 2024; 25(5):2885. https://doi.org/10.3390/ijms25052885
Chicago/Turabian StyleArya, Rakesh, Bimal Prasad Jit, Vijay Kumar, and Jong Joo Kim. 2024. "Exploring the Potential of Exosomes as Biomarkers in Tuberculosis and Other Diseases" International Journal of Molecular Sciences 25, no. 5: 2885. https://doi.org/10.3390/ijms25052885
APA StyleArya, R., Jit, B. P., Kumar, V., & Kim, J. J. (2024). Exploring the Potential of Exosomes as Biomarkers in Tuberculosis and Other Diseases. International Journal of Molecular Sciences, 25(5), 2885. https://doi.org/10.3390/ijms25052885






