The Molecular Mechanism of the Response of Rice to Arsenic Stress and Effective Strategies to Reduce the Accumulation of Arsenic in Grain
Abstract
1. Introduction
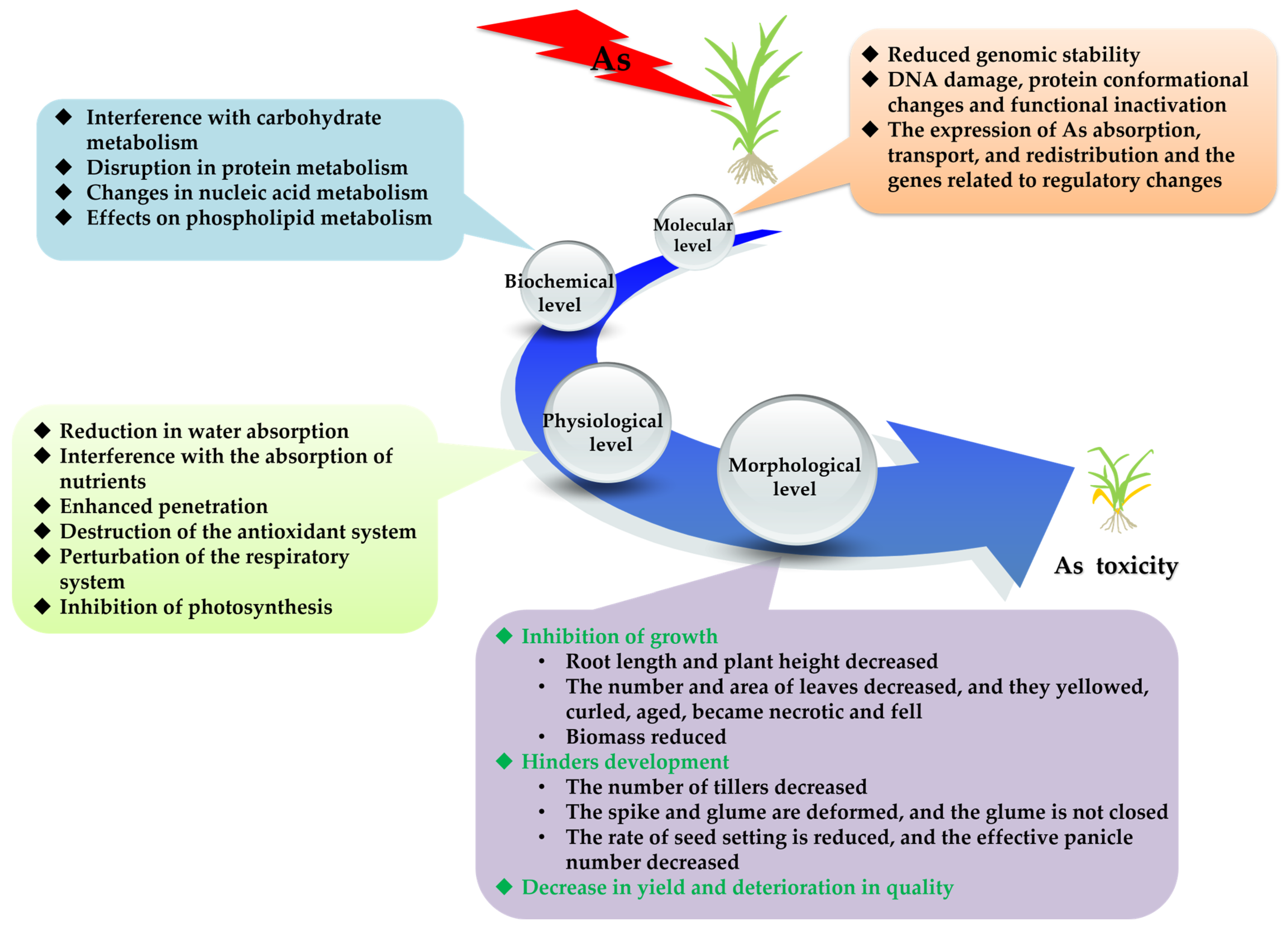
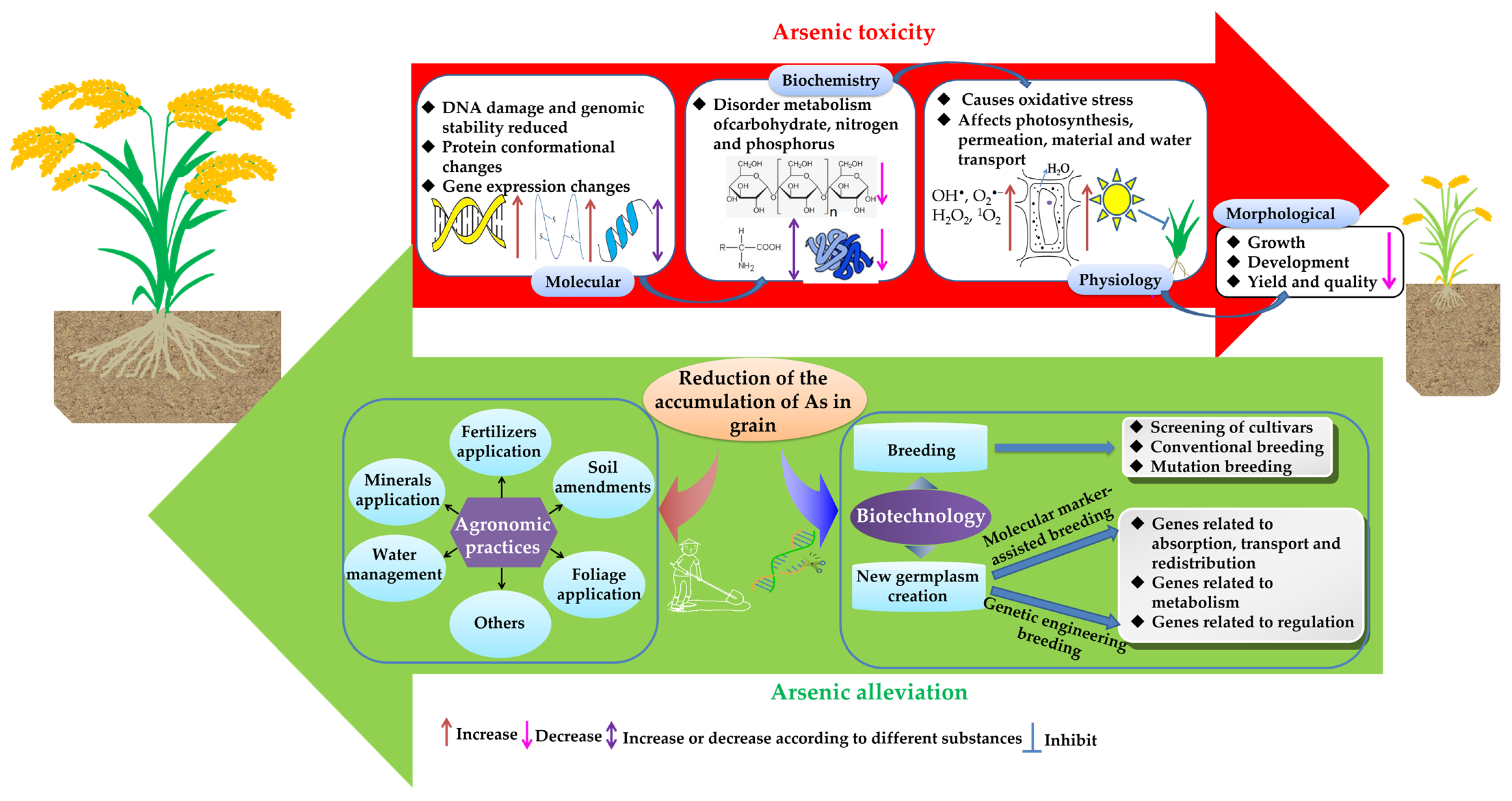
2. Effects of As on the Growth, Development and Metabolism of Rice
3. Absorption, Transport and Redistribution of As(III) in Rice
3.1. Absorption of As(III)
3.2. Transport of As(III)
3.3. Redistribution of As(III)
4. Absorption, Translocation and Redistribution of As(V) in Rice
4.1. Absorption of As(V)
4.2. Transport of As(V)
4.3. Redistribution of As(V)
5. Absorption, Transport and Redistribution of Organic As in Rice
5.1. Absorption of Organic As
5.2. Transport of Organic As
5.3. Redistribution of Organic As
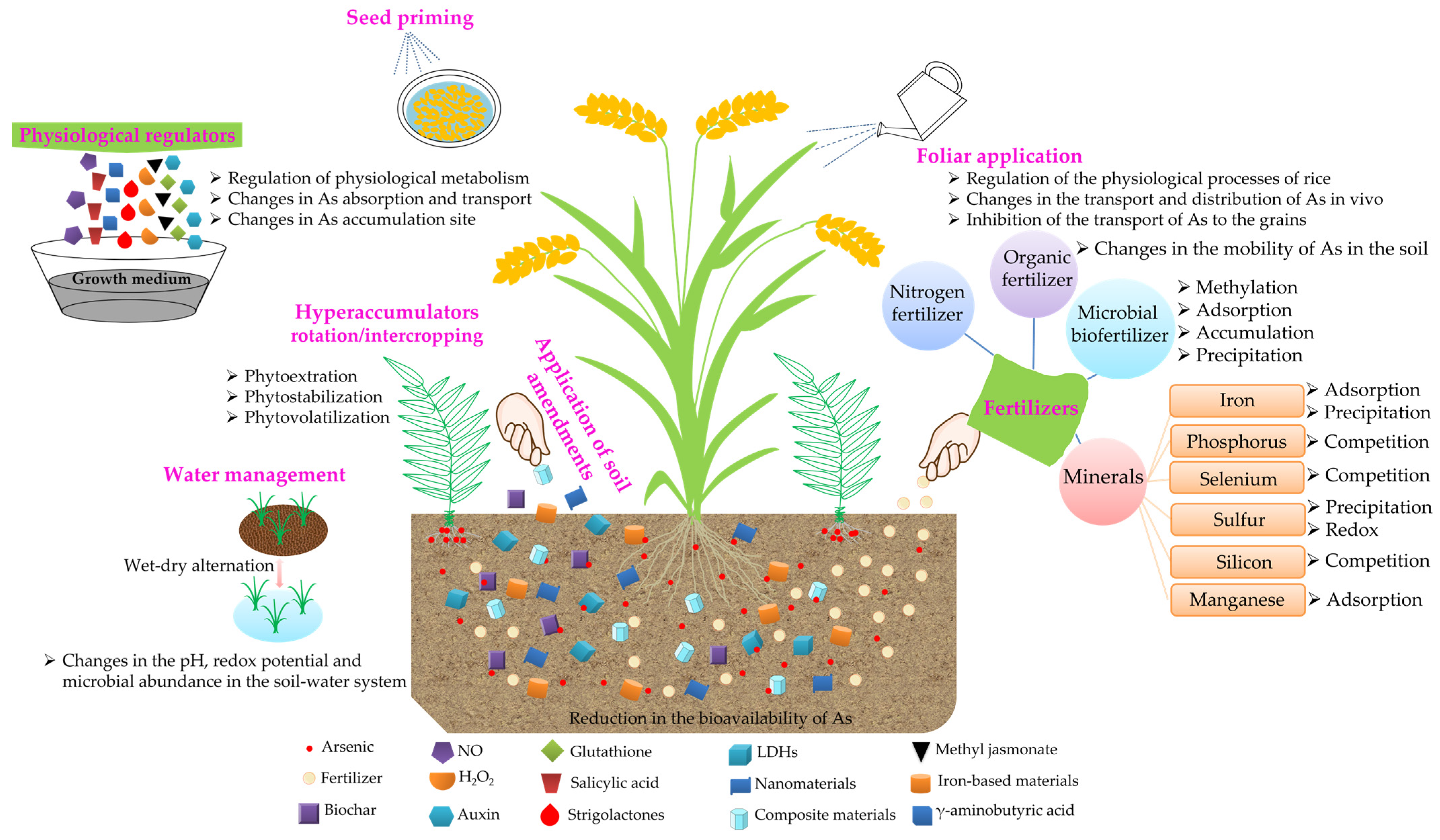
6. The Primary Strategies to Reduce the Accumulation of As in Rice
6.1. Agronomic Practices
6.1.1. Application of Minerals
Iron
Phosphorus
Sulfur
Silicon
Selenium
Manganese
6.1.2. Application of Soil Amendments
Modified Biochar
Modified Iron-Based Materials
Nanoparticles
Composite Amendments
LDHs
6.1.3. Application of Fertilizers
Nitrogen Fertilizer
Organic Fertilizer
Microbial Biofertilizer
6.1.4. Foliar Application
6.1.5. Water Management
6.1.6. Others
Physiological Regulators
Hyperaccumulators
Seed Priming
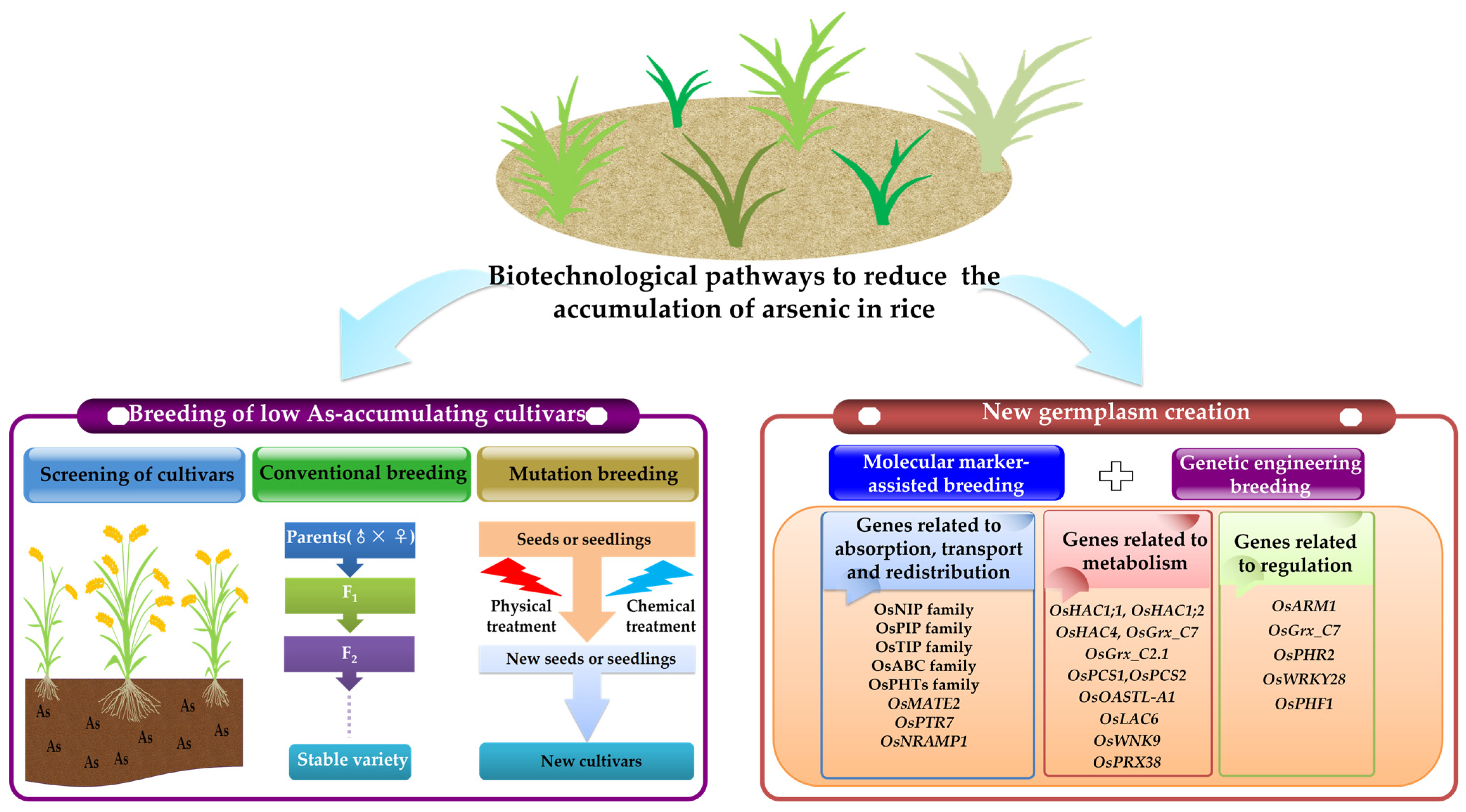
6.2. Biotechnological Pathways
6.2.1. Breeding of Rice Varieties That Accumulate Low Levels of As
6.2.2. Use of Genetic Engineering to Create New Rice Germplasm That Accumulates Low Levels of As
Genes Related to the Absorption, Transport, and Redistribution of As
Genes Related to Metabolism
Genes Related to Regulation
7. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| ABCC | C-TYPE ABC subfamily transporter |
| ABCG | ABC transporter G family member |
| ACA | P2B-type autoinhibited Ca2+-ATPase |
| ACR | arsenate reductase |
| ADH | alcohol dehydrogenase |
| ALA | P4-type aminophospholipid ATPase |
| ARM | ARSENITE-RESPONSIVE MYB |
| As | arsenic |
| As(III) | arsenite |
| As(III)-PC | As(III)-phytochelatin complex |
| As(V) | arsenate |
| AUX | auxin |
| CAT | catalase |
| CLT | CRT-like transporter |
| DMA | dimethylarsenic acid |
| DNA | deoxyribonucleic acid |
| Grx | glutaredoxin |
| Grx_C7 | glutaredoxin_C7 |
| GSH | glutathione |
| GSTU | glutathione-S-transferase |
| H2O2 | hydrogen peroxide |
| HAC | high arsenic content |
| HMA | heavy metal ATPase |
| IAA | Indole-3-Acetic Acid |
| Lsi | low silicon |
| MATE | multidrug and toxic compound extrusion |
| MIP | membrane intrinsic proteins |
| MMA | monomethylarsonic acid |
| MYB | v-myb avian myeloblastosis viral oncogene homolog |
| NIP | nodulin26-like intrinsic protein |
| NLA | nitrogen limitation adaptation |
| NO | nitric oxide |
| NRAMP | natural resistance macrophage protein transporter |
| OASTL-A1 | O-acetylserine(thiol) lyase 1 |
| PC | phytochelatin |
| PCS | phytochelatin synthase |
| PHF | phosphate transporter traffic facilitator |
| PHO | phosphate |
| PHR | phosphate starvation response |
| PIP | plasma membrane intrinsic protein |
| PRX | peroxidase |
| PTR | putative peptide transporter |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SA | salicylic acid |
| SH | sulfhydryl |
| Si | silicon |
| SIP | small basic intrinsic proteins |
| SLs | strigolactones |
| SOD | superoxide dismutase |
| Sultr(SULTR) | sulphate transporter |
| TIP | tonoplast intrinsic protein |
| TMA | trimethylarsine |
| WNK | with no lysine |
| WT | wild type |
| XIP | uncategorized intrinsic proteins |
References
- Murcott, S. Arsenic Contamination in the World: An International Sourcebook 2012; IWA Publishing: London, UK, 2012; Volume 11. [Google Scholar] [CrossRef]
- Vithanage, M.; Herath, I.; Joseph, S.; Bundschuh, J.; Bolan, N.; Ok, Y.S.; Kirkham, M.B.; Rinklebe, J. Interaction of arsenic with biochar in soil and water: A critical review. Carbon 2017, 113, 219–230. [Google Scholar] [CrossRef]
- Yuan, C.; Li, Q.; Sun, Z.; Zhang, W.; Chen, J.; Chen, Z.; Na, S.; Sun, H. Chemical oxidation of arsenic in the environment and its application in remediation: A mini review. Pedosphere 2023, 33, 185–193. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Ori, L.V.; Amacher, M.C.; Sedberry, J.E. Survey of the total arsenic content in soils in louisiana. Commun. Soil Sci. Plant Anal. 1993, 24, 2321–2332. [Google Scholar] [CrossRef]
- Hong, Y.S.; Song, K.H.; Chung, J.Y. Health effects of chronic arsenic exposure. J. Prev. Med. Public Health 2014, 47, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.X.; Speer, R.M.; Volk, L.; Hudson, L.G.; Liu, K.J. Arsenic co-carcinogenesis: Inhibition of DNA repair and interaction with zinc finger proteins. Semin. Cancer Biol. 2021, 76, 86–98. [Google Scholar] [CrossRef]
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Rahman, M.M.; Naidu, R.; Dong, Z.; Shahid, M.; Arshad, M. Unraveling Health Risk and Speciation of Arsenic from Groundwater in Rural Areas of Punjab, Pakistan. Int. J. Environ. Res. Public Health 2015, 12, 12371–12390. [Google Scholar] [CrossRef]
- Williams, P.; Villada, A.; Deacon, C.; Raab, A.; Figuerola, J.; Green, A.; Feldmann, J.; Meharg, A. Greatly Enhanced Arsenic Shoot Assimilation in Rice Leads to Elevated Grain Levels Compared to Wheat and Barley. Environ. Sci. Technol. 2007, 41, 6854–6859. [Google Scholar] [CrossRef]
- Niu, B.H. The Physiological Characteristics of Rice Seedling Responding to Inorganic Arsenic Stress. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2009. (In Chinese). [Google Scholar]
- Ohno, K.; Yanase, T.; Matsuo, Y.; Kimura, T.; Rahman, M.; Magara, Y.; Matsui, Y. Arsenic intake via water and food by a population living in an arsenic-affected area of Bangladesh. Sci. Total Environ. 2007, 381, 68–76. [Google Scholar] [CrossRef]
- Cervantes, C.; Ji, G.; Ramirez, J.; Silver, S. Resistance to arsenic compounds in microorganisms. FEMS Microbiol. Rev. 1994, 15, 355–367. [Google Scholar] [CrossRef]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.N.; Pedron, T.; Freire, B.M.; Pereira, R.M.; Batista, B.L. Arsenic in rice grain. In The Future of Rice Demand: Quality Beyond Productivity; Costa de Oliveira, A., Pegoraro, C., Ebeling Viana, V., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Y.; Gao, A.; Ji, Y.; Yang, B.; Wang, P.; Tang, Z.; Zhao, F. Dimethylarsinic acid is the causal agent inducing rice straighthead disease. J. Exp. Bot. 2020, 71, 5631–5644. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Thounaojam, T.C.; Upadhyaya, H. Arsenic stress in Rice (Oryza sativa) and its amelioration approaches. Plant Stress 2022, 4, 100076. [Google Scholar] [CrossRef]
- Tu, D.H.; Li, D.Q.; Song, S.Y.; Huang, F.; Zhang, L.; Chen, G.D. Differences in arsenic tolerance and screening of high arsenic-tolerant meterials of rice (Oryza sativa L.). Chin. J. Appl. Environ. Biol. 2018, 24, 1065–1072. (In Chinese) [Google Scholar] [CrossRef]
- Nath, S.; Panda, P.; Mishra, S.; Dey, M.; Choudhury, S.; Sahoo, L.; Panda, S.K. Arsenic stress in rice: Redox consequences and regulation by iron. Plant Physiol. Biochem. 2014, 80, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, R.X.; Fang, Z.; Zhou, X.B. Effect of foliage spraying Si and Se on As accumulation and photosynthetic parameters of rice. Soils 2022, 54, 547–555. (In Chinese) [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Mechanisms to cope with arsenic or cadmium excess in plants. Curr. Opin. Plant Biol. 2009, 12, 364–372. [Google Scholar] [CrossRef]
- Jha, A.B.; Dubey, R.S. Carbohydrate metabolism in growing rice seedlings under arsenic toxicity. J. Plant Physiol. 2004, 161, 867–872. [Google Scholar] [CrossRef]
- Upadhyay, A.K.; Singh, N.K.; Singh, R.; Rai, U.N. Amelioration of arsenic toxicity in rice: Comparative effect of inoculation of Chlorella vulgaris and Nannochloropsis sp. on growth, biochemical changes and arsenic uptake. Ecotoxicol. Environ. Saf. 2016, 124, 68–73. [Google Scholar] [CrossRef]
- Begum, M.C.; Islam, M.S.; Islam, M.; Amin, R.; Parvez, M.S.; Kabir, A.H. Biochemical and molecular responses underlying differential arsenic tolerance in rice (Oryza sativa L.). Plant Physiol. Biochem. 2016, 104, 266–277. [Google Scholar] [CrossRef]
- Dwivedi, S.; Mishra, A.; Tripathi, P.; Dave, R.; Kumar, A.; Srivastava, S.; Chakrabarty, D.; Trivedi, P.K.; Adhikari, B.; Norton, G.J.; et al. Arsenic affects essential and non-essential amino acids differentially in rice grains: Inadequacy of amino acids in rice based diet. Environ. Int. 2012, 46, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.; Khan, M.I.; Amjad, M.; Hussain, M.; Natasha, N. Arsenic Uptake, Toxicity, Detoxification, and Speciation in Plants: Physiological, Biochemical, and Molecular Aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.A.; Gaur, R.; Gupta, M. Comparative biochemical and RAPD analysis in two varieties of rice (Oryza sativa) under arsenic stress by using various biomarkers. J. Hazard. Mater. 2012, 217–218, 141–148. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, H.; Reinfelder, J.; Liang, X.; Sun, C.; Liu, C.; Li, F.; Yi, J. A transcriptomic (RNA-seq) analysis of genes responsive to both cadmium and arsenic stress in rice root. Sci. Total Environ. 2019, 666, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Awasthi, S.; Indoliya, Y.; Chauhan, A.S.; Mishra, S.; Agrawal, L.; Srivastava, S.; Dwivedi, S.; Singh, P.C.; Mallick, S.; et al. Transcriptome and proteome analyses reveal selenium mediated amelioration of arsenic toxicity in rice (Oryza sativa L.). J. Hazard. Mater. 2020, 390, 122122. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yingfeng, L.; Liao, B.; Xie, L.; Chen, L.; Xiao, S.; Li, J.; Hu, S.; Shu, W. Comparative transcriptome analysis of transporters, phytohormone and lipid metabolism pathways in response to arsenic stress in rice (Oryza sativa). New Phytol. 2012, 195, 97–112. [Google Scholar] [CrossRef]
- Mawia, A.M.; Hui, S.; Zhou, L.; Li, H.; Tabassum, J.; Lai, C.; Wang, J.; Shao, G.; Wei, X.; Tang, S.; et al. Inorganic arsenic toxicity and alleviation strategies in rice. J. Hazard. Mater. 2021, 408, 124751. [Google Scholar] [CrossRef]
- Castrillo, G.; Sánchez-Bermejo, E.; Lorenzo, L.; Crevillén, P.; Fraile-Escanciano, A.; TC, M.; Mouriz, A.; Catarecha, P.; Sobrino Plata, J.; Olsson, S.; et al. WRKY6 Transcription Factor Restricts Arsenate Uptake and Transposon Activation in Arabidopsis. Plant Cell 2013, 25, 2944–2957. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.; Rafiq, M.; Bakhat, H.; Imran, M.; Abbas, T.; Bibi, I.; Dumat, C. Arsenic Behaviour in Soil-Plant System: Biogeochemical Reactions and Chemical Speciation Influences. In Enhancing Cleanup of Environmental Pollutants; Volume 2: Non-Biological Approaches; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Sun, S.; Chen, Y.; Che, J.; Konishi, N.; Tang, Z.; Miller, A.; Zhao, F. Decreasing arsenic accumulation in rice by overexpressing OsNIP1;1 and OsNIP3;3 through disrupting arsenite radial transport in roots. New Phytol. 2018, 219, 641–653. [Google Scholar] [CrossRef]
- Katsuhara, M.; Sasano, S.; Horie, T.; Matsumoto, T.; Rhee, J.; Shibasaka, M. Functional and molecular characteristics of rice and barley NIP aquaporins transporting water, hydrogen peroxide and arsenite. Plant Biotechnol. 2014, 31, 213–219. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, S.; Tang, Z.; Liu, G.; Moore, K.; Maathuis, F.; Miller, A.; Mcgrath, S.; Zhao, F. The Nodulin 26-like intrinsic membrane protein OsNIP3;2 is involved in arsenite uptake by lateral roots in rice. J. Exp. Bot. 2017, 68, 3007–3016. [Google Scholar] [CrossRef]
- Tiwari, M.; Sharma, D.; Dwivedi, S.; Singh, M.; Tripathi, D.; Trivedi, P. Expression in Arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP1, in arsenic transport and tolerance. Plant Cell Environ. 2013, 37, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, H.; Mukhopadhyay, R.; Thiyagarajan, S.; Rosen, B. Aquaglyceroporins: Ancient channels for metalloids. J. Biol. 2008, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.E.; Pittman, J.K.; Hall, J.L. Emerging mechanisms for heavy metal transport in plants. Biochim. Biophys. Acta Biomembr. 2000, 1465, 104–126. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Trivedi, P. Glutathione S-Transferases: Role in Combating Abiotic Stresses Including Arsenic Detoxification in Plants. Front. Plant Sci. 2018, 9, 751. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Moore, K.; Miller, A.; Mcgrath, S.; Zhao, F. The role of nodes in arsenic storage and distribution in rice. J. Exp. Bot. 2015, 66, 3717–3724. [Google Scholar] [CrossRef]
- Song, W.; Yamaki, T.; Yamaji, N.; Ko, D.; Jung, K.; Fujii-Kashino, M.; An, G.; Martinoia, E.; Lee, Y. A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc. Natl. Acad. Sci. USA 2014, 111, 15699–15704. [Google Scholar] [CrossRef]
- Verma, G.; Srivastava, D.; Narayan, S.; Shirke, P.; Chakrabarty, D. Exogenous application of methyl jasmonate alleviates arsenic toxicity by modulating its uptake and translocation in rice (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2020, 201, 110735. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, Y.; Miller, A.; Zhao, F. The C-type ATP-Binding Cassette Transporter OsABCC7 Is Involved in the Root-to-Shoot Translocation of Arsenic in Rice. Plant Cell Physiol. 2019, 60, 1525–1535. [Google Scholar] [CrossRef]
- Yamaji, N.; Mitani, N.; Xu, X.; Su, Y.; Mcgrath, S.; Zhao, F. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef]
- Mosa, K.; Kumar, K.; Chhikara, S.; Mcdermott, J.; Liu, Z.; Musante, C.; White, J.; Dhankher, O.P. Members of rice plasma membrane intrinsic proteins subfamily are involved in arsenite permeability and tolerance in plants. Transgenic Res. 2012, 21, 1265–1277. [Google Scholar] [CrossRef]
- Yang, J.; Gao, M.; Hu, H.; Ding, X.; Lin, H.; Wang, L.; Xu, J.; Mao, C.; Zhao, F.; Wu, Z. OsCLT1, a CRT-like transporter 1, is required for glutathione homeostasis and arsenic tolerance in rice. New Phytol. 2016, 211, 658–670. [Google Scholar] [CrossRef]
- Verma, P.; Verma, S.; Pande, V.; Mallick, S.; Tripathi, R.; Dhankher, O.P.; Chakrabarty, D. Overexpression of Rice Glutaredoxin OsGrx_C7 and OsGrx_C2.1 Reduces Intracellular Arsenic Accumulation and Increases Tolerance in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 740. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Basu, S.; Rishu, A.K.; Kumar, G. Revisiting the mechanisms of arsenic uptake, transport and detoxification in plants. Environ. Exp. Bot. 2022, 194, 104730. [Google Scholar] [CrossRef]
- Hayashi, S.; Kuramata, M.; Abe, T.; Takagi, H.; Ozawa, K.; Ishikawa, S. Phytochelatin synthase OsPCS1 plays a crucial role in reducing arsenic levels in rice grains. Plant J. Cell Mol. Biol. 2017, 91, 840–848. [Google Scholar] [CrossRef]
- Yamazaki, S.; Ueda, Y.; Mukai, A.; Ochiai, K.; Matoh, T. Rice phytochelatin synthases OsPCS1 and OsPCS2 make different contributions to cadmium and arsenic tolerance. Plant Direct 2018, 2, e34. [Google Scholar] [CrossRef]
- Kidwai, M.; Dhar, Y.V.; Gautam, N.; Tiwari, M.; Ahmad, I.Z.; Asif, M.H.; Chakrabarty, D. Oryza sativa class III peroxidase (OsPRX38) overexpression in Arabidopsis thaliana reduces arsenic accumulation due to apoplastic lignification. J. Hazard. Mater. 2019, 362, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Khare, R.; Trivedi, P.K. Arsenic-responsive high-affinity rice sulphate transporter, OsSultr1;1, provides abiotic stress tolerance under limiting sulphur condition. J. Hazard. Mater. 2019, 373, 753–762. [Google Scholar] [CrossRef]
- Manuka, R.; Saddhe, A.A.; Srivastava, A.K.; Kumar, K.; Penna, S. Overexpression of rice OsWNK9 promotes arsenite tolerance in transgenic Arabidopsis plants. J. Biotechnol. 2021, 332, 114–125. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Guo, Y.; Liu, Q.B. Role of laccase gene OsLAC6 in response to arsenite stress in rice. J. Henan Agric. Sci. 2022, 51, 27–33. (In Chinese) [Google Scholar] [CrossRef]
- Hayashi, S.; Kuramata, M.; Abe, T.; Yamaguchi, N.; Takagi, H.; Tanikawa, H.; Iino, M.; Sugimoto, K.; Ishikawa, S. Deficiency in alcohol dehydrogenase 2 reduces arsenic in rice grains by suppressing silicate transporters. Plant Physiol. 2021, 186, 611–623. [Google Scholar] [CrossRef]
- Wang, F.; Chen, M.; Yu, L.; Xie, L.; Yuan, L.; Qi, H.; Xiao, M.; Guo, W.; Chen, Z.; Yi, K.; et al. OsARM1, an R2R3 MYB Transcription Factor, Is Involved in Regulation of the Response to Arsenic Stress in Rice. Front. Plant Sci. 2017, 8, 1868. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.K.; Verma, S.; Tripathi, R.D.; Chakrabarty, D. A rice glutaredoxin regulate the expression of aquaporin genes and modulate root responses to provide arsenic tolerance. Ecotoxicol. Environ. Saf. 2020, 195, 110471. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Kidwai, M.; Dutta, P.; Narayan, S.; Gautam, N.; Chawda, K.; Shirke, P.A.; Mishra, A.K.; Chakrabarty, D. A tau class glutathione-S-transferase (OsGSTU5) confers tolerance against arsenic toxicity in rice by accumulating more arsenic in root. J. Hazard. Mater. 2022, 426, 128100. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Yi, J.; Li, F.; Li, X.; Liu, C.; Wu, W.; Tao, T. Dynamics of gene expression associated with arsenic uptake and transport in rice during the whole growth period. BMC Plant Biol. 2020, 20, 133. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N. Further characterization of a rice silicon efflux transporter, Lsi2. Soil Sci. Plant Nutr. 2011, 57, 259–264. [Google Scholar] [CrossRef]
- Suriyagoda, L.D.B.; Dittert, K.; Lambers, H. Mechanism of arsenic uptake, translocation and plant resistance to accumulate arsenic in rice grains. Agric. Ecosyst. Environ. 2018, 253, 23–37. [Google Scholar] [CrossRef]
- Shri, M.; Singh, P.; Kidwai, M.; Gautam, N.; Dubey, S.; Verma, G.; Chakrabarty, D. Recent advances in arsenic metabolism in plants: Current status, challenges and highlighted biotechnological intervention to reduce grain arsenic in rice. Metallomics 2019, 11, 519–532. [Google Scholar] [CrossRef]
- Carey, A.; Scheckel, K.; Lombi, E.; Newville, M.; Choi, Y.; Norton, G.; Charnock, J.; Feldmann, J.; Price, A.; Meharg, A. Grain Unloading of Arsenic Species in Rice. Plant Physiol. 2009, 152, 309–319. [Google Scholar] [CrossRef]
- Kumari, P.; Rastogi, A.; Shukla, A.; Srivastava, S.; Yadav, S. Prospects of genetic engineering utilizing potential genes for regulating arsenic accumulation in plants. Chemosphere 2018, 211, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Islam, M.; Duan, G.; Uraguchi, S.; Fujiwara, T. Phosphate deficiency signaling pathway is a target of arsenate and phosphate transporter OsPT1 is involved in As accumulation in shoots of rice. Soil Sci. Plant Nutr. 2013, 59, 580–590. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, D.; Ai, H.; Mei, H.; Liu, X.; Sun, S.; Xu, G.; Liu, Y.; Chen, Y. Knocking Out OsPT4 Gene Decreases Arsenate Uptake by Rice Plants and Inorganic Arsenic Accumulation in Rice Grains. Environ. Sci. Technol. 2017, 51, 12131–12138. [Google Scholar] [CrossRef] [PubMed]
- Peitong, W.; Zhang, W.; Mao, C.; Xu, G.; Zhao, F. The role of OsPT8 in arsenate uptake and varietal difference in arsenate tolerance in rice. J. Exp. Bot. 2016, 67, 6051–6059. [Google Scholar] [CrossRef]
- Wu, Z.; Ren, H.; Mcgrath, S.; Wu, P.; Zhao, F. Investigating the Contribution of the Phosphate Transport Pathway to Arsenic Accumulation in Rice. Plant Physiol. 2011, 157, 498–508. [Google Scholar] [CrossRef]
- Peitong, W.; Xu, X.; Tang, Z.; Zhang, W.; Huang, X.; Zhao, F. OsWRKY28 Regulates Phosphate and Arsenate Accumulation, Root System Architecture and Fertility in Rice. Front. Plant Sci. 2018, 9, 1330. [Google Scholar] [CrossRef]
- Abedi, T.; Mojiri, A. Arsenic Uptake and Accumulation Mechanisms in Rice Species. Plants 2020, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Paszkowski, U.; Kroken, S.; Roux, C.; Briggs, S. Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2002, 99, 13324–13329. [Google Scholar] [CrossRef]
- Ai, P.; Sun, S.; Zhao, J.; Fan, X.; Xin, W.; Guo, Q.; Yu, L.; Shen, Q.; Wu, P.; Miller, A.; et al. Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. Plant J. Cell Mol. Biol. 2008, 57, 798–809. [Google Scholar] [CrossRef]
- Chen, X.W.; Wu, F.Y.; Li, H.; Chan, W.F.; Wu, C.; Wu, S.C.; Wong, M.H. Phosphate transporters expression in rice (Oryza sativa L.) associated with arbuscular mycorrhizal fungi (AMF) colonization under different levels of arsenate stress. Environ. Exp. Bot. 2013, 87, 92–99. [Google Scholar] [CrossRef]
- Zhao, F.; Ago, Y.; Mitani, N.; Li, R.; Su, Y.; Yamaji, N.; Mcgrath, S. The role of the rice aquaporin Lsi1 in arsenite efflux from roots. New Phytol. 2010, 186, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, T.; Chen, Z.; Tang, Z.; Wu, Z.; Salt, D.; Chao, D.; Zhao, F. OsHAC1;1 and OsHAC1;2 Function as Arsenate Reductases and Regulate Arsenic Accumulation. Plant Physiol. 2016, 172, 1708–1719. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Chen, Y.; Chen, J.; Shi, S.; Chen, Z.; Wang, C.; Danku, J.; Zhao, F.; Salt, D. Genome-wide Association Mapping Identifies a New Arsenate Reductase Enzyme Critical for Limiting Arsenic Accumulation in Plants. PLoS Biol. 2014, 12, e1002009. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shi, S.; Wang, L.; Tang, Z.; Lv, T.; Zhu, X.; Ding, X.; Wang, Y.; Zhao, F.; Wu, Z. OsHAC4 is critical for arsenate tolerance and regulates arsenic accumulation in rice. New Phytol. 2017, 215, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Zhou, Y.; Tong, Y.; Mukhopadhyay, R.; Rosen, B. A CDC25 homologue from rice functions as an arsenate reductase. New Phytol. 2007, 174, 311–321. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, L.; Tang, Z.; Sun, S.; Huang, X.; Zhao, F. OsOASTL-A1 functions as a cytosolic cysteine synthase and affects arsenic tolerance in rice. J. Exp. Bot. 2020, 71, 3678–3689. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Tian, Z.; Yang, X.; Liu, B.; Yang, J.; Lin, H. The role of OsNLA1 in regulating arsenate uptake and tolerance in rice. J. Plant Physiol. 2019, 236, 15–22. [Google Scholar] [CrossRef]
- Das, N.; Bhattacharya, S.; Bhattacharyya, S.; Maiti, M. Expression of rice MATE family transporter OsMATE2 modulates arsenic accumulation in tobacco and rice. Plant Mol. Biol. 2018, 98, 101–120. [Google Scholar] [CrossRef]
- Smith, P.G.; Koch, I.; Reimer, K.J. An investigation of arsenic compounds in fur and feathers using X-ray absorption spectroscopy speciation and imaging. Sci. Total Environ. 2008, 390, 198–204. [Google Scholar] [CrossRef]
- Ye, W.; Zhang, J.; Fan, T.; Lu, H.; Chen, H.; Li, X.; Hua, R. Arsenic speciation in the phloem exudates of rice and its role in arsenic accumulation in rice grains. Ecotoxicol. Environ. Saf. 2017, 143, 87–91. [Google Scholar] [CrossRef]
- Zheng, M.; Li, G.; Sun, G.; Shim, H.; Cai, C. Differential toxicity and accumulation of inorganic and methylated arsenic in rice. Plant Soil 2013, 365, 227–238. [Google Scholar] [CrossRef]
- Ajees, A.; Marapakala, K.; Packianathan, C.; Sankaran, B.; Rosen, B. Structure of an As(III) S-Adenosylmethionine Methyltransferase: Insights into the Mechanism of Arsenic Biotransformation. Biochemistry 2012, 51, 5476–5485. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Rosen, B.; Zhang, Y.; Wang, G.; Franke, S.; Rensing, C. Arsenic Detoxification and Evolution of Trimethylarsine Gas by a Microbial Arsenite S-Adenosylmethionine Methyltransferase. Proc. Natl. Acad. Sci. USA 2006, 103, 2075–2080. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Huang, H.; Zhong, M.; Wang, F.; Zhang, L.; Zhu, Y. Microbial Arsenic Methylation in Soil and Rice Rhizosphere. Environ. Sci. Technol. 2013, 47, 3141–3148. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, L.; Huang, K.; Zhang, J.; Xie, W.; Lu, Y.; Dong, X.; Zhao, F. Sulfate-reducing bacteria and methanogens are involved in arsenic methylation and demethylation in paddy soils. ISME J. 2019, 13, 2523–2535. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ago, Y.; Liu, W.; Mitani, N.; Feldmann, J.; Mcgrath, S.; Zhao, F. The Rice Aquaporin Lsi1 Mediates Uptake of Methylated Arsenic Species. Plant Physiol. 2009, 150, 2071–2080. [Google Scholar] [CrossRef] [PubMed]
- Pimpão, C.; Wragg, D.; da Silva, I.V.; Casini, A.; Soveral, G. Aquaglyceroporin modulators as emergent pharmacological molecules for human diseases. Front. Mol. Biosci. 2022, 9, 845237. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Kadohashi, K.; Maki, T.; Hasegawa, H. Transport of DMAA and MMAA into rice (Oryza sativa L.) roots. Environ. Exp. Bot. 2011, 72, 41–46. [Google Scholar] [CrossRef]
- Raab, A.; Williams, P.; Meharg, A.; Feldmann, J. Uptake and translocation of inorganic and methylated arsenic species by plants. Environ. Chem. 2007, 4, 197–203. [Google Scholar] [CrossRef]
- Abedin, J.; Feldmann, J.; Meharg, A. Uptake Kinetics of Arsenic Species in Rice Plants. Plant Physiol. 2002, 128, 1120–1128. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, Y.; Chen, F.; Ji, Y.; Zhao, F. OsPTR7 (OsNPF8.1), a Putative Peptide Transporter in Rice, is Involved in Dimethylarsenate Accumulation in Rice Grain. Plant Cell Physiol. 2017, 58, 904–913. [Google Scholar] [CrossRef]
- Lomax, C.; Liu, W.; Wu, L.; Xue, K.; Zhou, J.; Mcgrath, S.; Meharg, A.; Miller, A.; Zhao, F. Methylated arsenic species in plants originate from soil microorganisms. New Phytol. 2011, 193, 665–672. [Google Scholar] [CrossRef]
- Sarwar, T.; Khan, S.; Muhammad, S.; Amin, S. Arsenic speciation, mechanisms, and factors affecting rice uptake and potential human health risk: A systematic review. Environ. Technol. Innov. 2021, 22, 101392. [Google Scholar] [CrossRef]
- Meharg, A.A.; Williams, P.N.; Adomako, E.; Lawgali, Y.; Deacon, C.; Villada, A.; Cambell, R.C.J.; Sun, G.; Feldmann, J.; Raab, A.; et al. Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ. Sci Technol. 2009, 43, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Arao, T.; Kawasaki, A.; Baba, K.; Matsumoto, S. Effects of Arsenic Compound Amendment on Arsenic Speciation in Rice Grain. Environ. Sci. Technol. 2011, 45, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.; Norton, G.; Deacon, C.; Scheckel, K.; Lombi, E.; Punshon, T.; Guerinot, M.; Lanzirotti, A.; Newville, M.; Choi, Y.; et al. Phloem transport of arsenic species from flag leaf to grain during grain filling. New Phytol. 2011, 192, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Kasuga, J.; Makino, T.; Arao, T. Evaluation of the effects of application of iron materials on the accumulation and speciation of arsenic in rice grain grown on uncontaminated soil with relatively high levels of arsenic. Environ. Exp. Bot. 2016, 125, 42–51. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Q.; Duan, G.; Huang, Y. Influence of sulphur on arsenic accumulation and metabolism in rice seedlings. Environ. Exp. Bot. 2011, 72, 34–40. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, J.; Teng, M.; Wang, L. Arsenic uptake is suppressed in a rice mutant defective in silicon uptake. J. Plant Nutr. Soil Sci. 2009, 172, 867–874. [Google Scholar] [CrossRef]
- Kumar, N.; Dubey, A.; Jaiswal, P.; Sahu, N.; Behera, S.; Tripathi, R.; Mallick, S. Selenite supplementation reduces arsenate uptake greater than phosphate but compromises the phosphate level and physiological performance in hydroponically grown Oryza sativa L. Environ. Toxicol. Chem. 2016, 35, 163–172. [Google Scholar] [CrossRef]
- Li, B.; Zhou, S.; Wei, D.; Long, J.; Peng, L.; Tie, B.; Williams, P.N.; Lei, M. Mitigating arsenic accumulation in rice (Oryza sativa L.) from typical arsenic contaminated paddy soil of southern China using nanostructured α-MnO2: Pot experiment and field application. Sci. Total Environ. 2019, 650, 546–556. [Google Scholar] [CrossRef]
- Hu, L.; Zeng, M.; Lei, M.; Liao, B.; Zhou, H. Effect of Zero-Valent Iron on Arsenic Uptake by Rice (Oryza sativa L.) and its Relationship with Iron, Arsenic, and Phosphorus in Soil and Iron Plaque. Water Air Soil Pollut. 2020, 231, 481. [Google Scholar] [CrossRef]
- Li, T.; Li, J.; Zhan, X.; Wang, X.; He, B.; Cao, F.; Liao, C.; Yu, Y.; Zhang, Z.; Zhang, J.; et al. Application of Exogenous Iron Alters the Microbial Community Structure and Reduces the Accumulation of Cadmium and Arsenic in Rice (Oryza sativa L.). Nanomaterials 2022, 12, 1311. [Google Scholar] [CrossRef] [PubMed]
- Miretzky, P.; Cirelli, A. Remediation of Arsenic-Contaminated Soils by Iron Amendments: A Review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 93–115. [Google Scholar] [CrossRef]
- Fendorf, S.; Eick, M.; Grossl, P.; Sparks, D. Arsenate and Chromate Retention Mechanisms on Goethite. 1. Surface Structure. Environ. Sci. Technol. 1997, 31, 321–326. [Google Scholar] [CrossRef]
- Luong, V.T.; Cañas Kurz, E.E.; Hellriegel, U.; Luu, T.L.; Hoinkis, J.; Bundschuh, J. Iron-based subsurface arsenic removal technologies by aeration: A review of the current state and future prospects. Water Res. 2018, 133, 110–122. [Google Scholar] [CrossRef]
- Liu, C.; Yu, H.; Liu, C.; Li, F.; Xu, X.; Wang, Q. Arsenic availability in rice from a mining area: Is amorphous iron oxide-bound arsenic a source or sink? Environ. Pollut. 2015, 199, 95–101. [Google Scholar] [CrossRef]
- Ultra, V.; NAKAYAMA, A.; TANAKA, S.; Kang, Y.; Sakurai, K.; Iwasaki, K. Potential for the alleviation of arsenic toxicity in paddy rice using amorphous iron-(hydr)oxide amendments. Soil Sci. Plant Nutr. 2009, 55, 160–169. [Google Scholar] [CrossRef]
- Majumder, S.; Powell, M.A.; Kumar Biswas, P.; Banik, P. The role of agronomic factors (rice cultivation practices and soil amendments) on Arsenic fractionation: A strategy to minimise Arsenic uptake by rice, with some observations related to cadmium. Catena 2021, 206, 105556. [Google Scholar] [CrossRef]
- Rahaman, S.; Sinha, A.C.; Mukhopadhyay, D. Effect of water regimes and organic matters on transport of arsenic in summer rice (Oryza sativa L.). J. Environ. Sci. 2011, 23, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; Singh, A.; Kasote, D.; Sen, I.; Regina, A. Effect of Phosphorus Application on Arsenic Species Accumulation and Co-Deposition of Polyphenols in Rice Grain: Phyto and Food Safety Evaluation. Plants 2021, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- WANG, L.; DUAN, G. Effect of external and internal phosphate status on arsenic toxicity and accumulation in rice seedlings. J. Environ. Sci. 2009, 21, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wu, C.; Syu, C.; Jiang, P.; Huang, C.; Lee, D. Effects of phosphorous application on arsenic toxicity to and uptake by rice seedlings in As-contaminated paddy soils. Geoderma 2016, 270, 60–67. [Google Scholar] [CrossRef]
- Geng, C.; Zhu, Y.; Liu, W.; Smith, S.E. Arsenate uptake and translocation in seedlings of two genotypes of rice is affected by external phosphate concentrations. Aquat. Bot. 2005, 83, 321–331. [Google Scholar] [CrossRef]
- Bolan, N.; Mahimairaja, S.; Kunhikrishnan, A.; Choppala, G. Phosphorus–arsenic interactions in variable-charge soils in relation to arsenic mobility and bioavailability. Sci. Total Environ. 2013, 463–464, 1154–1162. [Google Scholar] [CrossRef]
- Sahoo, P.; Kim, K. A review of the arsenic concentration in paddy rice from the perspective of geoscience. Geosci. J. 2013, 17, 107–122. [Google Scholar] [CrossRef]
- Jacobs, L.W.; Syers, J.K.; Keeney, D.R. Arsenic Sorption by Soils. Soil Sci. Soc. Am. J. 1970, 34, 750–754. [Google Scholar] [CrossRef]
- Dang, F.; Zhong, H.; Zhao, X.; Zhou, D.; Wang, Y. Effects of phosphate on trace element accumulation in rice (Oryza sativa L.): A 5-year phosphate application study. J. Soils Sediments 2016, 16, 1440–1447. [Google Scholar] [CrossRef]
- SURIYAGODA, L.D.B.; DITTERT, K.; LAMBERS, H. Arsenic in Rice Soils and Potential Agronomic Mitigation Strategies to Reduce Arsenic Bioavailability: A Review. Pedosphere 2018, 28, 363–382. [Google Scholar] [CrossRef]
- Sahrawat, K. Redox Potential and pH as Major Drivers of Fertility in Submerged Rice Soils: A Conceptual Framework for Management. Commun. Soil Sci. Plant Anal. 2015, 46, 250505978. [Google Scholar] [CrossRef]
- Burton, E.; Johnston, S.; Kocar, B. Arsenic Mobility During Flooding of Contaminated Soil: The Effect of Microbial Sulfate Reduction. Environ. Sci. Technol. 2014, 48, 13660–13667. [Google Scholar] [CrossRef] [PubMed]
- Bostick, B.; Fendorf, S.; Brown, G. I>In situ analysis of thioarsenite complexes in neutral to alkaline arsenic sulphide solutions. Miner. Mag. 2005, 69, 781–795. [Google Scholar] [CrossRef]
- Hu, Z.; Zhu, Y.; Li, M.; Zhang, L.; Cao, Z.; Smith, F.A. Sulfur (S)-induced enhancement of iron plaque formation in the rhizosphere reduces arsenic accumulation in rice (Oryza sativa L.) seedlings. Environ. Pollut. 2007, 147, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Dixit, G.; Singh, A.P.; Kumar, A.; Dwivedi, S.; Deeba, F.; Kumar, S.; Suman, S.; Adhikari, B.; Shukla, Y.; Trivedi, P.K.; et al. Sulfur alleviates arsenic toxicity by reducing its accumulation and modulating proteome, amino acids and thiol metabolism in rice leaves. Sci. Rep. 2015, 5, 16205. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Hu, Y.; Liu, W.; Kneer, R.; Zhao, F.; Zhu, Y. Evidence for a role of phytochelatins in regulating arsenic accumulation in rice grain. Environ. Exp. Bot. 2011, 71, 416–421. [Google Scholar] [CrossRef]
- Kerl, C.F.; Rafferty, C.; Clemens, S.; Planer-Friedrich, B. Monothioarsenate Uptake, Transformation, and Translocation in Rice Plants. Environ. Sci. Technol. 2018, 52, 9154–9161. [Google Scholar] [CrossRef] [PubMed]
- Fleck, A.; Mattusch, J.; Schenk, M. Silicon decreases the arsenic level in rice grain by limiting arsenite transport. J. Plant Nutr. Soil Sci. 2013, 176, 785–794. [Google Scholar] [CrossRef]
- Wu, C.; Zou, Q.; Xue, S.; Mo, J.; Pan, W.; Lou, L.; Wong, M.H. Effects of silicon (Si) on arsenic (As) accumulation and speciation in rice (Oryza sativa L.) genotypes with different radial oxygen loss (ROL). Chemosphere 2015, 138, 447–453. [Google Scholar] [CrossRef]
- Li, G.; Zheng, M.; Tang, J.; Shim, H.; Cai, C. Effect of Silicon on Arsenic Concentration and Speciation in Different Rice Tissues. Pedosphere 2018, 28, 511–520. [Google Scholar] [CrossRef]
- Lee, C.; Huang, H.; Syu, C.; Lin, T.; Lee, D. Increase of As release and phytotoxicity to rice seedlings in As-contaminated paddy soils by Si fertilizer application. J. Hazard. Mater. 2014, 276, 253–261. [Google Scholar] [CrossRef]
- Seyfferth, A.; Fendorf, S. Silicate Mineral Impacts on the Uptake and Storage of Arsenic and Plant Nutrients in Rice (Oryza sativa L.). Environ. Sci. Technol. 2012, 46, 13176–13183. [Google Scholar] [CrossRef]
- Wang, H.; Wen, S.; Chen, P.; Zhang, L.; Cen, K.; Sun, G. Mitigation of cadmium and arsenic in rice grain by applying different silicon fertilizers in contaminated fields. Environ. Sci. Pollut. Res. Int. 2016, 23, 3781–3788. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef]
- Chauhan, R.; Awasthi, S.; Tripathi, P.; Mishra, S.; Dwivedi, S.; Niranjan, A.; Mallick, S.; Tripathi, P.; Pande, V.; Tripathi, R. Selenite modulates the level of phenolics and nutrient element to alleviate the toxicity of arsenite in rice (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2016, 138, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Awasthi, G.; Singh, A.; Dwivedi, S.; Srivastava, S.; Mishra, K.; Tripathi, R. Selenate mitigates arsenite toxicity in rice (Oryza sativa L.) by reducing arsenic uptake and ameliorates amino acid content and thiol metabolism. Ecotoxicol. Environ. Saf. 2016, 133, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, C.; Wang, P.; Kretzschmar, R.; Zhao, F. Control of arsenic mobilization in paddy soils by manganese and iron oxides. Environ. Pollut. 2017, 231, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Komárek, M.; Vaněk, A.; Ettler, V. Chemical stabilization of metals and arsenic in contaminated soils using oxides—A review. Environ. Pollut. 2013, 172, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Takagi, D.; Ishiyama, K.; Suganami, M.; Ushijima, T.; Fujii, T.; Tazoe, Y.; Kawasaki, M.; Noguchi, K.; Makino, A. Manganese toxicity disrupts indole acetic acid homeostasis and suppresses the CO2 assimilation reaction in rice leaves. Sci. Rep. 2021, 11, 20922. [Google Scholar] [CrossRef] [PubMed]
- Rayen, M.; Reyes-Díaz, M.; Ivanov, A.; Mora, M.L.; Alberdi, M. Manganese as Essential and Toxic Element for Plants: Transport, Accumulation and Resistance Mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 476–494. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; Da Silva, E.; De Oliveira, L.; Chen, Y. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Seyfferth, A.; Morris, A.; Gill, R.; Kearns, K.; Mann, J.; Paukett, M.; Leskanic, C. Soil-incorporation of silica-rich rice husk decreases inorganic As in rice grain. J. Agric. Food. Chem. 2016, 64, 3760–3766. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gao, B.; Zimmerman, A.; Chen, H.; Zhang, M.; Cao, X. Biochar-supported zerovalent iron for removal of various contaminants from aqueous solutions. Bioresour. Technol. 2013, 152, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, H.; Yan, X.; Xiao, Y.; Huang, K.; Liu, J.; Li, L.; Zhang, J.; Gu, J.; Zhou, Y.; et al. The Fe3O4-modified biochar reduces arsenic availability in soil and arsenic accumulation in indica rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2021, 28, 18050–18061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, B. Removal of arsenic, methylene blue, and phosphate by biochar/AlOOH nanocomposite. Chem. Eng. J. 2013, 226, 286–292. [Google Scholar] [CrossRef]
- Liang, T.; Li, L. Arsenic Immobilization for Paddy Field and Improvement of Rice (Oryza sativa L.) Growth through Cerium–Manganese Modified Wheat Straw Biochar Application. Sustainability 2023, 15, 16161. [Google Scholar] [CrossRef]
- Lin, L.; Gao, M.; Liu, X.; Qiu, W.; Song, Z. Effect of Fe–Mn–La-modified biochar composites on arsenic volatilization in flooded paddy soil. Environ. Sci. Pollut. Res. 2021, 28, 49889–49898. [Google Scholar] [CrossRef]
- Khan, S.; Reid, B.; Li, G.; Zhu, Y.G. Application of biochar to soil reduces cancer risk via rice consumption: A case study in Miaoqian village, Longyan, China. Environ. Int. 2014, 68, 154–161. [Google Scholar] [CrossRef]
- Wang, N.; Xue, X.M.; Juhasz, A.; Chang, Z.; Li, H. Biochar increases arsenic release from an anaerobic paddy soil due to enhanced microbial reduction of iron and arsenic. Environ. Pollut. 2016, 220, 514–522. [Google Scholar] [CrossRef]
- Lin, L.; Gao, M.; Qiu, W.; Wang, D.; Huang, Q.; Song, Z. Reduced arsenic accumulation in indica rice (Oryza sativa L.) cultivar with ferromanganese oxide impregnated biochar composites amendments. Environ. Pollut. 2017, 231, 479–486. [Google Scholar] [CrossRef]
- Wu, J.; Li, Z.; Huang, D.; Liu, X.; Tang, C.; Parikh, S.; Xu, J. A novel calcium-based magnetic biochar is effective in stabilization of arsenic and cadmium co-contamination in aerobic soils. J. Hazard. Mater. 2020, 387, 122010. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Tang, X.; Wu, J.Z.; Zhao, K.; Ye, Z. Simultaneous immobilization of arsenic, lead, and cadmium in paddy soils using two iron-based materials. Environ. Sci. 2021, 42, 3535–3548. (In Chinese) [Google Scholar] [CrossRef]
- Tang, X.; Shen, H.; Chen, M.; Yang, X.; Yang, D.; Wang, F.; Chen, Z.; Liu, X.; Wang, H.; Xu, J. Achieving the safe use of Cd- and As-contaminated agricultural land with an Fe-based biochar: A field study. Sci. Total Environ. 2019, 706, 135898. [Google Scholar] [CrossRef]
- Wu, C.; Cui, M.; Xue, S.; Li, W.; Huang, L.; Jiang, X.; Qian, Z. Remediation of arsenic-contaminated paddy soil by iron-modified biochar. Environ. Sci. Pollut. Res. 2018, 25, 20792–20801. [Google Scholar] [CrossRef]
- Fresno, T.; Moreno-Jiménez, E.; Zornoza, P.; Peñalosa, J. Aided phytostabilisation of As- and Cu-contaminated soils using white lupin and combined iron and organic amendments. J. Environ. Manag. 2018, 205, 142–150. [Google Scholar] [CrossRef]
- Liu, W.; Smith, F.; Smith, S. Do phosphorus nutrition and iron plaque alter arsenate (As) uptake by rice seedlings in hydroponic culture? New Phytol. 2004, 162, 481–488. [Google Scholar] [CrossRef]
- Lata, S.; Samadder, S. Removal of arsenic from water using nano adsorbents and challenges: A review. J. Environ. Manag. 2015, 166, 387–406. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.H.; Zhao, D. Environmental dynamics of metal oxide nanoparticles in heterogeneous systems: A review. J. Hazard. Mater. 2017, 322, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Noman, M.; Manzoor, N.; Shahid, M.; Hussaini, K.M.; Rizwan, M.; Ali, S.; Maqsood, A.; Li, B. Green magnesium oxide nanoparticles-based modulation of cellular oxidative repair mechanisms to reduce arsenic uptake and translocation in rice (Oryza sativa L.) plants. Environ. Pollut. 2021, 288, 117785. [Google Scholar] [CrossRef]
- Ma, X.; Sharifan, H.; Dou, F.; Sun, W. Simultaneous reduction of arsenic (As) and cadmium (Cd) accumulation in rice by zinc oxide nanoparticles. Chem. Eng. J. 2020, 384, 123802. [Google Scholar] [CrossRef]
- Wang, X.; Sun, W.; Ma, X. Differential impacts of copper oxide nanoparticles and Copper(II) ions on the uptake and accumulation of arsenic in rice (Oryza sativa). Environ. Pollut. 2019, 252, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Peng, L.; Lei, M.; Pan, Y.; Lan, D. Control of As soil-to-rice transfer (Oryza sativa L.) with nano-manganese dioxide. Huanjing Kexue Xuebao/Acta Sci. Circumstantiae 2015, 35, 855–861. [Google Scholar] [CrossRef]
- Liu, C.; Wei, L.; Zhang, S.; Xu, X.; Li, F. Effects of Nanoscale Silica Sol Foliar Application on Arsenic Uptake, Distribution and Oxidative Damage Defense in Rice (Oryza sativa L.) under Arsenic Stress. RSC Adv. 2014, 4, 57227–57234. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, Q.; Lin, L.; Li, F.; Han, Y.; Song, Z. Reduction of arsenic toxicity in two rice cultivar seedlings by different nanoparticles. Ecotoxicol. Environ. Saf. 2018, 159, 261–271. [Google Scholar] [CrossRef]
- He, Y.; Fang, T.; Wang, J.; Liu, X.; Yan, Z.; Lin, H.; Li, F.; Guo, G. Insight into the stabilization mechanism and long-term effect on As, Cd, and Pb in soil using zeolite-supported nanoscale zero-valent iron. J. Clean. Prod. 2022, 355, 131634. [Google Scholar] [CrossRef]
- Wu, X.; Hu, J.; Wu, F.; Zhang, X.; Wang, B.; Yang, Y.; Shen, G.; Liu, J.; Tao, S.; Wang, X. Application of TiO2 nanoparticles to reduce bioaccumulation of arsenic in rice seedlings (Oryza sativa L.): A mechanistic study. J. Hazard. Mater. 2021, 405, 124047. [Google Scholar] [CrossRef]
- Wang, X.; Sun, W.; Zhang, S.; Sharifan, H.; Ma, X. Elucidating the Effects of Cerium Oxide Nanoparticles and Zinc Oxide Nanoparticles on Arsenic Uptake and Speciation in Rice (Oryza sativa) in a Hydroponic System. Environ. Sci. Technol. 2018, 52, 10040–10047. [Google Scholar] [CrossRef]
- Rossi, L.; Sharifan, H.; Zhang, W.; Schwab, A.; Ma, X. Mutual effects and: In planta accumulation of co-existing cerium oxide nanoparticles and cadmium in hydroponically grown soybean (Glycine max (L.) Merr.). Environ. Sci. Nano 2018, 5, 150–157. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Wan, Y.; Mi, Z.; Wang, Q.; Wang, Q.; Li, H. The fate of arsenic in rice plants (Oryza sativa L.): Influence of different forms of selenium. Chemosphere 2021, 264, 128417. [Google Scholar] [CrossRef]
- Lebrun, M.; Miard, F.; Nandillon, R.; Morabito, D.; Bourgerie, S. Effect of biochar, iron sulfate and poultry manure application on the phytotoxicity of a former tin mine. Int. J. Phytoremediation 2021, 23, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.F.; Zhou, H.; Yang, W.T.; Zen, M.; Peng, P.Q.; Zhang, P.; Liao, B.H. Effect of combined soil amendment regulating chemical forms of cadmium and arsenic in paddy soil and their bioaccumulation and translocation in rice. Acta Pedol. Sin. 2016, 53, 1576–1585. (In Chinese) [Google Scholar]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Palmer, S.; Frost, R. Effect of pH on the uptake of arsenate and vanadate by hydrotalcites in alkaline solutions: A Raman spectroscopic study. J. Raman Spectrosc. 2011, 42, 224–229. [Google Scholar] [CrossRef]
- Bagherifam, S.; Komarneni, S.; Lakzian, A.; Fotovat, A.; Khorassani, R.; Huang, W.; Ma, J.; Wang, Y. Evaluation of Zn–Al–SO4 layered double hydroxide for the removal of arsenite and arsenate from a simulated soil solution: Isotherms and kinetics. Appl. Clay Sci. 2014, 95, 119–125. [Google Scholar] [CrossRef]
- Zhou, J.; Shu, W.; Gao, Y.; Cao, Z.; Zhang, J.; Hou, H.; Zhao, J.; Chen, X.; Pan, Y.; Guangren, Q. Enhanced arsenite immobilization via ternary layered double hydroxides and application to paddy soil remediation. RSC Adv. 2017, 7, 20320–20326. [Google Scholar] [CrossRef]
- Chen, G.; Du, Y.; Fang, L.; Wang, X.; Liu, C.; Yu, H.; Feng, M.; Chen, X.; Li, F. Distinct arsenic uptake feature in rice reveals the importance of N fertilization strategies. Sci. Total Environ. 2022, 854, 158801. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hong, M.; Kappler, A.; Xu, Y. Effects of different forms of nitrogen fertilizers on arsenic uptake by rice plants. Environ. Toxicol. Chem. Int. J. 2008, 27, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Zhang, H.; Davison, W.; Meharg, A.; Hossain, M.; Norton, G.; Brammer, H.; Islam, M. Organic matter-solid phase interactions are critical for predicting arsenic release and plant uptake in bangladesh paddy soils. Environ. Sci. Technol. 2011, 45, 6080–6087. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Sur, P.; Das, K. Mobilisation of arsenic in soils and in rice (Oryza sativa L.) plants affected by organic matter and zinc application in irrigation water contaminated with arsenic. Plant Soil Environ. 2008, 54, 30–37. [Google Scholar] [CrossRef]
- Ma, R.; Shen, J.; Wu, J.; Tang, Z.; Shen, Q.; Zhao, F. Impact of agronomic practices on arsenic accumulation and speciation in rice grain. Environ. Pollut. 2014, 194, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Syu, C.; Wu, P.; Lee, C.; Juang, K.; Lee, D. Arsenic phytotoxicity and accumulation in rice seedlings grown in arsenic-contaminated soils as influenced by the characteristics of organic matter amendments and soils. J. Plant. Nutr. Soil. Sci. 2018, 182, 60–71. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Samal, A.; Majumdar, J.; Santra, S. Accumulation of arsenic and its distribution in rice plant (Oryza sativa L.) in Gangetic West Bengal, India. Paddy Water Environ. 2010, 8, 63–70. [Google Scholar] [CrossRef]
- Norton, G.; Adomako, E.; Deacon, C.; Carey, A.; Price, A.; Meharg, A. Effect of organic matter amendment, arsenic amendment and water management regime on rice grain arsenic species. Environ. Pollut. 2013, 177, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, M.; Bi, X.; He, Y.; Ren, L.; Xiang, W.; Qiao, S.; Yan, S.; Li, Z.; Ma, Z. Occurrence of arsenic in brown rice and its relationship to soil properties from Hainan Island, China. Environ. Pollut. 2011, 159, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Das, S.; Jean, J.; Chakraborty, S.; Liu, C. Arsenic in the water–soil–plant system and the potential health risks in the coastal part of Chianan Plain, Southwestern Taiwan. J. Asian Earth Sci. 2013, 77, 295–302. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sharma, P.; Mitra, S.; Mallick, I.; Ghosh, A. Arsenic Uptake and Bioaccumulation in Plants: A Review on Remediation and Socio-Economic Perspective in Southeast Asia. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100430. [Google Scholar] [CrossRef]
- Dey, U.; Chatterjee, S.; Mondal, N. Isolation and characterization of arsenic-resistant bacteria and possible application in bioremediation. Biotechnol. Rep. 2016, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, J. Impact of Microorganisms on Arsenic Biogeochemistry: A Review. Water Air Soil Pollut. 2014, 225, 1848. [Google Scholar] [CrossRef]
- Chen, X.W.; Li, H.; Chan, W.; Wu, C.; Wu, F.; Wu, S.; Wong, M. Arsenite transporters expression in rice (Oryza sativa L.) associated with arbuscular mycorrhizal fungi (AMF) colonization under different levels of arsenite stress. Chemosphere 2012, 89, 1248–1254. [Google Scholar] [CrossRef]
- Smith, S.; Christophersen, H.; Pope, S.; Smith, F. Arsenic uptake and toxicity in plants: Integrating mycorrhizal influences. Plant Soil. 2009, 327, 1–21. [Google Scholar] [CrossRef]
- Karn, S.; Pan, X.; Jenkinson, I. Bio-transformation and stabilization of arsenic (As) in contaminated soil using arsenic oxidizing bacteria and FeCl3 amendment. 3 Biotech 2017, 7, 50. [Google Scholar] [CrossRef]
- Du, Z.W.; Li, H.R.; Gu, T.Y. A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 2007, 25, 464–482. [Google Scholar] [CrossRef]
- Gustave, W.; Yuan, Z.; Ren, Y.; Raju, S.; Zhang, J.; Chen, Z. Arsenic alleviation in rice by using paddy soil microbial fuel cells. Plant Soil 2019, 441, 111–127. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.; Tripathi, D.; Singh, V.; Chauhan, D.; Prasad, S. Reactive Oxygen Species (ROS): Beneficial Companions of Plants? Developmental Processes. Front. Plant Sci. 2016, 7, 13052. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ye, Z.; Chan, W.F.; Chen, X.W.; Wu, F.; Wu, S.; Wong, M. Can arbuscular mycorrhizal fungi improve grain yield, As uptake and tolerance of rice grown under aerobic conditions? Environ. Pollut. 2011, 159, 2537–2545. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.W.; Wong, M.H. Arbuscular mycorrhizal fungi reduced the ratios of inorganic/organic arsenic in rice grains. Chemosphere 2016, 145, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, J.; GC, A.; Meharg, A.A. Copper and arsenic induced oxidative stress in Holcus lanatus L. clones with differential sensitivity. Plant Cell Environ. 2001, 24, 713–722. [Google Scholar] [CrossRef]
- Purchase, D.; Pantoja Munoz, L.; Jones, H.; Feldmann, J.; Garelick, H. Enhanced determination of As-phytochelatin complexes in Chlorella vulgaris using focused sonication for extraction of water-soluble species. Anal. Methods 2014, 6, 791–797. [Google Scholar] [CrossRef]
- Dwivedi, S.; Kumar, A.; Mishra, S.; Sharma, P.; Sinam, G.; Bahadur, L.; Goyal, V.; Jain, N.; Rudra; Tripathi, D. Orthosilicic acid (OSA) reduced grain arsenic accumulation and enhanced yield by modulating the level of trace element, antioxidants, and thiols in rice. Environ. Sci. Pollut. Res. Int. 2020, 27, 24025–24038. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, Y.; Jin, Q.; Li, F. Silica nanoparticles inhibit arsenic uptake into rice suspension cells via improving pectin synthesis and the mechanical force of the cell wall. Environ. Sci. Nano 2020, 7, 162–171. [Google Scholar] [CrossRef]
- Zhang, S.; Geng, L.; Fan, L.; Zhang, M.; Zhao, Q.; Xue, P.; Liu, W. Spraying silicon to decrease inorganic arsenic accumulation in rice grain from arsenic-contaminated paddy soil. Sci. Total Environ. 2020, 704, 135239. [Google Scholar] [CrossRef]
- Pan, D.; Huang, G.; Yi, J.; Cui, J.; Liu, C.; Li, F.; Li, X. Foliar application of silica nanoparticles alleviates arsenic accumulation in rice grain: Co-localization of silicon and arsenic in nodes. Environ. Sci. Nano 2022, 9, 1271–1281. [Google Scholar] [CrossRef]
- Syu, C.; Huang, C.; Jiang, P.; Chien, P.; Wang, H.; Su, J.; Lee, D. Effects of foliar and soil application of sodium silicate on arsenic toxicity and accumulation in rice (Oryza sativa L.) seedlings grown in As-contaminated paddy soils. Soil Sci. Plant Nutr. 2015, 62, 357–366. [Google Scholar] [CrossRef]
- Yang, X.; Wang, C.; Huang, Y.; Liu, B.; Liu, Z.; Huang, Y.; Cheng, L.; Huang, Y.; Zhang, C. Foliar application of the sulfhydryl compound 2,3-dimercaptosuccinic acid inhibits cadmium, lead, and arsenic accumulation in rice grains by promoting heavy metal immobilization in flag leaves. Environ. Pollut. 2021, 285, 117355. [Google Scholar] [CrossRef] [PubMed]
- Jia-Yi, Y.; Meng-Qiang, S.; Zhi-Liang, C.; Yu-Tang, X.; Hang, W.; Jian-Qiang, Z.; Ling, H.; Qi, Z. Effect of foliage applied chitosan-based silicon nanoparticles on arsenic uptake and translocation in rice (Oryza sativa L.). J. Hazard. Mater. 2022, 433, 128781. [Google Scholar] [CrossRef] [PubMed]
- Avellan, A.; Yun, J.; Morais, B.; Clement, E.; Rodrigues, S.; Lowry, G. Critical Review: Role of Inorganic Nanoparticle Properties on Their Foliar Uptake and in Planta Translocation. Environ. Sci. Technol. 2021, 55, 13417–13431. [Google Scholar] [CrossRef] [PubMed]
- He, C. Effects of furrow irrigation on the growth, production, and water use efficiency of direct sowing rice. Sci. World J. 2010, 10, 1483–1497. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Huang, J.; Ouyang, Y.; Wu, L.; Song, J.; Wang, S.; Li, Z.; Han, C.; Zhou, L.; Huang, Y.; et al. Water management affects arsenic and cadmium accumulation in different rice cultivars. Environ. Geochem. Health 2013, 35, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Pan, J.; Yang, Y.; Cao, Z.; Xu, P.; Chen, M.; Guan, M. Water management affects arsenic uptake and translocation by regulating arsenic bioavailability, transporter expression and thiol metabolism in rice (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2020, 206, 111208. [Google Scholar] [CrossRef]
- Majumdar, A.; Upadhyay, M.K.; Giri, B.; Yadav, P.; Moulick, D.; Sarkar, S.; Thakur, B.K.; Sahu, K.; Srivastava, A.K.; Buck, M.; et al. Sustainable water management in rice cultivation reduces arsenic contamination, increases productivity, microbial molecular response, and profitability. J. Hazard. Mater. 2024, 466, 133610. [Google Scholar] [CrossRef]
- Harine, I.; Islam, M.; Hossain, M.; Afroz, H.; Jahan, R.; Siddique, A.; Uddin, S.; Hossain, M.A.; Alamri, S.; Siddiqui, M.; et al. Arsenic accumulation in rice grain as influenced by water management: Human health risk assessment. Agronomy 2021, 11, 1741. [Google Scholar] [CrossRef]
- Shehzad, M.T.; Sabir, M.; Saifullah; Siddique, A.B.; Rahman, M.M.; Naidu, R. Impact of water regimes on minimizing the accumulation of arsenic in rice (Oryza sativa L.). Water Air Soil Pollut. 2022, 233, 383. [Google Scholar] [CrossRef]
- Majumdar, A.; Upadhyay, M.K.; Giri, B.; Srivastava, S.; Srivastava, A.K.; Jaiswal, M.K.; Bose, S. Arsenic dynamics and flux assessment under drying-wetting irrigation and enhanced microbial diversity in paddy soils: A four year study in Bengal delta plain. J. Hazard. Mater. 2021, 409, 124443. [Google Scholar] [CrossRef] [PubMed]
- Carrijo, D.R.; Akbar, N.; Reis, A.F.B.; Li, C.; Gaudin, A.C.M.; Parikh, S.J.; Green, P.G.; Linquist, B.A. Impacts of variable soil drying in alternate wetting and drying rice systems on yields, grain arsenic concentration and soil moisture dynamics. Field Crops Res. 2018, 222, 101–110. [Google Scholar] [CrossRef]
- Syu, C.; Yu, C.; Lee, D. Effect of applying calcium peroxide on the accumulation of arsenic in rice plants grown in arsenic-elevated paddy soils. Environ. Pollut. 2020, 266, 115140. [Google Scholar] [CrossRef]
- Ma, X.; Sun, K.; Dou, F.G.; Li, X.; Wang, X.; Sun, W. Impact of Elevated Nitrate and Perchlorate in Irrigation Water on the Uptake, Speciation, and Accumulation of Arsenic in Rice (Oryza sativa L.). Water Air Soil Pollut. 2020, 231, 309. [Google Scholar] [CrossRef]
- Kumar, N.; Dubey, A.; Upadhyay, A.; Gautam, A.; Ranjan, R.; Saripella, S.; Sahu, N.; Behera, S.; Mallick, S. GABA accretion reduces Lsi-1 and Lsi-2 gene expressions and modulates physiological responses in Oryza sativa to provide tolerance towards arsenic. Sci. Rep. 2017, 7, 8786. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Dixit, G.; Kumar, A.; Mishra, S.; Kumar, N.; Dixit, S.; Singh, P.; Dwivedi, S.; Trivedi, P.; Pandey, V.; et al. A protective role for nitric oxide and salicylic acid for arsenite phytotoxicity in rice (Oryza sativa L.). Plant Physiol. Biochem. 2017, 115, 163–173. [Google Scholar] [CrossRef]
- Singh, A.; Awasthi, G.; Kumar, A.; Mishra, S.; Singh, P.; Dwivedi, S.; Trivedi, P.; Chakrabarty, D.; Mallick, S.; Pandey, V.; et al. Nitric Oxide Alleviated Arsenic Toxicity by Modulation of Antioxidants and Thiol Metabolism in Rice (Oryza sativa L.). Front. Plant Sci. 2015, 6, 1272. [Google Scholar] [CrossRef]
- Singh, P.K.; Indoliya, Y.; Chauhan, A.S.; Singh, S.P.; Singh, A.P.; Dwivedi, S.; Tripathi, R.D.; Chakrabarty, D. Nitric oxide mediated transcriptional modulation enhances plant adaptive responses to arsenic stress. Sci. Rep. 2017, 7, 3592. [Google Scholar] [CrossRef]
- Yadav, P.; Srivastava, S. Assessing the role of glutathione in arsenic toxicity amelioration in rice (Oryza sativa L.) during early seedling growth. Res. J. Chem. Environ. 2019, 23, 1–6. [Google Scholar]
- .Khan, M.I.R.; Jahan, B.; AlAjmi, M.F.; Rehman, M.T.; Iqbal, N.; Irfan, M.; Sehar, Z.; Khan, N.A. Crosstalk of plant growth regulators protects photosynthetic performance from arsenic damage by modulating defense systems in rice. Ecotoxicol. Environ. Saf. 2021, 222, 112535. [Google Scholar] [CrossRef]
- Asgher, D.M.; Choudhary, S.A.; Sehar, Z.; Gautam, H.; Gandhi, S.; Khan, N. Hydrogen peroxide modulates activity and expression of antioxidant enzymes and protects photosynthetic activity from arsenic damage in rice (Oryza sativa L.). J. Hazard. Mater. 2020, 401, 123365. [Google Scholar] [CrossRef]
- Pandey, C.; Gupta, M. Selenium and auxin mitigates arsenic stress in rice (Oryza sativa L.) by combining the role of stress indicators, modulators and genotoxicity assay. J. Hazard. Mater. 2015, 287, 384–391. [Google Scholar] [CrossRef]
- Mostofa, M.; Rahman, M.; Nguyen, K.; Li, W.; Watanabe, Y.; Cuong, T.; Zhang, M.; Itouga, M.; Fujita, M.; Tran, L. Strigolactones regulate arsenate uptake, vacuolar-sequestration and antioxidant defense responses to resist arsenic toxicity in rice roots. J. Hazard. Mater. 2021, 415, 125589. [Google Scholar] [CrossRef]
- Placek, A.; Grobelak, A.; Kacprzak, M. Improving the phytoremediation of heavy metals contaminated soil by use of sewage sludge. Int. J. Phytoremediation 2016, 18, 605–618. [Google Scholar] [CrossRef]
- Raj, A.; Singh, N. Phytoremediation of arsenic contaminated soil by arsenic accumulators: A three year study. Bull. Environ. Contam. Toxicol. 2015, 94, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Khan, M.; Mcgrath, S.; Zhao, F. Phytoremediation of arsenic contaminated paddy soils with Pteris vittata markedly reduces arsenic uptake by rice. Environ. Pollut. 2011, 159, 3739–3743. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhao, Y.H.; Zhang, X.; Zhang, W.; Sun, D.; Feng, H.Y.; Tang, Y.T.; Qiu, R.L. Research progress on phytoextraction technology of arsenic contaminated soil in farmland. Environ. Eng. 2022, 16, 4037–4048. (In Chinese) [Google Scholar]
- Tanou, G.; Fotopoulos, V.; Molassiotis, A. Priming against environmental challenges and proteomics in plants: Update and agricultural perspectives. Front Plant Sci. 2012, 3, 216. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Dey, S.; Kundu, R. Seed priming: An emerging tool towards sustainable agriculture. Plant Growth Regul. 2022, 97, 215–234. [Google Scholar] [CrossRef]
- Moulick, D.; Samanta, S.; Sarkar, S.; Mukherjee, A.; Pattnaik, B.; Saha, S.; Awasthi, J.; Bhowmick, S.; Ghosh, D.; Samal, A.; et al. Arsenic contamination, impact and mitigation strategies in rice agro-environment: An inclusive insight. Sci. Total Environ. 2021, 800, 149477. [Google Scholar] [CrossRef] [PubMed]
- Moulick, D.; Ghosh, D.; Santra, S. Evaluation of effectiveness of seed priming with selenium in rice during germination under arsenic stress. Plant Physiol. Biochem. 2016, 109, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Moulick, D.; Mazumder, M.; Pattnaik, B.; Ghosh, D.; Vemireddy, L.; Aldhahrani, A.; Soliman, M.; Gaber, A.; Hossain, A. An in vitro and in silico perspective study of seed priming with zinc on the phytotoxicity and accumulation pattern of arsenic in rice seedlings. Antioxidants 2022, 11, 1500. [Google Scholar] [CrossRef] [PubMed]
- Norton, G.; Islam, M.; Deacon, C.; Zhao, F.; Stroud, J.; Mcgrath, S.; Islam, S.; Jahiruddin, M.; Feldmann, J.; Price, A.; et al. Identification of low inorganic and total grain arsenic rice cultivars from Bangladesh. Environ. Sci. Technol. 2009, 43, 6070–6075. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Hasegawa, H.; Rahman, M.; Rahman, M.; Miah, M.M. Accumulation of arsenic in tissues of rice plant (Oryza sativa L.) and its distribution in fractions of rice grain. Chemosphere 2007, 69, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Shi, C.; Wu, J. Genotypic differences in arsenic, mercury, lead and cadmium in milled rice (Oryza sativa L.). Int. J. Food Sci. Nutr. 2011, 63, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Q.; Liao, X.; Li, X.; Zheng, S.; Zhao, F. Phytoexclusion of heavy metals using low heavy metal accumulating cultivars: A green technology. J. Hazard. Mater. 2021, 413, 12547. [Google Scholar] [CrossRef]
- Chen, G.; Du, R.; Wang, X. Genetic Regulation Mechanism of cadmium accumulation and its utilization in rice breeding. Int. J. Mol. Sci. 2023, 24, 1247. [Google Scholar] [CrossRef]
- Lim, S.; Lee, S.; Choi, S.; Lee, J.; Hwang, S.; Jang, C. A TILLING rice ATT1 enhances arsenic tolerance by increasing vacuolar sequestration capacity of arsenic. Environ. Exp. Bot. 2020, 175, 104057. [Google Scholar] [CrossRef]
- Ye, Y.; Li, P.; Xu, T.; Zeng, L.; Cheng, D.; Yang, M.; Luo, J.; Lian, X. OsPT4 contributes to arsenate uptake and transport in rice. Front. Plant Sci. 2017, 8, 2197. [Google Scholar] [CrossRef]
- Modareszadeh, M.; Bahmani, R.; Kim, D.; Hwang, S. Decreases in arsenic accumulation by the plasma membrane intrinsic protein PIP2;2 in Arabidopsis and yeast. Environ. Pollut. 2021, 275, 116646. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhang, L.; Tang, Z.; Huang, X.; Zhao, F. Producing cadmium-free Indica rice by overexpressing OsHMA3. Environ. Int. 2019, 126, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Kamiya, T.; Ishikawa, S.; Arao, T.; Fujiwara, T. Expressing ScACR3 in Rice Enhanced Arsenite Efflux and Reduced Arsenic Accumulation in Rice Grains. Plant Cell Physiol. 2011, 53, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Shri, M.; Dave, R.; Diwedi, S.; Shukla, D.; Kesari, R.; Tripathi, R.; Trivedi, P.; Chakrabarty, D. Heterologous expression of Ceratophyllum demersum phytochelatin synthase, CdPCS1, in rice leads to lower arsenic accumulation in grain. Sci. Rep. 2014, 4, 5784. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Ni, J.; Wang, Y.; Bai, Y.; Shi, J.; Gan, J.; Wu, Z.; Wu, P. OsPHF1 regulates the plasma membrane localization of low- and high-affinity inorganic phosphate transporters and determines inorganic phosphate uptake and translocation in rice. Plant Physiol. 2011, 157, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Olyaie, E.; Banejad, H.; Afkhami, A.; Rahmani, A.; Khodaveisi, J. Development of a cost-effective technique to remove the arsenic contamination from aqueous solutions by calcium peroxide nanoparticles. Sep. Purif. Technol. 2012, 95, 10–15. [Google Scholar] [CrossRef]
- Hochella, M.F.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M.; et al. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363, eaau8299. [Google Scholar] [CrossRef]
- Suri, S.S.; Fenniri, H.; Singh, B. Nanotechnology-based drug delivery systems. J. Occup. Med. Toxicol. 2007, 2, 16. [Google Scholar] [CrossRef]
| Function | Gene Name | Locus ID | Mechanism | Refs. |
|---|---|---|---|---|
| Absorption and transport | OsABCC1 | LOC_Os04g52900 | Promotes the transport of As(III) to the vacuole for sequestration | [42] |
| OsABCC2 | LOC_Os01g67580 | Transports As-PC complexes | [43] | |
| OsABCC7 | LOC_Os04g49900 | Promotes the loading of As(III) inside the xylem | [44] | |
| OsLsi2 | LOC_Os03g01700 | Effluxes As(III) from the root to xylem and phloem | [45] | |
| OsNIP1;1 | LOC_Os02g13870 | Provides a route for As(III) to leak out of the stele, thus, restricting As(III) loading into the xylem | [34,45] | |
| OsNIP2;1 (OsLsi1) | LOC_Os02g51110 | Transports As(III) into the root | [45] | |
| OsNIP2;2 (OsLsi6) | LOC_Os06g12310 | |||
| OsNIP3;1 | LOC_Os10g36924 | Slightly absorbs As(III) in rice | [45] | |
| OsNIP3;2 | LOC_Os08g05590 | Promotes the absorption of As(III) by lateral roots | [36] | |
| OsNIP3;3 | LOC_Os08g05600 | Provides a route for As(III) to leak out of the stele, thus, restricting As(III) loading into the xylem | [34] | |
| OsNRAMP1 | LOC_Os07g15460 | Promotes xylem loading for the root-to-shoot mobilization | [37] | |
| OsPIP2;4 | LOC_Os07g26630 | Influxes and effluxes of As(III) in the roots | [46] | |
| OsPIP2;6 | LOC_Os04g16450 | |||
| OsPIP2;7 | LOC_Os09g36930 | |||
| Metabolism | OsCLT1 | LOC_Os01g72570 | Maintains glutathione homeostasis and a higher accumulation of As(III) in the roots | [47] |
| OsGrx_C2.1 | LOC_Os02g40500 | Involved in redox regulation and protection under oxidative stress/Reduces the accumulation of intracellular As | [48] | |
| OsGrx_C2.2 | LOC_Os04g42930 | Maintains cellular redox homeostasis | [49] | |
| OsPCS1 | LOC_Os05g34290 | Promotes PC synthesis | [50] | |
| OsPCS2 | LOC_Os06g01260 | [51] | ||
| OsPRX38 | LOC_Os03g13210 | Activates the signaling network of different antioxidant systems under As stress conditions and enhances tolerance by reducing the accumulation of As due to apoplastic lignification | [52] | |
| OsSultr1;1 | LOC_Os03g09970 | Enhances the activity of antioxidants | [53] | |
| OsWNK9 | LOC_Os12g06490 | Increases antioxidant capacity, proline and reduces the levels of hydrogen peroxide | [54] | |
| OsLAC6 | LOC_Os01g62600 | Increases the activity of laccase in the leaves, decreases the lignin content in the roots, and negatively regulates tolerance to As(III) | [55] | |
| Regulation | OsADH2 | LOC_Os11g10510 | Regulates silicate transporters to influence the contents of As(III) in the aerial tissues of rice | [56] |
| OsARM1 | LOC_Os05g37060 | Regulates the absorption and root-to-shoot translocation of As(III) | [57] | |
| OsGrx_C7 | LOC_Os01g27140 | Regulates the levels of expression of the aquaporins, which reduce the translocation of As(III) to the roots | [48,58] | |
| OsGSTU5 | LOC_Os09g20220 | Promotes the chelation of As with GSH and sequesters it into the root vacuole using the OsABCC1 transporter and thus, limits the upward translocation of As toward the shoot and maintains the ROS homeostasis, physiological and biochemical activities. | [59] |
| Function | Gene Name | Gene ID | Mechanism | Refs. |
|---|---|---|---|---|
| Absorption and transport | OsPT1 | LOC_Os03g05620 | Promotes the absorption and translocation of As(V) from the roots to shoots | [66] |
| OsPT4 | LOC_Os09g37200 | [67] | ||
| OsPT8 | LOC_Os10g30790 | Promotes the absorption of As(V) | [68,69] | |
| Metabolism | OsHAC1;1 | LOC_Os02g01220 | Promotes the reduction of As(V) to As(III) | [76,77] |
| OsHAC1;2 | LOC_Os04g17660 | [76] | ||
| OsHAC4 | LOC_Os02g06290 | [78] | ||
| OsACR2.1 | LOC_Os10g39860 | Can act as arsenate reductases | [79] | |
| OsACR2.2 | LOC_Os03g01770 | |||
| OsOASTL-A1 | LOC_Os03g53650 | Plays an important role in the biosynthesis of non-protein thiols in the roots to detoxify As | [80] | |
| Regulation | OsPHF1 | LOC_Os07g09000 | Regulates OsPT8 for the absorption and transport of As(V) | [69] |
| OsPHR2 | LOC_Os07g25710 | Regulates Pi transporters to affect the transport of As(V) to the roots and xylem | [69] | |
| OsNLA1 | LOC_Os07g47590 | Primarily regulates As(V) absorption and tolerance by regulating the amount of Pi transporters | [81] | |
| OsWRKY28 | LOC_Os06g44010 | Regulates the accumulation of As(V) in the shoots | [70] | |
| OsCLT1 | LOC_Os01g72570 | Maintains GSH homeostasis by mediating the export of γ-glutamylcysteine and GSH from plastids to the cytoplasm, which, in turn, affects As detoxification in rice | [47] | |
| OsMATE2 | LOC_Os05g48040 | Modulates the accumulation of As in rice grains | [82] |
| Function | Gene Name | Locus ID | Mechanism | Ref. |
|---|---|---|---|---|
| Absorption and transport | OsNIP2;1 (OsLsi1) | LOC_Os02g51110 | Transports of DMA and MMA into the root and upward | [90] |
| OsPTR7 | LOC_Os01g04950 | Involves in the long-distance transport of DMA | [95] | |
| Redistribution | OsPTR7 | LOC_Os01g04950 | Involves in the accumulation of DMA in the rice grains | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, A.; Lian, W.; Wang, Y.; Liu, M.; Zhang, Y.; Wang, X.; Chen, G. The Molecular Mechanism of the Response of Rice to Arsenic Stress and Effective Strategies to Reduce the Accumulation of Arsenic in Grain. Int. J. Mol. Sci. 2024, 25, 2861. https://doi.org/10.3390/ijms25052861
Geng A, Lian W, Wang Y, Liu M, Zhang Y, Wang X, Chen G. The Molecular Mechanism of the Response of Rice to Arsenic Stress and Effective Strategies to Reduce the Accumulation of Arsenic in Grain. International Journal of Molecular Sciences. 2024; 25(5):2861. https://doi.org/10.3390/ijms25052861
Chicago/Turabian StyleGeng, Anjing, Wenli Lian, Yihan Wang, Minghao Liu, Yue Zhang, Xu Wang, and Guang Chen. 2024. "The Molecular Mechanism of the Response of Rice to Arsenic Stress and Effective Strategies to Reduce the Accumulation of Arsenic in Grain" International Journal of Molecular Sciences 25, no. 5: 2861. https://doi.org/10.3390/ijms25052861
APA StyleGeng, A., Lian, W., Wang, Y., Liu, M., Zhang, Y., Wang, X., & Chen, G. (2024). The Molecular Mechanism of the Response of Rice to Arsenic Stress and Effective Strategies to Reduce the Accumulation of Arsenic in Grain. International Journal of Molecular Sciences, 25(5), 2861. https://doi.org/10.3390/ijms25052861







