Abstract
Mycoplasma gallisepticum is one of the smallest self-replicating organisms. It causes chronic respiratory disease, leading to significant economic losses in poultry industry. Following M. gallisepticum invasion, the pathogen can persist in the host owing to its immune evasion, resulting in long-term chronic infection. The strategies of immune evasion by mycoplasmas are very complex and recent research has unraveled these sophisticated mechanisms. The antigens of M. gallisepticum exhibit high-frequency changes in size and expression cycle, allowing them to evade the activation of the host humoral immune response. M. gallisepticum can invade non-phagocytic chicken cells and also regulate microRNAs to modulate cell proliferation, inflammation, and apoptosis in tracheal epithelial cells during the disease process. M. gallisepticum has been shown to transiently activate the inflammatory response and then inhibit it by suppressing key inflammatory mediators, avoiding being cleared. The regulation and activation of immune cells are important for host response against mycoplasma infection. However, M. gallisepticum has been shown to interfere with the functions of macrophages and lymphocytes, compromising their defense capabilities. In addition, the pathogen can cause immunological damage to organs by inducing an inflammatory response, cell apoptosis, and oxidative stress, leading to immunosuppression in the host. This review comprehensively summarizes these evasion tactics employed by M. gallisepticum, providing valuable insights into better prevention and control of mycoplasma infection.
1. Introduction
Mycoplasma gallisepticum is a pathogen that mainly affects chickens and turkeys and causes severe economic losses to the poultry industry across the globe. M. gallisepticum infection causes chronic respiratory disease (CRD) and infectious sinusitis in turkeys, affecting feed conversion rate, egg production, hatchability, and mortality in chickens [1,2]. This disease usually develops slowly and leads to chronic infection in poultry.
M. gallisepticum lacks cell walls and is surrounded by a cytoplasmic membrane containing lipoproteins. It does not contain toxins and its lipoproteins play an important role in pathogen invasion, adsorption, and the induction of inflammatory responses and immune escape. In the one to two weeks after M. gallisepticum infection, there is an acute phase characterized as immune dysregulation. During this period, mycoplasma lipoproteins activate pattern recognition molecules (PRMs), such as TLR2/4/7/15, inducing the expression of cytokines and chemokines, including C-X-C motif chemokine ligand (CXCL)13, CXCL14, C-C motif chemokine ligand 5 (CCL5/RANTES), and interleukin 1β (IL-1β) [3,4]. Large numbers of lymphocytes infiltrate the tracheal tissues, causing local inflammation, but phagocytosis is not fully activated, especially in the absence of specific antibodies [5,6,7]. Mycoplasma may suppress the immune response to some extent, thus allowing the persistence of the infection. It was found that CD8+ T cells were absent in the acute phase [3,8] and that the predominant type of antibody secreted by B cells as well as the amount and pattern of B cell infiltration differed between unvaccinated and vaccinated chickens [3,9,10]. The suppression of the adaptive immune response by M. gallisepticum may be crucial to the progression of the disease as local antibodies and cell-mediated immunity are effective in clearing and preventing M. gallisepticum infection [11]. Immune evasion, therefore, presents significant challenges to the control and clearance of the pathogen.
In most cases, immunization of flocks with vaccines is an essential means of the prevention and control of infectious diseases. However, the current vaccines against M. gallisepticum provide only limited protection and partial or temporary immunity against M. gallisepticum infection. More effective vaccines against M. gallisepticum infection are in urgent demand. However, the development of novel vaccines requires guidance from a deep understanding of the strategies employed by M. gallisepticum for its survival and immune evasion in the host. In recent years, considerable progress has been made in the understanding of the pathogenesis of M. gallisepticum infection. Therefore, this review focuses on our current understanding of the interaction of M. gallisepticum with the host, in particular, the immune evasion of M. gallisepticum from the host immune response. This information may contribute to the development of potential therapeutic approaches for the control of M. gallisepticum infection.
2. Invasion and Transmission
Although M. gallisepticum is not highly transmissible, it can spread vertically and horizontally and its infection of flocks on farms is difficult to completely eradicate, often persisting for a long time [12]. The mechanism of mycoplasma cell infection can be summarized as gliding, adhesion, and invasion. M. gallisepticum lacks flagella or pili but could form a membrane protrusion composed of bleb and infrableb, allowing it to glide [13] and work cooperatively with adhesin complexes, namely CrmA and GapA [14,15]. This gliding ability enables them to reach epithelial surfaces and to breach certain physical defenses of the host such as ciliary activity and the mucin layer in the respiratory tract [16].
M. gallisepticum has a high affinity towards chicken respiratory epithelial cells by binding to host cells through adhesins, inducing the expressions of a series of pro-inflammatory cytokines and causing apoptosis or necroptosis in cells [7,17,18]. M. gallisepticum hemagglutinin protein pMGA1.2 interacts with cellular protein ApoA-I to establish infection [19]. Other surface proteins, such as hemadsorption-mediating proteins GapA and CrmA or variable surface membrane proteins (p30, p48, p50, and p80), are able to adhere to cells and serve as prerequisite adhesion components for cell invasion [20]. GapA, CrmA, and Mgc2 have been shown to be involved in the gliding ability of M. gallisepticum, necessary for effective adhesion and movement of the microbe from the cilia tips to the apical cell membrane, leading to cell invasion [21].
M. gallisepticum also utilizes extracellular matrix (ECM) proteins as a secondary anchoring system [22] as well as its surface protein PlpA; Hlp3 has been shown to bind to the gelatin/heparin-binding structural domain of fibronectin [23]. It was suggested that the M. gallisepticum strain Rhigh [24,25] has reduced cytadherence capacities [20] due to the lack of major cytadherence proteins GapA and CrmA [21], which improves cell adhesion rates in Hela cells when preincubated with plasminogen, fibronectin, and plasmin. It seems that the invading process of mycoplasma is much more complicated than predicted.
Cytadherence is a prerequisite for mycoplasma cell invasion and some studies suggest that mycoplasma entry into cells may be via lipid rafts [22]. However, the mechanism of M. gallisepticum is less certain. It was reported that M. gallisepticum synthesizes hydrogen peroxide to initiate lipid peroxidation of host cell membrane, thereby compromising the integrity and permeability of the eukaryotic cell membrane and facilitating bacterial entry [26]. Other mycoplasmas are diverse and may involve cellular pathways or endocytic mechanisms [27,28].
M. gallisepticum adheres to the tracheal mucosa, causing damage to ciliated cells. This impairs the cell’s ability to expel foreign materials and sticky secretions from the trachea, resulting in lung lesions such as tracheitis and airsacculitis that affect normal breathing. Once the lungs are colonized with M. gallisepticum, the invader spreads to all organs of the body and its presence can be detected in the heart, brain, liver, spleen, and kidneys [29]. This may be related to the fact that M. gallisepticum invades non-phagocytic chicken cells, such as red blood cells, tracheal epithelium, and embryonic fibroblasts, and reaches the distal limbs through the blood [30,31]. In some cases, M. gallisepticum infection is also associated with arthritis [32], salpingitis [33], conjunctivitis [34], and fatal encephalopathy [35]. Thus, M. gallisepticum infection could impact multiple organs or tissues, causing considerable economic losses to the stakeholders.
M. gallisepticum has acquired a perfect transport system, which benefits from the ability to invade the host’s erythrocytes during infection and cross the mucosal barrier to spread systemically in vivo [31]. This allows M. gallisepticum to settle in tissues at a distance while being protected by the host immune system and then possibly escape by lysing the erythrocytes with the help of membrane-bound hemolysin activity [36]. Thus, mycoplasma takes full advantage of host erythrocytes to benefit its survival and spread.
3. Genetic and Protein Characteristics of M. gallisepticum
Following mycoplasma invasion, the host initiates an immune response that suppresses M. gallisepticum infection. However, many mycoplasmas, including M. gallisepticum, are able to establish successful infection in a wide range of hosts and often cause chronic disease, even in the presence of a specific immune response. Their successful colonization and survival in hosts may contribute to the ability of pathogens to evade host immunity by rapidly changing the phase and size of antigens on their surfaces. For example, the pMGA gene family of M. gallisepticum adhesion proteins produces antigenic variants by switching the expression of different pMGA genes [37,38].
Mycoplasmas possess many complex systems that control the mechanisms directed toward the phase and size variation of surface lipoproteins with a high-frequency variation [39,40]. This allows one variant to prevail when faced with unpredictable challenges. So far, two possible mechanisms for the induction of variants have been described: one is based on spontaneous mutations in regions prone to DNA slippage due to nucleotide insertions/deletions in homologous or heterologous polynucleotide tracts or short tandem repeats [41,42], occuring mainly in M. hyorhinis and M. gallisepticum, and the other involves chromosomal rearrangements, which are utilized, for example, by M. bovis and M. synoviae [43,44]. It seems that the mechanism for induction of variants of mycoplasma is related to the host to some extent.
M. gallisepticum contains up to 50 members related to genes for putative adhesion molecules (pMGA) [45]. The expression of the pMGA genes of M. gallisepticum is controlled by variation in the GAA trinucleotide repeat lengths within the 5′ non-coding regions [42]. As shown in Figure 1, there are 12 GAA trinucleotide motifs upstream of the −35 boxes of the pMGA1.1 gene. The downstream pMGA gene is only expressed when the number of repeats of upstream GAA trinucleotide is 12. Interestingly, the expression of one member of the pMGA gene family is accompanied by the cessation of other pMGA transcription and usually only one pMGA gene is predominantly expressed in any given strain [46]. High-frequency alterations in 5′ trinucleotide repeat numbers are the predominant reason for the switch from producing pMGA1.1 to producing alternative pMGA1.9 on the surface of M. gallisepticum strain S6 in vitro culture in the presence of specific antibodies against pMGA1.1 in the growth medium [47]. It was reported that the pMGA phenotype of M. gallisepticum could reversibly switch from the expression of pMGA1.1 to pMGA1.2 in vivo infection and this rapid and reversible switch was also detected in vitro when co-cultured with pMGA1.1 antibody. But in vivo, the switching of pMGA protein occurs before host pMGA antibody production, suggesting that factors other than antibodies could influence M. gallisepticum population conversion and that changes in the pMGA gene may be necessary for M. gallisepticum colonization, infection, or escape from host immunity [38].
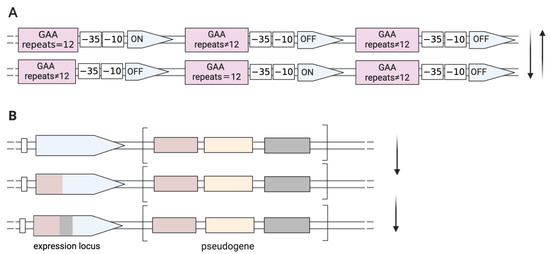
Figure 1.
Genetic mechanisms directed towards phase and size variation of surface proteins with a high frequency. (A) ON/OFF switching by DNA slippage in M. gallisepticum. Spontaneously high-frequency mutations occur in the GAA trinucleotide motifs located upstream of the −35 boxes of the pMGA gene. Transcription of any pMGA gene occurs only when there are twelve GAA trinucleotide repeats. (B) Antigenic variations in M. synoviae occur through unidirectional recombination (gene conversion) that generates a chimeric-expressed gene, occurring between an expressed locus and pseudogenes, which are the reservoir sequence pools located in relatively closed clusters.
4. The Innate Immune Response to M. gallisepticum
Innate immunity is the first line of host defense against pathogenic infection and a strong inflammatory response is closely associated with M. gallisepticum-caused CRD [48]. M. gallisepticum attaches and colonizes at the respiratory epithelium and these events occur nearly without classical invasion of tracheal epithelial cells or breach of the mucosal barrier. However, it can mediate severe inflammation and a marked subepithelial infiltration initially by heterophils and macrophages, followed by infiltration of lymphocytes, including B and T cells, into the mucosa [49]. Macrophages and other inflammatory cells are recruited into tracheal mucosa along with the upregulation of chemokines, inflammatory factors, and Toll-like receptor (TLR)-mediated signal activation, including IL-1, IL-8, CXCL13, RANTES, and macrophage inflammatory protein-1β (MIP-1β/CCL4) [4], subsequently recruiting and activating naive B and T lymphocytes into tissues, resulting in more profound dysregulation of the subsequent adaptive immune response.
After M. gallisepticum infection, it was found that the expression of cytokines was upregulated in samples, irrespective of whether the specimens were taken from whole tracheal structures [4] or lavage fluid [6]. A rapid host response was observed 24 h after infection with M. gallisepticum, with more than 2500 differentially expressed genes on the peak day of infection, followed by a gradual decrease in differences [50]. Many of these genes are involved in immune-related signaling pathways, including TLR, the mitogen-activated protein kinase (MAPK) pathway, Janus kinase/signal transducer and activator of transcription (Jak-STAT), the NOD-like receptor signaling pathway [50], injury response, metabolism, cell adhesion and remodeling, extracellular matrix (ECM) degradation, and membrane transport [6]; the gene downregulation is associated with impaired ciliary body movement and intercellular connections in infected M. gallisepticum strain Ap3AS [5], which ultimately contributes to the pathological damages of the epithelial barrier.
TLRs play important roles in inflammation and innate immune response [51], the expression of many cell surface receptors increased post mycoplasma infection, such as TLR2, TLR4, and TLR15 [50] as well as TLR7 [52]. Their expressions may involve severe immune dysregulation and the development of diseases. TLRs could recognize multiple components of pathogens, including bacteria, viruses, fungi, and protozoa [53]. Bacterial lipoproteins serve as pathogen-associated molecular patterns (PAMPs) that are recognized by the heterodimerization of TLR1/2 or TLR2/6 [54,55]. Most mycoplasma lipopeptides are diacylated and recognized by TLR2/6 and triacylated lipopeptides of mycoplasma are recognized by TLR1/2 [56]. Additionally, TLR15 also recognizes M. synoviae diacylated lipopeptide based on the N-terminal sequence of MSPB [57]. Recognition of PAMPs by TLRs activates the NF-κB-mediated cascade inflammatory responses, leading to the expression of proinflammatory cytokines, such as IL-1β, IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) [58], which are associated with inflammatory lung injury [59].
M. gallisepticum infection triggers the nuclear factor kappa B (NF-κB) signaling pathway through TLRs including TLR-2/TLR-4/TLR-6 and nod-like receptors (NLRs) [60,61,62]. It was reported that M. gallisepticum -HS infection upregulates TLR6 and stimulates IL-2/IL-6-mediated inflammatory responses in DF-1 cells via the TLR6-MyD88-NF-κB signaling pathway [60]. M. gallisepticum lipid-associated membrane proteins (LAMPs) induce the expressions of IL-1β, IL-6, IL-8, IL-12p40, CCL-20, and nitric oxide synthase-2 (NOS-2) in epithelial cells when treated both in vitro and ex vivo [63]. It was found that M. gallisepticum LAMPs-induced IL-1β expression involved the NF-κB signaling pathway via TLR2 in DF-1 cells and chicken tracheal epithelial cells [64]. IL-12p40 mRNA is usually up-regulated but declined rapidly thereafter [63]. The inflammatory response is triggered by M. gallisepticum infection in macrophages via the TLR-2-NF-κB-mediated NLRP3-inflammasome pathway [65]. TLR2 can also activate autophagy through the extracellular signal-related kinase (ERK1/2) signaling pathway, which helps resist M. gallisepticum invasion. Although M. gallisepticum does not possess lipopolysaccharide (LPS), it can increase the expression of TLR4, which is possibly due to increased numbers of immune cells [66,67]. Recently, it has been found that damage-associated molecular pattern (DAMP)-family molecules, such as high-mobility group box 1 (HMGB1), act as inflammatory mediators secreted by infected macrophages, which could stimulate nearby immune cells such as monocytes, dendritic cells, and monophagocytes [68]. Extracellular HMGB1 upregulates the expression of pro-inflammatory factors including IL-6, IL-1β, IL-12, and TNF-α through the activation of TLR2/4, causing cytokine storms, immune disorders, and severe apoptosis in the spleen, thymus, and bursa of Fabricius [69].
In general, the infection of chickens with M. gallisepticum sensed by TLRs triggers the NF-κB signaling pathway, causing inflammatory responses. If the inflammatory response is uncontrollable, excessive proinflammatory cytokines, such as IL-6, IL-1β, IL-12, and TNF-α would be produced, resulting in cytokine storms associated with immune damage or even sepsis. Although inflammation is necessary to combat the spread of microbes, excessive inflammation can still cause tissue damage and is one of the key features of M. gallisepticum-induced CRD [70]. However, suppression of the innate immune response may have exacerbated M. gallisepticum infection [71]. This will be discussed in detail in the following section (Section 5).
5. Escape from Innate Immune Response
5.1. Regulation of the Inflammatory and Chemokine Cytokines
The inflammatory response is modulated by a number of transcription factors and cellular pathways [72], which promote the production of ROS and pro-inflammatory mediators [73]. Some key inflammatory cytokines, such as TNF-α, IL-1 and IL-6, are capable of initiating cell recruitment to facilitate the clearance of pathogens [74]. It was demonstrated using in vitro assays that M. gallisepticum is able to interfere with the inflammatory response. IL-6 and TNF-α did not change significantly up to 8 days after M. gallisepticum strain Rlow infection, except that IL-1 was significantly up-regulated at 3 days post-infection [4]. Similarly, the expression of the tumor necrosis factor (TNF) gene and the CCL20 gene was downregulated two weeks after infection with M. gallisepticum strain Ap3AS [5], which may be attributed to the immunomodulatory effects of M. gallisepticum [4]. The mRNA levels of IL-1β, IL-6, and CCL20, which are known to trigger an inflammatory response, peaked at 6 h following infection with M. gallisepticum in HD-11 cells and tracheal epithelial cells, then gradually decreased and reached the baseline 24 h post-infection [7]. Eight hours after M. gallisepticum infection in chicken primary alveolar type II epithelial cells, large amounts of TNF-α and IL-6 were produced and inflammation was suppressed 24 h after infection [71]. M. gallisepticum causes a strong inflammatory response but does not stimulate certain classical cytokines and it has been shown to transiently activate and then inhibit the inflammatory response [75], which may be one of the reasons for immune dysregulation and evasion of premature clearance by the organism. In addition, several studies suggest that the functions performed by macrophages are not fully activated because TNF-α and IL-12 tended to be downregulated [4,5,6]. Phagocytosis by the macrophage could be observed 2 weeks post-infection, after the phase of immune dysregulation [5]. The interaction of M. gallisepticum with host respiratory epithelial cells prompts macrophage chemotaxis and activation, which leads to the expression of a unique set of inflammatory and chemokine genes. However, key cytokines such as IL-12p40 failed to be up-regulated, which may lead to incomplete macrophage activation and thus reduced mycoplasma clearance efficiency [7]. Recent studies have shown that extracellular vesicles (EVs) can influence the downregulation of inflammatory factors and the activation of macrophages in vitro. It has been observed that while M. gallisepticum infection alone upregulates pro-inflammatory factors such as TNF-α, IL-1β and IL-6, the presence of M. gallisepticum-derived EVs results in a dose-dependent suppression of these factors in HD11 cells at 24 h post-infection [76].
Mycoplasmas can regulate the inflammatory response by activating the nuclear factor erythroid 2-related factor-2 (Nrf2) [77,78]. The lipopeptide MALP-2 (macrophage-activating lipopeptide) from M. fermentans is the first lipopeptide known to bind Toll-like receptors (TLR2/6) [79,80]. Its activation leads to an increase in the expression of heme oxygenase-1 (HO-1) [81], an enzyme involved in cellular defense against oxidative stress [82]. By upregulating HO-1, the release of pro-inflammatory molecules such as IL-6, IL-8, ROS, NO, and PGE2 was negatively regulated in the M. pneumoniae LAMPs-stimulated cells, thereby helping Mycoplasma evade the immune system and suppress the overall inflammatory response [77,83]. In vitro experiments reveal that the membrane lipoproteins (LAMPs), along with lipoprotein derivatives (lipopeptide MALP-2) in mycoplasmas, induce a “cross-talk” between the pro-inflammatory and anti-inflammatory signaling pathways, including NF-κB and Nrf2/ARE [83]. A homolog of macrophage-activating lipoprotein (MALP-2) in M. gallisepticum has been characterized and was found to have no effect on attachment, growth, or pathogenicity in tracheal organ cultures [84] and the exact role of P47 in regulating inflammation is still unclear. Moreover, Nrf2 and HO-1mRNA and protein expression levels did not significantly change in the M. gallisepticum infected group compared to the control group [85]. Overall, mycoplasmas may interfere with the inflammatory response to evade the immune system but the exact mechanism requires further study.
5.2. Effects of M. gallisepticum at an RNA Level
MicroRNAs (miRNAs) are small noncoding RNAs of 20 to 24 nucleotides that regulate eukaryotic gene expression post transcriptionally by affecting the degradation and translation of target mRNAs [86]. They can bind to the mRNA 3′UTR to promote mRNA degradation or inhibit translation [87]. It was reported that respiratory infectious diseases are closely related to miRNAs [88,89]. After M. gallisepticum infection, miRNAs were differentially expressed in the lungs of chicken embryos, with 36 down-regulated and 9 up-regulated miRNAs belonging to a family of 31 miRNAs detected at 3 days post-infection, while 50 down-regulated and 18 up-regulated miRNAs were found at 10 days post-infection. These altered miRNAs target genes in diverse pathways, such as the MAPK pathway, focal adhesion, Wnt pathway, endocytosis, Jak/STAT pathway, phosphatidylinositol pathway, and adherens junctions [62]. Additionally, about 30 microRNAs derived from CP-II cell-derived exosomes were significantly differentially expressed post M. gallisepticum infection. Those exosome-miRNAs exerted influence on both neighboring and distal cells by regulating cellular pathways, particularly in the cell cycle, Toll-like receptor signaling pathway, and MAPK signaling pathway [90]. Furthermore, miRNAs play a pivotal role in regulating these cellular processes, which in turn impacts the replication of M. gallisepticum, as illustrated in Figure 2.
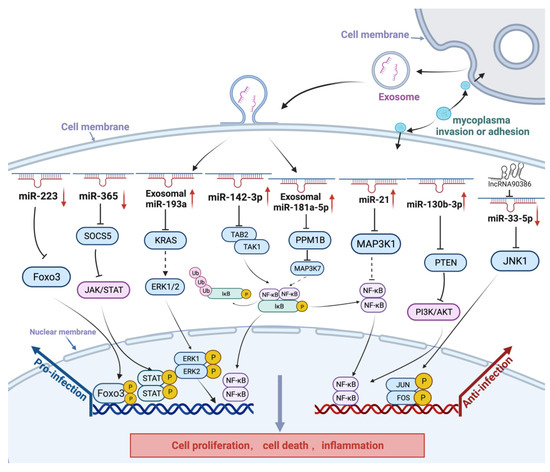
Figure 2.
Schematic diagram of non-coding RNAs in the host response to M. gallisepticum infection. Upward arrows indicate an increase in the expression of miRNAs, while downward arrows denote a decrease in their expression. After M. gallisepticum infection, host cells inhibit M. gallisepticum replication, for example, by regulating the expression of miRNAs (e.g., miR-181a-5p, miR-21, and miR-130b-3p) to promote cell proliferation, inhibit cell apoptosis, and enhance the expression of inflammatory cytokines. In addition, M. gallisepticum can regulate miRNAs (e.g., miR-223, miR-365-3p, miR-193a, and miR-142-3p) to promote self-replication. In the early stage of M. gallisepticum infection in cells, gga-miR-365-3p was upregulated and activated the JAK/STAT signaling pathway to suppress the expression of pMGA1.2. At the later stage of infection, gga-miR-365-3p was downregulated through a negative feedback mechanism, thereby promoting mycoplasma adhesion or invasion.
It has been found that M. gallisepticum infection inhibits the cell cycle by blocking the transition of DF-1 cells from G1 to S and G2 phases and promotes cell apoptosis [91,92]. Some miRNAs are involved in inhibiting M. gallisepticum replication, mainly by upregulating the expression of pro-inflammatory cytokines (such as TNF-α, IL-6, and IL-8), influencing the cell cycle, and activating signaling pathways. Upregulated expression of miR-130b-3p promoted cell proliferation and the inflammatory response through regulating the PI3K/AKT/NF-κB-mediated signaling pathway in M. gallisepticum-infected chicken embryo lungs and DF-1 cells by targeting phosphatase and tensin homolog (PTEN) expression in vivo and in vitro, which plays a role in inhibiting cell proliferation and inducing G1 arrest [93]. Furthermore, it was shown that gga-miR-21 negatively regulated M. gallisepticum propagation by promoting the proliferation of M. gallisepticum infected cells, enhancing the expression of inflammatory cytokines and inhibiting cell apoptosis, which is mediated by activating MAPKs and NF-κB signaling pathways via targeting MAP3K1 in the M. gallisepticum infected DF-1 cells [94]. It was found that miR-181a-5p is transported into DF-1 cells through exosomes secreted by CP-II cells and targets PPM1B to activate the TLR2/MYD88/NF-κB signaling pathway, leading to the promotion of cell proliferation, inflammatory response, and inhibition of apoptosis triggered by M. gallisepticum-HS infection [95]. Similarly, gga-miR-99a and gga-miR-19a were also found that can trigger the transition from G1 to S and G2 phases of the cell cycle by targeting genes post M. gallisepticum infection [96,97]. In addition, some microRNAs promoted cell proliferation and attenuated the inflammatory response and apoptosis to resist M. gallisepticum infection. It was found that upon M. gallisepticum infection, Lnc90386 sponged miR-33-5p to negatively regulate the c-Jun N-terminal kinase (JNK) pathway and inhibited M. gallisepticum pMGA1.2 expression, promoting cell proliferation and inhibiting inflammatory damage and apoptosis caused by M. gallisepticum infection [98].
In chronic respiratory diseases in chickens, aberrant expression of some miRNAs is involved in inhibiting M. gallisepticum replication. Host miRNAs affect the replication of pathogenic microorganisms and these microorganisms develop highly complex mechanisms to evade the host immune response. Gga-miR-142-3p, which mainly suppresses the expression of pro-inflammatory cytokines (such as TNF-α, IL-1 and IL-6), influences the cell cycle and activates signaling pathways, thereby influencing M. gallisepticum infection [99]. FOXO3 belongs to the O subclass of the forkhead family of transcription factors and plays a crucial role in cell proliferation and apoptosis [100]. It was found that M. gallisepticum infection downregulated gga-miR-223 in host cells, which targets FOXO3 to downregulate the expression of cycle genes (CDK1, CDK6, and CCND1) and promotes the expression of apoptosis genes (BIM, FASL, and TRAIL), finally exacerbating M. gallisepticum infection [101].
Following M. gallisepticum infection, exosomes secreted from M. gallisepticum-infected alveolar epithelial cells can cause or exacerbate lung inflammation and significantly increase TNF-α and IL-1β protein levels in DF-1 cells [95]. Gga-miR-193a content increased in exosomes to disturb distal cell proliferation, apoptosis, and cytokine production by targeting the KRAS/ERK signaling pathway [102] and gga-miR-451 was significantly reduced in exosomes, enhancing the inflammatory response in DF-1 cells, although gga-miR-451 expression was highly significantly upregulated in M. gallisepticum-infected CP-II cells [90]. In addition, the inflammatory response is critical in modulating the host’s immune response to pathogens such as bacteria, viruses, and parasites but a strong inflammatory immune response is an important cause of CRD. In the early stage of M. gallisepticum infection in cells, gga-miR-365-3p was upregulated and activated the JAK/STAT signaling pathway through inhibition of SOCS5 (suppressor of cytokine signaling 5), thus promoting the secretion of inflammatory factors, such as TNF-α and IL-6, and suppressing the expression of pMGA1.2. At the later stage of M. gallisepticum infection of cells, SOCS5 downregulates the expression of intracellular gga-miR-365-3p through a negative feedback mechanism, thereby reducing cellular inflammatory damage while promoting mycoplasma adhesion or invasion [71].
Although there is a wealth of research on M. gallisepticum pathogenesis at the RNA level, comprehensive summaries are limited. It seems that M. gallisepticum utilizes microRNAs to both directly and indirectly influence inflammatory processes, apoptosis, and cell proliferation, altering host cell functions and shaping inflammatory responses to support their survival and proliferation. The host response to M. gallisepticum infection is also closely related to miRNAs. Further investigation into the role of miRNAs in the host response to M. gallisepticum infection will contribute to a complete understanding of the pathogenesis of M. gallisepticum infection at an RNA level.
6. The Adaptive Immune Response to M. gallisepticum
6.1. Mucosal Immunity
M. gallisepticum initially colonizes the upper respiratory tract, firmly adheres to the surface of epithelial cells to resist host clearance from mucosal epithelial cell cilia and phagocytosis by phagocytic cells, and then spreads to the lower respiratory trachea, causing bronchitis, airsacculitis, and pneumonia [33]. Therefore, the immunity of the respiratory trachea is crucial to inhibit M. gallisepticum infection. The respiratory immune system is associated with various structures connected to the mucosal surface that is exposed to polluted air, including the Harderian gland (HG), conjunctiva-associated lymphoid tissue (CALT), paranasal glands (PG), and bronchus-associated lymphoid tissue (BALT) [103]. They are functionally important components of local immunity, especially in the upper airways.
Although chickens are genetically deficient in lymph nodes, infection of chickens with M. gallisepticum strongly activates the tracheal mucosal immune response, causing inflammatory lesions in local tissues; the severity of immune damage is age-related [104]. The different degrees of damage caused by M. gallisepticum infection are marked by the development of thickened and debilitated mucosal epithelium due to the large number of lymphocytes, histiocytes, and plasma cells infiltrating the lamina propria, accompanied by a small number of heterophils diffusely distributed in the epithelium and luminal exudate [10]. Thus, the diffuse tertiary lymphoid tissues along the respiratory trachea are involved in local immune response as well as the pathogenesis of M. gallisepticum infection.
It was reported that there are large numbers of B cells and CD4+ and CD8+ T cells infiltrating the tracheal mucosa of chickens post M. gallisepticum infection, as shown in Figure 3. In five-week-old birds infected with M. gallisepticum (Rlow), B cells were found to be the major lymphocyte population infiltrating the mucosa from one day after infection [10]. In another study, B cells were detected three weeks after infection with the AP3AS strain of M. gallisepticum in eight-week-old birds and only CD8+ and CD4+ T cells were observed in the first week [11]. Recently, it was found that B cell recruitment into the mucosa in mature birds occurred later than that in young birds and is generally detectable two weeks after infection [3]. Birds under four weeks of age likely have a stronger immune response and more severe damage than mature birds, with greater concentrations of M. gallisepticum in the trachea, which may be related to the maturity of the immune system [104], especially BALT, whose structure, morphology, and ability to perform defense functions in birds are largely age-dependent. Mature BALT is covered with a layer of fragile epithelium called follicle-associated epithelium (FAE), which harbors numerous lymphocytes [105,106]. These tertiary lymphoid tissues play a major role in the tracheal mucosal immune response. B lymphocytes were not detected until 3 weeks after infection and their appearance coincided with a decrease in the concentration of mycoplasma DNA detectable in the trachea [11]. In general, B cells should appear in local inflammatory sites; more efforts will be required to instigate the involvement of immune cells in tissues with M. gallisepticum infection.
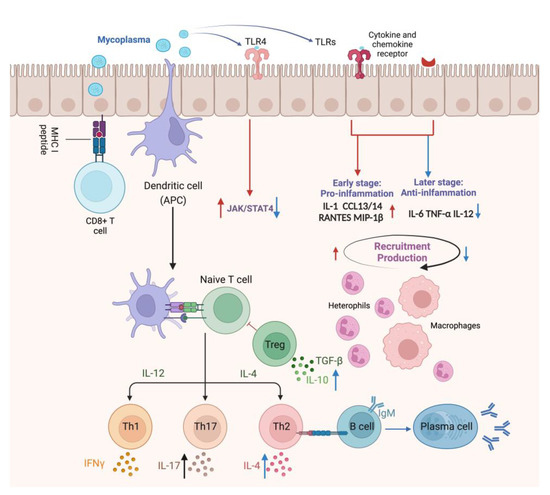
Figure 3.
The immune response of the tracheal mucosa to M. gallisepticum infection in the respiratory tract. The red arrows indicate the modulation of early-stage immune mechanisms initiated upon infection, highlighting the induction of inflammation and recruitment of immune cells to the site of infection. M. gallisepticum attaches and colonizes the airway epithelium and is recognized by epithelial TLRs, initiating a TLR-mediated signaling pathway that regulates the expression of pro-inflammatory factors, including IL-1, CXCL13, CXCL14, RANTES, and MIP-1β, followed by the recruitment of macrophages and inflammatory cells to the local tissue. These recruited immune cells release inflammatory mediators to enhance host response and the secreted chemokines recruit naive B and T lymphocytes to the local tissue, where they are activated for subsequent adaptive immune responses. Conversely, the blue arrows illustrate the late stage of infection when M. gallisepticum acts to suppress inflammatory mediators and modulate the immune response, potentially suppressing inflammatory mediators such as IL-6 and TNF-α, influencing Th1 immune responses through the modulation of JAK/STAT4 pathway and cytokines correlated with cell-mediated immunity, such as IL-2 and IL-4, which prevents the host immune system from clearing the pathogen prematurely.
6.2. Cell-Mediated Immunity
The cell-mediated immune response to M. gallisepticum in the tracheal mucosa of vaccinated and unvaccinated chickens during the acute and chronic stages of the disease plays a crucial role in clearing the pathogen. Previous studies have used immunohistochemistry to determine the number of lymphocytes in the upper, middle, and lower trachea of chickens [29]. It was found that both CD8+ and CD4+ cells were observed in the first week of M. gallisepticum infection, notably with an elevated prevalence of CD8+ cells and the formation of follicular aggregates within the tracheal mucosa. However, the vaccinated birds did not have significantly greater numbers of CD4+ or CD8+ lymphocytes in their tracheal mucosa [10,107]. In unvaccinated chicken, CD8+ cells peaked at 1 week and then declined significantly in the tracheal mucosa [3] and similar changes in CD8+ cell number and distribution were observed in the tracheal mucosa of infected turkeys [107]. However, these cells were shown to be CD8+TCR− cells by double immunohistochemical staining of TCR and CD8 and are likely to be NK or NK-like cells, which are involved in the initial, possibly non-specific, inflammatory response and may be related to the pathogenesis of M. gallisepticum in the trachea [11], as increased expression of various chemokines and proinflammatory cytokines was observed one week post-M. gallisepticum infection [4]. The numbers of CD8+TCR+ cells increased over 6 weeks after infection [11], suggesting a subsequent cytotoxic T-cell response to M. gallisepticum infection.
The number of CD4+ cells was low in the first two weeks and then remained high from the third week post-infection. The proportion of CD4+TCRαβ1+ cells increased over the first 3 weeks and then subsequently decreased, while the proportion of CD4+TCRαβ2+ cells constantly increased during the 6-week experimental period [11]. The shift in CD4+ cells in the trachea from CD4+TCRαβ2+ to CD4+TCRαβ1+ during the infection suggests a change in T-cell immunity in the trachea after infection.
7. Escape from the Adaptive Immune Response in Trachea
Local immunity for tracheal mucosa plays an important role in preventing mycoplasma attachment to respiratory epithelia. Unvaccinated chickens had several defined B-cell clusters with many interspersed CD4+ cells [10] and these B cells are Th-dependent and mainly secrete lgA. The number of IgA+ cells is high in unvaccinated chickens after infection but does not contribute to accelerated pathogen clearance and recovery from infection, probably due to the immune escape of the pathogen and the fact that mucosal immunity exists mainly on the mucosal surface and covers a small area [10]. In contrast, GT5-vaccinated chickens were found to have lower numbers of infiltrating lymphocytes, with more B-cells organized in a typical follicular pattern one week after infection. Chickens vaccinated with ts304 showed a similar situation, with no CD3+, CD4+, or CD8+ cells detected in the tracheal mucosa two weeks after challenge at 60 weeks of age [3]. B cells without help from CD4+ cells are commonly Th-independent and mainly secrete IgM, which promotes M. gallisepticum clearance and reduces the severity of the damage. Several vaccines, such as ts-11, GT5, and ts304, have been shown to effectively prevent the development of the disease in birds after challenge [104].
In general, the regulation of mucosal immunity by M. gallisepticum includes the following. First, M. gallisepticum infection leads to damage of mucosal epithelial cells, which inhibits the generation and impaired function of mucosal immunity. Second, the different types of antibodies produced by B cells and the degree of lymphocyte infiltration may have an impact on mucosal immunity. Third, pMGA phenotypic variation in M. gallisepticum occurs by selection pressures induced by antibodies and this might be an essential tactic employed by the pathogen for immune evasion [108]. Furthermore, CysP (cysteine protease) is involved in the cleavage of IgG by M. synoviae and M. gallisepticum. The IgG cleavage pattern is similar to that of papain, a cysteine protease that cleaves IgG into Fab and Fc, but the effect of this on immune escape is not yet known [109]. CD8+ cell depletion may be a result of immune dysregulation caused by M. gallisepticum, which contributes to their replication and survival [8]. Similar signs of immune dysregulation have been found in recent studies in the bursa of Fabricius [8,110]. Immune dysregulation after M. gallisepticum infection may be related to Th cell differentiation and proliferation, which could be cross-regulated by the effector cytokines produced by other Th subsets. CD4+ Treg cells secreting IFN-γ and IL-17 can dampen Th2 responses, including the IL-13 response and the serum antibody response to chronic infections with M. pulmonis [111]. Moreover, an upregulation of IFN-γ and IL-17 was also found two weeks after mycoplasma infection and at the same time, the downregulation of IL-2 also corroborates the suppression of the cellular immune response [3]. However, the exact mechanism underlying mycoplasma-induced immune compromise requires further investigation.
Recent studies suggest that M. gallisepticum induced immune dysregulation may be associated with TLR4-mediated regulation of the JAK/STAT signaling pathway, exerting opposite effects of promoting or inhibiting Th subpopulation polarization at different times of infection [75]. It was shown that on day 3 after M. gallisepticum infection, TLR4 mediated the activation of IL-12Rβ2 and TYK2, leading to STAT4 phosphorylation and the release of proinflammatory factors. However, the expression of these genes tended to decrease and the activation of the JAK/STAT signaling pathway was inhibited on days 5 and 7 post-infection, suggesting that M. gallisepticum regulates the transition from the Th1 to Th2 type of adaptive immunity. This may explain the depletion of CD8+ cells in the early phase of M. gallisepticum infection in the trachea.
8. Immune Organ Damage Caused by M. gallisepticum Infection
Bursa of Fabricius (BF) is the central immune organ of birds and the primary site for the generation, development, and maturation of B lymphocytes, which play a crucial role in host immunity against M. gallisepticum infection [112]. Previous studies have demonstrated that M. gallisepticum infection impairs the structural integrity and T cell-mediated immunity to clear pathogens in BF within 48 h post-M. gallisepticum infection [113]. The number of CD8+ lymphocytes significantly decreased in chicken BF at day 7 post-M. gallisepticum infection and apoptosis and oxygen species (ROS) increased in BF tissues after M. gallisepticum infection [8]. Additionally, the mRNA and protein expression levels of autophagy-related genes were significantly reduced and the level of the mTOR gene, as evaluated, showed that autophagy was inhibited [110]. Autophagy has been known as the cell homeostatic mechanism removing pathogens or damaged organelles and protecting cells by reducing DAMPs or PAMPs [114]. Studies have shown that baicalin upregulates autophagy levels, thereby attenuating tissue damage caused by M. gallisepticum and reducing its load [110].
The thymus is a primary lymphoid organ and a critical site for the generation, differentiation, and maturation of T cells. A thymic injury may lead to severe immunocompromised pathology [115]. Recently, studies have gradually revealed the underlying molecular mechanisms by which M. gallisepticum infection induces immune damage to the thymus. It was found that there was structural damage in the thymus of chickens with M. gallisepticum infection, including lymphocytosis, discrete cell arrangement, and accumulated nuclear debris [116]. Furthermore, the number of CD8+ lymphocytes has significantly decreased in the M. gallisepticum infection group and typical apoptotic features including mitochondrial swelling, chromatin shrinkage and condensation, and DNA fragmentation were found as examined by ultrastructure observation and analysis [117]. Meanwhile, M. gallisepticum infection induced oxidative stress and mitochondrial dynamic imbalance, triggered the inflammatory response through the TLR-2/MyD88/NF-κB signaling pathway, activated the NLRP3 inflammasome, and induced the secretion of IL-1β [117,118], which is closely related to cell death, immune disorders, and inflammatory diseases [50,119,120]. For example, inflammasome activation in COVID-19-infected macrophages drives a cytokine storm and inflammatory damage and inhibitors of NLRP3 could attenuate COVID-19 pathology [121].
It was reported that M. gallisepticum infection could induce autophagy in RAW264.7 cells through the extracellular regulated protein kinase (ERK) signaling pathway [61], which can decrease the accumulation of DAMPs or PAMPs and reduce mitochondrial damage [122]. On the contrary, autophagy was inhibited in primary immune organs after M. gallisepticum infection [110,118]. Andrographolide (AG) attenuates the inflammatory response by inhibiting the PI3K/Akt pathway to regulate autophagy in HD11 cells [123]. Notably, the antioxidant effect of Baicalin can efficiently alleviate oxidative stress and apoptosis in the thymus after M. gallisepticum infection through the Nrf2/HO-1 signaling axis [116]. Further studies will be needed to explore the molecular mechanism of autophagy associated with M. gallisepticum infection, which might be of help to the development of effective strategies for the prevention and control of M. gallisepticum infection.
9. Prevention of M. gallisepticum
With the increase in antimicrobial resistance and the diminishing efficacy of antibiotics for M. gallisepticum control, the improvement in existing vaccines and the development of new ones have become increasingly important. Both inactivated and live vaccines have been widely used for M. gallisepticum prevention. Inactivated vaccines typically induce a high level of humoral immune response and lower the risk of reversion to a pathogenic form of the vaccine. Live vaccines, such as the F strain [124] and ts-11 strain [125], have been proven effective in preventing air sac infection and respiratory diseases caused by M. gallisepticum and in reducing losses in production performance. In general, live vaccines derived from F strains induce higher levels of antibodies against M. gallisepticum than those derived from ts-11 strains [126]. With technological advancements, new generations of M. gallisepticum vaccine candidates like GT5 [127], MG 7 [128], K-strain [129], and ts-304 [130] have been researched to address concerns about existing vaccine strains. GT5 and MG 7 originated from the virulent Rlow strain, while the K-strain has shown effectiveness in the upper respiratory tract for about five months [131], protecting chickens from tracheal lesions. The ts-304, a variant of the ts-11 strain, offers a higher protection [132].
Genetically engineered vaccines, such as recombinant vaccines, are increasingly recognized as crucial in combating M. gallisepticum infections. For example, a recombinant Fowl Pox vector has been developed to express M. gallisepticum 40k and mgc genes, offering a new avenue for vaccination strategies [133]. Additionally, the use of recombinant adenovirus vectors for expressing the S1 spike glycoprotein of the infectious bronchitis virus (IBV) and the TM-1 protein of M. gallisepticum in HEK293 cells has shown promise in reducing clinical signs and lesions associated with these infections [134]. Recent studies have focused on designing multi-epitope vaccines using immunoinformatics, targeting antigenic proteins of M. gallisepticum [135,136]. These candidate vaccines, comprising cytotoxic T-cell (CTL), helper T-cell (HTL), and B-cell epitopes, show promising results in structural stability, specificity, and immunogenic response, predicting effective binding with chicken Toll-like receptors and indicating strong immune induction potential but which need to be validated in animal experiments. In addition to traditional bacterial, yeast, and mammalian expression systems, future vaccine development includes the use of plants as biofactories for vaccine production [137]. A notable example is the stable expression of the TM-1 gene of M. gallisepticum in wheat seed tissues, providing a safe, scalable, and cost-effective vaccine option. This plant-made vaccine, when orally delivered, elicited an effective antibody response in chickens, comparable to commercially available inactivated vaccines against CRD [138]. These vaccines, as well as those developed in the future, are expected to elicit a strong immune response and provide a safe, highly specific, and stable preventive option.
10. Conclusions
Avian mycoplasmas tactically evade innate and adaptive immune responses via different mechanisms. It is clear that M. gallisepticum induces an inflammatory response in the host, causing immune damage. Currently, it is known that M. gallisepticum infection of the airway elicits a strong inflammatory response, recruiting lymphocytes, macrophages, and heterophils to the mucosal lamina propria, which produce a large number of cytokines and chemokines that modulate the immune response while causing pathological damage. In addition, M. gallisepticum infection can regulate cellular microRNAs to facilitate its replication and survival. As M. gallisepticum can reach different organs of the whole body after infection, causing inflammatory responses and immune damage, it seems that the pathogen can successfully evade the host’s immune response. However, the mechanism by which hosts combat M. gallisepticum or the latter evades the former seems much more complicated than previously thought. Further studies will be required to investigate the mechanism that regulates M. gallisepticum infection, disease progression, or host responses to M. gallisepticum infection. No doubt, the elucidation of the pathogenesis of M. gallisepticum infection will be of great help to the development of effective strategies for the prevention and control of M. gallisepticum infection.
Author Contributions
Conceived and designed, S.J.Z.; writing—original draft preparation, Y.L.; revised the paper, S.J.Z. and Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Key Research and Development Program of China (grant number 2022YFD 1300100) and the earmarked fund for CARS-40, China.
Acknowledgments
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Adler, H.E. Mycoplasma, the cause of chronic respiratory disease. Ann. N. Y. Acad. Sci. 1960, 79, 703–712. [Google Scholar] [CrossRef]
- Mohammed, H.O.; Carpenter, T.E.; Yamamoto, R. Economic impact of Mycoplasma gallisepticum and M. synoviae in commercial layer flocks. Avian Dis. 1987, 31, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Kulappu Arachchige, S.N.; Wawegama, N.K.; Coppo, M.J.C.; Derseh, H.B.; Vaz, P.K.; Kanci Condello, A.; Omotainse, O.S.; Noormohammadi, A.H.; Browning, G.F. Mucosal immune responses in the trachea after chronic infection with Mycoplasma gallisepticum in unvaccinated and vaccinated mature chickens. Cell. Microbiol. 2021, 23, e13383. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, J.; Frasca, S., Jr.; Cecchini, K.; Rood, D.; Nyaoke, A.C.; Geary, S.J.; Silbart, L.K. Chemokine and cytokine gene expression profiles in chickens inoculated with Mycoplasma gallisepticum strains Rlow or GT5. Vaccine 2007, 25, 8611–8621. [Google Scholar] [CrossRef] [PubMed]
- Kulappu Arachchige, S.N.; Young, N.D.; Shil, P.K.; Legione, A.R.; Kanci Condello, A.; Browning, G.F.; Wawegama, N.K. Differential Response of the Chicken Trachea to Chronic Infection with Virulent Mycoplasma gallisepticum Strain Ap3AS and Vaxsafe MG (Strain ts-304): A Transcriptional Profile. Infect. Immun. 2020, 88, e00053-20. [Google Scholar] [CrossRef] [PubMed]
- Beaudet, J.; Tulman, E.R.; Pflaum, K.; Canter, J.A.; Silbart, L.K.; Geary, S.J. Immunologic Pathways in Protective versus Maladaptive Host Responses to Attenuated and Pathogenic Strains of Mycoplasma gallisepticum. Infect. Immun. 2019, 87, e00613-18. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Silbart, L.K. Interaction of Mycoplasma gallisepticum with Chicken Tracheal Epithelial Cells Contributes to Macrophage Chemotaxis and Activation. Infect. Immun. 2016, 84, 266–274. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Zhang, Q.; Waqas Ali Shah, S.; Wu, Z.; Wang, J.; Ishfaq, M.; Li, J. Mycoplasma gallisepticum Infection Impaired the Structural Integrity and Immune Function of Bursa of Fabricius in Chicken: Implication of Oxidative Stress and Apoptosis. Front. Vet. Sci. 2020, 7, 225. [Google Scholar] [CrossRef]
- Papazisi, L.; Silbart, L.K.; Frasca, S.; Rood, D.; Liao, X.; Gladd, M.; Javed, M.A.; Geary, S.J. A modified live Mycoplasma gallisepticum vaccine to protect chickens from respiratory disease. Vaccine 2002, 20, 3709–3719. [Google Scholar] [CrossRef]
- Javed, M.A.; Frasca, S., Jr.; Rood, D.; Cecchini, K.; Gladd, M.; Geary, S.J.; Silbart, L.K. Correlates of immune protection in chickens vaccinated with Mycoplasma gallisepticum strain GT5 following challenge with pathogenic M. gallisepticum strain R(low). Infect. Immun. 2005, 73, 5410–5419. [Google Scholar] [CrossRef]
- Gaunson, J.E.; Philip, C.J.; Whithear, K.G.; Browning, G.F. The cellular immune response in the tracheal mucosa to Mycoplasma gallisepticum in vaccinated and unvaccinated chickens in the acute and chronic stages of disease. Vaccine 2006, 24, 2627–2633. [Google Scholar] [CrossRef]
- Mohammed, H.O.; Carpenter, T.E.; Yamamoto, R. Evaluation of factors associated with infection of commercial layers with Mycoplasma gallisepticum and M. synoviae. Avian Dis. 1987, 31, 470–476. [Google Scholar] [CrossRef]
- Nakane, D.; Miyata, M. Cytoskeletal asymmetrical dumbbell structure of a gliding mycoplasma, Mycoplasma gallisepticum, revealed by negative-staining electron microscopy. J. Bacteriol. 2009, 191, 3256–3264. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M.; Miyata, M. Behaviors and Energy Source of Mycoplasma gallisepticum Gliding. J. Bacteriol. 2019, 201, e00397-19. [Google Scholar] [CrossRef]
- Indikova, I.; Vronka, M.; Szostak, M.P. First identification of proteins involved in motility of Mycoplasma gallisepticum. Vet. Res. 2014, 45, 99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hawley, D.M.; Sydenstricker, K.V.; Kollias, G.V.; Dhondt, A.A. Genetic diversity predicts pathogen resistance and cell-mediated immunocompetence in house finches. Biol. Lett. 2005, 1, 326–329. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, L.; Chen, Y.; Li, Y.; Wang, Z.; Li, G.; Wang, G.; Xin, J. GroEL Protein (Heat Shock Protein 60) of Mycoplasma gallisepticum Induces Apoptosis in Host Cells by Interacting with Annexin A2. Infect. Immun. 2019, 87, e00248-19. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ishfaq, M.; Li, J. Baicalin ameliorates Mycoplasma gallisepticum-induced inflammatory injury in the chicken lung through regulating the intestinal microbiota and phenylalanine metabolism. Food Funct. 2021, 12, 4092–4104. [Google Scholar] [CrossRef]
- Hu, F.; Zhao, C.; Bi, D.; Tian, W.; Chen, J.; Sun, J.; Peng, X. Mycoplasma gallisepticum (HS strain) surface lipoprotein pMGA interacts with host apolipoprotein A-I during infection in chicken. Appl. Microbiol. Biotechnol. 2016, 100, 1343–1354. [Google Scholar] [CrossRef]
- Papazisi, L.; Troy, K.E.; Gorton, T.S.; Liao, X.; Geary, S.J. Analysis of cytadherence-deficient, GapA-negative Mycoplasma gallisepticum strain R. Infect. Immun. 2000, 68, 6643–6649. [Google Scholar] [CrossRef][Green Version]
- Ruger, N.; Szostak, M.P.; Rautenschlein, S. The expression of GapA and CrmA correlates with the Mycoplasma gallisepticum in vitro infection process in chicken TOCs. Vet. Res. 2022, 53, 66. [Google Scholar] [CrossRef]
- Furnkranz, U.; Siebert-Gulle, K.; Rosengarten, R.; Szostak, M.P. Factors influencing the cell adhesion and invasion capacity of Mycoplasma gallisepticum. Acta Vet. Scand. 2013, 55, 63. [Google Scholar] [CrossRef] [PubMed]
- May, M.; Papazisi, L.; Gorton, T.S.; Geary, S.J. Identification of fibronectin-binding proteins in Mycoplasma gallisepticum strain R. Infect. Immun. 2006, 74, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Levisohn, S.; Dykstra, M.J.; Lin, M.Y.; Kleven, S.H. Comparison of in vivo and in vitro methods for pathogenicity evaluation for Mycoplasma gallisepticum in respiratory infection. Avian Pathol. 1986, 15, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Y.; Kleven, S.H. Evaluation of attenuated strains of Mycoplasma gallisepticum as vaccines in young chickens. Avian Dis. 1984, 28, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Matyushkina, D.; Pobeguts, O.; Butenko, I.; Vanyushkina, A.; Anikanov, N.; Bukato, O.; Evsyutina, D.; Bogomazova, A.; Lagarkova, M.; Semashko, T.; et al. Phase Transition of the Bacterium upon Invasion of a Host Cell as a Mechanism of Adaptation: A Mycoplasma gallisepticum Model. Sci. Rep. 2016, 6, 35959. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Chen, Z.; Tian, Y.; Yang, M.; Dong, Q.; Yang, Y.; Ding, H. Incomplete autophagy promotes the proliferation of Mycoplasma hyopneumoniae through the JNK and Akt pathways in porcine alveolar macrophages. Vet. Res. 2022, 53, 62. [Google Scholar] [CrossRef] [PubMed]
- Raymond, B.B.A.; Turnbull, L.; Jenkins, C.; Madhkoor, R.; Schleicher, I.; Uphoff, C.C.; Whitchurch, C.B.; Rohde, M.; Djordjevic, S.P. Mycoplasma hyopneumoniae resides intracellularly within porcine epithelial cells. Sci. Rep. 2018, 8, 17697. [Google Scholar] [CrossRef] [PubMed]
- Gaunson, J.E.; Philip, C.J.; Whithear, K.G.; Browning, G.F. Lymphocytic infiltration in the chicken trachea in response to Mycoplasma gallisepticum infection. Microbiology 2000, 146 Pt 5, 1223–1229. [Google Scholar] [CrossRef]
- Winner, F.; Rosengarten, R.; Citti, C. In vitro cell invasion of Mycoplasma gallisepticum. Infect. Immun. 2000, 68, 4238–4244. [Google Scholar] [CrossRef]
- Vogl, G.; Plaickner, A.; Szathmary, S.; Stipkovits, L.; Rosengarten, R.; Szostak, M.P. Mycoplasma gallisepticum invades chicken erythrocytes during infection. Infect. Immun. 2008, 76, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kerr, K.M.; Olson, N.O. Cardiac pathology associated with viral and mycoplasmal arthritis in chickens. Ann. N. Y. Acad. Sci. 1967, 143, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Nunoya, T.; Kanai, K.; Yagihashi, T.; Hoshi, S.; Shibuya, K.; Tajima, M. Natural case of salpingitis apparently caused by Mycoplasma gallisepticum in chickens. Avian Pathol. 1997, 26, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Mikaelian, I.; Ley, D.H.; Claveau, R.; Lemieux, M.; Berube, J.P. Mycoplasmosis in evening and pine grosbeaks with conjunctivitis in Quebec. J. Wildl. Dis. 2001, 37, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Clyde, W.A., Jr.; Thomas, L. Tropism of Mycoplasma gallisepticum for arterial walls. Proc. Natl. Acad. Sci. USA 1973, 70, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Minion, F.C.; Jarvill-Taylor, K. Membrane-associated hemolysin activities in mycoplasmas. FEMS Microbiol. Lett. 1994, 116, 101–106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bearson, S.M.; Collier, S.D.; Bearson, B.L.; Branton, S.L. Induction of a Mycoplasma gallisepticum pMGA gene in the chicken tracheal ring organ culture model. Avian Dis. 2003, 47, 745–749. [Google Scholar] [CrossRef]
- Glew, M.D.; Browning, G.F.; Markham, P.F.; Walker, I.D. pMGA phenotypic variation in Mycoplasma gallisepticum occurs in vivo and is mediated by trinucleotide repeat length variation. Infect. Immun. 2000, 68, 6027–6033. [Google Scholar] [CrossRef]
- Wise, K.S.; Kim, M.F.; Theiss, P.M.; Lo, S.C. A family of strain-variant surface lipoproteins of Mycoplasma fermentans. Infect. Immun. 1993, 61, 3327–3333. [Google Scholar] [CrossRef]
- Razin, S.; Yogev, D.; Naot, Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 1998, 62, 1094–1156. [Google Scholar] [CrossRef]
- Yogev, D.; Rosengarten, R.; Watson-McKown, R.; Wise, K.S. Molecular basis of Mycoplasma surface antigenic variation: A novel set of divergent genes undergo spontaneous mutation of periodic coding regions and 5’ regulatory sequences. EMBO J. 1991, 10, 4069–4079. [Google Scholar] [CrossRef]
- Glew, M.D.; Baseggio, N.; Markham, P.F.; Browning, G.F.; Walker, I.D. Expression of the pMGA genes of Mycoplasma gallisepticum is controlled by variation in the GAA trinucleotide repeat lengths within the 5’ noncoding regions. Infect. Immun. 1998, 66, 5833–5841. [Google Scholar] [CrossRef]
- Noormohammadi, A.H.; Markham, P.F.; Kanci, A.; Whithear, K.G.; Browning, G.F. A novel mechanism for control of antigenic variation in the haemagglutinin gene family of mycoplasma synoviae. Mol. Microbiol. 2000, 35, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Lysnyansky, I.; Sachse, K.; Rosenbusch, R.; Levisohn, S.; Yogev, D. The vsp locus of Mycoplasma bovis: Gene organization and structural features. J. Bacteriol. 1999, 181, 5734–5741. [Google Scholar] [CrossRef]
- Markham, P.F.; Glew, M.D.; Sykes, J.E.; Bowden, T.R.; Pollocks, T.D.; Browning, G.F.; Whithear, K.G.; Walker, I.D. The organisation of the multigene family which encodes the major cell surface protein, pMGA, of Mycoplasma gallisepticum. FEBS Lett. 1994, 352, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Glew, M.D.; Markham, P.F.; Browning, G.F.; Walker, I.D. Expression studies on four members of the pMGA multigene family in Mycoplasma gallisepticum S6. Microbiology 1995, 141 Pt 11, 3005–3014. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Markham, P.F.; Glew, M.D.; Browning, G.F.; Whithear, K.G.; Walker, I.D. Expression of two members of the pMGA gene family of Mycoplasma gallisepticum oscillates and is influenced by pMGA-specific antibodies. Infect. Immun. 1998, 66, 2845–2853. [Google Scholar] [CrossRef]
- Rottem, S.; Naot, Y. Subversion and exploitation of host cells by mycoplasmas. Trends Microbiol. 1998, 6, 436–440. [Google Scholar] [CrossRef]
- Hickman-Davis, J.M. Role of innate immunity in respiratory mycoplasma infection. Front. Biosci. 2002, 7, d1347–d1355. [Google Scholar] [CrossRef]
- Beaudet, J.; Tulman, E.R.; Pflaum, K.; Liao, X.; Kutish, G.F.; Szczepanek, S.M.; Silbart, L.K.; Geary, S.J. Transcriptional Profiling of the Chicken Tracheal Response to Virulent Mycoplasma gallisepticum Strain R(low). Infect. Immun. 2017, 85, e00343-17. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Wang, T.; Wang, Y.; Luo, R.; Sun, Y.; Peng, X. Comparative Transcriptome Analysis Reveals the Innate Immune Response to Mycoplasma gallisepticum Infection in Chicken Embryos and Newly Hatched Chicks. Animals 2023, 13, 1667. [Google Scholar] [CrossRef] [PubMed]
- Farrokhi, S.; Abbasirad, N.; Movahed, A.; Khazaei, H.A.; Pishjoo, M.; Rezaei, N. TLR9-based immunotherapy for the treatment of allergic diseases. Immunotherapy 2017, 9, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kida, Y.; Kuwano, K. Lipid-associated membrane proteins of Mycoplasma fermentans and M. penetrans activate human immunodeficiency virus long-terminal repeats through Toll-like receptors. Immunology 2004, 113, 121–129. [Google Scholar] [CrossRef]
- Brightbill, H.D.; Libraty, D.H.; Krutzik, S.R.; Yang, R.B.; Belisle, J.T.; Bleharski, J.R.; Maitland, M.; Norgard, M.V.; Plevy, S.E.; Smale, S.T.; et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 1999, 285, 732–736. [Google Scholar] [CrossRef]
- Shimizu, T.; Kida, Y.; Kuwano, K. Triacylated lipoproteins derived from Mycoplasma pneumoniae activate nuclear factor-kappaB through toll-like receptors 1 and 2. Immunology 2007, 121, 473–483. [Google Scholar] [CrossRef]
- Oven, I.; Resman Rus, K.; Dusanic, D.; Bencina, D.; Keeler, C.L., Jr.; Narat, M. Diacylated lipopeptide from Mycoplasma synoviae mediates TLR15 induced innate immune responses. Vet. Res. 2013, 44, 99. [Google Scholar] [CrossRef]
- Yeh, D.W.; Huang, L.R.; Chen, Y.W.; Huang, C.F.; Chuang, T.H. Interplay between Inflammation and Stemness in Cancer Cells: The Role of Toll-Like Receptor Signaling. J. Immunol. Res. 2016, 2016, 4368101. [Google Scholar] [CrossRef]
- Jiang, D.; Li, D.; Cao, L.; Wang, L.; Zhu, S.; Xu, T.; Wang, C.; Pan, D. Positive feedback regulation of proliferation in vascular smooth muscle cells stimulated by lipopolysaccharide is mediated through the TLR 4/Rac1/Akt pathway. PLoS ONE 2014, 9, e92398. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhao, C.; Hu, Q.; Sun, J.; Peng, X. Roles of Toll-like receptors 2 and 6 in the inflammatory response to Mycoplasma gallisepticum infection in DF-1 cells and in chicken embryos. Dev. Comp. Immunol. 2016, 59, 39–47. [Google Scholar] [CrossRef]
- Lu, Z.; Xie, D.; Chen, Y.; Tian, E.; Muhammad, I.; Chen, X.; Miao, Y.; Hu, W.; Wu, Z.; Ni, H.; et al. TLR2 mediates autophagy through ERK signaling pathway in Mycoplasma gallisepticum-infected RAW264.7 cells. Mol. Immunol. 2017, 87, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hou, Y.; Zhang, K.; Yuan, B.; Peng, X. Identification of differentially expressed miRNAs through high-throughput sequencing in the chicken lung in response to Mycoplasma gallisepticum HS. Comp. Biochem. Physiol. Part D Genom. Proteom. 2017, 22, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Zappulla, F.; Silbart, L.K. Mycoplasma gallisepticum lipid associated membrane proteins up-regulate inflammatory genes in chicken tracheal epithelial cells via TLR-2 ligation through an NF-kappaB dependent pathway. PLoS ONE 2014, 9, e112796. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, Y.; Wang, Y.; Li, Y.; Zhang, L.; Xin, J. TLR2/MyD88/NF-kappaB signaling pathway regulates IL-1beta production in DF-1 cells exposed to Mycoplasma gallisepticum LAMPs. Microb. Pathog. 2018, 117, 225–231. [Google Scholar] [CrossRef]
- Ishfaq, M.; Wu, Z.; Wang, J.; Li, R.; Chen, C.; Li, J. Baicalin alleviates Mycoplasma gallisepticum-induced oxidative stress and inflammation via modulating NLRP3 inflammasome-autophagy pathway. Int. Immunopharmacol. 2021, 101, 108250. [Google Scholar] [CrossRef] [PubMed]
- Erridge, C. Lysozyme promotes the release of Toll-like receptor-2 stimulants from gram-positive but not gram-negative intestinal bacteria. Gut Microbes 2010, 1, 383–387. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kelsh, R.M.; McKeown-Longo, P.J. Topographical changes in extracellular matrix: Activation of TLR4 signaling and solid tumor progression. Trends Cancer Res. 2013, 9, 1–13. [Google Scholar] [PubMed]
- Chen, R.; Kang, R.; Tang, D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Hu, F.; Zou, M.; Luo, R.; Sun, Y.; Wang, T.; Guo, Q.; Peng, X. Extracellular HMGB1 as Inflammatory Mediator in the Progression of Mycoplasma gallisepticum Infection. Cells 2022, 11, 2817. [Google Scholar] [CrossRef]
- Wang, J.; Ishfaq, M.; Li, J. Lactobacillus salivarius ameliorated Mycoplasma gallisepticum-induced inflammatory injury and secondary Escherichia coli infection in chickens: Involvement of intestinal microbiota. Vet. Immunol. Immunopathol. 2021, 233, 110192. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, Y.; Wang, L.; Zou, M.; Sun, Y.; Sun, H.; Guo, Q.; Peng, X. Mycoplasma gallisepticum escapes the host immune response via gga-miR-365-3p/SOCS5/STATs axis. Vet. Res. 2022, 53, 103. [Google Scholar] [CrossRef]
- Prawan, A.; Saw, C.L.; Khor, T.O.; Keum, Y.S.; Yu, S.; Hu, L.; Kong, A.N. Anti-NF-kappaB and anti-inflammatory activities of synthetic isothiocyanates: Effect of chemical structures and cellular signaling. Chem. Biol. Interact. 2009, 179, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, N.R.; Vasudevan, P.; Zhong, L.; Kim, G.; Samant, S.; Parekh, V.; Pillai, V.B.; Ravindra, P.V.; Gupta, M.; Jeevanandam, V.; et al. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat. Med. 2012, 18, 1643–1650. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef]
- Miao, Y.; Niu, D.; Wang, Z.; Wang, J.; Wu, Z.; Bao, J.; Hu, W.; Guo, Y.; Li, R.; Ishfaq, M.; et al. Mycoplasma gallisepticum induced inflammation-mediated Th1/Th2 immune imbalance via JAK/STAT signaling pathway in chicken trachea: Involvement of respiratory microbiota. Vet. Microbiol. 2022, 265, 109330. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, S.; Wang, T.; Zou, M.; Peng, X. Extracellular Vesicles from Mycoplasma gallisepticum: Modulators of Macrophage Activation and Virulence. J. Infect. Dis. 2023, 2023, jiad486. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, C.; Ou, G.; You, X.; Tan, T.; Hu, X.; Zeng, Y.; Yu, M.; Zhu, C. Nrf2 regulates the inflammatory response, including heme oxygenase-1 induction, by mycoplasma pneumoniae lipid-associated membrane proteins in THP-1 cells. Pathog. Dis. 2017, 75, ftx044. [Google Scholar] [CrossRef]
- He, L.; You, X.; Li, G.; Zeng, Y.; Li, R.; Zhu, C.; Yu, M.; Wu, Y. Mycoplasma genitalium-derived lipid-associated membrane proteins negatively regulate cytokine secretion by inducing HO-1 expression in placental trophoblast cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2015, 31, 194–198. [Google Scholar]
- Muhlradt, P.F.; Meyer, H.; Jansen, R. Identification of S-(2,3-dihydroxypropyl)cystein in a macrophage-activating lipopeptide from Mycoplasma fermentans. Biochemistry 1996, 35, 7781–7786. [Google Scholar] [CrossRef] [PubMed]
- Wilde, I.; Lotz, S.; Engelmann, D.; Starke, A.; van Zandbergen, G.; Solbach, W.; Laskay, T. Direct stimulatory effects of the TLR2/6 ligand bacterial lipopeptide MALP-2 on neutrophil granulocytes. Med. Microbiol. Immunol. 2007, 196, 61–71. [Google Scholar] [CrossRef]
- You, X.; Liu, L.; Zeng, Y.; Li, R.; He, J.; Ma, X.; Jiang, C.; Zhu, C.; Chen, L.; Yu, M.; et al. Macrophage-activating lipopeptide-2 requires Mal and PI3K for efficient induction of heme oxygenase-1. PLoS ONE 2014, 9, e103433. [Google Scholar] [CrossRef]
- Soares, M.P.; Bach, F.H. Heme oxygenase-1: From biology to therapeutic potential. Trends Mol. Med. 2009, 15, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Chernov, V.M.; Chernova, O.A.; Mouzykantov, A.A.; Lopukhov, L.V.; Trushin, M.V. Mycoplasmas and Novel HO-1 Inducers: Recent Advances. Curr. Pharm. Des. 2018, 24, 2236–2240. [Google Scholar] [CrossRef]
- Markham, P.F.; Kanci, A.; Czifra, G.; Sundquist, B.; Hains, P.; Browning, G.F. Homologue of macrophage-activating lipoprotein in Mycoplasma gallisepticum is not essential for growth and pathogenicity in tracheal organ cultures. J. Bacteriol. 2003, 185, 2538–2547. [Google Scholar] [CrossRef] [PubMed]
- Ishfaq, M.; Chen, C.; Bao, J.; Zhang, W.; Wu, Z.; Wang, J.; Liu, Y.; Tian, E.; Hamid, S.; Li, R.; et al. Baicalin ameliorates oxidative stress and apoptosis by restoring mitochondrial dynamics in the spleen of chickens via the opposite modulation of NF-kappaB and Nrf2/HO-1 signaling pathway during Mycoplasma gallisepticum infection. Poult. Sci. 2019, 98, 6296–6310. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, W.; Xu, H.; Liu, J.; Deng, Y.; Cheng, H.; Zhan, T.; Lu, X.; Liao, T.; Guo, L.; et al. Gga-miR-19b-3p Inhibits Newcastle Disease Virus Replication by Suppressing Inflammatory Response via Targeting RNF11 and ZMYND11. Front. Microbiol. 2019, 10, 2006. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Zhai, Y.; Zhang, L.; Cui, P.; Feng, L.; Yan, W.; Fu, X.; Tian, Y.; Wang, H.; et al. Gga-miR-30d regulates infectious bronchitis virus infection by targeting USP47 in HD11 cells. Microb. Pathog. 2020, 141, 103998. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fu, Y.; Zou, M.; Sun, Y.; Yin, X.; Niu, L.; Gong, Y.; Peng, X. Analysis of deep sequencing exosome-microRNA expression profile derived from CP-II reveals potential role of gga-miRNA-451 in inflammation. J. Cell. Mol. Med. 2020, 24, 6178–6190. [Google Scholar] [CrossRef]
- Zhang, K.; Han, Y.; Wang, Z.; Zhao, Y.; Fu, Y.; Peng, X. gga-miR-146c Activates TLR6/MyD88/NF-kappaB Pathway through Targeting MMP16 to Prevent Mycoplasma gallisepticum (HS Strain) Infection in Chickens. Cells 2019, 8, 501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Han, Y.; Zhao, Y.; Sun, Y.; Zou, M.; Fu, Y.; Peng, X. Upregulated gga-miR-16-5p Inhibits the Proliferation Cycle and Promotes the Apoptosis of MG-Infected DF-1 Cells by Repressing PIK3R1-Mediated the PI3K/Akt/NF-kappaB Pathway to Exert Anti-Inflammatory Effect. Int. J. Mol. Sci. 2019, 20, 1036. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Zou, M.; Zhao, Y.; Zhang, K.; Sun, Y.; Peng, X. Up-Regulation of miR-130b-3p Activates the PTEN/PI3K/AKT/NF-kappaB Pathway to Defense against Mycoplasma gallisepticum (HS Strain) Infection of Chicken. Int. J. Mol. Sci. 2018, 19, 2172. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, M.; Sun, Y.; Zhang, K.; Peng, X. gga-miR-21 modulates Mycoplasma gallisepticum (HS strain)-Induced inflammation via targeting MAP3K1 and activating MAPKs and NF-kappaB pathways. Vet. Microbiol. 2019, 237, 108407. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Y.; Zhao, Y.; Zou, M.; Peng, X. Exosomal miR-181a-5p reduce Mycoplasma gallisepticum (HS strain) infection in chicken by targeting PPM1B and activating the TLR2-mediated MyD88/NF-kappaB signaling pathway. Mol. Immunol. 2021, 140, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Z.; Hou, Y.; Zhang, K.; Peng, X. gga-miR-99a targets SMARCA5 to regulate Mycoplasma gallisepticum (HS strain) infection by depressing cell proliferation in chicken. Gene 2017, 627, 239–247. [Google Scholar] [CrossRef]
- Hu, Q.; Zhao, Y.; Wang, Z.; Hou, Y.; Bi, D.; Sun, J.; Peng, X. Chicken gga-miR-19a Targets ZMYND11 and Plays an Important Role in Host Defense against Mycoplasma gallisepticum (HS Strain) Infection. Front. Cell. Infect. Microbiol. 2016, 6, 102. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Y.; Zou, M.; Wang, T.; Wang, L.; Peng, X. Lnc90386 Sponges miR-33-5p to Mediate Mycoplasma gallisepticum-Induced Inflammation and Apoptosis in Chickens via the JNK Pathway. Front. Immunol. 2022, 13, 887602. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Y.; Zou, M.; Deng, G.; Peng, X. gga-miR-142-3p negatively regulates Mycoplasma gallisepticum (HS strain)-induced inflammatory cytokine production via the NF-kappaB and MAPK signaling by targeting TAB2. Inflamm. Res. 2021, 70, 1217–1231. [Google Scholar] [CrossRef]
- Evans-Anderson, H.J.; Alfieri, C.M.; Yutzey, K.E. Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ. Res. 2008, 102, 686–694. [Google Scholar] [CrossRef]
- Yin, X.; Wang, Y.; Sun, Y.; Han, Y.; Sun, H.; Zou, M.; Luo, R.; Peng, X. Down-regulated gga-miR-223 inhibits proliferation and induces apoptosis of MG-infected DF-1 cells by targeting FOXO3. Microb. Pathog. 2021, 155, 104927. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Fu, Y.; Zhao, Y.; Sun, Y.; Yin, X.; Peng, X. Mycoplasma gallisepticum induced exosomal gga-miR-193a to disturb cell proliferation, apoptosis, and cytokine production by targeting the KRAS/ERK signaling pathway. Int. Immunopharmacol. 2022, 111, 109090. [Google Scholar] [CrossRef] [PubMed]
- Smialek, M.; Tykalowski, B.; Stenzel, T.; Koncicki, A. Local immunity of the respiratory mucosal system in chickens and turkeys. Pol. J. Vet. Sci. 2011, 14, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Gaunson, J.E.; Philip, C.J.; Whithear, K.G.; Browning, G.F. Age related differences in the immune response to vaccination and infection with Mycoplasma gallisepticum. Vaccine 2006, 24, 1687–1692. [Google Scholar] [CrossRef]
- Befus, D.; Bienenstock, J. Induction and expression of mucosal immune responses and inflammation to parasitic infections. Contemp. Top. Immunobiol. 1984, 12, 71–108. [Google Scholar] [CrossRef] [PubMed]
- Fagerland, J.A.; Arp, L.H. Structure and development of bronchus-associated lymphoid tissue in conventionally reared broiler chickens. Avian Dis. 1993, 37, 10–18. [Google Scholar] [CrossRef]
- Wijesurendra, D.S.; Kanci, A.; Tivendale, K.A.; Devlin, J.M.; Wawegama, N.K.; Bacci, B.; Noormohammadi, A.H.; Markham, P.F.; Browning, G.F. Immune responses to vaccination and infection with Mycoplasma gallisepticum in turkeys. Avian Pathol. 2017, 46, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Ishfaq, M.; Hu, W.; Khan, M.Z.; Ahmad, I.; Guo, W.; Li, J. Current status of vaccine research, development, and challenges of vaccines for Mycoplasma gallisepticum. Poult. Sci. 2020, 99, 4195–4202. [Google Scholar] [CrossRef]
- Cizelj, I.; Bercic, R.L.; Dusanic, D.; Narat, M.; Kos, J.; Dovc, P.; Bencina, D. Mycoplasma gallisepticum and Mycoplasma synoviae express a cysteine protease CysP, which can cleave chicken IgG into Fab and Fc. Microbiology 2011, 157, 362–372. [Google Scholar] [CrossRef][Green Version]
- Ishfaq, M.; Zhang, W.; Liu, Y.; Wang, J.; Wu, Z.; Shah, S.W.; Li, R.; Miao, Y.; Chen, C.; Li, J. Baicalin attenuated Mycoplasma gallisepticum-induced immune impairment in chicken bursa of fabricius through modulation of autophagy and inhibited inflammation and apoptosis. J. Sci. Food Agric. 2021, 101, 880–890. [Google Scholar] [CrossRef]
- Odeh, A.N.; Simecka, J.W. Regulatory CD4+CD25+ T Cells Dampen Inflammatory Disease in Murine Mycoplasma Pneumonia and Promote IL-17 and IFN-gamma Responses. PLoS ONE 2016, 11, e0155648. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, H.A.; Bailey, M.; Kaiser, P. An overview of infectious bursal disease. Arch. Virol. 2012, 157, 2047–2057. [Google Scholar] [CrossRef] [PubMed]
- Manafi, M.; Pirany, N.; Noor Ali, M.; Hedayati, M.; Khalaji, S.; Yari, M. Experimental pathology of T-2 toxicosis and mycoplasma infection on performance and hepatic functions of broiler chickens. Poult. Sci. 2015, 94, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Seveau, S.; Turner, J.; Gavrilin, M.A.; Torrelles, J.B.; Hall-Stoodley, L.; Yount, J.S.; Amer, A.O. Checks and Balances between Autophagy and Inflammasomes during Infection. J. Mol. Biol. 2018, 430, 174–192. [Google Scholar] [CrossRef] [PubMed]
- Raviola, E.; Karnovsky, M.J. Evidence for a blood-thymus barrier using electron-opaque tracers. J. Exp. Med. 1972, 136, 466–498. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qiao, Z.; Hu, W.; Zhang, W.; Shah, S.W.A.; Ishfaq, M. Baicalin mitigated Mycoplasma gallisepticum-induced structural damage and attenuated oxidative stress and apoptosis in chicken thymus through the Nrf2/HO-1 defence pathway. Vet. Res. 2019, 50, 83. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, W.; Shah, S.W.A.; Ishfaq, M.; Li, J. Mycoplasma gallisepticum infection triggered histopathological changes, oxidative stress and apoptosis in chicken thymus and spleen. Dev. Comp. Immunol. 2021, 114, 103832. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.; Zhang, W.; Shah, S.W.A.; Ishfaq, M. Mycoplasma gallisepticum triggers immune damage in the chicken thymus by activating the TLR-2/MyD88/NF-kappaB signaling pathway and NLRP3 inflammasome. Vet. Res. 2020, 51, 52. [Google Scholar] [CrossRef]
- Levisohn, S.; Kleven, S.H. Avian mycoplasmosis (Mycoplasma gallisepticum). Rev. Sci. Tech. 2000, 19, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Brydges, S.D.; Broderick, L.; McGeough, M.D.; Pena, C.A.; Mueller, J.L.; Hoffman, H.M. Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. J. Clin. Investig. 2013, 123, 4695–4705. [Google Scholar] [CrossRef]
- Leal, V.N.C.; Andrade, M.M.S.; Teixeira, F.M.E.; Cambui, R.A.G.; Roa, M.; Marra, L.G.; Yamada, S.M.; Alberca, R.W.; Gozzi-Silva, S.C.; Yendo, T.M.; et al. Severe COVID-19 patients show a dysregulation of the NLRP3 inflammasome in circulating neutrophils. Scand. J. Immunol. 2023, 97, e13247. [Google Scholar] [CrossRef]
- Joosten, L.A.; Netea, M.G.; Dinarello, C.A. Interleukin-1beta in innate inflammation, autophagy and immunity. Semin. Immunol. 2013, 25, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xiao, Y.; Luo, R.; Wang, Y.; Zou, M.; Sun, Y.; Wang, L.; Guo, Q.; Peng, X. Host resistance to Mycoplasma gallisepticum infection is enhanced by inhibiting PI3K/Akt pathway in Andrographolide-treating chickens. Int. Immunopharmacol. 2022, 113, 109419. [Google Scholar] [CrossRef] [PubMed]
- Cummings, T.S.; Kleven, S.H. Evaluation of protection against Mycoplasma gallisepticum infection in chickens vaccinated with the F strain of M. gallisepticum. Avian Dis. 1986, 30, 169–171. [Google Scholar] [CrossRef]
- Whithear, K.G.; Soeripto; Harrigan, K. E.; Ghiocas, E. Immunogenicity of a temperature sensitive mutant Mycoplasma gallisepticum vaccine. Aust. Vet. J. 1990, 67, 168–174. [Google Scholar] [CrossRef]
- Abd-el-Motelib, T.Y.; Kleven, S.H. A comparative study of Mycoplasma gallisepticum vaccines in young chickens. Avian Dis. 1993, 37, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Gates, A.E.; Frasca, S.; Nyaoke, A.; Gorton, T.S.; Silbart, L.K.; Geary, S.J. Comparative assessment of a metabolically attenuated Mycoplasma gallisepticum mutant as a live vaccine for the prevention of avian respiratory mycoplasmosis. Vaccine 2008, 26, 2010–2019. [Google Scholar] [CrossRef]
- Papazisi, L.; Frasca, S., Jr.; Gladd, M.; Liao, X.; Yogev, D.; Geary, S.J. GapA and CrmA coexpression is essential for Mycoplasma gallisepticum cytadherence and virulence. Infect. Immun. 2002, 70, 6839–6845. [Google Scholar] [CrossRef] [PubMed]
- Ferguson-Noel, N.; Cookson, K.; Laibinis, V.A.; Kleven, S.H. The efficacy of three commercial Mycoplasma gallisepticum vaccines in laying hens. Avian Dis. 2012, 56, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Kanci, A.; Wijesurendra, D.S.; Wawegama, N.K.; Underwood, G.J.; Noormohammadi, A.H.; Markham, P.F.; Browning, G.F. Evaluation of Mycoplasma gallisepticum (MG) ts-304 vaccine as a live attenuated vaccine in turkeys. Vaccine 2018, 36, 2487–2493. [Google Scholar] [CrossRef] [PubMed]
- Ferguson-Noel, N.M.; Laibinis, V.A.; Kleven, S.H. Evaluation of Mycoplasma gallisepticum K-strain as a live vaccine in chickens. Avian Dis. 2012, 56, 44–50. [Google Scholar] [CrossRef]
- Kanci Condello, A.; Kulappu Arachchige, S.N.; Shil, P.K.; Underwood, G.J.; Noormohammadi, A.H.; Markham, P.F.; Wawegama, N.K.; Browning, G.F. Duration of protective immunity induced by Mycoplasma gallisepticum strain ts-304 vaccine in chickens. Vet. Microbiol. 2020, 251, 108883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Z.; Zhang, R.; Zhao, H.L.; Wang, X.T.; Zhang, S.P.; Li, X.J.; Qin, C.Z.; Lv, C.M.; Zhao, J.X.; Zhou, J.F. A safety assessment of a fowlpox-vectored Mycoplasma gallisepticum vaccine in chickens. Poult. Sci. 2010, 89, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Long, Y.; Li, M.; Gong, J.; Li, X.; Lin, J.; Meng, J.; Gao, K.; Zhao, R.; Jin, T. Development and evaluation of novel recombinant adenovirus-based vaccine candidates for infectious bronchitis virus and Mycoplasma gallisepticum in chickens. Avian Pathol. 2018, 47, 213–222. [Google Scholar] [CrossRef]
- Mugunthan, S.P.; Harish, M.C. Multi-epitope-Based Vaccine Designed by Targeting Cytoadherence Proteins of Mycoplasma gallisepticum. ACS Omega 2021, 6, 13742–13755. [Google Scholar] [CrossRef] [PubMed]
- Mugunthan, S.P.; Mani Chandra, H. A Computational Reverse Vaccinology Approach for the Design and Development of Multi-Epitopic Vaccine Against Avian Pathogen Mycoplasma gallisepticum. Front. Vet. Sci. 2021, 8, 721061. [Google Scholar] [CrossRef] [PubMed]
- Shim, B.S.; Hong, K.J.; Maharjan, P.M.; Choe, S. Plant factory: New resource for the productivity and diversity of human and veterinary vaccines. Clin. Exp. Vaccine Res. 2019, 8, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Habibi, P.; Haq, A.N.U.; Saeed, M.; Gulghutay Amjad, N.; Khan, I. Seed-Based System for Cost-Effective Production of Vaccine Against Chronic Respiratory Disease in Chickens. Mol. Biotechnol. 2023, 65, 570–580. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).