Osteostatin Mitigates Gouty Arthritis through the Inhibition of Caspase-1 Activation and Upregulation of Nrf2 Expression
Abstract
1. Introduction
2. Results
2.1. Effects of Osteostatin on Peritoneal Macrophages
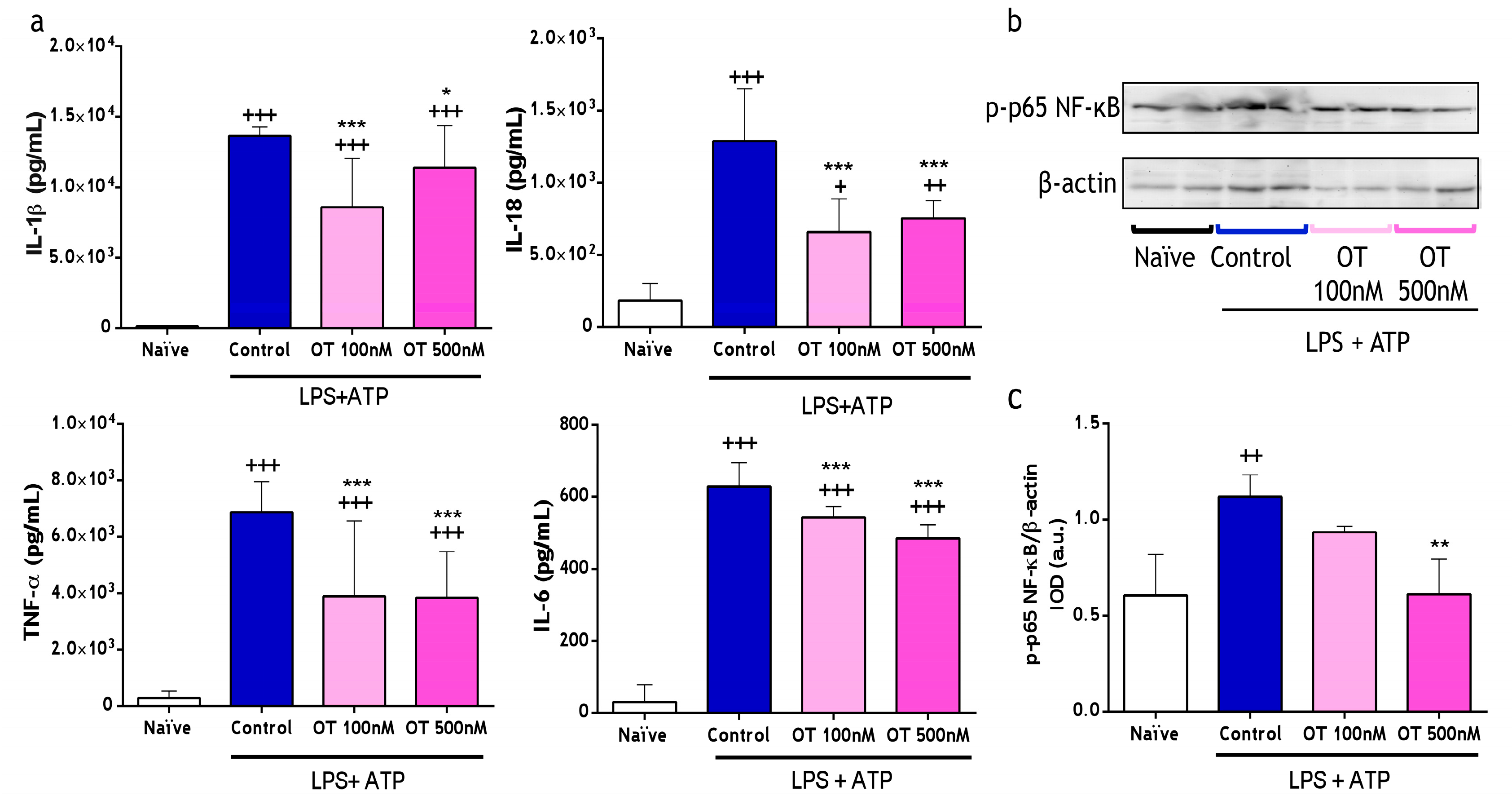
2.1.1. Effect of Osteostatin on Cytokine Levels and NF-κB Activation
2.1.2. Effect of Osteostatin on Caspase-1 Activation and Lactate Dehydrogenase
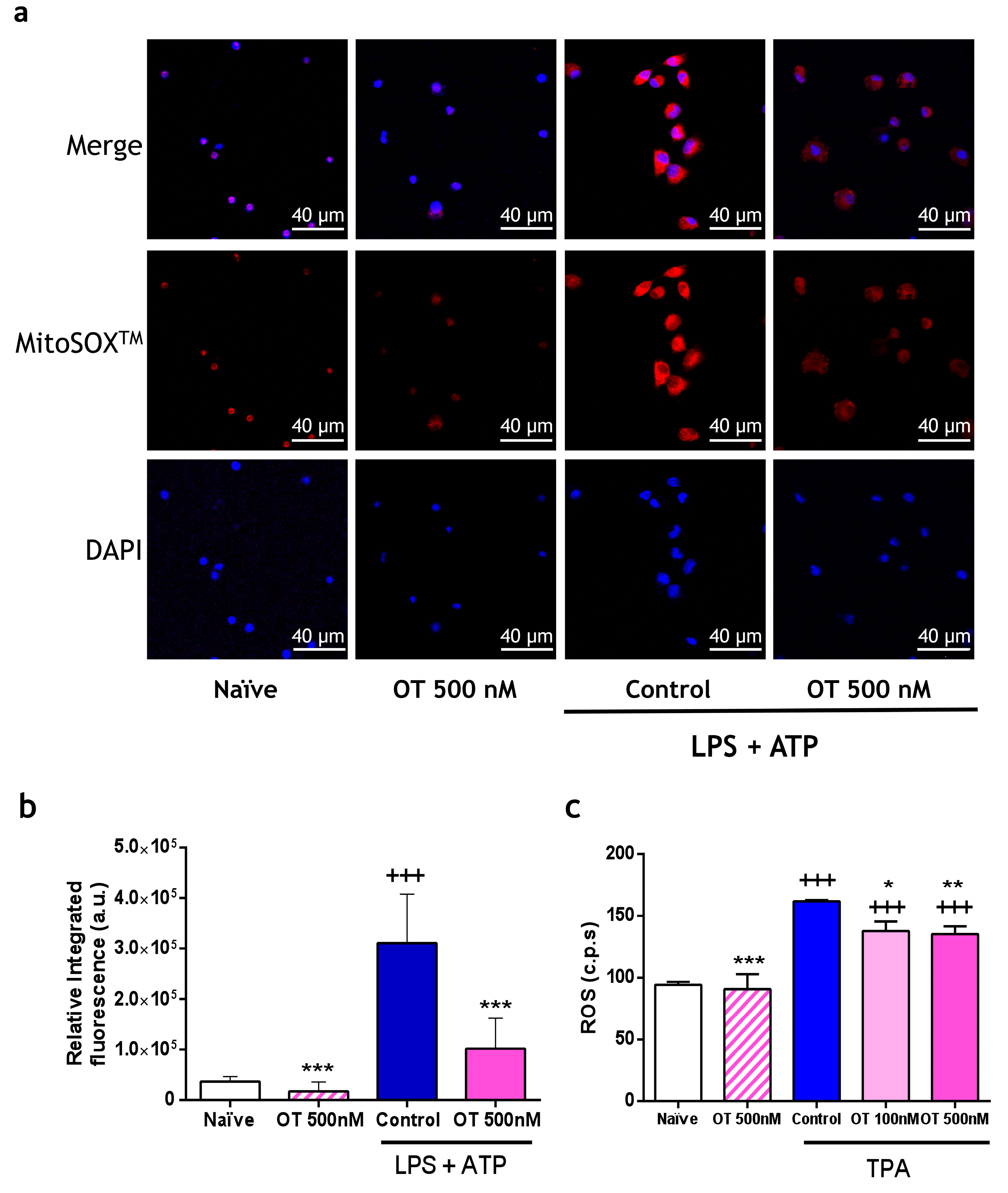
2.1.3. Effect of Osteostatin on Extracellular and Mitochondrial Reactive Oxygen Species (ROS) Production
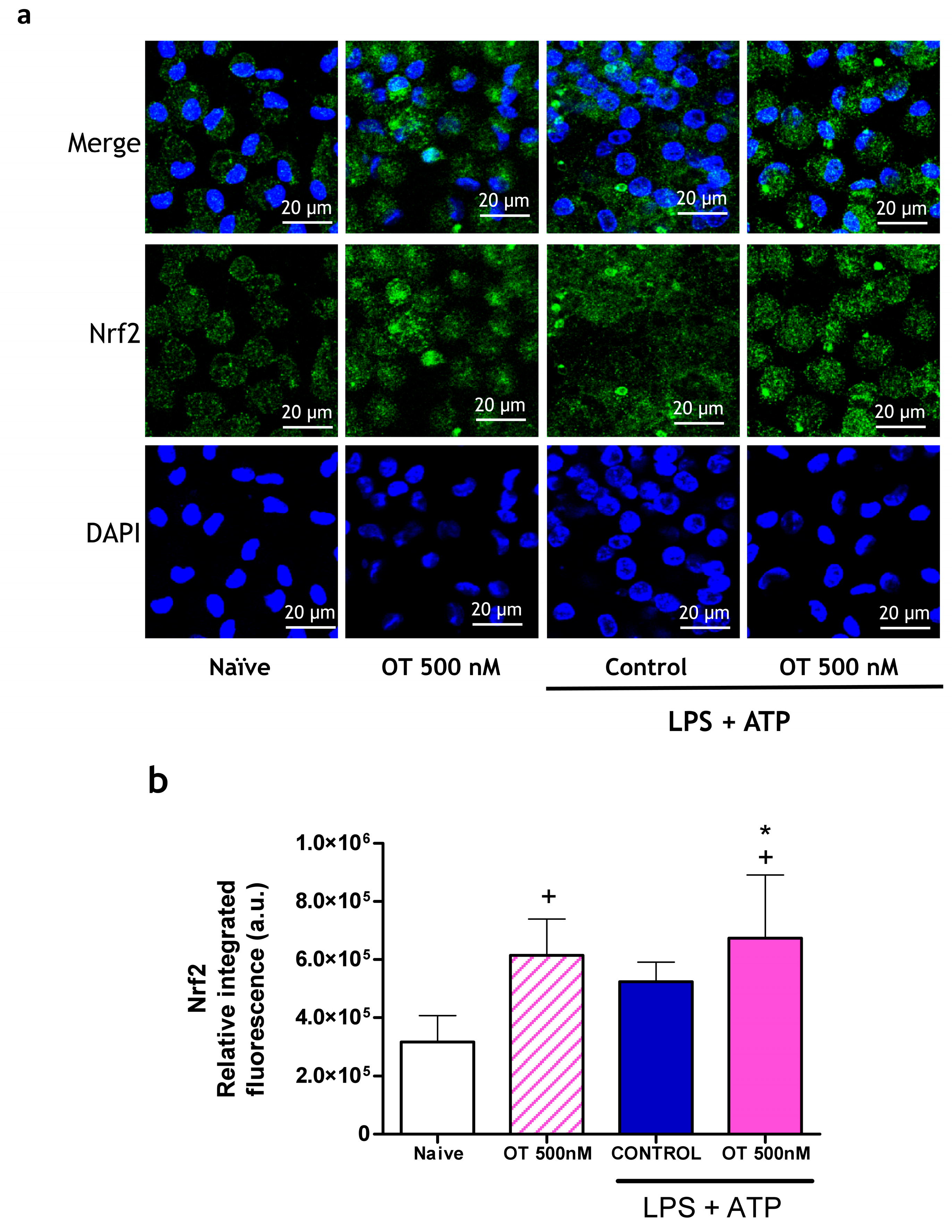
2.1.4. Effect of Osteostatin on Nrf2 Expression
2.2. Effect of Osteostatin on Calcium Pyrophosphate Dihydrate (CPPD) Crystal-Induced Mouse Air Pouch (MAP) Model
2.2.1. Effect of Osteostatin on Cell Migration, Myeloperoxidase (MPO) and Cytokines Release on Air Pouch Exudates
2.2.2. Effect of Osteostatin on Caspase-1 and NF-κB Activation
2.3. Effect of Osteostatin on Monosodium Urate (MSU) Crystal-Induced Gouty Arthritis Model
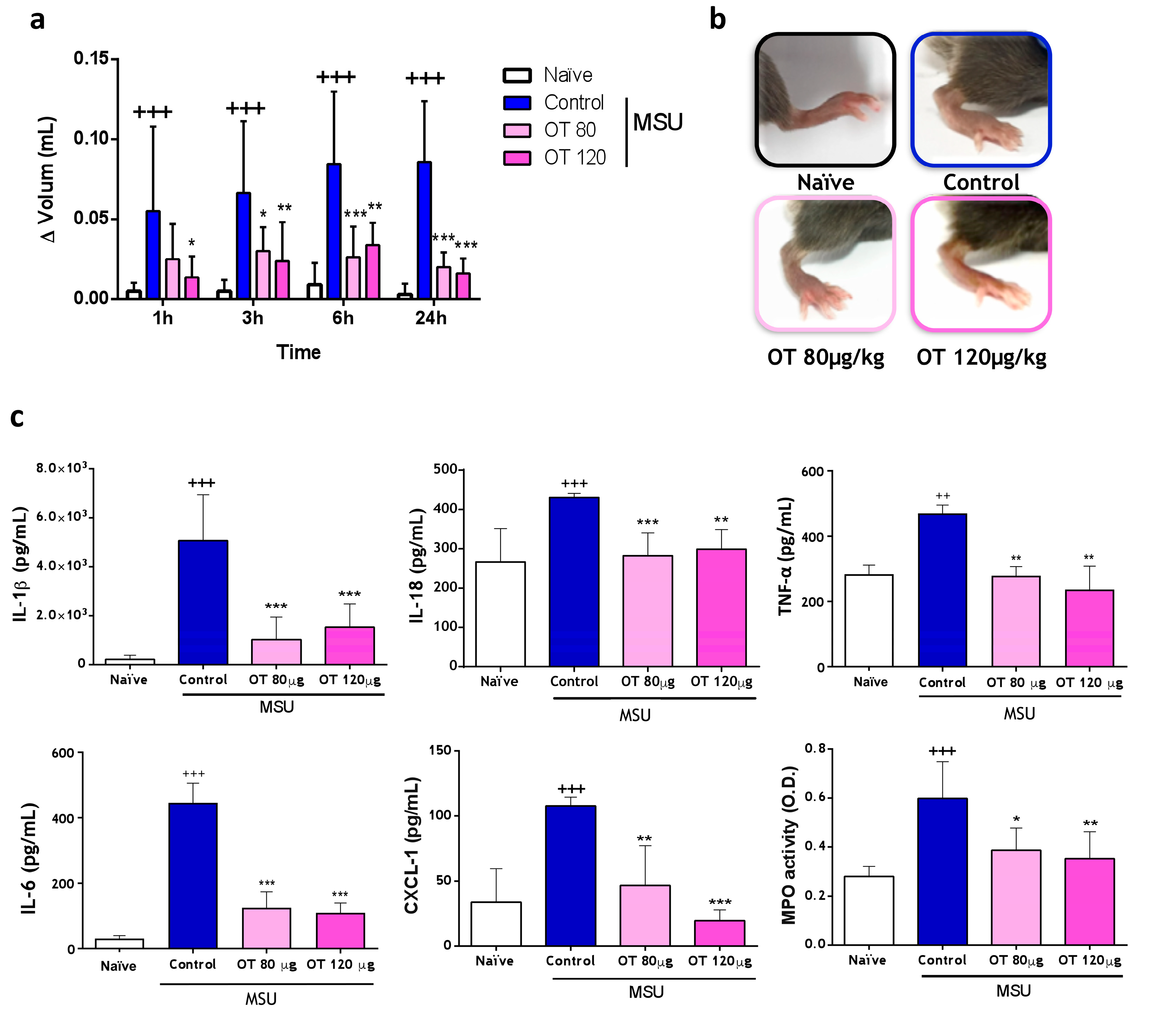
2.3.1. Effects of Osteostatin on Plantar Edema and Inflammatory Mediators
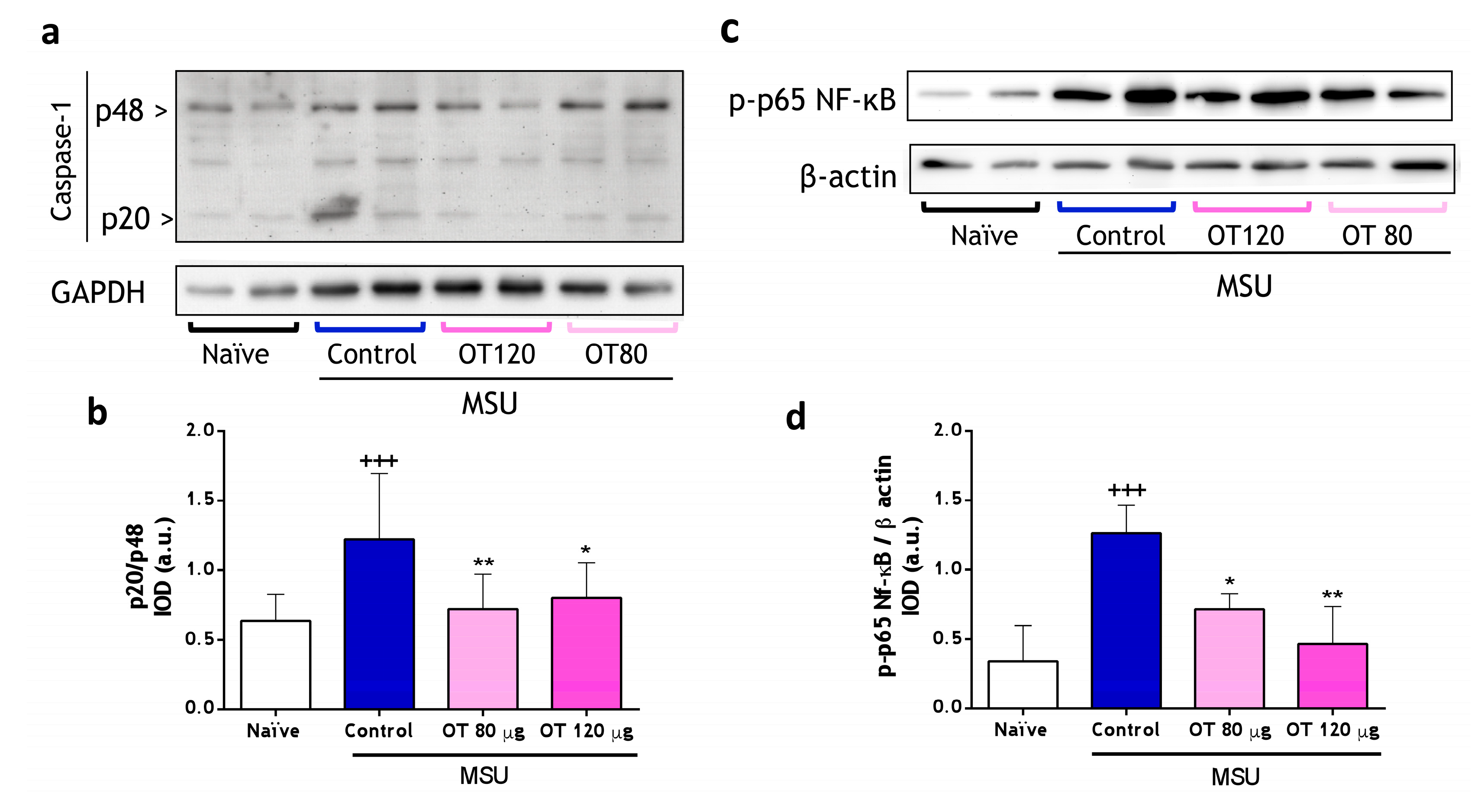
2.3.2. Effects of Osteostatin on Caspase-1 and NF-κB Activation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Isolation and Culture of Peritoneal Macrophages
4.3. LDH Assay
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Western Blotting
4.6. Determination of Mitochondrial ROS with MitoSOXTM
4.7. Determination of Extracellular ROS by Chemiluminescence
4.8. Determination of Nrf2 Translocation in the Nucleus by Immunofluorescence
4.9. Mouse Air Pouch Model Induced by Calcium Pyrophosphate Dihydrate (CPPD) Crystals
4.10. Myeloperoxidase Activity Determination
4.11. Mouse Model of Gouty Arthritis Induced by Monosodium Urate (MSU) Crystals
4.12. Determination of Mediators in Paw Homogenates
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fenando, A.; Rednam, M.; Gujarathi, R.; Widrich, J. Gout. StatPearls 2023. Available online: http://www.ncbi.nlm.nih.gov/pubmed/27380294 (accessed on 26 September 2023).
- Garrote Corral, S.; Zegarra Mondragón, S.; Guillen Astete, C.; Bachiller Corral, F.J. Artritis microcristalinas. Med.—Programa De Form. Médica Contin. Acreditado 2017, 12, 1574–1585. [Google Scholar] [CrossRef]
- Zamudio-Cuevas, Y.; Martínez-Flores, K.; Fernández-Torres, J.; Loissell-Baltazar, Y.A.; Medina-Luna, D.; López-Macay, A.; Camacho-Galindo, J.; Hernández-Díaz, C.; Santamaría-Olmedo, M.G.; López-Villegas, E.O.; et al. Monosodium urate crystals induce oxidative stress in human synoviocytes. Arthritis Res. Ther. 2016, 18, 117. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Dalbeth, N.; Merriman, T.R.; Stamp, L.K. Gout. Lancet 2016, 388, 2039–2052. [Google Scholar] [CrossRef] [PubMed]
- Richette, P.; Doherty, M.; Pascual, E.; Barskova, V.; Becce, F.; Castañeda-Sanabria, J.; Coyfish, M.; Guillo, S.; Jansen, T.L.; Janssens, H.; et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann. Rheum. Dis. 2017, 76, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Orloff, J.J.; Reddy, D.; De Papp, A.E.; Yang, K.H.; Soifer, N.E.; Stewart, A.F. Parathyroid Hormone-Related Protein as a Prohormone: Posttranslational Processing and Receptor Interactions. Endocr. Rev. 1994, 15, 40–60. [Google Scholar] [CrossRef] [PubMed]
- Wysolmerski, J.J.; Stewart, A.F. The physiology of parathyroid hormone-related protein: An emerging role as a developmental factor. Annu. Rev. Physiol. 1998, 60, 431–460. [Google Scholar] [CrossRef]
- Librizzi, M.; Naselli, F.; Abruscato, G.; Luparello, C.; Caradonna, F. Parathyroid Hormone Related Protein (PTHrP)-Associated Molecular Signatures in Tissue Differentiation and Non-Tumoral Diseases. Biology 2023, 12, 950. [Google Scholar] [CrossRef]
- García-Martín, A.; Acitores, A.; Maycas, M.; Villanueva-Peñacarrillo, M.L.; Esbrit, P. Src kinases mediate VEGFR2 transactivation by the osteostatin domain of PTHrP to modulate osteoblastic function. J. Cell Biochem. 2013, 114, 1404–1413. [Google Scholar] [CrossRef]
- Wu, D.; Liu, L.; Fu, S.; Zhang, J. Osteostatin improves the Osteogenic differentiation of mesenchymal stem cells and enhances angiogenesis through HIF-1α under hypoxia conditions in vitro. Biochem. Biophys. Res. Commun. 2022, 606, 100–107. [Google Scholar] [CrossRef]
- Lozano, D.; Trejo, C.G.; Gómez-Barrena, E.; Manzano, M.; Doadrio, J.C.; Salinas, A.J.; Vallet-Regí, M.; García-Honduvilla, N.; Esbrit, P.; Buján, J. Osteostatin-loaded onto mesoporous ceramics improves the early phase of bone regeneration in a rabbit osteopenia model. Acta Biomater. 2012, 8, 2317–2323. [Google Scholar] [CrossRef]
- Trejo, C.G.; Lozano, D.; Manzano, M.; Doadrio, J.C.; Salinas, A.J.; Dapía, S.; Gómez-Barrena, E.; Vallet-Regí, M.; García-Honduvilla, N.; Buján, J.; et al. The osteoinductive properties of mesoporous silicate coated with osteostatin in a rabbit femur cavity defect model. Biomaterials 2010, 31, 8564–8573. [Google Scholar] [CrossRef]
- Portal-Núñez, S.; Ardura, J.A.; Lozano, D.; De Toda, I.M.; De la Fuente, M.; Herrero-Beaumont, G.; Largo, R.; Esbrit, P. Parathyroid hormone-related protein exhibits antioxidant features in osteoblastic cells through its N-terminal and osteostatin domains. Bone Jt. Res. 2018, 7, 58–68. [Google Scholar] [CrossRef]
- Platas, J.; Guillén, M.I.; Gomar, F.; Castejón, M.A.; Esbrit, P.; Alcaraz, M.J. Anti-senescence and Anti-inflammatory Effects of the C-terminal Moiety of PTHrP Peptides in OA Osteoblasts. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 72, 624–631. [Google Scholar] [CrossRef]
- Ibáñez, L.; Nácher-Juan, J.; Terencio, M.C.; Ferrándiz, M.L.; Alcaraz, M.J. Osteostatin Inhibits M-CSF+RANKL-Induced Human Osteoclast Differentiation by Modulating NFATc1. Int. J. Mol. Sci. 2022, 23, 8551. [Google Scholar] [CrossRef]
- Nácher-Juan, J.; Terencio, M.C.; Alcaraz, M.J.; Ferrándiz, M.L. Osteostatin Inhibits Collagen-Induced Arthritis by Regulation of Immune Activation, Pro-Inflammatory Cytokines, and Osteoclastogenesis. Int. J. Mol. Sci. 2019, 20, 3845. [Google Scholar] [CrossRef] [PubMed]
- Myers, L.K.; Rosloniec, E.F.; Cremer, M.A.; Kang, A.H. Collagen-induced arthritis, an animal model of autoimmunity. Life Sci. 1997, 61, 1861–1878. [Google Scholar] [CrossRef] [PubMed]
- So, A.K.; Martinon, F. Inflammation in gout: Mechanisms and therapeutic targets. Nat. Rev. Rheumatol. 2017, 13, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Ferrándiz, M.L.; Nacher-Juan, J.; Alcaraz, M.J. Nrf2 as a therapeutic target for rheumatic diseases. Biochem. Pharmacol. 2018, 152, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Campillo-Gimenez, L.; Renaudin, F.; Jalabert, M.; Gras, P.; Gosset, M.; Rey, C.; Sarda, S.; Collet, C.; Cohen-Solal, M.; Combes, C.; et al. Inflammatory Potential of Four Different Phases of Calcium Pyrophosphate Relies on NF-κB Activation and MAPK Pathways. Front. Immunol. 2018, 9, 2248. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.; Varalakshmi, P. Suppressive effect of Withania somnifera root powder on experimental gouty arthritis: An in vivo and in vitro study. Chem. Biol. Interact. 2006, 164, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Stamp, L.K.; Turner, R.; Khalilova, I.S.; Zhang, M.; Drake, J.; Forbes, L.V.; Kettle, A.J. Myeloperoxidase and oxidation of uric acid in gout: Implications for the clinical consequences of hyperuricaemia. Rheumatology 2014, 53, 1958–1965. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Conforti-Andreoni, C.; Ricciardi-Castagnoli, P.; Mortellaro, A. The inflammasomes in health and disease: From genetics to molecular mechanisms of autoinflammation and beyond. Cell Mol. Immunol. 2011, 8, 135–145. [Google Scholar] [CrossRef]
- Winkler, S.; Rösen-Wolff, A. Caspase-1: An integral regulator of innate immunity. Semin. Immunopathol. 2015, 37, 419–427. [Google Scholar] [CrossRef]
- Bolívar, B.E.; Vogel, T.P.; Bouchier-Hayes, L. Inflammatory caspase regulation: Maintaining balance between inflammation and cell death in health and disease. FEBS J. 2019, 286, 2628–2644. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Abais, J.M.; Xia, M.; Zhang, Y.; Boini, K.M.; Li, P.-L. Redox Regulation of NLRP3 Inflammasomes: ROS as Trigger or Effector? Antioxid. Redox Signal 2015, 22, 1111–1129. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Ding, Y.; Zhou, W.; Tao, L.; Lu, P.; Wang, Y.; Hu, R. Nuclear Factor E2-Related Factor-2 Negatively Regulates NLRP3 Inflammasome Activity by Inhibiting Reactive Oxygen Species-Induced NLRP3 Priming. Antioxid. Redox Signal 2017, 26, 28–43. [Google Scholar] [CrossRef]
- Hennig, P.; Garstkiewicz, M.; Grossi, S.; Di Filippo, M.; French, L.; Beer, H.-D. The Crosstalk between Nrf2 and Inflammasomes. Int. J. Mol. Sci. 2018, 19, 562. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Z.; Zhang, B.; Jiang, Z.; Guo, F.; Yang, T. Cichorium intybus L. Extract Suppresses Experimental Gout by Inhibiting the NF-κB and NLRP3 Signaling Pathways. Int. J. Mol. Sci. 2019, 20, 4921. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.C.; de Cássia Lemos Lima, R.; Schimith Ferraz Filha, Z.; Barros, C.H.; de Paula Michel Araújo, M.C.; Antunes Saúde-Guimarães, D. Effects of Pimenta pseudocaryophyllus extracts on gout: Anti-inflammatory activity and anti-hyperuricemic effect through xantine oxidase and uricosuric action. J. Ethnopharmacol. 2016, 180, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F. Update on Biology: Uric Acid and the Activation of Immune and Inflammatory Cells. Curr. Rheumatol. Rep. 2010, 12, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Jones, J.D.G.; Dangl, J.L. Reactive Oxygen Species Signaling in Response to Pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Guma, M.; Ronacher, L.; Liu-Bryan, R.; Takai, S.; Karin, M.; Corr, M. Caspase 1–independent activation of interleukin-1β in neutrophil-predominant inflammation. Arthritis Rheum. 2009, 60, 3642–3650. [Google Scholar] [CrossRef] [PubMed]
- Posadas, I.; De Rosa, S.; Carmen Terencio, M.; Payá, M.; José Alcaraz, M. Cacospongionolide B suppresses the expression of inflammatory enzymes and tumour necrosis factor-α by inhibiting nuclear factor-κB activation. Br. J. Pharmacol. 2003, 138, 1571–1579. [Google Scholar] [CrossRef]
- Rayamajhi, M.; Zhang, Y.; Miao, E.A. Detection of Pyroptosis by Measuring Released Lactate Dehydrogenase Activity. In The Inflammasome. Methods in Molecular Biology; De Nardo, C., Latz, E., Eds.; Humana Press: Totowa, NJ, USA, 2013; Volume 1040, pp. 85–90. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Young, L.M.; Kheifets, J.B.; Ballaron, S.J.; Young, J.M. Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions 1989, 26, 335–341. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalán, L.; Carceller, M.C.; Terencio, M.C.; Alcaraz, M.J.; Ferrándiz, M.L.; Montesinos, M.C. Osteostatin Mitigates Gouty Arthritis through the Inhibition of Caspase-1 Activation and Upregulation of Nrf2 Expression. Int. J. Mol. Sci. 2024, 25, 2752. https://doi.org/10.3390/ijms25052752
Catalán L, Carceller MC, Terencio MC, Alcaraz MJ, Ferrándiz ML, Montesinos MC. Osteostatin Mitigates Gouty Arthritis through the Inhibition of Caspase-1 Activation and Upregulation of Nrf2 Expression. International Journal of Molecular Sciences. 2024; 25(5):2752. https://doi.org/10.3390/ijms25052752
Chicago/Turabian StyleCatalán, Laura, María Carmen Carceller, María Carmen Terencio, María José Alcaraz, María Luisa Ferrándiz, and María Carmen Montesinos. 2024. "Osteostatin Mitigates Gouty Arthritis through the Inhibition of Caspase-1 Activation and Upregulation of Nrf2 Expression" International Journal of Molecular Sciences 25, no. 5: 2752. https://doi.org/10.3390/ijms25052752
APA StyleCatalán, L., Carceller, M. C., Terencio, M. C., Alcaraz, M. J., Ferrándiz, M. L., & Montesinos, M. C. (2024). Osteostatin Mitigates Gouty Arthritis through the Inhibition of Caspase-1 Activation and Upregulation of Nrf2 Expression. International Journal of Molecular Sciences, 25(5), 2752. https://doi.org/10.3390/ijms25052752






