Bioluminescence of (R)-Cypridina Luciferin with Cypridina Luciferase
Abstract
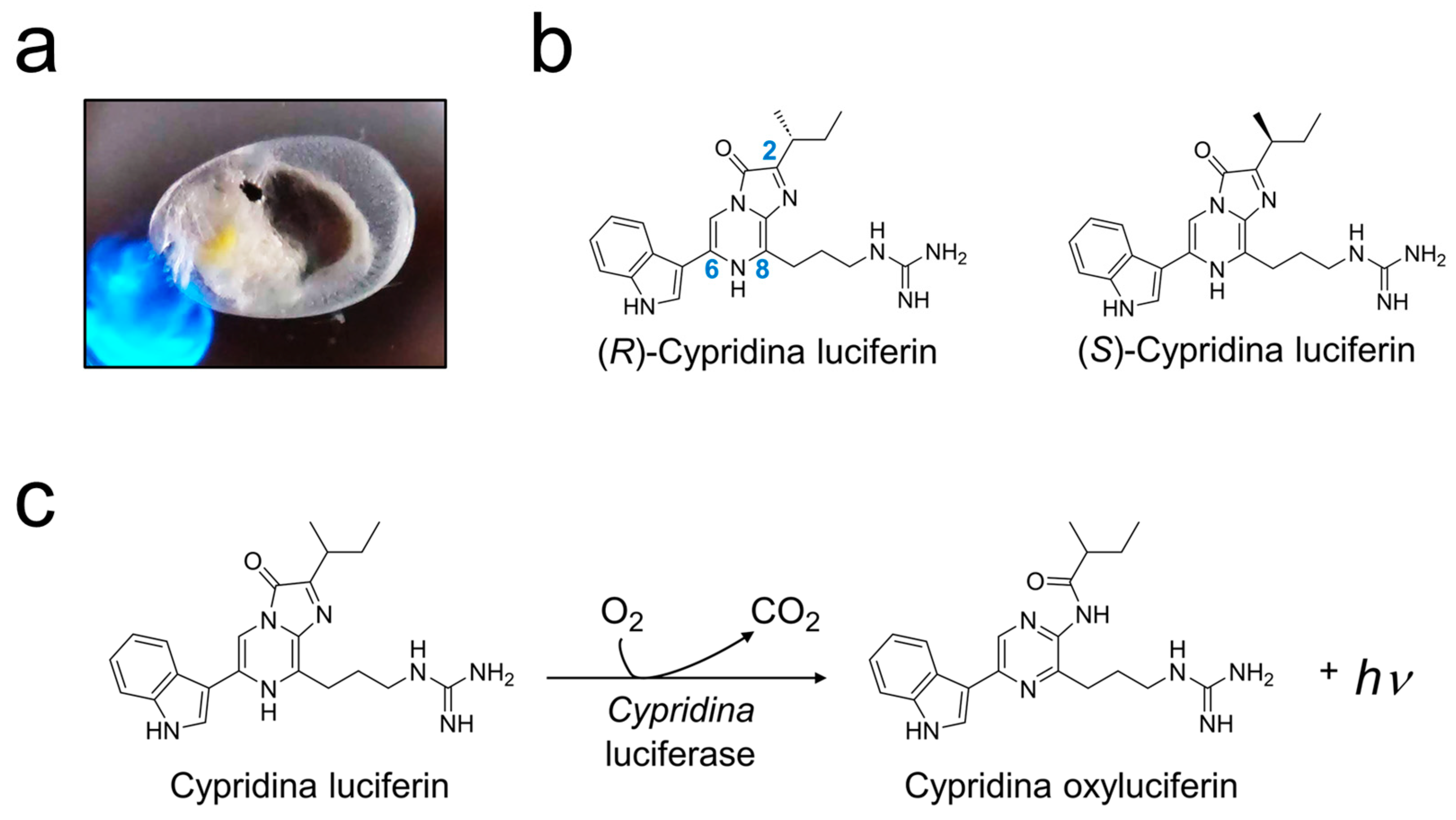
1. Introduction
2. Results
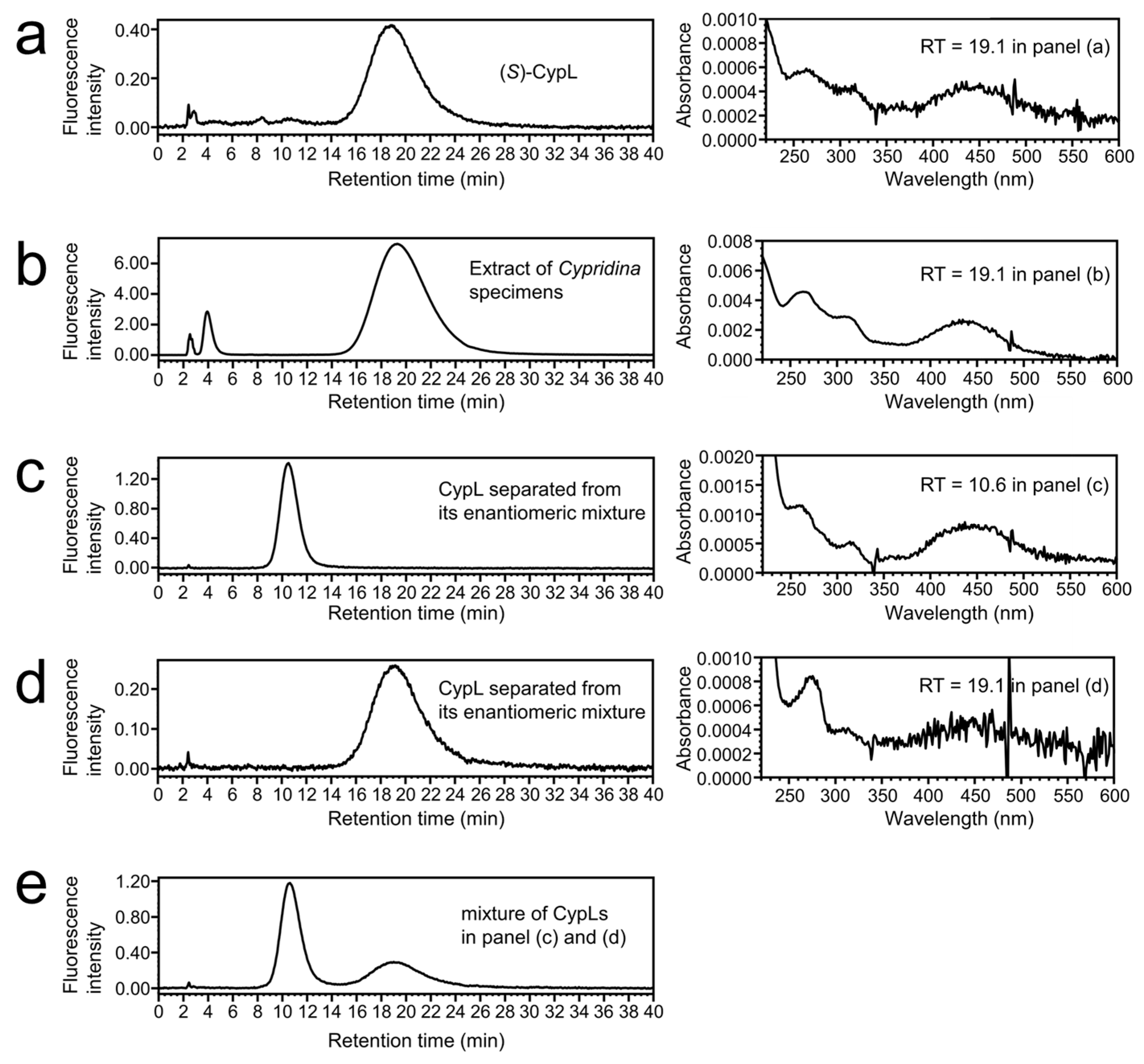
2.1. Chiral Separation of (R)-CypL from the Enantiomeric Mixture
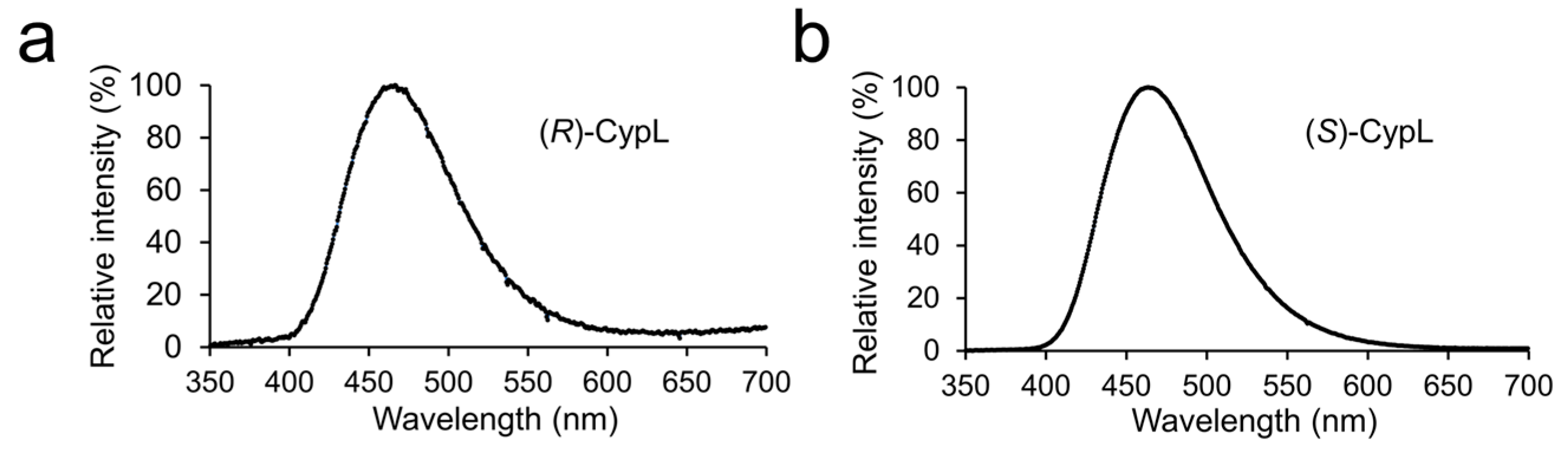
2.2. Luminescence Spectrum of (R)-CypL with CypLase
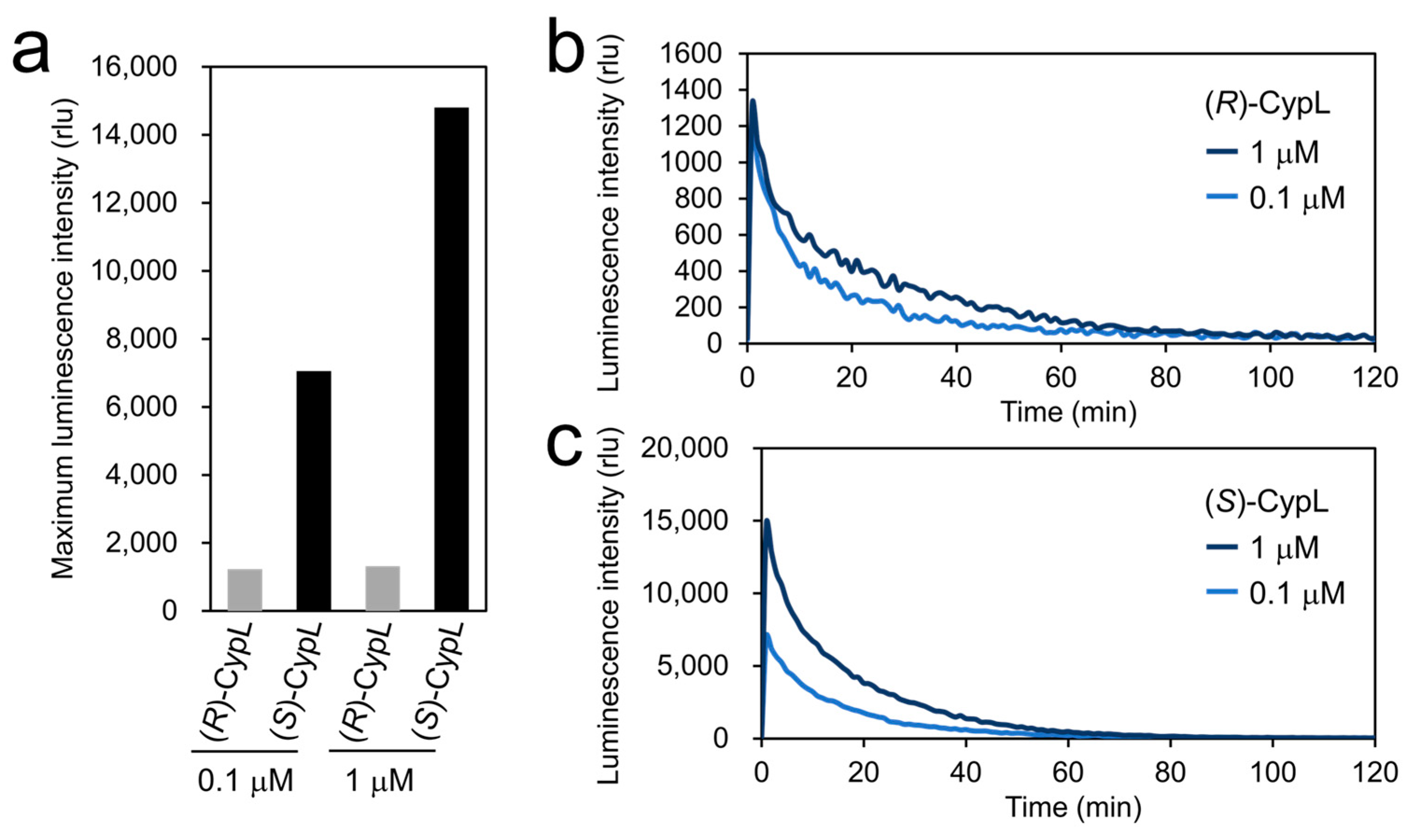
2.3. Luminescence Intensity of (R)-CypL with CypLase
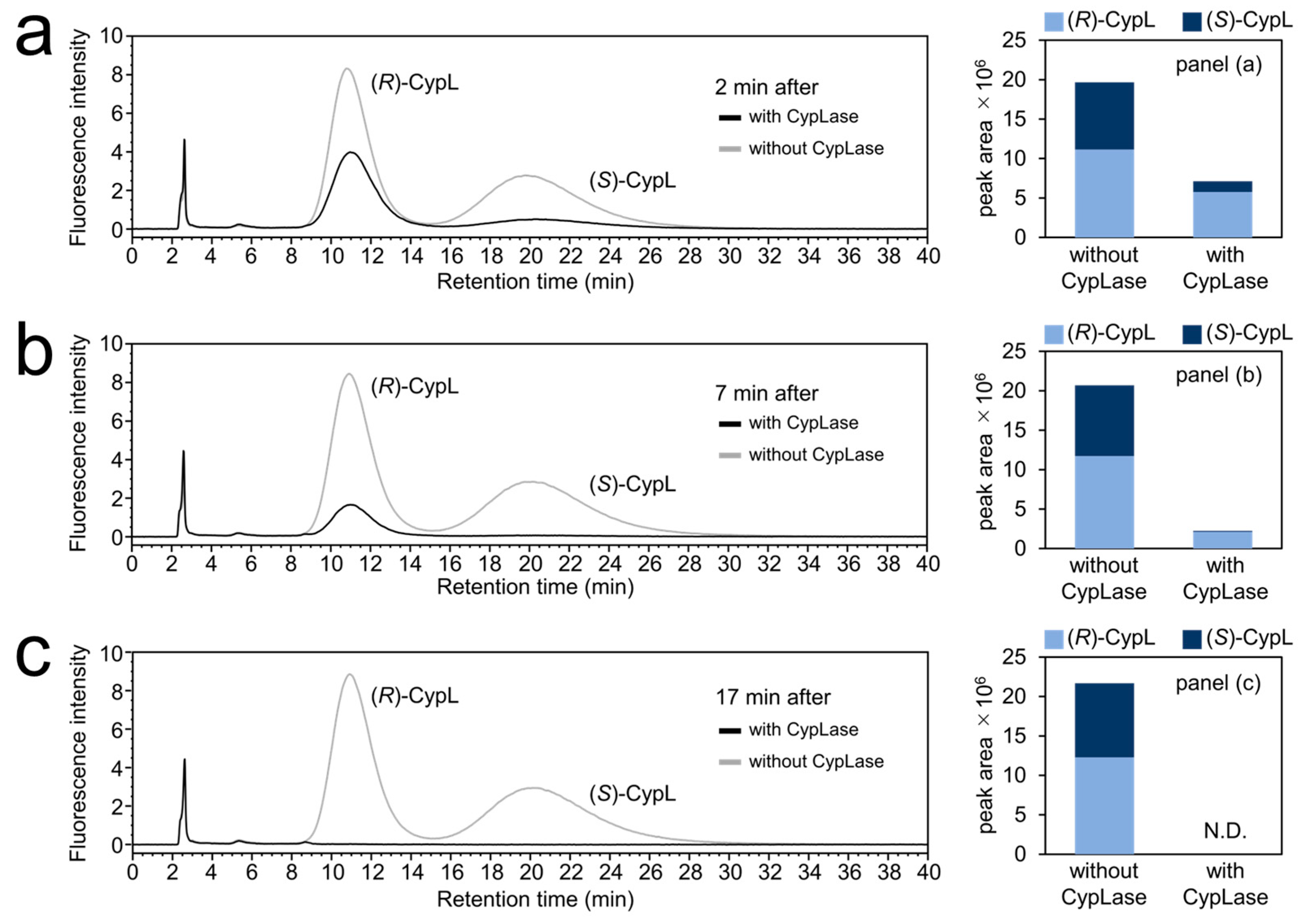
2.4. Turnover Rate of CypLase for (R)- and (S)-CypL
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Chiral HPLC Separation of Enantiomers of CypL
4.3. Chiral HPLC Analysis of Chiral-Separated CypLs
4.4. Measurement of Luminescence Emission Spectra
4.5. Measurement of the Luminescence Intensity of CypLs with CypLase
4.6. Measurement of Luminescence Intensity of CypLs with Human AGP
4.7. Kinetic Analysis of CypLase
4.8. Chiral HPLC Analysis of the Reaction Mixtures of Racemic CypL and CypLase
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGP | Alpha 1-acid glycoprotein |
| CCD | Charge-coupled device |
| CypLase | Cypridina luciferase |
| CypL | Cypridina luciferin |
| HCl | Hydrochloric acid |
| HPLC | High-performance liquid chromatography |
| NMWL | Nominal molecular weight limit |
| CypOxyL | Cypridina oxyluciferin |
| rlu | Relative light unit |
| RT | Retention time |
| Tris | Tris(hydroxymethyl)aminomethane |
Appendix A


| Concentration of the Substrate | Substrate | The Ratio of the Partially Integrated Luminescence Intensity to the Integration over 120 min | |
|---|---|---|---|
| 0–12 min | 12–120 min | ||
| 0.1 μM | (R)-CypL | 43.0% | 57.0% |
| (S)-CypL | 51.2% | 48.8% | |
| 1 μM | (R)-CypL | 35.6% | 64.4% |
| (S)-CypL | 49.5% | 50.5% | |
| Substrate | Km Value of CypLase |
|---|---|
| (R)-CypL | 0.23 μM |
| (S)-CypL | 0.75 μM |
| Concentration of the Substrate | Substrate | The Ratio of the Partially Integrated Luminescence Intensity to the Integration over 180 min | |
|---|---|---|---|
| 0–18 min | 18–180 min | ||
| 0.1 μM | (R)-CypL | 28.7% | 71.3% |
| (S)-CypL | 29.5% | 70.5% | |
| 1 μM | (R)-CypL | 26.6% | 73.4% |
| (S)-CypL | 26.6% | 73.4% | |
| Reaction Time | CypLase | The Ratio of the Peak Area of (R)- and (S)-CypL to the Total CypL | |
|---|---|---|---|
| (R)-CypL | (S)-CypL | ||
| 2 min | + | 80.9% | 19.1% |
| - | 56.7% | 43.3% | |
| 7 min | + | 94.6% | 5.4% |
| - | 56.7% | 43.3% | |
| 17 min | + | N.D. 1 | N.D. 1 |
| - | 56.7% | 43.3% | |
References
- Johnson, F.H.; Shimomura, O.; Saiga, Y.; Gershman, L.C.; Reynolds, G.T.; Waters, J.R. Quantum efficiency of Cypridina luminescence, with a note on that of Aequorea. J. Cell. Comp. Physiol. 1962, 60, 85–103. [Google Scholar] [CrossRef]
- Shimomura, O.; Johnson, F.H. Mechanisms in Quantum Yield of Cypridina Bioluminescence. Photochem. Photobiol. 1970, 12, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Morin, J.G. Based on a review of the data, use of the term ‘cypridinid’ solves the Cypridina/Vargula dilemma for naming the constituents of the luminescent system of ostracods in the family Cypridinidae. Luminescence 2011, 26, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Johnson, F.H.; Shimomura, O. Introduction to the Cypridina System. Methods Enzymol. 1978, 57, 331–364. [Google Scholar] [CrossRef]
- Thompson, E.M.; Nagata, S.; Tsuji, F.I. Cloning and expression of cDNA for the luciferase from the marine ostracod Vargula hilgendorfii. Proc. Natl. Acad. Sci. USA 1989, 86, 6567–6571. [Google Scholar] [CrossRef] [PubMed]
- Kazami, J.; Nakamura, H.; Goto, T. Luciferase, Luciferase-Coding Gene, and Process for Preparing Luciferase. PCT International Application No. WO90/01542, 22 February 1990. [Google Scholar]
- Haddock, S.H.D.; Moline, M.A.; Case, J.F. Bioluminescence in the sea. Ann. Rev. Mar. Sci. 2010, 2, 443–493. [Google Scholar] [CrossRef]
- Kaskova, Z.M.; Tsarkova, A.S.; Yampolsky, I.V. 1001 lights: Luciferins, luciferases, their mechanisms of action and applications in chemical analysis, biology and medicine. Chem. Soc. Rev. 2016, 45, 6048–6077. [Google Scholar] [CrossRef]
- Bessho-Uehara, M.; Yamamoto, N.; Shigenobu, S.; Mori, H.; Kuwata, K.; Oba, Y. Kleptoprotein bioluminescence: Parapriacanthus fish obtain luciferase from ostracod prey. Sci. Adv. 2020, 6, eaax4942. [Google Scholar] [CrossRef]
- Inouye, S. Multiple Cypridina Luciferase Genes in the Genome of Individual Ostracods, Vargula hilgendorfii (Cypridina hilgendorfii). Photochem. Photobiol. 2022, 98, 1293–1302. [Google Scholar] [CrossRef]
- Hensley, N.M.; Ellis, E.A.; Leung, N.Y.; Coupart, J.; Mikhailovsky, A.; Taketa, D.A.; Tessler, M.; Gruber, D.F.; De Tomaso, A.W.; Mitani, Y.; et al. Selection, drift, and constraint in cypridinid luciferases and the diversification of bioluminescent signals in sea fireflies. Mol. Ecol. 2021, 30, 1864–1879. [Google Scholar] [CrossRef]
- Inouye, S.; Sahara, Y. Soluble protein expression in E. coli cells using IgG-binding domain of protein A as a solubilizing partner in the cold induced system. Biochem. Biophys. Res. Commun. 2008, 376, 448–453. [Google Scholar] [CrossRef]
- Hunt, E.A.; Moutsiopoulou, A.; Broyles, D.; Head, T.; Dikici, E.; Daunert, S.; Deo, S.K. Expression of a soluble truncated Vargula luciferase in Escherichia coli. Protein Expr. Purif. 2017, 132, 68–74. [Google Scholar] [CrossRef]
- Mitani, Y.; Oshima, Y.; Mitsuda, N.; Tomioka, A.; Sukegawa, M.; Fujita, M.; Kaji, H.; Ohmiya, Y. Efficient production of glycosylated Cypridina luciferase using plant cells. Protein Expr. Purif. 2017, 133, 102–109. [Google Scholar] [CrossRef]
- Mitani, Y.; Yasuno, R.; Kihira, K.; Chung, K.M.; Mitsuda, N.; Kanie, S.; Tomioka, A.; Kaji, H.; Ohmiya, Y. Host-Dependent Producibility of Recombinant Cypridina noctiluca Luciferase with Glycosylation Defects. Front. Bioeng. Biotechnol. 2022, 10, 774786. [Google Scholar] [CrossRef]
- Maeda, Y.; Ueda, H.; Kazami, J.; Kawano, G.; Suzuki, E.; Nagamune, T. Truncation of Vargula luciferase still results in retention of luminescence. J. Biochem. 1996, 119, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Nishimiya, Y.; Morita, Y.; Wu, C.; Ohyama, Y.; Tochigi, Y.; Okuzawa, T.; Sakashita, M.; Asakawa, A.; Irie, T.; Ohmiya, Y.; et al. Molecular evolution of Cypridina noctiluca secretory luciferase for production of spectrum-shifted luminescence-emitting mutants and their application in nuclear receptor-reporter assays. Photochem. Photobiol. 2023; early view. [Google Scholar] [CrossRef]
- Goto, T. Chemistry of bioluminescence. Pure Appl. Chem. 1968, 17, 421–441. [Google Scholar] [CrossRef]
- Nakamura, H.; Aizawa, M.; Takeuchi, D.; Murai, A.; Shimoura, O. Convergent and short-step syntheses of dl-Cypridina luciferin and its analogues based on Pd-mediated cross couplings. Tetrahedron Lett. 2000, 41, 2185–2188. [Google Scholar] [CrossRef]
- Wu, C.; Kawasaki, K.; Ohgiya, S.; Ohmiya, Y. Syntheses and evaluation of the bioluminescent activity of (S)-Cypridina luciferin and its analogs. Tetrahedron Lett. 2006, 47, 753–756. [Google Scholar] [CrossRef]
- Harvey, E.N. Bioluminescence; Academic Press: New York, NY, USA, 1952. [Google Scholar]
- Haneda, Y.; Johnson, F.H.; Masuda, Y.; Saiga, Y.; Shimomura, O.; Sie, H.-C.; Sugiyama, N.; Takatsuki, I. Crystalline luciferin from live Cypridina. J. Cell. Comp. Physiol. 1961, 57, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, O.; Goto, T.; Hirata, Y. Crystalline Cypridina Luciferin. Bull. Chem. Soc. Jpn. 1957, 30, 929–933. [Google Scholar] [CrossRef]
- Kishi, Y.; Goto, T.; Hirata, Y.; Shimomura, O.; Johnson, F.H. Cypridina bioluminescence I Sructure of Cypridina luciferin. Tetrahedron Lett. 1966, 7, 3427–3436. [Google Scholar] [CrossRef]
- Kishi, Y.; Goto, T.; Eguchi, S.; Hirata, Y.; Watanabe, E.; Aoyama, T. Cypridina bioluminescence II structural studies of Cypridina luciferin by means of a high resolution mass spectrometer and an amino acid analyzer. Tetrahedron Lett. 1966, 7, 3437–3444. [Google Scholar] [CrossRef]
- Kishi, Y.; Goto, T.; Inoue, S.; Sugiura, S.; Kishimoto, H. Cypridina bioluminescence III total synthesis of Cypridina luciferin. Tetrahedron Lett. 1966, 7, 3445–3450. [Google Scholar] [CrossRef]
- Hoffmann, R.W. Classical Methods in Structure Elucidation of Natural Products; Wiley-VHCA: Zürich, Switzerland, 2018. [Google Scholar]
- Sugiura, S.; Inoue, S.; Goto, T. Synthesis of Cypridina Luciferin and Related Compounds. VIII. Improvement in the Synthesis of Cypridina Luciferin. Yakugaku Zasshi 1970, 90, 707–710. [Google Scholar] [CrossRef][Green Version]
- Oba, Y.; Kato, S.; Ojika, M.; Inouye, S. Biosynthesis of luciferin in the sea firefly, Cypridina hilgendorfii: L-tryptophan is a component in Cypridina luciferin. Tetrahedron Lett. 2002, 43, 2389–2392. [Google Scholar] [CrossRef]
- Kato, S.; Oba, Y.; Ojika, M.; Inouye, S. Identification of the biosynthetic units of Cypridina luciferin in Cypridina (Vargula) hilgendorfii by LC/ESI-TOF-MS. Tetrahedron 2004, 60, 11427–11434. [Google Scholar] [CrossRef]
- Kato, S.; Oba, Y.; Ojika, M.; Inouye, S. Stereoselective incorporation of isoleucine into Cypridina luciferin in Cypridina hilgendorfii (Vargula hilgendorfii). Biosci. Biotechnol. Biochem. 2006, 70, 1528–1532. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kato, S.; Oba, Y.; Ojika, M.; Inouye, S. Biosynthesis of Cypridina luciferin in Cypridina noctiluca. Heterocycles 2007, 72, 673–676. [Google Scholar]
- Kanie, S.; Komatsu, M.; Mitani, Y. Luminescence of Cypridina Luciferin in the Presence of Human Plasma Alpha 1-Acid Glycoprotein. Int. J. Mol. Sci. 2020, 21, 7516. [Google Scholar] [CrossRef] [PubMed]
- Niwa, K.; Nakamura, M.; Ohmiya, Y. Stereoisomeric bio-inversion key to biosynthesis of firefly D-luciferin. FEBS Lett. 2006, 580, 5283–5287. [Google Scholar] [CrossRef] [PubMed]
- Maeda, J.; Kato, D.; Okuda, M.; Takeo, M.; Negoro, S.; Arima, K.; Ito, Y.; Niwa, K. Biosynthesis-inspired deracemizative production of D-luciferin by combining luciferase and thioesterase. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 2112–2118. [Google Scholar] [CrossRef] [PubMed]
- Kanie, S.; Nakai, R.; Ojika, M.; Oba, Y. 2-S-Cysteinylhydroquinone is an intermediate for the firefly luciferin biosynthesis that occurs in the pupal stage of the Japanese firefly, Luciola lateralis. Bioorg. Chem. 2018, 80, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Oba, Y.; Murai, A. Synthesis and Absolute Configuration of the Ozonolysis Product of Krill Fluorescent Compound F. Tetrahedron Lett. 1993, 34, 2779–2782. [Google Scholar] [CrossRef]
- Tsarkova, A.S.; Kaskova, Z.M.; Yampolsky, I.V. A Tale Of Two Luciferins: Fungal and Earthworm New Bioluminescent Systems. Acc. Chem. Res. 2016, 49, 2372–2380. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, O.; Yampolsky, I.V. Bioluminescence: Chemical Principles and Methods, 3rd ed.; World Scientific: Hackensack, NJ, USA, 2019. [Google Scholar]
- Bolt, Y.V.; Dubinnyi, M.A.; Litvinenko, V.V.; Kotlobay, A.A.; Belozerova, O.A.; Zagitova, R.I.; Shmygarev, V.I.; Yatskin, O.N.; Guglya, E.B.; Kublitski, V.S.; et al. Total Synthesis of Racemic Thieno[3,2-f]thiochromene Tricarboxylate, a Luciferin from Marine Polychaeta Odontosyllis undecimdonta. Org. Lett. 2023, 25, 4892–4897. [Google Scholar] [CrossRef] [PubMed]
- Seliger, H.H.; McElroy, W.D.; Field, G.F.; White, E.H. Stereospecificity and firefly bioluminescence, a comparison of natural and synthetic luciferins. Proc. Natl. Acad. Sci. USA 1961, 47, 1129–1134. [Google Scholar] [CrossRef]
- Lembert, N. Firefly luciferase can use L-luciferin to produce light. Biochem. J. 1996, 317, 273–277. [Google Scholar] [CrossRef]
- Goto, T.; Fukatsu, H. Cypridina bioluminescence VII. chemiluminescence in micelle solutions—A model system for cypridina bioluminescence. Tetrahedron Lett. 1969, 10, 4299–4302. [Google Scholar] [CrossRef]
- Goto, T.; Kubota, I.; Suzuki, N.; Kishi, Y.; Inoue, S. Aspects of the Mechanism of Bioluminescence. In Chemiluminescence and Bioluminescence; Cormier, M.J., Hercules, D.M., Lee, J., Eds.; Springer: Boston, MA, USA, 1973; pp. 325–335. [Google Scholar]
- Hirano, T.; Takahashi, Y.; Kondo, H.; Maki, S.; Kojima, S.; Ikeda, H.; Niwa, H. The reaction mechanism for the high quantum yield of Cypridina (Vargula) bioluminescence supported by the chemiluminescence of 6-aryl-2-methylimidazo[1,2-a]pyrazin-3(7H)-ones (Cypridina luciferin analogues). Photochem. Photobiol. Sci. 2008, 7, 197–207. [Google Scholar] [CrossRef]
- Ishii, Y.; Hayashi, C.; Suzuki, Y.; Hirano, T. Chemiluminescent 2,6-diphenylimidazo[1,2-a]pyrazin-3(7H)-ones: A new entry to Cypridina luciferin analogues. Photochem. Photobiol. Sci. 2014, 13, 182–189. [Google Scholar] [CrossRef]
- Ding, B.W.; Naumov, P.; Liu, Y.J. Mechanistic Insight into Marine Bioluminescence: Photochemistry of the Chemiexcited Cypridina (Sea Firefly) Lumophore. J. Chem. Theory Comput. 2015, 11, 591–599. [Google Scholar] [CrossRef]
- da Silva, L.P.; Pereira, R.F.J.; Magalhaes, C.M.; da Silva, J. Mechanistic Insight into Cypridina Bioluminescence with a Combined Experimental and Theoretical Chemiluminescent Approach. J. Phys. Chem. B 2017, 121, 7862–7871. [Google Scholar] [CrossRef]
- Vacher, M.; Galvan, I.F.; Ding, B.W.; Schramm, S.; Berraud-Pache, R.; Naumov, P.; Ferre, N.; Liu, Y.J.; Navizet, I.; Roca-Sanjuan, D.; et al. Chemi- and Bioluminescence of Cyclic Peroxides. Chem. Rev. 2018, 118, 6927–6974. [Google Scholar] [CrossRef]
- Min, C.G.; Liu, Q.B.; Leng, Y.; Magalhaes, C.M.; Huang, S.J.; Liu, C.X.; Yang, X.K.; da Silva, L.P. Mechanistic Insight into the Chemiluminescent Decomposition of Cypridina Dioxetanone and the Chemiluminescent, Fluorescent Properties of the Light Emitter of Cypridina Bioluminescence. J. Chem. Inf. Model. 2019, 59, 4393–4401. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, O.; Johnson, F.H.; Masugi, T. Cypridina Bioluminescence: Light-Emitting Oxyluciferin-Luciferase Complex. Science 1969, 164, 1299–1300. [Google Scholar] [CrossRef] [PubMed]
- Ooka, H.; Chiba, Y.; Nakamura, R. Thermodynamic principle to enhance enzymatic activity using the substrate affinity. Nat. Commun. 2023, 14, 4860. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Nakamura, M.; Suzuki, T.; Ishizaka, N.; Sato, J.; Inouye, S. Identification of 3-enol sulfate of 72 Cypridina luciferin, Cypridina luciferyl sulfate, in the sea-firefly Cypridina (Vargula) hilgendorfii. Tetrahedron 2014, 70, 2161–2168. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanie, S.; Wu, C.; Kihira, K.; Yasuno, R.; Mitani, Y.; Ohmiya, Y. Bioluminescence of (R)-Cypridina Luciferin with Cypridina Luciferase. Int. J. Mol. Sci. 2024, 25, 2699. https://doi.org/10.3390/ijms25052699
Kanie S, Wu C, Kihira K, Yasuno R, Mitani Y, Ohmiya Y. Bioluminescence of (R)-Cypridina Luciferin with Cypridina Luciferase. International Journal of Molecular Sciences. 2024; 25(5):2699. https://doi.org/10.3390/ijms25052699
Chicago/Turabian StyleKanie, Shusei, Chun Wu, Kiyohito Kihira, Rie Yasuno, Yasuo Mitani, and Yoshihiro Ohmiya. 2024. "Bioluminescence of (R)-Cypridina Luciferin with Cypridina Luciferase" International Journal of Molecular Sciences 25, no. 5: 2699. https://doi.org/10.3390/ijms25052699
APA StyleKanie, S., Wu, C., Kihira, K., Yasuno, R., Mitani, Y., & Ohmiya, Y. (2024). Bioluminescence of (R)-Cypridina Luciferin with Cypridina Luciferase. International Journal of Molecular Sciences, 25(5), 2699. https://doi.org/10.3390/ijms25052699








