Abstract
Since its inception, induced pluripotent stem cell (iPSC) technology has been hailed as a powerful tool for comprehending disease etiology and advancing drug screening across various domains. While earlier iPSC-based disease modeling and drug assessment primarily operated at the cellular level, recent years have witnessed a significant shift towards organoid-based investigations. Organoids derived from iPSCs offer distinct advantages, particularly in enabling the observation of disease progression and drug metabolism in an in vivo-like environment, surpassing the capabilities of iPSC-derived cells. Furthermore, iPSC-based cell therapy has emerged as a focal point of clinical interest. In this review, we provide an extensive overview of non-integrative reprogramming methods that have evolved since the inception of iPSC technology. We also deliver a comprehensive examination of iPSC-derived organoids, spanning the realms of the nervous system, cardiovascular system, and oncology, as well as systematically elucidate recent advancements in iPSC-related cell therapies.
1. Background
The remarkable ability of pluripotent stem cells (PSCs) to self-renew and develop into any type of somatic cell is what sets them apart. These cells can originate from embryonic cells or adult somatic cells. Ethical constraints have limited the clinical application of embryonic stem cells (ESCs), which are derived from embryos [1]. This limitation has led to the emergence of iPSCs, which share similarities with ESCs but bypass ethical concerns by utilizing patient-specific somatic cells. The inception of human iPSC technology in 2007 marked a pivotal moment [2]. While initial reprogramming methods suffered from low efficiency, subsequent refinements have enabled robust and efficient iPSC generation without the genomic integration of transgenes. Conventional iPSC reprogramming often relies on integrating viral vectors, carrying the risk of insertional mutagenesis. To mitigate this risk, non-integrative reprogramming methods, such as adenovirus, endai virus (SeV), and protein-based approaches, have gained favor among researchers.
Organoids, self-organizing cell aggregates derived from human stem cells, closely mimic the cell types and organizational properties of native organs [3]. iPSCs play a pivotal role in organoid generation, yielding diverse and physiologically relevant models adaptable to normal and disease conditions. The inability of traditional two-dimensional (2D) cell cultures to replicate the cellular processes found in tissues is due to their lack of cellular variety, hierarchical structure, and cell-matrix or cell-cell interactions [4]. iPSC-derived three-dimensional (3D) organoids, with their multicellular and multifunctional nature, replicate organ-like properties. Moreover, researchers can manipulate the 3D culture system by adjusting growth factors and differentiation protocols to create a spectrum of organoids mirroring normal or disease states [4]. Thus, iPSCs offer a versatile platform for organoid development.
In this paper, we explore iPSC reprogramming techniques, emphasizing iPSC-derived organoids and their potential applications in disease therapy. These advancements hold promise in advancing our understanding of disease mechanisms and facilitating drug screening and therapy development.
2. Nonintegrating Way of iPSC Reprogramming
Since the successful isolation of human ESCs in 1998, significant efforts have been directed towards differentiating ESCs into mature tissues, particularly for disease modeling and the long-term goal of allogeneic cell therapy [5]. However, prior to the development of gene targeting techniques, genome editing in human ESCs was plagued by remarkably low efficiency, and establishing clear genotype-phenotype correlations within ESC-based models was challenging [6]. Consequently, obtaining specific mutations in ESCs proved to be a formidable task. More importantly, the advent of reprogramming technology marked a pivotal shift, facilitating the utilization of disease-specific iPSCs within established directed differentiation protocols. This breakthrough overcame the limitations associated with disease modeling in ESCs [5].
While iPSCs were officially reported in 2006, the foundation for reprogramming had been laid long before. iPSCs offer diverse sources compared to other PSCs with various reprogramming methods at our disposal [7]. For example, PSCs have been generated from human and mouse somatic cells by expressing transcription factors such as Oct4, Sox2, and Klf4 by viruses. Nevertheless, a significant disadvantage of this strategy is the employment of viruses that integrate into the genome, which increases the likelihood of tumor formation since viral transgenes may reactivate [8]. Lentiviral vectors, another gene transfer method, integrate the transgene into the host chromosome, theoretically ensuring long-term transgene expression. Nevertheless, this approach may yield unstable transgene expression or unexpected side effects, such as overexpression or inactivation of unrelated genes [9]. To circumvent potential insertional mutagenesis, transient expression via non-integrating vectors has emerged as a promising alternative [10]. The subsequent sections briefly introduce several non-integrating reprogramming methods.
2.1. Adenovirus
Adenoviral vectors are widely employed in both experimental research and clinical trials due to their ability to efficiently transfer genes into various somatic, stem, and cancer cell types. iPSCs can be generated from both mouse and human somatic cells using adenoviral vectors, enabling transient and robust expression of exogenous genes without genomic integration [8,11].
2.2. SeV
Another widely employed reprogramming method utilizes SeV-derived particles [12]. SeV, an RNA virus primarily affecting respiratory tissues in rodents, readily infects various murine and human cells by binding to sialic acid residues expressed on target cells [12]. Since SeV does not replicate during the nuclear phase, there’s no chance of host genome modification or gene silencing [13]. Consequently, SeV is extensively used as a vector in stem cell research, enabling efficient reprogramming of cells. Compared to other integrative strategies, SeV has demonstrated high reliability, superior efficiency in generating iPSCs free from viral contamination, and reduced workload [13]. For instance, SeVdp-302L, delivering a single-stranded RNA genome with multiple transgenes into somatic cells for iPSC generation, ensures stable transgene expression and includes an auto-erasable feature responsive to stem cell-specific microRNA-302 [14].
2.3. Protein
The notion of using proteins as a means to induce reprogramming for generating footprint-free iPSCs was proposed over a decade ago [15]. Cho’s pioneering study showed that pluripotent state reprogramming may be achieved without forcing the expression of ectopic transgenes with a single transfer of proteins obtained from embryonic stem cells into predominantly cultivated adult mouse fibroblasts. This discovery unveiled the remarkable potential of protein-iPSCs, showcasing their ability to undergo in vivo differentiation, as evidenced by well-differentiated teratoma formation. Furthermore, these protein-iPSCs displayed developmental potential by enabling the generation of chimeric mice and participating in tetraploid blastocyst complementation experiments [16]. Despite its advantages, it’s worth noting that synthesizing large quantities of bioactive proteins capable of efficiently crossing the plasma membrane has presented technical challenges.
2.4. mRNA/miRNA
mRNA reprogramming is now widely adopted due to its transient vector characteristics, which enable rapid and efficient iPSC generation. It offers precise control over reprogramming factors, including dosing, stoichiometry, and timing [17]. In late 2010, Warren published a seminal study demonstrating iPSC induction via mRNA transfection is feasible [17,18]. Compared to traditional mRNA reprogramming, modified mRNA (modRNA) addresses key limitations, such as the short persistence of protein expression after mRNA transfection and rapid cytoplasmic degradation. ModRNA exhibits improved stability and reduced immunogenicity [19]. One notable challenge in realizing mRNA reprogramming is the potent activation of broadly expressed antiviral defense pathways in mammalian cells upon synthetic mRNA delivery. This activation suppresses protein translation from exogenous transcripts and triggers cytotoxic and cytostatic responses detrimental to reprogramming [17]. Consequently, extensive research is required to overcome these hurdles.
2.5. PiggyBac
The PiggyBac (PB) transposon system is recognized for its demonstrated effectiveness in the genomic engineering of mammalian cells, playing a pivotal role in various preclinical applications. This versatile tool has facilitated gene discovery, multiplexed genome modification, animal transgenesis, and in vivo gene transfer, ensuring sustained gene expression in animals. Additionally, it has been instrumental in genetically modifying clinically relevant cell types, including iPSCs and human T lymphocytes [20]. One remarkable advantage of this non-viral approach is its ability to simplify and reduce the cost of producing the four-factor vector [20]. Notably, PB also allows for precise excision of reprogramming factors from iPSCs by re-expressing the PB transposase when introduced on a transposon [20]. Woltjen uncovers the successful and effective reprogramming of human and mouse embryonic fibroblasts with PB transposition-delivered doxycycline-inducible transcription factors [21]. More recently, numerous researchers have leveraged CRISPR/Cas9 technology to edit the PB transposon, enabling iPSC reprogramming [22,23,24].
2.6. Episomal Plasmids
A decade ago, lasmid vectors encoding Oct3/4, Sox2, Klf4, L-MYC, and Lin28 have been successfully used to induce human iPSCs from peripheral blood mononuclear cells (PBMCs) [25]. Remarkably, they could establish hundreds of iPSC lines from as few as 1 × 106 PBMCs isolated from donors ranging in age from their 20s to their 60s [25]. Additionally, combing episomal plasmids with the small chemical sodium butyrate also enhances the effectiveness of reprogramming when generating integration-free human iPSCs from differentiated adult fibroblasts [26]. In conclusion, this attractive footprint-free method is simple to produce iPSCs from a single transfection in a variety of cell types. It shows promise for upcoming therapeutic applications and is well-suited for the production of iPSCs tailored to individual patients. Plasmids reprogramming has proven effective in generating footprint-free iPSCs, with the main limitation being the need for modified cell culture methods to achieve acceptable reprogramming efficiency in fibroblasts. The efficiency, difficulty, and carrier removal of the above reprogramming methods are summarized, as shown in Table 1.

Table 1.
Different reprogramming methods.
3. Construction of Organoids Based on iPSC Technology
Human organoids, 3D cellular structures cultured from adult or PSCs, faithfully recapitulate key morphological, functional, and transcriptomic aspects of human organs [32,33,34,35]. These organoids are essential tools for understanding the mechanisms behind a variety of diseases, regardless of whether they were created to carry mutations that cause the disease or were taken directly from patient cells [32,36,37]. They offer a more precise reflection of the intricate cellular ecosystems found in complex tissues, making them ideal for disease modeling and drug screening.
Traditionally, translating drug trial results from experimental animal models to humans has been fraught with challenges due to species-specific variations in biological responses. A small number of new medications that the US Food and Drug Administration approves for clinical use each year is proof that this mismatch frequently leads to significant failure rates [38]. However, recent advancements in scientific methods, particularly the development of 3D organoid cultures and 3D-printed scaffolds, are revolutionizing this landscape [39]. This technological breakthrough in 3D organoid models holds great promise, enabling a comprehensive exploration of human disease complexities and facilitating in-depth investigations into disease pathogenesis [39]. However, with the expanding applications of iPSC technology, iPSC-derived organoid cultures are emerging as a dominant trend. These organoids are invaluable for mimicking a wide range of human maladies, including infectious diseases, genetic abnormalities, and cancer, since they realistically replicate the complex cellular ecosystems of complex tissues, in conjunction with human-rodent chimeras [39]. Particularly, patient-derived iPSC-derived organoids that can truly reflect the pathological characteristics of each individual, avoid possible adverse reactions, and make personalized treatment more accurate.
3.1. Derivation of Neural Organoids
Considering the human brain is complex, it’s difficult to recreate in mice, which makes an in vitro human brain model necessary to investigate many brain illnesses in model organisms. Consequently, a significant body of research has been dedicated to developing a 3D organoid culture system derived from human iPSCs.
The innate immune cells of the central nervous system, known as microglia, are essential for tissue homeostasis and neurodevelopment [40,41,42,43,44,45,46,47,48]. They are responsible for tasks such as phagocytizing apoptotic cells, pruning redundant synapses, and regulating neurogenesis and axonal growth [49]. Chronic inflammation is a key characteristic of neurological disorders, and modeling neuroinflammation-related diseases cannot be done without microglia. A growing amount of research has also demonstrated the connection between microglial dysfunction and a variety of neurological conditions, such as traumatic brain injury, Alzheimer’s disease (AD), and schizophrenia [49,50]. The lack of microglia, which presents the capability to reorganize brain networks and engulf dead cells and detritus, is a significant shortcoming for the neural organoid systems that are already in use. There are two ways to obtain microglia-containing organoids, one is to incorporate homologous microglia into the completed organoids, and the other is to generate microglia-containing brain organoids directly based on iPSCs. The former physically attaches microglia to the surface of organoids by means of co-culture. For instance, in the process of midbrain organoid differentiation, a coculture medium containing 186,000 freshly harvested macrophage precursor cells per organoid was substituted for the culture medium of midbrain organoids and assembloids starts on day 15 of dopaminergic (DA) differentiation [51]. By contrast, the latter obtained organoid models containing microglia by co-culturing iPSC-derived progenitors and progenitors of macrophages. According to recent studies, iPSCs taken from healthy individuals were used to generate primitive neural and macrophage progenitor cells. These progenitors were then co-cultured with 7000 neural and 3000 macrophage progenitors to create brain organoids that contained microglia [49]. Although the above studies actually obtained microglia-containing organoids, presently, iPSC-derived neural organoids containing microglia are relatively few, and there’s still a certain gap between these organoids and the real brain in terms of the distribution and quantity of microglia. Microglia only physically cover the surface of the organoids and do not really recapitulate the physiological processes in the body, which needs to be further addressed in the future.
Additionally, amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD), and AD can all be effectively modeled by iPSC-derived organoids. While challenges related to differentiation efficiency, maturation periods, and recapitulating sporadic or late-onset disease phenotypes persist, recent research suggests that iPSC-based organoids hold significant promise for advancing research and discovery in the field of neurodegenerative diseases [52,53,54,55,56,57].
3.2. Derivation of Liver Organoids
The evolving comprehension of liver organogenesis mechanisms has paved the way for innovative strategies in personalized liver disease modeling and treatment through human iPSC-derived hepatic sources and stromal cellular compositions [58]. The common method of differentiation of iPSCs into hepatocytes and organoids is shown in Figure 1. A multitude of researchers are currently focused on developing various liver organoids tailored to address numerous liver conditions [59,60,61,62], including fatty liver [63], hepatic steatosis [64], hepatitis, liver fibrosis [65], and irreversibly damaged liver.
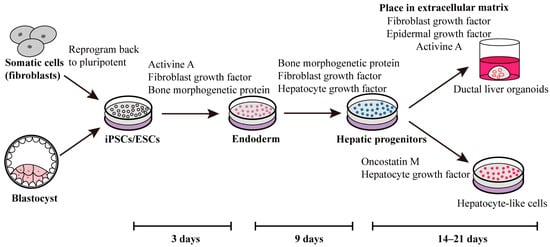
Figure 1.
One approach involves employing self-renewal factors to reprogram fibroblasts or blastocysts into iPSCs or ESCs. During the initial three days, activin A, fibroblast growth factor, and bone morphogenetic protein are introduced to prompt stem cell differentiation towards the endoderm lineage. Subsequently, over the following six days, fibroblast growth factor, bone morphogenetic protein, and hepatocyte growth factor are administered to facilitate the differentiation of hepatic progenitors. Ultimately, this process yields ducted liver organoids or hepatocyte-like cells, achieved through the influence of various stimulating factors [66,67,68,69,70].
While it’s interesting to obtain liver organoids from iPSCs, it’s also important to improve existing methods. A strategy of direct dialysis-based media conditioning was employed to induce the differentiation of human liver organoids [71]. By optimizing the accumulation of growth factors, they significantly enhanced hepatic differentiation of human iPSCs at high cell densities. The resultant human liver organoids showed hepatobiliary physiology and hepatic indicators that were on par with or superior to those that had been differentiated at lower cell densities in suspension cultures with regular daily media replacement [71].
Indeed, up to a third of adult liver transplants and 70% of pediatric liver transplants are caused by problems with the biliary system, which moves bile from the liver to the duodenum [72]. Therefore, it’s urgent to obtain organoids with hepatobiliary characteristics. The process of causing human iPSCs to develop into 3D hepatobiliary organoids has advanced. Researchers achieved this by supplementing hepatic differentiation medium with 25% mTeSRTM (STEMCELL Technologies, Cat:85850, Vancouver, Canada) culture medium and 10% cholesterol+ MIX to induce endodermal and mesodermal commitment, leading to the formation of mature hepatobiliary organoids [73]. These organoids demonstrated appropriate secretion abilities (albumin and urea) and drug metabolic capacity (CYP3A4 activity and inducibility). Furthermore, they exhibited remarkable survivability in immune-deficient mice for over 8 weeks and proved valuable for in vitro studies investigating the molecular mechanisms of liver development, holding substantial potential for liver disease therapy [73].
Additionally, maturity has always been one of the major factors that hinder the application of organoids. Thus, to obtain mature liver organoids, the researchers have built liver organoids with a rich vascular structure. Takebe demonstrated a groundbreaking achievement in generating vascularized and functional human liver tissue from human iPSCs through the transplantation of in vitro-created liver buds. Certain hepatocytes were autonomously arranged into 3D iPSC liver buds by mimicking the organogenetic interactions between endothelial cells and mesenchymal cells [74]. By adhering to host capillaries, these transplanted human iPSC liver buds successfully established functional vasculatures. Without the need for recipient liver replacement, the highly metabolic iPSC-derived tissue carried out liver-specific tasks such as protein synthesis and human-specific drug metabolism [74]. Additionally, in multi-organ systems generated from iPSCs, it has been demonstrated that intertissue communication promotes organ maturation [75]. For example, in co-cultivation, liver and islet organoids generated from iPSCs consistently exhibit robust growth and tissue-specific function [76]. In addition, it has recently been demonstrated that human liver-pancreatic islet axis recapitulation in both healthy and diseased conditions is possible in terms of using microfluidic multi-organ systems [77]. The technology allows for the 3D co-culture of human islet and liver organoids produced from iPSCs for up to 30 days under circulatory perfusion conditions. It’s made up of two divided zones connected by a network of microchannels [77]. By offering precise biomechanical cues, biochemical signals, and organ-organ interactions, organoids-on-a-chip devices provide a regulated tissue microenvironment [78,79]. Consequently, it’s possible to produce a large number of organoids with highly similar composition structures, indicating that the differences between the organoids obtained from the same batch production are smaller, by utilizing the controlled culture conditions of chip technology, the features of diversified stimulation, and continuous parameter readout to enhance the mutual communication between iPSC-derived organoids. However, the differentiation parameters for providing individual organoids in multi-organ chips have not been reported.
The field will still confront a number of obstacles in the future, despite these impressive advances. These include the following: (1) creating strategies to modify the intrinsic cellular composition, distribution, and proportion within liver organoids; (2) identifying their unique functions in the development and pathophysiology of the liver; (3) clarifying the biochemical characteristics of extracellular matrices (ECM) specific to the liver; and (4) incorporating ECM hydrogels or microparticles that are clinically compatible. It will be essential to address these issues if liver organoids’ physiological properties are to be preserved throughout modeling [58].
3.3. Derivation of Cardiac Organoids
Heart disease, a leading global cause of death, imposes a substantial burden on healthcare systems. Given its diverse manifestations and high mortality rates, there’s an urgent need for a deeper understanding of its various mechanisms.
The development of human heart organoid models for cardiovascular disease research has been slow and trails much behind that of other organs, despite the significance of comprehending human cardiovascular illnesses for treatment and prevention. There’s a great need to close this knowledge and technical gap because creating more accurate in vitro human heart models would help scientists and clinicians better understand both healthy and sick states for research and translational applications (Figure 2).
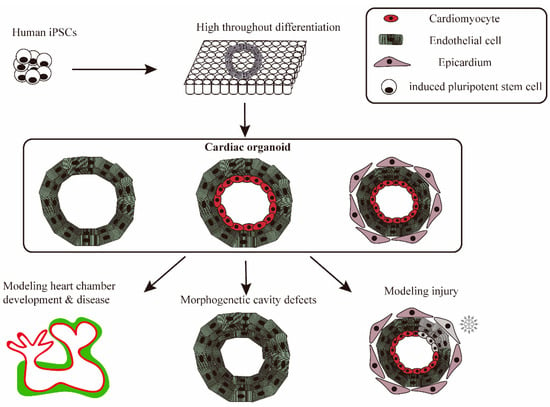
Figure 2.
iPSC-derived cardiac organoids mimics heart disease and physiological and pathological processes. iPSCs are widely differentiated into cardiac organoids in pore plates, which are used to simulate heart disease. Cardiac organoids with different cellular compositions are used for the study and exploration of different disease mechanisms or normal physiological processes, including injury, heart chamber development, and morphogenetic cavity defects.
Hence, many methods and techniques for generating cardiac organoids have emerged. For instance, human cardiac fibroblasts (HCFs), human cardiac microvascular endothelial cells (HCMECs), and human iPSC-derived cardiomyocytes (iPSC-CMs) are mixed in a 3:5:2 cell ratio. Then, in a 60-well plate, a 20 µL cell suspension containing about 100,000 cells was pipetted in each well and organoids are harvested three days later [80]. Furthermore, the potential of a microgel-based biphasic (MB) bioink is disclosed, which exhibits shear-thinning and self-healing behavior, both as an excellent bioink and a suspension medium for embedded 3D printing. When human iPSCs were encapsulated in the MB bioink, substantial stem cell proliferation and cardiac differentiation were able to generate cardiac tissues and organoids [81].
However, methods for generating cardiac organoids with native heart morphology based on iPSC technology are still lacking. The WNT signaling pathway, an important signaling pathway that regulates cardiomyocyte differentiation and cardiac development [82,83,84,85], can alleviate the above problems. Stated differently, during the routine manufacturing and differentiation of cardiac organoids, WNT inhibitors and activators are added at specific times, resulting in the generation of significant heart-like structures with respect to structure, organization, function, the complexity of cardiac cell types, ECM composition, and vascularization. For instance, on the 0 day of organoid differentiation, CHIR99021 (activator of WNT signaling pathway) was added for 24 h, and then the old medium in the pore plate was removed and the new medium was added. On the second day of organoid differentiation, WNT-C59 (nhibitors of the WNT signaling pathway) was added for 48 h, and then the organoids with heart-like structures could be obtained by continuing culture with normal medium [86]. As an alternative, mesodermal WNT-BMP signal axis targeting can potentially impart a hollow structure to heart organoids. Furthermore, studies reveal that the high level of WNT signaling during mesoderm induction promotes cavity growth in the later cardiac mesoderm stage [87], yet iPSC-derived cardiac organoids have not yet used this method. Organs produced from iPSCs therefore have greater development potential.
Organoids can mimic many physiological aspects of tissue development in vitro, but they usually lack the diverse multilineage progenitors necessary for the development of complex organs like the heart, as well as the cooperative paracrine interactions between adjacent tissues that characterize embryogenesis. It was thus shown that paracrine communication between tissues in close proximity helps to the determination of their respective cell fates and maturation into functioning organs. Researchers also disclosed a human multilineage iPSC-derived organoid. To establish concentric cardio-pulmonary micro-Tissues, for instance, a novel stepwise strategy is used to direct the simultaneous induction of both mesoderm-derived cardiac and endoderm-derived lung epithelial lineages within a single differentiation of human iPSCs via temporal specific tuning of WNT and nodal signaling in the absence of exogenous growth factors. This organoid quickly matured alveoli in the presence of cardiac accompaniment [88]. There has also been the production of another multi-lineage iPSC-derived organoid that, over extended periods of time, recapitulates the co-development, differentiation, and maturation of two distinct tissues (the gut and the heart) and shows enhanced physiological maturation of cardiac tissue, notably of atrial/nodal cardiomyocytes [89]. Multilineage organoids are a natural next step towards more physiological in vitro models of human development, but more work needs to be done to expand the opportunities for simulating complicated multi-organ disorders or disorders of tissue morphogenesis or maturation ex vivo.
3.4. Derivation of Cancer Organoids
Model systems are used in both translational and basic cancer research to replicate the malignant state at the molecular, cellular, tissue, organ, and organism levels. The scientific community’s interest in creating patient-derived cancer models has been reignited due to growing worries about the low rate at which fundamental research findings are applied and the fact that cancer is a far more complex disease than previously thought. In addition to current developments in genome editing, xenotransplantation, bioengineering, and the processing and culture of human tissue, iPSCs provide a number of novel possibilities for the research of human cancer. iPSC-derived cancer cells may offer a fresh approach to the field of cancer research. During the process of reprogramming, these cancer cells may take on characteristics of cancer stem cells. Alternatively, the differentiation of cancer-iPSCs by teratoma development or organoid culturing may imitate the tumorigenic process. The use of the cancer-iPSC model could shed light on a few molecular processes linked to the advancement of cancer [90].
With the change of modern lifestyle, the incidence of colitis has gradually increased, and the prone population is very wide, difficult to cure, and the cancer rate is high, so it’s listed as one of the modern difficult diseases by the World Health Organization [91]. Long-term colitis and adenomatous polyposis can lead to contractions, perforations, and even bowel cancer [92,93,94,95,96,97,98,99,100]. Colon cancer organoids can be derived based on iPSC technology [101]. Crespo tried to create a successful method for obtaining differentiated human iPSCs that would allow for the production of colonic organoids (Cos), which would be used to simulate diseases of the large intestine in humans [102]. In this study, extensive gene and immunohistochemical profiling confirmed that the derived Cos represent the colon rather than the small intestine, the author applied this strategy to iPSCs derived from patients with familial adenomatous polyposis harboring germline mutations in the WNT-signaling-pathway-regulator gene encoding adenomatous polyposis coli (APC), and Cos exhibits enhanced WNT activity and increased epithelial cell proliferation, which was used as a platform for colorectal cancer model and drug testing [102]. The iPSC-derived colon cancer model is highly similar to the traditional colon cancer model. iPSC-derived organoids, on the other hand, differ from patient-derived organoids in the case of gastrointestinal cancer. There are a few obvious distinctions between the two: The first is that iPSC-derived organoids require a lot longer to mature than organoids obtained from patients; the second is that iPSC-derived organoids have a far more complicated maturation process with numerous layers and stroma [103]. However, the stromal compartments seen in iPSC-derived organoids may be very helpful for researching cancer, as it has been observed that pancreatic cancer cells’ stromal compartments do not function normally. Because stromal compartments are present in iPSC-derived organoids, it’s feasible to monitor and control intracellular communication to influence probable illness causes or more effectively administer chemotherapy and other cancer-targeting drugs [103]. Furthermore, genetically modified iPSC-derived organoids are helpful for studying how the microenvironment of cancer stem cells is regulated, which is important for determining how cancer tissues, normal cell organoids, and cancer organoids differ from cancerous regions [104]. iPSC-derived organoids are also different from organoids produced by tissue stem cells. iPSC-derived organoids are produced by sequential exposure to a series of signaling cues, which can provide possible mechanisms for the development and trajectory of disease. It has been accepted that iPSC-derived organoids are diverse. Tissue stem cells are derived from both normal and diseased tissues, providing the possibility to study specific tissues [105]. For instance, it’s interesting to use CRISPR-Cas9 technology to induce genetic mutations in colon organoids of normal tissue origin to study the genetic profile of colorectal tumors [106]. Moreover, organoids derived from tissue stem cells also have the potential for long-term expansion in vitro [107].
Retinoblastoma is a childhood cancer of the developing retina that starts with biallelic inactivation of the retinoblastoma gene 1 (RB1) gene [108,109]. Children with germline mutations in RB1 have an increased risk of developing retinoblastoma and other cancers later in life. Early on, several researchers tried to use R1 gene mutations to imitate retinoblastoma in mice, but they were unable to cause retinal tumors in Rb+/− mice. The subsequent conditional inactivation of both copies of R1 in the embryonic murine retina similarly failed to create retinoblastoma, since species-specific intrinsic genetic redundancy and compensation among Rb family members prevent retinoblastoma in mice [108]. Consequently, a few researchers created iPSCs from fifteen individuals who had germline RB1 mutations or deletions. Multiple clones from each iPSC line were then confirmed to retain the germline mutation and exposed to molecular profiling [110]. Subsequently, representative clones of every participant were created, developed into retinal organoids, separated, and injected intraocularly into the eyes of immunocompromised mice to track the development of tumors. This approach offers a fresh model for researching malignancies. Retinoblastoma, on the other hand, can further damage the eye structure, resulting in conjunctival congestion and corneal damage. iPSCs can also self-organize into autonomous multizone ectoderm of ocular cells, and then form functional corneal epithelial cell sheets [111]. Corneal epithelial cells obtained in this way successfully ameliorated corneal barrier dysfunction and can also ameliorate corneal damage caused by retinoblastoma.
One common reason of epithelial malignancies is the mutant Kirsten rats sarcoma viral oncogene homolog gene (KRAS). However, little is known on the molecular alterations that take place in epithelial cells immediately following the activation of oncogenic KRAS. In addition to patient samples and the genetically engineered mouse model, the development of organoid systems from primary mouse and human iPSC-derived lung epithelial cells to simulate lung adenocarcinoma has become a reality and demonstrated the utility of in vitro organoid approaches for uncovering the early consequences of oncogenic KRAS expression [112].
A bottleneck in the process of generating organoids from iPSCs is that traditional differentiation methods can only differentiate iPSCs into a specific type of cell, rather than the multiple cell types that make up tissues and organs. In order to achieve organ-level cellular variety, current techniques for creating cerebral, renal, retinal, and other organoids frequently call for extended culture durations, ranging from a few weeks to several months [113]. By simultaneously co-differentiating human iPSCs into distinct cell types via the forced overexpression of transcription factors, independent of culture-media composition, it’s possible to generate patterned organoids and bio-printed tissues with controlled composition and organization, but it will still require a significant financial and time investment [113].
4. Screening Drugs and Toxicity Detection of iPSC-Derived Organoids
Traditional iPSC-derived 2D cell culture systems have been shown to be an incredibly useful resource, yielding vital insights and lowering the cost of disease modeling techniques [114]. However, the primary drawback of iPSC-derived 2D cell lines is that they are unable to replicate the cellular functions found in tissues due to their lack of cellular variety, dimensionality, hierarchical structure, and cell–cell or cell–matrix interactions [115]. Scientists contend that the complexity of human organs is not captured by 2D models, necessitating the development of more medically accurate models [115]. Over the past decade, iPSC technology has advanced significantly. When coupled with recent breakthroughs in gene editing and 3D organoid development, iPSC-based platforms have gained enhanced capabilities for drug screening. As shown in Figure 3, this approach is gaining widespread popularity, driven by the growing interest in phenotypic screening and the distinct advantages offered by human iPSCs compared to traditional cellular screening methods.
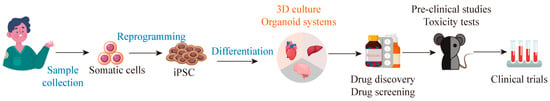
Figure 3.
iPSC-derived organoids can be used for drug screening. iPSCs derived from somatic cells can differentiate into 3D organoids, which can provide a disease model that replicates disease pathophysiology at the tissue and organ level for drug screening.
4.1. Neural Organoids
Owing to the fact that iPSCs are able to differentiate into 3D organoids, a model that authentically simulates tissue and organ-level disease pathophysiology for drug screening can be created that nearly mimics the complexity of the brain [116,117,118,119,120,121]. Compared to conventional 2D cultures, organoids display a series of advantages. Their cellular makeup is almost physiologic, and they can grow large in culture without losing genetic integrity, which can enable them to be useful for high-throughput testing. Given that 3D organoids have a morphology and structure more similar to genuine tissues than iPSC-derived cells or 2D cultures, they can more accurately represent disease and physiological states at the tissue and organ level. As such, for the purposes of drug screening and disease modeling, iPSC-derived 3D organoids perform better than iPSC-derived 2D cells.
Therefore, a variety of iPSC-derived neural organoids are used as platforms to screen for effective drugs. To speed up the search for therapeutics for diseases including schizophrenia, autism, and epilepsy, researchers tend to implement robotics and artificial intelligence-based phenotypic screening on iPSC-derived neural organoids. These researchers created motor neurons from iPSCs derived from familial ALS patients, which displayed cytosolic aggregates resembling those observed in ALS patients’ postmortem tissue [122]. Another example is that the researchers evaluated the therapeutic effects of four chemical compounds on ALS and identified anacardic acid, a histone acetyltransferase inhibitor, as a potential rescue agent for the abnormal ALS motor neuron phenotype [123]. Recently, ropinirole, a novel medication, has just started a phase 1/2a clinical trial. This medication prolongs ALS patients’ disease-progression-free survival by successfully reducing the expression of neurofilament light chain protein in the cerebrospinal fluid of ALS patients. An in vitro model of cytoplasmic aggregates produced by neurons originating from iPSCs was used to choose this medication [124]. This strongly implies that medication candidates can be successfully screened using ALS disease models produced from iPSCs.
However, despite the versatility of iPSC-derived neural organoids, their effective application in large-scale drug screening still faces challenges of heterogeneity, scalability, repeatability, and maturity [122]. Effectively solving these problems is the key to the current breakthrough. For instance, a differentiation technique from iPSC-derived expandable primitive neural stem cells is established, enabling the rapid generation of simplified brain organoids within two weeks, consisting of mature neurons and astrocytes [125]. This addresses the problem of organoid scalability. Immunochemistry and single-cell studies confirmed that the cytoarchitecture and transcriptional characteristics of these 3D cultures were uniform [125]. Taken together, more efforts are needed to make breakthroughs in organoid maturation and heterogeneity.
Additionally, recent advances in tissue stem cell research have also been significant. Hendriks D and co-workers successfully used fetal brain tissue to self-organize and form complex brain organoids in vitro [107]. This organoid contained a variety of cell types in brain tissue, including neurons, neural stem cells, and various progenitor cells, and was highly heterogeneous [107]. Because of this, it had the characteristics of long-term expansion and better organizational similarity. Compared with this kind of organoid, iPSC-derived organoids exhibited worse heterogeneity. If researchers want to obtain organoids with good heterogeneity from iPSCs, more complex signaling interventions are required in the process. Nevertheless, the sources of iPSCs are extensive and the ways to obtain them are varied. For the above brain organoids, the source may only be fetal brain tissue, which is very limited. However, it’s still a great innovation and a major technological breakthrough.
4.2. Liver Organoids
Developing more effective and repeatable procedures to generate liver organoids that can be utilized for drug screening has attracted increasing attention ever since the first reports of creating hepatocyte-like cells from iPSCs [126,127,128,129].
Currently used to prepare liver organoids, the classic Matrigel dome approach lacks precise microenvironment control and produces liver organoids with significant variances [130,131]. This is not conducive to the screening of drugs that have a therapeutic effect on liver disease, but research has been done on this problem. For instance, a culture technique that enables fine control over the size, density, and mass of individual liver organoids has been reported recently, along with a method for manufacturing high throughput liver organoids [130]. Without the use of Matrigel, human iPSCs are first developed into human foregut stem cells (hFSCs), which are subsequently further differentiated into liver organoids inside agarose-based microfabricated hexagonal arrays [130]. This liver organoid serves as a preclinical model to evaluate the liver’s response to acute hepatotoxicity caused by acetaminophen (APAP) exposure [130]. It’s possible to create a novel liver organoid that closely mimics important aspects of human liver development during the fetal stage by using human iPSC technology and micropatterning techniques. By virtue of this technique, bioengineered fetal liver organoids with deterministic size and position in a multi-well plate and consistent morphology may be created in a repeatable, high-throughput manner [132]. This organoid effectively demonstrates the hepatotoxicity of APAP and its metabolism in the liver. In addition, Shinozawa developed a reproducible method to generate human liver organoids from storable foregut progenitors derived from PSCs lines. These organoids were utilized to examine the toxicity of drugs since they had structures resembling functional bile canaliculi. A novel organoid-based assay was developed by the researchers, which demonstrated strong predictive values for 238 commercially available medications based on multiplexed readouts evaluating survivability, cholestatic, and/or mitochondrial toxicity [133].
Nevertheless, low cytochrome P450 (CYP450) enzyme expression and activity is responsible for the diminished drug metabolic function of the current in vitro liver models, which are widely utilized for toxicity analysis and drug development. Kim produced human iPSC-derived hepatic organoids (hHOs) from human iPSCs for long-term expansion and drug testing to solve this difficulty. These organoids feature multicellular compositions, cellular polarity, hepatobiliary structures, and remarkable CYP450 activity, faithfully recapitulating metabolic clearance, CYP450-mediated drug toxicity, and metabolism [134]. The organoid was validated for hepatotoxicity caused by seven drugs and elucidated drug metabolism pathways, including those for amitriptyline, chlorpromazine, and diclofenac. Hence, human in vitro hepatic models that accurately replicate liver function are crucial for translational research and medication discovery [70,134].
4.3. Cardiac Organoids
Fewer new cardiovascular drugs are successful through clinical trials, mainly due to the lack of clarity about drug toxicity and potential drug effects. Thus, the demand for drug toxicity assessment and new drugs for heart disease is still steadily increasing [135,136,137].
Cardiac organoids derived from human iPSCs are now widely used for drug toxicity testing. Human iPSC-derived cardiomyocytes can elucidate the role of ceramides and mitochondrial autophagy in cardiotoxicity [138], as well as detect drug-induced cardiotoxicity for various drugs including anthracyclines [139]. However, given that 2D cell cultures cannot perfectly replicate in vivo conditions, researchers are turning to iPSCs to create 3D heart models. A simple method to generate human 3D heart microtissue from iPSCs has been proposed, which can be generated scaffold-free, cultured for extended periods, and exhibit multiple cell types found in the human heart. They preserve coordinated contractile activity for several months and respond functionally to chemotherapy drugs [140]. Additionally, iPSC-derived cardiac organoids can also be used to screen therapeutic drugs after heart transplantation, simulate the body’s rejection reaction to drugs, and explore the potential adverse reactions of drugs. For example, the potential adverse effects of immunosuppressants like calcineurin inhibitors (tacrolimus) and proliferative signaling inhibitors (sirolimus) on cardiac function post-transplantation need further investigation. Cardiac organoids from iPSC-derived cardiomyocytes, fibroblasts, and endothelial cells were created [141], and combined with single-cell RNA sequencing to examine the cardiovascular effects of these drugs, revealing favorable cardiac remodeling with proliferation signal inhibitors compared to calcineurin inhibitors [141].
However, when screening drugs for heart disease treatment, researchers often employ gene editing to create 2D in vitro models for drug screening, but few construct highly mimetic 3D organoids to replicate the internal environment for drug discovery. One of the reasons is the lack of a high-throughput screening system and homogeneous cardiac organoids, which makes drug screening based on iPSC-derived organoids a nonsense. Hence, this direction could shape the future of heart drug screening, requiring innovation and further investigation to overcome challenges in this area.
5. Cell Therapy Based on iPSC Technology
Although there has been a lot of excitement about using iPSCs to create tissues to treat a range of diseases, such as leukemia, PD, and liver cirrhosis, it’s still elusive how beneficial iPSC-derived cells will be in the long run for regenerative medicine. The scientific community was thrilled by the discovery of iPSC technology and the prospect of customized cell treatment. Researchers benefit from iPSCs’ ability to produce a variety of cell types on a large scale by removing barriers related to processes like apheresis, which involves removing certain blood components and returning the remaining components to the donor’s circulation in order to obtain human cells [142]. Thus, below we elaborate and summarize around this topic.
5.1. The Advent of iPSC-Derived DA Neurons Offers an Opportunity to Treat Neurodegenerative Disease
To date, the usage of iPSCs to model neurodegenerative diseases has become increasingly popular, and relatively few studies have been conducted using cells derived from them for treatment. Therefore, here we take PD as an example to illustrate the significance of iPSC-related cell therapy in this field.
PD, a neurodegenerative disorder affecting around 1% of the population over 60, has long relied on dopamine-replacement therapy as its primary treatment [143]. The gradual degradation of DA neurons at the significant nigra in the midbrain is the pathological hallmark of PD. Only DA neurons are harmed in PD, and the lost cells are limited by space, only in the midbrain. iPSC-derived autologous DA neurons offer an opportunity to solve the problems. Recently, most of the studies have shown that iPSC-derived DA neurons effectively restore motor function and effectively reinnervate the host brain without the potential threat of tumor formation [143,144,145,146,147,148,149,150,151,152]. For instance, researchers employed small-molecule compounds to direct human iPSCs differentiation into nigral DA neurons that closely resembled in vivo counterparts [153]. Functionally, they demonstrated autoreceptor-dependent control of dopamine release and autonomous pacemaking based on L-type voltage-dependent Ca2+ channels [153]. The iPSC-derived DA neurons show strong survival and axon expansion when transplanted into the striatum of 6-Hydroxydopamine hydrochloride (6-OHDA)-lesioned athymic rats. This improves motor impairments in the rat PD model [153].
Nevertheless, the application of human iPSC-based cell therapy for PD faces numerous significant obstacles. Firstly, there’s a great deal of variation in the differentiation potentials of distinct human iPSC lines, most likely due to our incomplete understanding on the reprogramming process. Secondly, there’s still much to learn about the safety of human iPSC-based cell therapy. It’s also significant to fully remove some human iPSCs from the cell mixture that are undifferentiated or have tumorigenic mutations because they have the potential to develop into cancer.
5.2. iPSC-Derived Hepatocytes and Immune Cells Are Effective in the Treatment of Liver Diseases
Chronic liver disease is a significant global health concern, often leading to fibrosis as a response to persistent liver injury, resulting in both healing and scar formation [154]. In certain clinical situations, hepatocyte transplantation has been indicated as a substitute for whole organ transplantation in order to stabilize and extend the lives of patients. Among them, iPSC played a crucial role as an important treatment method. iPSC-derived hepatocyte-like cells expressed hepatocyte-specific genes and proteins and displayed high levels of hepatocyte growth factor and IL-10 expression [155,156,157]. By transplanting these generated hepatocyte-like cells, abnormal liver function can be improved, and thioacetamide-induced liver fibrosis and apoptosis are dramatically alleviated [155]. Additionally, iPSCs can also differentiate into immune cells involved in the treatment of chronic liver disease. For example, macrophages derived from human iPSCs can be directed into M1 or M2 phenotypes [158]. These human iPSC-derived macrophages, in particular the M2 subtype, dramatically diminish the expression levels of fibrogenic genes and related histological markers when used to treat liver fibrosis, providing a promising cell-based method to lessen fibrosis [158]. Alternatively, iPSCs can also be directly used to treat liver disease. iPSCs naturally produce and release extracellular vesicles, which, when internalized by hepatic stellate cells, modify their profibrogenic phenotype by reducing the expression of profibrogenic markers [154]. Thus, these studies have explored the liver-regenerating potential of iPSC-derived cells, providing a solid foundation for further investigations into cell therapy as an alternative treatment for liver disorders in humans [155].
5.3. iPSC-Derived Cardiac Patches and Extracellular Vesicles May Be a New Approach to Treating Heart Failure
Heart failure has a significant morbidity, mortality, and cost component, making it a serious and rapidly expanding global public health concern. With approximately 5500 transplants performed annually, heart transplantation is still the major therapeutic strategy for patients with end-stage heart failure and is a challenging issue in the medical industry [141]. The challenges of heart transplantation have been the overwhelming demand and the ideal of immunosuppression-free strategies. Cardiac stem cell therapy has recently been developed and extensively investigated as an alternative therapeutic strategy for heart failure. Among them, the combination of iPSCs and drug dosage forms is a relatively novel treatment. A novel cardiac patch for cardiac function recovery was produced using 3D bioprinting technology combined with a co-culture of cardiomyocytes, fibroblasts, and endothelial cells from human iPSCs [159,160]. It was shown that rats treated with patches showed enhanced diastolic function indices determined from echocardiography, lowered left ventricular-end diastolic pressure and the time constant of left ventricular relaxation, and increased anterior wall thickness in diastole [160]. In the heart that had been infarcted, the patch enhanced the expression of genes associated with vascular endothelial growth factor, angiopoietin 1, gap junction α-1 protein, β-myosin heavy 7, and insulin growth factor-1 [160]. Alternatively, extracellular vesicles of iPSC-CMs were used for cardiac treatment [161]. To get mitochondria-rich extracellular vesicles (M-EVs), the iPSC-CMs-conditioned media was ultracentrifuged. The therapeutic benefits of M-EVs were then examined using an in vivo murine myocardial infarction model (MI) [161]. As early as 3 h following treatment, therapy with 1.0 × 108/mL M-EVs significantly enhanced the contractile characteristics and intracellular adenosine triphosphate synthesis of hypoxia-injured iPSC-CMs [161]. Intramyocardial injection of M-EVs enhanced post-MI cardiac function in vivo and reduced the risk of heart failure [161,162]. Moreover, in addition to the above treatment methods, cardiac fibroblasts also have the potential to play a role in cardiac therapy. Given the possible contribution of cutaneous or human cardiac fibroblasts to cardiac repair, reprogramming these fibroblasts into iPSCs and collecting and purifying the exosomes released from these cells may lead to a therapeutic tool to support the development of cardiomyocytes [163].
5.4. iPSC-Derived Immune Cells Are a New Method of Tumor Immunotherapy
Adoptive cell therapy, which infuses immune cells into the patient’s body, has shown unique efficacy in the treatment of refractory malignancies. Most of the immune cells in the immune system are involved in the progression of tumors, and these cells deserve to be further studied and explored. Here, we focus on elucidating the role of some immune cells in tumor progression, including T cells, Natural killer (NK) cells, and macrophages.
5.4.1. T Cells
T cells are the main force in the fight against tumors and can recognize MHC molecules on the cell surface. These immune cells can be divided into two broad classes according to the different mechanism of effect, namely, CD4+ T cells and CD8+ T cells. CD4+ T cells recognize MHC Class II molecules to regulate the immune system. By contrast, CD8+ T cells recognize MHC Class I molecules and exert cytotoxicity directly, killing alien cells. Clinically, T cell adoptive cell therapy can treat some cancers [164,165,166,167,168], however, for patients with refractory tumors, the therapeutic effect of this approach is minimal. In order to solve this problem, researchers have long found that engineered T cells are a new strategy for the treatment of recurrent and refractory tumors [169,170,171], especially chimeric antigen receptor T cells (CAR-T) [172,173,174,175,176]. Chimeric antigen receptors (CARs) are engineered proteins designed to guide T cells to target cancer cells. (The structure of the chimeric antigen receptor is shown in Figure 4 below). In preclinical models, adoptive transfer of stem-like CAR-T cells produced improved tumor control and resistance to tumor rechallenge [177]. In xenograft models, CAR-CD3+CD4−CD8− T cells demonstrated efficient infiltration and tumor suppression against lung cancer genetically engineered to produce CD19, as well as antigen-specific cytotoxicity against B cell acute lymphoblastic leukemia [176]. Thus, the design of CAR-T cell therapy is feasible and effective in clinical cancer treatment.
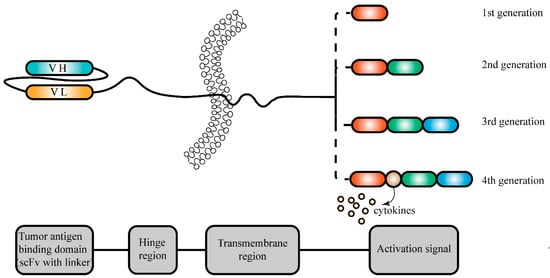
Figure 4.
Structural composition of chimeric antigen receptors for tumor treatment. Chimeric antigen receptors (CARs) are engineered proteins designed to guide T cells to target cancer cells. Typically, a CAR comprises an antigen recognition domain from a monoclonal antibody (mAb), a hinge region, a transmembrane domain, a costimulatory domain, and a T-cell receptor activation domain. The antigen recognition domain usually contains a single-chain variable fragment (scFv) derived from antibodies, enabling specific recognition of tumor antigens on cancer cells. Different generations of chimeric antigen receptors result in different activation signal regions.
However, T cell adoptive cell therapy and CAR T cell therapy utilizes a person’s own T lymphocytes, making it challenging for patients with progressive diseases and leukemia [178]. Additionally, the time-consuming process of generating autologous CAR T cells due to the limited availability of T lymphocytes and its inapplicability for third-party patients creates significant obstacles [178]. The fusion of iPSC and CAR technologies presents a promising avenue in oncology and significantly streamlines cell-based cancer therapy [178]. The unlimited production of CAR T cells from human iPSCs offers a compelling “off-the-shelf” CAR T cell immunotherapy approach [179]. CD8αβ+CAR T cells with typical characteristics can be obtained from iPSCs, and these expanded CAR-T derived from iPSCs (iPSC-CAR T cells) exhibited potent in vivo antitumor activity, extending the survival of mice with human tumor xenografts [179,180,181,182]. Furthermore, the use of gene editing technology may enable iPSC-CAR T cells to have higher anti-tumor activity. Of note, it has been demonstrated that EZH1 suppression promotes T cell maturation and differentiation from iPSCs in vitro. iPSC-T cells generated in a stroma-free, serum-free environment following silence of EZH1 exhibit strong antitumor effects when transduced with CARs both in vitro and in xenograft models [183]. It has been reported that transducing cells with genes encoding for membrane-bound intereleukin-15 (IL-15) and its receptor subunit IL-15α, as well as genetically knocking out diacylglycerol kinase, which inhibits antigen-receptor signaling, can increase the proliferation and persistency of CD8αβ+ cytotoxic CAR T cells in solid tumors [184]. Moreover, iPSCs produced from LMP2-specific cytotoxic T lymphocytes were treated with latent membrane protein 1 (LMP-1)-CAR to generate rejuvenated cytotoxic T lymphocytes. These engineered cells, which targeted CD19 and LMP-2 antigens, also displayed a strong tumor suppressive effect and clearly conferred a survival advantage [185]. Thus, editable and readily available therapeutically altered T cells can be produced using iPSCs, a new source with limitless potential.
5.4.2. NK Cells
Compared to T cells, NK cells may be more suitable for engineering to participate in tumor therapy, because they can release perforin or granzyme directly to kill tumor cells without presenting antigens via MHC molecules [186]. Such cells play a crucial role in the immune response against viral infections and tumors [187,188,189], which have the capacity to identify and eliminate “stressed cells”, including virus-infected, allogeneic, and tumor cells. Its effector functions are governed by a balance of signals from activating and inhibitory receptors, along with cytokines such as IL-2, IL-12, IL-15, and IL-18 [190]. Activated NK cells can directly destroy tumor cells or engage in antibody-dependent cellular cytotoxicity through the CD16 membrane receptor [191].
Even though these cells are capable of identifying and eliminating tumor cells, they only make up 5% of the lymphocyte pool in peripheral blood, which implies that in order to acquire enough cells for a single therapeutic dosage, extensive donor apheresis and expensive sample processing are required [192]. iPSC, a helpful tool for creating a large number of allogeneic NK cells, effectively solves this issue [193,194,195]. It also provides a special platform for genetic modifications at the clonal level and the subsequent generation of a large number of genetically engineered NK cells derived from a standardized starting cell source. As illustrated in Figure 5. For instance, cytokine-inducible SH2-containing protein (CISH) can be easily knocked out during the differentiation phase of iPSCs, and further induction of differentiation of such iPSCs can produce standardized CISH knockout NK cells [196]. Since CARs have the potential to overcome tumor cell escape strategies and target NK cells to tumor cells carrying specific antigens, which has good implications for cancer therapy and are easily inserted into iPSC. iPSC-CAR NK cells demonstrate strong cell viability and are effective in eliminating various tumor cells [197,198,199,200]. For example, a novel CAR targeting the conserved α3 domain of MHC class I chain-related protein A (MICA) and MHC class I chain-related protein B (MICB) is incorporated into a multiplexed-engineered iPSC-derived NK cell exhibiting antigen-specific anti-tumor reactivity across an expansive library of human cancer cell lines [201]. For targeted immunotherapy, the improved human iPSC-based approach may provide a workable way to produce CAR NK cells with an immunological memory-like nature.
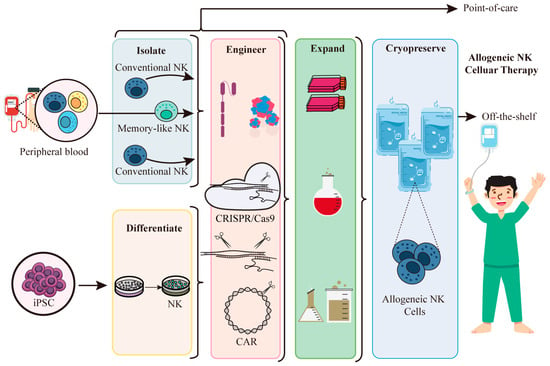
Figure 5.
iPSC-derived NK cells are used for allogeneic cell therapy. NK cells can be isolated from the peripheral blood, but they represent only a small fraction of the peripheral blood lymphocyte pool. This requires extensive donor beading and extensive sample handling. iPSC is a valuable resource for producing NK cells for allogeneic therapy. Acquired NK cells can be genetically modified at the clonal level, as is done with NK cells isolated from peripheral blood, and then a large number of genetically engineered NK cells can be obtained from a standardized source of initial cells for allogeneic cellular therapy.
However, CAR also has deficiencies in cancer treatment. The capacity of CAR-NK cells to interact with their target is hampered by the decreased tumor antigen density caused by the transfer of the CAR cognate antigen from the tumor to NK cells, as demonstrated by a recent study [202]. A dual-CAR system might counteract the phenomenon. The researchers use NK self-recognizing inhibitory CAR, which controls NK cells by sending them a “don’t kill me” signal, in addition to activating CAR against the cognate tumor antigen [203].
5.4.3. Macrophages
Macrophages play also an important role in cancer development and metastasis [204,205,206,207,208,209,210]. There are significant challenges in expanding the use of CAR T therapy across various cancer types, primarily due to its limited effectiveness caused by the intricate tumor microenvironment. It’s possible that manipulating macrophages to modify the tumor immune microenvironment is a more effective tumor therapeutic strategy. iPSC-derived macrophage cells are a fantastic source of macrophage cells because immortalized macrophage cell lines are not suitable for use in clinical settings and primary macrophages produced from bone marrow or PBMCs are not effectively engineered [211]. By producing CAR-expressing macrophage cells from iPSCs, the procedure has advanced significantly [212]. These modified macrophages have been illustrated to have better functions, including higher cytokine production and expression, polarization toward a pro-inflammatory/anti-tumor state, greater tumor cell phagocytosis, and in vivo anticancer effectiveness (Figure 6). Hence, macrophages derived from iPSCs have potential applications in cancer treatment.
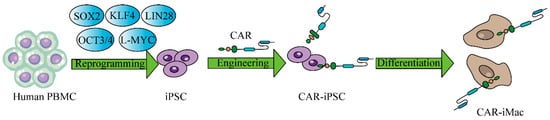
Figure 6.
CAR-expressing iPSCs can differentiate into CAR-macrophage cells. The overview of deriving CAR-iMacs from CAR-iPSCs.
6. Conclusions and Perspectives
The choice of reprogramming method depends on factors such as efficiency and safety, which are critical considerations depending on the purpose of generating iPSCs. For basic research studies where safety is less of a concern, the choice of method is more flexible. However, for clinical applications, safety is paramount, regardless of efficiency. So far, iPSC research leans more towards applications rather than reprogramming methods. This suggests that reprogramming methods are still in the early stages of iPSC development. While modern advanced technology may lead to breakthroughs in reprogramming methods, it’s essential to consider the balance between research costs and benefits. Since the initial description of diseased iPSCs, models have evolved from simple differentiated 2D cultures of single lineage cells with cellular-level outputs to more complex 3D organoids and in vivo chimeras. These advancements now incorporate interactions at the cell-tissue and tissue-organ levels into disease models. The emergence of human-specific modalities that require in vitro models of human cells and molecular biology has accelerated the humanization of drug discovery [213]. Perhaps, continuous improvements in iPSC differentiation protocols and chimeric models have the potential to revolutionize various fields, including infectious diseases, oncology, and tissue transplantation.
There’s a wide variety of cells that can be derived from iPSCs, and the advanced methods used in this process hold great promise. Autologous iPSC-derived cells are considered the safest in terms of immune rejection. While many iPSC-derived cells have shown therapeutic effects on diseases, their application remains mostly in the laboratory. Clinical transformation takes time and incurs relatively high costs. During the differentiation of iPSCs into specific cells, mutations can occur that interfere with treatment, leading to cases where patients have had to discontinue surgeries. Additionally, there’s a shortage of allogeneic iPSCs available for use. A significant challenge is ensuring that stored cells can serve a wide range of individuals. Many institutions are building inventories of iPSCs from healthy donors, specifically generated from the blood of human leukocyte antigen homozygous donors to minimize the risk of tissue rejection following transplantation. Once the inventory is built, it will greatly advance human medicine.
To date, site-specific genome editing tools like CRISPR/Cas9 have enabled the correction of target gene mutations. The combination of iPSC technology and CRISPR/Cas9 gene editing may be able to better improve the treatment of major diseases such as cancer, thereby ushering in a new era of treatment. Furthermore, chip technology has gained popularity, allowing for controllable culture conditions, diverse stimulations, and continuous parameter read-outs. If a new multi-organ chip based on human iPSCs is developed, it may effectively address the major problems such as organoid heterogeneity. Therefore, generating iPSC-derived organoids on chips or exploring interactions between iPSC-derived organoids and other organoids via chips may represent a promising avenue for future research.
Author Contributions
T.Z.: Article writing. C.Q.: Revision of article. M.S.: Collection of materials. Y.T.: Collection of materials. Y.Z. (Yueke Zhou): Collection of materials. G.D.: Collection of materials. Q.S.: Collection of materials. W.C.: Collection of materials. A.W.: Collection of materials. S.S.: Revision of article. Y.Z. (Yang Zhao): revision of article, writing—review and editing. Y.L.: revision of article, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Jiangsu Specially Appointed Professorship Foundation (013038021001) to Y.Z. (Yang Zhao) and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX23-2036) to T.Z.
Conflicts of Interest
The authors declare no competing interest.
References
- Yoshida, Y.; Yamanaka, S. Induced Pluripotent Stem Cells 10 Years Later: For Cardiac Applications. Circ. Res. 2017, 120, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Okano, H.; Morimoto, S. iPSC-based disease modeling and drug discovery in cardinal neurodegenerative disorders. Cell Stem Cell 2022, 29, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.G.; Kriegstein, A.R. Challenges of Organoid Research. Annu. Rev. Neurosci. 2022, 45, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Y.; Wu, S.; Wang, D.; Chu, C.; Hong, Y.; Tao, M.; Hu, H.; Xu, M.; Guo, X.; Liu, Y. Human organoids in basic research and clinical applications. Signal Transduct. Target. Ther. 2022, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.G.; Daley, G.Q. Induced pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Genet. 2019, 20, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Halevy, T.; Urbach, A. Comparing ESC and iPSC-Based Models for Human Genetic Disorders. J. Clin. Med. 2014, 3, 1146–1162. [Google Scholar] [CrossRef]
- Karagiannis, P.; Takahashi, K.; Saito, M.; Yoshida, Y.; Okita, K.; Watanabe, A.; Inoue, H.; Yamashita, J.K.; Todani, M.; Nak-agawa, M.; et al. Induced Pluripotent Stem Cells and Their Use in Human Models of Disease and Development. Physiol. Rev. 2019, 99, 79–114. [Google Scholar] [CrossRef]
- Stadtfeld, M.; Nagaya, M.; Utikal, J.; Weir, G.; Hochedlinger, K. Induced pluripotent stem cells generated without viral inte-gration. Science 2008, 322, 945–949. [Google Scholar] [CrossRef]
- Mitsui, K.; Takahashi, T.; Ide, K.; Matsuda, E.; Kosai, K.I. Optimization of adenoviral gene transfer in human pluripotent stem cells. Biochem. Biophys. Res. Commun. 2021, 541, 78–83. [Google Scholar] [CrossRef]
- Bayart, E.; Cohen-Haguenauer, O. Technological overview of iPS induction from human adult somatic cells. Curr. Gene Ther. 2013, 13, 73–92. [Google Scholar] [CrossRef]
- Zhou, W.; Freed, C.R. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells 2009, 27, 2667–2674. [Google Scholar] [CrossRef] [PubMed]
- Schlaeger, T.M. Nonintegrating Human Somatic Cell Reprogramming Methods. Adv. Biochem. Eng. Biotechnol. 2018, 163, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Haridhasapavalan, K.K.; Borgohain, M.P.; Dey, C.; Saha, B.; Narayan, G.; Kumar, S.; Thummer, R.P. An insight into non-integrative gene delivery approaches to generate transgene-free induced pluripotent stem cells. Gene 2019, 686, 146–159. [Google Scholar] [CrossRef]
- Okumura, T.; Horie, Y.; Lai, C.Y.; Lin, H.T.; Shoda, H.; Natsumoto, B.; Fujio, K.; Kumaki, E.; Okano, T.; Ono, S.; et al. Robust and highly efficient hiPSC generation from patient non-mobilized peripheral blood-derived CD34(+) cells using the auto-erasable Sendai virus vector. Stem Cell Res. Ther. 2019, 10, 185. [Google Scholar] [CrossRef]
- Seo, B.J.; Hong, Y.J.; Do, J.T. Cellular Reprogramming Using Protein and Cell-Penetrating Peptides. Int. J. Mol. Sci. 2017, 18, 552. [Google Scholar] [CrossRef]
- Cho, H.J.; Lee, C.S.; Kwon, Y.W.; Paek, J.S.; Lee, S.H.; Hur, J.; Lee, E.J.; Roh, T.Y.; Chu, I.S.; Leem, S.H.; et al. Induction of pluripotent stem cells from adult somatic cells by protein-based reprogramming without genetic manipulation. Blood 2010, 116, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.; Lin, C. mRNA-Based Genetic Reprogramming. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.; Manos, P.D.; Ahfeldt, T.; Loh, Y.H.; Li, H.; Lau, F.; Ebina, W.; Mandal, P.K.; Smith, Z.D.; Meissner, A.; et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010, 7, 618–630. [Google Scholar] [CrossRef]
- Wang, A.Y.L. Application of Modified mRNA in Somatic Reprogramming to Pluripotency and Directed Conversion of Cell Fate. Int. J. Mol. Sci. 2021, 22, 8148. [Google Scholar] [CrossRef]
- Woodard, L.E.; Wilson, M.H. piggyBac-ing models and new therapeutic strategies. Trends Biotechnol. 2015, 33, 525–533. [Google Scholar] [CrossRef]
- Woltjen, K.; Michael, I.P.; Mohseni, P.; Desai, R.; Mileikovsky, M.; Hämäläinen, R.; Cowling, R.; Wang, W.; Liu, P.; Gertsenstein, M.; et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 2009, 458, 766–770. [Google Scholar] [CrossRef]
- Wang, G.; Yang, L.; Grishin, D.; Rios, X.; Ye, L.Y.; Hu, Y.; Li, K.; Zhang, D.; Church, G.M.; Pu, W.T. Efficient, footprint-free human iPSC genome editing by consolidation of Cas9/CRISPR and piggyBac technologies. Nat. Protoc. 2017, 12, 88–103. [Google Scholar] [CrossRef]
- Itoh, M.; Kawagoe, S.; Tamai, K.; Nakagawa, H.; Asahina, A.; Okano, H.J. Footprint-free gene mutation correction in induced pluripotent stem cell (iPSC) derived from recessive dystrophic epidermolysis bullosa (RDEB) using the CRISPR/Cas9 and pig-gyBac transposon system. J. Dermatol. Sci. 2020, 98, 163–172. [Google Scholar] [CrossRef]
- Rodriguez-Polo, I.; Mißbach, S.; Petkov, S.; Mattern, F.; Maierhofer, A.; Grządzielewska, I.; Tereshchenko, Y.; Urrutia-Cabrera, D.; Haaf, T.; Dressel, R.; et al. A piggyBac-based platform for genome editing and clonal rhesus macaque iPSC line derivation. Sci. Rep. 2021, 11, 15439. [Google Scholar] [CrossRef]
- Okita, K.; Nakagawa, M.; Hong, H.J.; Ichisaka, T.; Yamanaka, S. Generation of Mouse Induced Pluripotent Stem Cells without Viral Vectors. Science 2008, 322, 949–953. [Google Scholar] [CrossRef]
- Hu, W.; He, Y.; Xiong, Y.; Lu, H.; Chen, H.; Hou, L.; Qiu, Z.; Fang, Y.; Zhang, S. Derivation, Expansion, and Motor Neuron Differentiation of Human-Induced Pluripotent Stem Cells with Non-Integrating Episomal Vectors and a Defined Xenogene-ic-free Culture System. Mol. Neurobiol. 2016, 53, 1589–1600. [Google Scholar] [CrossRef]
- Yue, G.; Liao, J.; Wang, Y.; He, L.; Wang, T.; Zhou, G.; Lei, B. Quality evaluation of induced pluripotent stem cell colonies by fusing multi-source features. Comput. Methods Programs Biomed. 2021, 208, 106235. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Yamanaka, S. Induced pluripotent stem cells: Opportunities and challenges. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2011, 366, 2198–2207. [Google Scholar] [CrossRef] [PubMed]
- Maali, A.; Maroufi, F.; Sadeghi, F.; Atashi, A.; Kouchaki, R.; Moghadami, M.; Azad, M. Induced pluripotent stem cell tech-nology: Trends in molecular biology, from genetics to epigenetics. Epigenomics 2021, 13, 631–647. [Google Scholar] [CrossRef] [PubMed]
- VandenDriessche, T.; Ivics, Z.; Izsvák, Z.; Chuah, M.K. Emerging potential of transposons for gene therapy and generation of induced pluripotent stem cells. Blood 2009, 114, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Hu, K. Vectorology and factor delivery in induced pluripotent stem cell reprogramming. Stem Cells Dev. 2014, 23, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Cowan, C.S.; Renner, M.; De Gennaro, M.; Gross-Scherf, B.; Goldblum, D.; Hou, Y.; Munz, M.; Rodrigues, T.M.; Krol, J.; Szikra, T.; et al. Cell Types of the Human Retina and Its Organoids at Single-Cell Resolution. Cell 2020, 182, 1623–1640.e34. [Google Scholar] [CrossRef] [PubMed]
- Human brain ‘organoids’ grafted into rats take note of light and pattern. Nature 2023, 614, 198. [CrossRef] [PubMed]
- Eisenstein, M. Organoids open fresh paths to biomedical advances. Nature 2022, 612, S34–S35. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Maynard, A.; Jain, A.; Gerber, T.; Petri, R.; Lin, H.C.; Santel, M.; Ly, K.; Dupré, J.S.; Sidow, L.; et al. Lineage recording in human cerebral organoids. Nat. Methods 2022, 19, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Bender, E. How organoids are advancing the understanding of chronic kidney disease. Nature 2023, 615, S10–S11. [Google Scholar] [CrossRef] [PubMed]
- Pașca, S.P.; Arlotta, P.; Bateup, H.S.; Camp, J.G.; Cappello, S.; Gage, F.H.; Knoblich, J.A.; Kriegstein, A.R.; Lancaster, M.A.; Ming, G.L.; et al. A nomenclature consensus for nervous system organoids and assembloids. Nature 2022, 609, 907–910. [Google Scholar] [CrossRef]
- Doss, M.X.; Sachinidis, A. Current Challenges of iPSC-Based Disease Modeling and Therapeutic Implications. Cells 2019, 8, 403. [Google Scholar] [CrossRef]
- Rauth, S.; Karmakar, S.; Batra, S.K.; Ponnusamy, M.P. Recent advances in organoid development and applications in disease modeling. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188527. [Google Scholar] [CrossRef]
- Prinz, M.; Jung, S.; Priller, J. Microglia Biology: One Century of Evolving Concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and non-immune functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.A.; Boddeke, H.W.; Kettenmann, H. Microglia in Physiology and Disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef] [PubMed]
- Wright-Jin, E.C.; Gutmann, D.H. Microglia as Dynamic Cellular Mediators of Brain Function. Trends Mol. Med. 2019, 25, 967–979. [Google Scholar] [CrossRef]
- Umpierre, A.D.; Wu, L.J. How microglia sense and regulate neuronal activity. Glia 2021, 69, 1637–1653. [Google Scholar] [CrossRef]
- Ghosh, S.; Castillo, E.; Frias, E.S.; Swanson, R.A. Bioenergetic regulation of microglia. Glia 2018, 66, 1200–1212. [Google Scholar] [CrossRef]
- Augusto-Oliveira, M.; Arrifano, G.P.; Delage, C.I.; Tremblay, M.; Crespo-Lopez, M.E.; Verkhratsky, A. Plasticity of microglia. Biol. Rev. Camb. Philos. Soc. 2022, 97, 217–250. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Xu, R.; Boreland, A.J.; Li, X.; Erickson, C.; Jin, M.; Atkins, C.; Pang, Z.P.; Daniels, B.P.; Jiang, P. Developing human pluripotent stem cell-based cerebral organoids with a controllable microglia ratio for modeling brain development and pathology. Stem Cell Rep. 2021, 16, 1923–1937. [Google Scholar] [CrossRef]
- Abud, E.M.; Ramirez, R.N.; Martinez, E.S.; Healy, L.M.; Nguyen, C.H.H.; Newman, S.A.; Yeromin, A.V.; Scarfone, V.M.; Marsh, S.E.; Fimbres, C.; et al. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron 2017, 94, 278–293.e9. [Google Scholar] [CrossRef]
- Sabate-Soler, S.; Nickels, S.L.; Saraiva, C.; Berger, E.; Dubonyte, U.; Barmpa, K.; Lan, Y.J.; Kouno, T.; Jarazo, J.; Robertson, G.; et al. Microglia integration into human midbrain organoids leads to increased neuronal maturation and functionality. Glia 2022, 70, 1267–1288. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Ting, H.C.; Liu, C.A.; Su, H.L.; Chiou, T.W.; Lin, S.Z.; Harn, H.J.; Ho, T.J. Induced Pluripotent Stem Cell (iPSC)-Based Neurodegenerative Disease Models for Phenotype Recapitulation and Drug Screening. Molecules 2020, 25, 2000. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Ra, E.A.; Kweon, S.H.; Seo, B.A.; Ko, H.S.; Oh, Y.; Lee, G. Advanced human iPSC-based preclinical model for Par-kinson’s disease with optogenetic alpha-synuclein aggregation. Cell Stem Cell 2023, 30, 973–986.e11. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.G.; Jung, D.J.; Jung, Y.K.; Kang, K.S. The Effect of a Novel Mica Nanoparticle, STB-MP, on an Alzheimer’s Disease Patient-Induced PSC-Derived Cortical Brain Organoid Model. Nanomaterials 2023, 13, 893. [Google Scholar] [CrossRef] [PubMed]
- Oun, A.; Sabogal-Guaqueta, A.M.; Galuh, S.; Alexander, A.; Kortholt, A.; Dolga, A.M. The multifaceted role of LRRK2 in Parkinson’s disease: From human iPSC to organoids. Neurobiol. Dis. 2022, 173, 105837. [Google Scholar] [CrossRef] [PubMed]
- Tejchman, A.; Znój, A.; Chlebanowska, P.; Frączek-Szczypta, A.; Majka, M. Carbon Fibers as a New Type of Scaffold for Midbrain Organoid Development. Int. J. Mol. Sci. 2020, 21, 5959. [Google Scholar] [CrossRef]
- Kim, H.; Park, H.J.; Choi, H.; Chang, Y.; Park, H.; Shin, J.; Kim, J.; Lengner, C.J.; Lee, Y.K.; Kim, J. Modeling G2019S-LRRK2 Sporadic Parkinson’s Disease in 3D Midbrain Organoids. Stem Cell Rep. 2019, 12, 518–531. [Google Scholar] [CrossRef]
- Cao, D.; Ge, J.Y.; Wang, Y.; Oda, T.; Zheng, Y.W. Hepatitis B virus infection modeling using multi-cellular organoids derived from human induced pluripotent stem cells. World J. Gastroenterol. 2021, 27, 4784–4801. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Kimura, M.; Yoshizawa, E.; Ayano, S.; Koido, M.; Funayama, S.; Nakanishi, N.; Hisai, T.; Kobayashi, T.; et al. Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Rep. 2017, 21, 2661–2670. [Google Scholar] [CrossRef]
- Guo, J.; Duan, L.; He, X.; Li, S.; Wu, Y.; Xiang, G.; Bao, F.; Yang, L.; Shi, H.; Gao, M.; et al. A Combined Model of Human iPSC-Derived Liver Organoids and Hepatocytes Reveals Ferroptosis in DGUOK Mutant mtDNA Depletion Syndrome. Adv. Sci. 2021, 8, 2004680. [Google Scholar] [CrossRef] [PubMed]
- Kulkeaw, K.; Tubsuwan, A.; Tongkrajang, N.; Whangviboonkij, N. Generation of human liver organoids from pluripotent stem cell-derived hepatic endoderms. PeerJ 2020, 8, e9968. [Google Scholar] [CrossRef]
- Nguyen, R.; Da Won Bae, S.; Qiao, L.; George, J. Developing liver organoids from induced pluripotent stem cells (iPSCs): An alternative source of organoid generation for liver cancer research. Cancer Lett. 2021, 508, 13–17. [Google Scholar] [CrossRef]
- Mun, S.J.; Ryu, J.S.; Lee, M.O.; Son, Y.S.; Oh, S.J.; Cho, H.S.; Son, M.Y.; Kim, D.S.; Kim, S.J.; Yoo, H.J.; et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J. Hepatol. 2019, 71, 970–985. [Google Scholar] [CrossRef]
- Ouchi, R.; Togo, S.; Kimura, M.; Shinozawa, T.; Koido, M.; Koike, H.; Thompson, W.; Karns, R.A.; Mayhew, C.N.; McGrath, P.S.; et al. Modeling Steatohepatitis in Humans with Pluripotent Stem Cell-Derived Organoids. Cell Metab. 2019, 30, 374–384.e6. [Google Scholar] [CrossRef]
- Guan, Y.; Enejder, A.; Wang, M.; Fang, Z.; Cui, L.; Chen, S.Y.; Wang, J.; Tan, Y.; Wu, M.; Chen, X.; et al. A human multi-lineage hepatic organoid model for liver fibrosis. Nat. Commun. 2021, 12, 6138. [Google Scholar] [CrossRef]
- Toivonen, S.; Lundin, K.; Balboa, D.; Ustinov, J.; Tamminen, K.; Palgi, J.; Trokovic, R.; Tuuri, T.; Otonkoski, T. Activin A and Wnt-dependent specification of human definitive endoderm cells. Exp. Cell Res. 2013, 319, 2535–2544. [Google Scholar] [CrossRef]
- Schmidt, C.; Bladt, F.; Goedecke, S.; Brinkmann, V.; Zschiesche, W.; Sharpe, M.; Gherardi, E.; Birchmeier, C. Scatter fac-tor/hepatocyte growth factor is essential for liver development. Nature 1995, 373, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, A.; Kinoshita, T.; Ito, Y.; Matsui, T.; Morikawa, Y.; Senba, E.; Nakashima, K.; Taga, T.; Yoshida, K.; Kishimoto, T.; et al. Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer. EMBO J. 1999, 18, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- Roy-Chowdhury, N.; Wang, X.; Guha, C.; Roy-Chowdhury, J. Hepatocyte-like cells derived from induced pluripotent stem cells. Hepatol. Int. 2017, 11, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Olgasi, C.; Cucci, A.; Follenzi, A. iPSC-Derived Liver Organoids: A Journey from Drug Screening, to Disease Modeling, Arriving to Regenerative Medicine. Int. J. Mol. Sci. 2020, 21, 6215. [Google Scholar] [CrossRef]
- Torizal, F.G.; Utami, T.; Lau, Q.Y.; Inamura, K.; Nishikawa, M.; Sakai, Y. Dialysis based-culture medium conditioning improved the generation of human induced pluripotent stem cell derived-liver organoid in a high cell density. Sci. Rep. 2022, 12, 20774. [Google Scholar] [CrossRef]
- Sampaziotis, F.; Muraro, D.; Tysoe, O.C.; Sawiak, S.; Beach, T.E.; Godfrey, E.M.; Upponi, S.S.; Brevini, T.; Wesley, B.T.; Gar-cia-Bernardo, J.; et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science 2021, 371, 839–846. [Google Scholar] [CrossRef]
- Wu, F.; Wu, D.; Ren, Y.; Huang, Y.; Feng, B.; Zhao, N.; Zhang, T.; Chen, X.; Chen, S.; Xu, A. Generation of hepatobiliary or-ganoids from human induced pluripotent stem cells. J. Hepatol. 2019, 70, 1145–1158. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Rajan, S.A.P.; Aleman, J.; Wan, M.; Pourhabibi Zarandi, N.; Nzou, G.; Murphy, S.; Bishop, C.E.; Sadri-Ardekani, H.; Shupe, T.; Atala, A.; et al. Probing prodrug metabolism and reciprocal toxicity with an integrated and humanized multi-tissue or-gan-on-a-chip platform. Acta Biomater. 2020, 106, 124–135. [Google Scholar] [CrossRef]
- Koike, H.; Iwasawa, K.; Ouchi, R.; Maezawa, M.; Giesbrecht, K.; Saiki, N.; Ferguson, A.; Kimura, M.; Thompson, W.L.; Wells, J.M.; et al. Modelling human hepato-biliary-pancreatic organogenesis from the foregut-midgut boundary. Nature 2019, 574, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Deng, P.; Wang, Y.; Zhang, X.; Guo, Y.; Chen, W.; Qin, J. Microengineered Multi-Organoid System from hiPSCs to Recapitulate Human Liver-Islet Axis in Normal and Type 2 Diabetes. Adv. Sci. 2022, 9, e2103495. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Shupe, T.; Atala, A. Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modeling. Drug Discov. Today 2016, 21, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Takebe, T.; Zhang, B.; Radisic, M. Synergistic Engineering: Organoids Meet Organs-on-a-Chip. Cell Stem Cell 2017, 21, 297–300. [Google Scholar] [CrossRef]
- Filippo Buono, M.; von Boehmer, L.; Strang, J.; Hoerstrup, S.P.; Emmert, M.Y.; Nugraha, B. Human Cardiac Organoids for Modeling Genetic Cardiomyopathy. Cells 2020, 9, 1733. [Google Scholar] [CrossRef]
- Fang, Y.; Guo, Y.; Wu, B.; Liu, Z.; Ye, M.; Xu, Y.; Ji, M.; Chen, L.; Lu, B.; Nie, K.; et al. Expanding Embedded 3D Bioprinting Capability for Engineering Complex Organs with Freeform Vascular Networks. Adv. Mater. 2023, 35, e2205082. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, S.; Neves, C.; Bonner, R.J.; Bernardo, A.S.; Docherty, K.; Hoppler, S. Distinctive Roles of Canonical and Noncanonical Wnt Signaling in Human Embryonic Cardiomyocyte Development. Stem Cell Rep. 2016, 7, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sun, J.; Zhong, T.P. Wnt Signaling in Heart Development and Regeneration. Curr. Cardiol. Rep. 2022, 24, 1425–1438. [Google Scholar] [CrossRef]
- Di Sante, M.; Antonucci, S.; Pontarollo, L.; Cappellaro, I.; Segat, F.; Deshwal, S.; Greotti, E.; Grilo, L.F.; Menabò, R.; Di Lisa, F.; et al. Monoamine oxidase A-dependent ROS formation modulates human cardiomyocyte differentiation through AKT and WNT activation. Basic Res. Cardiol. 2023, 118, 4. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.H.; Kim, H.J.; Pyun, J.H.; Choi, K.S.; Lee, D.I.; Solhjoo, S.; O’Rourke, B.; Tomaselli, G.F.; Jeong, D.S.; Cho, H.; et al. Cdon deficiency causes cardiac remodeling through hyperactivation of WNT/β-catenin signaling. Proc. Natl. Acad. Sci. USA 2017, 114, E1345–E1354. [Google Scholar] [CrossRef]
- Lewis-Israeli, Y.R.; Wasserman, A.H.; Gabalski, M.A.; Volmert, B.D.; Ming, Y.; Ball, K.A.; Yang, W.; Zou, J.; Ni, G.; Pajares, N.; et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat. Commun. 2021, 12, 5142. [Google Scholar] [CrossRef]
- Hofbauer, P.; Jahnel, S.M.; Papai, N.; Giesshammer, M.; Deyett, A.; Schmidt, C.; Penc, M.; Tavernini, K.; Grdseloff, N.; Meledeth, C.; et al. Cardioids reveal self-organizing principles of human cardiogenesis. Cell 2021, 184, 3299–3317.e22. [Google Scholar] [CrossRef]
- Ng, W.H.; Johnston, E.K.; Tan, J.J.; Bliley, J.M.; Feinberg, A.W.; Stolz, D.B.; Sun, M.; Wijesekara, P.; Hawkins, F.; Kotton, D.N.; et al. Recapitulating human cardio-pulmonary co-development using simultaneous multilineage differentiation of pluripotent stem cells. eLife 2022, 11, e67872. [Google Scholar] [CrossRef]
- Silva, A.C.; Matthys, O.B.; Joy, D.A.; Kauss, M.A.; Natarajan, V.; Lai, M.H.; Turaga, D.; Blair, A.P.; Alexanian, M.; Bruneau, B.G.; et al. Co-emergence of cardiac and gut tissues promotes cardiomyocyte maturation within human iPSC-derived organoids. Cell Stem Cell 2021, 28, 2137–2152.e6. [Google Scholar] [CrossRef]
- Chao, H.M.; Chern, E. Patient-derived induced pluripotent stem cells for models of cancer and cancer stem cell research. J. Formos. Med. Assoc. Taiwan Yi Zhi 2018, 117, 1046–1057. [Google Scholar] [CrossRef]
- Kuenzig, M.E.; Fung, S.G.; Marderfeld, L.; Mak, J.W.Y.; Kaplan, G.G.; Ng, S.C.; Wilson, D.C.; Cameron, F.; Henderson, P.; Kotze, P.G.; et al. Twenty-first Century Trends in the Global Epidemiology of Pediatric-Onset Inflammatory Bowel Disease: Systematic Review. Gastroenterology 2022, 162, 1147–1159.e4. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.L.; Neufeld, K.L. Elevated adenomatous polyposis coli in goblet cells is associated with inflammation in mouse and human colon. Exp. Physiol. 2020, 105, 2154–2167. [Google Scholar] [CrossRef] [PubMed]
- Bopanna, S.; Ananthakrishnan, A.N.; Kedia, S.; Yajnik, V.; Ahuja, V. Risk of colorectal cancer in Asian patients with ulcerative colitis: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Lin, H.; Fu, Y.; Zeng, F.; Gu, F.; Niu, G.; Fang, J.; Gu, B. Identification of gene signatures associated with ulcerative colitis and the association with immune infiltrates in colon cancer. Front. Immunol. 2023, 14, 1086898. [Google Scholar] [CrossRef]
- Yadav, A.; Jena, A.; Arpitha, K.; Shah, J.; Vaiphei, K.; Dutta, U.; Singh, A.K. Colon Cancer in Ulcerative Colitis: A Mimicker of a Flare of Disease. Inflamm. Bowel Dis. 2022, 28, e116–e117. [Google Scholar] [CrossRef]
- Naydenov, N.G.; Lechuga, S.; Zalavadia, A.; Mukherjee, P.K.; Gordon, I.O.; Skvasik, D.; Vidovic, P.; Huang, E.; Rieder, F.; Ivanov, A.I. P-Cadherin Regulates Intestinal Epithelial Cell Migration and Mucosal Repair, but Is Dispensable for Colitis As-sociated Colon Cancer. Cells 2022, 11, 1467. [Google Scholar] [CrossRef] [PubMed]
- Devall, M.A.; Eaton, S.; Ali, M.W.; Dampier, C.H.; Weisenberger, D.; Powell, S.M.; Li, L.; Casey, G. DNA methylation analysis of normal colon organoids from familial adenomatous polyposis patients reveals novel insight into colon cancer development. Clin. Epigenet. 2022, 14, 104. [Google Scholar] [CrossRef]
- Devall, M.A.; Eaton, S.; Ali, M.W.; Powell, S.M.; Li, L.; Casey, G. Insights into Early Onset Colorectal Cancer through Analysis of Normal Colon Organoids of Familial Adenomatous Polyposis Patients. Cancers 2022, 14, 4138. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Li, A.; Tang, X. A pan-cancer analysis on the carcinogenic effect of human adenomatous polyposis coli. PLoS ONE 2022, 17, e0265655. [Google Scholar] [CrossRef]
- Richards, S.; Walker, J.; Nakanishi, M.; Belghasem, M.; Lyle, C.; Arinze, N.; Napoleon, M.A.; Ravid, J.D.; Crossland, N.; Zhao, Q.; et al. Haploinsufficiency of Casitas B-Lineage Lymphoma Augments the Progression of Colon Cancer in the Background of Adenomatous Polyposis Coli Inactivation. Am. J. Pathol. 2020, 190, 602–613. [Google Scholar] [CrossRef]
- Janssen, A.W.F.; Duivenvoorde, L.P.M.; Rijkers, D.; Nijssen, R.; Peijnenburg, A.; van der Zande, M.; Louisse, J. Cytochrome P450 expression, induction and activity in human induced pluripotent stem cell-derived intestinal organoids and comparison with primary human intestinal epithelial cells and Caco-2 cells. Arch. Toxicol. 2021, 95, 907–922. [Google Scholar] [CrossRef]
- Crespo, M.; Vilar, E.; Tsai, S.Y.; Chang, K.; Amin, S.; Srinivasan, T.; Zhang, T.; Pipalia, N.H.; Chen, H.J.; Witherspoon, M.; et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat. Med. 2017, 23, 878–884. [Google Scholar] [CrossRef]
- Hirshorn, S.T.; Steele, N.; Zavros, Y. Modeling pancreatic pathophysiology using genome editing of adult stem cell-derived and induced pluripotent stem cell (iPSC)-derived organoids. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G1142–G1150. [Google Scholar] [CrossRef]
- Wuputra, K.; Ku, C.C.; Kato, K.; Wu, D.C.; Saito, S.; Yokoyama, K.K. Translational models of 3-D organoids and cancer stem cells in gastric cancer research. Stem Cell Res. Ther. 2021, 12, 492. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Matano, M.; Date, S.; Shimokawa, M.; Takano, A.; Fujii, M.; Ohta, Y.; Watanabe, T.; Kanai, T.; Sato, T. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat. Med. 2015, 21, 256–262. [Google Scholar] [CrossRef]
- Hendriks, D.; Pagliaro, A.; Andreatta, F.; Ma, Z.; van Giessen, J.; Massalini, S.; López-Iglesias, C.; van Son, G.J.F.; DeMartino, J.; Damen, J.M.A.; et al. Human fetal brain self-organizes into long-term expanding organoids. Cell 2024, 187, 712–732.e38. [Google Scholar] [CrossRef]
- Norrie, J.L.; Nityanandam, A.; Lai, K.; Chen, X.; Wilson, M.; Stewart, E.; Griffiths, L.; Jin, H.; Wu, G.; Orr, B.; et al. Reti-noblastoma from human stem cell-derived retinal organoids. Nat. Commun. 2021, 12, 4535. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Iwagawa, T.; Fukushima, M.; Watanabe, S. Characterization of human-induced pluripotent stem cells carrying homozygous RB1 gene deletion. Genes Cells Devoted Mol. Cell. Mech. 2020, 25, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Rozanska, A.; Cerna-Chavez, R.; Queen, R.; Collin, J.; Zerti, D.; Dorgau, B.; Beh, C.S.; Davey, T.; Coxhead, J.; Hussain, R.; et al. pRB-Depleted Pluripotent Stem Cell Retinal Organoids Recapitulate Cell State Transitions of Retinoblastoma Development and Suggest an Important Role for pRB in Retinal Cell Differentiation. Stem Cells Transl. Med. 2022, 11, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Ishikawa, Y.; Sasamoto, Y.; Katori, R.; Nomura, N.; Ichikawa, T.; Araki, S.; Soma, T.; Kawasaki, S.; Sekiguchi, K.; et al. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature 2016, 531, 376–380. [Google Scholar] [CrossRef]
- Dost, A.F.M.; Moye, A.L.; Vedaie, M.; Tran, L.M.; Fung, E.; Heinze, D.; Villacorta-Martin, C.; Huang, J.; Hekman, R.; Kwan, J.H.; et al. Organoids Model Transcriptional Hallmarks of Oncogenic KRAS Activation in Lung Epithelial Progenitor Cells. Cell Stem Cell 2020, 27, 663–678.e8. [Google Scholar] [CrossRef] [PubMed]
- Skylar-Scott, M.A.; Huang, J.Y.; Lu, A.; Ng, A.H.M.; Duenki, T.; Liu, S.; Nam, L.L.; Damaraju, S.; Church, G.M.; Lewis, J.A. Orthogonally induced differentiation of stem cells for the programmatic patterning of vascularized organoids and bioprinted tissues. Nat. Biomed. Eng. 2022, 6, 449–462. [Google Scholar] [CrossRef]
- Centeno, E.G.Z.; Cimarosti, H.; Bithell, A. 2D versus 3D human induced pluripotent stem cell-derived cultures for neuro-degenerative disease modelling. Mol. Neurodegener. 2018, 13, 27. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, H.; Ming, G.L. Genetics of human brain development. Nat. Rev. Genet. 2024, 25, 26–45. [Google Scholar] [CrossRef]
- Villa, C.; Combi, R.; Conconi, D.; Lavitrano, M. Patient-Derived Induced Pluripotent Stem Cells (iPSCs) and Cerebral Organoids for Drug Screening and Development in Autism Spectrum Disorder: Opportunities and Challenges. Pharmaceutics 2021, 13, 280. [Google Scholar] [CrossRef]
- Lu, K.; Hong, Y.; Tao, M.; Shen, L.; Zheng, Z.; Fang, K.; Yuan, F.; Xu, M.; Wang, C.; Zhu, D.; et al. Depressive patient-derived GABA interneurons reveal abnormal neural activity associated with HTR2C. EMBO Mol. Med. 2023, 15, e16364. [Google Scholar] [CrossRef]
- Velasco, S.; Kedaigle, A.J.; Simmons, S.K.; Nash, A.; Rocha, M.; Quadrato, G.; Paulsen, B.; Nguyen, L.; Adiconis, X.; Regev, A.; et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 2019, 570, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.A.; Gonçalves, J.T.; Bloyd, C.W.; Li, H.; Fernandes, S.; Quang, D.; Johnston, S.; Parylak, S.L.; Jin, X.; Gage, F.H. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 2018, 36, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Yuan, X.; Jones, Z.; Griffin, K.; Zhou, Y.; Ma, T.; Li, Y. Assembly of Human Stem Cell-Derived Cortical Spheroids and Vascular Spheroids to Model 3-D Brain-like Tissues. Sci. Rep. 2019, 9, 5977. [Google Scholar] [CrossRef]
- Bagley, J.A.; Reumann, D.; Bian, S.; Lévi-Strauss, J.; Knoblich, J.A. Fused cerebral organoids model interactions between brain regions. Nat. Methods 2017, 14, 743–751. [Google Scholar] [CrossRef]
- Costamagna, G.; Comi, G.P.; Corti, S. Advancing Drug Discovery for Neurological Disorders Using iPSC-Derived Neural Or-ganoids. Int. J. Mol. Sci. 2021, 22, 2659. [Google Scholar] [CrossRef]
- Egawa, N.; Kitaoka, S.; Tsukita, K.; Naitoh, M.; Takahashi, K.; Yamamoto, T.; Adachi, F.; Kondo, T.; Okita, K.; Asaka, I.; et al. Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci. Transl. Med. 2012, 4, 145ra104. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, S.; Takahashi, S.; Ito, D.; Daté, Y.; Okada, K.; Kato, C.; Nakamura, S.; Ozawa, F.; Chyi, C.M.; Nishiyama, A.; et al. Phase 1/2a clinical trial in ALS with ropinirole, a drug candidate identified by iPSC drug discovery. Cell Stem Cell 2023, 30, 766–780.e9. [Google Scholar] [CrossRef]
- Ha, J.; Kang, J.S.; Lee, M.; Baek, A.; Kim, S.; Chung, S.K.; Lee, M.O.; Kim, J. Simplified Brain Organoids for Rapid and Robust Modeling of Brain Disease. Front. Cell Dev. Biol. 2020, 8, 594090. [Google Scholar] [CrossRef]
- Pasqua, M.; Di Gesù, R.; Chinnici, C.M.; Conaldi, P.G.; Francipane, M.G. Generation of Hepatobiliary Cell Lineages from Human Induced Pluripotent Stem Cells: Applications in Disease Modeling and Drug Screening. Int. J. Mol. Sci. 2021, 22, 8227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Meyer, S.R.; O’Meara, M.J.; Huang, S.; Capeling, M.M.; Ferrer-Torres, D.; Childs, C.J.; Spence, J.R.; Fontana, R.J.; Sexton, J.Z. A human liver organoid screening platform for DILI risk prediction. J. Hepatol. 2023, 78, 998–1006. [Google Scholar] [CrossRef]
- Broutier, L.; Mastrogiovanni, G.; Verstegen, M.M.; Francies, H.E.; Gavarró, L.M.; Bradshaw, C.R.; Allen, G.E.; Arnes-Benito, R.; Sidorova, O.; Gaspersz, M.P.; et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 2017, 23, 1424–1435. [Google Scholar] [CrossRef]
- Koui, Y.; Kido, T.; Ito, T.; Oyama, H.; Chen, S.W.; Katou, Y.; Shirahige, K.; Miyajima, A. An In Vitro Human Liver Model by iPSC-Derived Parenchymal and Non-parenchymal Cells. Stem Cell Rep. 2017, 9, 490–498. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, F.; Jin, M.; Wang, Z.; Xu, X.; Zhou, Y.; Wang, J.; Gu, L.; Fan, H.; Fan, Y.; et al. Development of a high-throughput micropatterned agarose scaffold for consistent and reproducible hPSC-derived liver organoids. Biofabrication 2022, 15, 015006. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Han, S.; Sekyi, M.T.; Su, T.; Mattis, A.N.; Chang, T.T. Self-Assembled Matrigel-Free iPSC-Derived Liver Organoids Demonstrate Wide-Ranging Highly Differentiated Liver Functions. Stem Cells 2023, 41, 126–139. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, S.; Gu, L.; Li, B.; Xu, F.; Li, C.; Chen, P. High-throughput bioengineering of homogenous and functional hu-man-induced pluripotent stem cells-derived liver organoids via micropatterning technique. Front. Bioeng. Biotechnol. 2022, 10, 937595. [Google Scholar] [CrossRef]
- Shinozawa, T.; Kimura, M.; Cai, Y.; Saiki, N.; Yoneyama, Y.; Ouchi, R.; Koike, H.; Maezawa, M.; Zhang, R.R.; Dunn, A.; et al. High-Fidelity Drug-Induced Liver Injury Screen Using Human Pluripotent Stem Cell-Derived Organoids. Gastroenterology 2021, 160, 831–846.e10. [Google Scholar] [CrossRef]
- Kim, H.; Im, I.; Jeon, J.S.; Kang, E.H.; Lee, H.A.; Jo, S.; Kim, J.W.; Woo, D.H.; Choi, Y.J.; Kim, H.J.; et al. Development of human pluripotent stem cell-derived hepatic organoids as an alternative model for drug safety assessment. Biomaterials 2022, 286, 121575. [Google Scholar] [CrossRef]
- Cantillon, D.; Goff, A.; Taylor, S.; Salehi, E.; Fidler, K.; Stoneham, S.; Waddell, S.J. Searching for new therapeutic options for the uncommon pathogen Mycobacterium chimaera: An open drug discovery approach. Lancet Microbe 2022, 3, e382–e391. [Google Scholar] [CrossRef] [PubMed]
- Horlock, D.; Kaye, D.M.; Winbanks, C.E.; Gao, X.M.; Kiriazis, H.; Donner, D.G.; Gregorevic, P.; McMullen, J.R.; Bernardo, B.C. Old Drug, New Trick: Tilorone, a Broad-Spectrum Antiviral Drug as a Potential Anti-Fibrotic Therapeutic for the Diseased Heart. Pharmaceuticals 2021, 14, 263. [Google Scholar] [CrossRef] [PubMed]
- Ang, Y.S.; Rajamani, S.; Haldar, S.M.; Hüser, J. A New Therapeutic Framework for Atrial Fibrillation Drug Development. Circ. Res. 2020, 127, 184–201. [Google Scholar] [CrossRef] [PubMed]
- Bekhite, M.; González-Delgado, A.; Hübner, S.; Haxhikadrija, P.; Kretzschmar, T.; Müller, T.; Wu, J.M.F.; Bekfani, T.; Franz, M.; Wartenberg, M.; et al. The role of ceramide accumulation in human induced pluripotent stem cell-derived cardiomyocytes on mitochondrial oxidative stress and mitophagy. Free Radic. Biol. Med. 2021, 167, 66–80. [Google Scholar] [CrossRef]
- Magdy, T.; Jouni, M.; Kuo, H.H.; Weddle, C.J.; Lyra-Leite, D.; Fonoudi, H.; Romero-Tejeda, M.; Gharib, M.; Javed, H.; Fajardo, G.; et al. Identification of Drug Transporter Genomic Variants and Inhibitors That Protect Against Doxorubicin-Induced Cardio-toxicity. Circulation 2022, 145, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Ergir, E.; Oliver-De La Cruz, J.; Fernandes, S.; Cassani, M.; Niro, F.; Pereira-Sousa, D.; Vrbský, J.; Vinarský, V.; Perestrelo, A.R.; Debellis, D.; et al. Generation and maturation of human iPSC-derived 3D organotypic cardiac microtissues in long-term culture. Sci. Rep. 2022, 12, 17409. [Google Scholar] [CrossRef]
- Sallam, K.; Thomas, D.; Gaddam, S.; Lopez, N.; Beck, A.; Beach, L.; Rogers, A.J.; Zhang, H.; Chen, I.Y.; Ameen, M.; et al. Modeling Effects of Immunosuppressive Drugs on Human Hearts Using Induced Pluripotent Stem Cell-Derived Cardiac Or-ganoids and Single-Cell RNA Sequencing. Circulation 2022, 145, 1367–1369. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, M.W.; Ting, C.Y.; Chan, D.Z.H.; Cheng, Y.C.; Lee, Y.C.; Hsu, C.C.; Huang, C.Y.; Hsieh, P.C.H. Utility of iPSC-Derived Cells for Disease Modeling, Drug Development, and Cell Therapy. Cells 2022, 11, 1853. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Cha, Y.; Ko, S.; Jeon, J.; Lee, N.; Seo, H.; Park, K.J.; Lee, I.H.; Lopes, C.; Feitosa, M.; et al. Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson’s disease models. J. Clin. Investig. 2020, 130, 904–920. [Google Scholar] [CrossRef] [PubMed]
- Hiller, B.M.; Marmion, D.J.; Thompson, C.A.; Elliott, N.A.; Federoff, H.; Brundin, P.; Mattis, V.B.; McMahon, C.W.; Kordower, J.H. Optimizing maturity and dose of iPSC-derived dopamine progenitor cell therapy for Parkinson’s disease. NPJ Regen. Med. 2022, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, J.S.; Song, B.; Herrington, T.M.; Park, T.Y.; Lee, N.; Ko, S.; Jeon, J.; Cha, Y.; Kim, K.; Li, Q.; et al. Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. N. Engl. J. Med. 2020, 382, 1926–1932. [Google Scholar] [CrossRef]
- Kikuchi, T.; Morizane, A.; Doi, D.; Magotani, H.; Onoe, H.; Hayashi, T.; Mizuma, H.; Takara, S.; Takahashi, R.; Inoue, H.; et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature 2017, 548, 592–596. [Google Scholar] [CrossRef]
- Doi, D.; Magotani, H.; Kikuchi, T.; Ikeda, M.; Hiramatsu, S.; Yoshida, K.; Amano, N.; Nomura, M.; Umekage, M.; Morizane, A.; et al. Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson’s disease. Nat. Commun. 2020, 11, 3369. [Google Scholar] [CrossRef]
- Fernandes, H.J.R.; Patikas, N.; Foskolou, S.; Field, S.F.; Park, J.E.; Byrne, M.L.; Bassett, A.R.; Metzakopian, E. Single-Cell Transcriptomics of Parkinson’s Disease Human In Vitro Models Reveals Dopamine Neuron-Specific Stress Responses. Cell Rep. 2020, 33, 108263. [Google Scholar] [CrossRef]
- Lyu, Y.; Su, Z.; Ye, G.; He, X.; Liu, Y.; Yin, Q.; Xie, F.; Xu, L.; Chen, Y.; Long, D. MiR-210-5p promotes the differentiation of human induced pluripotent stem cells into dopaminergic neural precursors by targeting SMAD4 and SUFU and treats par-kinsonian rats. Exp. Gerontol. 2023, 179, 112243. [Google Scholar] [CrossRef]
- Brot, S.; Thamrin, N.P.; Bonnet, M.L.; Francheteau, M.; Patrigeon, M.; Belnoue, L.; Gaillard, A. Long-Term Evaluation of In-tranigral Transplantation of Human iPSC-Derived Dopamine Neurons in a Parkinson’s Disease Mouse Model. Cells 2022, 11, 1596. [Google Scholar] [CrossRef]
- Xu, Z.; Chu, X.; Jiang, H.; Schilling, H.; Chen, S.; Feng, J. Induced dopaminergic neurons: A new promise for Parkinson’s disease. Redox Biol. 2017, 11, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.C.; Krolewski, R.C.; Lucumi Moreno, E.; Blank, J.; Holton, K.M.; Ahfeldt, T.; Furlong, M.; Yu, Y.; Cockburn, M.; Thompson, L.K.; et al. A pesticide and iPSC dopaminergic neuron screen identifies and classifies Parkinson-relevant pesticides. Nat. Commun. 2023, 14, 2803. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, H.; Li, H.; Li, L.; Yan, Z.; Feng, J. Generation of human A9 dopaminergic pacemakers from induced pluripotent stem cells. Mol. Psychiatry 2022, 27, 4407–4418. [Google Scholar] [CrossRef] [PubMed]
- Povero, D.; Pinatel, E.M.; Leszczynska, A.; Goyal, N.P.; Nishio, T.; Kim, J.; Kneiber, D.; de Araujo Horcel, L.; Eguchi, A.; Or-donez, P.M.; et al. Human induced pluripotent stem cell-derived extracellular vesicles reduce hepatic stellate cell activation and liver fibrosis. JCI Insight 2019, 4, e125652. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Jeong, I.S.; Park, Y.J.; Kim, S.W. HGF and IL-10 expressing ALB::GFP reporter cells generated from iPSCs show robust anti-fibrotic property in acute fibrotic liver model. Stem Cell Res. Ther. 2020, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Monckton, C.P.; Brougham-Cook, A.; Underhill, G.H.; Khetani, S.R. Modulation of human iPSC-derived hepatocyte phenotype via extracellular matrix microarrays. Acta Biomater. 2022, 153, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Cai, J.; Liu, Y.; Zhao, D.; Yong, J.; Duo, S.; Song, X.; Guo, Y.; Zhao, Y.; Qin, H.; et al. Efficient generation of hepato-cyte-like cells from human induced pluripotent stem cells. Cell Res. 2009, 19, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Pouyanfard, S.; Meshgin, N.; Cruz, L.S.; Diggle, K.; Hashemi, H.; Pham, T.V.; Fierro, M.; Tamayo, P.; Fanjul, A.; Kisseleva, T.; et al. Human induced pluripotent stem cell-derived macrophages ameliorate liver fibrosis. Stem Cells 2021, 39, 1701–1717. [Google Scholar] [CrossRef]
- Yeung, E.; Fukunishi, T.; Bai, Y.; Bedja, D.; Pitaktong, I.; Mattson, G.; Jeyaram, A.; Lui, C.; Ong, C.S.; Inoue, T.; et al. Cardiac regeneration using human-induced pluripotent stem cell-derived biomaterial-free 3D-bioprinted cardiac patch in vivo. J. Tissue Eng. Regen. Med. 2019, 13, 2031–2039. [Google Scholar] [CrossRef]
- Lancaster, J.J.; Sanchez, P.; Repetti, G.G.; Juneman, E.; Pandey, A.C.; Chinyere, I.R.; Moukabary, T.; LaHood, N.; Daugherty, S.L.; Goldman, S. Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Patch in Rats with Heart Failure. Ann. Thorac. Surg. 2019, 108, 1169–1177. [Google Scholar] [CrossRef]
- Ikeda, G.; Santoso, M.R.; Tada, Y.; Li, A.M.; Vaskova, E.; Jung, J.H.; O’Brien, C.; Egan, E.; Ye, J.; Yang, P.C. Mitochondria-Rich Extracellular Vesicles From Autologous Stem Cell-Derived Cardiomyocytes Restore Energetics of Ischemic Myocardium. J. Am. Coll. Cardiol. 2021, 77, 1073–1088. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, Z.; Dong, M. Cardiac repair in a murine model of myocardial infarction with human induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 2020, 11, 297. [Google Scholar] [CrossRef]
- Kurtzwald-Josefson, E.; Zeevi-Levin, N.; Rubchevsky, V.; Bechar Erdman, N.; Schwartz Rohaker, O.; Nahum, O.; Hochhauser, E.; Ben-Avraham, B.; Itskovitz-Eldor, J.; Aravot, D.; et al. Cardiac Fibroblast-Induced Pluripotent Stem Cell-Derived Exosomes as a Potential Therapeutic Mean for Heart Failure. Int. J. Mol. Sci. 2020, 21, 7215. [Google Scholar] [CrossRef]
- Dudley, M.E.; Wunderlich, J.R.; Robbins, P.F.; Yang, J.C.; Hwu, P.; Schwartzentruber, D.J.; Topalian, S.L.; Sherry, R.; Restifo, N.P.; Hubicki, A.M.; et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lym-phocytes. Science 2002, 298, 850–854. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; Hughes, M.S.; Phan, G.Q.; Citrin, D.E.; Restifo, N.P.; Robbins, P.F.; Wunderlich, J.R.; et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 4550–4557. [Google Scholar] [CrossRef]
- Dudley, M.E.; Yang, J.C.; Sherry, R.; Hughes, M.S.; Royal, R.; Kammula, U.; Robbins, P.F.; Huang, J.; Citrin, D.E.; Leitman, S.F.; et al. Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 5233–5239. [Google Scholar] [CrossRef]
- Li, X.; Halldórsdóttir, H.R.; Weller, S.; Colliander, A.; Bak, M.; Kempen, P.; Clergeaud, G.; Andresen, T.L. Enhancing Adoptive Cell Therapy by T Cell Loading of SHP2 Inhibitor Nanocrystals before Infusion. ACS Nano 2022, 16, 10918–10930. [Google Scholar] [CrossRef]
- Mardiana, S.; Solomon, B.J.; Darcy, P.K.; Beavis, P.A. Supercharging adoptive T cell therapy to overcome solid tumor-induced immunosuppression. Sci. Transl. Med. 2019, 11, eaaw2293. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Lowery, F.J.; Copeland, A.R.; Bahadiroglu, E.; Mukherjee, R.; Jia, L.; Anibal, J.T.; Sachs, A.; Adebola, S.O.; Gu-rusamy, D.; et al. Stem-like CD8 T cells mediate response of adoptive cell immunotherapy against human cancer. Science 2020, 370, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, E.E.; Sekhri, P.; Telaraja, D.; Chen, J.; Chin, S.J.; Chiappinelli, K.B.; Sanchez, C.E.; Bollard, C.M.; Cruz, C.R.Y.; Fer-nandes, R. Engineered tumor-specific T cells using immunostimulatory photothermal nanoparticles. Cytotherapy 2023, 25, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Potenza, A.; Balestrieri, C.; Spiga, M.; Albarello, L.; Pedica, F.; Manfredi, F.; Cianciotti, B.C.; De Lalla, C.; Botrugno, O.A.; Faccani, C.; et al. Revealing and harnessing CD39 for the treatment of colorectal cancer and liver metastases by engineered T cells. Gut 2023, 72, 1887–1903. [Google Scholar] [CrossRef]
- Heitzeneder, S.; Bosse, K.R.; Zhu, Z.; Zhelev, D.; Majzner, R.G.; Radosevich, M.T. CAR T Cells Engineered to Target Low-Density Antigens Show Efficacy and Safety. Cancer Discov. 2022, 12, 595. [Google Scholar] [CrossRef]
- Ma, L.; Hostetler, A.; Morgan, D.M.; Maiorino, L.; Sulkaj, I.; Whittaker, C.A.; Neeser, A.; Pires, I.S.; Yousefpour, P.; Gregory, J.; et al. Vaccine-boosted CAR T crosstalk with host immunity to reject tumors with antigen heterogeneity. Cell 2023, 186, 3148–3165.e20. [Google Scholar] [CrossRef]
- Stein-Thoeringer, C.K.; Saini, N.Y.; Zamir, E.; Blumenberg, V.; Schubert, M.L.; Mor, U.; Fante, M.A.; Schmidt, S.; Hayase, E.; Hayase, T.; et al. A non-antibiotic-disrupted gut microbiome is associated with clinical responses to CD19-CAR-T cell cancer immunotherapy. Nat. Med. 2023, 29, 906–916. [Google Scholar] [CrossRef]
- Good, Z.; Spiegel, J.Y.; Sahaf, B.; Malipatlolla, M.B.; Ehlinger, Z.J.; Kurra, S.; Desai, M.H.; Reynolds, W.D.; Wong Lin, A.; Vandris, P.; et al. Post-infusion CAR T(Reg) cells identify patients resistant to CD19-CAR therapy. Nat. Med. 2022, 28, 1860–1871. [Google Scholar] [CrossRef] [PubMed]
- Vasic, D.; Lee, J.B.; Leung, Y.; Khatri, I.; Na, Y.; Abate-Daga, D.; Zhang, L. Allogeneic double-negative CAR-T cells inhibit tumor growth without off-tumor toxicities. Sci. Immunol. 2022, 7, eabl3642. [Google Scholar] [CrossRef] [PubMed]
- Meyran, D.; Zhu, J.J.; Butler, J.; Tantalo, D.; MacDonald, S.; Nguyen, T.N.; Wang, M.; Thio, N.; D’Souza, C.; Qin, V.M.; et al. T(STEM)-like CAR-T cells exhibit improved persistence and tumor control compared with conventional CAR-T cells in pre-clinical models. Sci. Transl. Med. 2023, 15, eabk1900. [Google Scholar] [CrossRef]
- Sadeqi Nezhad, M.; Abdollahpour-Alitappeh, M.; Rezaei, B.; Yazdanifar, M.; Seifalian, A.M. Induced Pluripotent Stem Cells (iPSCs) Provide a Potentially Unlimited T Cell Source for CAR-T Cell Development and Off-the-Shelf Products. Pharm. Res. 2021, 38, 931–945. [Google Scholar] [CrossRef]
- Wang, Z.; McWilliams-Koeppen, H.P.; Reza, H.; Ostberg, J.R.; Chen, W.; Wang, X.; Huynh, C.; Vyas, V.; Chang, W.C.; Starr, R.; et al. 3D-organoid culture supports differentiation of human CAR(+) iPSCs into highly functional CAR T cells. Cell Stem Cell 2022, 29, 515–527.e8. [Google Scholar] [CrossRef]
- Kaneko, S. Successful organoid-mediated generation of iPSC-derived CAR-T cells. Cell Stem Cell 2022, 29, 493–495. [Google Scholar] [CrossRef]
- Ueda, T.; Kaneko, S. In Vitro Differentiation of T Cell: From CAR-Modified T-iPSC. Methods Mol. Biol. 2019, 2048, 85–91. [Google Scholar] [CrossRef]
- van der Stegen, S.J.C.; Lindenbergh, P.L.; Petrovic, R.M.; Xie, H.; Diop, M.P.; Alexeeva, V.; Shi, Y.; Mansilla-Soto, J.; Hamieh, M.; Eyquem, J.; et al. Generation of T-cell-receptor-negative CD8αβ-positive CAR T cells from T-cell-derived induced pluripotent stem cells. Nat. Biomed. Eng. 2022, 6, 1284–1297. [Google Scholar] [CrossRef]
- Jing, R.; Scarfo, I.; Najia, M.A.; Lummertz da Rocha, E.; Han, A.; Sanborn, M.; Bingham, T.; Kubaczka, C.; Jha, D.K.; Falchetti, M.; et al. EZH1 repression generates mature iPSC-derived CAR T cells with enhanced antitumor activity. Cell Stem Cell 2022, 29, 1181–1196.e6. [Google Scholar] [CrossRef]
- Ueda, T.; Shiina, S.; Iriguchi, S.; Terakura, S.; Kawai, Y.; Kabai, R.; Sakamoto, S.; Watanabe, A.; Ohara, K.; Wang, B.; et al. Optimization of the proliferation and persistency of CAR T cells derived from human induced pluripotent stem cells. Nat. Biomed. Eng. 2023, 7, 24–37. [Google Scholar] [CrossRef]
- Harada, S.; Ando, M.; Ando, J.; Ishii, M.; Yamaguchi, T.; Yamazaki, S.; Toyota, T.; Ohara, K.; Ohtaka, M.; Nakanishi, M.; et al. Dual-antigen targeted iPSC-derived chimeric antigen receptor-T cell therapy for refractory lymphoma. Mol. Ther. J. Am. Soc. Gene Ther. 2022, 30, 534–549. [Google Scholar] [CrossRef]
- Myers, J.A.; Miller, J.S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100. [Google Scholar] [CrossRef]
- Saetersmoen, M.L.; Hammer, Q.; Valamehr, B.; Kaufman, D.S.; Malmberg, K.J. Off-the-shelf cell therapy with induced plu-ripotent stem cell-derived natural killer cells. Semin. Immunopathol. 2019, 41, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.L.; Finlay, D.K. Immunometabolism and natural killer cell responses. Nat. Rev. Immunol. 2019, 19, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.S.; Rezvani, K. Chimeric Antigen Receptor Expressing Natural Killer Cells for the Immunotherapy of Cancer. Front. Immunol. 2018, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Cui, H.; Caligiuri, M.A.; Yu, J. Chimeric antigen receptor-engineered natural killer cells for cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 168. [Google Scholar] [CrossRef]
- Cichocki, F.; van der Stegen, S.J.C.; Miller, J.S. Engineered and banked iPSCs for advanced NK- and T-cell immunotherapies. Blood 2023, 141, 846–855. [Google Scholar] [CrossRef]
- Cichocki, F.; Bjordahl, R.; Gaidarova, S.; Mahmood, S.; Abujarour, R.; Wang, H.; Tuininga, K.; Felices, M.; Davis, Z.B.; Bendzick, L.; et al. iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and an-ti-PD-1 therapy. Sci. Transl. Med. 2020, 12, eaaz5618. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.; Xie, J.; Zhou, Y.; Wu, Q.; Lu, B.; Zhou, S.; Zhao, X.; Li, Y. Leveraging CD16 fusion receptors to remodel the immune response for enhancing anti-tumor immunotherapy in iPSC-derived NK cells. J. Hematol. Oncol. 2023, 16, 62. [Google Scholar] [CrossRef]
- Chiu, E.; Felices, M.; Cichocki, F.; Davis, Z.; Wang, H.; Tuninga, K.; Vallera, D.A.; Lee, T.; Bjordahl, R.; Malmberg, K.J.; et al. Anti-NKG2C/IL-15/anti-CD33 killer engager directs primary and iPSC-derived NKG2C+ NK cells to target myeloid leukemia. Mol. Ther. J. Am. Soc. Gene Ther. 2021, 29, 3410–3421. [Google Scholar] [CrossRef]
- Zhu, H.; Blum, R.H.; Bernareggi, D.; Ask, E.H.; Wu, Z.; Hoel, H.J.; Meng, Z.; Wu, C.; Guan, K.L.; Malmberg, K.J.; et al. Metabolic Reprograming via Deletion of CISH in Human iPSC-Derived NK Cells Promotes In Vivo Persistence and Enhances Anti-tumor Activity. Cell Stem Cell 2020, 27, 224–237.e6. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Kumagai, A.; Iriguchi, S.; Yasui, Y.; Miyasaka, T.; Nakagoshi, K.; Nakane, K.; Saito, K.; Takahashi, M.; Sasaki, A.; et al. Non-clinical efficacy, safety and stable clinical cell processing of induced pluripotent stem cell-derived anti-glypican-3 chimeric antigen receptor-expressing natural killer/innate lymphoid cells. Cancer Sci. 2020, 111, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hermanson, D.L.; Moriarity, B.S.; Kaufman, D.S. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell 2018, 23, 181–192.e5. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, F.; Goodridge, J.P.; Bjordahl, R.; Mahmood, S.; Davis, Z.B.; Gaidarova, S.; Abujarour, R.; Groff, B.; Witty, A.; Wang, H.; et al. Dual antigen-targeted off-the-shelf NK cells show durable response and prevent antigen escape in lymphoma and leu-kemia. Blood 2022, 140, 2451–2462. [Google Scholar] [CrossRef]
- Klaihmon, P.; Kang, X.; Issaragrisil, S.; Luanpitpong, S. Generation and Functional Characterization of Anti-CD19 Chimeric Antigen Receptor-Natural Killer Cells from Human Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2023, 24, 10508. [Google Scholar] [CrossRef]
- Goulding, J.; Yeh, W.I.; Hancock, B.; Blum, R.; Xu, T.; Yang, B.H.; Chang, C.W.; Groff, B.; Avramis, E.; Pribadi, M.; et al. A chimeric antigen receptor uniquely recognizing MICA/B stress proteins provides an effective approach to target solid tumors. Med 2023, 4, 457–477.e8. [Google Scholar] [CrossRef]
- Hamieh, M.; Dobrin, A.; Cabriolu, A.; van der Stegen, S.J.C.; Giavridis, T.; Mansilla-Soto, J.; Eyquem, J.; Zhao, Z.; Whitlock, B.M.; Miele, M.M.; et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 2019, 568, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Basar, R.; Wang, G.; Liu, E.; Moyes, J.S.; Li, L.; Kerbauy, L.N.; Uprety, N.; Fathi, M.; Rezvan, A.; et al. KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape. Nat. Med. 2022, 28, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, L.; Guo, H.; Zhang, W. Macrophage membrane-coated nanovesicles for dual-targeted drug delivery to inhibit tumor and induce macrophage polarization. Bioact. Mater. 2023, 23, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Sun, B.; Duan, S.; Han, J.; Barr, T.; Zhang, J.; Bissonnette, M.B.; Kortylewski, M.; He, C.; Chen, J.; et al. YTHDF2 or-chestrates tumor-associated macrophage reprogramming and controls antitumor immunity through CD8+ T cells. Nat. Immunol. 2023, 24, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Wenes, M.; Shang, M.; Di Matteo, M.; Goveia, J.; Martín-Pérez, R.; Serneels, J.; Prenen, H.; Ghesquière, B.; Carmeliet, P.; Mazzone, M. Macrophage Metabolism Controls Tumor Blood Vessel Morphogenesis and Metastasis. Cell Metab. 2016, 24, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Puttock, E.H.; Tyler, E.J.; Manni, M.; Maniati, E.; Butterworth, C.; Burger Ramos, M.; Peerani, E.; Hirani, P.; Gauthier, V.; Liu, Y.; et al. Extracellular matrix educates an immunoregulatory tumor macrophage phenotype found in ovarian cancer metastasis. Nat. Commun. 2023, 14, 2514. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Qiu, G.; Zhu, X.; Wang, Q.; Zhu, C.; Fang, C.; Liu, J.; Zhang, K.; Liu, Y. Macrophage-inherited exosome excise tumor immunosuppression to expedite immune-activated ferroptosis. J. Immunother. Cancer 2023, 11, e006516. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Yu, M.; Chen, T.; Liu, Y.; Yi, Y.; Huang, C.; Tang, J.; Li, H.; Ou, M.; Wang, T.; et al. Polypyrrole Nanoenzymes as Tumor Microenvironment Modulators to Reprogram Macrophage and Potentiate Immunotherapy. Adv. Sci. 2022, 9, e2201703. [Google Scholar] [CrossRef] [PubMed]
- Goossens, P.; Rodriguez-Vita, J.; Etzerodt, A.; Masse, M.; Rastoin, O.; Gouirand, V.; Ulas, T.; Papantonopoulou, O.; Van Eck, M.; Auphan-Anezin, N.; et al. Membrane Cholesterol Efflux Drives Tumor-Associated Macrophage Reprogramming and Tumor Progression. Cell Metab. 2019, 29, 1376–1389.e4. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, C.; Shah, R.; Bermingham, K.; Hinkle, C.C.; Li, W.; Rodrigues, A.; Tabita-Martinez, J.; Millar, J.S.; Cuchel, M.; et al. Functional analysis and transcriptomic profiling of iPSC-derived macrophages and their application in modeling Mendelian disease. Circ. Res. 2015, 117, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tian, L.; Dai, X.; Yu, H.; Wang, J.; Lei, A.; Zhu, M.; Xu, J.; Zhao, W.; Zhu, Y.; et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J. Hematol. Oncol. 2020, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Kleiman, R.J.; Engle, S.J. Human inducible pluripotent stem cells: Realization of initial promise in drug discovery. Cell Stem Cell 2021, 28, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).