Targeted Suicide Gene Therapy with Retroviral Replicating Vectors for Experimental Canine Cancers
Abstract
1. Introduction
2. Results
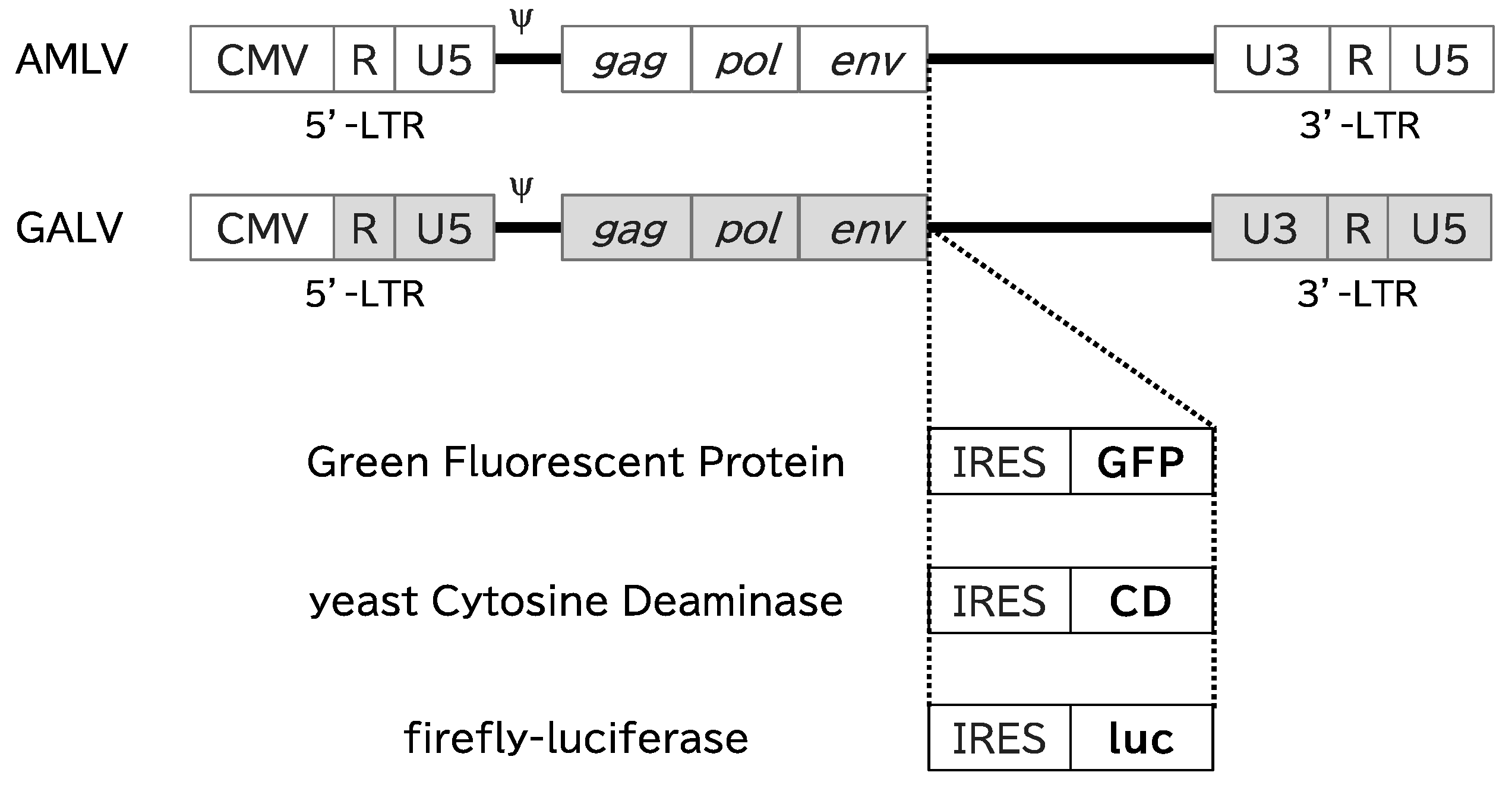
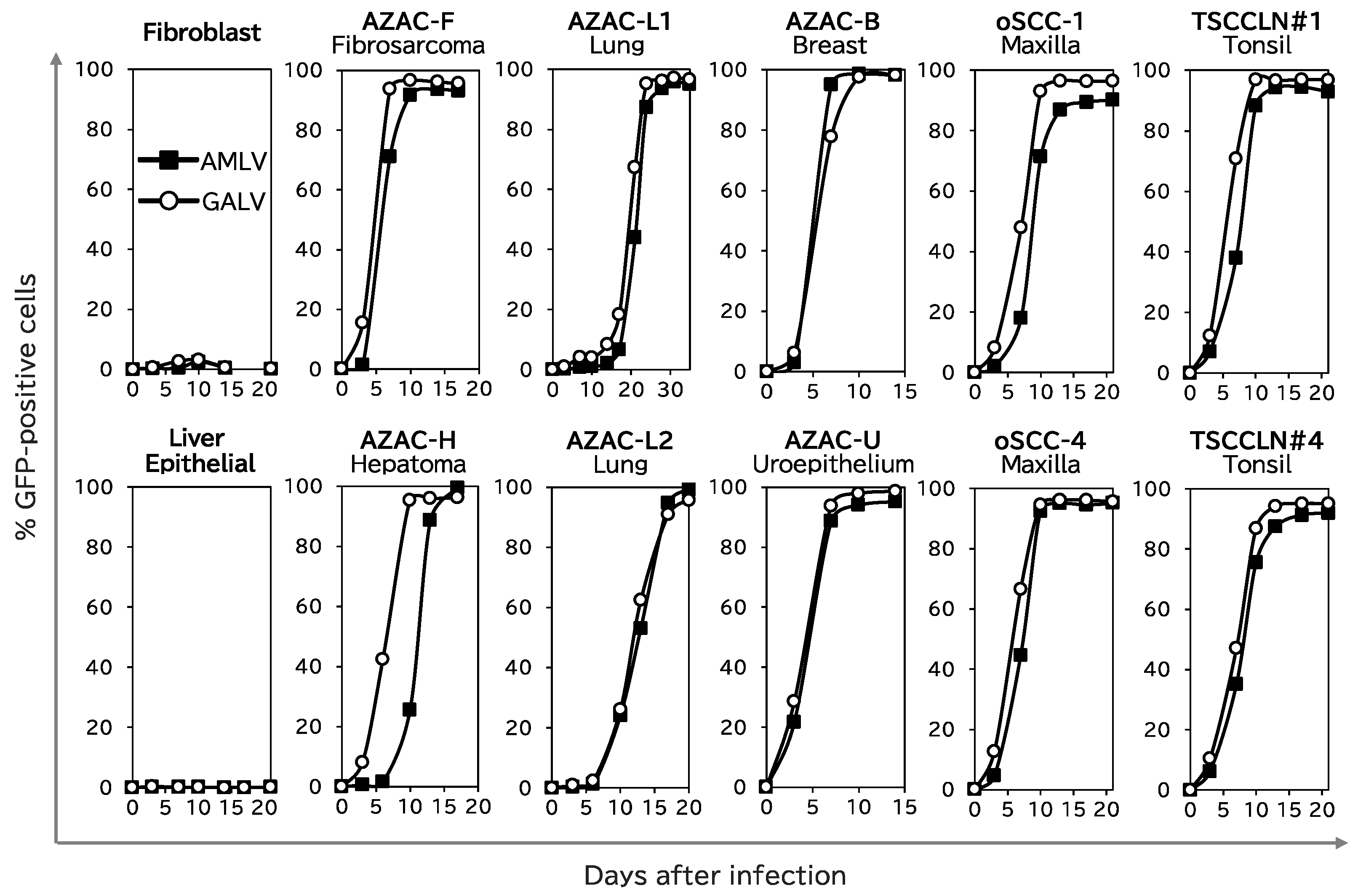
2.1. RRVs Replicate and Spread Efficiently in Canine Cancer Cell Lines
2.2. RRVs Replicate and Spread Efficiently in Subcutaneous Canine Xenograft Tumors in Nude Mice
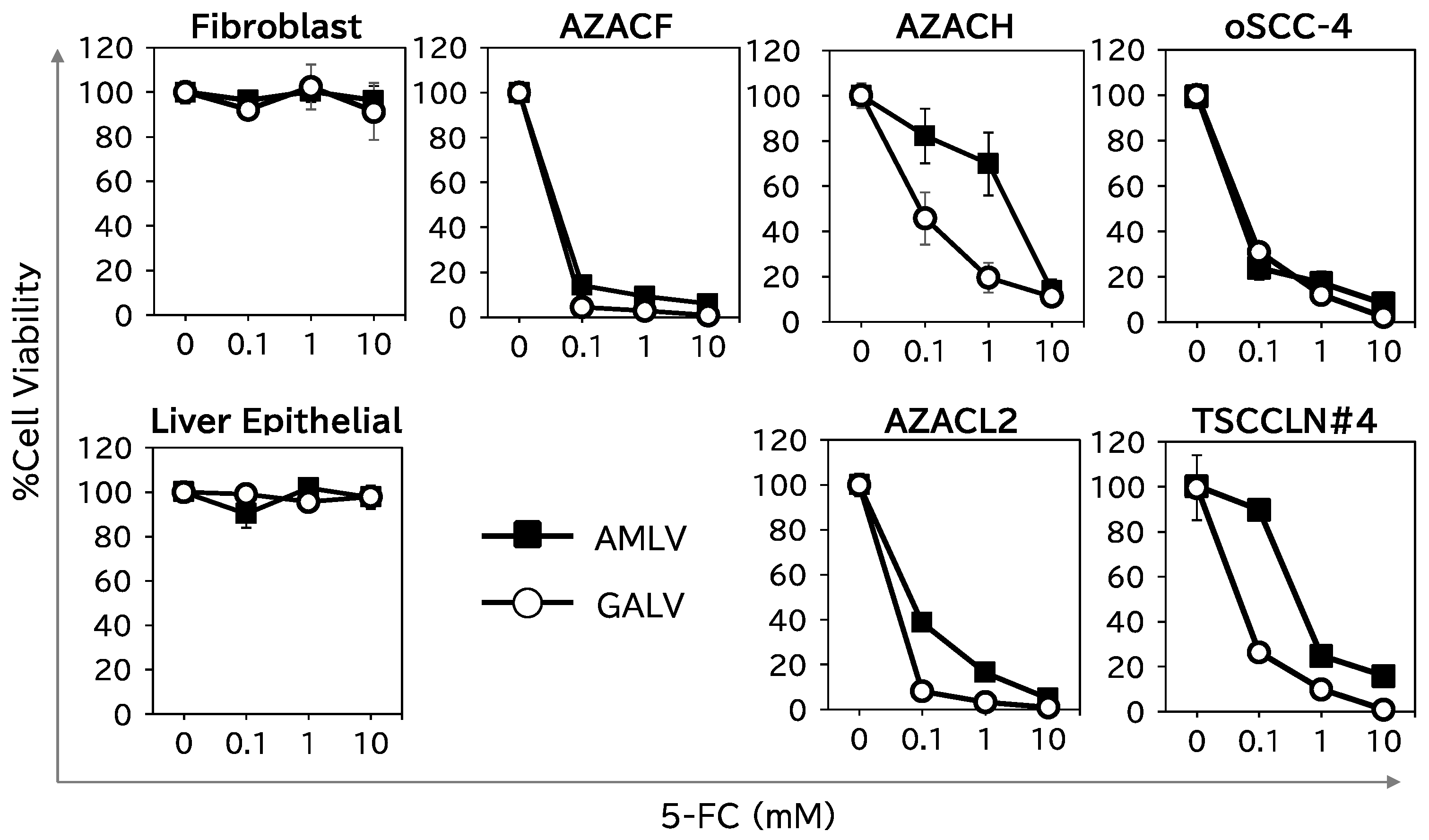
2.3. Suicide-Gene-Mediated Tumor-Cell-Specific Killing Effect of RRVs on Canine Cancer Cells
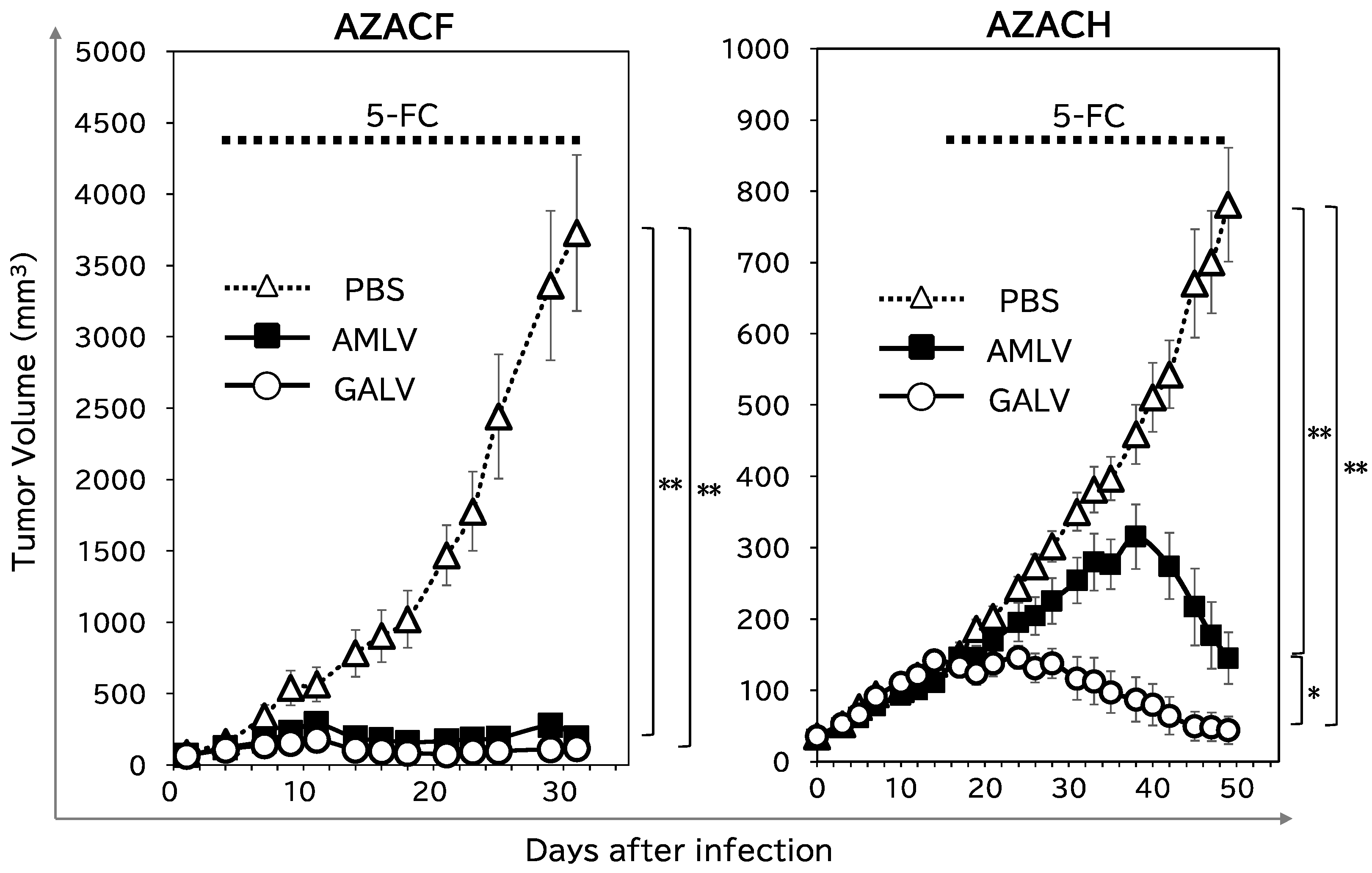
2.4. RRV–CD/5-FC Suicide Gene Therapy Exerts Potent Antitumor Effects on Subcutaneous Canine Xenograft Mouse Models
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Viral Vector Plasmids and Virus Production
4.3. Analysis of RRV Replication Kinetics In Vitro
4.4. RRV Replication Kinetics in a Subcutaneous Tumor Model
4.5. In Vitro Cytotoxicity Assay
4.6. Subcutaneous Xenograft Models of Canine Tumors
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mizuno, T. Spontaneously occurring canine cancer as a relevant animal model for developing novel treatments for human cancers. Transl. Regul. Sci. 2021, 3, 51–59. [Google Scholar] [CrossRef]
- Sanchez, D.; Cesarman-Maus, G.; Amador-Molina, A.; Lizano, M. Oncolytic Viruses for Canine Cancer Treatment. Cancers 2018, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Thamm, D.H. Canine Cancer: Strategies in Experimental Therapeutics. Front. Oncol. 2019, 9, 1257. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zuo, M.; Zhou, Q.; Wang, Y. Oncolytic virotherapy in cancer treatment: Challenges and optimization prospects. Front. Immunol. 2023, 14, 1308890. [Google Scholar] [CrossRef] [PubMed]
- Volovat, S.R.; Scripcariu, D.V.; Vasilache, I.A.; Stolniceanu, C.R.; Volovat, C.; Augustin, I.G.; Volovat, C.C.; Ostafe, M.R.; Andreea-Voichița, S.G.; Bejusca-Vieriu, T.; et al. Oncolytic Virotherapy: A New Paradigm in Cancer Immunotherapy. Int. J. Mol. Sci. 2024, 25, 1180. [Google Scholar] [CrossRef] [PubMed]
- van Putten, E.H.P.; Kleijn, A.; van Beusechem, V.W.; Noske, D.; Lamers, C.H.J.; de Goede, A.L.; Idema, S.; Hoefnagel, D.; Kloezeman, J.J.; Fueyo, J.; et al. Convection Enhanced Delivery of the Oncolytic Adenovirus Delta24-RGD in Patients with Recurrent GBM: A Phase I Clinical Trial Including Correlative Studies. Clin. Cancer Res. 2022, 28, 1572–1585. [Google Scholar] [CrossRef]
- Andtbacka, R.H.; Ross, M.; Puzanov, I.; Milhem, M.; Collichio, F.; Delman, K.A.; Amatruda, T.; Zager, J.S.; Cranmer, L.; Hsueh, E.; et al. Patterns of Clinical Response with Talimogene Laherparepvec (T-VEC) in Patients with Melanoma Treated in the OPTiM Phase III Clinical Trial. Ann. Surg. Oncol. 2016, 23, 4169–4177. [Google Scholar] [CrossRef]
- Fong, Y.; Kim, T.; Bhargava, A.; Schwartz, L.; Brown, K.; Brody, L.; Covey, A.; Karrasch, M.; Getrajdman, G.; Mescheder, A.; et al. A herpes oncolytic virus can be delivered via the vasculature to produce biologic changes in human colorectal cancer. Mol. Ther. 2009, 17, 389–394. [Google Scholar] [CrossRef]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: A phase 2 trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef]
- Nawrocki, S.T.; Olea, J.; Villa Celi, C.; Dadrastoussi, H.; Wu, K.; Tsao-Wei, D.; Colombo, A.; Coffey, M.; Fernandez Hernandez, E.; Chen, X.; et al. Comprehensive Single-Cell Immune Profiling Defines the Patient Multiple Myeloma Microenvironment Following Oncolytic Virus Therapy in a Phase Ib Trial. Clin. Cancer Res. 2023, 29, 5087–5103. [Google Scholar] [CrossRef]
- Cejalvo, T.; Perise-Barrios, A.J.; Del Portillo, I.; Laborda, E.; Rodriguez-Milla, M.A.; Cubillo, I.; Vazquez, F.; Sardon, D.; Ramirez, M.; Alemany, R.; et al. Remission of Spontaneous Canine Tumors after Systemic Cellular Viroimmunotherapy. Cancer Res. 2018, 78, 4891–4901. [Google Scholar] [CrossRef]
- Hwang, C.C.; Igase, M.; Sakurai, M.; Haraguchi, T.; Tani, K.; Itamoto, K.; Shimokawa, T.; Nakaichi, M.; Nemoto, Y.; Noguchi, S.; et al. Oncolytic reovirus therapy: Pilot study in dogs with spontaneously occurring tumours. Vet. Comp. Oncol. 2018, 16, 229–238. [Google Scholar] [CrossRef]
- Ilyinskaya, G.V.; Mukhina, E.V.; Soboleva, A.V.; Matveeva, O.V.; Chumakov, P.M. Oncolytic Sendai Virus Therapy of Canine Mast Cell Tumors (A Pilot Study). Front. Vet. Sci. 2018, 5, 116. [Google Scholar] [CrossRef]
- Henson, M.S.; Suter, S.E.; von Messling, V.A.; Cattaneo, R.; Fielding, A.K. The Effects of Intratumoral Injection of a Replicating Morbillivirus in a Canine Model of Naturally Occurring Lymphoma. Mol. Ther. 2005, 11, S312. [Google Scholar] [CrossRef]
- Sanchez, D.; Pelayo, R.; Medina, L.A.; Vadillo, E.; Sanchez, R.; Nunez, L.; Cesarman-Maus, G.; Sarmiento-Silva, R.E. Newcastle Disease Virus: Potential Therapeutic Application for Human and Canine Lymphoma. Viruses 2015, 8, 3. [Google Scholar] [CrossRef]
- Shin, D.H.; Nguyen, T.; Ozpolat, B.; Lang, F.; Alonso, M.; Gomez-Manzano, C.; Fueyo, J. Current strategies to circumvent the antiviral immunity to optimize cancer virotherapy. J. Immunother. Cancer 2021, 9, e002086. [Google Scholar] [CrossRef]
- Collins, S.A.; Shah, A.H.; Ostertag, D.; Kasahara, N.; Jolly, D.J. Clinical development of retroviral replicating vector Toca 511 for gene therapy of cancer. Expert. Opin. Biol. Ther. 2021, 21, 1199–1214. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Takagi-Kimura, M.; Logg, C.R.; Kasahara, N. Highly efficient tumor transduction and antitumor efficacy in experimental human malignant mesothelioma using replicating gibbon ape leukemia virus. Cancer Gene Ther. 2013, 20, 671–677. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Logg, C.R.; Robbins, J.M.; Jolly, D.J.; Gruber, H.E.; Kasahara, N. Retroviral replicating vectors in cancer. Methods Enzym. 2012, 507, 199–228. [Google Scholar] [CrossRef]
- Inoko, K.; Hiraoka, K.; Inagaki, A.; Takahashi, M.; Kushibiki, T.; Hontani, K.; Takano, H.; Sato, S.; Takeuchi, S.; Nakamura, T.; et al. Therapeutic activity of retroviral replicating vector-mediated prodrug activator gene therapy for pancreatic cancer. Cancer Gene Ther. 2018, 25, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Kushiya, H.; Hiraoka, K.; Suzuki, T.; Inoko, K.; Inagaki, A.; Niwa, H.; Sasaki, K.; Nakamura, T.; Tsuchikawa, T.; Shichinohe, T.; et al. Retroviral Replicating Vector Toca 511 (Vocimagene Amiretrorepvec) for Prodrug Activator Gene Therapy of Lung Cancer. Cancers 2022, 14, 5820. [Google Scholar] [CrossRef]
- Wang, W.J.; Tai, C.K.; Kasahara, N.; Chen, T.C. Highly efficient and tumor-restricted gene transfer to malignant gliomas by replication-competent retroviral vectors. Hum. Gene Ther. 2003, 14, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Sun, J.M.; Chen, B.M.; Lin, S.C.; Chang, H.F.; Collins, S.; Chang, D.; Wu, S.F.; Lu, Y.C.; Wang, W.; et al. Efficient Prodrug Activator Gene Therapy by Retroviral Replicating Vectors Prolongs Survival in an Immune-Competent Intracerebral Glioma Model. Int. J. Mol. Sci. 2020, 21, 1433. [Google Scholar] [CrossRef]
- Cloughesy, T.F.; Landolfi, J.; Vogelbaum, M.A.; Ostertag, D.; Elder, J.B.; Bloomfield, S.; Carter, B.; Chen, C.C.; Kalkanis, S.N.; Kesari, S.; et al. Durable complete responses in some recurrent highgrade glioma patients treated with Toca 511+Toca FC. Neuro-Oncol. 2018, 20, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Petrecca, K.; Walbert, T.; Butowski, N.; Salacz, M.; Perry, J.; Damek, D.; Bota, D.; Bettegowda, C.; Zhu, J.J.; et al. Effect of Vocimagene Amiretrorepvec in Combination with Flucytosine vs Standard of Care on Survival Following Tumor Resection in Patients with Recurrent High-Grade Glioma: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1939–1946. [Google Scholar] [CrossRef]
- Fujino, H.; Sonoda-Fukuda, E.; Isoda, L.; Kawabe, A.; Takarada, T.; Kasahara, N.; Kubo, S. Retroviral Replicating Vectors Mediated Prodrug Activator Gene Therapy in a Gastric Cancer Model. Int. J. Mol. Sci. 2023, 24, 14823. [Google Scholar] [CrossRef] [PubMed]
- Isoda, L.; Sonoda-Fukuda, E.; Fujino, H.; Takarada, T.; Hasegawa, K.; Kasahara, N.; Kubo, S. Therapeutic Efficacy of Prodrug Activator Gene Therapy Using Retroviral Replicating Vectors for Human Ovarian Cancer. Anticancer. Res. 2023, 43, 5311–5317. [Google Scholar] [CrossRef]
- Kaur, B.; Kaur, J.; Kashyap, N.; Arora, J.S.; Mukhopadhyay, C.S. A comprehensive review of genomic perspectives of canine diseases as a model to study human disorders. Can. J. Vet. Res. 2023, 87, 3–8. [Google Scholar]
- Ostertag, D.; Amundson, K.K.; Lopez Espinoza, F.; Martin, B.; Buckley, T.; Galvao da Silva, A.P.; Lin, A.H.; Valenta, D.T.; Perez, O.D.; Ibanez, C.E.; et al. Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neuro Oncol. 2012, 14, 145–159. [Google Scholar] [CrossRef]
- Cloughesy, T.F.; Landolfi, J.; Hogan, D.J.; Bloomfield, S.; Carter, B.; Chen, C.C.; Elder, J.B.; Kalkanis, S.N.; Kesari, S.; Lai, A.; et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci. Transl. Med. 2016, 8, 341ra375. [Google Scholar] [CrossRef]
- Tai, C.K.; Wang, W.J.; Chen, T.C.; Kasahara, N. Single-shot, multicycle suicide gene therapy by replication-competent retrovirus vectors achieves long-term survival benefit in experimental glioma. Mol. Ther. 2005, 12, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Eiden, M.V. The receptors for gibbon ape leukemia virus and amphotropic murine leukemia virus are not downregulated in productively infected cells. Retrovirology 2011, 8, 53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miller, D.G.; Adam, M.A.; Miller, A.D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol. Cell Biol. 1990, 10, 4239–4242. [Google Scholar] [CrossRef] [PubMed]
- Overbaugh, J.; Miller, A.D.; Eiden, M.V. Receptors and entry cofactors for retroviruses include single and multiple transmembrane-spanning proteins as well as newly described glycophosphatidylinositol-anchored and secreted proteins. Microbiol. Mol. Biol. Rev. 2001, 65, 371–389, table of contents. [Google Scholar] [CrossRef]
- Noguchi, S.; Hattori, A.; Tanimoto, N.; Nishida, R.; Hirano, K.; Wada, Y.; Matsuyama, S.; Shimada, T.; Akiyoshi, H. Establishing cell lines for canine tonsillar and non-tonsillar oral squamous cell carcinoma and identifying characteristics associated with malignancy. Tissue Cell 2020, 67, 101408. [Google Scholar] [CrossRef]

| Cell Names | Origin | Breed | Age (Year) | Source |
|---|---|---|---|---|
| Fibroblast | Head skin | Miniature Dachshund | 11 | Dr. Noguchi (Osaka Metropolitan University) |
| Liver Epithelial | Liver | Beagle | U/N | Cell Biologics, Inc. |
| AZACF | Fibrosarcoma | U/N | U/N | Cosmo Bio Co., Ltd. |
| AZACH | Hepatoma | Maltese | U/N | Cosmo Bio Co., Ltd. |
| AZACL1 | Lung Carcinoma | Shetland Sheepdog | U/N | Cosmo Bio Co., Ltd. |
| AZACL2 | Lung Carcinoma | U/N | U/N | Cosmo Bio Co., Ltd. |
| AZACB | Breast Tumor | U/N | U/N | Cosmo Bio Co., Ltd. |
| AZACU | Urothelial Carcinoma | U/N | U/N | Cosmo Bio Co., Ltd. |
| oSCC-1 | Maxillary squamous carcinoma | Toy Poodle | 10 | Dr. Noguchi (Osaka Metropolitan University) [35] |
| oSCC-4 | Maxillary squamous carcinoma | Toy Poodle | 10 | Dr. Noguchi (Osaka Metropolitan University) [35] |
| TSCCLN#1 | Tonsillar squamous carcinoma | Miniature Schnauzer | 13 | Dr. Noguchi (Osaka Metropolitan University) [35] |
| TSCCLN#4 | Tonsillar squamous carcinoma | Miniature Schnauzer | 13 | Dr. Noguchi (Osaka Metropolitan University) [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonoda-Fukuda, E.; Takeuchi, Y.; Ogawa, N.; Noguchi, S.; Takarada, T.; Kasahara, N.; Kubo, S. Targeted Suicide Gene Therapy with Retroviral Replicating Vectors for Experimental Canine Cancers. Int. J. Mol. Sci. 2024, 25, 2657. https://doi.org/10.3390/ijms25052657
Sonoda-Fukuda E, Takeuchi Y, Ogawa N, Noguchi S, Takarada T, Kasahara N, Kubo S. Targeted Suicide Gene Therapy with Retroviral Replicating Vectors for Experimental Canine Cancers. International Journal of Molecular Sciences. 2024; 25(5):2657. https://doi.org/10.3390/ijms25052657
Chicago/Turabian StyleSonoda-Fukuda, Emiko, Yuya Takeuchi, Nao Ogawa, Shunsuke Noguchi, Toru Takarada, Noriyuki Kasahara, and Shuji Kubo. 2024. "Targeted Suicide Gene Therapy with Retroviral Replicating Vectors for Experimental Canine Cancers" International Journal of Molecular Sciences 25, no. 5: 2657. https://doi.org/10.3390/ijms25052657
APA StyleSonoda-Fukuda, E., Takeuchi, Y., Ogawa, N., Noguchi, S., Takarada, T., Kasahara, N., & Kubo, S. (2024). Targeted Suicide Gene Therapy with Retroviral Replicating Vectors for Experimental Canine Cancers. International Journal of Molecular Sciences, 25(5), 2657. https://doi.org/10.3390/ijms25052657






