Extracellular Vesicles as Novel Diagnostic and Therapeutic Agents for Non-Melanoma Skin Cancer: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Study Protocol
2.2. Search Strategy
2.3. Eligibility of Relevant Studies
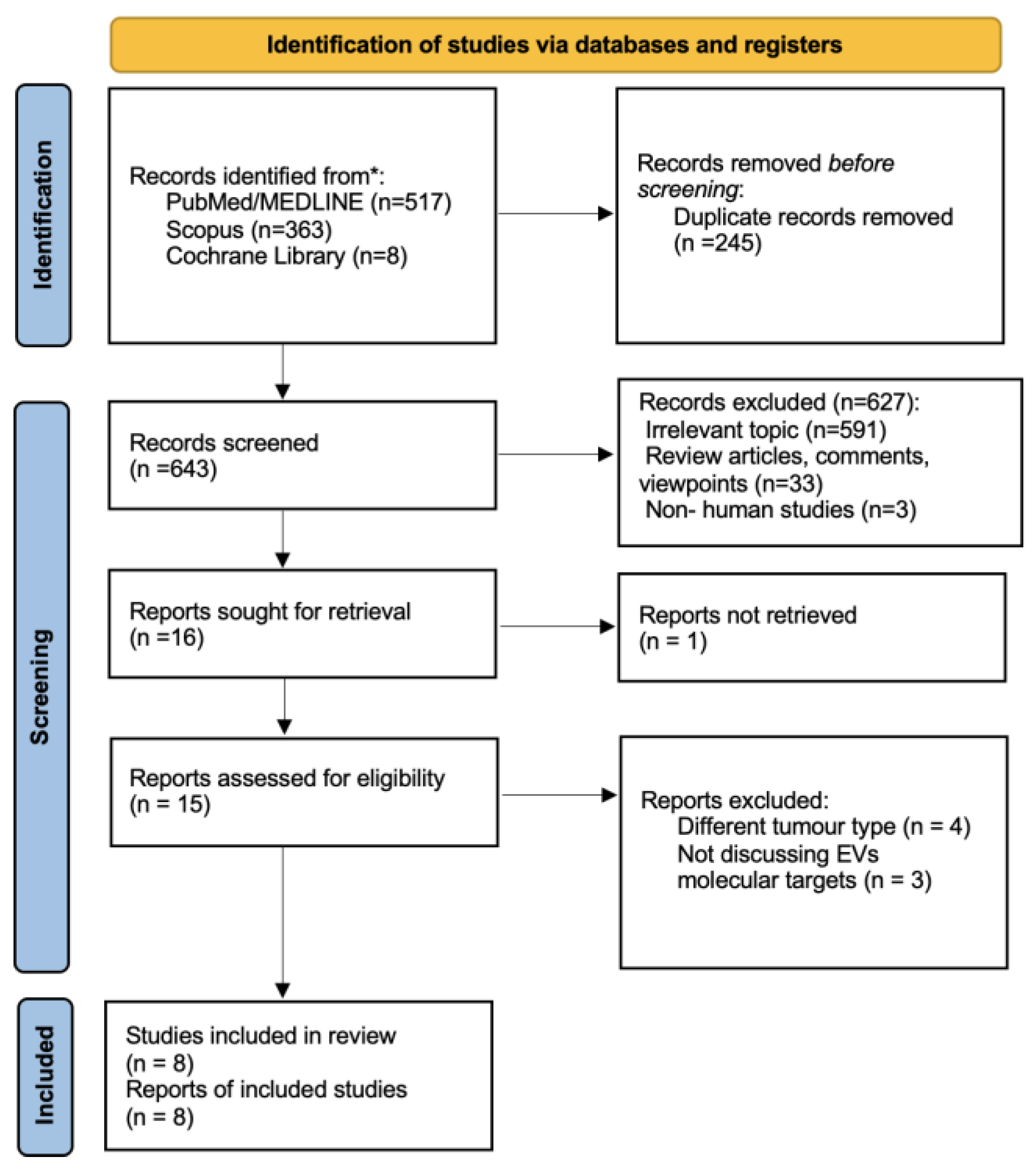
2.4. Study Selection
2.5. Data Collection and Risk of Bias Assessment
2.6. Data Synthesis and Analysis
3. Results
3.1. General Study Characteristics
3.2. EV Structure and Function
3.3. EVs Role in Tumorigenesis
3.4. EVs as Therapeutic Targets in cSCC
3.4.1. Circ-CYP24A1
3.4.2. Desmoglein 2 (Dsg2)
3.4.3. p38 Inhibited Cutaneous Squamous Cell Carcinoma-Associated lincRNA (PICSAR)
3.4.4. miRNA
3.5. EVs as Diagnostic Biomarkers
3.6. EVs’ Role in Prognosis
3.7. EVs as Drug Delivery Systems
3.8. Prospects for EVs in NMSC Anticancer Therapy: The Promising Role of Exosomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zelin, E.; Maronese, C.A.; Dri, A.; Toffoli, L.; Di Meo, N.; Nazzaro, G.; Zalaudek, I. Identifying Candidates for Immunotherapy among Patients with Non-Melanoma Skin Cancer: A Review of the Potential Predictors of Response. J. Clin. Med. 2022, 11, 3364. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Yang, Y.; Zhang, H.-B.; Zheng, X.-H.; Li, H.-R.; Wen, J. Identification of CDK1 as a Candidate Marker in Cutaneous Squamous Cell Carcinoma by Integrated Bioinformatics Analysis. Transl. Cancer Res. 2021, 10, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, H.; Yang, W.; Li, J. Exosomal Circular RNA RNA-Seq Profiling and the Carcinogenic Role of Exosomal Circ-CYP24A1 in Cutaneous Squamous Cell Carcinoma. Front. Med. 2021, 8, 675842. [Google Scholar] [CrossRef] [PubMed]
- Shalhout, S.Z.; Emerick, K.S.; Kaufman, H.L.; Miller, D.M. Immunotherapy for Non-Melanoma Skin Cancer. Curr. Oncol. Rep. 2021, 23, 125. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Akhtar, S.; Prabhu, K.S.; Zarif, L.; Khan, R.; Alam, M.; Buddenkotte, J.; Ahmad, A.; Steinhoff, M.; Uddin, S. Exosomes: Emerging Diagnostic and Therapeutic Targets in Cutaneous Diseases. Int. J. Mol. Sci. 2020, 21, 9264. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Gao, L.; Loveless, R.; Rodrigo, J.P.; Strojan, P.; Willems, S.M.; Nathan, C.A.; Mäkitie, A.A.; Saba, N.F.; Ferlito, A. The Hidden Link of Exosomes to Head and Neck Cancer. Cancers 2021, 13, 5802. [Google Scholar] [CrossRef] [PubMed]
- Overmiller, A.M.; Pierluissi, J.A.; Wermuth, P.J.; Sauma, S.; Martinez-Outschoorn, U.; Tuluc, M.; Luginbuhl, A.; Curry, J.; Harshyne, L.A.; Wahl, J.K.; et al. Desmoglein 2 Modulates Extracellular Vesicle Release from Squamous Cell Carcinoma Keratinocytes. FASEB J. 2017, 31, 3412–3424. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, J.; Wang, H.; Zhao, J.; Yan, M.; He, H.; Yu, S. Exosomes: Potential Biomarkers and Functions in Head and Neck Squamous Cell Carcinoma. Front. Mol. Biosci. 2022, 9, 881794. [Google Scholar] [CrossRef]
- Wang, W.-M.; Wu, C.; Jin, H.-Z. Exosomes in Chronic Inflammatory Skin Diseases and Skin Tumors. Exp. Dermatol. 2019, 28, 213–218. [Google Scholar] [CrossRef]
- Cumpston, M.; Chandler, J. Chapter IV: Updating a Review. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Updated February 2022; Cochrane: London, UK, 2022; Available online: www.training.cochrane.org/handbook (accessed on 17 February 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, L4898. [Google Scholar] [CrossRef]
- Chang, J.; Tran, D.C.; Zhu, G.A.; Li, R.; Whitson, R.; Kim, Y.H.; Gupta, A.; Afshari, A.; Antes, T.; Spitale, R.C.; et al. Initial in Vitro Functional Characterization of Serum Exosomal MicroRNAs from Patients with Metastatic Basal Cell Carcinoma. Br. J. Dermatol. 2017, 177, e187–e190. [Google Scholar] [CrossRef]
- Sun, Y.; Woess, K.; Kienzl, M.; Leb-Reichl, V.M.; Feinle, A.; Wimmer, M.; Zauner, R.; Wally, V.; Luetz-Meindl, U.; Mellerio, J.E.; et al. Extracellular Vesicles as Biomarkers for the Detection of a Tumor Marker Gene in Epidermolysis Bullosa-Associated Squamous Cell Carcinoma. J. Investig. Dermatol. 2018, 138, 1197–1200. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, H.; Zeng, Q.; Wang, P.; Zhang, G.; Ji, J.; Li, M.; Shen, S.; Wang, X. Exosomes from 5-Aminolevulinic Acid Photodynamic Therapy-Treated Squamous Carcinoma Cells Promote Dendritic Cell Maturation. Photodiagnosis Photodyn. Ther. 2020, 30, 101746. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhou, X.; Yin, J.; Zhou, Y. Lnc-PICSAR Contributes to Cisplatin Resistance by MiR-485-5p/REV3L Axis in Cutaneous Squamous Cell Carcinoma. Open Life Sci. 2020, 15, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Flemming, J.P.; Hill, B.L.; Haque, M.W.; Raad, J.; Bonder, C.S.; Harshyne, L.A.; Rodeck, U.; Luginbuhl, A.; Wahl, J.K.; Tsai, K.Y.; et al. MiRNA- and Cytokine-Associated Extracellular Vesicles Mediate Squamous Cell Carcinomas. J. Extracell. Vesicles 2020, 9, 1790159. [Google Scholar] [CrossRef] [PubMed]
- Zauner, R.; Wimmer, M.; Atzmueller, S.; Proell, J.; Niklas, N.; Ablinger, M.; Reisenberger, M.; Lettner, T.; Illmer, J.; Dorfer, S.; et al. Biomarker Discovery in Rare Malignancies: Development of a MiRNA Signature for RDEB-CSCC. Cancers 2023, 15, 3286. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Tang, F.; Li, J.; Yu, H.; Wu, M.; Wu, Y.; Zeng, H.; Hou, K.; Zhang, Q. Tumor-Derived Exosomes: The Emerging Orchestrators in Melanoma. Biomed. Pharmacother. 2022, 149, 112832. [Google Scholar] [CrossRef] [PubMed]
- Panvongsa, W.; Pegtel, D.M.; Voortman, J. More than a Bubble: Extracellular Vesicle MicroRNAs in Head and Neck Squamous Cell Carcinoma. Cancers 2022, 14, 1160. [Google Scholar] [CrossRef] [PubMed]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.L.; Vader, P. Extracellular Vesicles as Drug Delivery Systems: Why and How? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Ma, J.; Sun, T.; Zhou, Q.; Wang, W.; Wang, G.; Wu, P.; Wang, H.; Jiang, L.; et al. Exosomal CircRNAs: Biogenesis, Effect and Application in Human Diseases. Mol. Cancer 2019, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.-L.; Shen, C.-H.; Tsai, F.-C.; Chen, C.-B.; Ma, K.S.-K. Cancer-Derived Extracellular Vesicles as Biomarkers for Cutaneous Squamous Cell Carcinoma: A Systematic Review. Cancers 2022, 14, 5098. [Google Scholar] [CrossRef]
- Lesnik, J.; Antes, T.; Kim, J.; Griner, E.; Pedro, L. Registered Report: Melanoma Exosomes Educate Bone Marrow Progenitor Cells toward a pro-Metastatic Phenotype through MET. eLife 2016, 5, e07383. [Google Scholar] [CrossRef] [PubMed]
- Di Modica, M.; Regondi, V.; Sandri, M.; Iorio, M.V.; Zanetti, A.; Tagliabue, E.; Casalini, P.; Triulzi, T. Breast Cancer-Secreted MiR-939 Downregulates VE-Cadherin and Destroys the Barrier Function of Endothelial Monolayers. Cancer Lett. 2017, 384, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Morello, M.; Minciacchi, V.R.; De Candia, P.; Yang, J.; Posadas, E.; Kim, H.; Griffiths, D.; Bhowmick, N.; Chung, L.W.K.; Gandellini, P.; et al. Large Oncosomes Mediate Intercellular Transfer of Functional MicroRNA. Cell Cycle 2013, 12, 3526–3536. [Google Scholar] [CrossRef] [PubMed]
- Cazzoli, R.; Buttitta, F.; Di Nicola, M.; Malatesta, S.; Marchetti, A.; Rom, W.N.; Pass, H.I. MicroRNAs Derived from Circulating Exosomes as Noninvasive Biomarkers for Screening and Diagnosing Lung Cancer. J. Thorac. Oncol. 2013, 8, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Jia, E.; Ren, N.; Shi, X.; Zhang, R.; Yu, H.; Yu, F.; Qin, S.; Xue, J. Extracellular Vesicle Biomarkers for Pancreatic Cancer Diagnosis: A Systematic Review and Meta-Analysis. BMC Cancer 2022, 22, 573. [Google Scholar] [CrossRef]
- Rodríguez, M.; Silva, J.; López-Alfonso, A.; López-Muñiz, M.B.; Peña, C.; Domínguez, G.; García, J.M.; López-Gónzalez, A.; Méndez, M.; Provencio, M.; et al. Different Exosome Cargo from Plasma/Bronchoalveolar Lavage in Non-Small-Cell Lung Cancer. Genes Chromosom. Cancer 2014, 53, 713–724. [Google Scholar] [CrossRef]
- Theodoraki, M.-N.; Laban, S.; Jackson, E.K.; Lotfi, R.; Schuler, P.J.; Brunner, C.; Hoffmann, T.K.; Whiteside, T.L.; Hofmann, L. Changes in Circulating Exosome Molecular Profiles Following Surgery/(Chemo)Radiotherapy: Early Detection of Response in Head and Neck Cancer Patients. Br. J. Cancer 2021, 125, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Massey, A.E.; Malik, S.; Sikander, M.; Doxtater, K.A.; Tripathi, M.K.; Khan, S.; Yallapu, M.M.; Jaggi, M.; Chauhan, S.C.; Hafeez, B.B. Clinical Implications of Exosomes: Targeted Drug Delivery for Cancer Treatment. Int. J. Mol. Sci. 2021, 22, 5278. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Zhu, Y.; Ali, D.J.; Tian, T.; Xu, H.; Si, K.; Sun, B.; Chen, B.; Xiao, Z. Engineered Exosomes for Targeted Co-Delivery of MiR-21 Inhibitor and Chemotherapeutics to Reverse Drug Resistance in Colon Cancer. J Nanobiotechnol. 2020, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Wen, Z.; Chen, H.; Duan, Y. Exosomes as Carriers for Drug Delivery in Cancer Therapy. Pharm. Res. 2023, 40, 873–887. [Google Scholar] [CrossRef]
- Ohno, S.I.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor Microrna to Breast Cancer Cells. Mol. Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef]
- Schmults, C.D.; Blitzblau, R.; Aasi, S.Z.; Alam, M.; Andersen, J.S.; Baumann, B.C.; Bordeaux, J.; Chen, P.-L.; Chin, R.; Contreras, C.M.; et al. NCCN Guidelines® Insights: Squamous Cell Skin Cancer, Version 1.2022. J. Natl. Compr. Cancer Netw. 2021, 19, 1382–1394. [Google Scholar] [CrossRef]
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; Bataille, V.; Bastholt, L.; Dreno, B.; Fargnoli, M.C.; Forsea, A.M.; Frenard, C.; et al. European Interdisciplinary Guideline on Invasive Squamous Cell Carcinoma of the Skin: Part 1. Epidemiology, Diagnostics and Prevention. Eur. J. Cancer 2020, 128, 60–82. [Google Scholar] [CrossRef]
- Seretis, K.; Thomaidis, V.; Karpouzis, A.; Tamiolakis, D.; Tsamis, I. Epidemiology of Surgical Treatment of Nonmelanoma Skin Cancer of the Head and Neck in Greece. Dermatol. Surg. 2010, 36, 15–22. [Google Scholar] [CrossRef]
- Seretis, K.; Bounas, N.; Lykoudis, E.G. An Algorithmic Approach for the Reconstruction of Extended Upper-Third Auricular Soft Tissue Defects. J. Craniofacial Surg. 2022, 33, e452–e453. [Google Scholar] [CrossRef]
- Thomaidis, V.; Seretis, K.; Fiska, A.; Tamiolakis, D.; Karpouzis, A.; Tsamis, I. The Scalping Forehead Flap in Nasal Reconstruction: Report of 2 Cases. J. Oral Maxillofac. Surg. 2007, 65, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Seretis, K.; Boptsi, A.; Boptsi, E.; Lykoudis, E.G. The “Facelift” Flap Revisited. J. Craniofacial Surg. 2023, 34, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Seretis, K.; Bounas, N.; Lykoudis, E.G. Repair of a Large Defect Involving the Cheek and Ear. Dermatol. Surg. 2023, 49, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Ascierto, P.A.; Basset-Seguin, N.; Dréno, B.; Garbe, C.; Gutzmer, R.; Hauschild, A.; Krattinger, R.; Lear, J.T.; Malvehy, J.; et al. Sonidegib and Vismodegib in the Treatment of Patients with Locally Advanced Basal Cell Carcinoma: A Joint Expert Opinion. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1944–1956. [Google Scholar] [CrossRef]
- Villani, A.; Potestio, L.; Fabbrocini, G.; Scalvenzi, M. New Emerging Treatment Options for Advanced Basal Cell Carcinoma and Squamous Cell Carcinoma. Adv. Ther. 2022, 39, 1164–1178. [Google Scholar] [CrossRef]
- Nieuwland, R.; Siljander, P.R.M.; Falcón-Pérez, J.M.; Witwer, K.W. Reproducibility of extracellular vesicle research. Eur. J. Cell Biol. 2022, 101, 151226. [Google Scholar] [CrossRef]
- Lewis, S.J.; Gardner, M.; Higgins, J.; Holly, J.M.P.; Gaunt, T.R.; Perks, C.M.; Turner, S.D.; Rinaldi, S.; Thomas, S.; Harrison, S.; et al. Developing the WCRF International/University of Bristol Methodology for Identifying and Carrying out Systematic Reviews of Mechanisms of Exposure–Cancer Associations. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Target Molecule | Source | Study Group | Control Group | Incubation | Exp. Method | Analysis Method | Outcome | Function |
|---|---|---|---|---|---|---|---|---|---|
| Overmiller A. et al. (2017) [7] | Dsg-2-CTF | Cell lines | - | - | A431/GFP, A431-Dsg2/GFP, HaCaT/GFP, HaCaT-Dsg2/GFP, primary NHK | In vitro | Western blot | Overexpression in SCC-derived EVs resulted in increased EVs secretion; inhibition of proteolysis of Dsg2 resulted in reduced EVs secretion | Therapeutic target |
| Chang et al. (2017) [14] | miR197 | Serum | 9 MBCC patients | 9 non MBCC patients | NHK, human skin fibroblasts | In vitro | PCR | SS upregulation in MBCC patients; no impact on proliferation noted in fibroblasts and keratinocytes | Therapeutic target, prognostic biomarker |
| Sun et al. (2018) [15] | Ct-SLCO1B3 | Tissue | RDEB-SCC patients | - | RDEB, RDEB-SCC, NHK | In vitro and in vivo | PCR | Expression of Ct-SLCO1B3 only in RDEB-SCC derived EVs | Diagnostic biomarker |
| Zhao Z. et al. (2020) [16] | ALA-PDT exosomes | Cell lines | - | - | SCCs (human A431, mouse PECA, primary mice SCCs), fibroblasts 3T3, DCs | In vitro | Western blot | Stimulation of DCs maturation and fibroblasts’ TGF-β1 secretion, leading to an anti-tumor immune response | Treatment |
| Wang et al. (2020) [17] | lnc-PICSAR | Serum | 30 cSCC patients | 30 healthy patients | NHEK, A431, HSC-5 cells | In vitro and in vivo | PCR | Elevated in cSCC cells and DDP-resistant cSCC cells | Prognostic biomarker, therapeutic target |
| Flemming J. et al. (2020) [18] | Dsg-2 | Cell lines | - | - | A431/GFP, A431-Dsg2/GFP, A432-Dsg2cacs/GFP | In vitro and in vivo | Western blot | Inhibited palmitoylation of Dsg-2 corellates with reduced sEVs secretion and attenuated tumor development | Therapeutic target |
| Zhang Z. et al. (2021) [3] | circ-CYP24A1 | Serum | 5 cSCC patients | 5 healty patients | A431, SCL-1 cells | In vitro | PCR | Upregulated in cSCC EVs; inhibition leads to attenuation of the tumor’s metastatic dynamic | Therapeutic target, diagnostic biomarker |
| Zauner R et al. (2023) [19] | miRNA (expr. profile) | Tissue | 6 RDEB-cSCC | 4 healthy patients, 5 RDEB | - | In vitro | PCR | 51 miRNAS found significantly up-regulated and 74 down-regulated in RDEB-cSCC compared to RDEB | Diagnostic biomarker |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seretis, K.; Boptsi, E.; Boptsi, A. Extracellular Vesicles as Novel Diagnostic and Therapeutic Agents for Non-Melanoma Skin Cancer: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 2617. https://doi.org/10.3390/ijms25052617
Seretis K, Boptsi E, Boptsi A. Extracellular Vesicles as Novel Diagnostic and Therapeutic Agents for Non-Melanoma Skin Cancer: A Systematic Review. International Journal of Molecular Sciences. 2024; 25(5):2617. https://doi.org/10.3390/ijms25052617
Chicago/Turabian StyleSeretis, Konstantinos, Eleni Boptsi, and Anastasia Boptsi. 2024. "Extracellular Vesicles as Novel Diagnostic and Therapeutic Agents for Non-Melanoma Skin Cancer: A Systematic Review" International Journal of Molecular Sciences 25, no. 5: 2617. https://doi.org/10.3390/ijms25052617
APA StyleSeretis, K., Boptsi, E., & Boptsi, A. (2024). Extracellular Vesicles as Novel Diagnostic and Therapeutic Agents for Non-Melanoma Skin Cancer: A Systematic Review. International Journal of Molecular Sciences, 25(5), 2617. https://doi.org/10.3390/ijms25052617






