Dual Emissive Zn(II) Naphthalocyanines: Synthesis, Structural and Photophysical Characterization with Theory-Supported Insights towards Soluble Coordination Compounds with Visible and Near-Infrared Emission
Abstract
1. Introduction
2. Results and Discussion
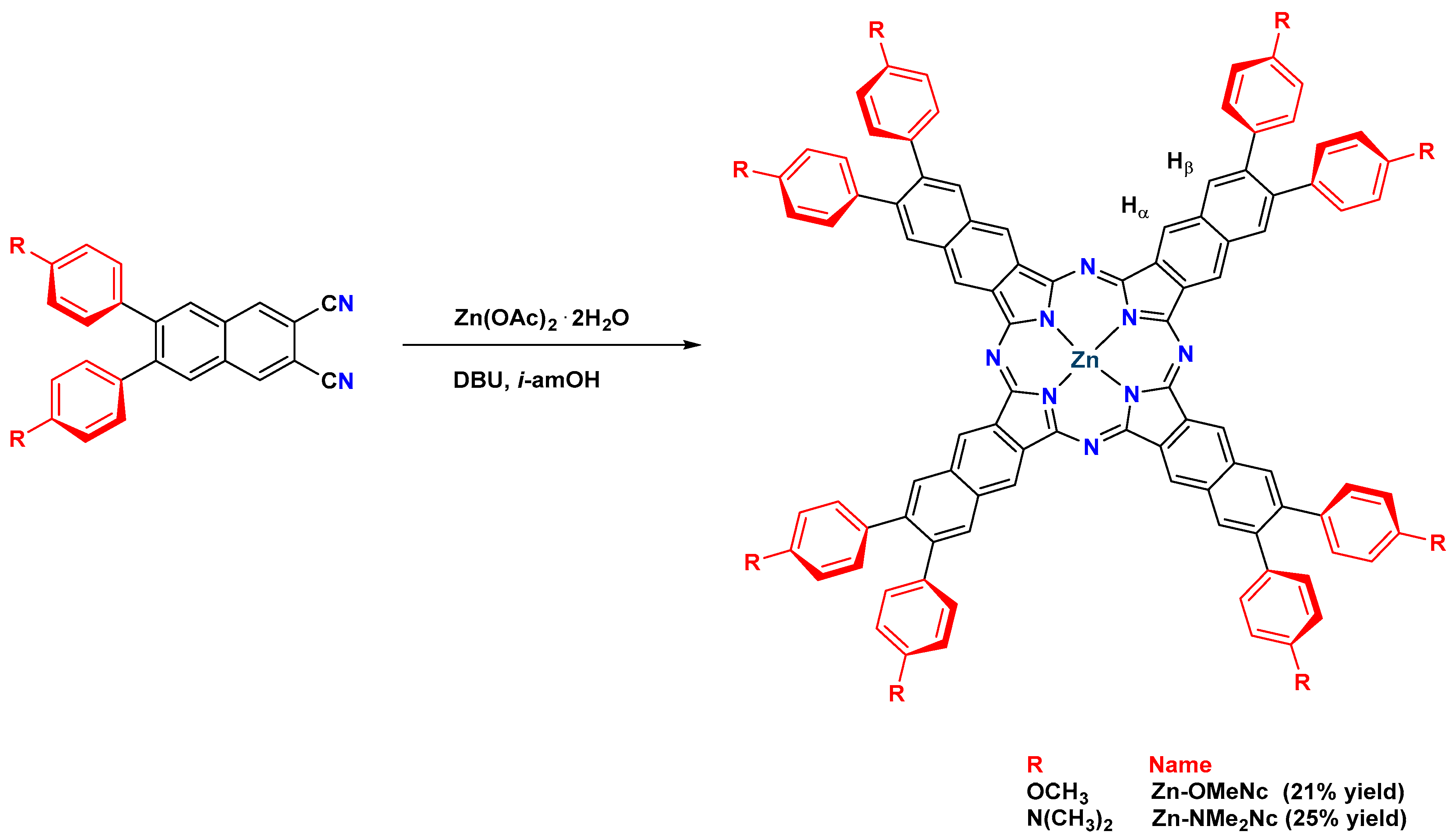
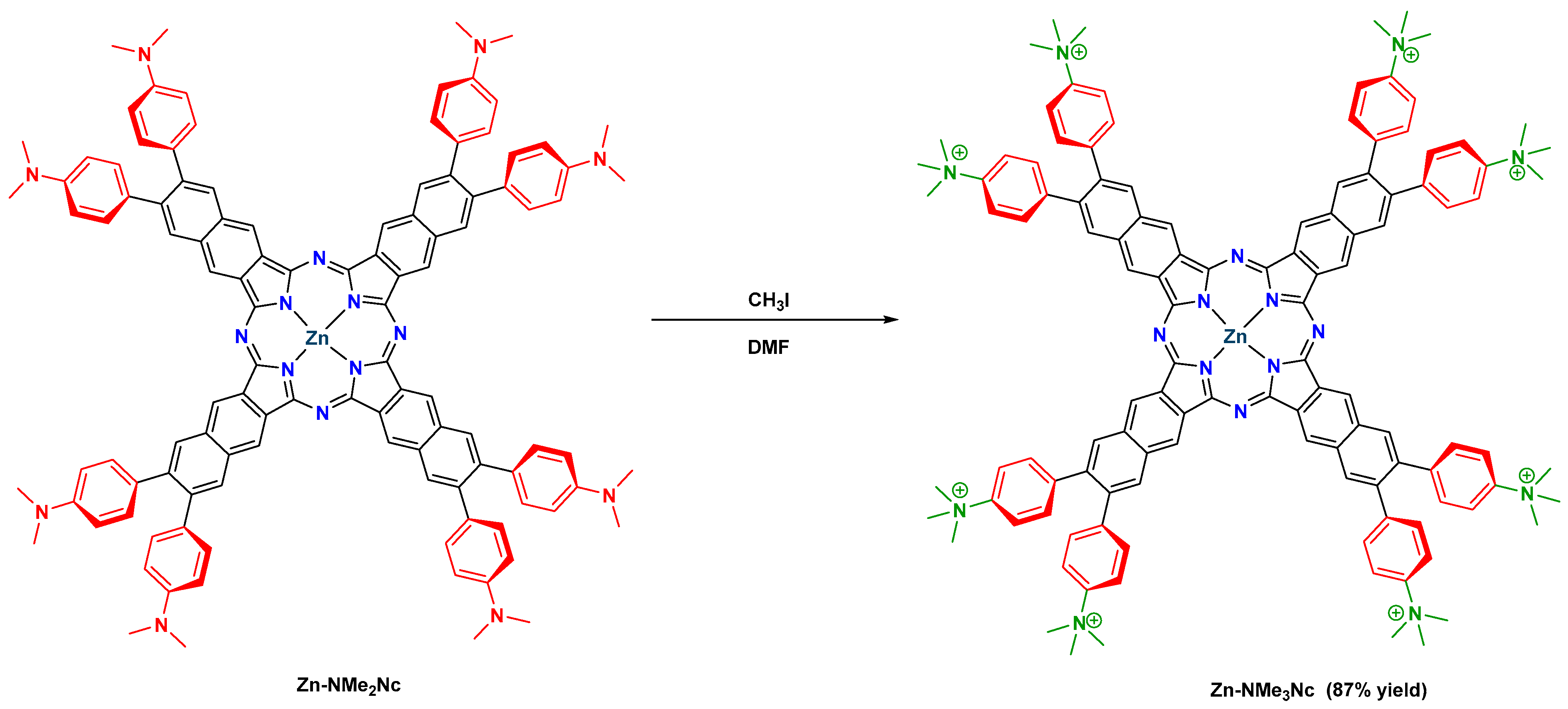
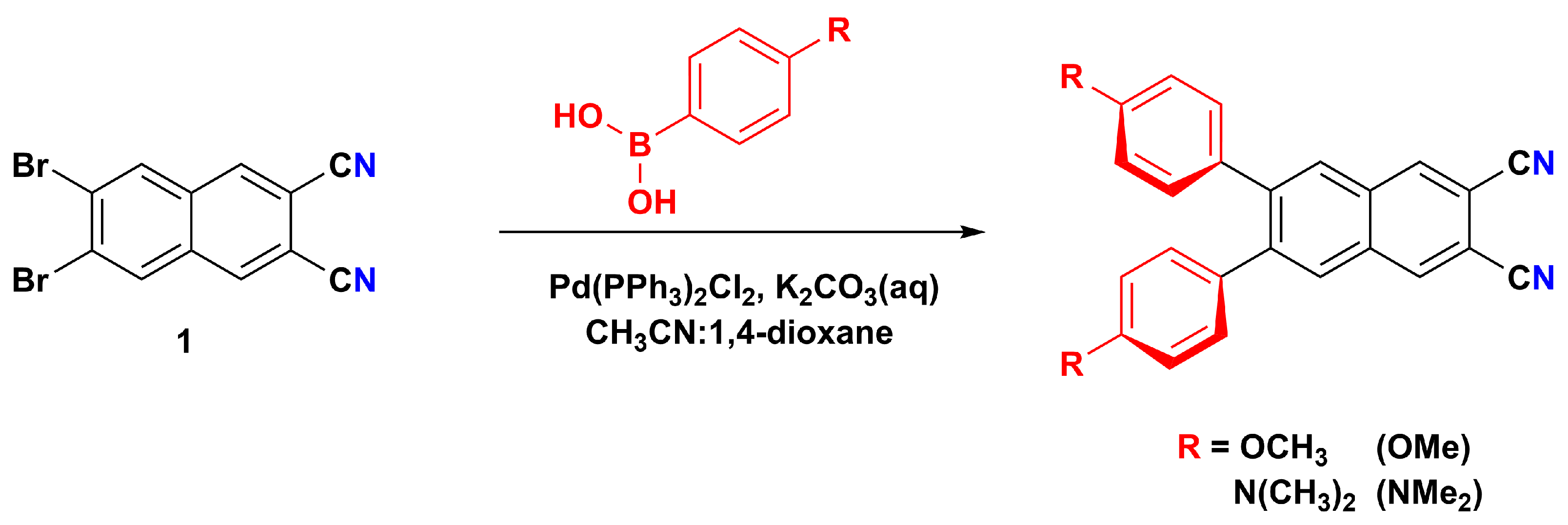
2.1. Design, Synthesis, Purification, and Structural Characterization
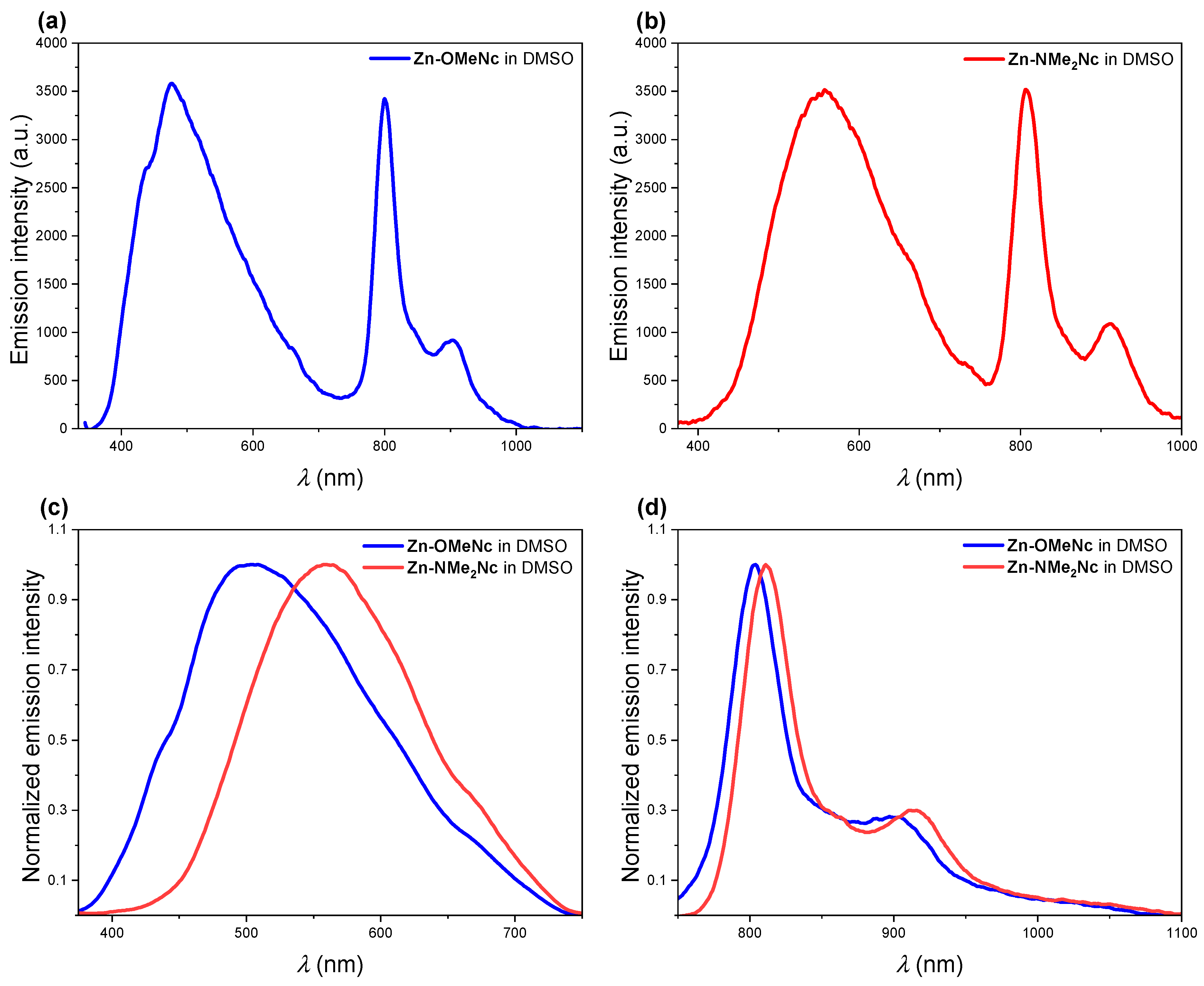
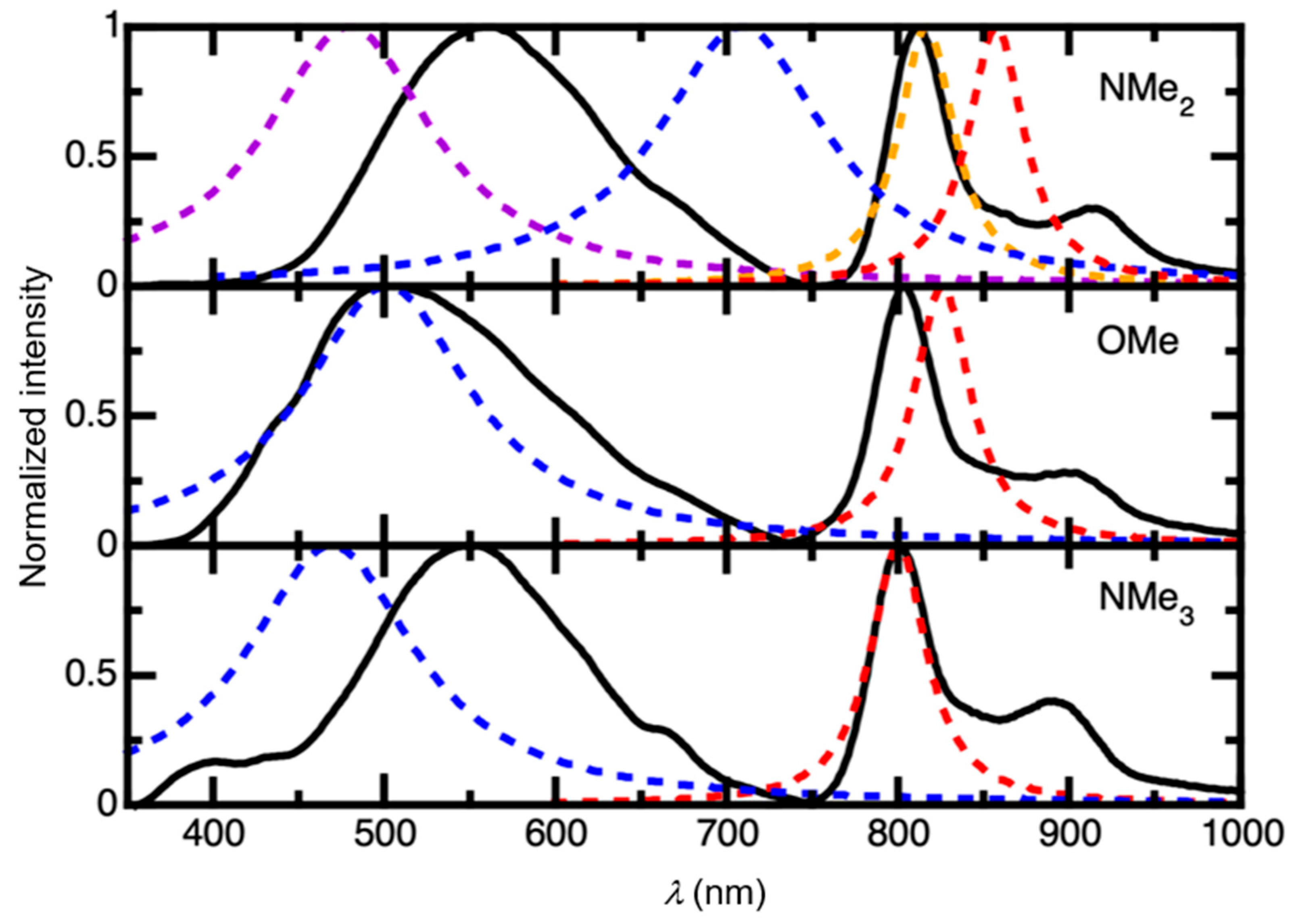
2.2. UV-Vis Absorption, Steady-State, and Time-Resolved Photoluminescence Spectroscopies
2.3. TD-DFT Calculations
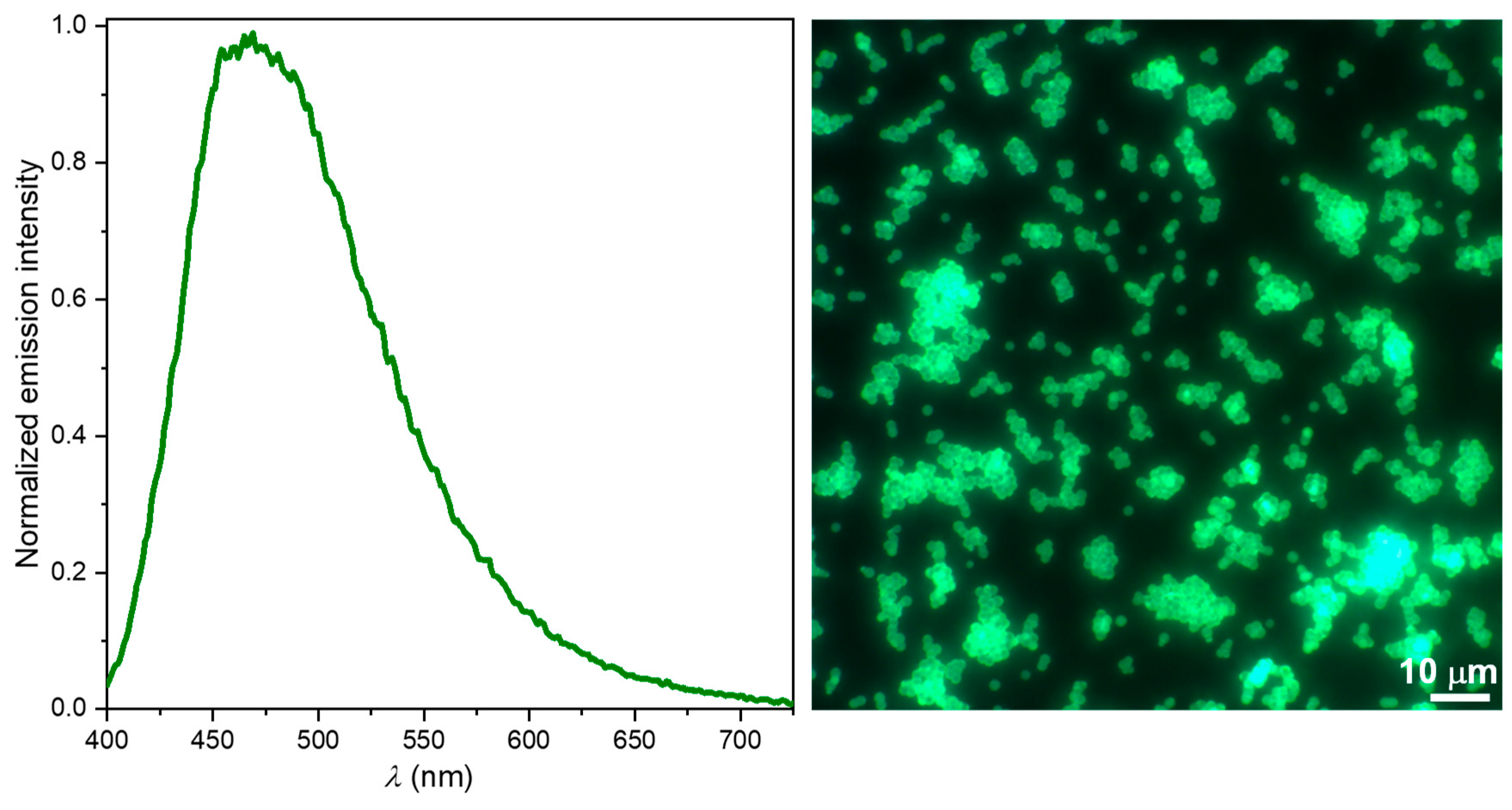
2.4. MSOT Imaging in Gel Phantoms
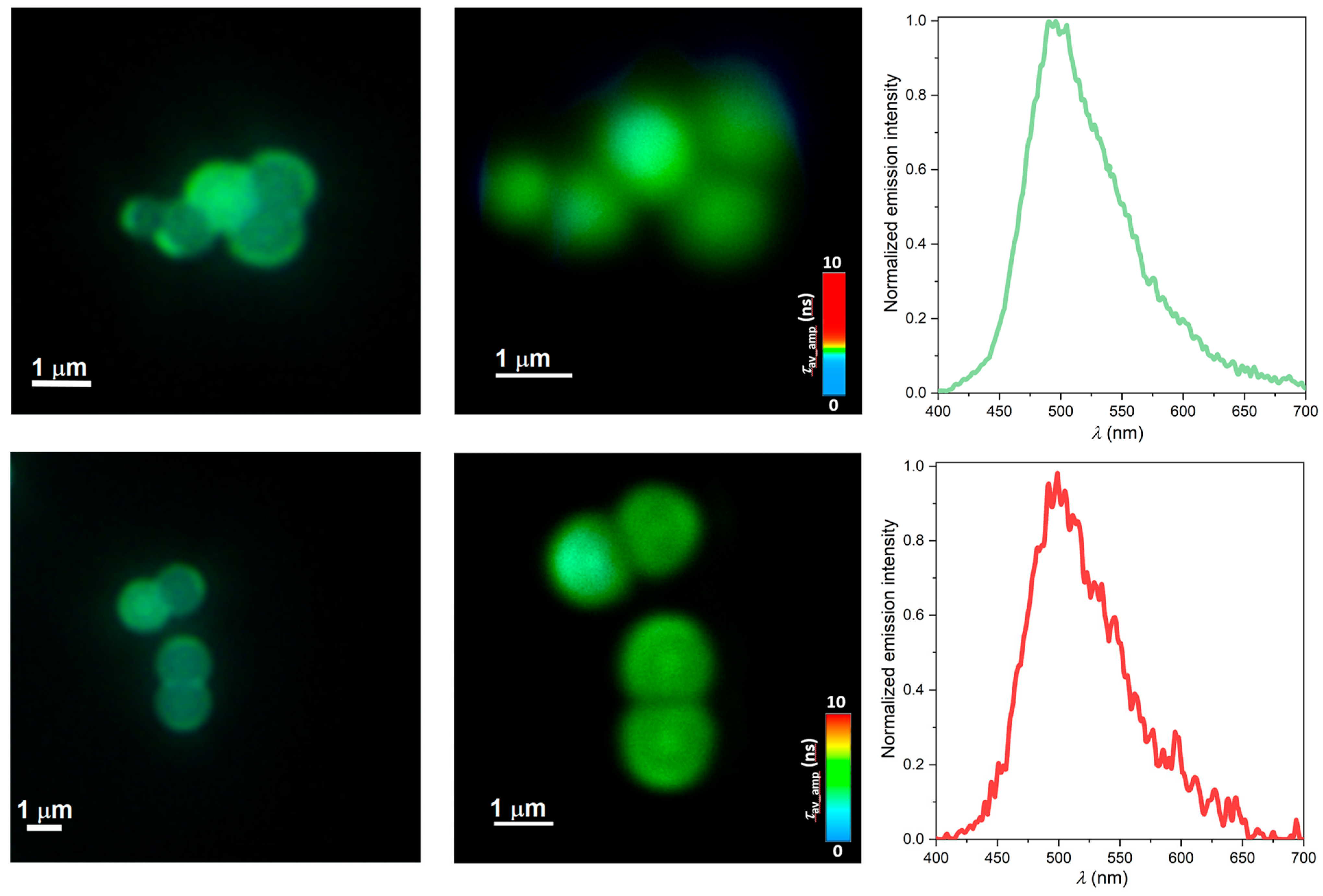
2.5. Time-Resolved Multiphoton Micro(spectro)scopy
3. Materials and Methods
3.1. Synthesis and Characterization of 6,7-Disubstituted Naphthalene-2,3-dicarbonitriles and Zinc(II) Naphthalocyanines
3.1.1. General Procedure for the Synthesis of 6,7-Disubstituted Naphthalene-2,3-dicarbonitriles
3.1.2. Synthesis of 6,7-Bis(4-methoxyphenyl)naphthalene-2,3-dicarbonitrile (OMe)
3.1.3. Synthesis of 6,7-Bis(4-(dimethylamino)phenyl)naphthalene-2,3-dicarbonitrile (NMe2)
3.1.4. General Procedure for the Synthesis of Zinc(II) Naphthalocyanines
3.1.5. Synthesis of 3,4,12,13,21,22,30,31-Octakis(4-methoxyphenyl) Naphthalocyaninato Zinc(II) (Zn-OMeNc)
3.1.6. Synthesis of 3,4,12,13,21,22,30,31-Octakis(4-(N,N-dimethylamino)phenyl) Naphthalocyaninato Zinc(II) (Zn-NMe2Nc)
3.1.7. Synthesis of 3,4,12,13,21,22,30,31-Octakis(4-(N,N,N-trimethylammonium)phenyl) naphthalocyaninato Zinc(II) Octaiodide (Zn-NMe3Nc)
3.1.8. ZnNMe2Nc Loaded onto Polystyrene Microparticles (PSMPs)
3.2. NMR Spectroscopy and Mass Spectrometry
3.3. Absorption and Fluorescence Spectroscopy
3.4. Computational Details
3.5. MSOT Photoacoustic Imaging
3.6. Fluorescence Lifetime Imaging Microscopy (FLIM) by Time-Resolved Multiphoton Micro(spectro)scopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kukhta, N.A.; Bryce, M.R. Dual Emission in Purely Organic Materials for Optoelectronic Applications. Mater. Horiz. 2021, 8, 33–55. [Google Scholar] [CrossRef] [PubMed]
- Sung, R.; Sung, K. TICT Excited States of o- and p-N,N-Dimethylamino Analogues of GFP Chromophore. J. Phys. Org. Chem. 2018, 31, e3791. [Google Scholar] [CrossRef]
- Lippert, E.; Lüder, W.; Moll, F.; Nägele, W.; Boos, H.; Prigge, H.; Seibold-Blankenstein, I. Umwandlung von Elektronenanregungsenergie. Angew. Chem. 1961, 73, 695–706. [Google Scholar] [CrossRef]
- Atsbeha, T.; Mohammed, A.M.; Redi-Abshiro, M. Excitation Wavelength Dependence of Dual Fluorescence of DMABN in Polar Solvents. J. Fluoresc. 2010, 20, 1241–1248. [Google Scholar] [CrossRef]
- Chou, P.-T.; Pu, S.-C.; Cheng, Y.-M.; Yu, W.-S.; Yu, Y.-C.; Hung, F.-T.; Hu, W.-P. Femtosecond Dynamics on Excited-State Proton/Charge-Transfer Reaction in 4’-N,N-Diethylamino-3-Hydroxyflavone. The Role of Dipolar Vectors in Constructing a Rational Mechanism. J. Phys. Chem. A 2005, 109, 3777–3787. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-H.; Chen, Z.-B.; Jiang, Y.-B. Intramolecular Charge Transfer with 4-(N-Phenylamino)Benzoic Acid. The N-Phenyl Amino Conjugation Effect. Chem. Phys. Lett. 2003, 372, 104–113. [Google Scholar] [CrossRef]
- Murali, S.; Rettig, W. TICT Formation in Para- and Meta-Derivatives of N-Phenylpyrrole. J. Phys. Chem. A 2006, 110, 28–37. [Google Scholar] [CrossRef]
- Pereira, R.V.; Garcia Ferreira, A.P.; Gehlen, M.H. Excited-State Intramolecular Charge Transfer in 9-Aminoacridine Derivative. J. Phys. Chem. A 2005, 109, 5978–5983. [Google Scholar] [CrossRef]
- Bag, S.S.; Kundu, R. Sensing of Micellar Microenvironment with Dual Fluorescent Probe, Triazolylpyrene (TNDMBPy). J. Fluoresc. 2013, 23, 929–938. [Google Scholar] [CrossRef]
- Etaiw, S.E.-D.H.; Fayed, T.A.; Saleh, N.Z. Photophysics of Benzazole Derived Push–Pull Butadienes: A Highly Sensitive Fluorescence Probes. J. Photochem. Photobiol. A Chem. 2006, 177, 238–247. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Tsai, H.-Y. Synthesis, X-Ray Structure, Spectroscopic Properties and DFT Studies of a Novel Schiff Base. Int. J. Mol. Sci. 2014, 15, 18706–18724. [Google Scholar] [CrossRef]
- Gómez, I.; Mercier, Y.; Reguero, M. Theoretical Investigation of Luminescence Behavior as a Function of Alkyl Chain Size in 4-Aminobenzonitrile Alicyclic Derivatives. J. Phys. Chem. A 2006, 110, 11455–11461. [Google Scholar] [CrossRef]
- Lippert, E.; Lüder, W.; Boos, H. Advances in Molecular Spectroscopy. In Proceedings of the European Conference on Molecular Spectroscopy, Bologna, Italy, 1959; Macmillan: New York, NY, USA, 1962; Volume 2. [Google Scholar]
- Bosshard, C. Organic Nonlinear Optical Materials; CRC Press: Boca Raton, FL, USA, 2020; ISBN 0429114095. [Google Scholar]
- Kippelen, B.; Lackritz, H.S.; Claus, R.O. Organic Nonlinear Optical Materials and Devices: Symposium Held 6–9 April 1999, San Francisco, CA, USA; Materials Research Society: Warrendale, PA, USA, 1999; ISBN 1558994688. [Google Scholar]
- Zhang, Y.; Lovell, J.F. Recent Applications of Phthalocyanines and Naphthalocyanines for Imaging and Therapy. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1420. [Google Scholar] [CrossRef]
- Santos, K.L.M.; Barros, R.M.; da Silva Lima, D.P.; Nunes, A.M.A.; Sato, M.R.; Faccio, R.; de Lima Damasceno, B.P.G.; Oshiro-Junior, J.A. Prospective Application of Phthalocyanines in the Photodynamic Therapy against Microorganisms and Tumor Cells: A Mini-Review. Photodiagn. Photodyn. Ther. 2020, 32, 102032. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Calvete, M.J.F. Phthalocyanines: An Old Dog Can Still Have New (Photo)Tricks! Molecules 2021, 26, 2823. [Google Scholar] [CrossRef] [PubMed]
- Dubinina, T.V.; Moiseeva, E.O.; Astvatsaturov, D.A.; Borisova, N.E.; Tarakanov, P.A.; Trashin, S.A.; De Wael, K.; Tomilova, L.G. Novel 2-Naphthyl Substituted Zinc Naphthalocyanine: Synthesis, Optical, Electrochemical and Spectroelectrochemical Properties. New J. Chem. 2020, 44, 7849–7857. [Google Scholar] [CrossRef]
- Leem, D.-S.; Lee, K.-H.; Li, N.; Park, B.W.; Choi, T.; Ro, T.; Kwon, O.K.; Kwon, Y.-N.; Ng, T.N.; Kim, S. Highly Responsive and Thermally Reliable Near-Infrared Organic Photodiodes Utilizing Naphthalocyanine Molecules Tuned with Axial Ligands. Adv. Opt. Mater. 2021, 9, 2001682. [Google Scholar] [CrossRef]
- Taratula, O.; Doddapaneni, B.S.; Schumann, C.; Li, X.; Bracha, S.; Milovancev, M.; Alani, A.W.G.; Taratula, O. Naphthalocyanine-Based Biodegradable Polymeric Nanoparticles for Image-Guided Combinatorial Phototherapy. Chem. Mater. 2015, 27, 6155–6165. [Google Scholar] [CrossRef]
- Huang, H.; Wang, D.; Zhang, Y.; Zhou, Y.; Geng, J.; Chitgupi, U.; Cook, T.R.; Xia, J.; Lovell, J.F. Axial PEGylation of Tin Octabutoxy Naphthalocyanine Extends Blood Circulation for Photoacoustic Vascular Imaging. Bioconjug. Chem. 2016, 27, 1574–1578. [Google Scholar] [CrossRef]
- Dubinina, T.V.; Trashin, S.A.; Borisova, N.E.; Boginskaya, I.A.; Tomilova, L.G.; Zefirov, N.S. Phenyl-Substituted Planar Binuclear Phthalo- and Naphthalocyanines: Synthesis and Investigation of Physicochemical Properties. Dye. Pigment. 2012, 93, 1471–1480. [Google Scholar] [CrossRef]
- Dubinina, T.V.; Kosov, A.D.; Petrusevich, E.F.; Borisova, N.E.; Trigub, A.L.; Mamin, G.V.; Gilmutdinov, I.F.; Masitov, A.A.; Tokarev, S.V.; Pushkarev, V.E.; et al. Sandwich Double-Decker Er(III) and Yb(III) Complexes Containing Naphthalocyanine Moiety: Synthesis and Investigation of the Effect of a Paramagnetic Metal Center. Dalton Trans. 2019, 48, 13413–13422. [Google Scholar] [CrossRef]
- Dubinina, T.V.; Tychinsky, P.I.; Borisova, N.E.; Maklakov, S.S.; Sedova, M.V.; Kosov, A.D.; Tomilova, L.G.; Zefirov, N.S. Zinc Complexes of 3-(Ethylthio)Phenyl-Substituted Phthalocyanines and Naphthalocyanine: Synthesis and Investigation of Physicochemical Properties. Dye. Pigment. 2017, 144, 41–47. [Google Scholar] [CrossRef]
- Dubinina, T.V.; Paramonova, K.V.; Trashin, S.A.; Borisova, N.E.; Tomilova, L.G.; Zefirov, N.S. Novel Near-IR Absorbing Phenyl-Substituted Phthalo- and Naphthalocyanine Complexes of Lanthanide(III): Synthesis and Spectral and Electrochemical Properties. Dalton Trans. 2014, 43, 2799–2809. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.A.; Pawle, R.; Selfridge, S.; Contreras, L.; Xavierselvan, M.; Nguyen, C.D.; Mallidi, S.; Hasan, T. Optimizing Axial and Peripheral Substitutions in Si-Centered Naphthalocyanine Dyes for Enhancing Aqueous Solubility and Photoacoustic Signal Intensity. Int. J. Mol. Sci. 2023, 24, 2241. [Google Scholar] [CrossRef] [PubMed]
- Vagin, S.; Hanack, M. Synthesis and Characterization of Soluble Octaaryl- and Octaaryloxy-Substituted Metal-Naphthalocyanines. Eur. J. Org. Chem. 2003, 2003, 2661–2669. [Google Scholar] [CrossRef]
- Luan, L.; Ding, L.; Zhang, W.; Shi, J.; Yu, X.; Liu, W. A Naphthalocyanine Based Near-Infrared Photosensitizer: Synthesis and in vitro Photodynamic Activities. Bioorg. Med. Chem. Lett. 2013, 23, 3775–3779. [Google Scholar] [CrossRef] [PubMed]
- Mitra, K.; Hartman, M.C.T. Silicon Phthalocyanines: Synthesis and Resurgent Applications. Org. Biomol. Chem. 2021, 19, 1168–1190. [Google Scholar] [CrossRef]
- Kakade, S.; Ghosh, R.; Palit, D.K. Excited State Dynamics of Zinc–Phthalocyanine Nanoaggregates in Strong Hydrogen Bonding Solvents. J. Phys. Chem. C 2012, 116, 15155–15166. [Google Scholar] [CrossRef]
- Safonova, E.A.; Polovkova, M.A.; Martynov, A.G.; Gorbunova, Y.G.; Tsivadze, A.Y. Crown-Substituted Naphthalocyanines: Synthesis and Supramolecular Control over Aggregation and Photophysical Properties. Dalton Trans. 2018, 47, 15226–15231. [Google Scholar] [CrossRef]
- de Souza, T.; Antonio, F.; Zanotto, M.; Homem-de-Mello, P.; Ribeiro, A. Photophysical and Photochemical Properties and Aggregation Behavior of Phthalocyanine and Naphthalocyanine Derivatives. J. Braz. Chem. Soc. 2017, 29, 1199–1209. [Google Scholar] [CrossRef]
- Dubinina, T.V.; Maklakov, S.S.; Petrusevich, E.F.; Borisova, N.E.; Trashin, S.A.; De Wael, K.; Tomilova, L.G. Photoactive Layers for Photovoltaics Based on Near-Infrared Absorbing Aryl-Substituted Naphthalocyanine Complexes: Preparation and Investigation of Properties. New J. Chem. 2021, 45, 14815–14821. [Google Scholar] [CrossRef]
- Dubinina, T.V.; Borisova, N.E.; Paramonova, K.V.; Tomilova, L.G. Synthesis and Spectral Properties of Dodecaphenyl-Substituted Planar Binuclear Naphthalocyanine Magnesium Complex Sharing a Common Benzene Ring. Mendeleev Commun. 2011, 21, 165–167. [Google Scholar] [CrossRef]
- Keleş, T.; Barut, B.; Özel, A.; Biyiklioglu, Z. Design, Synthesis and Biological Evaluation of Water Soluble and Non-Aggregated Silicon Phthalocyanines, Naphthalocyanines against A549, SNU-398, SK-MEL128, DU-145, BT-20 and HFC Cell Lines as Potential Anticancer Agents. Bioorg. Chem. 2021, 107, 104637. [Google Scholar] [CrossRef] [PubMed]
- Biyiklioglu, Z.; Alp, H. Synthesis and Electropolymerization Studies of Non-Aggregated (4-{3-[3-(Dimethylamino,diethylamino)phenoxy]propoxy}phenyl)propanoxy Substituted Silicon Naphthalocyanines. J. Coord. Chem. 2017, 70, 2359–2370. [Google Scholar] [CrossRef]
- Wheeler, B.L.; Nagasubramanian, G.; Bard, A.J.; Schechtman, L.A.; Kenney, M.E. A Silicon Phthalocyanine and a Silicon Naphthalocyanine: Synthesis, Electrochemistry, and Electrogenerated Chemiluminescence. J. Am. Chem. Soc. 1984, 106, 7404–7410. [Google Scholar] [CrossRef]
- Sato, K.; Ando, K.; Okuyama, S.; Moriguchi, S.; Ogura, T.; Totoki, S.; Hanaoka, H.; Nagaya, T.; Kokawa, R.; Takakura, H.; et al. Photoinduced Ligand Release from a Silicon Phthalocyanine Dye Conjugated with Monoclonal Antibodies: A Mechanism of Cancer Cell Cytotoxicity after Near-Infrared Photoimmunotherapy. ACS Cent. Sci. 2018, 4, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Choi, W.; Kim, J.; Kong, W.H.; Kim, K.S.; Kim, C.; Hahn, S.K. Multifunctional Nanodroplets Encapsulating Naphthalocyanine and Perfluorohexane for Bimodal Image-Guided Therapy. Biomacromolecules 2019, 20, 3767–3777. [Google Scholar] [CrossRef] [PubMed]
- Marchenko, I.V.; Borodina, T.N.; Trushina, D.B.; Nabatov, B.V.; Logachev, V.V.; Plotnikov, G.S.; Baranov, A.N.; Saletskii, A.M.; Ryabova, A.V.; Bukreeva, T.V. Incorporation of Naphthalocyanine into Shells of Polyelectrolyte Capsules and Their Disruption under Laser Radiation. Colloid J. 2018, 80, 399–406. [Google Scholar] [CrossRef]
- Li, H.; Jensen, T.J.; Fronczek, F.R.; Vicente, M.G.H. Syntheses and Properties of a Series of Cationic Water-Soluble Phthalocyanines. J. Med. Chem. 2008, 51, 502–511. [Google Scholar] [CrossRef]
- Huang, J.-D.; Wang, S.; Lo, P.-C.; Fong, W.-P.; Ko, W.-H.; Ng, D.K.P. Halogenated Silicon(IV) Phthalocyanines with Axial Poly(Ethylene Glycol) Chains. Synthesis, Spectroscopic Properties, Complexation with Bovine Serum Albumin and in vitro Photodynamic Activities. New J. Chem. 2004, 28, 348–354. [Google Scholar] [CrossRef]
- Lo, P.-C.; Huang, J.-D.; Cheng, D.Y.Y.; Chan, E.Y.M.; Fong, W.-P.; Ko, W.-H.; Ng, D.K.P. New Amphiphilic Silicon(IV) Phthalocyanines as Efficient Photosensitizers for Photodynamic Therapy: Synthesis, Photophysical Properties, and in vitro Photodynamic Activities. Chem.—A Eur. J. 2004, 10, 4831–4838. [Google Scholar] [CrossRef]
- Lee, P.P.S.; Lo, P.-C.; Chan, E.Y.M.; Fong, W.-P.; Ko, W.-H.; Ng, D.K.P. Synthesis and in vitro Photodynamic Activity of Novel Galactose-Containing Phthalocyanines. Tetrahedron Lett. 2005, 46, 1551–1554. [Google Scholar] [CrossRef]
- Huang, J.-D.; Lo, P.-C.; Chen, Y.-M.; Lai, J.C.; Fong, W.-P.; Ng, D.K.P. Preparation and in vitro Photodynamic Activity of Novel Silicon(IV) Phthalocyanines Conjugated to Serum Albumins. J. Inorg. Biochem. 2006, 100, 946–951. [Google Scholar] [CrossRef]
- Liu, W.; Jensen, T.J.; Fronczek, F.R.; Hammer, R.P.; Smith, K.M.; Vicente, M.G.H. Synthesis and Cellular Studies of Nonaggregated Water-Soluble Phthalocyanines. J. Med. Chem. 2005, 48, 1033–1041. [Google Scholar] [CrossRef]
- Yang, Y.C.; Ward, J.R.; Seiders, R.P. Dimerization of Cobalt(II) Tetrasulfonated Phthalocyanine in Water and Aqueous Alcoholic Solutions. Inorg. Chem. 1985, 24, 1765–1769. [Google Scholar] [CrossRef]
- Oleinick, N.L.; Antunez, A.R.; Clay, M.E.; Rihter, B.D.; Kenney, M.E. New Phthalocyanine Photosensitizers for Photodynamic Therapy. Photochem. Photobiol. 1993, 57, 242–247. [Google Scholar] [CrossRef]
- Li, Y.-S.; Zaidi, S.I.A.; Rodgers, M.A.J.; Mukhtar, H.; Kenney, M.E.; Oleinick, N.L.; He, J.; Larkin, H.E.; Rihter, B.D. The Synthesis, Photophysical and Photobiological Properties and in vitro Structure-Activity Relationships of a Set of Silicon Phthalocyanine PDT Photosensitizers. Photochem. Photobiol. 1997, 65, 581–586. [Google Scholar] [CrossRef]
- Lee, P.P.S.; Ngai, T.; Huang, J.-D.; Wu, C.; Fong, W.-P.; Ng, D.K.P. Synthesis, Characterization, Biodegradation, and in vitro Photodynamic Activities of Silicon(IV) Phthalocyanines Conjugated Axially with Poly(ε-Caprolactone). Macromolecules 2003, 36, 7527–7533. [Google Scholar] [CrossRef]
- Lee, P.P.S.; Ngai, T.; Yang, C.; Wu, C.; Ng, D.K.P. Synthesis, Characterization, and Degradation of Silicon(IV) Phthalocyanines Conjugated Axially with Poly(sebacic anhydride). J. Polym. Sci. Part A Polym. Chem. 2005, 43, 837–843. [Google Scholar] [CrossRef]
- Zhu, Y.-J.; Huang, J.-D.; Jiang, X.-J.; Sun, J.-C. Novel Silicon Phthalocyanines Axially Modified by Morpholine: Synthesis, Complexation with Serum Protein and in vitro Photodynamic Activity. Inorg. Chem. Commun. 2006, 9, 473–477. [Google Scholar] [CrossRef]
- Wöhrle, D.; Iskander, N.; Graschew, G.; Sinn, H.; Friedrich, E.A.; Maier-Borst, W.; Stern, J.; Schlag, P. Synthesis of Positively Charged Phthalocyanines and Their Activity in the Photodynamic Therapy of Cancer Cells. Photochem. Photobiol. 1990, 51, 351–356. [Google Scholar] [CrossRef]
- Michelsen, U.; Schnurpfeil, G.; Sobbi, A.K.; Wöhrle, D.; Kliesch, H. Unsymmetrically Substituted Benzonaphthoporphyrazines: A New Class of Cationic Photosensitizers for the Photodynamic Therapy of Cancer*. Photochem. Photobiol. 1996, 64, 694–701. [Google Scholar] [CrossRef]
- Sobbi, A.K.; Wöhrle, D.; Schlettwein, D. Photochemical Stability of Various Porphyrins in Solution and as Thin Film Electrodes. J. Chem. Soc. Perkin Trans. 1993, 2, 481–488. [Google Scholar] [CrossRef]
- Brewis, M.; Helliwell, M.; McKeown, N.B. Phthalocyanine-Centred and Naphthalocyanine-Centred Aryl Ether Dendrimers with Oligo(Ethyleneoxy) Surface Groups. Tetrahedron 2003, 59, 3863–3872. [Google Scholar] [CrossRef]
- Lioret, V.; Saou, S.; Berrou, A.; Lernerman, L.; Arnould, C.; Decréau, R.A. Water Soluble Octa-Imidazolium Zinc Phthalocyanine for Nucleus/Nucleolus Cell Fluorescence Microscopy and Photodynamic Therapy. Photochem. Photobiol. Sci. 2023, 22, 303–309. [Google Scholar] [CrossRef]
- Iqbal, Z.; Lyubimtsev, A.; Hanack, M.; Ziegler, T. Anomerically Glycosylated Zinc(II) Naphthalocyanines. Tetrahedron Lett. 2009, 50, 5681–5685. [Google Scholar] [CrossRef]
- Thulaseedharan Nair Sailaja, S.; Maisuls, I.; Kösters, J.; Hepp, A.; Faust, A.; Voskuhl, J.; Strassert, C.A. Naphthalonitriles Featuring Efficient Emission in Solution and in the Solid State. Beilstein J. Org. Chem. 2020, 16, 2960–2970. [Google Scholar] [CrossRef]
- Esenpınar, A.A.; Bulut, M. Synthesis and Characterization of Novel α- or β-Tetra[6,7-dihexyloxy-3-(4-oxyphenyl)coumarin]-Substituted Metal-Free and Metallo Phthalocyanines. Polyhedron 2009, 28, 3129–3137. [Google Scholar] [CrossRef]
- Bıyıklıoğlu, Z.; Kantekin, H. Synthesis and Spectroscopic Properties of a Series of Octacationic Water-Soluble Phthalocyanines. Synth. Met. 2011, 161, 943–948. [Google Scholar] [CrossRef]
- Ghazal, B.; Azizi, K.; Ewies, E.F.; Youssef, A.S.A.; Mwalukuku, V.M.; Demadrille, R.; Torres, T.; Makhseed, S. Push–Pull Zinc Phthalocyanine Bearing Hexa-Tertiary Substituted Carbazolyl Donor Groups for Dye-Sensitized Solar Cells. Molecules 2020, 25, 1692. [Google Scholar] [CrossRef] [PubMed]
- Ince, M.; Cardinali, F.; Yum, J.-H.; Martínez-Díaz, M.V.; Nazeeruddin, M.K.; Grätzel, M.; Torres, T. Convergent Synthesis of Near-Infrared Absorbing, “Push–Pull”, Bisthiophene-Substituted, Zinc(II) Phthalocyanines and Their Application in Dye-Sensitized Solar Cells. Chem.—A Eur. J. 2012, 18, 6343–6348. [Google Scholar] [CrossRef]
- Kazak, A.V.; Marchenkova, M.A.; Dubinina, T.V.; Smirnova, A.I.; Tomilova, L.G.; Rogachev, A.V.; Chausov, D.N.; Stsiapanau, A.A.; Usol’tseva, N.V. Self-Organization of Octa-Phenyl-2,3-Naphthalocyaninato Zinc Floating Layers. New J. Chem. 2020, 44, 3833–3837. [Google Scholar] [CrossRef]
- Kutlu, Ö.D.; Avcil, D.; Erdoğmuş, A. New Water-Soluble Cationic Indium (III) Phthalocyanine Bearing Thioquinoline Moiety; Synthesis, Photophysical and Photochemical Studies with High Singlet Oxygen Yield. Main Group Chem. 2019, 18, 139–151. [Google Scholar] [CrossRef]
- Strassert, C.A.; Bilmes, G.M.; Awruch, J.; Dicelio, L.E. Comparative Photophysical Investigation of Oxygen and Sulfur as Covalent Linkers on Octaalkylamino Substituted Zinc(II) Phthalocyanines. Photochem. Photobiol. Sci. 2008, 7, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Bézière, N.; Ntziachristos, V. Optoacoustic Imaging of Naphthalocyanine: Potential for Contrast Enhancement and Therapy Monitoring. J. Nucl. Med. 2015, 56, 323–328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duffy, M.J.; Planas, O.; Faust, A.; Vogl, T.; Hermann, S.; Schäfers, M.; Nonell, S.; Strassert, C.A. Towards Optimized Naphthalocyanines as Sonochromes for Photoacoustic Imaging in vivo. Photoacoustics 2018, 9, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Gujrati, V.; Malekzadeh-Najafabadi, J.; Werner, J.P.F.; Klemm, U.; Tang, L.; Chen, Z.; Prakash, J.; Huang, Y.; Stiel, A.; et al. Croconaine-Based Nanoparticles Enable Efficient Optoacoustic Imaging of Murine Brain Tumors. Photoacoustics 2021, 22, 100263. [Google Scholar] [CrossRef] [PubMed]
- Biyiklioglu, Z.; Bas, H.; Alp, H. Non-Aggregated Axially Disubstituted Silicon Phthalocyanines Bearing Electropolymerizable Ligands and Their Aggregation, Electropolymerizaton and Thermal Properties. Dalton Trans. 2015, 44, 14054–14062. [Google Scholar] [CrossRef] [PubMed]
- Mbambisa, G.; Nyokong, T. Synthesis and Electrochemical Characterisation of a near Infrared Absorbing Oxo Vanadium(IV) Octapentylthio-Phthalocyanine. Polyhedron 2008, 27, 2799–2804. [Google Scholar] [CrossRef]
- Christie, R.M. Colour and Constitution Relationships in Organic Pigments. Part 5: The Influence of Solvents, the Central Metal Atom and Substituents on the Electronic Spectra of Phthalocyanines. Dye. Pigment. 1995, 27, 35–43. [Google Scholar] [CrossRef]
- Tian, M.; Zhang, Y.; Wada, T.; Sasabe, H. Syntheses of Novel Non-Aggregated Binuclear Phthalocyaninato Vanadyl Complexes Using a Palladium-Catalyzed Cross-Coupling Reaction. Dye. Pigment. 2003, 58, 135–143. [Google Scholar] [CrossRef]
- Lee, J.-H.; Gomez, I.J.; Sitterle, V.B.; Meredith, J.C. Dye-Labeled Polystyrene Latex Microspheres Prepared via a Combined Swelling-Diffusion Technique. J. Colloid Interface Sci. 2011, 363, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Gvozdev, D.A.; Solovchenko, A.E.; Martynov, A.G.; Yagodin, A.V.; Strakhovskaya, M.G.; Gorbunova, Y.G.; Maksimov, E.G. Fluorescence Quenching of Carboxy-Substituted Phthalocyanines Conjugated with Nanoparticles under High Stoichiometric Ratios. Photonics 2022, 9, 668. [Google Scholar] [CrossRef]
- Bush, P.G.; Wokosin, D.L.; Hall, A.C. Two-versus One Photon Excitation Laser Scanning Microscopy: Critical Importance of Excitation Wavelength. Front. Biosci. 2007, 12, 2646–2657. [Google Scholar] [CrossRef]
- Dubinina, T.V.; Ivanov, A.V.; Borisova, N.E.; Trashin, S.A.; Gurskiy, S.I.; Tomilova, L.G.; Zefirov, N.S. Synthesis and Investigation of Spectral and Electrochemical Properties of Alkyl-Substituted Planar Binuclear Phthalocyanine Complexes Sharing a Common Naphthalene Ring. Inorg. Chim. Acta 2010, 363, 1869–1878. [Google Scholar] [CrossRef]
- Song, C.J.; Jang, C.K.; Yao, W.; Jaung, J.Y. Synthesis and Spectral Characterisation of Dicyanopyrazine-Related Cyanoheterocycles. J. Chem. Res. 2013, 37, 268–272. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09, Revision, D. 01, Programme; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a Full Periodic Table Force Field for Molecular Mechanics and Molecular Dynamics Simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-Adjustedab Initio Pseudopotentials for the Second and Third Row Transition Elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Dunning, T.H.; Hay, P.J. Modern Theoretical Chemistry; Plenum: New York, NY, USA, 1977; Volume 3, pp. 1–28. [Google Scholar]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange–Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]


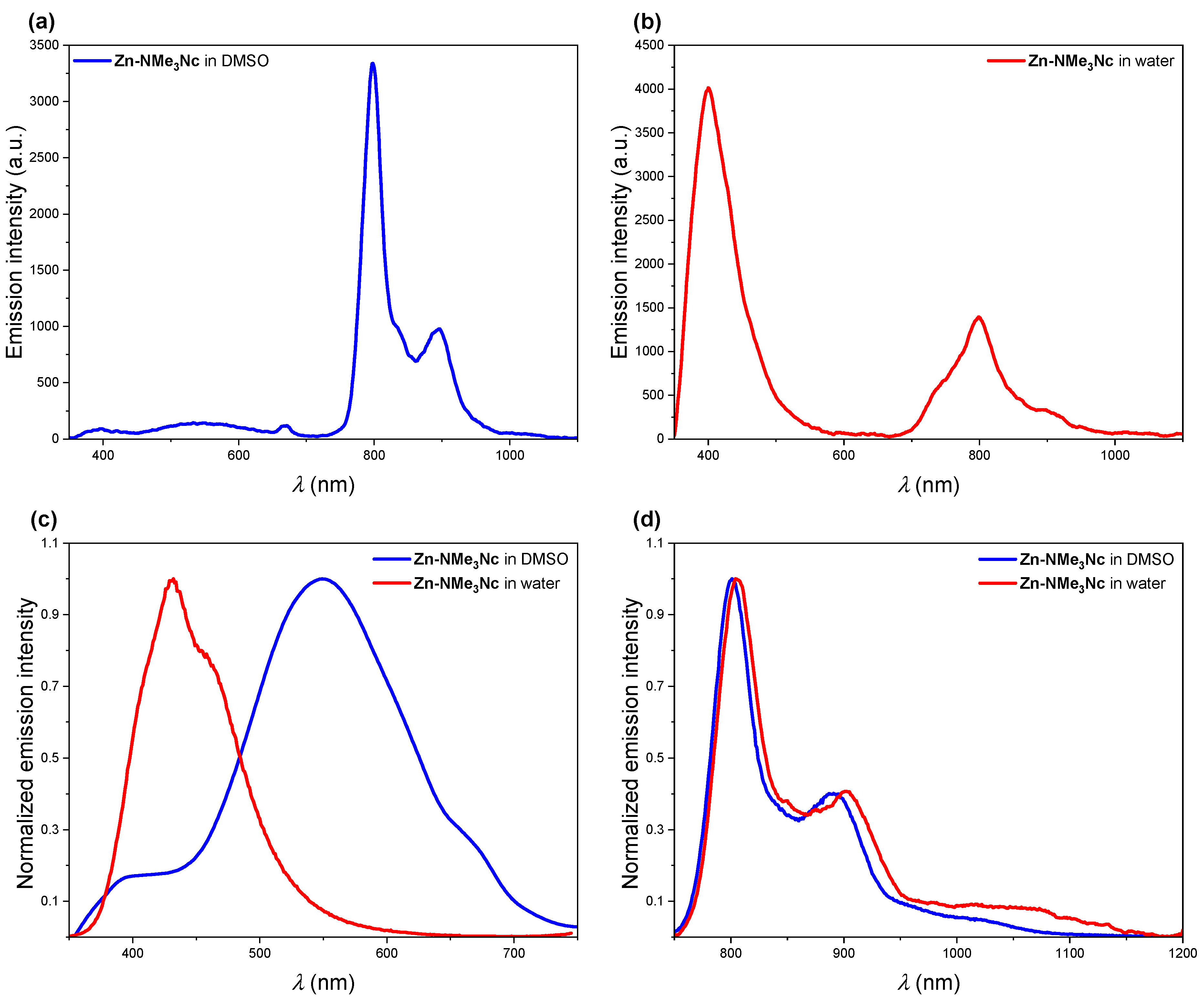
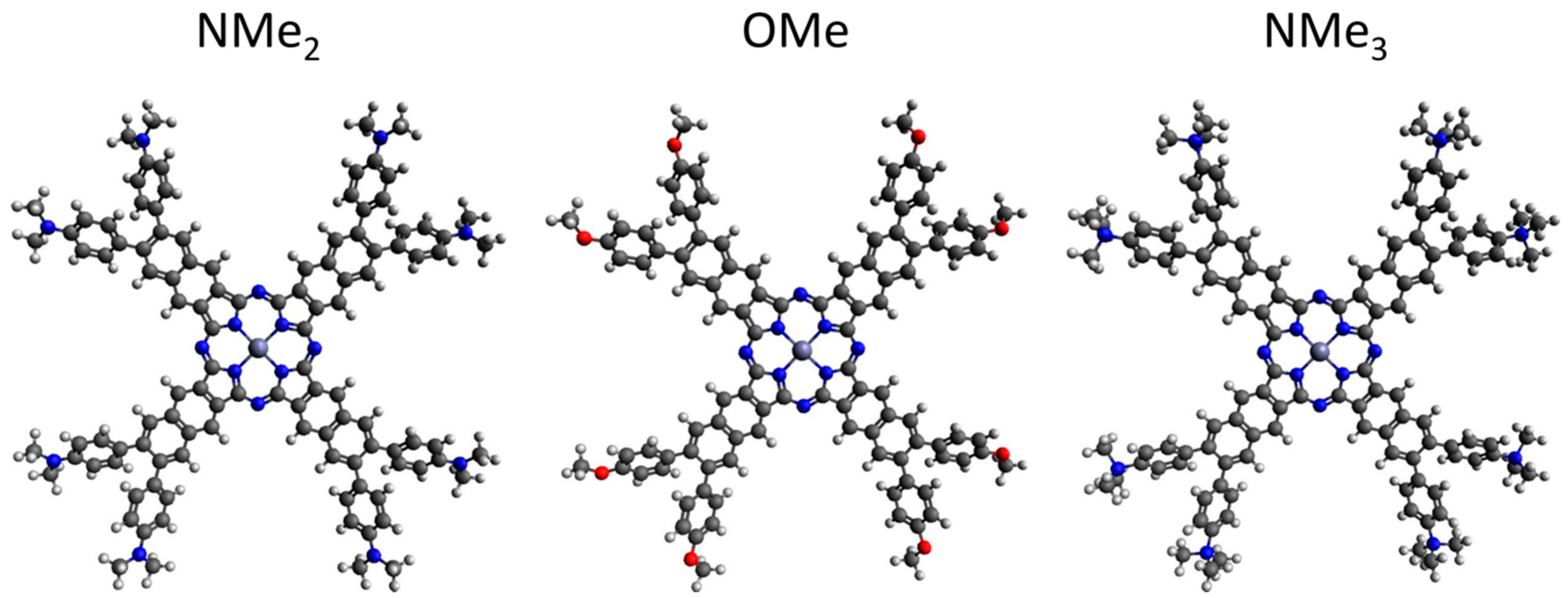
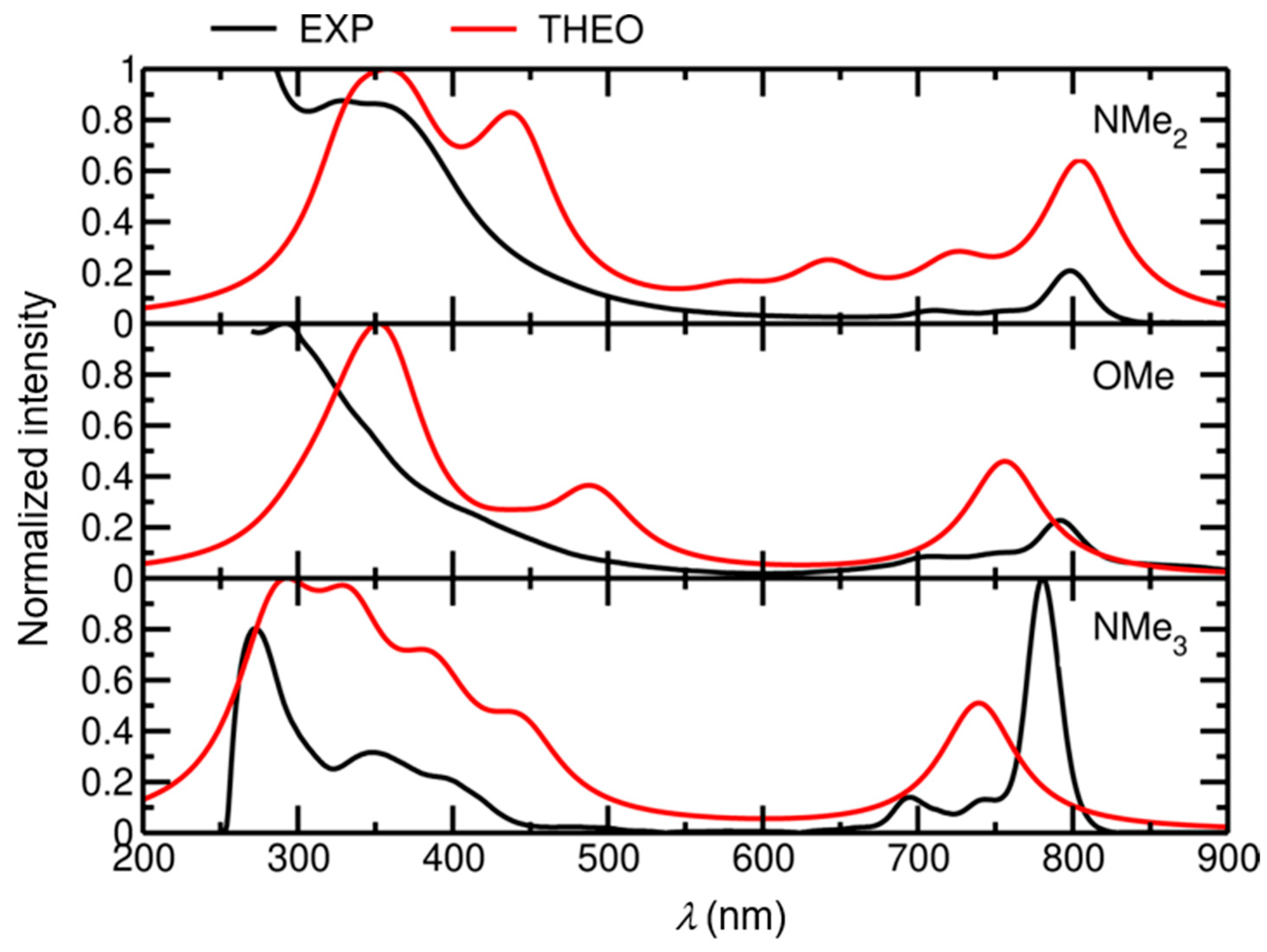
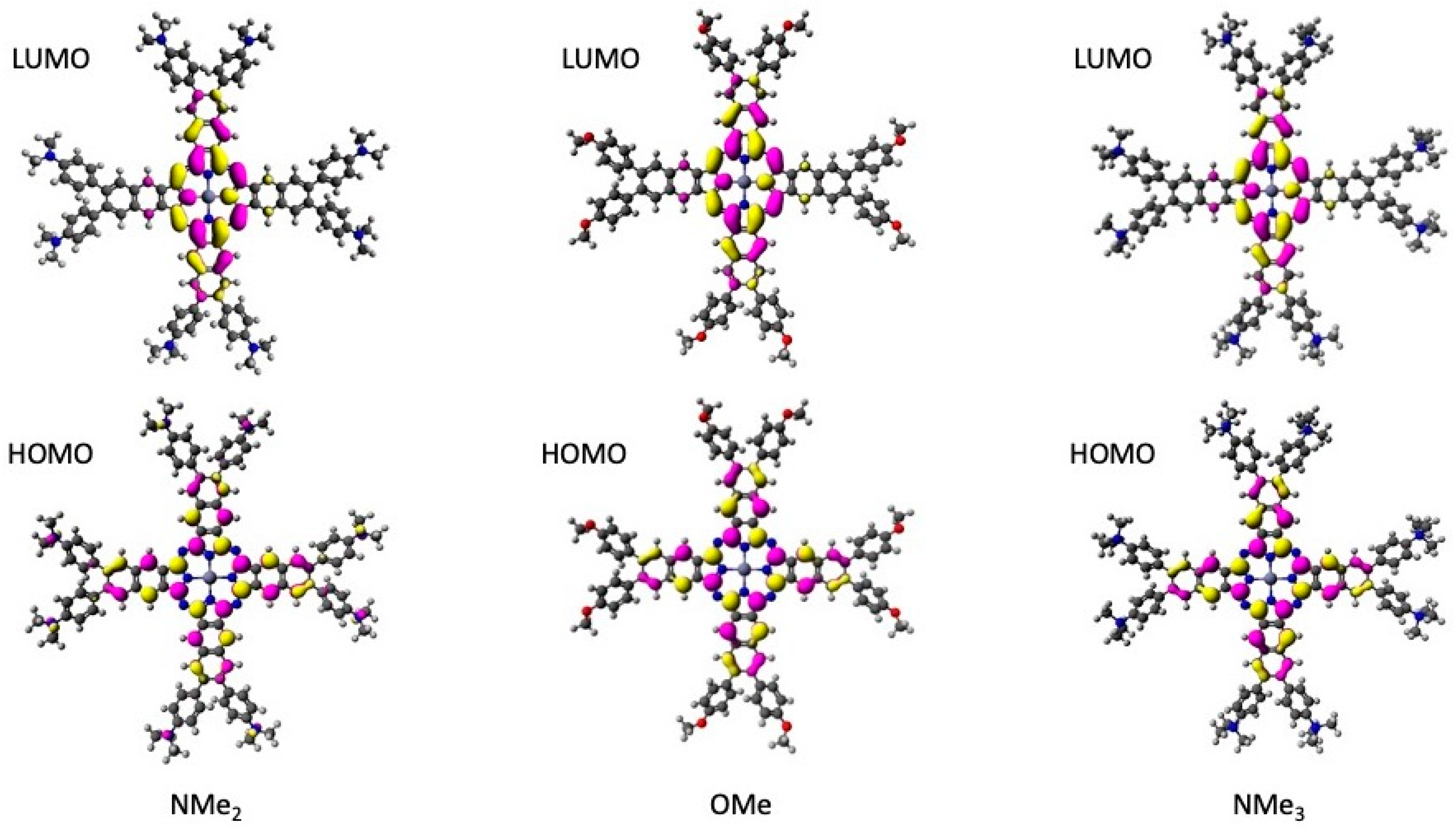



| Compound/ Solvent | Q-Band (λmax/nm) [ε/104 M−1 cm−1] | B-Band (λmax/nm) [ε/105 M−1 cm−1] |
|---|---|---|
| Zn-OMeNc/DMSO | 794 [4.4] | 293 [1.9] |
| Zn-NMe2Nc/DMSO | 800 [5.3] | 350 [2.0] |
| Zn-NMe3Nc/DMSO | 781 [55] | 350 [1.8] |
| Zn-NMe3Nc/H2O | 732, 784 [22], [18] | 340 [2.7] |
| Compound/ Solvent | λmax/nm P1/P2 | τ/ns [P1] | τ/ns [P2] |
|---|---|---|---|
| Zn-OMeNc/DMSO | 508/804 | τ1 = 8.2 ± 0.3 (21%) τ2 = 2.7 ± 0.1 (79%) τav_amp = 3.8 ± 0.1 | τ1 = 2.05 ± 0.03 (68%) τ2 = 1.0 ± 0.1 (32%) τav_amp = 1.7 ± 0.1 |
| Zn-NMe2Nc/DMSO | 560/811 | τ1 = 8.4 ± 0.2 (34%) τ2 = 2.3 ± 0.2 (61%) τav_amp = 4.6 ± 0.1 | τ1 = 1.80 ± 0.08 (21%) τ2 = 0.61 ± 0.03 (79%) τav_amp = 0.86 ± 0.04 |
| Zn-NMe3Nc/DMSO | 550/801 | τ1 = 9.1 ± 0.2 (55%) τ2 = 3.4 ± 0.4 (45%) τav_amp = 6.6 ± 0.2 | τ = 5.69 ± 0.02 |
| Zn-NMe3Nc/H2O | 432/804 | τ1 = 8.5 ± 0.4 (9%) τ2 = 5.2 ± 0.1 (91%) τav_amp = 5.51 ± 0.03 | τ1 = 5.43 ± 0.05 (28%) τ2 = 1.20 ± 0.08 (72%) τav_amp = 2.4 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sailaja, S.T.N.; Maisuls, I.; Hepp, A.; Brünink, D.; Doltsinis, N.L.; Faust, A.; Hermann, S.; Strassert, C.A. Dual Emissive Zn(II) Naphthalocyanines: Synthesis, Structural and Photophysical Characterization with Theory-Supported Insights towards Soluble Coordination Compounds with Visible and Near-Infrared Emission. Int. J. Mol. Sci. 2024, 25, 2605. https://doi.org/10.3390/ijms25052605
Sailaja STN, Maisuls I, Hepp A, Brünink D, Doltsinis NL, Faust A, Hermann S, Strassert CA. Dual Emissive Zn(II) Naphthalocyanines: Synthesis, Structural and Photophysical Characterization with Theory-Supported Insights towards Soluble Coordination Compounds with Visible and Near-Infrared Emission. International Journal of Molecular Sciences. 2024; 25(5):2605. https://doi.org/10.3390/ijms25052605
Chicago/Turabian StyleSailaja, Sidharth Thulaseedharan Nair, Iván Maisuls, Alexander Hepp, Dana Brünink, Nikos L. Doltsinis, Andreas Faust, Sven Hermann, and Cristian A. Strassert. 2024. "Dual Emissive Zn(II) Naphthalocyanines: Synthesis, Structural and Photophysical Characterization with Theory-Supported Insights towards Soluble Coordination Compounds with Visible and Near-Infrared Emission" International Journal of Molecular Sciences 25, no. 5: 2605. https://doi.org/10.3390/ijms25052605
APA StyleSailaja, S. T. N., Maisuls, I., Hepp, A., Brünink, D., Doltsinis, N. L., Faust, A., Hermann, S., & Strassert, C. A. (2024). Dual Emissive Zn(II) Naphthalocyanines: Synthesis, Structural and Photophysical Characterization with Theory-Supported Insights towards Soluble Coordination Compounds with Visible and Near-Infrared Emission. International Journal of Molecular Sciences, 25(5), 2605. https://doi.org/10.3390/ijms25052605