Gengricin®: A Nutraceutical Formulation for Appetite Control and Therapeutic Weight Management in Adults Who Are Overweight/Obese
Abstract
1. Introduction
2. Results
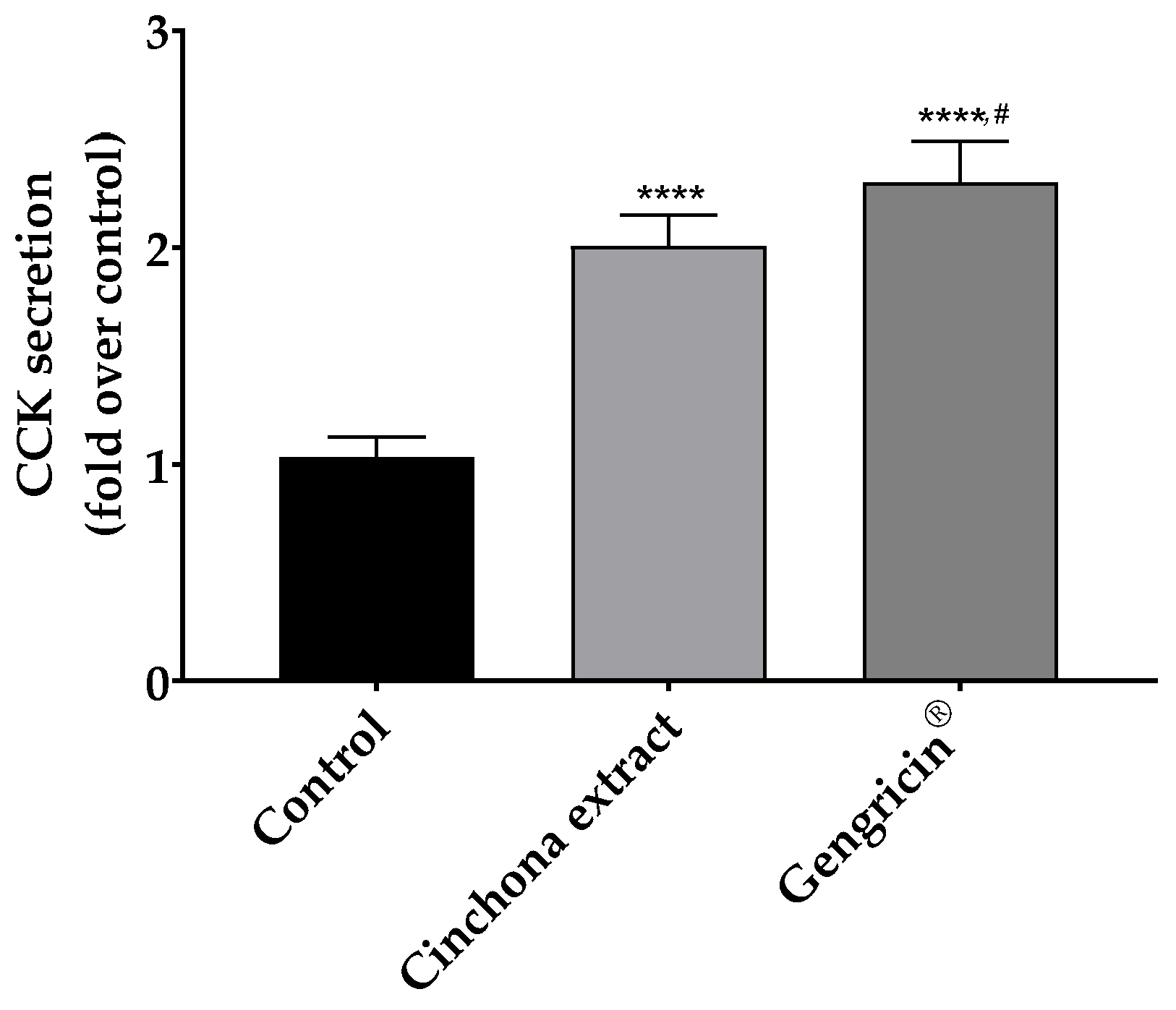
2.1. Cholecystokinin Release in CaCo-2 Cells
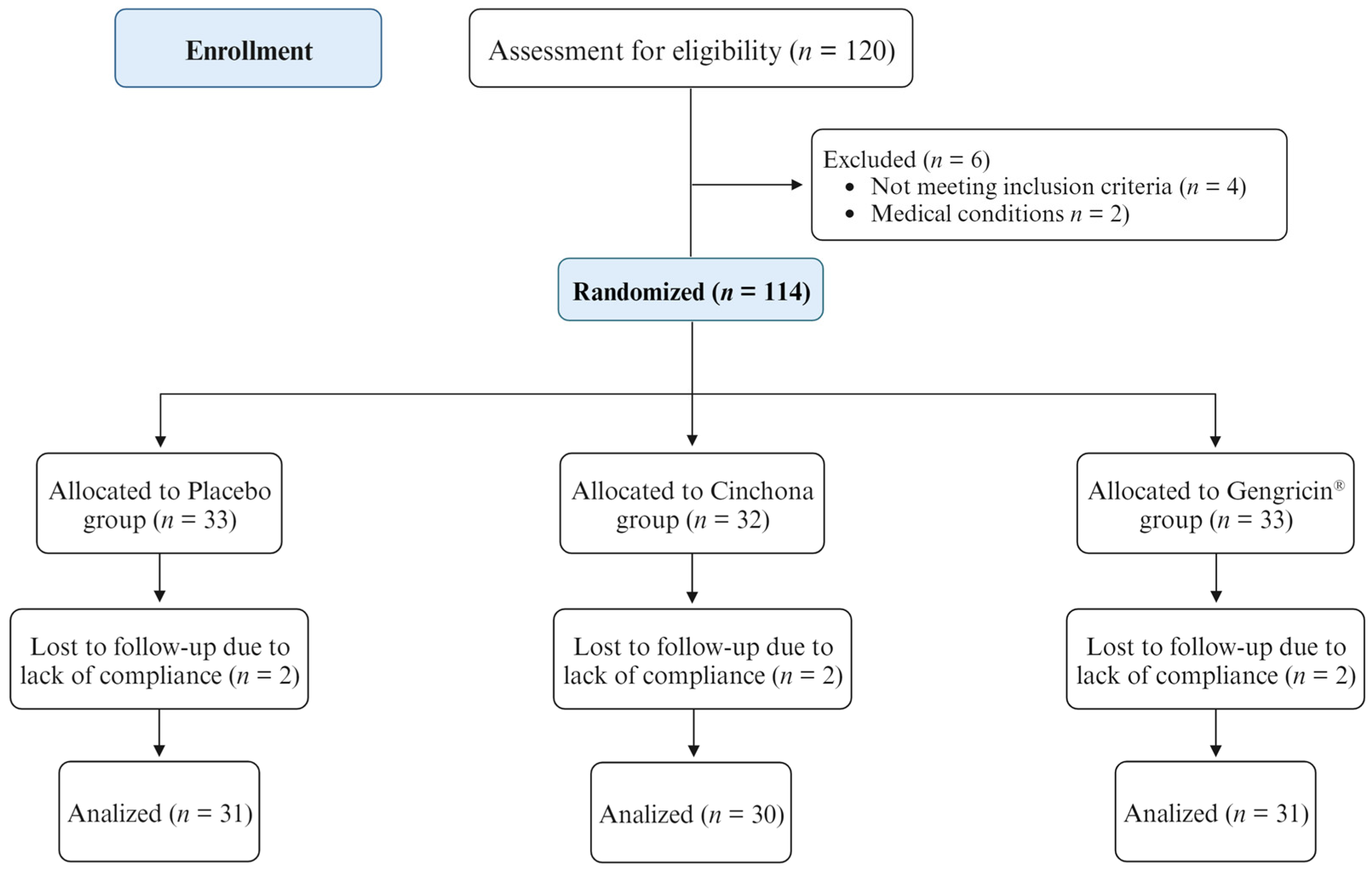
2.2. Randomized Controlled Trial (RCT)
2.2.1. Evaluation of Demographic and Biochemical Parameters
2.2.2. Nutritional and Satiety Assessment
2.2.3. Evaluation of Serum CCK and Ghrelin Levels
3. Discussion
4. Materials and Methods
4.1. Cell Culture Experiments
4.2. Randomized Controlled Trial (RCT)
4.2.1. Study Population and Design
4.2.2. Food Supplementation
4.2.3. Assessments
4.2.4. Sample Size and Statistical Power
4.3. Quantification of Serum CCK and Ghrelin Levels
4.4. Statistical Analysis
5. Conclusions and Future Perspectives
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef]
- Svačina, Š. Obesity and cardiovascular disease. Vnitr. Lek. 2020, 66, 89–91. [Google Scholar] [CrossRef]
- Li, Y.H.; Yang, L.H.; Sha, K.H.; Liu, T.G.; Zhang, L.G.; Liu, X.X. Efficacy of poly-unsaturated fatty acid therapy on patients with nonalcoholic steatohepatitis. World J. Gastroenterol. 2015, 21, 7008–7013. [Google Scholar] [CrossRef]
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef]
- Wharton, S.; Lau, D.C.W.; Vallis, M.; Sharma, A.M.; Biertho, L.; Campbell-Scherer, D.; Adamo, K.; Alberga, A.; Bell, R.; Boulé, N.; et al. Obesity in adults: A clinical practice guideline. Cmaj 2020, 192, E875–E891. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. J. Am. Coll. Cardiol. 2014, 63, 2985–3023. [Google Scholar] [CrossRef]
- Teixeira, P.J.; Marques, M.M. Health Behavior Change for Obesity Management. Obes. Facts 2018, 10, 666–673. [Google Scholar] [CrossRef]
- Gupta, S.; Chauhan, D.; Mehla, K.; Sood, P.; Nair, A. An overview of nutraceuticals: Current scenario. J. Basic Clin. Pharm. 2010, 1, 55–62. [Google Scholar]
- Maisto, M.; Annunziata, G.; Schiano, E.; Piccolo, V.; Iannuzzo, F.; Santangelo, R.; Ciampaglia, R.; Tenore, G.C.; Novellino, E.; Grieco, P. Potential functional snacks: Date fruit bars supplemented by different species of Lactobacillus spp. Foods 2021, 10, 1760. [Google Scholar] [CrossRef]
- Gohel, M.G.; Chacko, A.N. Serum GGT activity and hsCRP level in patients with type 2 diabetes mellitus with good and poor glycemic control: An evidence linking oxidative stress, inflammation and glycemic control. J. Diabetes Metab. Disord. 2013, 12, 56. [Google Scholar] [CrossRef]
- Yoshidan, R. Hormones and bioactive substances that affect peripheral taste sensitivity. J. Oral Biosci. 2012, 54, 67–72. [Google Scholar] [CrossRef]
- Descamps-Solà, M.; Vilalta, A.; Jalsevac, F.; Blay, M.T.; Rodríguez-Gallego, E.; Pinent, M.; Beltrán-Debón, R.; Terra, X.; Ardévol, A. Bitter taste receptors along the gastrointestinal tract: Comparison between humans and rodents. Front. Nutr. 2023, 10, 1215889. [Google Scholar] [CrossRef]
- Janssen, S.; Laermans, J.; Verhulst, P.J.; Thijs, T.; Tack, J.; Depoortere, I. Bitter taste receptors and α-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc. Natl. Acad. Sci. USA 2011, 108, 2094–2099. [Google Scholar] [CrossRef]
- Wooding, S.P.; Ramirez, V.A.; Behrens, M. Bitter taste receptors, Genes, evolution and health. Evol. Med. Public Health 2021, 9, 431–447. [Google Scholar] [CrossRef]
- Avau, B.; Rotondo, A.; Thijs, T.; Andrews, C.N.; Janssen, P.; Tack, J.; Depoortere, I. Targeting extra-oral bitter taste receptors modulates gastrointestinal motility with effects on satiation. Sci. Rep. 2015, 5, 15985. [Google Scholar] [CrossRef]
- Tenore, G.C.; Bottone, S.; Riccio, G.; Badolati, N.; Stornaiuolo, M.; Novellino, E. A Crosstalk between Melatonin and Taste-Receptors’ Signaling Tunes Quinine-Induced Gut Hormone Secretion in Mice. J. Nutr. Food Sci. 2018, 8, 1. [Google Scholar] [CrossRef]
- Kilic, U.; Yilmaz, B.; Ugur, M.; Yüksel, A.; Reiter, R.J.; Hermann, D.M.; Kilic, E. Evidence that membrane-bound G protein-coupled melatonin receptors MT1 and MT2 are not involved in the neuroprotective effects of melatonin in focal cerebral ischemia. J. Pineal Res. 2012, 52, 228–235. [Google Scholar] [CrossRef]
- Boratyński, P.J.; Zielińska-Błajet, M.; Skarżewski, J. Cinchona Alkaloids—Derivatives and Applications. Alkaloids Chem. Biol. 2019, 82, 29–145. [Google Scholar] [CrossRef]
- Chiurazzi, M.; De Conno, B.; Di Lauro, M.; Guida, B.; Nasti, G.; Schiano, E.; Stornaiuolo, M.; Tenore, G.C.; Colantuoni, A.; Novellino, E. The Effects of a Cinchona Supplementation on Satiety, Weight Loss and Body Composition in a Population of Overweight/Obese Adults: A Controlled Randomized Study. Nutrients 2023, 15, 5033. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Brockhoff, A.; Batram, C.; Kuhn, C.; Appendino, G.; Meyerhof, W. The human bitter taste receptor hTAS2R50 is activated by the two natural bitter terpenoids andrographolide and amarogentin. J. Agric. Food Chem. 2009, 57, 9860–9866. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, J.D.; Chakraborty, R.; Shaik, F.A.; Jaggupilli, A.; Bhullar, R.P.; Chelikani, P.C. The pharmacochaperone activity of quinine on bitter taste receptors. PLoS ONE 2016, 11, e0156347. [Google Scholar] [CrossRef]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses 2009, 35, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Sulli, C.; Davidson, E.; Berdougo, E.; Phillips, M.; Puffer, B.A.; Paes, C.; Doranz, B.J.; Rucker, J.B. The Bitter Taste Receptor TAS2R16 Achieves High Specificity and Accommodates Diverse Glycoside Ligands by using a Two-faced Binding Pocket. Sci. Rep. 2017, 7, 7753. [Google Scholar] [CrossRef]
- Wiener, A.; Shudler, M.; Levit, A.; Niv, M.Y. BitterDB: A database of bitter compounds. Nucleic Acids Res. 2012, 40, 413–419. [Google Scholar] [CrossRef]
- Lang, R.; Lang, T.; Dunkel, A.; Ziegler, F.; Behrens, M. Overlapping activation pattern of bitter taste receptors affect sensory adaptation and food perception. Front. Nutr. 2022, 9, 1–11. [Google Scholar] [CrossRef]
- Meyerhof, W.; Born, S.; Brockhoff, A.; Behrens, M. Molecular biology of mammalian bitter taste receptors. A review. Flavour Fragr. J. 2011, 26, 260–268. [Google Scholar] [CrossRef]
- Yamazaki, T.; Takahashi, C.; Taniguchi, Y.; Narukawa, M.; Misaka, T.; Ano, Y. Bitter taste receptor activation by hop-derived bitter components induces gastrointestinal hormone production in enteroendocrine cells. Biochem. Biophys. Res. Commun. 2020, 533, 704–709. [Google Scholar] [CrossRef]
- Yamada, T.; Kimura-Koyanagi, M.; Sakaguchi, K.; Ogawa, W.; Tamori, Y. Obesity and risk for its comorbidities diabetes, hypertension, and dyslipidemia in Japanese individuals aged 65 years. Sci. Rep. 2023, 13, 2346. [Google Scholar] [CrossRef]
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef]
- Paz-Filho, G.; Mastronardi, C.A. Interactions between the endocrine system and the gastrointestinal tract. Transl. Gastrointest. Cancer 2014, 4, 1–2. [Google Scholar] [CrossRef]
- Rehfeld, J.F. The Changing Concept of Gut Endocrinology. Endocr. Dev. 2017, 32, 8–19. [Google Scholar] [CrossRef]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef]
- Chakhtoura, M.; Haber, R.; Ghezzawi, M.; Rhayem, C.; Tcheroyan, R.; Mantzoros, C.S. Pharmacotherapy of obesity: An update on the available medications and drugs under investigation. eClinicalMedicine 2023, 58, 101882. [Google Scholar] [CrossRef]
- Xie, C.; Wang, X.; Young, R.L.; Horowitz, M.; Rayner, C.K.; Wu, T. Role of intestinal bitter sensing in enteroendocrine hormone secretion and metabolic control. Front. Endocrinol. 2018, 9, 576. [Google Scholar] [CrossRef]
- Gribble, F.M.; Reimann, F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat. Rev. Endocrinol. 2019, 15, 226–237. [Google Scholar] [CrossRef]
- Kamila, T.; Agnieszka, K. An update on extra-oral bitter taste receptors. J. Transl. Med. 2021, 19, 440. [Google Scholar]
- Kok, B.P.; Galmozzi, A.; Littlejohn, N.K.; Albert, V.; Godio, C.; Kim, W.; Kim, S.M.; Bland, J.S.; Grayson, N.; Fang, M.; et al. Intestinal bitter taste receptor activation alters hormone secretion and imparts metabolic benefits. Mol. Metab. 2018, 16, 76–87. [Google Scholar] [CrossRef]
- Schiano, E.; Maisto, M.; Piccolo, V.; Novellino, E.; Annunziata, G.; Ciampaglia, R.; Montesano, C.; Croce, M.; Caruso, G.; Iannuzzo, F.; et al. Beneficial Contribution to Glucose Homeostasis by an Agro-Food Waste Product Rich in Abscisic Acid: Results from a Randomized Controlled Trial. Foods 2022, 11, 2637. [Google Scholar] [CrossRef] [PubMed]
- Stuby, J.; Gravestock, I.; Wolfram, E.; Pichierri, G.; Steurer, J.; Burgstaller, J.M. Appetite-suppressing and satiety-increasing bioactive phytochemicals: A systematic review. Nutrients 2019, 11, 2238. [Google Scholar] [CrossRef]
- Andreozzi, P.; Sarnelli, G.; Pesce, M.; Zito, F.P.; D’Alessandro, A.; Verlezza, V.; Palumbo, I.; Turco, F.; Esposito, K.; Cuomo, R. The bitter taste receptor agonist quinine reduces calorie intake and increases the postprandial release of cholecystokinin in healthy subjects. J. Neurogastroenterol. Motil. 2015, 21, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.A.; Choi, M.; Kim, S.; Yu, R.; Park, T. Cinchonine prevents high-fat-diet-induced obesity through downregulation of adipogenesis and adipose inflammation. PPAR Res. 2012, 2012, 541204. [Google Scholar] [CrossRef]
- Zhang, W.L.; Zhu, L.; Jiang, J.G. Active ingredients from natural botanicals in the treatment of obesity. Obes. Rev. 2014, 15, 957–967. [Google Scholar] [CrossRef]
- Suh, H.W.; Lee, K.B.; Kim, K.S.; Yang, H.J.; Choi, E.K.; Shin, M.H.; Park, Y.S.; Na, Y.C.; Ahn, K.S.; Jang, Y.P.; et al. A bitter herbal medicine Gentiana scabra root extract stimulates glucagon-like peptide-1 secretion and regulates blood glucose in db/db mouse. J. Ethnopharmacol. 2015, 172, 219–226. [Google Scholar] [CrossRef]
- Mennella, I.; Fogliano, V.; Ferracane, R.; Arlorio, M.; Pattarino, F.; Vitaglione, P. Microencapsulated bitter compounds (from Gentiana lutea) reduce daily energy intakes in humans. Br. J. Nutr. 2016, 116, 1841–1850. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fouré, M.; Dugardin, C.; Foligné, B.; Hance, P.; Cadalen, T.; Delcourt, A.; Taminiau, B.; Daube, G.; Ravallec, R.; Cudennec, B.; et al. Chicory Roots for Prebiotics and Appetite Regulation: A Pilot Study in Mice. J. Agric. Food Chem. 2018, 66, 6439–6449. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.M.; Leidy, H.J. Novel Methodological Considerations Regarding the Use of Visual Analog Scale (VAS) Appetite Questionnaires in Tightly Controlled Feeding Trials. Curr. Dev. Nutr. 2019, 3, nzz061. [Google Scholar] [CrossRef]
- De Carvalho, T. Calorie restriction or dietary restriction: How far they can protect the brain against neurodegenerative diseases? Neural Regen. Res. 2022, 17, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Pala, V.; Sieri, S.; Palli, D.; Salvini, S.; Berrino, F.; Bellegotti, M.; Frasca, G.; Tumino, R.; Sacerdote, C.; Fiorini, L.; et al. Diet in the italian EPIC cohorts: Presentation of data and methodological issues. Tumori 2003, 89, 594–607. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.; Stewart, S.M. Validity of the international physical activity questionnaire short form. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef]

| Parameters | Placebo Group (n = 31) | Cinchona Group (n = 30) | Gengricin® Group (n = 31) | |||
|---|---|---|---|---|---|---|
| T0 | T90 | T0 | T90 | T0 | T90 | |
| Male, n° (%) | 17 (54.8) | - | 12 (40) | - | 16 (51.6) | - |
| Female, n° (%) | 14 (54.2) | - | 18 (60) | - | 15 (48.4) | - |
| Age (years) | 43 ± 6.2 | - | 47 ± 6.8 | - | 45 ± 5.9 | - |
| FBG (mg/dL) | 95.4 ± 4.6 | 91.2 ± 5.4 * | 94.4 ± 66 | 89.4 ± 7.4 ** | 96.1 ± 8.3 | 90.4 ± 4.5 ** |
| HDL-C (mg/dL) | 42.2 ± 1.8 | 41.0 ± 1.3 | 44.2 ± 4.7 | 44.0 ± 3.9 | 45.7 ± 3.5 | 46.6 ± 4.2 |
| LDL-C (mg/dL) | 103.4 ± 8.8 | 102.7 ± 6.5 | 109.5 ± 9.8 | 108.8 ± 11.1 | 114.5 ± 15.9 | 112.6 ± 13.8 |
| TC (mg/dL) | 188.4 ± 14.1 | 177.8 ± 17.9 * | 187.5 ± 14.2 | 176.5 ± 12.5 * | 190.5 ± 12.4 | 181.6 ± 14.3 * |
| TGs (mg/dL) | 134.3 ± 22.1 | 130.6 ± 21.9 | 122.7 ± 17.5 | 118.5 ± 18.5 | 137.5 ± 22.1 | 123.6 ± 13.2 * |
| AST (UI/L) | 25.6 ± 7.2 | 24.7 ± 6.2 | 21.5 ± 6.3 | 20.1 ± 5.6 | 23.5 ± 3.6 | 24.3 ± 2.8 |
| ALT (UI/L) | 18.9 ± 4.3 | 19.0 ± 2.3 | 19.3 ± 3.7 | 18.4 ± 3.6 | 20.8 ± 5.6 | 21.4 ± 5.5 |
| Cre (mg/dL) | 0.9 ± 0.1 | 0.9 ± 0.2 | 1.1 ± 0.1 | 1.2 ± 0.2 | 1.2 ± 0.4 | 1.1 ± 0.2 |
| Parameters | Placebo Group (n = 31) | Cinchona Group (n = 30) | Gengricin® Group (n = 31) | |||
|---|---|---|---|---|---|---|
| T0 | T90 | T0 | T90 | T0 | T90 | |
| Weight (kg) | 93.4 ± 7.0 | 88.4 ± 8.3 * | 94.1 ± 8.2 | 86.8 ± 7.3 ** | 95.4 ± 9.9 | 84.5 ± 8.4 **** |
| BMI (kg/m2) | 32.3 ± 6.2 | 30.6 ± 2.9 | 32.6 ± 5.7 | 30.0 ± 6.4 | 33.0 ± 6.7 | 29.2 ± 5.8 * |
| WC (cm) | 103.1 ± 7.5 | 98.9 ± 6.6 | 102.8 ± 6.8 | 96.7 ± 7.9 * | 103.5 ± 7.8 | 95.5 ± 8.6 ** |
| HC (cm) | 123.2 ± 4.5 | 118.9 ± 6.5 * | 122.4 ± 5.3 | 115.7 ± 4.8 **** | 124.2 ± 4.5 | 114.9 ± 7.2 **** |
| TBW (%) | 51.2 ± 3.5 | 52.9 ± 4.6 | 51.4 ± 5.3 | 52.7 ± 5.8 | 49.8 ± 6.5 | 53.4 ± 5.2 |
| FM (%) | 33.2 ± 3.4 | 31.5 ± 5.7 | 32.0 ± 4.8 | 27.8 ± 5.3 * | 33.4 ± 6.2 | 27.2 ± 7.4 ***,# |
| FFM (%) | 66.8 ± 3.4 | 68.5 ± 5.7 | 68.0 ± 4.8 | 72.2 ± 5.3 * | 66.6 ± 6.2 | 72.8 ± 7.4 ***,# |
| Placebo Group (n = 31) | Cinchona Group (n = 30) | Gengricin® Group (n = 31) | ||||
|---|---|---|---|---|---|---|
| T0 | T90 | T0 | T90 | T0 | T90 | |
| CCK (pg/mL) | 14.4 ± 2.9 | 12.3 ± 3.8 ** | 14.1 ± 2.2 | 13.8 ± 1.3 | 14.5 ± 1.9 | 15.8 ± 2.4 |
| Ghrelin (ng/mL) | 7.2 ± 1.1 | 7.1 ± 1.3 | 7.6 ± 2.5 | 7.5 ± 1. 4 | 7.2 ± 1.3 | 7.3 ± 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiano, E.; Iannuzzo, F.; Stornaiuolo, M.; Guerra, F.; Tenore, G.C.; Novellino, E. Gengricin®: A Nutraceutical Formulation for Appetite Control and Therapeutic Weight Management in Adults Who Are Overweight/Obese. Int. J. Mol. Sci. 2024, 25, 2596. https://doi.org/10.3390/ijms25052596
Schiano E, Iannuzzo F, Stornaiuolo M, Guerra F, Tenore GC, Novellino E. Gengricin®: A Nutraceutical Formulation for Appetite Control and Therapeutic Weight Management in Adults Who Are Overweight/Obese. International Journal of Molecular Sciences. 2024; 25(5):2596. https://doi.org/10.3390/ijms25052596
Chicago/Turabian StyleSchiano, Elisabetta, Fortuna Iannuzzo, Mariano Stornaiuolo, Fabrizia Guerra, Gian Carlo Tenore, and Ettore Novellino. 2024. "Gengricin®: A Nutraceutical Formulation for Appetite Control and Therapeutic Weight Management in Adults Who Are Overweight/Obese" International Journal of Molecular Sciences 25, no. 5: 2596. https://doi.org/10.3390/ijms25052596
APA StyleSchiano, E., Iannuzzo, F., Stornaiuolo, M., Guerra, F., Tenore, G. C., & Novellino, E. (2024). Gengricin®: A Nutraceutical Formulation for Appetite Control and Therapeutic Weight Management in Adults Who Are Overweight/Obese. International Journal of Molecular Sciences, 25(5), 2596. https://doi.org/10.3390/ijms25052596