Bactericidal Biodegradable Linear Polyamidoamines Obtained with the Use of Endogenous Polyamines
Abstract
1. Introduction
2. Results
2.1. Initial Attempts at the Synthesis of Polyamidoamines
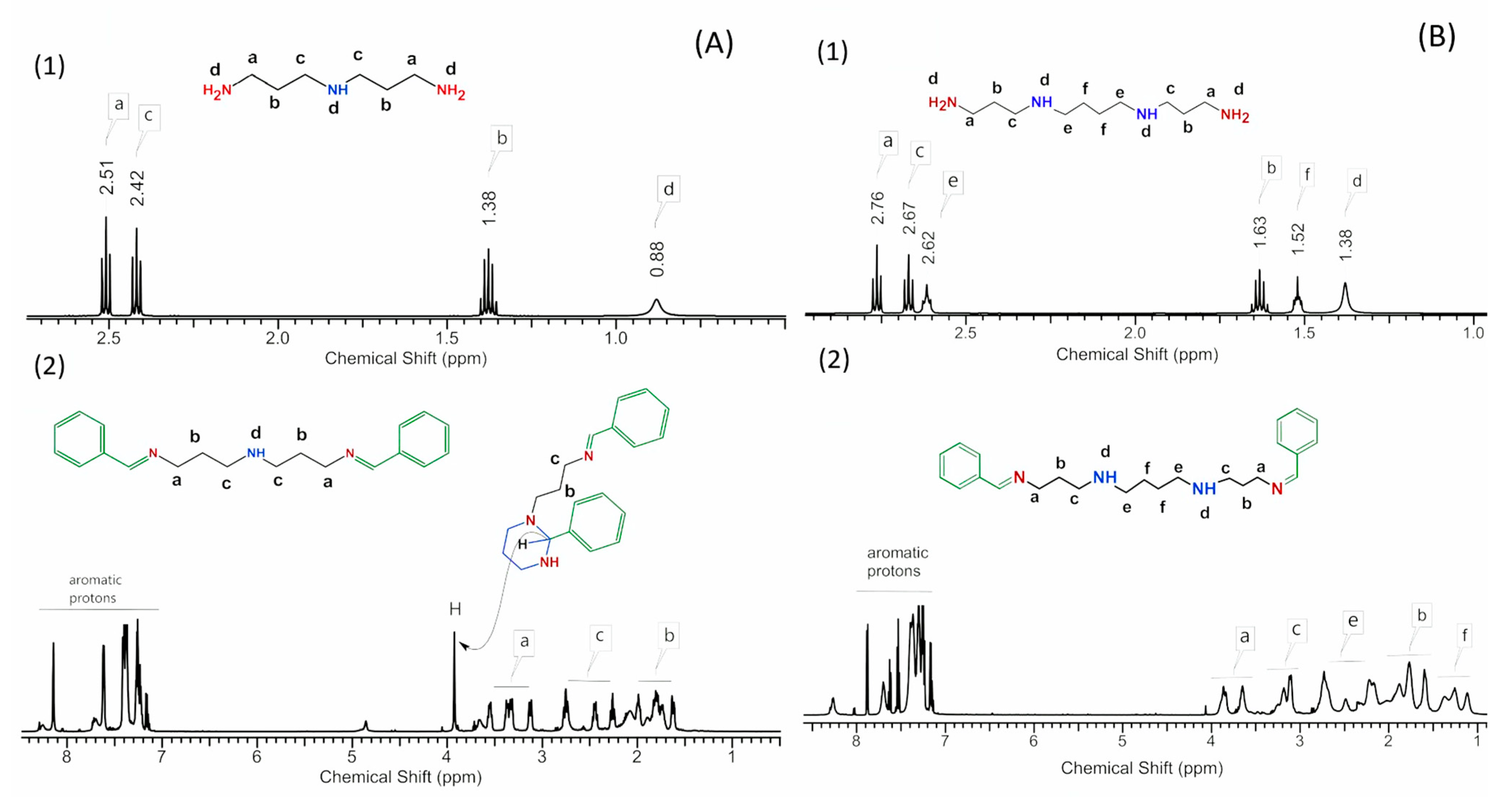
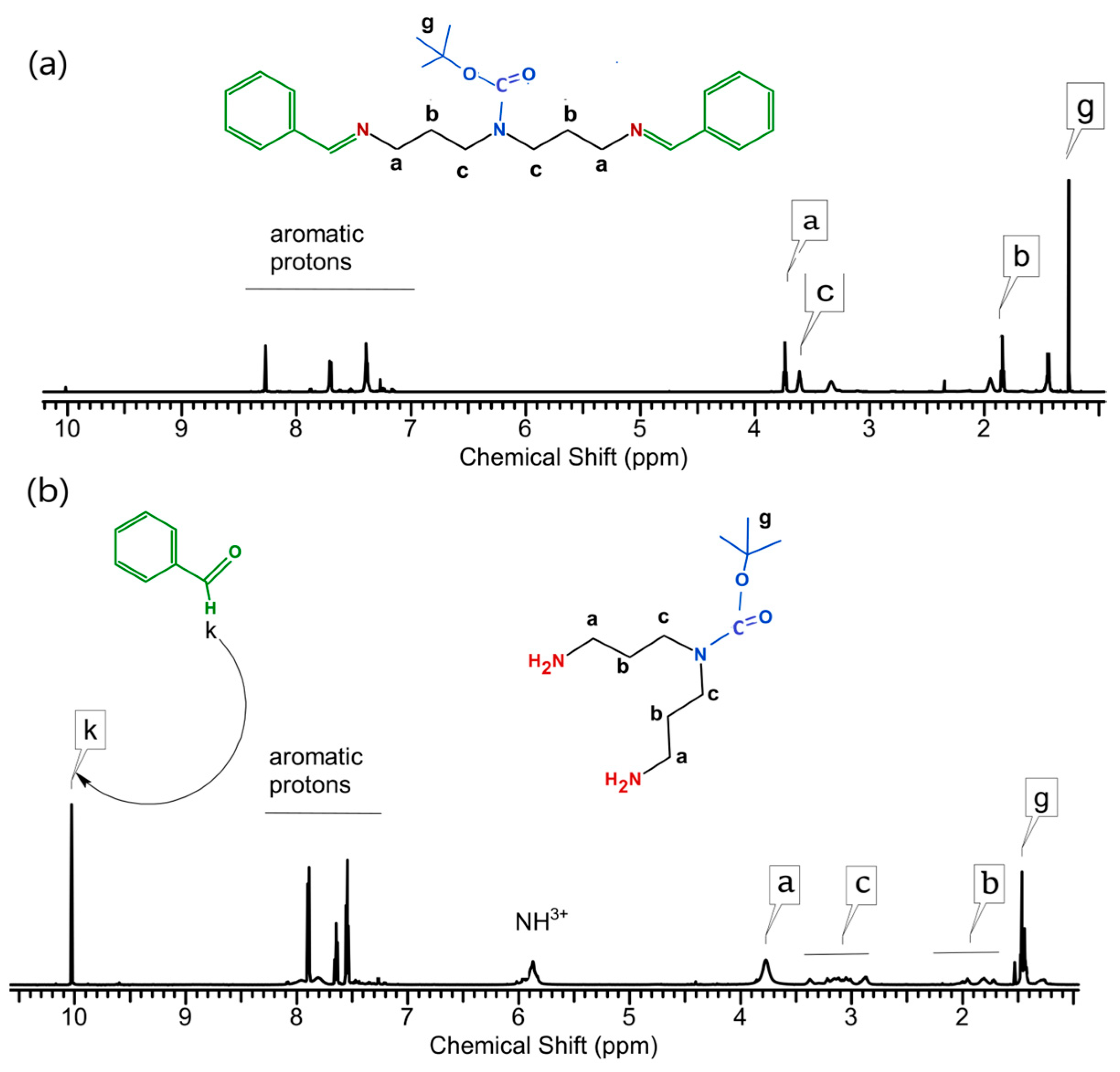
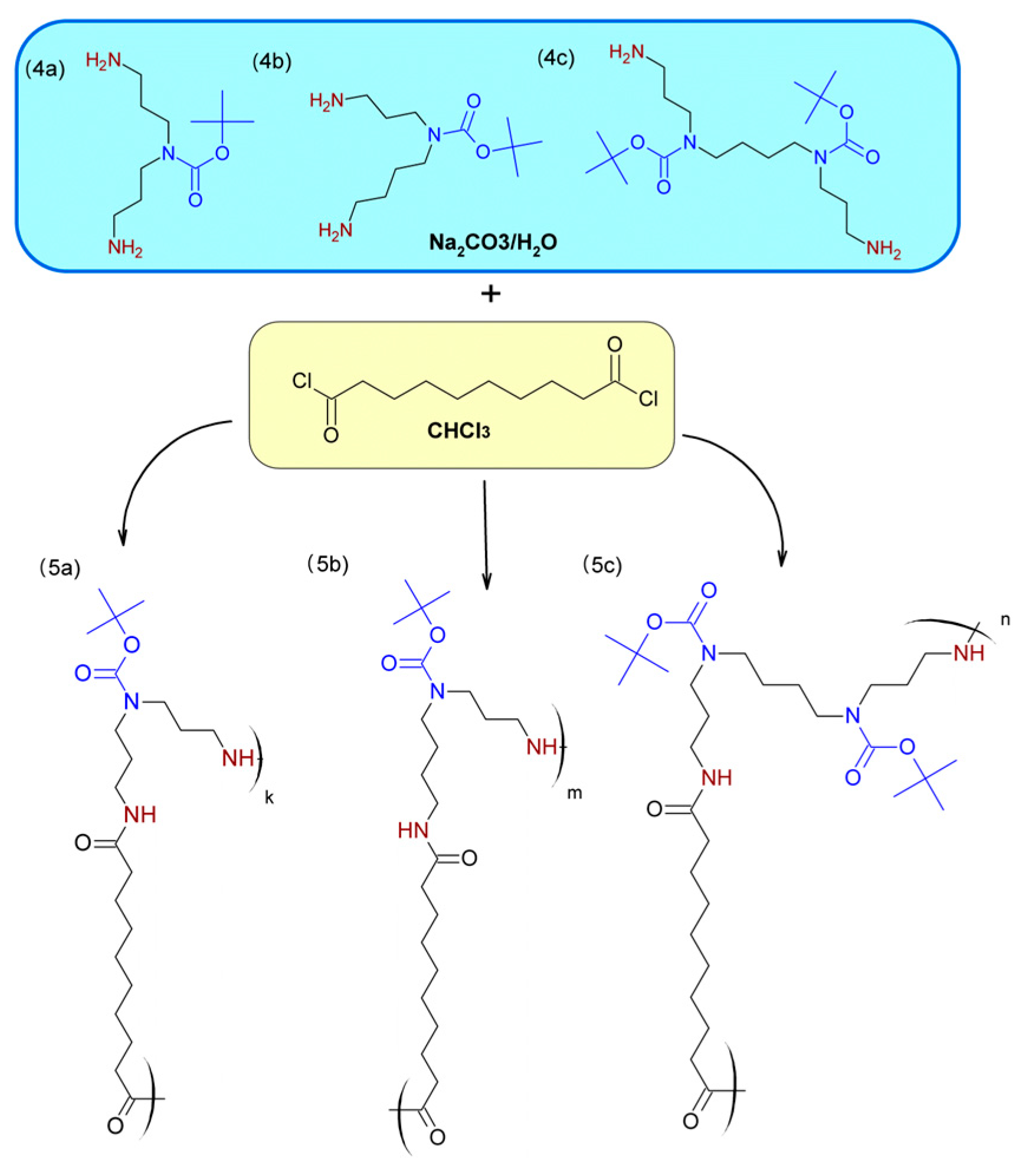
2.2. Preparation of Monomers—Synthesis of Polyamines with Protected Secondary Amine Groups
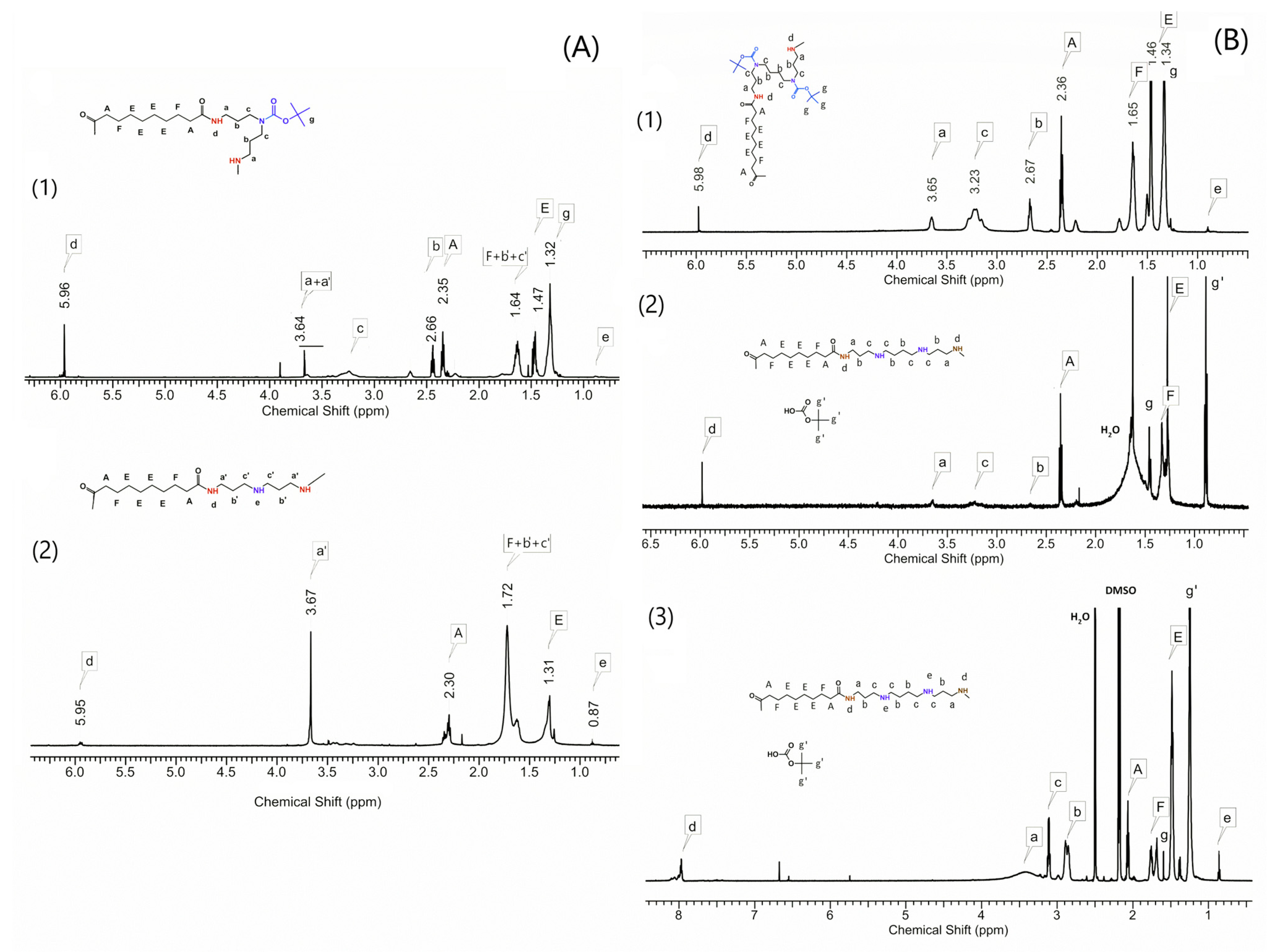
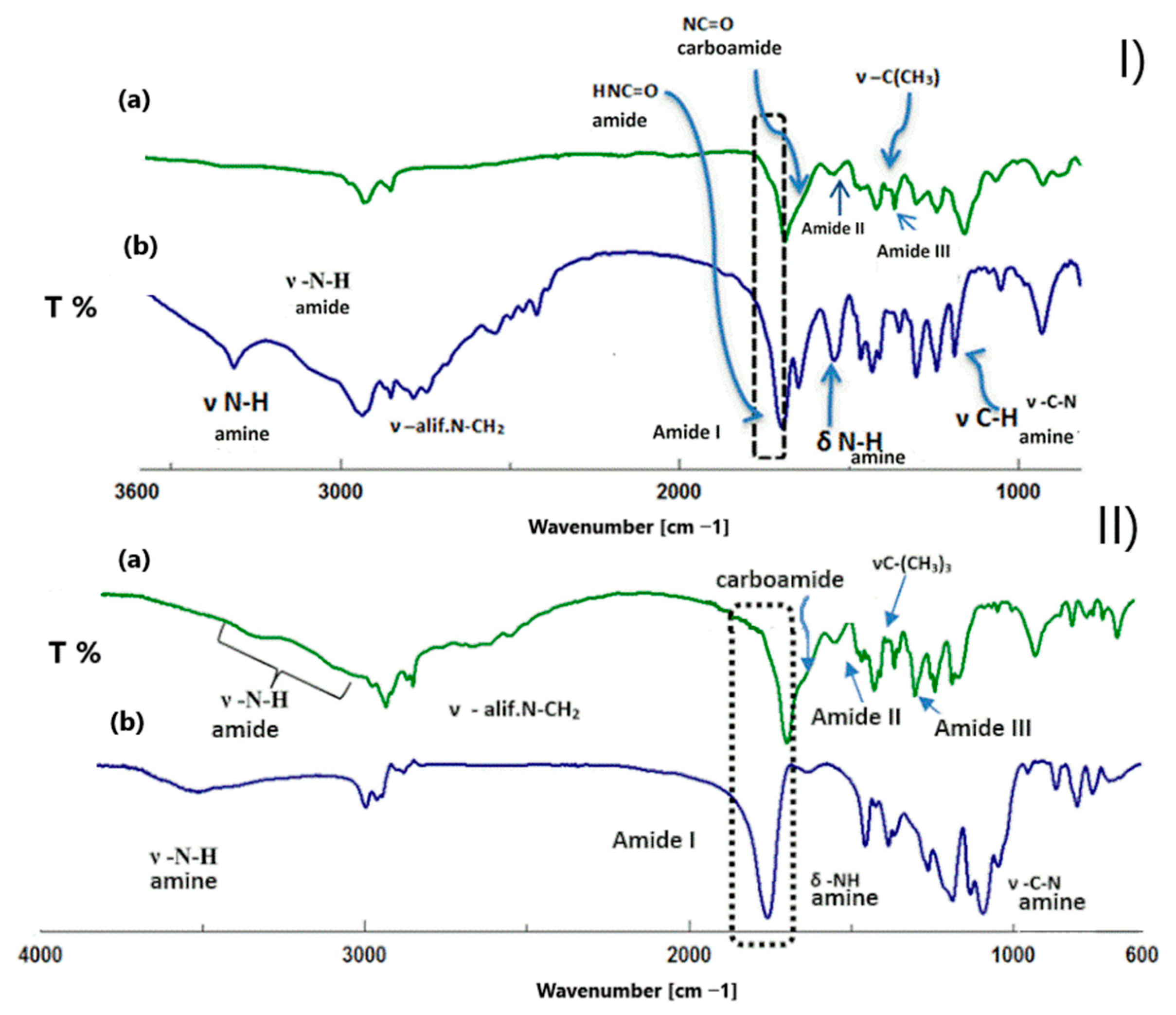
2.3. Synthesis and Properties of Linear Polyamidoamines
- -
- The presence of a signal associated with stretching vibrations in the range of the 2779 cm−1 characteristic band in aliphatic amines of secondary and tertiary origin;
- -
- The presence of the first amide band (from the second-row amide groups), with C=O deforming vibrations occurring around 1670 cm−1;
- -
- Signal decay 1663 cm−1 vibrations from carbamide groups after unblocking the amino groups;
- -
- Formation of a band at 1543 cm−1 originating from amines caused by N-H deforming vibrations. This is a low-intensity band that overlaps the band originating from the II amide band in the case of polyamines composed of spermine derivatives (in which there are two amine groups in the unit repeating) after unblocking. This increase in intensity is most visible;
- -
- The presence of a signal at 1188 cm−1, with C-N stretching vibrations characteristic of secondary aliphatic amines. The observed band at 1650 cm−1 is probably due to associated amines.
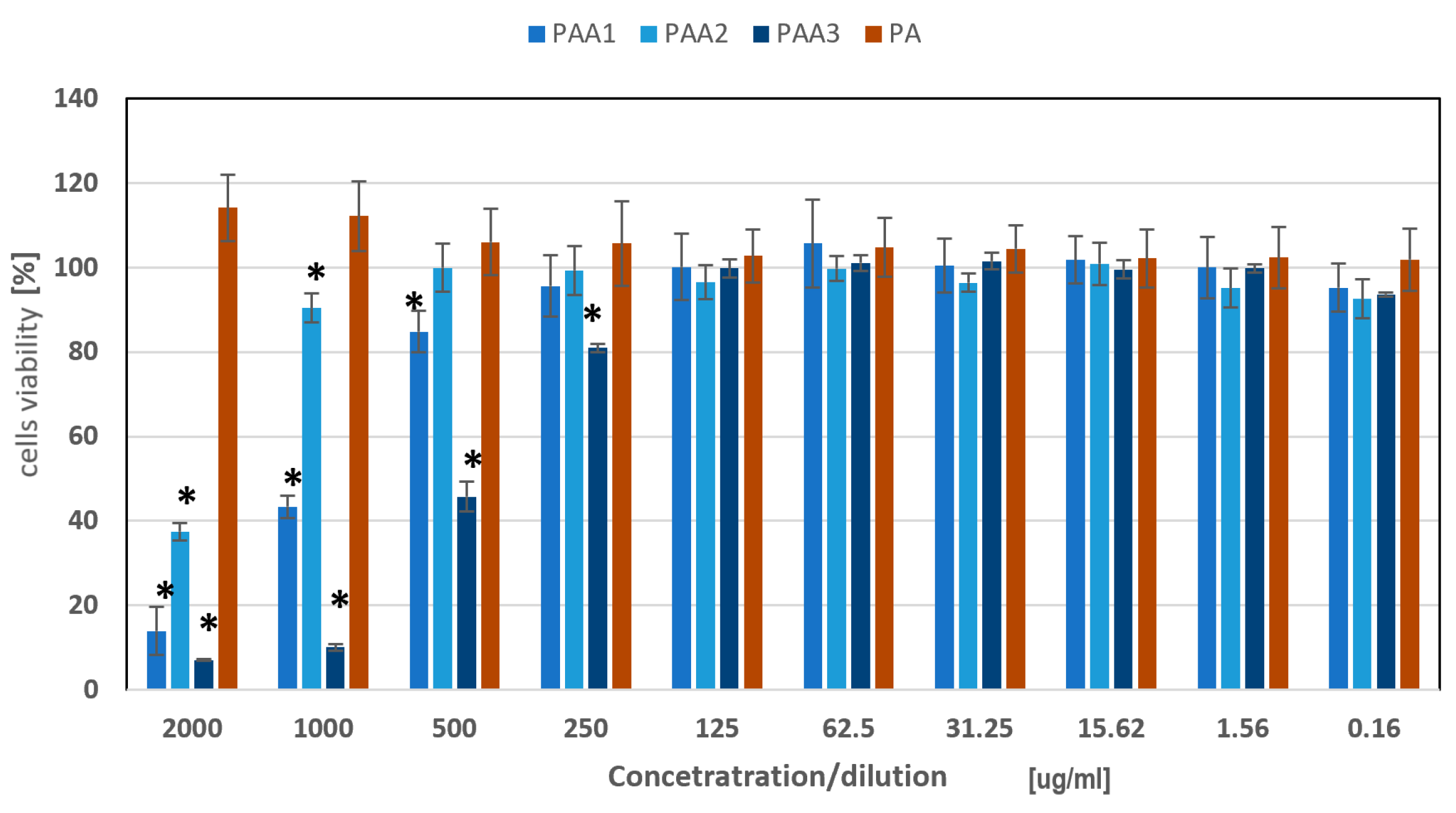
2.4. Assessment of Cytotoxicity of the Obtained Polyamidoamines towards Skin Cells
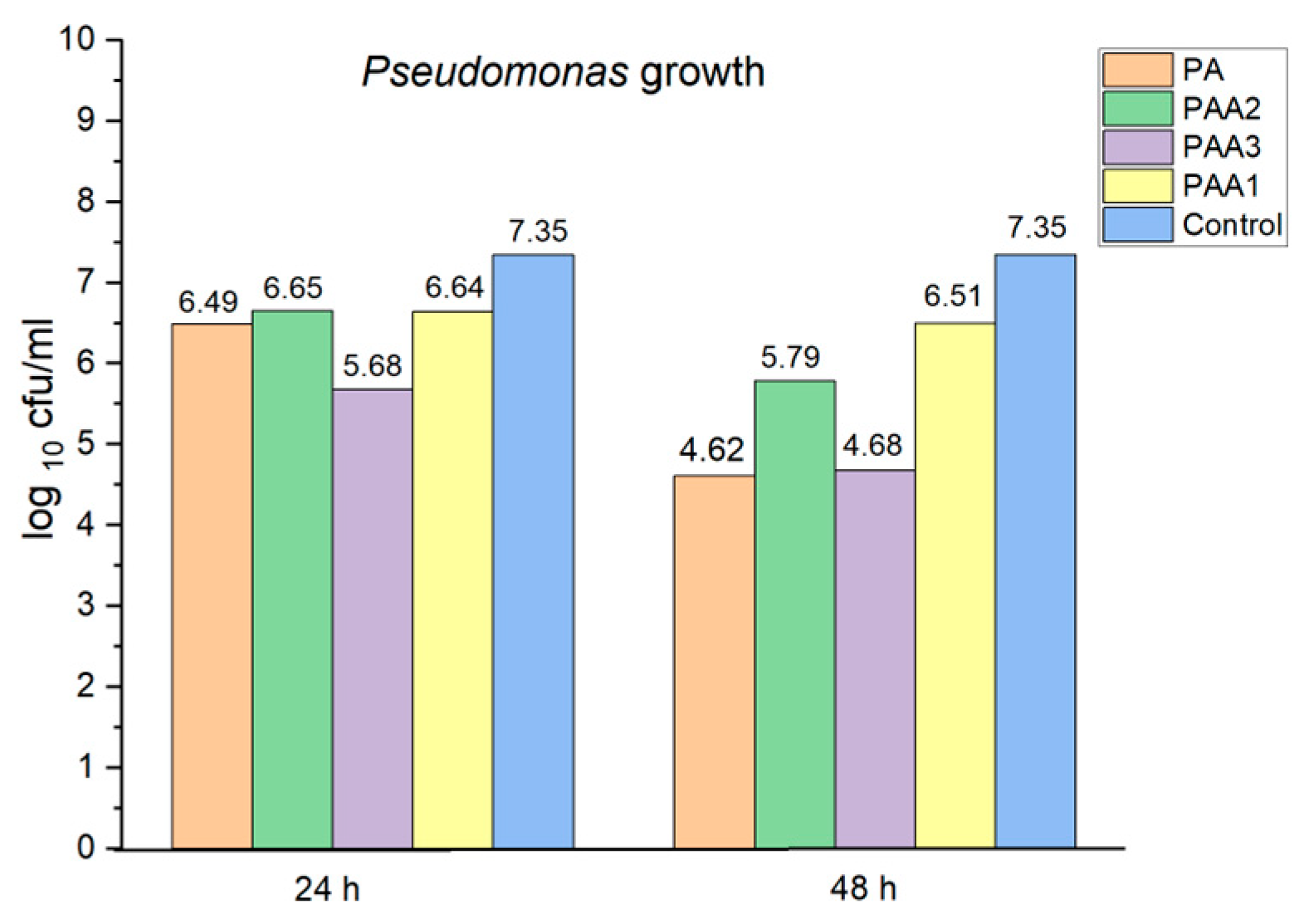
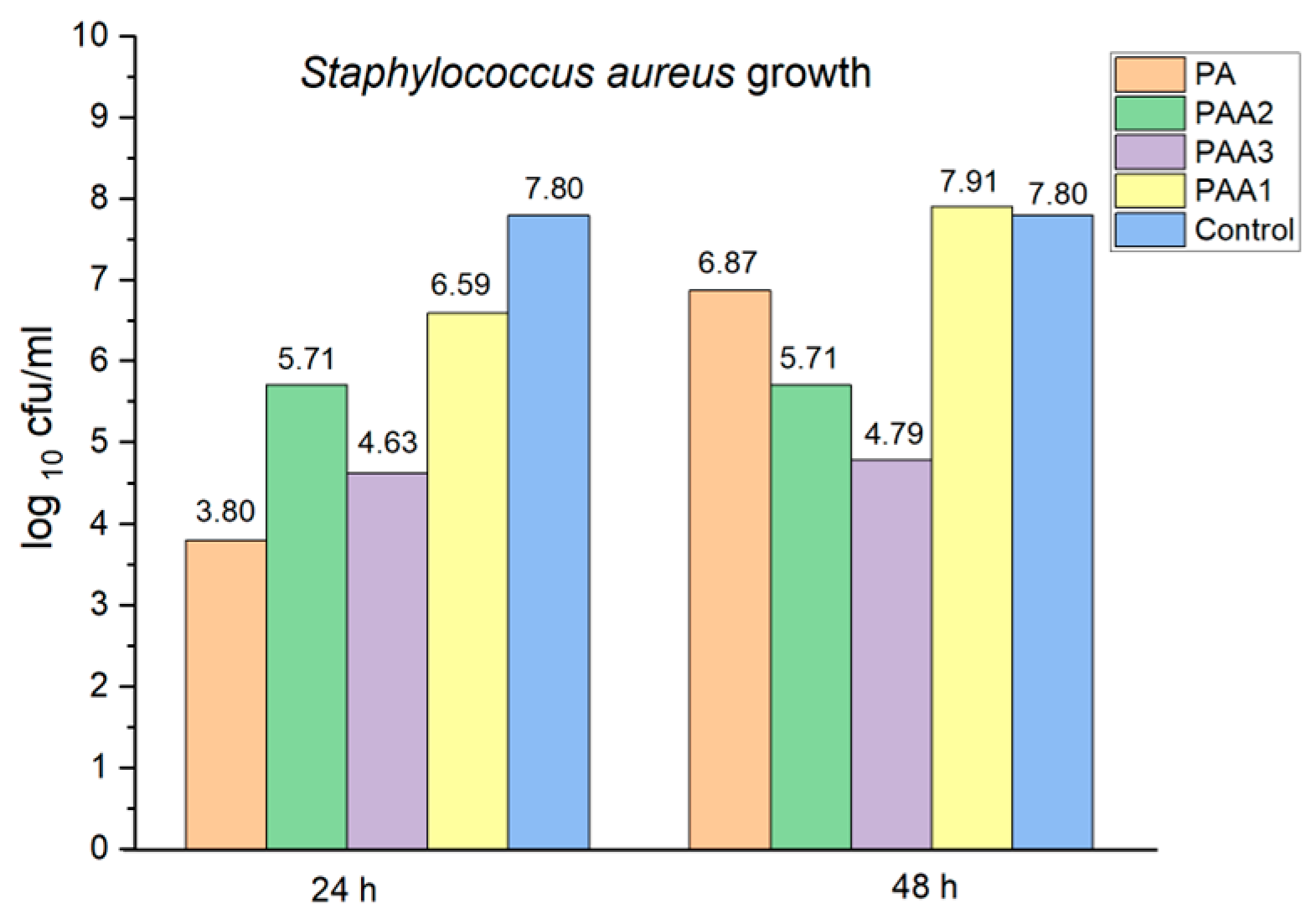
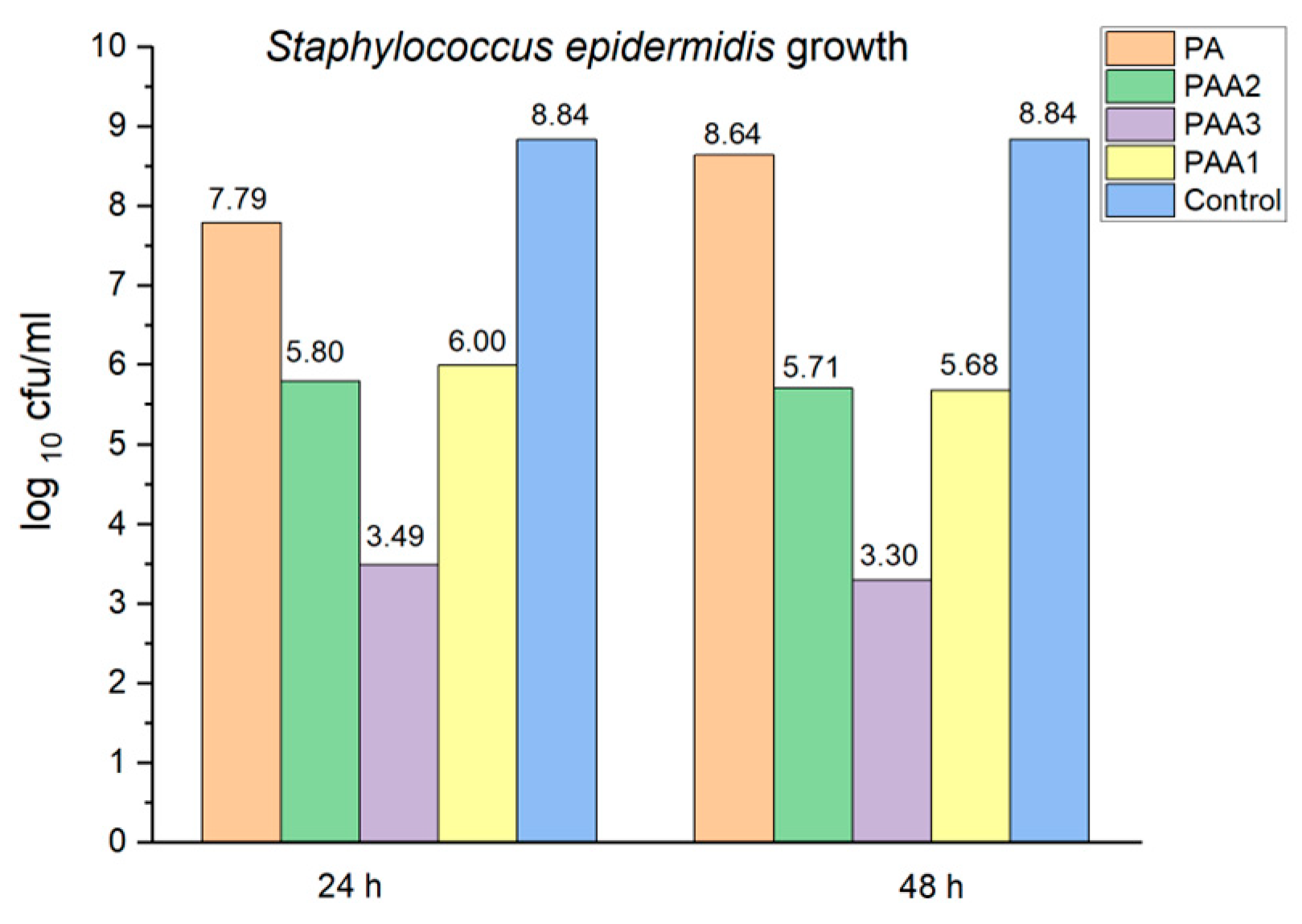
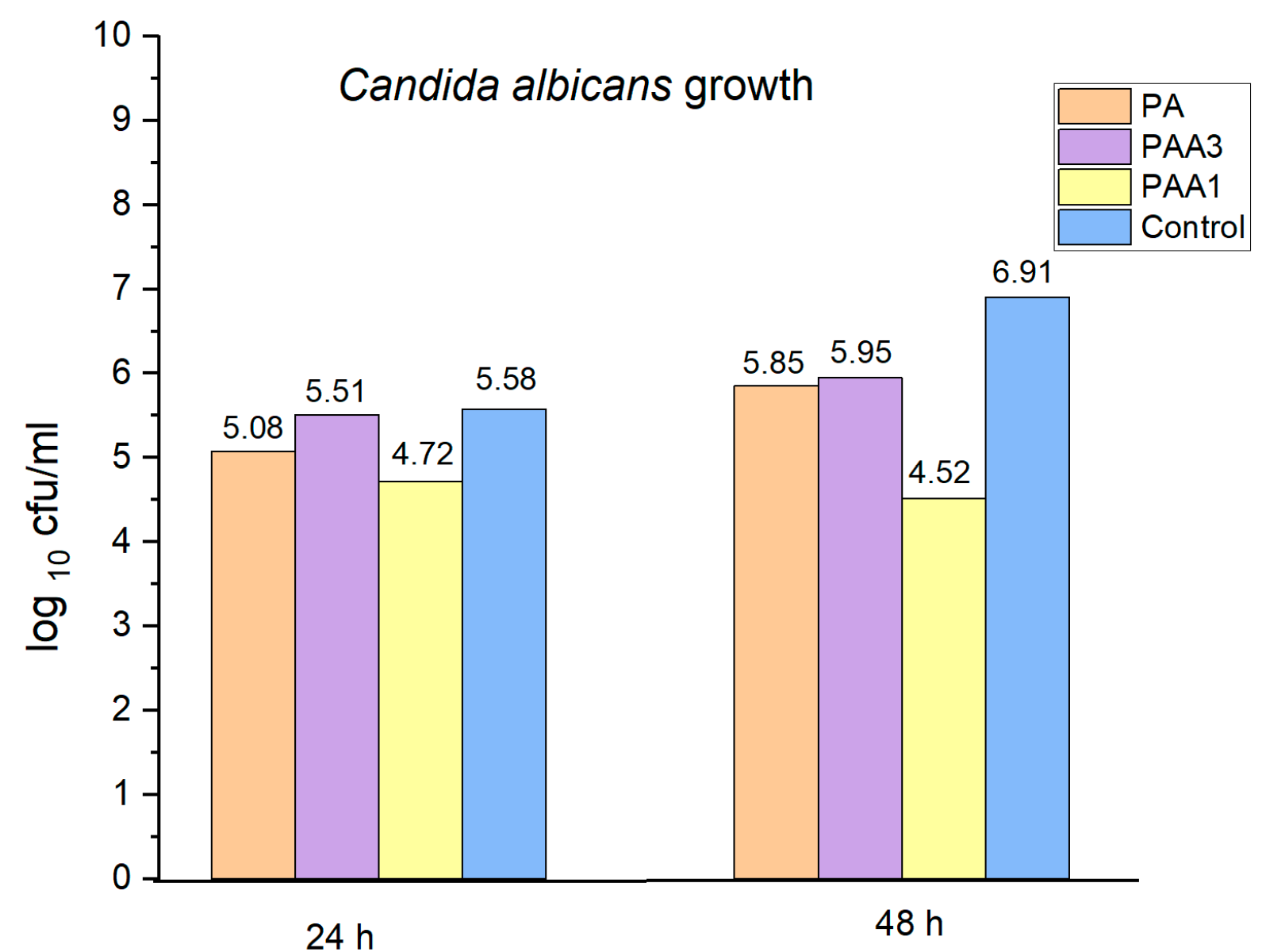
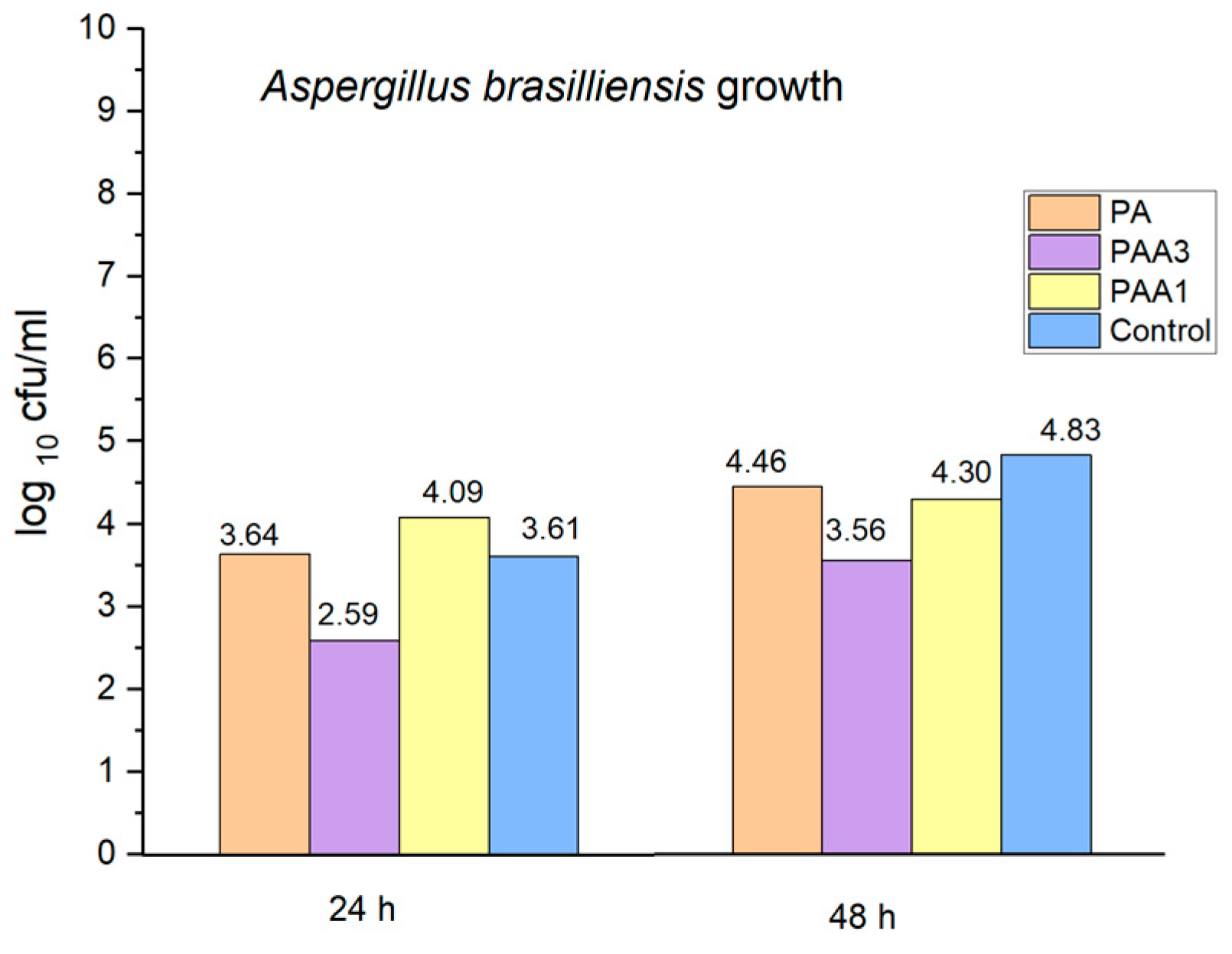
2.5. Preliminary Assessment of the Antibacterial and Antifungal Activities of the Obtained Polyamidoamines
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Polyamine Modification Procedures
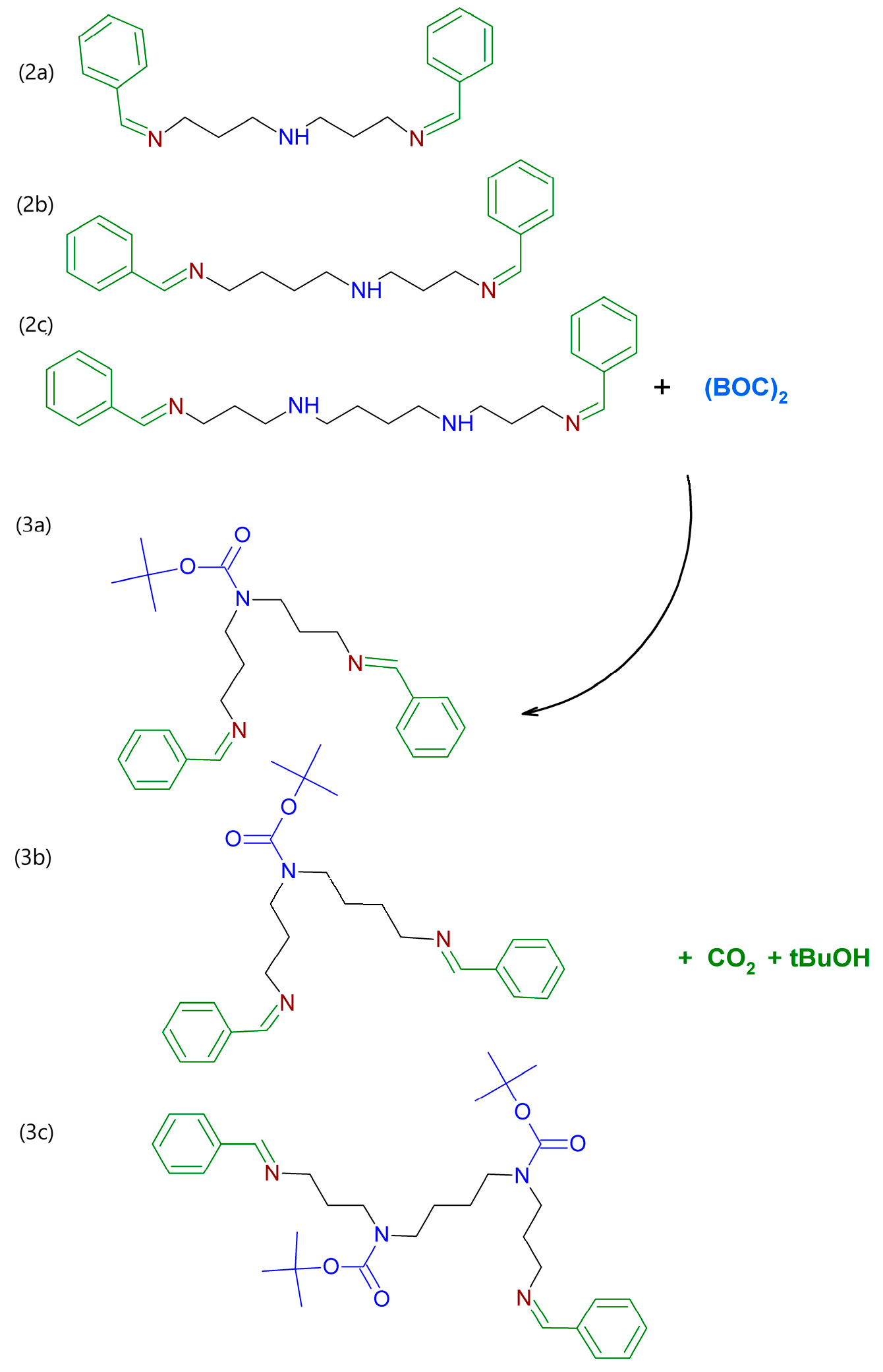
4.2.1. Procedures for the Protection of Primary Amine Groups in the Polyamines
4.2.2. Procedures for the Protection of Secondary Amine Groups in the Polyamines
- (2a)
- N-[(1E)-phenylmethylene]-N′-(3-{[(1E)-phenylmethylene]amino}propyl)propane-1,3-diamine;
- (2b)
- N-[(1E)-phenylmethylene]-N′-(3-{[(1Z)-phenylmethylene]amino}propyl)butane-1,4-diamine;
- (2c)
- N,N′-bis(3-{[(1E)-phenylmethylene]amino}propyl)butane-1,4-diamine.
4.2.3. Selective Deprotection of Primary Amine Groups in the Polyamines
4.3. Synthesis of Polyamidoamines
4.4. Procedures for Deprotecting Amino Groups in Polyamidoamines
4.5. Measurements
4.5.1. Nuclear Magnetic Resonance (NMR) Spectroscopy
4.5.2. Thermal Properties
4.5.3. Fourier Transform Infrared (FTIR) Spectroscopy
4.5.4. Wettability
4.5.5. Determination of Intrinsic Viscosity and Estimated Viscosity Average Molecular Mass
4.5.6. Assessment of Cytotoxicity of the Obtained Polyamidoamines
4.5.7. Assessment of Antibacterial and Antifungal Properties
- Gram-positive: Staphylococcus aureus NCTC 10788/ATCC 6538;
- Staphylococcus epidermidis NCTC 13360/ATCC 1222;
- Gram-negative: Escherichia coli NCTC 12923/ATCC 8739;
- Pseudomonas aeruginosa NCTC 12924/ATCC 9027;
- Fungi: Aspergillus brasiliensis NCPF 2275/ATC C 16404;
- Yeast: Can-dida albicans NCPF 3179/ATCC 10231.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ventola, C.L. The Antibiotic Resistance Crisis. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- Arslan, I. Trends in Antimicrobial Resistance in Healthcare-Associated Infections: A Global Concern. In Encyclopedia of Infection and Immunity; Rezaei, N., Ed.; Elsevier: Oxford, UK, 2022; pp. 652–661. ISBN 978-0-323-90303-5. [Google Scholar]
- Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 6 September 2023).
- Dong, J.; Wang, W.; Zhou, W.; Zhang, S.; Li, M.; Li, N.; Pan, G.; Zhang, X.; Bai, J.; Zhu, C. Immunomodulatory Biomaterials for Implant-Associated Infections: From Conventional to Advanced Therapeutic Strategies. Biomater. Res. 2022, 26, 72. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, S.; Wang, C.; Liu, W.; Zhang, Y.; Deng, L.; Zhang, J.; Huang, P.; Wang, W.; Dong, A. Combating Drug-Resistant Bacterial Infection Using Biodegradable Nanoparticles Assembled from Comb-like Polycarbonates Grafted with Amphiphilic Polyquaternium. J. Mater. Chem. B 2021, 9, 357–365. [Google Scholar] [CrossRef]
- Smola-Dmochowska, A.; Lewicka, K.; Macyk, A.; Rychter, P.; Pamuła, E.; Dobrzyński, P. Biodegradable Polymers and Polymer Composites with Antibacterial Properties. Int. J. Mol. Sci. 2023, 24, 7473. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Duan, S.; Ding, X.; Liu, R.; Xu, F.-J. Versatile Antibacterial Materials: An Emerging Arsenal for Combatting Bacterial Pathogens. Adv. Funct. Mater. 2018, 28, 1802140. [Google Scholar] [CrossRef]
- Casero, R.A.; Woster, P.M. Recent Advances in the Development of Polyamine Analogues as Antitumor Agents. J. Med. Chem. 2009, 52, 4551–4573. [Google Scholar] [CrossRef] [PubMed]
- Pelton, R. Polyvinylamine: A Tool for Engineering Interfaces. Langmuir 2014, 30, 15373–15382. [Google Scholar] [CrossRef] [PubMed]
- Mohammadifar, E.; Kharat, A.N.; Adeli, M. Polyamidoamine and Polyglycerol; Their Linear, Dendritic and Linear–Dendritic Architectures as Anticancer Drug Delivery Systems. J. Mater. Chem. B 2015, 3, 3896–3921. [Google Scholar] [CrossRef] [PubMed]
- Arioli, M.; Manfredi, A.; Alongi, J.; Ferruti, P.; Ranucci, E. Highlight on the Mechanism of Linear Polyamidoamine Degradation in Water. Polymers 2020, 12, 1376. [Google Scholar] [CrossRef] [PubMed]
- Ferruti, P. Poly(Amidoamine)s: Past, Present, and Perspectives. J. Polym. Sci. Part Polym. Chem. 2013, 51, 2319–2353. [Google Scholar] [CrossRef]
- Dai, F.; Li, Q.; Wang, Y.; Ge, C.; Feng, C.; Xie, S.; He, H.; Xu, X.; Wang, C. Design, Synthesis, and Biological Evaluation of Mitochondria-Targeted Flavone–Naphthalimide–Polyamine Conjugates with Antimetastatic Activity. J. Med. Chem. 2017, 60, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Dewa, T.; Asai, T.; Oku, N.; Nango, M.; Dewa, T.; Asai, T.; Oku, N.; Nango, M. Polyamine—Lipid Conjugates as Effective Gene Carriers: Chemical Structure, Morphology, and Gene Transfer Activity. In Non-Viral Gene Therapy; IntechOpen: London, UK, 2011; ISBN 978-953-307-538-9. [Google Scholar]
- Monsalve, L.N.; Kaniz Fatema, M.; Nonami, H.; Erra-Balsells, R.; Baldessari, A. Lipase-Catalyzed Synthesis and Characterization of a Novel Linear Polyamidoamine Oligomer. Polymer 2010, 51, 2998–3005. [Google Scholar] [CrossRef]
- Kanasty, R.L.; Vegas, A.J.; Ceo, L.M.; Maier, M.; Charisse, K.; Nair, J.K.; Langer, R.; Anderson, D.G. Sequence-Defined Oligomers from Hydroxyproline Building Blocks for Parallel Synthesis Applications. Angew. Chem. Int. Ed. 2016, 55, 9529–9533. [Google Scholar] [CrossRef] [PubMed]
- Thody, A.J.; Shuster, S. Control and Function of Sebaceous Glands. Physiol. Rev. 1989, 69, 383–416. [Google Scholar] [CrossRef]
- Yamaga, M.; Tani, H.; Yamaki, A.; Tatefuji, T.; Hashimoto, K. Metabolism and Pharmacokinetics of Medium Chain Fatty Acids after Oral Administration of Royal Jelly to Healthy Subjects. RSC Adv. 2019, 9, 15392–15401. [Google Scholar] [CrossRef]
- Morgan, P.W. Interfacial Polymerization. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; ISBN 978-0-471-44026-0. [Google Scholar]
- Culf, A.S.; Melanson, J.A.; Ouellette, R.J.; Briand, G.G. Bis-Imine Primary Amine Protection of the Dialkyltriamine, Norspermidine. Tetrahedron Lett. 2012, 53, 3301–3304. [Google Scholar] [CrossRef]
- Sanadhya, S.G.; Oswal, S.L.; Parmar, K.C. Synthesis and Characterization of Aliphatic-Aromatic Polyesters Using Interfacial Polycondensation Technique. J. Chem. Pharm. Res. 2014, 6, 705–714. [Google Scholar]
- Ali, M.A.; Kaneko, T. Polyamide Syntheses. In Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1750–1762. ISBN 978-3-642-29648-2. [Google Scholar]
- Weisskopf, K. Determination of Molecular Weight Averages and Molecular Weight Distribution by g.p.c. of N-Trifluoroacetylated Polyamides. Polymer 1985, 26, 1187–1190. [Google Scholar] [CrossRef]
- Baniasadi, H.; Seppälä, J. Novel Long-Chain Aliphatic Polyamide/Surface-Modified Silicon Dioxide Nanocomposites: In-Situ Polymerization and Properties. Mater. Today Chem. 2021, 20, 100450. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, P.; Qian, C.; Zhao, Y.; Wang, L.; Wang, D.; Dong, X. The Brill Transition in Long-Chain Aliphatic Polyamide 1012: The Role of Hydrogen-Bonding Organization. Macromolecules 2021, 54, 6835–6844. [Google Scholar] [CrossRef]
- Lu, A.; Petit, E.; Jelonek, K.; Orchel, A.; Kasperczyk, J.; Wang, Y.; Su, F.; Li, S. Self-Assembled Micelles Prepared from Bio-Based Hydroxypropyl Methyl Cellulose and Polylactide Amphiphilic Block Copolymers for Anti-Tumor Drug Release. Int. J. Biol. Macromol. 2020, 154, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Furó, I. NMR Spectroscopy of Micelles and Related Systems. J. Mol. Liq. 2005, 117, 117–137. [Google Scholar] [CrossRef]
- Millot, C.; Fillot, L.-A.; Lame, O.; Sotta, P.; Seguela, R. Assessment of Polyamide-6 Crystallinity by DSC. J. Therm. Anal. Calorim. 2015, 122, 307–314. [Google Scholar] [CrossRef]
- Douglas, E.J.A.; Alkhzem, A.H.; Wonfor, T.; Li, S.; Woodman, T.J.; Blagbrough, I.S.; Laabei, M. Antibacterial Activity of Novel Linear Polyamines against Staphylococcus Aureus. Front. Microbiol. 2022, 13, 948343. [Google Scholar] [CrossRef] [PubMed]
- Arya, N.R.; Rafiq, N.B. Candidiasis. In StatPearls [Internet]; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kanaujia, R.; Singh, S.; Rudramurthy, S.M. Aspergillosis: An Update on Clinical Spectrum, Diagnostic Schemes, and Management. Curr. Fungal Infect. Rep. 2023, 17, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Rogalsky, S.; Bardeau, J.-F.; Wu, H.; Lyoshina, L.; Bulko, O.; Tarasyuk, O.; Makhno, S.; Cherniavska, T.; Kyselov, Y.; Koo, J.H. Structural, Thermal and Antibacterial Properties of Polyamide 11/Polymeric Biocide Polyhexamethylene Guanidine Dodecylbenzenesulfonate Composites. J. Mater. Sci. 2016, 51, 7716–7730. [Google Scholar] [CrossRef]
- Saïhi, D.; El-Achari, A.; Vroman, I.; Périchaud, A. Antibacterial Activity of Modified Polyamide Fibers. J. Appl. Polym. Sci. 2005, 98, 997–1000. [Google Scholar] [CrossRef]
- Śmigiel-Gac, N.; Smola-Dmochowska, A.; Janeczek, H.; Dobrzyński, P. Biodegradable Block Poly(Ester Amine)s with Pendant Hydroxyl Groups for Biomedical Applications. Polymers 2023, 15, 1473. [Google Scholar] [CrossRef]
- Ding, X.; Wang, A.; Tong, W.; Xu, F.-J. Biodegradable Antibacterial Polymeric Nanosystems: A New Hope to Cope with Multidrug-Resistant Bacteria. Small 2019, 15, 1900999. [Google Scholar] [CrossRef]
- Xiong, M.-H.; Li, Y.-J.; Bao, Y.; Yang, X.-Z.; Hu, B.; Wang, J. Bacteria-Responsive Multifunctional Nanogel for Targeted Antibiotic Delivery. Adv. Mater. 2012, 24, 6175–6180. [Google Scholar] [CrossRef]
- Dey, P.; Mukherjee, S.; Das, G.; Ramesh, A. Micellar Chemotherapeutic Platform Based on a Bifunctional Salicaldehyde Amphiphile Delivers a “Combo-Effect” for Heightened Killing of MRSA. J. Mater. Chem. B 2018, 6, 2116–2125. [Google Scholar] [CrossRef] [PubMed]
- Dangerfield, E.M.; Plunkett, C.H.; Win-Mason, A.L.; Stocker, B.L.; Timmer, M.S.M. Protecting-Group-Free Synthesis of Amines: Synthesis of Primary Amines from Aldehydes via Reductive Amination. J. Org. Chem. 2010, 75, 5470–5477. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.L.S.; Grehn, L.; Ragnarsson, U. Selective Protection of Polyamines: Synthesis of Model Compounds and Spermidine Derivatives. J. Chem. Soc. Perkin 1 1988, 7, 1905–1911. [Google Scholar] [CrossRef]
- D’Agostino, L.; Di Luccia, A. Polyamines Interact with DNA as Molecular Aggregates. Eur. J. Biochem. 2002, 269, 4317–4325. [Google Scholar] [CrossRef]
- Nishio, T.; Yoshikawa, Y.; Shew, C.-Y.; Umezawa, N.; Higuchi, T.; Yoshikawa, K. Specific Effects of Antitumor Active Norspermidine on the Structure and Function of DNA. Sci. Rep. 2019, 9, 14971. [Google Scholar] [CrossRef]
- Allouche, A.R.; Graveron-Demilly, D.; Fauvelle, F.; Aubert-Frécon, M. Theoretical and Experimental Investigation of the 1H NMR Spectrum of Putrescine. Chem. Phys. Lett. 2008, 466, 219–222. [Google Scholar] [CrossRef]
- Standard Test Method for Assignment of the Glass Transition Temperatures by Differential Scanning Calorimetry (Withdrawn 2023). Available online: https://www.astm.org/e1356-08r14.html (accessed on 21 February 2024).
- A Practical Guide to ISO 10993-5: Cytotoxicity. Available online: https://www.mddionline.com/testing/a-practical-guide-to-iso-10993-5-cytotoxicity (accessed on 18 December 2023).
- Levis, J.S.; Weinstein, M.P.; Bobenchik, A.M.; Campeau, S.; Cullen, S.K.; Galas, M.F.; Gold, H.; Humphries, R.M.; Kirn, T.J.; Limbago, B.; et al. Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022. [Google Scholar]



| No. | Used Polyamine | Before Deprotection of Amine Groups | After Deprotection of Amine Groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mv [g/mol] | ηinh [dL/g] | Tg [°C] | Tm [°C] | ΔHm [J/g] | Contact Angle [°] | ηinh [dL/g] | Tg [°C] | Tm [°C] | ΔHm [J/g] | Contact Angle [°] | ||
| 1 | Putrescine | 25,200 | 0.25 | −13 | 231 | 68 | 78 | ----- | ---- | ----- | ---- | ---- |
| 2 | Boc-norspermidine | 20,000 | 0.18 | −25 | 108 | 89 | 79 | 0.15 | −20 | 131 | 164 | 21 |
| 3 | Boc-spermidine | 22,900 | 0.19 | −28 | 78; 115 | 84 | 78 | 0.17 | −7 | 130 | 161 | 20 |
| 4 | Boc2-spermine | 20,000 | 0.18 | −35 | 70; 116 | 80 | 81 | 0.15 | 2 | 130 | 83 | <10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śmigiel-Gac, N.; Smola-Dmochowska, A.; Jelonek, K.; Musiał-Kulik, M.; Barczyńska-Felusiak, R.; Rychter, P.; Lewicka, K.; Dobrzyński, P. Bactericidal Biodegradable Linear Polyamidoamines Obtained with the Use of Endogenous Polyamines. Int. J. Mol. Sci. 2024, 25, 2576. https://doi.org/10.3390/ijms25052576
Śmigiel-Gac N, Smola-Dmochowska A, Jelonek K, Musiał-Kulik M, Barczyńska-Felusiak R, Rychter P, Lewicka K, Dobrzyński P. Bactericidal Biodegradable Linear Polyamidoamines Obtained with the Use of Endogenous Polyamines. International Journal of Molecular Sciences. 2024; 25(5):2576. https://doi.org/10.3390/ijms25052576
Chicago/Turabian StyleŚmigiel-Gac, Natalia, Anna Smola-Dmochowska, Katarzyna Jelonek, Monika Musiał-Kulik, Renata Barczyńska-Felusiak, Piotr Rychter, Kamila Lewicka, and Piotr Dobrzyński. 2024. "Bactericidal Biodegradable Linear Polyamidoamines Obtained with the Use of Endogenous Polyamines" International Journal of Molecular Sciences 25, no. 5: 2576. https://doi.org/10.3390/ijms25052576
APA StyleŚmigiel-Gac, N., Smola-Dmochowska, A., Jelonek, K., Musiał-Kulik, M., Barczyńska-Felusiak, R., Rychter, P., Lewicka, K., & Dobrzyński, P. (2024). Bactericidal Biodegradable Linear Polyamidoamines Obtained with the Use of Endogenous Polyamines. International Journal of Molecular Sciences, 25(5), 2576. https://doi.org/10.3390/ijms25052576








