Biology and Therapeutic Properties of Mesenchymal Stem Cells in Leukemia
Abstract
1. Introduction of Mesenchymal Stem Cell (MSCs)
2. The Role of MSCs in BM Microenvironment
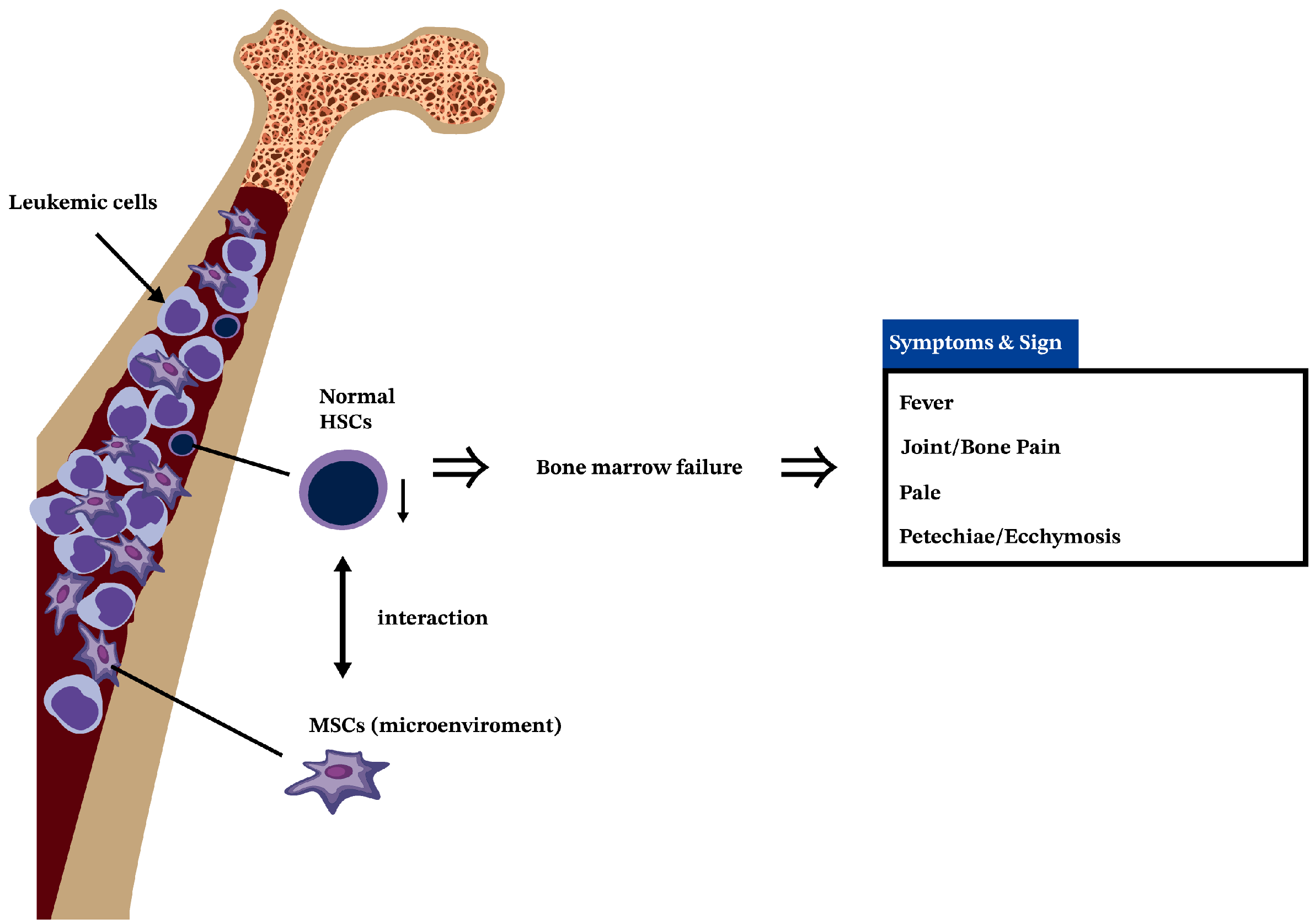
3. Leukemia Is a Cancer of HSCs
4. The Interactions of MSCs and HSCs in BM
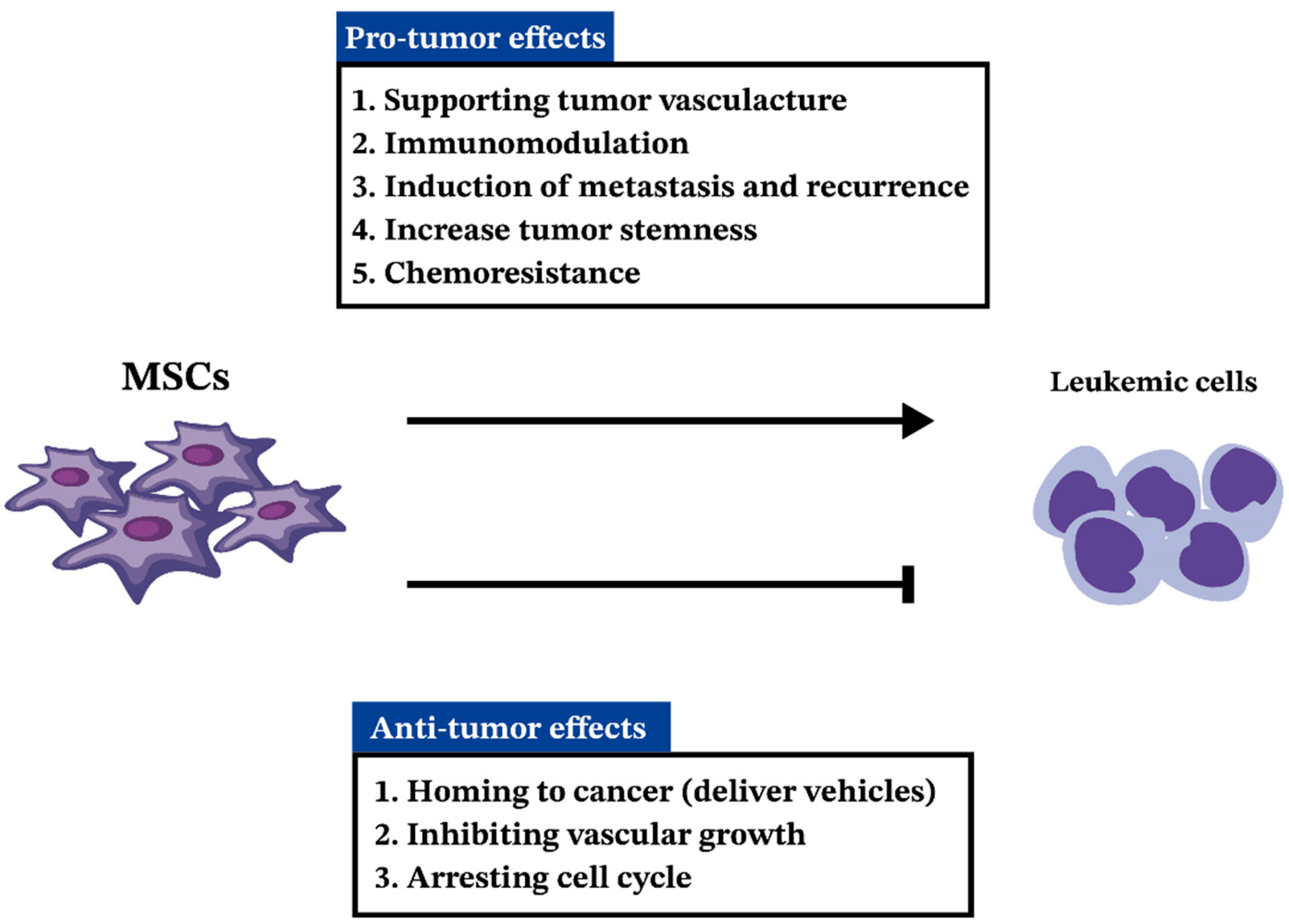
5. MSCs in AML
5.1. MSCs in Pathogenesis and Progression of AML
5.2. Anti-Tumorigenic Effects of MSCs in AML
6. MSCs in ALL
6.1. MSCs in B-ALL
6.1.1. MSCs in Pathogenesis and Progression of B-ALL
6.1.2. MSCs in Chemo-Resistance of B-ALL
6.2. MSCs in T-ALL
6.2.1. MSCs in Progression of T-ALL
6.2.2. The Role of MSCs in Chemo-Resistance of T-ALL Cells
7. MSCs in CML
7.1. MSCs in CML Pathogenesis and Progression
7.2. The Roles of MSCs in Promoting Resistance to TKIs in CML
7.3. The Multifaceted Impact of MSCs on CML
8. MSCs in CLL
8.1. MSCs in CLL Progression
8.2. The Role of MSCs in Chemo-Resistance of CLL
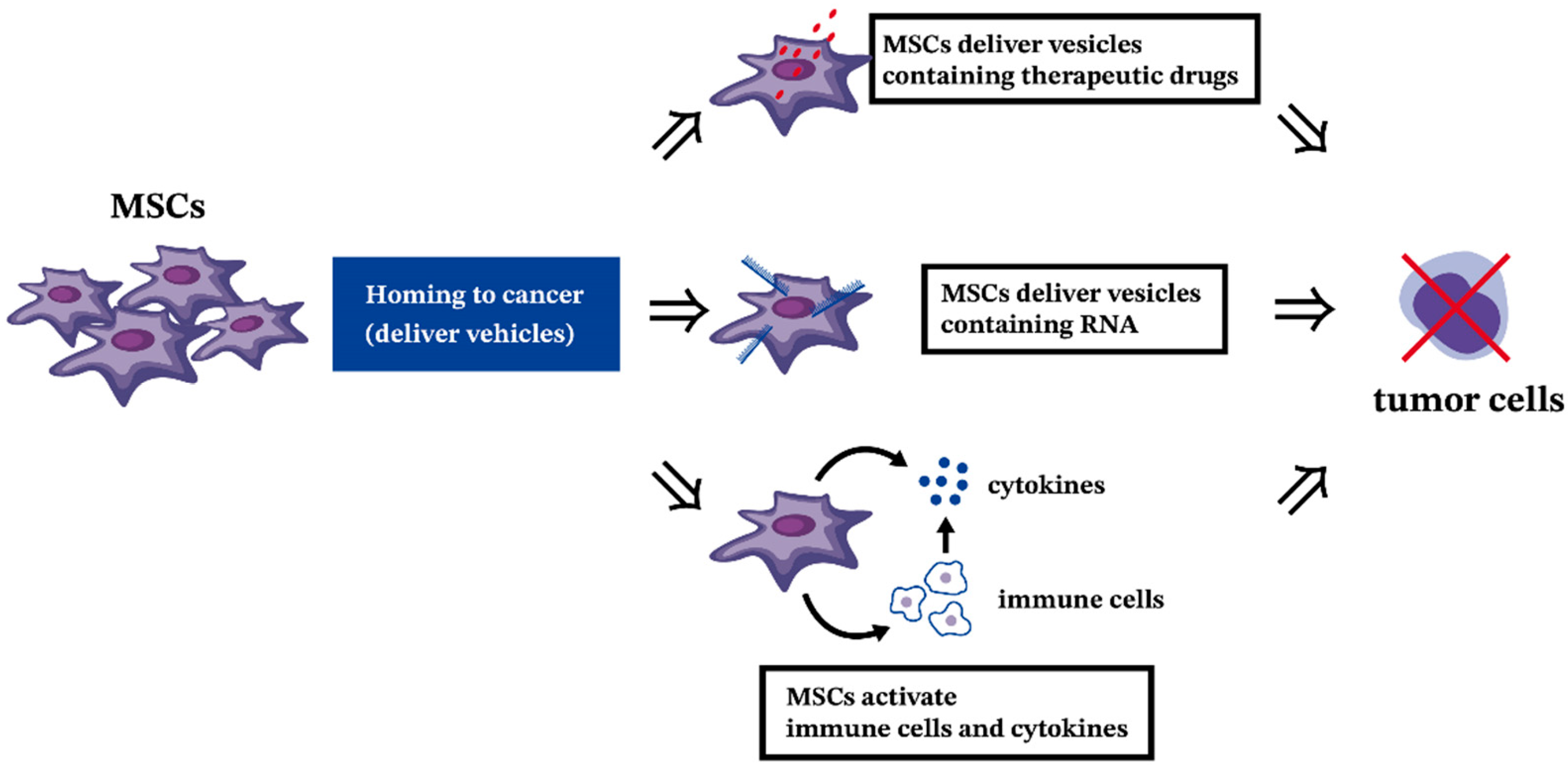
9. Potential Clinical Applications of MSCs in Leukemia
9.1. The Role of MSCs in Hematopoietic Stem Cell Transplantation for Leukemia
9.2. The Antitumor Effect of MSCs in Hematologic Malignancies
9.3. Disrupt the Chemo-Resistance from MSCs in Leukemia
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALL | Acute lymphoblastic leukemia |
| AML | Acute myeloid leukemia |
| BCR | B-cell antigen receptor |
| BM | Bone marrow |
| CAF | Cancer-associated fibroblast |
| CLL | Chronic lymphocytic leukemia |
| CML | Chronic myeloid leukemia |
| ECM | Extracellular matrix |
| EMT | Epithelial-mesenchymal transition |
| ESC | Embryonic stem cell |
| EV | Extracellular vesicle |
| FGF2 | Fibroblast growth factor 2 |
| FGFR2 | Fibroblast growth factor receptor 2 |
| GVHD | Graft-versus-host disease |
| HSC | Hematopoietic stem cells |
| HSCT | Hematopoietic stem cell transplantation |
| HSPC | Hematopoietic stem progenitor cell |
| LSC | Leukemia stem cell |
| MDSC | Myeloid-derived suppressor cell |
| MSC | Mesenchymal stem cell |
| PDGF | Platelet-derived growth factor |
| PTL | Parthenolide |
| rGSH | Reduced glutathione |
| ROS | Reactive oxygen species |
| TGF-β | Transforming growth factor-β |
| TKI | Tyrosine kinase inhibitor |
| TNT | Tunneling nanotube |
| USP16 | Ubiquitin-specific peptidase 16 |
| VEGF | Vascular endothelial growth factor |
References
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells-current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef]
- Asada, N.; Takeishi, S.; Frenette, P.S. Complexity of bone marrow hematopoietic stem cell niche. Int. J. Hematol. 2017, 106, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, Y.; Scadden, D.T. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell 2015, 16, 239–253. [Google Scholar] [CrossRef]
- Wei, Q.; Frenette, P.S. Niches for Hematopoietic Stem Cells and Their Progeny. Immunity 2018, 48, 632–648. [Google Scholar] [CrossRef] [PubMed]
- Barthes, J.; Özçelik, H.; Hindié, M.; Ndreu-Halili, A.; Hasan, A.; Vrana, N.E. Cell microenvironment engineering and monitoring for tissue engineering and regenerative medicine: The recent advances. Biomed. Res. Int. 2014, 2014, 921905. [Google Scholar] [CrossRef]
- Liu, J.; Gao, J.; Liang, Z.; Gao, C.; Niu, Q.; Wu, F.; Zhang, L. Mesenchymal stem cells and their microenvironment. Stem Cell Res. Ther. 2022, 13, 429. [Google Scholar] [CrossRef]
- Birbrair, A.; Frenette, P.S. Niche heterogeneity in the bone marrow. Ann. N. Y. Acad. Sci. 2016, 1370, 82–96. [Google Scholar] [CrossRef]
- Tan, L.; Liu, X.; Dou, H.; Hou, Y. Characteristics and regulation of mesenchymal stem cell plasticity by the microenvironment-specific factors involved in the regulation of MSC plasticity. Genes. Dis. 2022, 9, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Peta, K.T.; Ambele, M.A.; Pepper, M.S. Similarities between Tumour Immune Response and Chronic Wound Microenvironment: Influence of Mesenchymal Stromal/Stem Cells. J. Immunol. Res. 2021, 2021, 6649314. [Google Scholar] [CrossRef]
- Belson, M.; Kingsley, B.; Holmes, A. Risk factors for acute leukemia in children: A review. Environ. Health Perspect. 2007, 115, 138–145. [Google Scholar] [CrossRef]
- Zhou, H.S.; Carter, B.Z.; Andreeff, M. Bone marrow niche-mediated survival of leukemia stem cells in acute myeloid leukemia: Yin and Yang. Cancer Biol. Med. 2016, 13, 248–259. [Google Scholar] [CrossRef]
- Buss, E.C.; Ho, A.D. Leukemia stem cells. Int. J. Cancer 2011, 129, 2328–2336. [Google Scholar] [CrossRef]
- Yao, J.C.; Link, D.C. Concise Review: The Malignant Hematopoietic Stem Cell Niche. Stem Cells 2017, 35, 3–8. [Google Scholar] [CrossRef]
- Colmone, A.; Amorim, M.; Pontier, A.L.; Wang, S.; Jablonski, E.; Sipkins, D.A. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science 2008, 322, 1861–1865. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Brunning, R.D. Classification of acute leukemias. Semin. Diagn. Pathol. 2003, 20, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Vardiman, J.W. The World Health Organization (WHO) classification of tumors of the hematopoietic and lymphoid tissues: An overview with emphasis on the myeloid neoplasms. Chem. Biol. Interact. 2010, 184, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Belyavsky, A.V. Niches of Hematopoietic Stem Cells in Bone Marrow. Mol. Biol. 2019, 53, 1012–1019. [Google Scholar] [CrossRef]
- Kandarakov, O.; Belyavsky, A.; Semenova, E. Bone Marrow Niches of Hematopoietic Stem and Progenitor Cells. Int. J. Mol. Sci. 2022, 23, 4462. [Google Scholar] [CrossRef] [PubMed]
- Lee-Thedieck, C.; Schertl, P.; Klein, G. The extracellular matrix of hematopoietic stem cell niches. Adv. Drug Deliv. Rev. 2022, 181, 114069. [Google Scholar] [CrossRef]
- Cominal, J.G.; da Costa Cacemiro, M.; Pinto-Simões, B.; Kolb, H.J.; Malmegrim, K.C.R.; de Castro, F.A. Emerging Role of Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Pathogenesis of Haematological Malignancies. Stem Cells Int. 2019, 2019, 6854080. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Simmons, P.J.; Torok-Storb, B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood 1991, 78, 55–62. [Google Scholar] [CrossRef]
- Kuçi, S.; Kuçi, Z.; Kreyenberg, H.; Deak, E.; Pütsch, K.; Huenecke, S.; Amara, C.; Koller, S.; Rettinger, E.; Grez, M.; et al. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica 2010, 95, 651–659. [Google Scholar] [CrossRef]
- Méndez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef]
- Sacchetti, B.; Funari, A.; Michienzi, S.; Di Cesare, S.; Piersanti, S.; Saggio, I.; Tagliafico, E.; Ferrari, S.; Robey, P.G.; Riminucci, M.; et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007, 131, 324–336. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef]
- Boulais, P.E.; Frenette, P.S. Making sense of hematopoietic stem cell niches. Blood 2015, 125, 2621–2629. [Google Scholar] [CrossRef]
- Crippa, S.; Bernardo, M.E. Mesenchymal Stromal Cells: Role in the BM Niche and in the Support of Hematopoietic Stem Cell Transplantation. Hemasphere 2018, 2, e151. [Google Scholar] [CrossRef]
- Ehninger, A.; Trumpp, A. The bone marrow stem cell niche grows up: Mesenchymal stem cells and macrophages move in. J. Exp. Med. 2011, 208, 421–428. [Google Scholar] [CrossRef]
- Pontikoglou, C.; Deschaseaux, F.; Sensebé, L.; Papadaki, H.A. Bone marrow mesenchymal stem cells: Biological properties and their role in hematopoiesis and hematopoietic stem cell transplantation. Stem Cell Rev. Rep. 2011, 7, 569–589. [Google Scholar] [CrossRef] [PubMed]
- Taichman, R.S. Blood and bone: Two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood 2005, 105, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Taichman, R.S.; Emerson, S.G. The role of osteoblasts in the hematopoietic microenvironment. Stem Cells 1998, 16, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Zhi, W.; Deng, L.; Yang, Z. Role of osteoblasts in the hematopoietic microenvironment of bone marrow and regulatory pathways and mechanisms. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2007, 21, 517–522. [Google Scholar]
- Taichman, R.S.; Emerson, S.G. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J. Exp. Med. 1994, 179, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Visnjic, D.; Kalajzic, Z.; Rowe, D.W.; Katavic, V.; Lorenzo, J.; Aguila, H.L. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 2004, 103, 3258–3264. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Garrett, R.; Jung, Y.; Zhang, Y.; Kim, N.; Wang, J.; Joe, G.J.; Hexner, E.; Choi, Y.; Taichman, R.S.; et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood 2007, 109, 3706–3712. [Google Scholar] [CrossRef] [PubMed]
- Batsali, A.K.; Georgopoulou, A.; Mavroudi, I.; Matheakakis, A.; Pontikoglou, C.G.; Papadaki, H.A. The Role of Bone Marrow Mesenchymal Stem Cell Derived Extracellular Vesicles (MSC-EVs) in Normal and Abnormal Hematopoiesis and Their Therapeutic Potential. J. Clin. Med. 2020, 9, 856. [Google Scholar] [CrossRef] [PubMed]
- Naveiras, O.; Nardi, V.; Wenzel, P.L.; Hauschka, P.V.; Fahey, F.; Daley, G.Q. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 2009, 460, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Cocucci, E.; Meldolesi, J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Melzer, C.; Yang, Y.; Hass, R. Interaction of MSC with tumor cells. Cell Commun. Signal 2016, 14, 20. [Google Scholar] [CrossRef]
- Butler, J.T.; Abdelhamed, S.; Kurre, P. Extracellular vesicles in the hematopoietic microenvironment. Haematologica 2018, 103, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Timari, H.; Shamsasenjan, K.; Movassaghpour, A.; Akbarzadehlaleh, P.; Pashoutan Sarvar, D.; Aqmasheh, S. The Effect of Mesenchymal Stem Cell-Derived Extracellular Vesicles on Hematopoietic Stem Cells Fate. Adv. Pharm. Bull. 2017, 7, 531–546. [Google Scholar] [CrossRef]
- Zhou, Y.; Yamamoto, Y.; Xiao, Z.; Ochiya, T. The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. J. Clin. Med. 2019, 8, 1025. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, H.S.; Hong, I.S. Stem Cell-Derived Extracellular Vesicles as Immunomodulatory Therapeutics. Stem Cells Int. 2019, 2019, 5126156. [Google Scholar] [CrossRef]
- Park, K.S.; Bandeira, E.; Shelke, G.V.; Lässer, C.; Lötvall, J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 2019, 10, 288. [Google Scholar] [CrossRef]
- Gong, M.; Yu, B.; Wang, J.; Wang, Y.; Liu, M.; Paul, C.; Millard, R.W.; Xiao, D.S.; Ashraf, M.; Xu, M. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 2017, 8, 45200–45212. [Google Scholar] [CrossRef] [PubMed]
- De Kouchkovsky, I.; Abdul-Hay, M. Acute myeloid leukemia: A comprehensive review and 2016 update. Blood Cancer J. 2016, 6, e441. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, W.; Sun, H. A systematic review and meta-analysis on the risk factors of acute myeloid leukemia. Transl. Cancer Res. 2022, 11, 796–804. [Google Scholar] [CrossRef]
- Hartmann, L.; Metzeler, K.H. Clonal hematopoiesis and preleukemia-Genetics, biology, and clinical implications. Genes. Chromosomes Cancer 2019, 58, 828–838. [Google Scholar] [CrossRef]
- Kosmidou, A.; Tragiannidis, A.; Gavriilaki, E. Myeloid Leukemia of Down Syndrome. Cancers 2023, 15, 3265. [Google Scholar] [CrossRef]
- Thirman, M.J.; Larson, R.A. Therapy-related myeloid leukemia. Hematol. Oncol. Clin. N. Am. 1996, 10, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Ley, T.J.; Miller, C.; Ding, L.; Raphael, B.J.; Mungall, A.J.; Robertson, A.; Hoadley, K.; Triche, T.J., Jr.; Laird, P.W.; Baty, J.D.; et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar] [PubMed]
- Welch, J.S.; Ley, T.J.; Link, D.C.; Miller, C.A.; Larson, D.E.; Koboldt, D.C.; Wartman, L.D.; Lamprecht, T.L.; Liu, F.; Xia, J.; et al. The origin and evolution of mutations in acute myeloid leukemia. Cell 2012, 150, 264–278. [Google Scholar] [CrossRef]
- Moarii, M.; Papaemmanuil, E. Classification and risk assessment in AML: Integrating cytogenetics and molecular profiling. Hematology Am. Soc. Hematol. Educ. Program. 2017, 2017, 37–44. [Google Scholar] [CrossRef]
- Diaz de la Guardia, R.; Lopez-Millan, B.; Lavoie, J.R.; Bueno, C.; Castaño, J.; Gómez-Casares, M.; Vives, S.; Palomo, L.; Juan, M.; Delgado, J.; et al. Detailed Characterization of Mesenchymal Stem/Stromal Cells from a Large Cohort of AML Patients Demonstrates a Definitive Link to Treatment Outcomes. Stem Cell Rep. 2017, 8, 1573–1586. [Google Scholar] [CrossRef]
- Fracchiolla, N.S.; Fattizzo, B.; Cortelezzi, A. Mesenchymal Stem Cells in Myeloid Malignancies: A Focus on Immune Escaping and Therapeutic Implications. Stem Cells Int. 2017, 2017, 6720594. [Google Scholar] [CrossRef] [PubMed]
- Brenner, A.K.; Nepstad, I.; Bruserud, Ø. Mesenchymal Stem Cells Support Survival and Proliferation of Primary Human Acute Myeloid Leukemia Cells through Heterogeneous Molecular Mechanisms. Front. Immunol. 2017, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Azadniv, M.; Myers, J.R.; McMurray, H.R.; Guo, N.; Rock, P.; Coppage, M.L.; Ashton, J.; Becker, M.W.; Calvi, L.M.; Liesveld, J.L. Bone marrow mesenchymal stromal cells from acute myelogenous leukemia patients demonstrate adipogenic differentiation propensity with implications for leukemia cell support. Leukemia 2020, 34, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Kan, C.; Wong, M.; Sun, M.; Liu, Y.; Yang, F.; Wang, S.; Zheng, H. Regulation of Malignant Myeloid Leukemia by Mesenchymal Stem Cells. Front. Cell Dev. Biol. 2022, 10, 857045. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Ramirez, J.; Lavoie, J.R.; Maganti, H.B.; Stanford, W.L.; Ito, C.; Sabloff, M.; Brand, M.; Rosu-Myles, M.; Le, Y.; Allan, D.S. Micro-RNA Profiling of Exosomes from Marrow-Derived Mesenchymal Stromal Cells in Patients with Acute Myeloid Leukemia: Implications in Leukemogenesis. Stem Cell Rev. Rep. 2017, 13, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Y.; Li, X.; Ren, J.; Chen, L.; Chen, J.; Cao, Y. Exosomes from bone marrow mesenchymal stem cells decrease chemosensitivity of acute myeloid leukemia cells via delivering miR-10a. Biochem. Biophys. Res. Commun. 2022, 622, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Dong, Q.; Zhang, S.; Feng, Y.; Yang, J.; Zhao, L. Acute myeloid leukemia (AML)-derived mesenchymal stem cells induce chemoresistance and epithelial-mesenchymal transition-like program in AML through IL-6/JAK2/STAT3 signaling. Cancer Sci. 2023, 114, 3287–3300. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef]
- Luciano, M.; Krenn, P.W.; Horejs-Hoeck, J. The cytokine network in acute myeloid leukemia. Front. Immunol. 2022, 13, 1000996. [Google Scholar] [CrossRef]
- Lee, M.W.; Ryu, S.; Kim, D.S.; Lee, J.W.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Mesenchymal stem cells in suppression or progression of hematologic malignancy: Current status and challenges. Leukemia 2019, 33, 597–611. [Google Scholar] [CrossRef]
- Lee, H.Y.; Hong, I.S. Double-edged sword of mesenchymal stem cells: Cancer-promoting versus therapeutic potential. Cancer Sci. 2017, 108, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Kitagawa, Y. The role of mesenchymal stem cells in cancer development. Front. Genet. 2013, 4, 261. [Google Scholar] [CrossRef]
- Liang, R.; Huang, G.S.; Wang, Z.; Chen, X.Q.; Bai, Q.X.; Zhang, Y.Q.; Dong, B.X.; Wang, W.Q. Effects of human bone marrow stromal cell line (HFCL) on the proliferation, differentiation and apoptosis of acute myeloid leukemia cell lines U937, HL-60 and HL-60/VCR. Int. J. Hematol. 2008, 87, 152–166. [Google Scholar] [CrossRef]
- Fathi, E.; Farahzadi, R.; Valipour, B.; Sanaat, Z. Cytokines secreted from bone marrow derived mesenchymal stem cells promote apoptosis and change cell cycle distribution of K562 cell line as clinical agent in cell transplantation. PLoS ONE 2019, 14, e0215678. [Google Scholar] [CrossRef] [PubMed]
- Phetfong, J.; Tawonsawatruk, T.; Kamprom, W.; Ontong, P.; Tanyong, D.; Borwornpinyo, S.; Supokawej, A. Bone marrow-mesenchymal stem cell-derived extracellular vesicles affect proliferation and apoptosis of leukemia cells in vitro. FEBS Open Bio 2022, 12, 470–479. [Google Scholar] [CrossRef]
- Aravindhan, S.; Ejam, S.S.; Lafta, M.H.; Markov, A.; Yumashev, A.V.; Ahmadi, M. Mesenchymal stem cells and cancer therapy: Insights into targeting the tumour vasculature. Cancer Cell Int. 2021, 21, 158. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.G. Genetics and prognosis of ALL in children vs. adults. Hematol. Am. Soc. Hematol. Educ. Program 2018, 2018, 137–145. [Google Scholar] [CrossRef]
- Terwilliger, T.; Abdul-Hay, M. Acute lymphoblastic leukemia: A comprehensive review and 2017 update. Blood Cancer J. 2017, 7, e577. [Google Scholar] [CrossRef]
- Tebbi, C.K. Etiology of Acute Leukemia: A Review. Cancers 2021, 13, 2256. [Google Scholar] [CrossRef]
- Bhojwani, D.; Yang, J.J.; Pui, C.H. Biology of childhood acute lymphoblastic leukemia. Pediatr. Clin. N. Am. 2015, 62, 47–60. [Google Scholar] [CrossRef]
- Kakaje, A.; Alhalabi, M.M.; Ghareeb, A.; Karam, B.; Mansour, B.; Zahra, B.; Hamdan, O. Rates and trends of childhood acute lymphoblastic leukaemia: An epidemiology study. Sci. Rep. 2020, 10, 6756. [Google Scholar] [CrossRef]
- Yi, M.; Zhou, L.; Li, A.; Luo, S.; Wu, K. Global burden and trend of acute lymphoblastic leukemia from 1990 to 2017. Aging 2020, 12, 22869–22891. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, S.; Shi, Y.; Tang, Q.; Sun, J.; Bai, R.; Sun, Z.; Du, Z. The epidemic of acute lymphoid leukemia in China: Current trends and future prediction. Front. Oncol. 2023, 13, 1195065. [Google Scholar] [CrossRef]
- DeRenzo, C.; Krenciute, G.; Gottschalk, S. The Landscape of CAR T Cells Beyond Acute Lymphoblastic Leukemia for Pediatric Solid Tumors. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 830–837. [Google Scholar] [CrossRef]
- Ramos, K.N.; Ramos, I.N.; Zeng, Y.; Ramos, K.S. Genetics and epigenetics of pediatric leukemia in the era of precision medicine. F1000Res 2018, 7, F1000. [Google Scholar] [CrossRef]
- Valentin, R.; Grabow, S.; Davids, M.S. The rise of apoptosis: Targeting apoptosis in hematologic malignancies. Blood 2018, 132, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Fallati, A.; Di Marzo, N.; D’Amico, G.; Dander, E. Mesenchymal Stromal Cells (MSCs): An Ally of B-Cell Acute Lymphoblastic Leukemia (B-ALL) Cells in Disease Maintenance and Progression within the Bone Marrow Hematopoietic Niche. Cancers 2022, 14, 3303. [Google Scholar] [CrossRef] [PubMed]
- Tirado, H.A.; Balasundaram, N.; Laaouimir, L.; Erdem, A.; van Gastel, N. Metabolic crosstalk between stromal and malignant cells in the bone marrow niche. Bone Rep. 2023, 18, 101669. [Google Scholar] [CrossRef] [PubMed]
- Woods, K.; Guezguez, B. Dynamic Changes of the Bone Marrow Niche: Mesenchymal Stromal Cells and Their Progeny during Aging and Leukemia. Front. Cell Dev. Biol. 2021, 9, 714716. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.T.; Dolgalev, I.; Evensen, N.A.; Ma, C.; Chambers, T.; Roberts, K.G.; Sreeram, S.; Dai, Y.; Tikhonova, A.N.; Lasry, A.; et al. Extensive Remodeling of the Immune Microenvironment in B Cell Acute Lymphoblastic Leukemia. Cancer Cell 2020, 37, 867–882.e12. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.M.; Kuek, V.; Oommen, J.; Chua, G.A.; van Loenhout, M.; Malinge, S.; Kotecha, R.S.; Cheung, L.C. Characterization of mesenchymal stem cells in pre-B acute lymphoblastic leukemia. Front. Cell Dev. Biol. 2023, 11, 1005494. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.; Taurino, G.; Bianchi, M.G.; Bussolati, O. The Role of Amino Acids in the Crosstalk Between Mesenchymal Stromal Cells and Neoplastic Cells in the Hematopoietic Niche. Front. Cell Dev. Biol. 2021, 9, 714755. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.; Taurino, G.; Dander, E.; Bardelli, D.; Fallati, A.; Andreoli, R.; Bianchi, M.G.; Carubbi, C.; Pozzi, G.; Galuppo, L.; et al. ALL blasts drive primary mesenchymal stromal cells to increase asparagine availability during asparaginase treatment. Blood Adv. 2021, 5, 5164–5178. [Google Scholar] [CrossRef] [PubMed]
- Polak, R.; de Rooij, B.; Pieters, R.; den Boer, M.L. B-cell precursor acute lymphoblastic leukemia cells use tunneling nanotubes to orchestrate their microenvironment. Blood 2015, 126, 2404–2414. [Google Scholar] [CrossRef] [PubMed]
- De Rooij, B.; Polak, R.; Stalpers, F.; Pieters, R.; den Boer, M.L. Tunneling nanotubes facilitate autophagosome transfer in the leukemic niche. Leukemia 2017, 31, 1651–1654. [Google Scholar] [CrossRef]
- Burt, R.; Dey, A.; Aref, S.; Aguiar, M.; Akarca, A.; Bailey, K.; Day, W.; Hooper, S.; Kirkwood, A.; Kirschner, K.; et al. Activated stromal cells transfer mitochondria to rescue acute lymphoblastic leukemia cells from oxidative stress. Blood 2019, 134, 1415–1429. [Google Scholar] [CrossRef]
- Kihira, K.; Chelakkot, V.S.; Kainuma, H.; Okumura, Y.; Tsuboya, N.; Okamura, S.; Kurihara, K.; Iwamoto, S.; Komada, Y.; Hori, H. Close interaction with bone marrow mesenchymal stromal cells induces the development of cancer stem cell-like immunophenotype in B cell precursor acute lymphoblastic leukemia cells. Int. J. Hematol. 2020, 112, 795–806. [Google Scholar] [CrossRef]
- Manabe, A.; Coustan-Smith, E.; Behm, F.G.; Raimondi, S.C.; Campana, D. Bone marrow-derived stromal cells prevent apoptotic cell death in B-lineage acute lymphoblastic leukemia. Blood 1992, 79, 2370–2377. [Google Scholar] [CrossRef]
- Jacamo, R.; Chen, Y.; Wang, Z.; Ma, W.; Zhang, M.; Spaeth, E.L.; Wang, Y.; Battula, V.L.; Mak, P.Y.; Schallmoser, K.; et al. Reciprocal leukemia-stroma VCAM-1/VLA-4-dependent activation of NF-κB mediates chemoresistance. Blood 2014, 123, 2691–2702. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhao, X.; Deng, M.; Huang, Z.; Wang, J.; Wu, Y.; Cui, D.; Liu, Y.; Liu, R.; Ouyang, G. Bone Marrow Mesenchymal Stromal Cell-Derived Periostin Promotes B-ALL Progression by Modulating CCL2 in Leukemia Cells. Cell Rep. 2019, 26, 1533–1543.e4. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Li, Y.; Wang, L.; Si, J.; Zheng, Y.; Kang, J.; Wang, Y.; You, M.J.; Zheng, G. Blockade of FGF2/FGFR2 partially overcomes bone marrow mesenchymal stromal cells mediated progression of T-cell acute lymphoblastic leukaemia. Cell Death Dis. 2022, 13, 922. [Google Scholar] [CrossRef]
- Cowell, J.K.; Qin, H.; Hu, T.; Wu, Q.; Bhole, A.; Ren, M. Mutation in the FGFR1 tyrosine kinase domain or inactivation of PTEN is associated with acquired resistance to FGFR inhibitors in FGFR1-driven leukemia/lymphomas. Int. J. Cancer 2017, 141, 1822–1829. [Google Scholar] [CrossRef]
- Hu, T.; Wu, Q.; Chong, Y.; Qin, H.; Poole, C.J.; van Riggelen, J.; Ren, M.; Cowell, J.K. FGFR1 fusion kinase regulation of MYC expression drives development of stem cell leukemia/lymphoma syndrome. Leukemia 2018, 32, 2363–2373. [Google Scholar] [CrossRef]
- Chong, Y.; Liu, Y.; Lu, S.; Cai, B.; Qin, H.; Chang, C.S.; Ren, M.; Cowell, J.K.; Hu, T. Critical individual roles of the BCR and FGFR1 kinase domains in BCR-FGFR1-driven stem cell leukemia/lymphoma syndrome. Int. J. Cancer 2020, 146, 2243–2254. [Google Scholar] [CrossRef] [PubMed]
- Ramuta, T.; Kreft, M.E. Mesenchymal Stem/Stromal Cells May Decrease Success of Cancer Treatment by Inducing Resistance to Chemotherapy in Cancer Cells. Cancers 2022, 14, 3761. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Qiu, Y.; Shi, Y.; Cai, J.; Wang, B.; Wei, X.; Ke, Q.; Sui, X.; Wang, Y.; et al. Cell adhesion-mediated mitochondria transfer contributes to mesenchymal stem cell-induced chemoresistance on T cell acute lymphoblastic leukemia cells. J. Hematol. Oncol. 2018, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wang, J.; Huang, Y.; Wu, H.; Xia, T.; Xiao, J.; Chen, X.; Li, H.; Qiu, Y.; Wang, Y.; et al. ERK/Drp1-dependent mitochondrial fission is involved in the MSC-induced drug resistance of T-cell acute lymphoblastic leukemia cells. Cell Death Dis. 2016, 7, e2459. [Google Scholar] [CrossRef]
- Ede, B.C.; Asmaro, R.R.; Moppett, J.P.; Diamanti, P.; Blair, A. Investigating chemoresistance to improve sensitivity of childhood T-cell acute lymphoblastic leukemia to parthenolide. Haematologica 2018, 103, 1493–1501. [Google Scholar] [CrossRef]
- Balandrán, J.C.; Purizaca, J.; Enciso, J.; Dozal, D.; Sandoval, A.; Jiménez-Hernández, E.; Alemán-Lazarini, L.; Perez-Koldenkova, V.; Quintela-Núñez Del Prado, H.; Rios de Los Ríos, J.; et al. Pro-inflammatory-Related Loss of CXCL12 Niche Promotes Acute Lymphoblastic Leukemic Progression at the Expense of Normal Lymphopoiesis. Front. Immunol. 2016, 7, 666. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.E.G.; Deininger, M.W. Chronic Myeloid Leukemia: Modern therapies, current challenges and future directions. Blood Rev. 2021, 49, 100825. [Google Scholar] [CrossRef] [PubMed]
- Spiers, A.S.; Bain, B.J.; Turner, J.E. The peripheral blood in chronic granulocytic leukaemia. Study of 50 untreated Philadelphia-positive cases. Scand. J. Haematol. 1977, 18, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.; Kvasnicka, H.M.; Fischer, R. Bone marrow histopathology in chronic myelogenous leukemia (CML)—Evaluation of distinctive features with clinical impact. Histol. Histopathol. 1999, 14, 1241–1256. [Google Scholar]
- Faderl, S.; Talpaz, M.; Estrov, Z.; O’Brien, S.; Kurzrock, R.; Kantarjian, H.M. The biology of chronic myeloid leukemia. N. Engl. J. Med. 1999, 341, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, I.; Winston, K. Chronic Myeloid Leukemia, from Pathophysiology to Treatment-Free Remission: A Narrative Literature Review. J. Blood Med. 2023, 14, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Eide, C.A.; O’Hare, T. Chronic myeloid leukemia: Advances in understanding disease biology and mechanisms of resistance to tyrosine kinase inhibitors. Curr. Hematol. Malig. Rep. 2015, 10, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Mojtahedi, H.; Yazdanpanah, N.; Rezaei, N. Chronic myeloid leukemia stem cells: Targeting therapeutic implications. Stem Cell Res. Ther. 2021, 12, 603. [Google Scholar] [CrossRef] [PubMed]
- Rea, D.; Cayuela, J.M. Treatment-free remission in patients with chronic myeloid leukemia. Int. J. Hematol. 2018, 108, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Jootar, S.; Pornprasertsud, N.; Petvises, S.; Rerkamnuaychoke, B.; Disthabanchong, S.; Pakakasama, S.; Ungkanont, A.; Hongeng, S. Bone marrow derived mesenchymal stem cells from chronic myeloid leukemia t(9;22) patients are devoid of Philadelphia chromosome and support cord blood stem cell expansion. Leuk. Res. 2006, 30, 1493–1498. [Google Scholar] [CrossRef]
- Xishan, Z.; Guangyu, A.; Yuguang, S.; Hongmei, Z. The research on the immuno-modulatory defect of mesenchymal stem cell from Chronic Myeloid Leukemia patients. J. Exp. Clin. Cancer Res. 2011, 30, 47. [Google Scholar] [CrossRef]
- Tabe, Y.; Jin, L.; Iwabuchi, K.; Wang, R.Y.; Ichikawa, N.; Miida, T.; Cortes, J.; Andreeff, M.; Konopleva, M. Role of stromal microenvironment in nonpharmacological resistance of CML to imatinib through Lyn/CXCR4 interactions in lipid rafts. Leukemia 2012, 26, 883–892. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.; McDonald, T.; Holyoake, T.L.; Moon, R.T.; Campana, D.; Shultz, L.; Bhatia, R. Microenvironmental protection of CML stem and progenitor cells from tyrosine kinase inhibitors through N-cadherin and Wnt-β-catenin signaling. Blood 2013, 121, 1824–1838. [Google Scholar] [CrossRef] [PubMed]
- Vianello, F.; Villanova, F.; Tisato, V.; Lymperi, S.; Ho, K.K.; Gomes, A.R.; Marin, D.; Bonnet, D.; Apperley, J.; Lam, E.W.; et al. Bone marrow mesenchymal stromal cells non-selectively protect chronic myeloid leukemia cells from imatinib-induced apoptosis via the CXCR4/CXCL12 axis. Haematologica 2010, 95, 1081–1089. [Google Scholar] [CrossRef]
- Weisberg, E.; Wright, R.D.; McMillin, D.W.; Mitsiades, C.; Ray, A.; Barrett, R.; Adamia, S.; Stone, R.; Galinsky, I.; Kung, A.L.; et al. Stromal-mediated protection of tyrosine kinase inhibitor-treated BCR-ABL-expressing leukemia cells. Mol. Cancer Ther. 2008, 7, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tu, H.; Yang, Y.; Jiang, X.; Hu, X.; Luo, Q.; Li, J. Bone marrow-derived mesenchymal stromal cells promote resistance to tyrosine kinase inhibitors in chronic myeloid leukemia via the IL-7/JAK1/STAT5 pathway. J. Biol. Chem. 2019, 294, 12167–12179. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Sun, L.; Wang, Y.; Chen, B.; Wang, J.; Ge, X.; Lu, Y.; Yang, N.; Shen, P. Establishment of a novel mesenchymal stem cell-based regimen for chronic myeloid leukemia differentiation therapy. Cell Death Dis. 2021, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Giallongo, C.; Romano, A.; Parrinello, N.L.; La Cava, P.; Brundo, M.V.; Bramanti, V.; Stagno, F.; Vigneri, P.; Chiarenza, A.; Palumbo, G.A.; et al. Mesenchymal Stem Cells (MSC) Regulate Activation of Granulocyte-Like Myeloid Derived Suppressor Cells (G-MDSC) in Chronic Myeloid Leukemia Patients. PLoS ONE 2016, 11, e0158392. [Google Scholar] [CrossRef]
- Hallek, M.; Shanafelt, T.D.; Eichhorst, B. Chronic lymphocytic leukaemia. Lancet 2018, 391, 1524–1537. [Google Scholar] [CrossRef]
- Scarfò, L.; Ferreri, A.J.; Ghia, P. Chronic lymphocytic leukaemia. Crit. Rev. Oncol. Hematol. 2016, 104, 169–182. [Google Scholar] [CrossRef]
- Caligaris-Cappio, F.; Hamblin, T.J. B-cell chronic lymphocytic leukemia: A bird of a different feather. J. Clin. Oncol. 1999, 17, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Arp, E.W., Jr.; Wolf, P.H.; Checkoway, H. Lymphocytic leukemia and exposures to benzene and other solvents in the rubber industry. J. Occup. Med. 1983, 25, 598–602. [Google Scholar] [PubMed]
- Blair, A.; White, D.W. Leukemia cell types and agricultural practices in Nebraska. Arch. Environ. Health 1985, 40, 211–214. [Google Scholar] [CrossRef]
- Brown, L.M.; Gibson, R.; Blair, A.; Burmeister, L.F.; Schuman, L.M.; Cantor, K.P.; Fraumeni, J.F., Jr. Smoking and risk of leukemia. Am. J. Epidemiol. 1992, 135, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Khalade, A.; Jaakkola, M.S.; Pukkala, E.; Jaakkola, J.J. Exposure to benzene at work and the risk of leukemia: A systematic review and meta-analysis. Environ. Health 2010, 9, 31. [Google Scholar] [CrossRef]
- Marwick, C. Link found between Agent Orange and chronic lymphocytic leukaemia. BMJ 2003, 326, 242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schubauer-Berigan, M.K.; Daniels, R.D.; Fleming, D.A.; Markey, A.M.; Couch, J.R.; Ahrenholz, S.H.; Burphy, J.S.; Anderson, J.L.; Tseng, C.Y. Chronic lymphocytic leukaemia and radiation: Findings among workers at five US nuclear facilities and a review of the recent literature. Br. J. Haematol. 2007, 139, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Talibov, M.; Auvinen, A.; Weiderpass, E.; Hansen, J.; Martinsen, J.I.; Kjaerheim, K.; Tryggvadottir, L.; Pukkala, E. Occupational solvent exposure and adult chronic lymphocytic leukemia: No risk in a population-based case-control study in four Nordic countries. Int. J. Cancer 2017, 141, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Dühren-von Minden, M.; Übelhart, R.; Schneider, D.; Wossning, T.; Bach, M.P.; Buchner, M.; Hofmann, D.; Surova, E.; Follo, M.; Köhler, F.; et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature 2012, 489, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Ghia, P.; Caligaris-Cappio, F. Monoclonal B-cell lymphocytosis: Right track or red herring? Blood 2012, 119, 4358–4362. [Google Scholar] [CrossRef]
- Herishanu, Y.; Katz, B.Z.; Lipsky, A.; Wiestner, A. Biology of chronic lymphocytic leukemia in different microenvironments: Clinical and therapeutic implications. Hematol. Oncol. Clin. N. Am. 2013, 27, 173–206. [Google Scholar] [CrossRef]
- Sun, C.; Chen, Y.C.; Martinez Zurita, A.; Baptista, M.J.; Pittaluga, S.; Liu, D.; Rosebrock, D.; Gohil, S.H.; Saba, N.S.; Davies-Hill, T.; et al. The immune microenvironment shapes transcriptional and genetic heterogeneity in chronic lymphocytic leukemia. Blood Adv. 2023, 7, 145–158. [Google Scholar] [CrossRef]
- Dubois, N.; Crompot, E.; Meuleman, N.; Bron, D.; Lagneaux, L.; Stamatopoulos, B. Importance of Crosstalk Between Chronic Lymphocytic Leukemia Cells and the Stromal Microenvironment: Direct Contact, Soluble Factors, and Extracellular Vesicles. Front. Oncol. 2020, 10, 1422. [Google Scholar] [CrossRef]
- Binder, M.; Léchenne, B.; Ummanni, R.; Scharf, C.; Balabanov, S.; Trusch, M.; Schlüter, H.; Braren, I.; Spillner, E.; Trepel, M. Stereotypical chronic lymphocytic leukemia B-cell receptors recognize survival promoting antigens on stromal cells. PLoS ONE 2010, 5, e15992. [Google Scholar] [CrossRef]
- Trimarco, V.; Ave, E.; Facco, M.; Chiodin, G.; Frezzato, F.; Martini, V.; Gattazzo, C.; Lessi, F.; Giorgi, C.A.; Visentin, A.; et al. Cross-talk between chronic lymphocytic leukemia (CLL) tumor B cells and mesenchymal stromal cells (MSCs): Implications for neoplastic cell survival. Oncotarget 2015, 6, 42130–42149. [Google Scholar] [CrossRef] [PubMed]
- Plander, M.; Ugocsai, P.; Seegers, S.; Orsó, E.; Reichle, A.; Schmitz, G.; Hofstädter, F.; Brockhoff, G. Chronic lymphocytic leukemia cells induce anti-apoptotic effects of bone marrow stroma. Ann. Hematol. 2011, 90, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Nowakowski, G.S.; Knox, T.R.; Boysen, J.C.; Maas, M.L.; Schwager, S.M.; Wu, W.; Wellik, L.E.; Dietz, A.B.; Ghosh, A.K.; et al. Bi-directional activation between mesenchymal stem cells and CLL B-cells: Implication for CLL disease progression. Br. J. Haematol. 2009, 147, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, J.; Geng, Y. Exosomes derived from chronic lymphocytic leukaemia cells transfer miR-146a to induce the transition of mesenchymal stromal cells into cancer-associated fibroblasts. J. Biochem. 2020, 168, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Knox, T.R.; Tschumper, R.C.; Wu, W.; Schwager, S.M.; Boysen, J.C.; Jelinek, D.F.; Kay, N.E. Platelet-derived growth factor (PDGF)-PDGF receptor interaction activates bone marrow-derived mesenchymal stromal cells derived from chronic lymphocytic leukemia: Implications for an angiogenic switch. Blood 2010, 116, 2984–2993. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Noronha, N.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jiang, J.; Gu, Z.; Zhang, J.; Chen, Y.; Liu, X. Mesenchymal stromal cell therapies: Immunomodulatory properties and clinical progress. Stem Cell Res. Ther. 2020, 11, 345. [Google Scholar] [CrossRef]
- Studeny, M.; Marini, F.C.; Dembinski, J.L.; Zompetta, C.; Cabreira-Hansen, M.; Bekele, B.N.; Champlin, R.E.; Andreeff, M. Mesenchymal stem cells: Potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J. Natl. Cancer Inst. 2004, 96, 1593–1603. [Google Scholar] [CrossRef]
- Duchi, S.; Sotgiu, G.; Lucarelli, E.; Ballestri, M.; Dozza, B.; Santi, S.; Guerrini, A.; Dambruoso, P.; Giannini, S.; Donati, D.; et al. Mesenchymal stem cells as delivery vehicle of porphyrin loaded nanoparticles: Effective photoinduced in vitro killing of osteosarcoma. J. Control Release 2013, 168, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Tabata, Y.; Gao, J.Q. Mesenchymal stem cells as therapeutic agents and potential targeted gene delivery vehicle for brain diseases. J. Control Release 2012, 162, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, L.; Hu, J.; Sun, Y. Mesenchymal stem cells: A potential targeted-delivery vehicle for anti-cancer drug, loaded nanoparticles. Nanomedicine 2013, 9, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Porada, C.D.; Almeida-Porada, G. Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Von Bahr, L.; Sundberg, B.; Lönnies, L.; Sander, B.; Karbach, H.; Hägglund, H.; Ljungman, P.; Gustafsson, B.; Karlsson, H.; Le Blanc, K.; et al. Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol. Blood Marrow Transplant. 2012, 18, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Kadri, N.; Amu, S.; Iacobaeus, E.; Boberg, E.; Le Blanc, K. Current perspectives on mesenchymal stromal cell therapy for graft versus host disease. Cell Mol. Immunol. 2023, 20, 613–625. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, Q. The clinical application of mesenchymal stromal cells in hematopoietic stem cell transplantation. J. Hematol. Oncol. 2016, 9, 46. [Google Scholar] [CrossRef]
- Klopp, A.H.; Gupta, A.; Spaeth, E.; Andreeff, M.; Marini, F., 3rd. Concise review: Dissecting a discrepancy in the literature: Do mesenchymal stem cells support or suppress tumor growth? Stem Cells 2011, 29, 11–19. [Google Scholar] [CrossRef]
- Neuhuber, B.; Swanger, S.A.; Howard, L.; Mackay, A.; Fischer, I. Effects of plating density and culture time on bone marrow stromal cell characteristics. Exp. Hematol. 2008, 36, 1176–1185. [Google Scholar] [CrossRef]
- Ho, A.D.; Wagner, W.; Franke, W. Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy 2008, 10, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Du, J.; Xu, X.; Xu, C.; Song, W. IFN-γ-secreting-mesenchymal stem cells exert an antitumor effect in vivo via the TRAIL pathway. J. Immunol. Res. 2014, 2014, 318098. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, Y.; Huang, W.; Xu, H.; Chen, X.; Geng, Q.; Fan, H.; Tan, Y.; Xue, G.; Jiang, X. In Vitro effect of adenovirus-mediated human Gamma Interferon gene transfer into human mesenchymal stem cells for chronic myelogenous leukemia. Hematol. Oncol. 2006, 24, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lin, X.; Zhao, J.; Shi, W.; Zhang, H.; Wang, Y.; Kan, B.; Du, L.; Wang, B.; Wei, Y.; et al. A tumor-selective biotherapy with prolonged impact on established metastases based on cytokine gene-engineered MSCs. Mol. Ther. 2008, 16, 749–756. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Xu, W.; Qian, H.; Ye, S.; Zhu, W.; Cao, H.; Yan, Y.; Li, W.; Wang, M.; et al. Experimental therapy for lung cancer: Umbilical cord-derived mesenchymal stem cell-mediated interleukin-24 delivery. Curr. Cancer Drug Targets 2013, 13, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Gangadaran, P.; Rajendran, R.L.; Lee, H.W.; Kalimuthu, S.; Hong, C.M.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. Extracellular vesicles from mesenchymal stem cells activates VEGF receptors and accelerates recovery of hindlimb ischemia. J. Control Release 2017, 264, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.L.; Gangadaran, P.; Bak, S.S.; Oh, J.M.; Kalimuthu, S.; Lee, H.W.; Baek, S.H.; Zhu, L.; Sung, Y.K.; Jeong, S.Y.; et al. Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci. Rep. 2017, 7, 15560. [Google Scholar] [CrossRef]
- Hendijani, F.; Javanmard, S.H.; Sadeghi-aliabadi, H. Human Wharton’s jelly mesenchymal stem cell secretome display antiproliferative effect on leukemia cell line and produce additive cytotoxic effect in combination with doxorubicin. Tissue Cell 2015, 47, 229–234. [Google Scholar] [CrossRef]
- Rumpel, M.; Friedrich, T.; Deininger, M.W. Imatinib normalizes bone marrow vascularity in patients with chronic myeloid leukemia in first chronic phase. Blood 2003, 101, 4641–4643. [Google Scholar] [CrossRef]
- Otsu, K.; Das, S.; Houser, S.D.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood 2009, 113, 4197–4205. [Google Scholar] [CrossRef]
- Sauer, H.; Wartenberg, M. Reactive oxygen species as signaling molecules in cardiovascular differentiation of embryonic stem cells and tumor-induced angiogenesis. Antioxid. Redox Signal 2005, 7, 1423–1434. [Google Scholar] [CrossRef]
- Song, N.; Gao, L.; Qiu, H.; Huang, C.; Cheng, H.; Zhou, H.; Lv, S.; Chen, L.; Wang, J. Mouse bone marrow-derived mesenchymal stem cells inhibit leukemia/lymphoma cell proliferation in vitro and in a mouse model of allogeneic bone marrow transplant. Int. J. Mol. Med. 2015, 36, 139–149. [Google Scholar] [CrossRef]
- Ramasamy, R.; Lam, E.W.; Soeiro, I.; Tisato, V.; Bonnet, D.; Dazzi, F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: Impact on in vivo tumor growth. Leukemia 2007, 21, 304–310. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y.; Xu, Z.; Li, J.; Yang, J.; Li, Y.; Shang, Y.; Luo, J. Effect of bone marrow mesenchymal stem cells from blastic phase chronic myelogenous leukemia on the growth and apoptosis of leukemia cells. Oncol. Rep. 2013, 30, 1007–1013. [Google Scholar] [CrossRef]
- Sarmadi, V.H.; Tong, C.K.; Vidyadaran, S.; Abdullah, M.; Seow, H.F.; Ramasamy, R. Mesenchymal stem cells inhibit proliferation of lymphoid origin haematopoietic tumour cells by inducing cell cycle arrest. Med. J. Malaysia 2010, 65, 209–214. [Google Scholar]
- Wei, Z.; Chen, N.; Guo, H.; Wang, X.; Xu, F.; Ren, Q.; Lu, S.; Liu, B.; Zhang, L.; Zhao, H. Bone marrow mesenchymal stem cells from leukemia patients inhibit growth and apoptosis in serum-deprived K562 cells. J. Exp. Clin. Cancer Res. 2009, 28, 141. [Google Scholar] [CrossRef]
- Tian, K.; Yang, S.; Ren, Q.; Han, Z.; Lu, S.; Ma, F.; Zhang, L.; Han, Z. p38 MAPK contributes to the growth inhibition of leukemic tumor cells mediated by human umbilical cord mesenchymal stem cells. Cell Physiol. Biochem. 2010, 26, 799–808. [Google Scholar] [CrossRef]
- Kastan, M.B.; Bartek, J. Cell-cycle checkpoints and cancer. Nature 2004, 432, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Uy, G.L.; Rettig, M.P.; Motabi, I.H.; McFarland, K.; Trinkaus, K.M.; Hladnik, L.M.; Kulkarni, S.; Abboud, C.N.; Cashen, A.F.; Stockerl-Goldstein, K.E.; et al. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood 2012, 119, 3917–3924. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, G.; Zeng, Z.; Cortes, J.E.; Chen, H.C.; Huang, X.; Konopleva, M.; Ravandi, F.; Kadia, T.; Patel, K.P.; Daver, N.; et al. Phase 1 study of combinatorial sorafenib, G-CSF, and plerixafor treatment in relapsed/refractory, FLT3-ITD-mutated acute myelogenous leukemia patients. Am. J. Hematol. 2020, 95, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Mak, P.Y.; Mu, H.; Mak, D.H.; Zeng, Z.; Cortes, J.; Liu, Q.; Andreeff, M.; Carter, B.Z. Combined inhibition of β-catenin and Bcr-Abl synergistically targets tyrosine kinase inhibitor-resistant blast crisis chronic myeloid leukemia blasts and progenitors in vitro and in vivo. Leukemia 2017, 31, 2065–2074. [Google Scholar] [CrossRef]
- Jiang, X.; Mak, P.Y.; Mu, H.; Tao, W.; Mak, D.H.; Kornblau, S.; Zhang, Q.; Ruvolo, P.; Burks, J.K.; Zhang, W.; et al. Disruption of Wnt/β-Catenin Exerts Antileukemia Activity and Synergizes with FLT3 Inhibition in FLT3-Mutant Acute Myeloid Leukemia. Clin. Cancer Res. 2018, 24, 2417–2429. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.Z.; Mak, P.Y.; Wang, X.; Tao, W.; Ruvolo, V.; Mak, D.; Mu, H.; Burks, J.K.; Andreeff, M. An ARC-Regulated IL1β/Cox-2/PGE2/β-Catenin/ARC Circuit Controls Leukemia-Microenvironment Interactions and Confers Drug Resistance in AML. Cancer Res. 2019, 79, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Faderl, S.; Pagel, J.M.; Jung, C.W.; Yoon, S.S.; Pardanani, A.D.; Becker, P.S.; Lee, H.; Choi, J.; Lee, K.; et al. Phase 1 study of CWP232291 in patients with relapsed or refractory acute myeloid leukemia and myelodysplastic syndrome. Blood Adv. 2020, 4, 2032–2043. [Google Scholar] [CrossRef]
- Gutjahr, J.C.; Bayer, E.; Yu, X.; Laufer, J.M.; Höpner, J.P.; Tesanovic, S.; Härzschel, A.; Auer, G.; Rieß, T.; Salmhofer, A.; et al. CD44 engagement enhances acute myeloid leukemia cell adhesion to the bone marrow microenvironment by increasing VLA-4 avidity. Haematologica 2021, 106, 2102–2113. [Google Scholar] [CrossRef]
- Yu, X.; Munoz-Sagredo, L.; Streule, K.; Muschong, P.; Bayer, E.; Walter, R.J.; Gutjahr, J.C.; Greil, R.; Concha, M.L.; Müller-Tidow, C.; et al. CD44 loss of function sensitizes AML cells to the BCL-2 inhibitor venetoclax by decreasing CXCL12-driven survival cues. Blood 2021, 138, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- DeAngelo, D.J.; Jonas, B.A.; Liesveld, J.L.; Bixby, D.L.; Advani, A.S.; Marlton, P.; Magnani, J.L.; Thackray, H.M.; Feldman, E.J.; O’Dwyer, M.E.; et al. Phase 1/2 study of uproleselan added to chemotherapy in patients with relapsed or refractory acute myeloid leukemia. Blood 2022, 139, 1135–1146. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Hart, L.L.; Mace, J.R.; Arrowsmith, E.R.; Essell, J.H.; Owera, R.S.; Hainsworth, J.D.; Flinn, I.W. Phase I/II study of the combination of panobinostat and carfilzomib in patients with relapsed/refractory multiple myeloma. Haematologica 2015, 100, 670–676. [Google Scholar] [CrossRef]
- Advani, A.S.; Cooper, B.; Visconte, V.; Elson, P.; Chan, R.; Carew, J.; Wei, W.; Mukherjee, S.; Gerds, A.; Carraway, H.; et al. A Phase I/II Trial of MEC (Mitoxantrone, Etoposide, Cytarabine) in Combination with Ixazomib for Relapsed Refractory Acute Myeloid Leukemia. Clin. Cancer Res. 2019, 25, 4231–4237. [Google Scholar] [CrossRef]
- Wong, R.S. Mesenchymal stem cells: Angels or demons? J. Biomed. Biotechnol. 2011, 2011, 459510. [Google Scholar] [CrossRef]
- Qiao, L.; Xu, Z.L.; Zhao, T.J.; Ye, L.H.; Zhang, X.D. Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett. 2008, 269, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.L.; Yue, W.; Zhu, F.; Li, S.; Li, W. Human mesenchymal stem cells play a dual role on tumor cell growth in vitro and in vivo. J. Cell Physiol. 2011, 226, 1860–1867. [Google Scholar] [PubMed]
- Kidd, S.; Caldwell, L.; Dietrich, M.; Samudio, I.; Spaeth, E.L.; Watson, K.; Shi, Y.; Abbruzzese, J.; Konopleva, M.; Andreeff, M.; et al. Mesenchymal stromal cells alone or expressing interferon-beta suppress pancreatic tumors in vivo, an effect countered by anti-inflammatory treatment. Cytotherapy 2010, 12, 615–625. [Google Scholar] [CrossRef]
- Yu, J.M.; Jun, E.S.; Bae, Y.C.; Jung, J.S. Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth in vivo. Stem Cells Dev. 2008, 17, 463–473. [Google Scholar] [CrossRef]
- Lin, G.; Yang, R.; Banie, L.; Wang, G.; Ning, H.; Li, L.C.; Lue, T.F.; Lin, C.S. Effects of transplantation of adipose tissue-derived stem cells on prostate tumor. Prostate 2010, 70, 1066–1073. [Google Scholar] [CrossRef]
- Zhu, N.; Wang, H.; Wei, J.; Wang, B.; Shan, W.; Lai, X.; Zhao, Y.; Yu, J.; Huang, H. NR2F2 regulates bone marrow-derived mesenchymal stem cell-promoted proliferation of Reh cells. Mol. Med. Rep. 2016, 14, 1351–1356. [Google Scholar] [CrossRef][Green Version]
- Lin, H.D.; Fong, C.Y.; Biswas, A.; Choolani, M.; Bongso, A. Human Umbilical Cord Wharton’s Jelly Stem Cell Conditioned Medium Induces Tumoricidal Effects on Lymphoma Cells through Hydrogen Peroxide Mediation. J. Cell Biochem. 2016, 117, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.G.; Shuttleworth, C.A.; Kielty, C.M. Mesenchymal stem cells and neovascularization: Role of platelet-derived growth factor receptors. J. Cell Mol. Med. 2007, 11, 1012–1030. [Google Scholar] [CrossRef] [PubMed]
- Al-Khaldi, A.; Eliopoulos, N.; Martineau, D.; Lejeune, L.; Lachapelle, K.; Galipeau, J. Postnatal bone marrow stromal cells elicit a potent VEGF-dependent neoangiogenic response in vivo. Gene Ther. 2003, 10, 621–629. [Google Scholar] [CrossRef]
- Roorda, B.D.; ter Elst, A.; Kamps, W.A.; de Bont, E.S. Bone marrow-derived cells and tumor growth: Contribution of bone marrow-derived cells to tumor micro-environments with special focus on mesenchymal stem cells. Crit. Rev. Oncol. Hematol. 2009, 69, 187–198. [Google Scholar] [CrossRef]
- Liu, Y.; Han, Z.P.; Zhang, S.S.; Jing, Y.Y.; Bu, X.X.; Wang, C.Y.; Sun, K.; Jiang, G.C.; Zhao, X.; Li, R.; et al. Effects of inflammatory factors on mesenchymal stem cells and their role in the promotion of tumor angiogenesis in colon cancer. J. Biol. Chem. 2011, 286, 25007–25015. [Google Scholar] [CrossRef] [PubMed]
- Castro-Manrreza, M.E.; Montesinos, J.J. Immunoregulation by mesenchymal stem cells: Biological aspects and clinical applications. J. Immunol. Res. 2015, 2015, 394917. [Google Scholar] [CrossRef] [PubMed]
- Di Ianni, M.; Del Papa, B.; De Ioanni, M.; Moretti, L.; Bonifacio, E.; Cecchini, D.; Sportoletti, P.; Falzetti, F.; Tabilio, A. Mesenchymal cells recruit and regulate T regulatory cells. Exp. Hematol. 2008, 36, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, P.A.; Perez, S.A.; Gritzapis, A.D.; Baxevanis, C.N.; Papamichail, M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 2006, 24, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Potenta, S.; Zeisberg, E.; Kalluri, R. The role of endothelial-to-mesenchymal transition in cancer progression. Br. J. Cancer 2008, 99, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Chang, A.I.; Schwertschkow, A.H.; Nolta, J.A.; Wu, J. Involvement of mesenchymal stem cells in cancer progression and metastases. Curr. Cancer Drug Targets 2015, 15, 88–98. [Google Scholar] [CrossRef]
- Meleshina, A.V.; Cherkasova, E.I.; Shirmanova, M.V.; Klementieva, N.V.; Kiseleva, E.V.; Snopova, L.; Prodanets, N.N.; Zagaynova, E.V. Influence of mesenchymal stem cells on metastasis development in mice in vivo. Stem Cell Res. Ther. 2015, 6, 15. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, J.K.; Shiozawa, Y.; Wang, J.; Mishra, A.; Joseph, J.; Berry, J.E.; McGee, S.; Lee, E.; Sun, H.; et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat. Commun. 2013, 4, 1795. [Google Scholar] [CrossRef]
- Kabashima-Niibe, A.; Higuchi, H.; Takaishi, H.; Masugi, Y.; Matsuzaki, Y.; Mabuchi, Y.; Funakoshi, S.; Adachi, M.; Hamamoto, Y.; Kawachi, S.; et al. Mesenchymal stem cells regulate epithelial-mesenchymal transition and tumor progression of pancreatic cancer cells. Cancer Sci. 2013, 104, 157–164. [Google Scholar] [CrossRef]
- Corcoran, K.E.; Trzaska, K.A.; Fernandes, H.; Bryan, M.; Taborga, M.; Srinivas, V.; Packman, K.; Patel, P.S.; Rameshwar, P. Mesenchymal stem cells in early entry of breast cancer into bone marrow. PLoS ONE 2008, 3, e2563. [Google Scholar] [CrossRef]
- Trendowski, M. The inherent metastasis of leukaemia and its exploitation by sonodynamic therapy. Crit. Rev. Oncol. Hematol. 2015, 94, 149–163. [Google Scholar] [CrossRef]
- Wang, H.H.; Cui, Y.L.; Zaorsky, N.G.; Lan, J.; Deng, L.; Zeng, X.L.; Wu, Z.Q.; Tao, Z.; Guo, W.H.; Wang, Q.X.; et al. Mesenchymal stem cells generate pericytes to promote tumor recurrence via vasculogenesis after stereotactic body radiation therapy. Cancer Lett. 2016, 375, 349–359. [Google Scholar] [CrossRef]
- Ning, H.; Yang, F.; Jiang, M.; Hu, L.; Feng, K.; Zhang, J.; Yu, Z.; Li, B.; Xu, C.; Li, Y.; et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: Outcome of a pilot clinical study. Leukemia 2008, 22, 593–599. [Google Scholar] [CrossRef]
- Zhang, X.; Tu, H.; Yang, Y.; Wan, Q.; Fang, L.; Wu, Q.; Li, J. High IL-7 levels in the bone marrow microenvironment mediate imatinib resistance and predict disease progression in chronic myeloid leukemia. Int. J. Hematol. 2016, 104, 358–367. [Google Scholar] [CrossRef]
- Jin, L.; Tabe, Y.; Konoplev, S.; Xu, Y.; Leysath, C.E.; Lu, H.; Kimura, S.; Ohsaka, A.; Rios, M.B.; Calvert, L.; et al. CXCR4 up-regulation by imatinib induces chronic myelogenous leukemia (CML) cell migration to bone marrow stroma and promotes survival of quiescent CML cells. Mol. Cancer Ther. 2008, 7, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.Z.; Mak, P.Y.; Chen, Y.; Mak, D.H.; Mu, H.; Jacamo, R.; Ruvolo, V.; Arold, S.T.; Ladbury, J.E.; Burks, J.K.; et al. Anti-apoptotic ARC protein confers chemoresistance by controlling leukemia-microenvironment interactions through a NFκB/IL1β signaling network. Oncotarget 2016, 7, 20054–20067. [Google Scholar] [CrossRef]
- Xia, B.; Tian, C.; Guo, S.; Zhang, L.; Zhao, D.; Qu, F.; Zhao, W.; Wang, Y.; Wu, X.; Da, W.; et al. c-Myc plays part in drug resistance mediated by bone marrow stromal cells in acute myeloid leukemia. Leuk. Res. 2015, 39, 92–99. [Google Scholar] [CrossRef]
- Takam Kamga, P.; Bassi, G.; Cassaro, A.; Midolo, M.; Di Trapani, M.; Gatti, A.; Carusone, R.; Resci, F.; Perbellini, O.; Gottardi, M.; et al. Notch signalling drives bone marrow stromal cell-mediated chemoresistance in acute myeloid leukemia. Oncotarget 2016, 7, 21713–21727. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.N.; Zhao, Y.M.; He, Y.; Wang, B.S.; Du, K.L.; Fu, S.; Hu, K.M.; Zhang, L.F.; Liu, L.Z.; Hu, Y.X.; et al. Rapamycin interacts synergistically with idarubicin to induce T-leukemia cell apoptosis in vitro and in a mesenchymal stem cell simulated drug-resistant microenvironment via Akt/mammalian target of rapamycin and extracellular signal-related kinase signaling pathways. Leuk. Lymphoma 2014, 55, 668–676. [Google Scholar] [PubMed]
| Reference | The Pro-Tumorigenic Effects of MSCs in AML |
|---|---|
| Brenner, A.K. et al. [62] | Even though the effects of single cytokines derived from BM-MSCs on human AML cells differ among patients, the final cytokine-mediated effects of the MSCs during co-culture are growth enhancement and inhibition of apoptosis. |
| Azadniv, M. et al. [63] | AML-MSCs possess adipogenic potential, which may enhance support of leukemia progenitor cells. |
| Barrera-Ramirez, J. et al. [65] | MSC-derived exosomal miRNA represents a potential mechanism for influencing gene regulatory networks in AML. |
| Wu, J. et al. [66] | AML BM-MSCs released exosomes that delivered miR-10a to leukemia cells and downregulated RPRD1A, which activated Wnt/β-catenin that subsequently conferred protection of leukemia cells from chemotherapy. |
| Lu, J. et al. [67] | AML-MSCs induce the EMT-like characteristics in AML; this phenotypic change could be related to chemo-resistance progression. Additionally, AML-MSCs induce the EMT-like program in AML via IL-6/JAK2/STAT3 signaling, which could be a target to reverse chemo-resistance in AML. |
| Reference | The Anti-Tumorigenic Effects of MSCs in AML |
| Liang et al. [73] | Direct contact with HFCL stromal cells could inhibit the proliferation and induce the differentiation of AML cells. |
| Phetfong, J. et al. [75] | This study with NB4 and K562 enlightens the influence of MSC-EVs on some types of leukemic cell lines. The molecular mechanism causing cell apoptosis seemed to be different between both cell lines. |
| Reference | Main Findings |
|---|---|
| Fallati, A. et al. [87] | MSCs contribute to B-ALL pathogenesis and progression by creating a leukemia-supportive BM microenvironment rich in TGF-β molecules and inflammatory mediators. |
| Balandrán, J.C. et al. [101] | ALL tumor cells alter their environment, affecting MSCs and CXCL12 production. This change supports leukemic cell proliferation while hindering normal hematopoiesis. |
| Hughes, A.M. et al. [91] | BCR-ABL1+ B-ALL-associated MSCs exhibit reduced self-renewal capacity and extensive molecular alterations, indicating potential disruptions to important signaling pathways involved in inflammation, osteoblastogenesis, and ECM organization in vivo. |
| Chiu, M. et al. [93] | ALL blasts engage in an amino acid exchange with BM-MSCs, using glutamine to gain asparagine, aiding survival during L-asparaginase treatment. Blocking this pathway could enhance therapy effectiveness. |
| Polak, R. et al. [94] | TNT communication between B-ALL cells and MSCs in the BM influences cytokine release, impacting drug resistance and leukemia cell survival, suggesting TNT disruption as a potential therapeutic strategy. |
| Burt, R. et al. [96] | MSCs in ALL patient BM exhibit a cancer-associated fibroblast-like phenotype, secreting high levels of pro-inflammatory cytokines and affecting chemo-resistance, leading to a new hypothesis for preventing chemo-resistance in ALL. |
| Jacamo, R. et al. [99] | Leukemia cells modify BM-MSCs via NF-κB activation, enhancing chemo-resistance, suggesting that disrupting this interaction could improve leukemia treatment effectiveness. |
| Ma, Z. et al. [100] | Periostin, an extracellular component, enhances leukemia progression in B-ALL by increasing CCL2 expression via the integrin-ILK-NF-κB pathway, with reciprocal activation by CCL2, suggesting a key role in B-ALL cells-BM-MSC interactions. |
| Reference | Main Findings |
|---|---|
| Tian, C. et al. [102] | BM microenvironmental MSCs promote T-ALL cell growth via FGF2 and FGFR2 interaction, activating the PI3K/AKT/mTOR pathway. Targeting this interaction with FGF2 inhibition or FGFR2 antagonism suppresses T-ALL progression. |
| Wang, J. et al. [107] | T-ALL cells combat chemotherapy-induced stress by transferring mitochondria to MSCs via TNTs, often adhering via ICAM-1, suggesting targeting mitochondria transfer as a strategy against T-ALL chemo-resistance. |
| Cai, J. et al. [108] | MSCs in direct co-culture with T-ALL cells enhance leukemia chemo-resistance by activating the MAPK/ERK pathway, leading to changes in mitochondrial dynamics and metabolism, suggesting targeting these interactions as a new treatment strategy. |
| Ede, B.C. et al. [109] | PTL, effective against T-ALL in xenografts, faces resistance, possibly due to the BM microenvironment. MSCs protect T-ALL cells by mitigating PTL-induced ROS stress, with the xc system playing a key role. Targeting this system may enhance PTL’s effectiveness against leukemia. |
| Reference | Main Findings |
|---|---|
| Jootar, S. et al. [118] | MSCs from CML patient BM, free of the Ph chromosome and capable of differentiating into osteoblasts, can support cord blood stem cell expansion and reduce graft-versus-host disease (GVHD) in stem cell transplants. |
| Xishan, Z. et al. [119] | MSCs from CML patients have impaired immunomodulatory functions, affecting the hematopoietic environment. This impairment might limit the effectiveness of autologous MSC transplantation in CML treatment, pointing towards allogeneic transplantation as a more viable option. |
| Zhang, B. et al. [121] | TKI treatment for CML is effective but does not eliminate LSCs, which can cause relapse. The BM microenvironment, particularly MSCs, protects these LSCs via N-cadherin and Wnt/β-catenin signaling, suggesting new treatment targets. |
| Vianello, F. et al. [122] | Human MSCs shield CML cells from imatinib-induced apoptosis; targeting the CXCL12/CXCR4 axis with anti-CXCR4 antagonists could enhance treatment efficacy. |
| Zhang, X. et al. [124] | IL-7, secreted by MSCs, plays a crucial role in CML by inducing resistance to TKIs via JAK1/STAT5 activation, suggesting a combined approach of IL-7/JAK1/STAT5 inhibitors with TKIs for effective treatment. |
| Zuo, S. et al. [125] | MSCs enhance megakaryocytic differentiation in CML by restoring MPL signaling, and when combined with MPL agonist Eltrombopag, significantly reduce leukemia burden, offering a new therapeutic strategy. |
| Giallongo, C. et al. [126] | In CML, MSCs play a crucial role in creating an immune-suppressive microenvironment by driving MDSC activation, affecting T lymphocyte function, and contributing to leukemia immune evasion, highlighting their potential as a therapeutic target. |
| Reference | Main Findings |
|---|---|
| Binder, M. et al. [142] | BCRs on CLL cells recognize stromal cell antigens like vimentin, influencing apoptosis protection and potentially contributing to disease heterogeneity and progression. |
| Trimarco, V. et al. [143] | CLL B cell–MSC co-culture mimics BM conditions in vitro, reflecting CLL B cell response diversity. Kinase inhibitors like Bafetinib and Ibrutinib disrupt B cell–MSC interactions, crucial for targeting CLL treatment strategies. |
| Plander, M. et al. [144] | CLL cells alter BM-MSCs in the BM, enhancing their survival by upregulating specific adhesion molecules like ICAM-1, CD18, and CD49d and increasing secretion of cytokines like TNF-α, IL-6 and IL-8, which contribute to apoptosis resistance in CLL. |
| Ding, W. et al. [145] | In co-culture with MSCs, CLL B-cells show increased CD38, CD71, CD25, CD69, and CD70 markers, indicating disease progression. Soluble factors activate MSCs, leading to ERK and AKT phosphorylation and preventing both spontaneous and drug-induced apoptosis in CLL B-cells. |
| Yang, Y., Li, J. et al. [146] | In CLL, exosomes from CLL cells deliver miR-146a to BM-MSCs, inducing their transformation into CAFs by targeting USP16. |
| Ding, W. et al. [147] | The study demonstrates that PDGF secreted by CLL cells activates MSCs via the PDGFR-PI3K-Akt activation, increasing VEGF production and influencing leukemia progression, drug resistance, and angiogenesis in the CLL microenvironment. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-H.; Weng, T.-F.; Li, J.-P.; Wu, K.-H. Biology and Therapeutic Properties of Mesenchymal Stem Cells in Leukemia. Int. J. Mol. Sci. 2024, 25, 2527. https://doi.org/10.3390/ijms25052527
Wu C-H, Weng T-F, Li J-P, Wu K-H. Biology and Therapeutic Properties of Mesenchymal Stem Cells in Leukemia. International Journal of Molecular Sciences. 2024; 25(5):2527. https://doi.org/10.3390/ijms25052527
Chicago/Turabian StyleWu, Cheng-Hsien, Te-Fu Weng, Ju-Pi Li, and Kang-Hsi Wu. 2024. "Biology and Therapeutic Properties of Mesenchymal Stem Cells in Leukemia" International Journal of Molecular Sciences 25, no. 5: 2527. https://doi.org/10.3390/ijms25052527
APA StyleWu, C.-H., Weng, T.-F., Li, J.-P., & Wu, K.-H. (2024). Biology and Therapeutic Properties of Mesenchymal Stem Cells in Leukemia. International Journal of Molecular Sciences, 25(5), 2527. https://doi.org/10.3390/ijms25052527







