Enhanced Diaphragm Muscle Function upon Satellite Cell Transplantation in Dystrophic Mice
Abstract
1. Introduction
2. Results
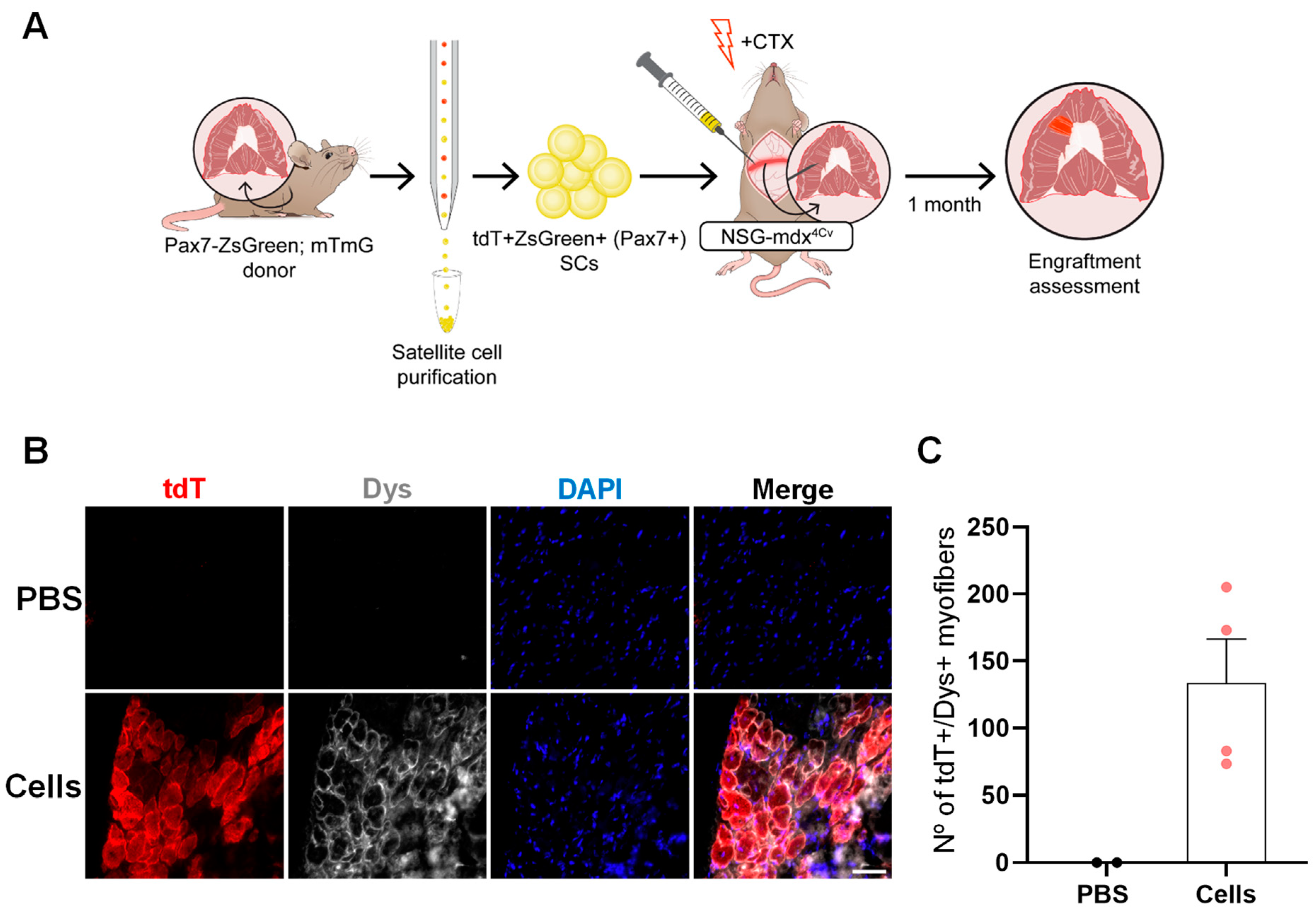
2.1. Optimization of a Method to Deliver Satellite Cells into the Diaphragm
2.2. SC Transplantation into the Diaphragm Muscle of FKRP-Mutant Dystrophic Mice
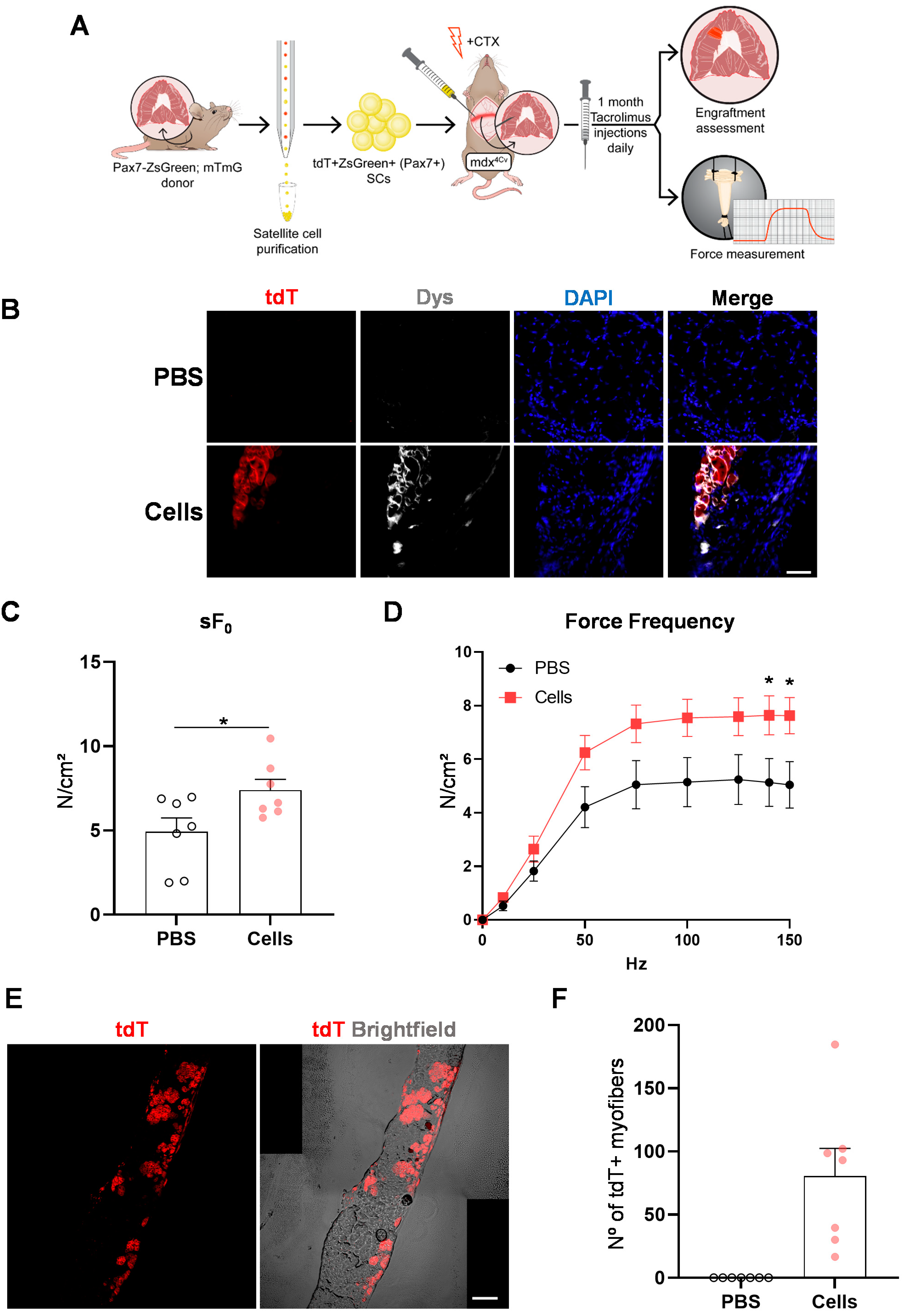
2.3. SC Transplantation Restores Dystrophin Expression in the DMD Mouse Diaphragm
2.4. Diaphragm Targeted Cell Therapy Improves Muscle Force
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. SC Isolation
4.3. Diaphragm Cell Transplantation
4.4. Immunofluorescence and H&E Staining
4.5. Force Measurements
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricoy, J.; Rodríguez-Núñez, N.; Álvarez-Dobaño, J.M.; Toubes, M.E.; Riveiro, V.; Valdés, L. Diaphragmatic dysfunction. Pulmonology 2019, 25, 223–235. [Google Scholar] [CrossRef]
- Mauro, A.L.; Aliverti, A. Physiology of respiratory disturbances in muscular dystrophies. Breathe 2016, 12, 318–327. [Google Scholar] [CrossRef]
- Richard, I.; Hogrel, J.; Stockholm, D.; Payan, C.A.M.; Fougerousse, F.; Eymard, B.; Mignard, C.; de Munain, A.L.; Fardeau, M.; Urtizberea, J.A.; et al. Natural history of LGMD2A for delineating outcome measures in clinical trials. Ann. Clin. Transl. Neurol. 2016, 3, 248–265. [Google Scholar] [CrossRef]
- Wohlgemuth, M.; van der Kooi, E.L.; van Kesteren, R.G.; van der Maarel, S.M.; Padberg, G.W. Ventilatory support in facioscapulohumeral muscular dystrophy. Neurology 2004, 63, 176–178. [Google Scholar] [CrossRef]
- Mathieu, J.; Allard, P.; Potvin, L.; Prévost, C.; Bégin, P. A 10-year study of mortality in a cohort of patients with myotonic dystrophy. Neurology 1999, 52, 1658–1662. [Google Scholar] [CrossRef]
- Wahlgren, L.; Kroksmark, A.-K.; Tulinius, M.; Sofou, K. One in five patients with Duchenne muscular dystrophy dies from other causes than cardiac or respiratory failure. Eur. J. Epidemiol. 2022, 37, 147–156. [Google Scholar] [CrossRef]
- Passamano, L.; Taglia, A.; Palladino, A.; Viggiano, E.; D’Ambrosio, P.; Scutifero, M.; Cecio, M.R.; Torre, V.; De Luca, F.; Picillo, E.; et al. Improvement of survival in Duchenne Muscular Dystrophy: Retrospective analysis of 835 patients. Acta Myol. 2012, 31, 121–125. [Google Scholar] [PubMed]
- Brockington, M.; Blake, D.J.; Prandini, P.; Brown, S.C.; Torelli, S.; Benson, M.A.; Ponting, C.P.; Estournet, B.; Romero, N.B.; Mercuri, E.; et al. Mutations in the fukutin-related protein gene (FKRP) cause a form of congenital muscular dystrophy with secondary laminin alpha2 deficiency and abnormal glycosylation of alpha-dystroglycan. Am. J. Hum. Genet. 2001, 69, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Valero de Bernabé, D.; Voit, T.; Longman, C.; Steinbrecher, A.; Straub, V.; Yuva, Y.; Herrmann, R.; Sperner, J.; Korenke, C.; Diesen, C.; et al. Mutations in the FKRP gene can cause muscle-eye-brain disease and Walker-Warburg syndrome. J. Med. Genet. 2004, 41, e61. [Google Scholar] [CrossRef]
- Brown, S.C.; Torelli, S.; Brockington, M.; Yuva, Y.; Jimenez, C.; Feng, L.; Anderson, L.; Ugo, I.; Kroger, S.; Bushby, K.; et al. Abnormalities in alpha-dystroglycan expression in MDC1C and LGMD2I muscular dystrophies. Am. J. Pathol. 2004, 164, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Poppe, M.; Bourke, J.; Eagle, M.; Frosk, P.; Wrogemann, K.; Greenberg, C.; Muntoni, F.; Voit, T.; Straub, V.; Hilton-Jones, D.; et al. Cardiac and respiratory failure in limb—Girdle muscular dystrophy 2I. Ann. Neurol. 2004, 56, 738–741. [Google Scholar] [CrossRef]
- Nelson, C.A.; Hunter, R.B.; Quigley, L.A.; Girgenrath, S.; Weber, W.D.; McCullough, J.A.; Dinardo, C.J.; Keefe, K.A.; Ceci, L.; Clayton, N.P.; et al. Inhibiting TGF-β activity improves respiratory function in mdx mice. Am. J. Pathol. 2011, 178, 2611–2621. [Google Scholar] [CrossRef]
- Heier, C.R.; Damsker, J.M.; Yu, Q.; Dillingham, B.C.; Huynh, T.; Van der Meulen, J.H.; Sali, A.; Miller, B.K.; Phadke, A.; Scheffer, L.; et al. VBP15, a novel anti-inflammatory and membrane-stabilizer, improves muscular dystrophy without side effects. EMBO Mol. Med. 2013, 5, 1569–1585. [Google Scholar] [CrossRef]
- Gorza, L.; Germinario, E.; Vitadello, M.; Guerra, I.; De Majo, F.; Gasparella, F.; Caliceti, P.; Vitiello, L.; Danieli-Betto, D. Curcumin Administration Improves Force of mdx Dystrophic Diaphragm by Acting on Fiber-Type Composition, Myosin Nitrotyrosination and SERCA1 Protein Levels. Antioxidants 2023, 12, 1181. [Google Scholar] [CrossRef]
- Burns, D.P.; Drummond, S.E.; Bolger, D.; Coiscaud, A.; Murphy, K.H.; Edge, D.; O’halloran, K.D. N-acetylcysteine Decreases Fibrosis and Increases Force-Generating Capacity of mdx Diaphragm. Antioxidants 2019, 8, 581. [Google Scholar] [CrossRef]
- Cataldi, M.P.; Lu, P.; Blaeser, A.; Lu, Q.L. Ribitol restores functionally glycosylated α-dystroglycan and improves muscle function in dystrophic FKRP-mutant mice. Nat. Commun. 2018, 9, 3448. [Google Scholar] [CrossRef]
- Wu, B.; Shah, S.N.; Lu, P.; Bollinger, L.E.; Blaeser, A.; Sparks, S.; Harper, A.D.; Lu, Q.L. Long-Term Treatment of Tamoxifen and Raloxifene Alleviates Dystrophic Phenotype and Enhances Muscle Functions of FKRP Dystroglycanopathy. Am. J. Pathol. 2018, 188, 1069–1080. [Google Scholar] [CrossRef]
- Ishizaki, M.; Maeda, Y.; Kawano, R.; Suga, T.; Uchida, Y.; Uchino, K.; Yamashita, S.; Kimura, E.; Uchino, M. Rescue From Respiratory Dysfunction by Transduction of Full-length Dystrophin to Diaphragm via the Peritoneal Cavity in Utrophin/Dystrophin Double Knockout Mice. Mol. Ther. 2011, 19, 1230–1235. [Google Scholar] [CrossRef]
- Potter, R.A.; Griffin, D.A.; Heller, K.N.; Peterson, E.L.; Clark, E.K.; Mendell, J.R.; Rodino-Klapac, L.R. Dose-Escalation Study of Systemically Delivered rAAVrh74.MHCK7.micro-dystrophin in the mdx Mouse Model of Duchenne Muscular Dystrophy. Hum. Gene Ther. 2021, 32, 375–389. [Google Scholar] [CrossRef]
- Birch, S.M.; Lawlor, M.W.; Conlon, T.J.; Guo, L.-J.; Crudele, J.M.; Hawkins, E.C.; Nghiem, P.P.; Ahn, M.; Meng, H.; Beatka, M.J.; et al. Assessment of systemic AAV-microdystrophin gene therapy in the GRMD model of Duchenne muscular dystrophy. Sci. Transl. Med. 2023, 15, eabo1815. [Google Scholar] [CrossRef]
- Vannoy, C.H.; Leroy, V.; Lu, Q.L. Dose-Dependent Effects of FKRP Gene-Replacement Therapy on Functional Rescue and Longevity in Dystrophic Mice. Mol. Ther. Methods Clin. Dev. 2018, 11, 106–120. [Google Scholar] [CrossRef]
- D’Ambrosio, E.S.; Mendell, J.R. Evolving Therapeutic Options for the Treatment of Duchenne Muscular Dystrophy. Neurotherapeutics 2023, 20, 1669–1681. [Google Scholar] [CrossRef]
- Boyer, O.; Butler-Browne, G.; Chinoy, H.; Cossu, G.; Galli, F.; Lilleker, J.B.; Magli, A.; Mouly, V.; Perlingeiro, R.C.R.; Previtali, S.C.; et al. Myogenic Cell Transplantation in Genetic and Acquired Diseases of Skeletal Muscle. Front. Genet. 2021, 12, 702547. [Google Scholar] [CrossRef]
- Montarras, D.; Morgan, J.; Collins, C.; Relaix, F.; Zaffran, S.; Cumano, A.; Partridge, T.; Buckingham, M. Direct isolation of satellite cells for skeletal muscle regeneration. Science 2005, 309, 2064–2067. [Google Scholar] [CrossRef]
- Sacco, A.; Doyonnas, R.; Kraft, P.; Vitorovic, S.; Blau, H.M. Self-renewal and expansion of single transplanted muscle stem cells. Nature 2008, 456, 502–506. [Google Scholar] [CrossRef]
- Darabi, R.; Gehlbach, K.; Bachoo, R.M.; Kamath, S.; Osawa, M.; E Kamm, K.; Kyba, M.; Perlingeiro, R.C.R. Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nat. Med. 2008, 14, 134–143. [Google Scholar] [CrossRef]
- Darabi, R.; Arpke, R.W.; Irion, S.; Dimos, J.T.; Grskovic, M.; Kyba, M.; Perlingeiro, R.C.R. Human ES- and iPS-Derived Myogenic Progenitors Restore DYSTROPHIN and Improve Contractility upon Transplantation in Dystrophic Mice. Cell Stem Cell 2012, 10, 610–619. [Google Scholar] [CrossRef]
- Arpke, R.W.; Darabi, R.; Mader, T.L.; Zhang, Y.; Toyama, A.; Lonetree, C.-L.; Nash, N.; Lowe, D.A.; Perlingeiro, R.C.R.; Kyba, M. A New Immuno-, Dystrophin-Deficient Model, the NSG-mdx 4Cv Mouse, Provides Evidence for Functional Improvement Following Allogeneic Satellite Cell Transplantation. Stem Cells 2013, 31, 1611–1620. [Google Scholar] [CrossRef]
- Hicks, M.R.; Hiserodt, J.; Paras, K.; Fujiwara, W.; Eskin, A.; Jan, M.; Xi, H.; Young, C.S.; Evseenko, D.; Nelson, S.F.; et al. ERBB3 and NGFR mark a distinct skeletal muscle progenitor cell in human development and hPSCs. Nat. Cell Biol. 2018, 20, 46–57. [Google Scholar] [CrossRef]
- Mitchell, K.J.; Pannérec, A.; Cadot, B.; Parlakian, A.; Besson, V.; Gomes, E.R.; Marazzi, G.; Sassoon, D.A. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat. Cell Biol. 2010, 12, 257–266. [Google Scholar] [CrossRef]
- Mizuno, Y.; Chang, H.; Umeda, K.; Niwa, A.; Iwasa, T.; Awaya, T.; Fukada, S.; Yamamoto, H.; Yamanaka, S.; Nakahata, T.; et al. Generation of skeletal muscle stem/progenitor cells from murine induced pluripotent stem cells. FASEB J. 2010, 24, 2245–2253. [Google Scholar] [CrossRef]
- Zhao, M.; Tazumi, A.; Takayama, S.; Takenaka-Ninagawa, N.; Nalbandian, M.; Nagai, M.; Nakamura, Y.; Nakasa, M.; Watanabe, A.; Ikeya, M.; et al. Induced Fetal Human Muscle Stem Cells with High Therapeutic Potential in a Mouse Muscular Dystrophy Model. Stem Cell Rep. 2020, 15, 80–94. [Google Scholar] [CrossRef]
- Torrente, Y.; Tremblay, J.-P.; Pisati, F.; Belicchi, M.; Rossi, B.; Sironi, M.; Fortunato, F.; El Fahime, M.; D’Angelo, M.G.; Caron, N.J.; et al. Intraarterial Injection of Muscle-derived CD34 Sca-1 Stem Cells Restores Dystrophin in mdx Mice. J. Cell Biol. 2001, 152, 335–348. [Google Scholar] [CrossRef]
- Matthias, N.; Hunt, S.D.; Wu, J.; Darabi, R. Skeletal muscle perfusion and stem cell delivery in muscle disorders using intra-femoral artery canulation in mice. Exp. Cell Res. 2015, 339, 103–111. [Google Scholar] [CrossRef]
- Filareto, A.; Parker, S.; Darabi, R.; Borges, L.; Iacovino, M.; Schaaf, T.; Mayerhofer, T.; Chamberlain, J.S.; Ervasti, J.M.; McIvor, R.S.; et al. An ex vivo gene therapy approach to treat muscular dystrophy using inducible pluripotent stem cells. Nat. Commun. 2013, 4, 1549. [Google Scholar] [CrossRef]
- Tedesco, F.S.; Gerli, M.F.M.; Perani, L.; Benedetti, S.; Ungaro, F.; Cassano, M.; Antonini, S.; Tagliafico, E.; Artusi, V.; Longa, E.; et al. Transplantation of Genetically Corrected Human iPSC-Derived Progenitors in Mice with Limb-Girdle Muscular Dystrophy. Sci. Transl. Med. 2012, 4, 140ra89. [Google Scholar] [CrossRef]
- Sampaolesi, M.; Torrente, Y.; Innocenzi, A.; Tonlorenzi, R.; D’Antona, G.; Pellegrino, M.A.; Barresi, R.; Bresolin, N.; De Angelis, M.G.C.; Campbell, K.P.; et al. Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science 2003, 301, 487–492. [Google Scholar] [CrossRef]
- Cossu, G.; Previtali, S.C.; Napolitano, S.; Cicalese, M.P.; Tedesco, F.S.; Nicastro, F.; Noviello, M.; Roostalu, U.; Sora, M.G.N.; Scarlato, M.; et al. Intra-arterial transplantation of HLA-matched donor mesoangioblasts in Duchenne muscular dystrophy. EMBO Mol. Med. 2015, 7, 1513–1528. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Sato, M.; Kuwahara, T.; Ebata, T.; Tabata, Y.; Sakurai, H. Transplantation of human iPSC-derived muscle stem cells in the diaphragm of Duchenne muscular dystrophy model mice. PLoS ONE 2022, 17, e0266391. [Google Scholar] [CrossRef] [PubMed]
- Siemionow, M.; Langa, P.; Harasymczuk, M.; Cwykiel, J.; Sielewicz, M.; Smieszek, J.; Heydemann, A. Human dystrophin expressing chimeric (DEC) cell therapy ameliorates cardiac, respiratory, and skeletal muscle’s function in Duchenne muscular dystrophy. Stem Cells Transl. Med. 2021, 10, 1406–1418. [Google Scholar] [CrossRef] [PubMed]
- Lessa, T.B.; Carvalho, R.C.; Franciolli, A.L.R.; de Oliveira, L.J.; Barreto, R.; Feder, D.; Bressan, F.F.; Miglino, M.A.; Ambrósio, C.E. Muscle reorganisation through local injection of stem cells in the diaphragm of mdx mice. Acta Vet. Scand. 2012, 54, 73. [Google Scholar] [CrossRef]
- Chang, N.C.; Rudnicki, M.A. Satellite cells: The architects of skeletal muscle. Curr. Top. Dev. Biol. 2014, 107, 161–181. [Google Scholar] [CrossRef]
- Morgan, J.E.; Hoffman, E.P.; Partridge, T.A. Normal Myogenic Cells from Newborn Mice Restore Normal Histology to Degenerating Muscles of the mdx Mouse. J. Cell. Biol. 1990, 111, 2437–2449. [Google Scholar] [CrossRef]
- Prakash, Y.S.; Mantilla, C.B.; Zhan, W.-Z.; Smithson, K.G.; Sieck, G.C. Phrenic motoneuron morphology during rapid diaphragm muscle growth. J. Appl. Physiol. 2000, 89, 563–572. [Google Scholar] [CrossRef]
- Mantilla, C.B.; Zhan, W.-Z.; Sieck, G.C. Retrograde labeling of phrenic motoneurons by intrapleural injection. J. Neurosci. Methods 2009, 182, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Muzumdar, M.D.; Tasic, B.; Miyamichi, K.; Li, L.; Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 2007, 45, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Incitti, T.; Magli, A.; Darabi, R.; Yuan, C.; Lin, K.; Arpke, R.W.; Azzag, K.; Yamamoto, A.; Stewart, R.; Thomson, J.A.; et al. Pluripotent stem cell-derived myogenic progenitors remodel their molecular signature upon in vivo engraftment. Proc. Natl. Acad. Sci. USA 2019, 116, 4346–4351. [Google Scholar] [CrossRef] [PubMed]
- Azzag, K.; Ortiz-Cordero, C.; Oliveira, N.A.J.; Magli, A.; Selvaraj, S.; Tungtur, S.; Upchurch, W.; Iaizzo, P.A.; Lu, Q.L.; Perlingeiro, R.C.R. Efficient engraftment of pluripotent stem cell-derived myogenic progenitors in a novel immunodeficient mouse model of limb girdle muscular dystrophy 2I. Skelet Muscle 2020, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Ervasti, J.M.; Campbell, K.P. Membrane organization of the dystrophin-glycoprotein complex. Cell 1991, 66, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Ervasti, J.M. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J. Cell Biol. 1993, 122, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.M.; Keramaris-Vrantsis, E.; Lidov, H.G.; Norton, J.H.; Zinchenko, N.; Gruber, H.E.; Thresher, R.; Blake, D.J.; Ashar, J.; Rosenfeld, J.; et al. Fukutin-related protein is essential for mouse muscle, brain and eye development and mutation recapitulates the wide clinical spectrums of dystroglycanopathies. Hum. Mol. Genet. 2010, 19, 3995–4006. [Google Scholar] [CrossRef] [PubMed]
- Bosnakovski, D.; Xu, Z.; Li, W.; Thet, S.; Cleaver, O.; Perlingeiro, R.C.R.; Kyba, M. Prospective Isolation of Skeletal Muscle Stem Cells with a Pax7 Reporter. Stem Cells 2008, 26, 3194–3204. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Matthias, N.; Lo, J.; Ortiz-Vitali, J.L.; Shieh, A.W.; Wang, S.H.; Darabi, R. A Myogenic Double-Reporter Human Pluripotent Stem Cell Line Allows Prospective Isolation of Skeletal Muscle Progenitors. Cell Rep. 2018, 25, 1966–1981.e4. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.M.; Tamaki, S.; Lee, S.; Wong, A.; Jose, A.; Dreux, J.; Kouklis, G.; Sbitany, H.; Seth, R.; Knott, P.D.; et al. High-Yield Purification, Preservation, and Serial Transplantation of Human Satellite Cells. Stem Cell Rep. 2018, 10, 1160–1174. [Google Scholar] [CrossRef]
- Greising, S.M.; Mantilla, C.B.; Gorman, B.A.; Ermilov, L.G.; Sieck, G.C. Diaphragm muscle sarcopenia in aging mice. Exp. Gerontol. 2013, 48, 881–887. [Google Scholar] [CrossRef]
- Smuder, A.J.; Falk, D.J.; Sollanek, K.J.; Nelson, W.B.; Powers, S.K. Delivery of Recombinant Adeno-Associated Virus Vectors to Rat Diaphragm Muscle via Direct Intramuscular Injection. Hum. Gene Ther. Methods 2013, 24, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Petrof, B.J.; Acsadi, G.; Jani, A.; Massie, B.; Bourdon, J.; Matusiewicz, N.; Yang, L.; Lochmüller, H.; Karpati, G. Efficiency and functional consequences of adenovirus-mediated in vivo gene transfer to normal and dystrophic (mdx) mouse diaphragm. Am. J. Respir. Cell Mol. Biol. 1995, 13, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Matecki, S.; Dudley, R.W.R.; Divangahi, M.; Gilbert, R.; Nalbantoglu, J.; Karpati, G.; Petrof, B.J. Therapeutic gene transfer to dystrophic diaphragm by an adenoviral vector deleted of all viral genes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L569–L576. [Google Scholar] [CrossRef]
- Shams, A.S.; Arpke, R.W.; Gearhart, M.D.; Weiblen, J.; Mai, B.; Oyler, D.; Bosnakovski, D.; Mahmoud, O.M.; Hassan, G.M.; Kyba, M. The chemokine receptor CXCR4 regulates satellite cell activation, early expansion, and self-renewal, in response to skeletal muscle injury. Front. Cell Dev. Biol. 2022, 10, 949532. [Google Scholar] [CrossRef]
- Arpke, R.W.; Shams, A.S.; Collins, B.C.; Larson, A.A.; Lu, N.; Lowe, D.A.; Kyba, M. Preservation of satellite cell number and regenerative potential with age reveals locomotory muscle bias. Skelet Muscle 2021, 11, 22. [Google Scholar] [CrossRef]
- Cerletti, M.; Jurga, S.; Witczak, C.A.; Hirshman, M.F.; Shadrach, J.L.; Goodyear, L.J.; Wagers, A.J. Highly Efficient, Functional Engraftment of Skeletal Muscle Stem Cells in Dystrophic Muscles. Cell 2008, 134, 37–47. [Google Scholar] [CrossRef]
- Sitzia, C.; Farini, A.; Jardim, L.; Razini, P.; Belicchi, M.; Cassinelli, L.; Villa, C.; Erratico, S.; Parolini, D.; Bella, P.; et al. Adaptive Immune Response Impairs the Efficacy of Autologous Transplantation of Engineered Stem Cells in Dystrophic Dogs. Mol. Ther. 2016, 24, 1949–1964. [Google Scholar] [CrossRef]
- Morgan, J.E.; Pagel, C.N.; Sherratt, T.; Partridge, T.A. Long-term persistence and migration of myogenic cells injected into pre-irradiated muscles of mdx mice. J. Neurol. Sci. 1993, 115, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Wallace, G.Q.; Lapidos, K.A.; Kenik, J.S.; McNally, E.M. Long-term survival of transplanted stem cells in immunocompetent mice with muscular dystrophy. Am. J. Pathol. 2008, 173, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Blaeser, A.; Awano, H.; Wu, B.; Lu, Q.-L. Progressive Dystrophic Pathology in Diaphragm and Impairment of Cardiac Function in FKRP P448L Mutant Mice. PLoS ONE 2016, 11, e0164187. [Google Scholar] [CrossRef]
- Giovarelli, M.; Arnaboldi, F.; Zecchini, S.; Cornaghi, L.B.; Nava, A.; Sommariva, M.; Clementi, E.G.I.; Gagliano, N. Characterisation of Progressive Skeletal Muscle Fibrosis in the Mdx Mouse Model of Duchenne Muscular Dystrophy: An In Vivo and In Vitro Study. Int. J. Mol. Sci. 2022, 23, 8735. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Lu, L.; Wu, S.; Zuo, L. Effects of Ionizing Irradiation on Mouse Diaphragmatic Skeletal Muscle. Front. Physiol. 2017, 8, 506. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzag, K.; Gransee, H.M.; Magli, A.; Yamashita, A.M.S.; Tungtur, S.; Ahlquist, A.; Zhan, W.-Z.; Onyebu, C.; Greising, S.M.; Mantilla, C.B.; et al. Enhanced Diaphragm Muscle Function upon Satellite Cell Transplantation in Dystrophic Mice. Int. J. Mol. Sci. 2024, 25, 2503. https://doi.org/10.3390/ijms25052503
Azzag K, Gransee HM, Magli A, Yamashita AMS, Tungtur S, Ahlquist A, Zhan W-Z, Onyebu C, Greising SM, Mantilla CB, et al. Enhanced Diaphragm Muscle Function upon Satellite Cell Transplantation in Dystrophic Mice. International Journal of Molecular Sciences. 2024; 25(5):2503. https://doi.org/10.3390/ijms25052503
Chicago/Turabian StyleAzzag, Karim, Heather M. Gransee, Alessandro Magli, Aline M. S. Yamashita, Sudheer Tungtur, Aaron Ahlquist, Wen-Zhi Zhan, Chiemelie Onyebu, Sarah M. Greising, Carlos B. Mantilla, and et al. 2024. "Enhanced Diaphragm Muscle Function upon Satellite Cell Transplantation in Dystrophic Mice" International Journal of Molecular Sciences 25, no. 5: 2503. https://doi.org/10.3390/ijms25052503
APA StyleAzzag, K., Gransee, H. M., Magli, A., Yamashita, A. M. S., Tungtur, S., Ahlquist, A., Zhan, W.-Z., Onyebu, C., Greising, S. M., Mantilla, C. B., & Perlingeiro, R. C. R. (2024). Enhanced Diaphragm Muscle Function upon Satellite Cell Transplantation in Dystrophic Mice. International Journal of Molecular Sciences, 25(5), 2503. https://doi.org/10.3390/ijms25052503





