Protective Effects of Inulin on Stress-Recurrent Inflammatory Bowel Disease
Abstract
1. Introduction
2. Results
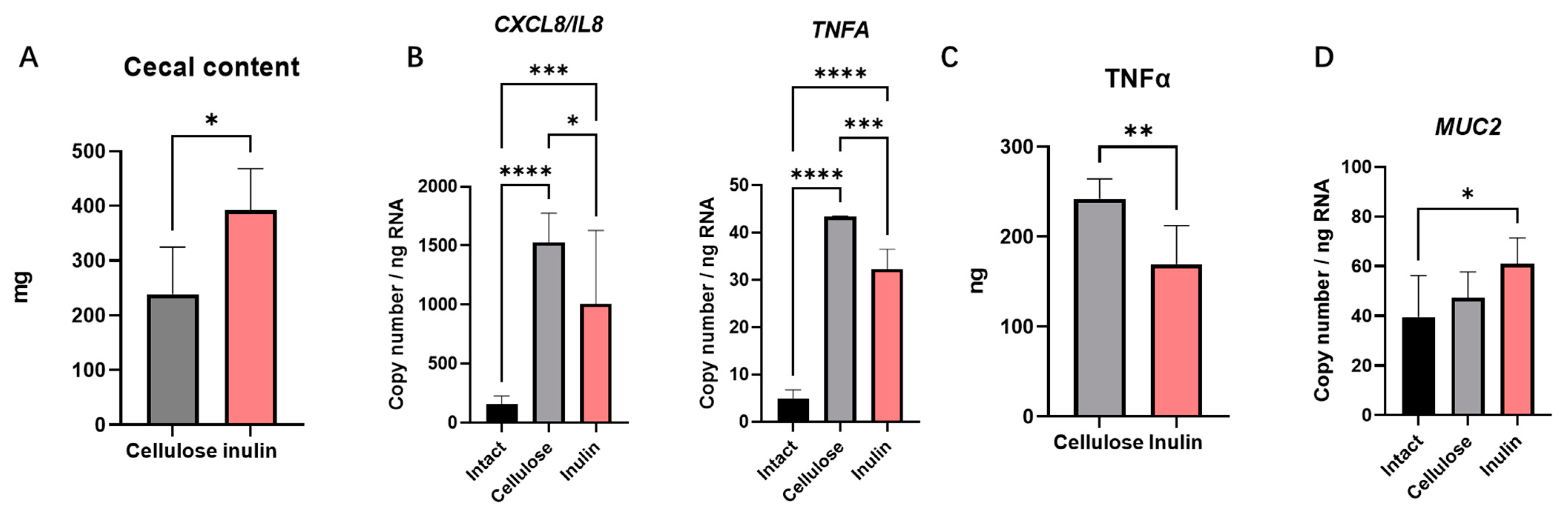
2.1. The Effect of Inulin Digesta on Immunity Reactions in Intestinal Epithelial Cells
2.2. The Effect of the Inulin Intake on Inflammation in Stress Model
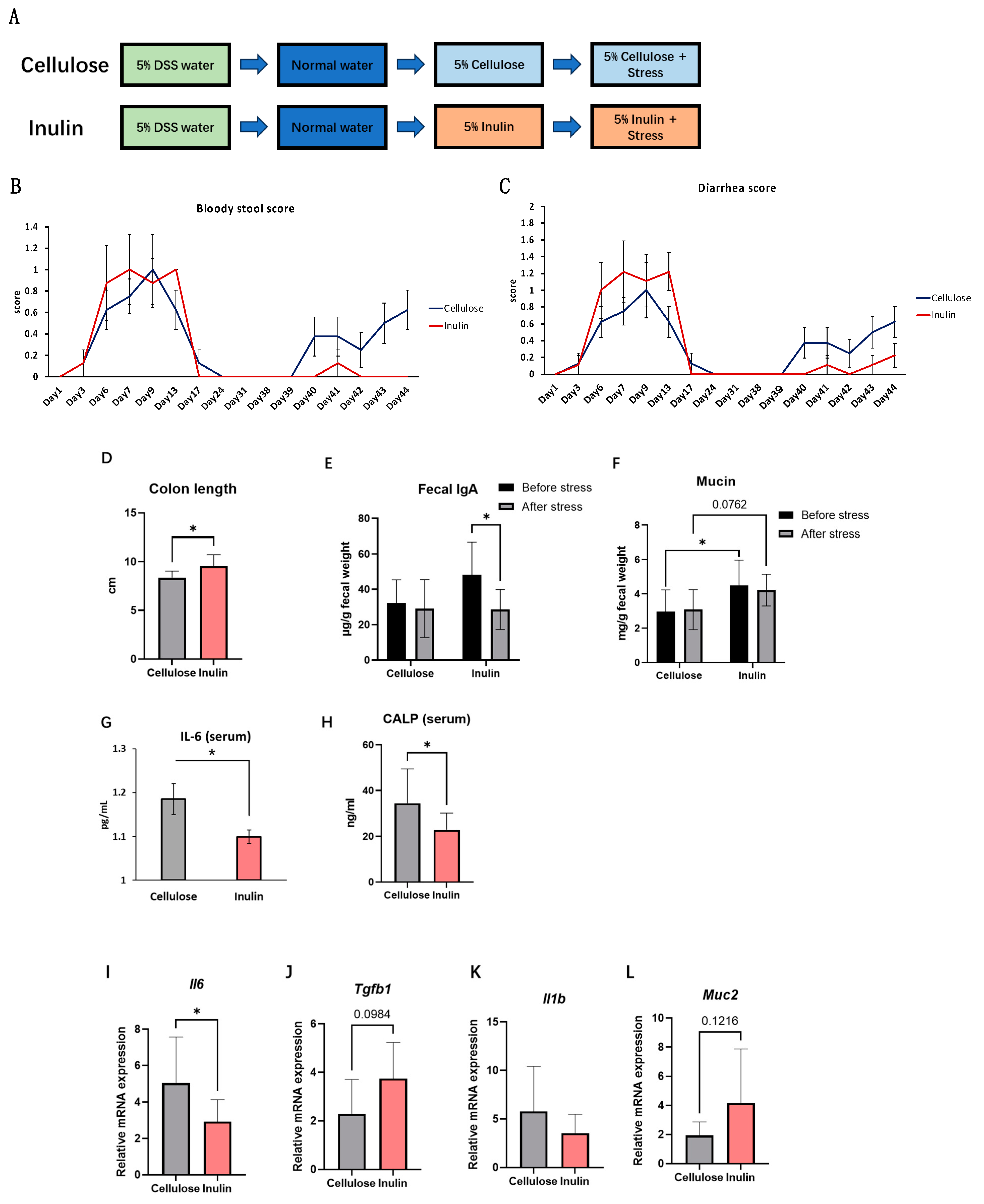
2.3. The Effect of Inulin on IBD Symptoms in Stress-Recurrent IBD Model
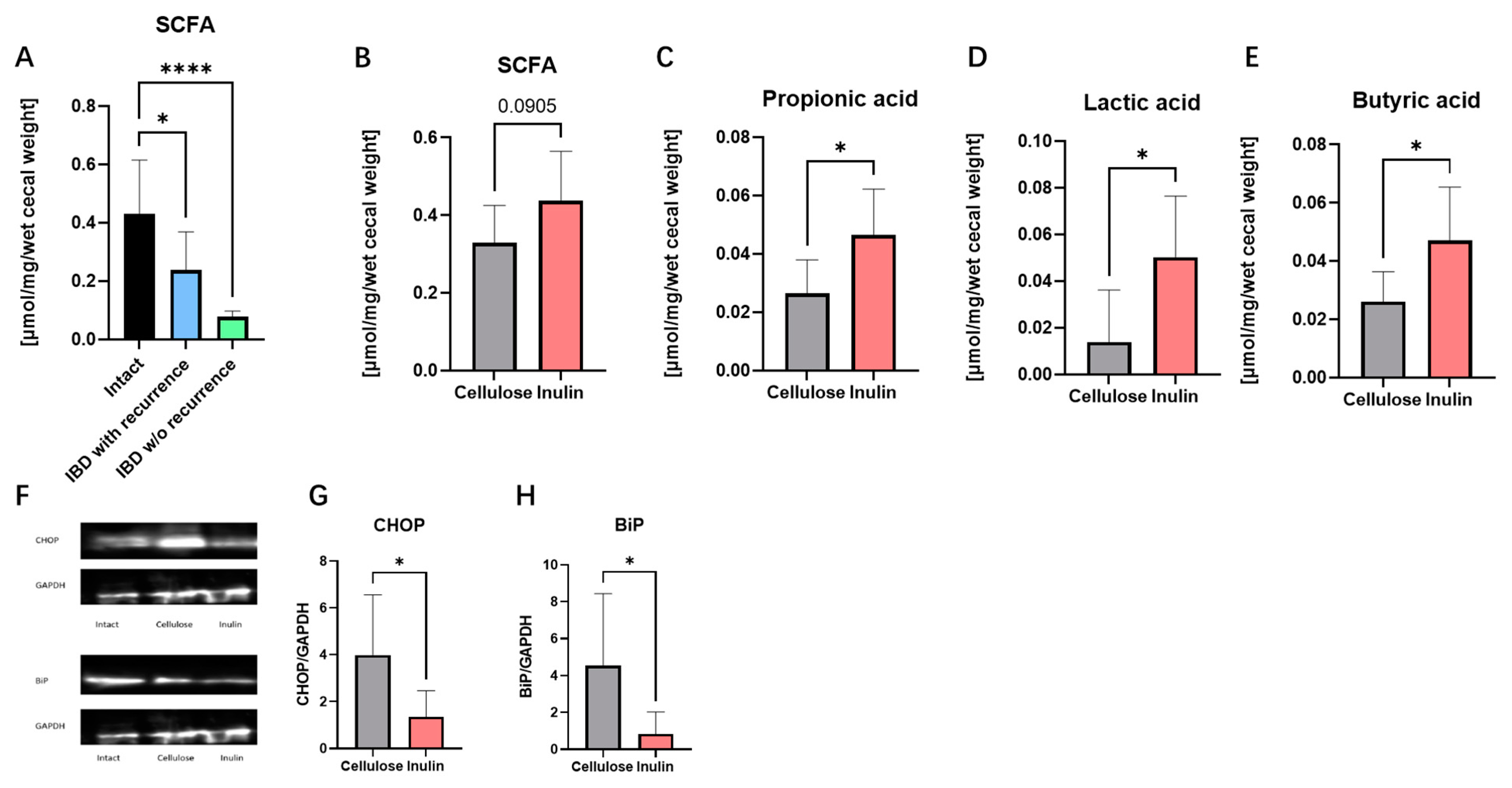
2.4. Inulin Improved IBD Symptoms by Augmenting SCFAs and Alleviating Endoplasmic Reticulum (ER) Stress
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Inulin and Cellulose Digesta Preparation
4.3. Total Cell RNA Extraction and Real-Time PCR
4.4. TNF-α ELISA
4.5. Animals
4.6. Restraint Stress
4.7. Measurements of Bloody Stool Score and Diarrhea Score
4.8. Stool Collection
4.9. IgA ELISA
4.10. Mucin ELISA
4.11. Blood Collection
4.12. Corticosterone ELISA
4.13. IL-6 ELISA
4.14. CALP ELISA
4.15. Tissue Collection
4.16. Isolation of Mice Total RNA and Real-Time RT-PCR
4.17. Western Blot
4.18. Gas Chromatography Analysis
4.19. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abraham, C.; Cho, J.H. Inflammatory Bowel Disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. IBD: In Food We Trust. J. Crohn’s Colitis 2016, 10, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.E.; Lichtiger, S.; Yajnik, V. Inflammatory Bowel Disease and Cancer: The Role of Inflammation, Immunosuppression, and Cancer Treatment. WJG 2016, 22, 4794. [Google Scholar] [CrossRef] [PubMed]
- Orihara, K.; Haraguchi, A.; Shibata, S. Crosstalk Among Circadian Rhythm, Obesity and Allergy. IJMS 2020, 21, 1884. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.A.C.; Wand, G. Stress and the HPA Axis: Role of Glucocorticoids in Alcohol Dependence. Alcohol. Res. 2012, 34, 468–483. [Google Scholar]
- Ponferrada, Á.; Caso, J.R.; Alou, L.; Colón, A.; Sevillano, D.; Moro, M.A.; Lizasoain, I.; Menchén, P.; Gómez-Lus, M.L.; Lorenzo, P.; et al. The Role of PPARγ on Restoration of Colonic Homeostasis After Experimental Stress-Induced Inflammation and Dysfunction. Gastroenterology 2007, 132, 1791–1803. [Google Scholar] [CrossRef]
- Sudo, N.; Oyama, N.; Yu, X.-N.; Kubo, C. Restraint Stress-Induced Elevation of Endogenous Glucocorticoids Decreases Peyer’s Patch Cell Numbers via Mechanisms That Are Either Dependent or Independent on Apoptotic Cell Death. Neuroimmunomodulation 2001, 9, 333–339. [Google Scholar] [CrossRef]
- Nukina, H.; Sudo, N.; Aiba, Y.; Oyama, N.; Koga, Y.; Kubo, C. Restraint Stress Elevates the Plasma Interleukin-6 Levels in Germ-Free Mice. J. Neuroimmunol. 2001, 115, 46–52. [Google Scholar] [CrossRef]
- Peña-Juárez, M.C.; Campos-Rodríguez, R.; Godínez-Victoria, M.; Cruz-Hernández, T.R.; Reyna-Garfias, H.; Barbosa-Cabrera, R.E.; Drago-Serrano, M.E. Effect of Bovine Lactoferrin Treatment Followed by Acute Stress on the IgA-Response in Small Intestine of BALB/c Mice. Immunol. Investig. 2016, 45, 652–667. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Greenberg, N.A.; Gassull, M.A.; Meier, R. Letters to the Editor. Nut. Clin. Pract. 2006, 21, 639–640. [Google Scholar] [CrossRef]
- Berni Canani, R.; Di Costanzo, M.; Leone, L. The Epigenetic Effects of Butyrate: Potential Therapeutic Implications for Clinical Practice. Clin. Epigenet. 2012, 4, 4. [Google Scholar] [CrossRef]
- Scharlau, D.; Borowicki, A.; Habermann, N.; Hofmann, T.; Klenow, S.; Miene, C.; Munjal, U.; Stein, K.; Glei, M. Mechanisms of Primary Cancer Prevention by Butyrate and Other Products Formed during Gut Flora-Mediated Fermentation of Dietary Fibre. Mutat. Res./Rev. Mutat. Res. 2009, 682, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Whelan, K. Prebiotic Inulin-type Fructans and Galacto-oligosaccharides: Definition, Specificity, Function, and Application in Gastrointestinal Disorders. J. Gastroenterol. Hepatol. 2017, 32, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Shiga, K.; Haraguchi, A.; Sasaki, H.; Tahara, Y.; Orihara, K.; Shibata, S. Effect of Circadian Clock and Claudin Regulations on Inulin-Induced Calcium Absorption in the Mouse Intestinal Tract. Biosci. Microbiota Food Health 2023, 42, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Neyrinck, A.M.; Van Kerckhoven, M.; Gianfrancesco, M.A.; Renguet, E.; Bertrand, L.; Cani, P.D.; Lanthier, N.; Cnop, M.; Paquot, N.; et al. Physical Activity Enhances the Improvement of Body Mass Index and Metabolism by Inulin: A Multicenter Randomized Placebo-Controlled Trial Performed in Obese Individuals. BMC Med. 2022, 20, 110. [Google Scholar] [CrossRef] [PubMed]
- Melgar, S.; Engström, K.; Jägervall, Å.; Martinez, V. Psychological Stress Reactivates Dextran Sulfate Sodium-Induced Chronic Colitis in Mice: Research Report. Stress 2008, 11, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Dragoş, D.; Tănăsescu, M.D. The Effect of Stress on the Defense Systems. J. Med. Life 2010, 3, 10–18. [Google Scholar]
- Yue, B.; Luo, X.; Yu, Z.; Mani, S.; Wang, Z.; Dou, W. Inflammatory Bowel Disease: A Potential Result from the Collusion between Gut Microbiota and Mucosal Immune System. Microorganisms 2019, 7, 440. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Neurath, M.F. Current and Emerging Therapeutic Targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 269–278. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Lan, Y.; Tuo, Y.; Ma, S.; Liu, X. Inulin Attenuates Blood–Brain Barrier Permeability and Alleviates Behavioral Disorders by Modulating the TLR4/MyD88/NF-κB Pathway in Mice with Chronic Stress. J. Agric. Food Chem. 2023, 71, 13325–13337. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Sjövall, H.; Hansson, G.C. The Gastrointestinal Mucus System in Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef]
- Sun, J.; Shen, X.; Li, Y.; Guo, Z.; Zhu, W.; Zuo, L.; Zhao, J.; Gu, L.; Gong, J.; Li, J. Therapeutic Potential to Modify the Mucus Barrier in Inflammatory Bowel Disease. Nutrients 2016, 8, 44. [Google Scholar] [CrossRef]
- Joossens, M.; Huys, G.; Cnockaert, M.; De Preter, V.; Verbeke, K.; Rutgeerts, P.; Vandamme, P.; Vermeire, S. Dysbiosis of the Faecal Microbiota in Patients with Crohn’s Disease and Their Unaffected Relatives. Gut 2011, 60, 631–637. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the Intestinal Microbiome in Inflammatory Bowel Disease and Treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The Promotion Mechanism of Prebiotics for Probiotics: A Review. Front. Nutr. 2022, 9, 1000517. [Google Scholar] [CrossRef]
- Schilderink, R.; Verseijden, C.; De Jonge, W.J. Dietary Inhibitors of Histone Deacetylases in Intestinal Immunity and Homeostasis. Front. Immunol. 2013, 4, 226. [Google Scholar] [CrossRef]
- Ratajczak, W.; Rył, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczyńska, M. Immunomodulatory Potential of Gut Microbiome-Derived Short-Chain Fatty Acids (SCFAs). Acta Biochim. Pol. 2019, 66, 1–12. [Google Scholar] [CrossRef]
- Willemsen, L.E.M. Short Chain Fatty Acids Stimulate Epithelial Mucin 2 Expression through Differential Effects on Prostaglandin E1 and E2 Production by Intestinal Myofibroblasts. Gut 2003, 52, 1442–1447. [Google Scholar] [CrossRef]
- Shenderov, K.; Riteau, N.; Yip, R.; Mayer-Barber, K.D.; Oland, S.; Hieny, S.; Fitzgerald, P.; Oberst, A.; Dillon, C.P.; Green, D.R.; et al. Cutting Edge: Endoplasmic Reticulum Stress Licenses Macrophages To Produce Mature IL-1β in Response to TLR4 Stimulation through a Caspase-8– and TRIF-Dependent Pathway. J. Immunol. 2014, 192, 2029–2033. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Eri, R.D.; Das, I.; Lourie, R.; Florin, T.H. ER Stress and the Unfolded Protein Response in Intestinal Inflammation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 298, G820–G832. [Google Scholar] [CrossRef]
- Pahl, H.L.; Baeuerle, P.A. A Novel Signal Transduction Pathway from the Endoplasmic Reticulum to the Nucleus Is Mediated by Transcription Factor NF-Kappa B. EMBO J. 1995, 14, 2580–2588. [Google Scholar] [CrossRef]
- Shkoda, A.; Ruiz, P.A.; Daniel, H.; Kim, S.C.; Rogler, G.; Sartor, R.B.; Haller, D. Interleukin-10 Blocked Endoplasmic Reticulum Stress in Intestinal Epithelial Cells: Impact on Chronic Inflammation. Gastroenterology 2007, 132, 190–207. [Google Scholar] [CrossRef]
- Deng, J.; Lu, P.D.; Zhang, Y.; Scheuner, D.; Kaufman, R.J.; Sonenberg, N.; Harding, H.P.; Ron, D. Translational Repression Mediates Activation of Nuclear Factor Kappa B by Phosphorylated Translation Initiation Factor 2. Mol. Cell. Biol. 2004, 24, 10161–10168. [Google Scholar] [CrossRef]
- Hu, P.; Han, Z.; Couvillon, A.D.; Kaufman, R.J.; Exton, J.H. Autocrine Tumor Necrosis Factor Alpha Links Endoplasmic Reticulum Stress to the Membrane Death Receptor Pathway through IRE1α-Mediated NF-κB Activation and Down-Regulation of TRAF2 Expression. Mol. Cell. Biol. 2006, 26, 3071–3084. [Google Scholar] [CrossRef]
- Uerlings, J.; Schroyen, M.; Willems, E.; Tanghe, S.; Bruggeman, G.; Bindelle, J.; Everaert, N. Differential Effects of Inulin or Its Fermentation Metabolites on Gut Barrier and Immune Function of Porcine Intestinal Epithelial Cells. J. Funct. Foods 2020, 67, 103855. [Google Scholar] [CrossRef]

| Gene Name | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| (Human) | ||
| GAPDH | CAGCCTCAGTACAGCAATCAAC | TAGGGGTCATAGGAGTCATTGG |
| CXCL8/IL8 | GGCAGCCTTCCTGATTTGTG | GCTTTACAATAATTTCTGTGTTGG |
| TNFA | GCTTTGATCCCTGACATCTGG | GGAAACATCTGGAGAGAGGAAG |
| MUC2 | GAAGTGAAGAGCAAGATGGTG | GGAGGAATAAACTGGAGAACC |
| (Mice) | ||
| 18srrna | CGAAAGCATTTGCCAAGAAT | GCGGGTCATGGGAATAAC |
| Pigr | AGTAACCGAGGCCTGTCCT | GTCACTCGGCAACTCAGGA |
| Claudin3 | ACCCACCAAGATCCTCTATT | TAGTCCTTGCGGTCGTAG |
| Claudin4 | GGGCAAGAGGGAAATG | TAGGGCTTGTCGTTGCTA |
| Occludin | CACACTTGCTTGGGACAG | GGGTCTGTATATCCGCCATA |
| Zo1 | GAAACTGATGCTGTGGATAGA | GGAATGTATGTGGAGA |
| Muc2 | CTCATCATGGACAGCCTATTC | CACTGGCACACTCCATATTAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Kusama, K.; Hama, K.; Chen, X.; Tahara, Y.; Kajiwara, S.; Shibata, S.; Orihara, K. Protective Effects of Inulin on Stress-Recurrent Inflammatory Bowel Disease. Int. J. Mol. Sci. 2024, 25, 2494. https://doi.org/10.3390/ijms25052494
Du Y, Kusama K, Hama K, Chen X, Tahara Y, Kajiwara S, Shibata S, Orihara K. Protective Effects of Inulin on Stress-Recurrent Inflammatory Bowel Disease. International Journal of Molecular Sciences. 2024; 25(5):2494. https://doi.org/10.3390/ijms25052494
Chicago/Turabian StyleDu, Yao, Kanta Kusama, Koki Hama, Xinyue Chen, Yu Tahara, Susumu Kajiwara, Shigenobu Shibata, and Kanami Orihara. 2024. "Protective Effects of Inulin on Stress-Recurrent Inflammatory Bowel Disease" International Journal of Molecular Sciences 25, no. 5: 2494. https://doi.org/10.3390/ijms25052494
APA StyleDu, Y., Kusama, K., Hama, K., Chen, X., Tahara, Y., Kajiwara, S., Shibata, S., & Orihara, K. (2024). Protective Effects of Inulin on Stress-Recurrent Inflammatory Bowel Disease. International Journal of Molecular Sciences, 25(5), 2494. https://doi.org/10.3390/ijms25052494






