Vaginal Lactobacilli Supernatants Protect from Herpes Simplex Virus Type 1 Infection in Cell Culture Models
Abstract
1. Introduction
2. Results
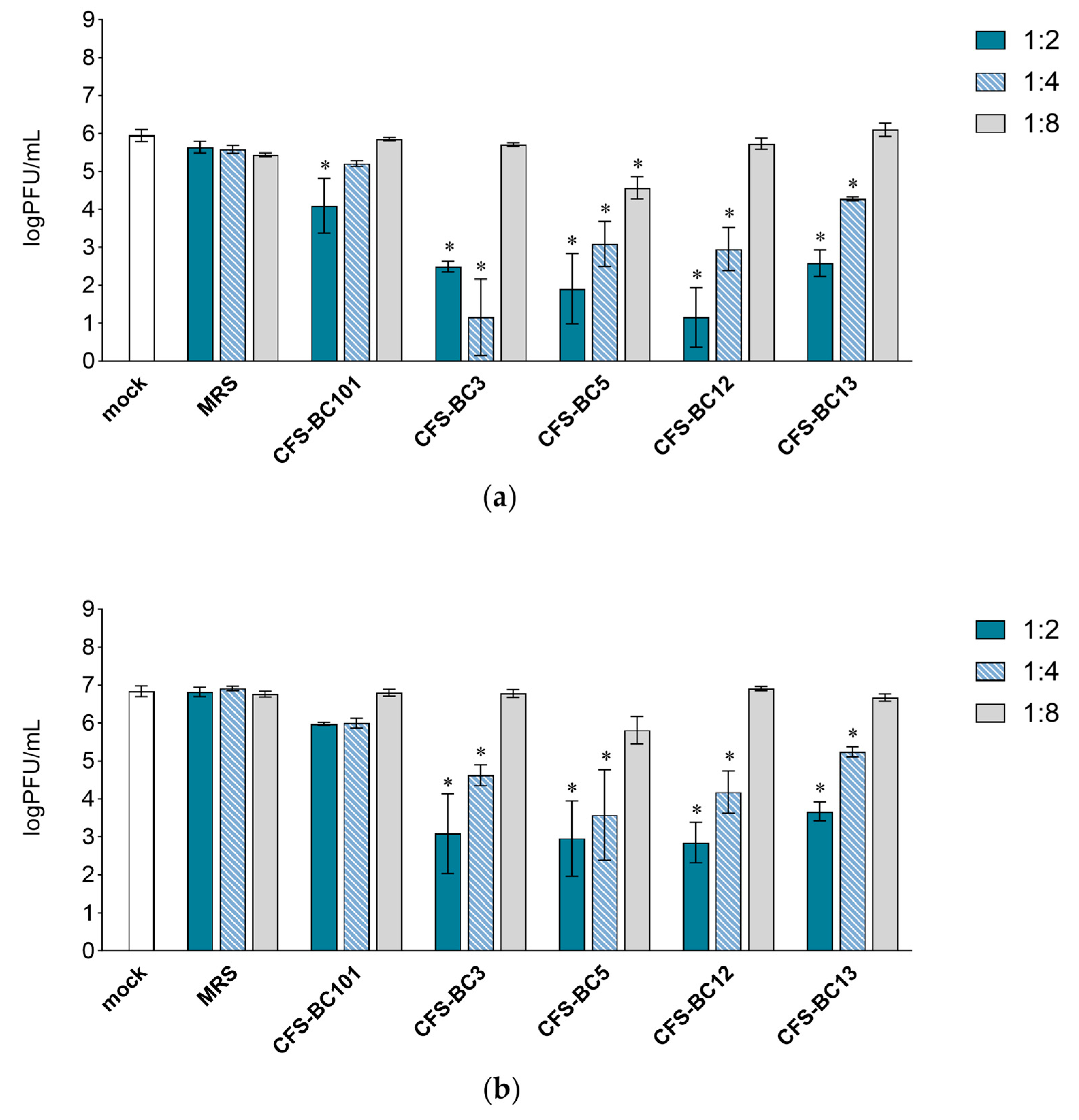
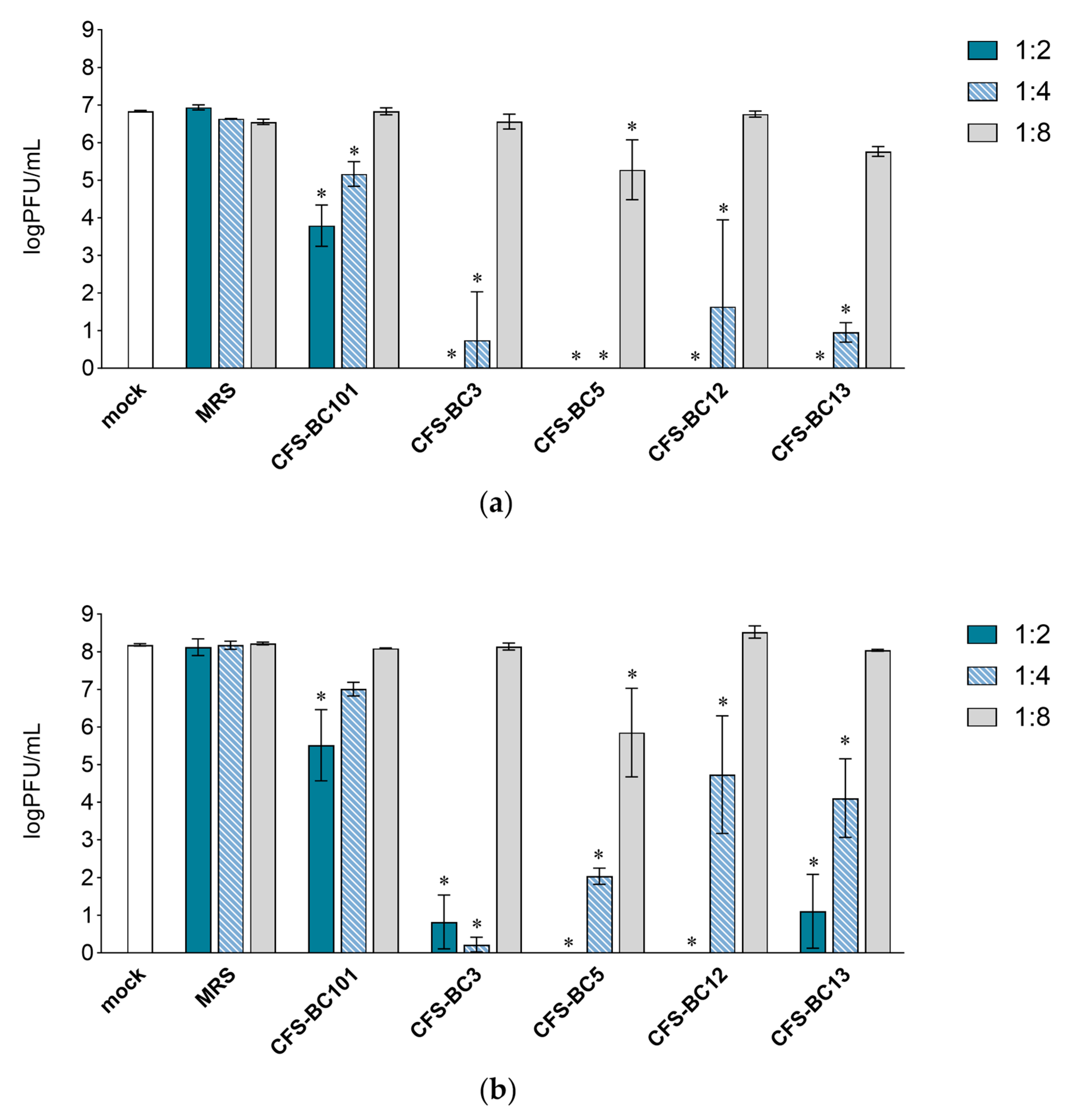
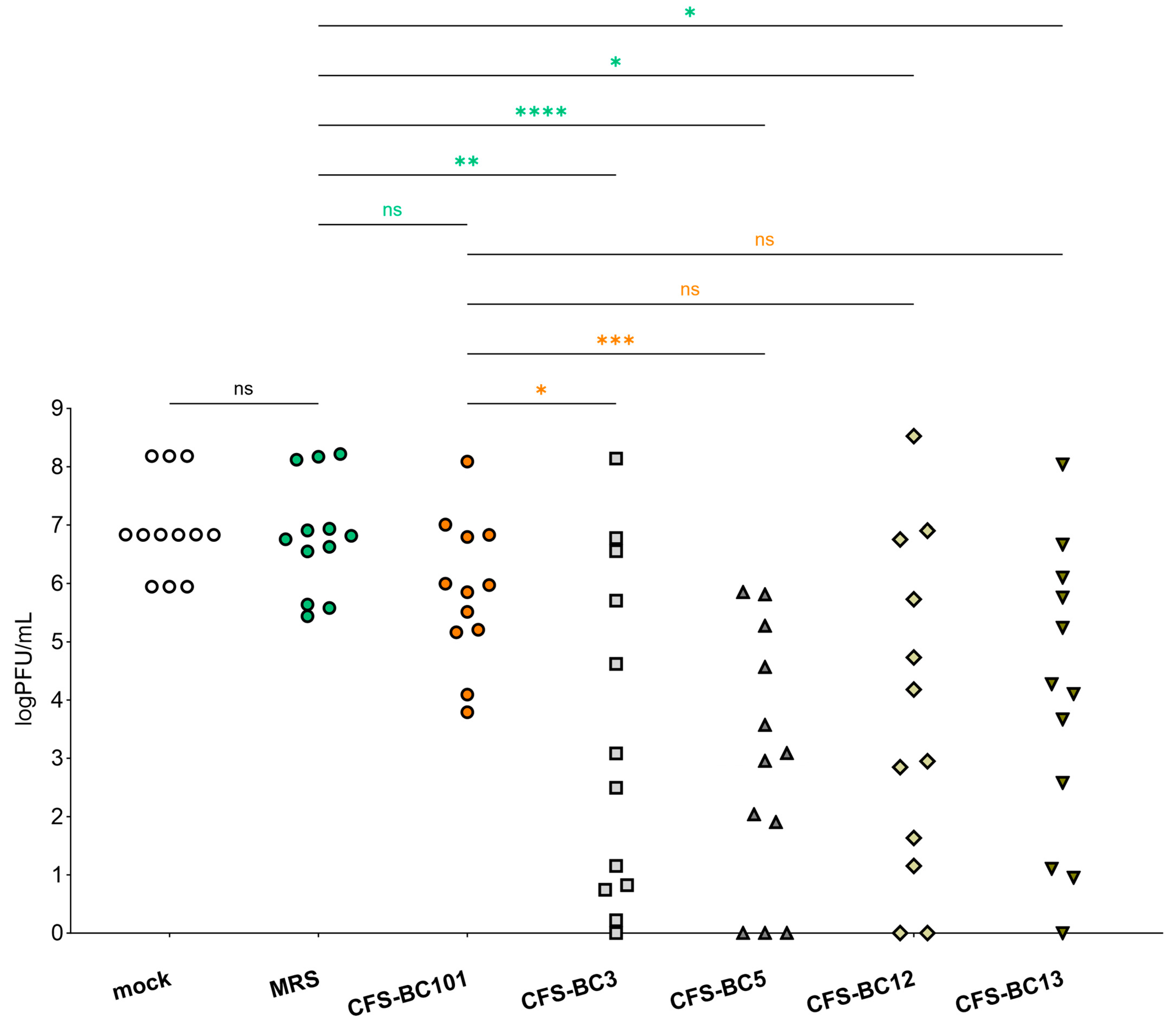
2.1. Lactobacilli Supernatants Reduce HSV-1 Replication
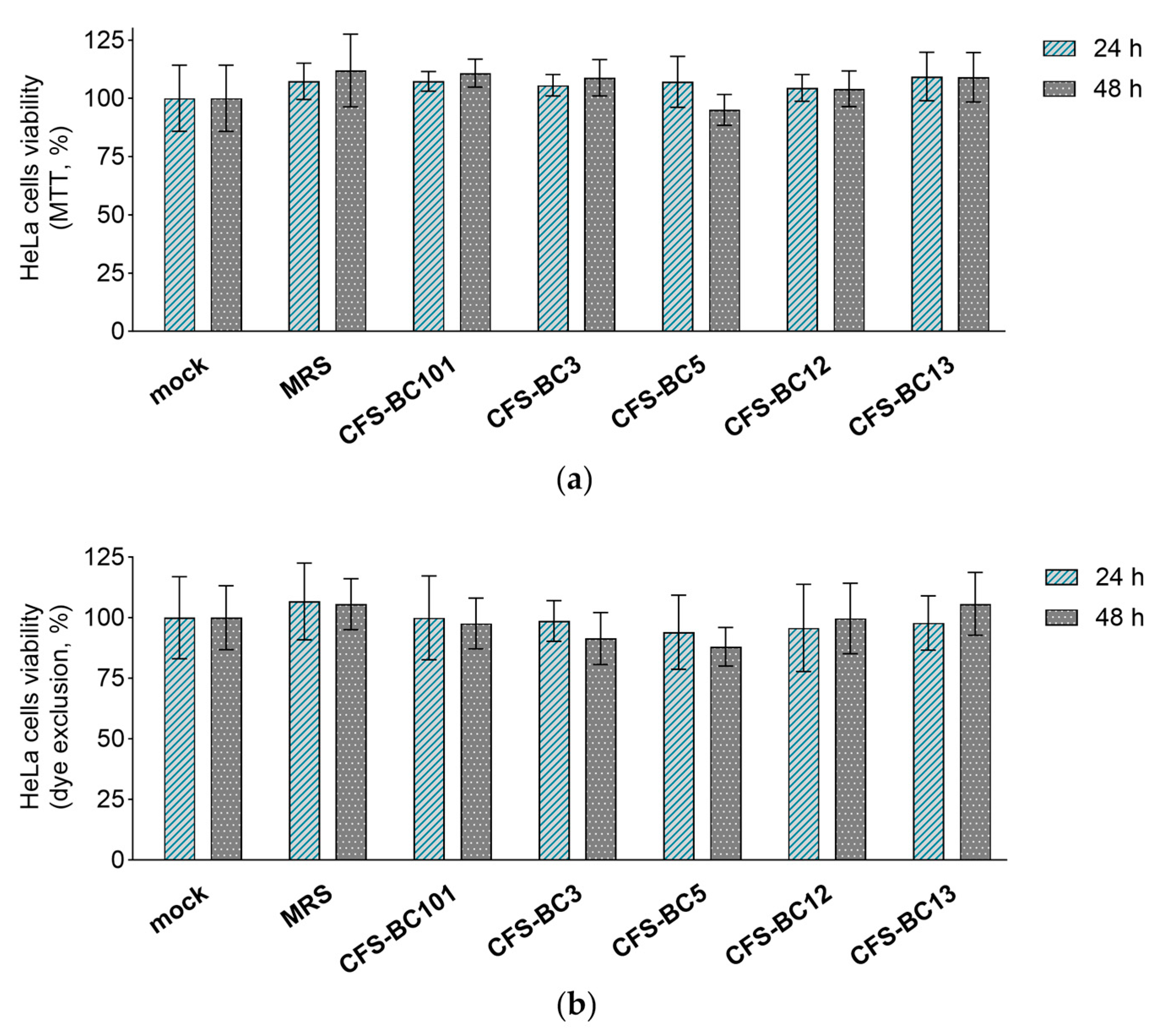
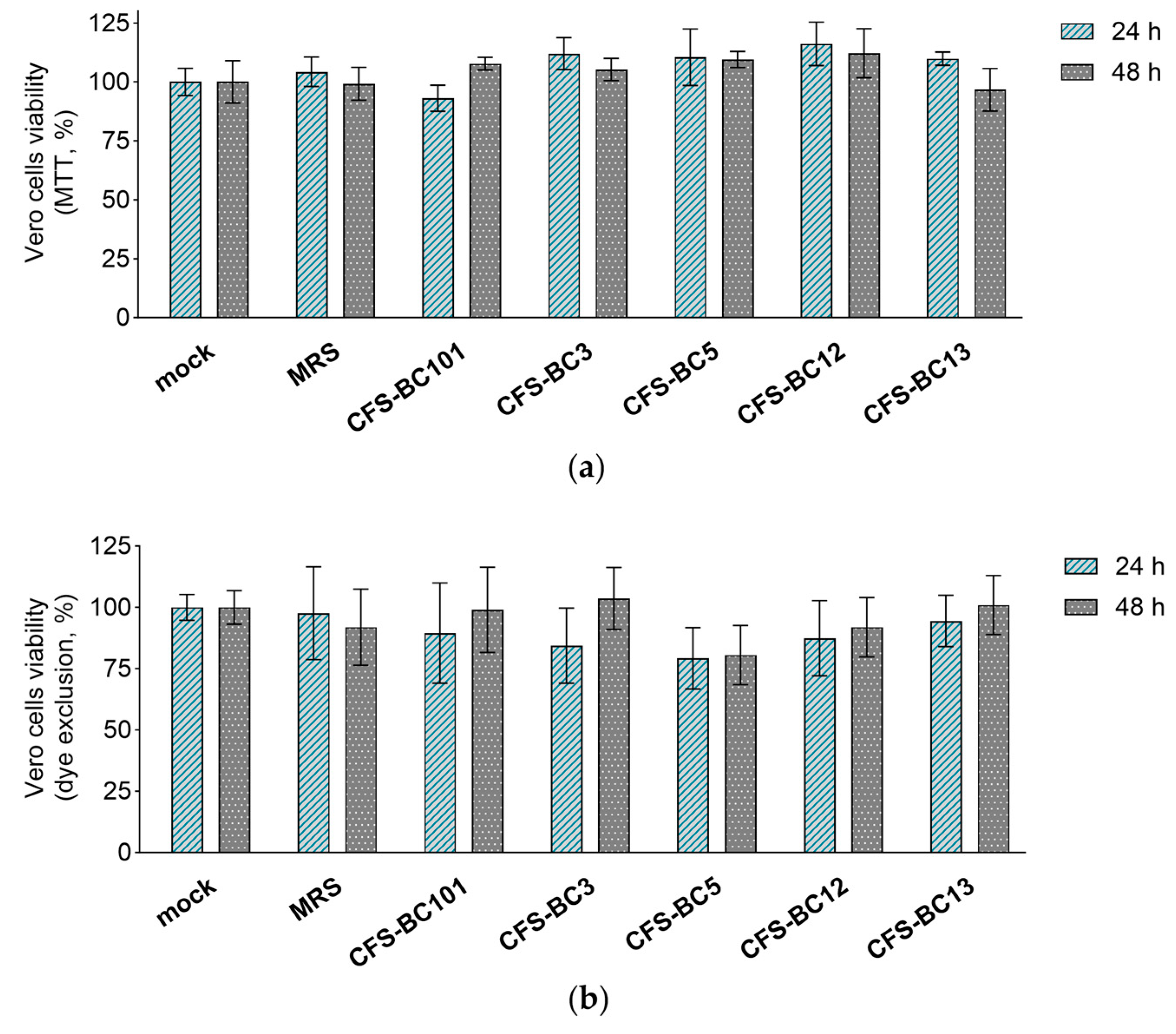
2.2. Lactobacilli Supernatants Do Not Influence the Viability of Mammalian Cells
2.3. Overview of CFS-Mediated Antiviral Activity
2.4. Metabolomic Analysis of Lactobacilli Supernatants
3. Discussion
4. Materials and Methods
4.1. Microorganisms and Culture Conditions
4.2. Preparation of Cell-Free Supernatants
4.3. Mammalian Cells and Virus
4.4. Antiviral Assay
4.5. Viability Assay on HeLa and Vero Cells
4.6. Data Analysis and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, Y.; Cai, X.; Ye, Y.; Wang, F.; Chen, F.; Zheng, C. The Role of Microbiota in Infant Health: From Early Life to Adulthood. Front. Immunol. 2021, 12, 708472. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef]
- Ottinger, S.; Robertson, C.M.; Branthoover, H.; Patras, K.A. The Human Vaginal Microbiota: From Clinical Medicine to Models to Mechanisms. Curr. Opin. Microbiol. 2024, 77, 102422. [Google Scholar] [CrossRef]
- France, M.; Alizadeh, M.; Brown, S.; Ma, B.; Ravel, J. Towards a Deeper Understanding of the Vaginal Microbiota. Nat. Microbiol. 2022, 7, 367–378. [Google Scholar] [CrossRef]
- Al Kassaa, I. The Antiviral Activity of Probiotic Metabolites. In New Insights on Antiviral Probiotics; Springer International Publishing: Cham, Switzerland, 2017; pp. 83–97. [Google Scholar]
- Biliavska, L.; Pankivska, Y.; Povnitsa, O.; Zagorodnya, S. Antiviral Activity of Exopolysaccharides Produced by Lactic Acid Bacteria of the Genera Pediococcus, Leuconostoc and Lactobacillus against Human Adenovirus Type 5. Medicina 2019, 55, 519. [Google Scholar] [CrossRef]
- Serkedjieva, J.; Danova, S.; Ivanova, I. Antiinfluenza Virus Activity of a Bacteriocin Produced by Lactobacillus Delbrueckii. Appl. Biochem. Biotechnol. 2000, 88, 285–298. [Google Scholar] [CrossRef]
- Younes, J.A.; Lievens, E.; Hummelen, R.; van der Westen, R.; Reid, G.; Petrova, M.I. Women and Their Microbes: The Unexpected Friendship. Trends Microbiol. 2018, 26, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.I.; Lievens, E.; Malik, S.; Imholz, N.; Lebeer, S. Lactobacillus Species as Biomarkers and Agents That Can Promote Various Aspects of Vaginal Health. Front. Physiol. 2015, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Dimitonova, S.P.; Danova, S.T.; Serkedjieva, J.P.; Bakalov, B.V. Antimicrobial Activity and Protective Properties of Vaginal Lactobacilli from Healthy Bulgarian Women. Anaerobe 2007, 13, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Al Kassaa, I.; Hober, D.; Hamze, M.; Caloone, D.; Dewilde, A.; Chihib, N.; Drider, D. Vaginal Lactobacillus Gasseri CMUL57 Can Inhibit Herpes Simplex Type 2 but Not Coxsackievirus B4E2. Arch. Microbiol. 2015, 197, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J.; Coombs, R.W. Viricidal Effect of Lactobacillus Acidophilus on Human Immunodeficiency Virus Type 1: Possible Role in Heterosexual Transmission. J. Exp. Med. 1991, 174, 289–292. [Google Scholar] [CrossRef]
- Ñahui Palomino, R.A.; Zicari, S.; Vanpouille, C.; Vitali, B.; Margolis, L. Vaginal Lactobacillus Inhibits HIV-1 Replication in Human Tissues Ex Vivo. Front. Microbiol. 2017, 8, 906. [Google Scholar] [CrossRef]
- Mastromarino, P.; Cacciotti, F.; Masci, A.; Mosca, L. Antiviral Activity of Lactobacillus Brevis towards Herpes Simplex Virus Type 2: Role of Cell Wall Associated Components. Anaerobe 2011, 17, 334–336. [Google Scholar] [CrossRef]
- Mousavi, E.; Makvandi, M.; Teimoori, A.; Ataei, A.; Ghafari, S.; Samarbaf-Zadeh, A. Antiviral Effects of Lactobacillus Crispatus against HSV-2 in Mammalian Cell Lines. J. Chin. Med. Assoc. 2018, 81, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Dicks, L.M.T.; Popov, I.V.; Karaseva, A.; Ermakov, A.M.; Suvorov, A.; Tagg, J.R.; Weeks, R.; Chikindas, M.L. Probiotics at War Against Viruses: What Is Missing from the Picture? Front. Microbiol. 2020, 11, 1877. [Google Scholar] [CrossRef]
- Ayoub, H.H.; Chemaitelly, H.; Abu-Raddad, L.J. Characterizing the Transitioning Epidemiology of Herpes Simplex Virus Type 1 in the USA: Model-Based Predictions. BMC Med. 2019, 17, 57. [Google Scholar] [CrossRef]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes Simplex Virus: Global Infection Prevalence and Incidence Estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Omarova, S.; Cannon, A.; Weiss, W.; Bruccoleri, A.; Puccio, J. Genital Herpes Simplex Virus-An Updated Review. Adv. Pediatr. 2022, 69, 149–162. [Google Scholar] [CrossRef]
- Groves, M.J. Genital Herpes: A Review. Am. Fam. Physician 2016, 93, 928–934. [Google Scholar]
- Vilhelmova_Ilieva, N.; Atanasov, G.; Simeonova, L.; Dobreva, L.; Mancheva, K.; Trepechova, M.; Danova, S. Anti-Herpes Virus Activity of Lactobacillus’ Postbiotics. Biomedicine 2022, 12, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Arduino, P.G.; Porter, S.R. Herpes Simplex Virus Type 1 Infection: Overview on Relevant Clinico-Pathological Features. J. Oral. Pathol. Med. 2007, 37, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Khani, S.; Motamedifar, M.; Golmoghaddam, H.; Hosseini, H.M.; Hashemizadeh, Z. In Vitro Study of the Effect of a Probiotic Bacterium Lactobacillus Rhamnosus against Herpes Simplex Virus Type 1. Braz. J. Infect. Dis. 2012, 16, 129–135. [Google Scholar] [CrossRef]
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and Virulence of Herpes Simplex Virus. Virulence 2021, 12, 2670–2702. [Google Scholar] [CrossRef]
- Gundamraj, V.; Hasbun, R. Viral Meningitis and Encephalitis: An Update. Curr. Opin. Infect. Dis. 2023, 36, 177–185. [Google Scholar] [CrossRef]
- Barzoki, M.G.; Malekshahi, S.S.; Shayestehpour, M. In Vitro Evaluation of Antiviral Activity of Shouchella Clausii Probiotic Strain and Bacterial Supernatant against Herpes Simplex Virus Type 1. Arch. Microbiol. 2022, 204, 522. [Google Scholar] [CrossRef] [PubMed]
- Coen, D.M. General Aspects of Virus Drug Resistance with Special Reference to Herpes Simplex Virus. J. Antimicrob. Chemother. 1986, 18, 1–10. [Google Scholar] [CrossRef]
- Upadhyayula, S.; Michaels, M.G. Ganciclovir, Foscarnet, and Cidofovir: Antiviral Drugs Not Just for Cytomegalovirus. J. Pediatr. Infect. Dis. Soc. 2013, 2, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Parolin, C.; Marangoni, A.; Laghi, L.; Foschi, C.; Ñahui Palomino, R.A.; Calonghi, N.; Cevenini, R.; Vitali, B. Isolation of Vaginal Lactobacilli and Characterization of Anti-Candida Activity. PLoS ONE 2015, 10, e0131220. [Google Scholar] [CrossRef]
- Tyssen, D.; Wang, Y.-Y.; Hayward, J.A.; Agius, P.A.; DeLong, K.; Aldunate, M.; Ravel, J.; Moench, T.R.; Cone, R.A.; Tachedjian, G. Anti-HIV-1 Activity of Lactic Acid in Human Cervicovaginal Fluid. mSphere 2018, 3, e00055-18. [Google Scholar] [CrossRef]
- Hearps, A.C.; Tyssen, D.; Srbinovski, D.; Bayigga, L.; Diaz, D.J.D.; Aldunate, M.; Cone, R.A.; Gugasyan, R.; Anderson, D.J.; Tachedjian, G. Vaginal Lactic Acid Elicits an Anti-Inflammatory Response from Human Cervicovaginal Epithelial Cells and Inhibits Production of pro-Inflammatory Mediators Associated with HIV Acquisition. Mucosal. Immunol. 2017, 10, 1480–1490. [Google Scholar] [CrossRef]
- AlMukdad, S.; Harfouche, M.; Farooqui, U.S.; Aldos, L.; Abu-Raddad, L.J. Epidemiology of Herpes Simplex Virus Type 1 in Canada: Systematic Review, Meta-Analyses, and Meta-Regressions. Front. Public Health 2023, 11, 1118249. [Google Scholar] [CrossRef]
- Gianni, T.; Campadelli-Fiume, G.; Menotti, L. Entry of Herpes Simplex Virus Mediated by Chimeric Forms of Nectin1 Retargeted to Endosomes or to Lipid Rafts Occurs through Acidic Endosomes. J. Virol. 2004, 78, 12268–12276. [Google Scholar] [CrossRef]
- Feng, C.; Jin, C.; Liu, K.; Yang, Z. Microbiota-Derived Short Chain Fatty Acids: Their Role and Mechanisms in Viral Infections. Biomed. Pharmacother. 2023, 160, 114414. [Google Scholar] [CrossRef]
- Ejercito, P.M.; Kieff, E.D.; Roizman, B. Characterization of Herpes Simplex Virus Strains Differing in Their Effects on Social Behaviour of Infected Cells. J. Gen. Virol. 1968, 2, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, C.R.; Burke, R.L.; Hoflack, B.; Ludwig, T.; Dingwell, K.S.; Johnson, D.C. Role of Mannose-6-Phosphate Receptors in Herpes Simplex Virus Entry into Cells and Cell-to-Cell Transmission. J. Virol. 1995, 69, 3517–3528. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avitabile, E.; Menotti, L.; Giordani, B.; Croatti, V.; Parolin, C.; Vitali, B. Vaginal Lactobacilli Supernatants Protect from Herpes Simplex Virus Type 1 Infection in Cell Culture Models. Int. J. Mol. Sci. 2024, 25, 2492. https://doi.org/10.3390/ijms25052492
Avitabile E, Menotti L, Giordani B, Croatti V, Parolin C, Vitali B. Vaginal Lactobacilli Supernatants Protect from Herpes Simplex Virus Type 1 Infection in Cell Culture Models. International Journal of Molecular Sciences. 2024; 25(5):2492. https://doi.org/10.3390/ijms25052492
Chicago/Turabian StyleAvitabile, Elisa, Laura Menotti, Barbara Giordani, Vanessa Croatti, Carola Parolin, and Beatrice Vitali. 2024. "Vaginal Lactobacilli Supernatants Protect from Herpes Simplex Virus Type 1 Infection in Cell Culture Models" International Journal of Molecular Sciences 25, no. 5: 2492. https://doi.org/10.3390/ijms25052492
APA StyleAvitabile, E., Menotti, L., Giordani, B., Croatti, V., Parolin, C., & Vitali, B. (2024). Vaginal Lactobacilli Supernatants Protect from Herpes Simplex Virus Type 1 Infection in Cell Culture Models. International Journal of Molecular Sciences, 25(5), 2492. https://doi.org/10.3390/ijms25052492









