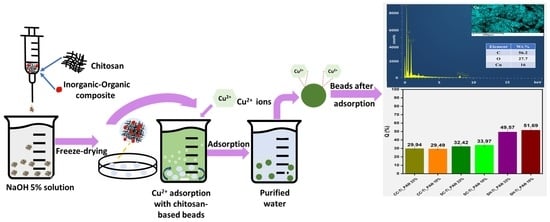
Chitosan-Based Beads Incorporating Inorganic–Organic Composites for Copper Ion Retention in Aqueous Solutions
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of Composite Beads
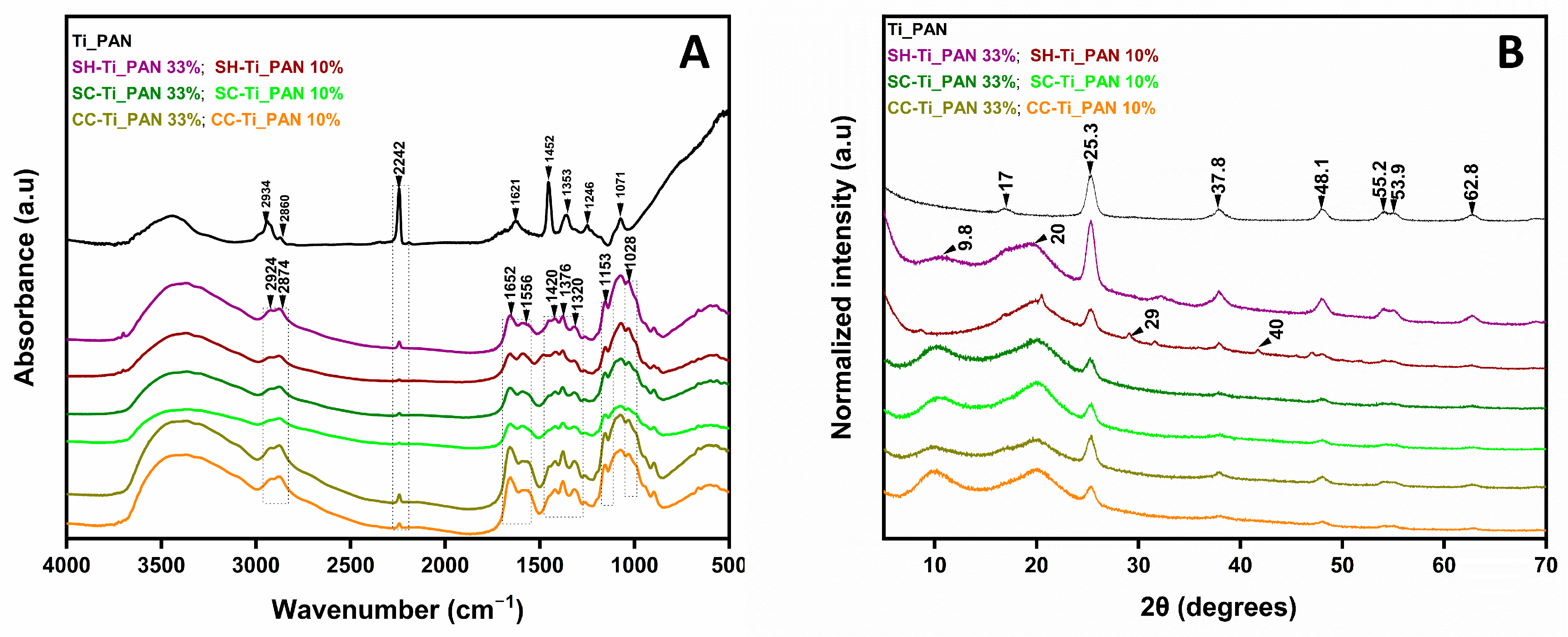
2.1.1. Structure Evaluation of Composite Beads
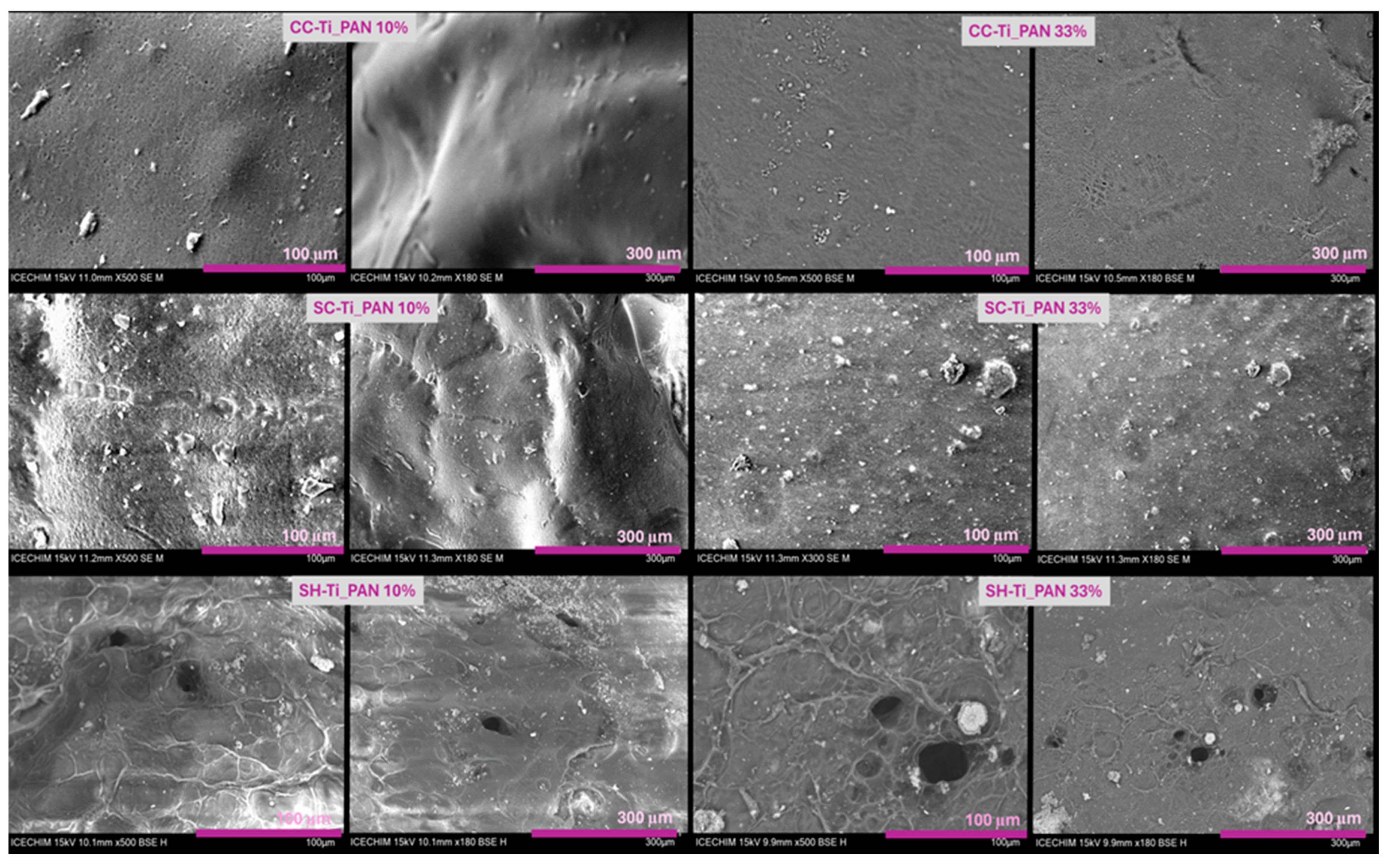
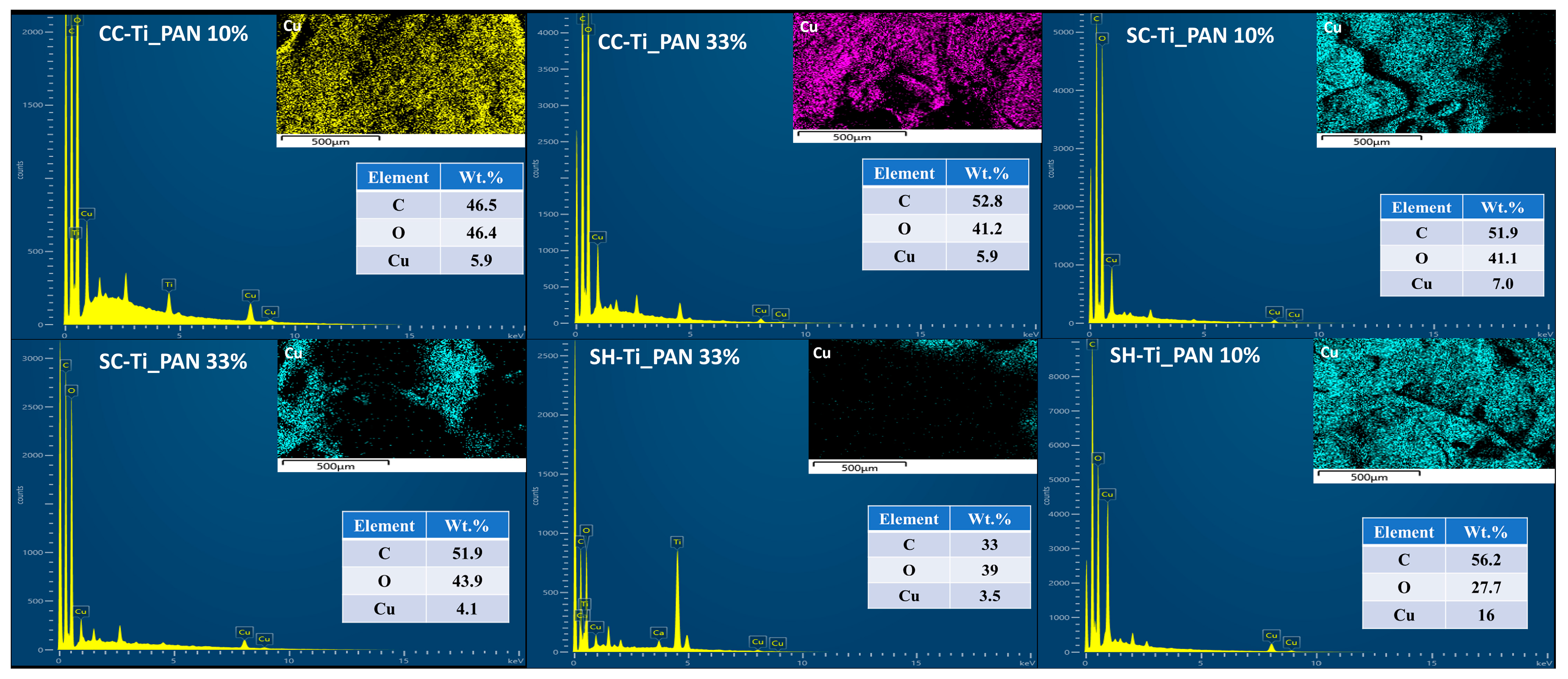
2.1.2. N2 Adsorption/Desorption Measurements and Morphology of Composite Beads
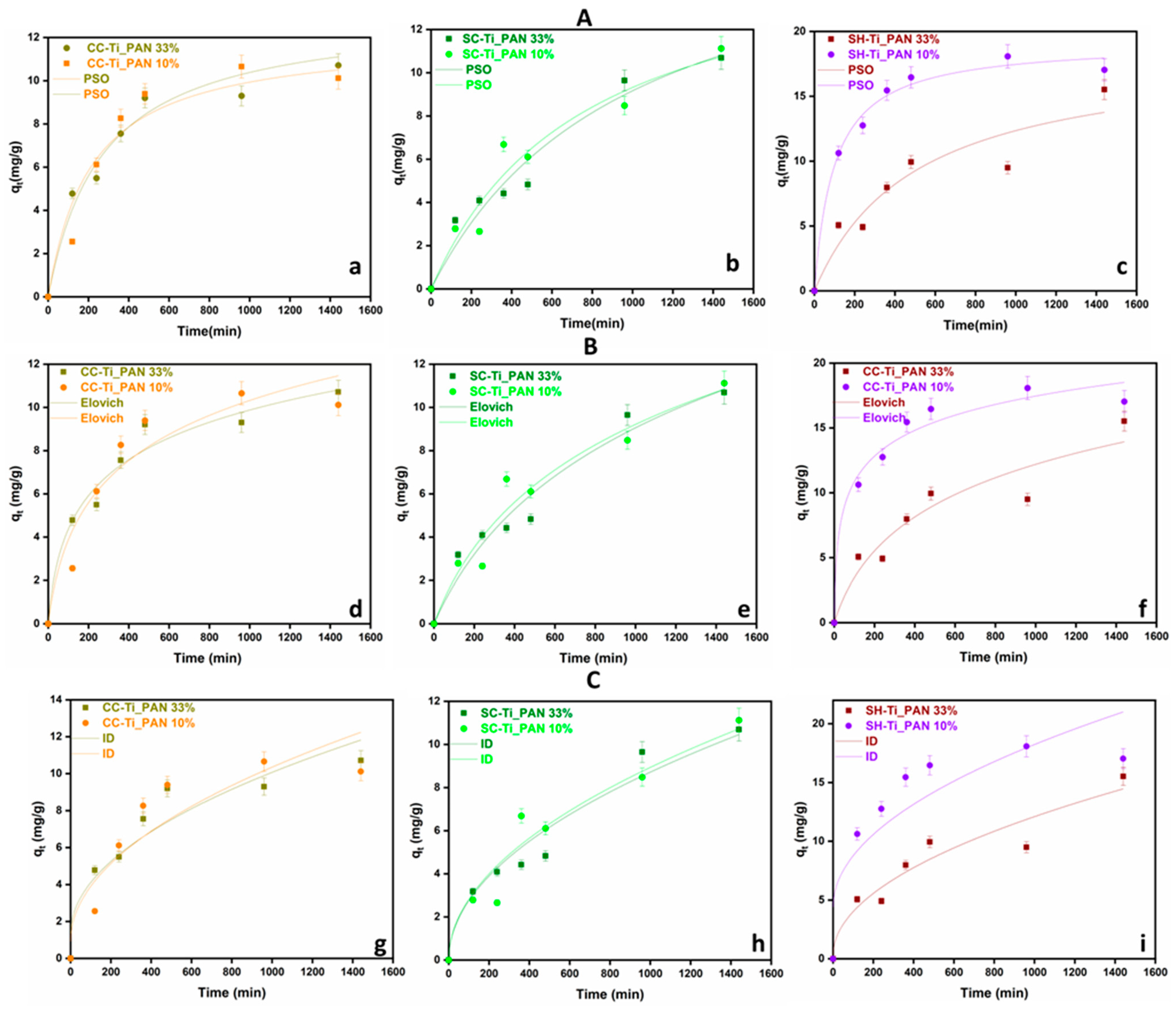
2.2. Adsorption Kinetics of Cu2+ Ions
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Composite Beads
3.3. Characterization Techniques
3.4. Adsorption Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azizullah, A.; Khattak, M.N.K.; Richter, P.; Häder, D.-P. Water Pollution in Pakistan and Its Impact on Public Health—A Review. Environ. Int. 2011, 37, 479–497. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A Review on Heavy Metal Pollution, Toxicity and Remedial Measures: Current Trends and Future Perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of Heavy Metal Pollution from Anthropogenic Activities and Remediation Strategies: A Review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Verma, R.K.; Chopade, R.L.; Pandit, P.P.; Nagar, V.; Aseri, V.; Choudhary, S.K.; Awasthi, G.; Awasthi, K.K.; et al. Heavy Metal Contamination of Water and Their Toxic Effect on Living Organisms. In The Toxicity of Environmental Pollutants; IntechOpen: London, UK, 2022; ISBN 978-1-80355-580-5. [Google Scholar]
- Wu, B.; Zhao, D.Y.; Jia, H.Y.; Zhang, Y.; Zhang, X.X.; Cheng, S.P. Preliminary Risk Assessment of Trace Metal Pollution in Surface Water from Yangtze River in Nanjing Section, China. Bull. Environ. Contam. Toxicol. 2009, 82, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.N.P.; Sousa Neto, V.O.; Oliveira, J.T.; Oliveira, T.C.; Melo, D.Q.; Silva, M.A.A.; Nascimento, R.F. Study on the Use of Roasted Barley Powder for Adsorption of Cu2+ Ions in Batch Experiments and in Fixed-Bed Columns. BioResources 2013, 8, 3556–3573. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Peng, Y.; Feng, L.; Li, X.; Zhao, C.; Sarfaraz, K. Novel Cationic Polymer Modified Magnetic Chitosan Beads for Efficient Adsorption of Heavy Metals and Dyes over a Wide pH Range. Int. J. Biol. Macromol. 2020, 156, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Sudha Rani, K.; Srinivas, B.; GouruNaidu, K.; Ramesh, K.V. Removal of Copper by Adsorption on Treated Laterite. Mater. Today Proc. 2018, 5, 463–469. [Google Scholar] [CrossRef]
- Ramos, V.C.; Utrilla, J.R.; Sánchez, A.R.; Ramón, M.V.L.; Polo, M.S. Marble Waste Sludges as Effective Nanomaterials for Cu (II) Adsorption in Aqueous Media. Nanomaterials 2021, 11, 2305. [Google Scholar] [CrossRef]
- Shehzad, H.; Ahmed, E.; Sharif, A.; Farooqi, Z.H.; Din, M.I.; Begum, R.; Liu, Z.; Zhou, L.; Ouyang, J.; Irfan, A.; et al. Modified Alginate-Chitosan-TiO2 Composites for Adsorptive Removal of Ni(II) Ions from Aqueous Medium. Int. J. Biol. Macromol. 2022, 194, 117–127. [Google Scholar] [CrossRef]
- Ariffin, N.; Abdullah, M.M.A.B.; Zainol, M.R.R.M.A.; Murshed, M.F.; Faris, M.A.; Bayuaji, R. Review on Adsorption of Heavy Metal in Wastewater by Using Geopolymer. MATEC Web Conf. 2017, 97, 01023. [Google Scholar] [CrossRef]
- Zaimee, M.Z.A.; Sarjadi, M.S.; Rahman, M.L. Heavy Metals Removal from Water by Efficient Adsorbents. Water 2021, 13, 2659. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Renu; Agarwal, M.; Singh, K. Heavy Metal Removal from Wastewater Using Various Adsorbents: A Review. J. Water Reuse Desalination 2017, 7, 387–419. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of Heavy Metal Ions by Various Low-Cost Adsorbents: A Review. Int. J. Environ. Anal. Chem. 2022, 102, 342–379. [Google Scholar] [CrossRef]
- Zito, P.; Shipley, H.J. Inorganic Nano-Adsorbents for the Removal of Heavy Metals and Arsenic: A Review. RSC Adv. 2015, 5, 29885–29907. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A.L. Carbon Based Materials: A Review of Adsorbents for Inorganic and Organic Compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M.; Anitha, K.L. Recent Advances in Heavy Metal Removal by Chitosan Based Adsorbents. Carbohydr. Polym. 2021, 251, 117000. [Google Scholar] [CrossRef]
- Awual, M.R. Novel Ligand Functionalized Composite Material for Efficient Copper(II) Capturing from Wastewater Sample. Compos. B Eng. 2019, 172, 387–396. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of Dyes and Heavy Metal Ions by Chitosan Composites: A Review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, X.; Tan, X.; Wang, X. Sorption of Heavy Metal Ions from Aqueous Solutions: A Review. J. Colloid Sci. 2010, 4, 19–31. [Google Scholar] [CrossRef]
- Jamshaid, A.; Iqbal, J.; Hamid, A.; Ghauri, M.; Muhammad, N.; Nasrullah, A.; Rafiq, S.; Shah, N.S. Fabrication and Evaluation of Cellulose-Alginate-Hydroxyapatite Beads for the Removal of Heavy Metal Ions from Aqueous Solutions. ZPC 2019, 233, 1351–1375. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; El-Bindary, A.A.; Kouta, E.Y.; Guibal, E. Functionalization of Polyacrylonitrile/Na-Y-Zeolite Composite with Amidoxime Groups for the Sorption of Cu(II), Cd(II) and Pb(II) Metal Ions. Chem. Eng. J. 2018, 332, 727–736. [Google Scholar] [CrossRef]
- . Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Su, C.; Berekute, A.K.; Yu, K.-P. Chitosan@TiO2 Composites for the Adsorption of Copper(II) and Antibacterial Applications. Sustain. Environ. Res. 2022, 32, 27. [Google Scholar] [CrossRef]
- Ablouh, E.; Hanani, Z.; Eladlani, N.; Rhazi, M.; Taourirte, M. Chitosan Microspheres/Sodium Alginate Hybrid Beads: An Efficient Green Adsorbent for Heavy Metals Removal from Aqueous Solutions. Sustain. Environ. Res. 2019, 29, 5. [Google Scholar] [CrossRef]
- Mohanasrinivasan, V.; Mishra, M.; Paliwal, J.S.; Singh, S.K.; Selvarajan, E.; Suganthi, V.; Subathra Devi, C. Studies on Heavy Metal Removal Efficiency and Antibacterial Activity of Chitosan Prepared from Shrimp Shell Waste. 3 Biotech 2014, 4, 167–175. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of Heavy Metal Ions Using Chitosan and Modified Chitosan: A Review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Begum, S.; Yuhana, N.Y.; Md Saleh, N.; Kamarudin, N.H.N.; Sulong, A.B. Review of Chitosan Composite as a Heavy Metal Adsorbent: Material Preparation and Properties. Carbohydr. Polym. 2021, 259, 117613. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Ghosh, T.K.; Viswanath, D.S.; Boddu, V.M. Dispersion of Chitosan on Perlite for Enhancement of Copper(II) Adsorption Capacity. J. Hazard. Mater. 2008, 152, 826–837. [Google Scholar] [CrossRef]
- Latorre, M.; Troncoso, R.; Uauy, R. Chapter 4—Biological Aspects of Copper. In Clinical and Translational Perspectives on WILSON DISEASE; Kerkar, N., Roberts, E.A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 25–31. ISBN 978-0-12-810532-0. [Google Scholar]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper Environmental Toxicology, Recent Advances, and Future Outlook: A Review. Environ. Sci. Pollut. Res. 2019, 26, 18003–18016. [Google Scholar] [CrossRef]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.-F.; Margaritis, I. Dietary Copper and Human Health: Current Evidence and Unresolved Issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Locovei, C.; Chiriac, A.-L.; Miron, A.; Iftimie, S.; Antohe, V.-A.; Sârbu, A.; Dumitru, A. Synthesis of Titanium Nitride via Hybrid Nanocomposites Based on Mesoporous TiO2/Acrylonitrile. Sci. Rep. 2021, 11, 5055. [Google Scholar] [CrossRef]
- Al Shaqsi, N.H.K.; Al Hoqani, H.A.S.; Hossain, M.A.; Al Sibani, M.A. Isolation, Characterization and Standardization of Demineralization Process for Chitin Polymer and Minerals from the Crabs Waste of Portunidae Segnis. Adv. Biomark. Sci. Technol. 2020, 2, 45–58. [Google Scholar] [CrossRef]
- Al-Sagheer, F.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M. Extraction and Characterization of Chitin and Chitosan from Marine Sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Kuczajowska-Zadrożna, M.; Filipkowska, U.; Jóźwiak, T. Adsorption of Cu (II) and Cd (II) from Aqueous Solutions by Chitosan Immobilized in Alginate Beads. J. Environ. Chem. Eng. 2020, 8, 103878. [Google Scholar] [CrossRef]
- Kudłacik-Kramarczyk, S.; Głąb, M.; Drabczyk, A.; Kordyka, A.; Godzierz, M.; Wróbel, P.S.; Krzan, M.; Uthayakumar, M.; Kędzierska, M.; Tyliszczak, B. Physicochemical Characteristics of Chitosan-Based Hydrogels Containing Albumin Particles and Aloe Vera Juice as Transdermal Systems Functionalized in the Viewpoint of Potential Biomedical Applications. Materials 2021, 14, 5832. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, L.; Yang, F.; Geng, L. The Synthesis of Polyacrylonitrile/Carbon Nanotube Microspheres by Aqueous Deposition Polymerization under Ultrasonication. Carbon 2010, 48, 688–695. [Google Scholar] [CrossRef]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase Characterization of TiO2 Powder by XRD and TEM. Agric. Nat. Resour. 2008, 42, 357–361. [Google Scholar]
- Miron, A.; Sarbu, A.; Zaharia, A.; Sandu, T.; Iovu, H.; Fierascu, R.C.; Neagu, A.-L.; Chiriac, A.-L.; Iordache, T.-V. A Top-Down Procedure for Synthesizing Calcium Carbonate-Enriched Chitosan from Shrimp Shell Wastes. Gels 2022, 8, 742. [Google Scholar] [CrossRef]
- Swain, S.K.; Dash, S.; Kisku, S.K.; Singh, R.K. Thermal and Oxygen Barrier Properties of Chitosan Bionanocomposites by Reinforcement of Calcium Carbonate Nanopowder. J. Mater. Sci. Technol. 2014, 30, 791–795. [Google Scholar] [CrossRef]
- Abebe, B.; Murthy, H.C.A.; Amare, E. Summary on Adsorption and Photocatalysis for Pollutant Remediation: Mini Review. J. Encapsulation Adsorpt. Sci. 2018, 08, 225–255. [Google Scholar] [CrossRef]
- Wan, M.-W.; Kan, C.-C.; Rogel, B.D.; Dalida, M.L.P. Adsorption of Copper (II) and Lead (II) Ions from Aqueous Solution on Chitosan-Coated Sand. Carbohydr. Polym. 2010, 80, 891–899. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, X.; Han, M.; Ma, W. Adsorption Kinetic Character of Copper Ions onto a Modified Chitosan Transparent Thin Membrane from Aqueous Solution. J. Hazard. Mater. 2010, 182, 408–415. [Google Scholar] [CrossRef]
- Orozco, C.I.; Freire, M.S.; Gómez-Díaz, D.; González-Álvarez, J. Removal of Copper from Aqueous Solutions by Biosorption onto Pine Sawdust. Sustain. Chem. Pharm. 2023, 32, 101016. [Google Scholar] [CrossRef]
- Emara, A.A.A.; Tawab, M.A.; El-ghamry, M.A.; Elsabee, M.Z. Metal Uptake by Chitosan Derivatives and Structure Studies of the Polymer Metal Complexes. Carbohydr. Polym. 2011, 83, 192–202. [Google Scholar] [CrossRef]
- Matias, P.M.C.; Sousa, J.F.M.; Bernardino, E.F.; Vareda, J.P.; Durães, L.; Abreu, P.E.; Marques, J.M.C.; Murtinho, D.; Valente, A.J.M. Reduced Chitosan as a Strategy for Removing Copper Ions from Water. Molecules 2023, 28, 4110. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, T.E.; Kozlov, V.A.; Telegin, F.Y. Chemisorption of Copper Ions in Aqueous Acidic Solutions by Modified Chitosan. Mater. Sci. Eng. B 2021, 263, 114778. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Characteristics of Elovich Equation Used for the Analysis of Adsorption Kinetics in Dye-Chitosan Systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Dos Santos, L.N.; Santos, A.S.; Das Graças Fernandes Dantas, K.; Ferreira, N.R. Adsorption of Cu (II) Ions Present in the Distilled Beverage (Sugar Cane Spirit) Using Chitosan Derived from the Shrimp Shell. Polymers 2022, 14, 573. [Google Scholar] [CrossRef]
- Chang, M.-Y.; Juang, R.-S. Equilibrium and Kinetic Studies on the Adsorption of Surfactant, Organic Acids and Dyes from Water onto Natural Biopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 35–46. [Google Scholar] [CrossRef]
- An, B. Cu(II) and As(V) Adsorption Kinetic Characteristic of the Multifunctional Amino Groups in Chitosan. Processes 2020, 8, 1194. [Google Scholar] [CrossRef]
- Hameed, B.H.; El-Khaiary, M.I. Malachite Green Adsorption by Rattan Sawdust: Isotherm, Kinetic and Mechanism Modeling. J. Hazard. Mater. 2008, 159, 574–579. [Google Scholar] [CrossRef]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Maghat, H. Critical of Linear and Nonlinear Equations of Pseudo-First Order and Pseudo-Second Order Kinetic Models. Karbala Int. J. Mod. Sci. 2018, 4, 244–254. [Google Scholar] [CrossRef]
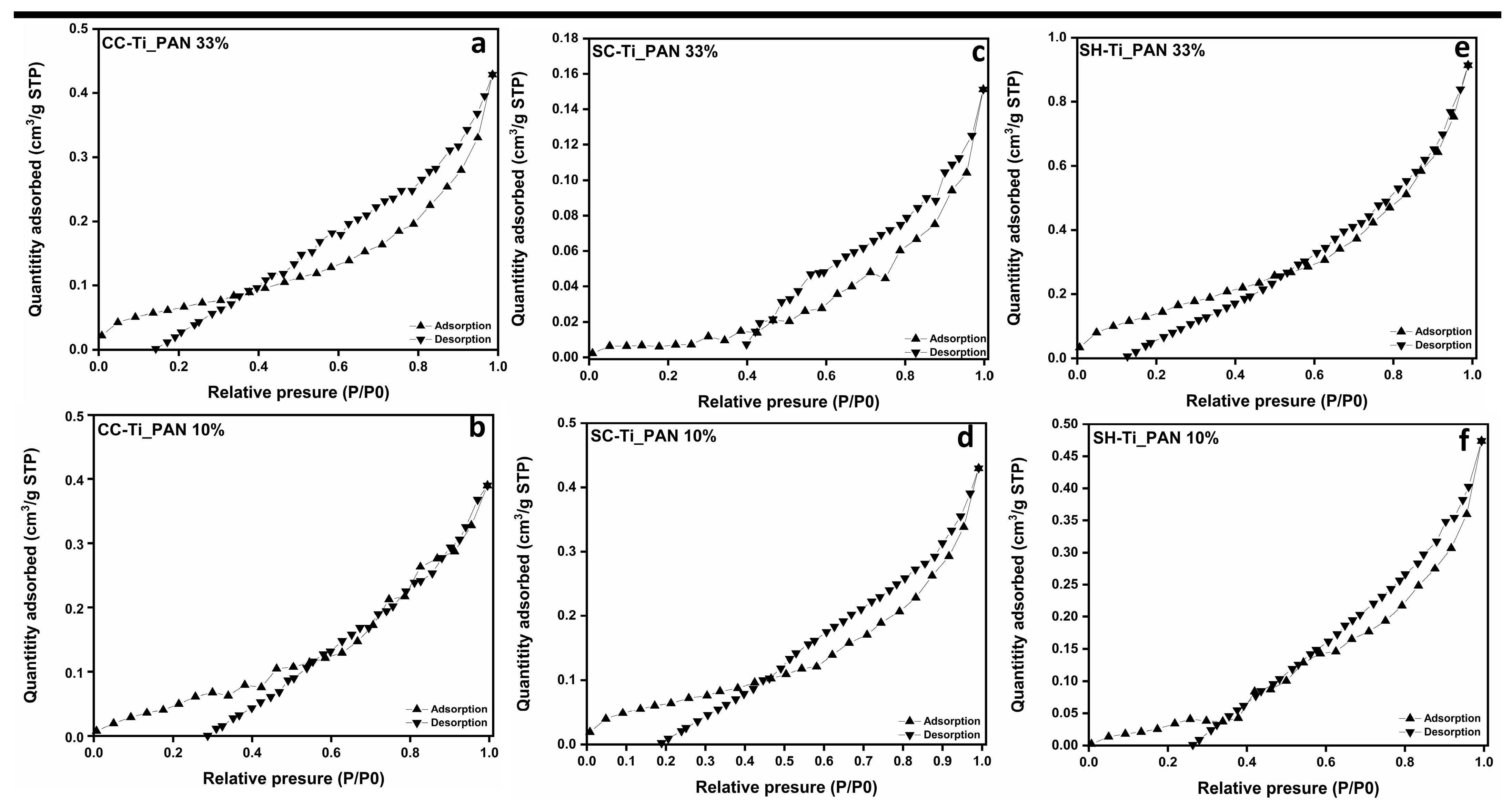

| Sample | BET Surface Area (m2 g−1) | Pore Surface Area (m2 g−1) | Maximum Pore Diameter 1 (nm) | Micropore Volume 2 (cm3 g−1) |
|---|---|---|---|---|
| CC-Ti_PAN 33% | 1.108 | 1.880 | 4.343 | 0.002 |
| CC-Ti_PAN 10% | 1.260 | 1.911 | 4.543 | 0.002 |
| SC-Ti_PAN 33% | 0.357 | 2.415 | 4.343 | 0.002 |
| SC-Ti_PAN 10% | 0.977 | 1.725 | 4.152 | 0.002 |
| SH-Ti_PAN 33% | 2.401 | 2.994 | 4.543 | 0.004 |
| SH-Ti_PAN 10% | 3.334 | 9.443 | 4.543 | 0.011 |
| Sample | Adsorption Capacity, q (mg/g) after 24 h | Retention Efficiency after 24 h (%) | Cu2+ Initial Concentration | Ref. |
|---|---|---|---|---|
| CC-Ti_PAN 33% | 10.7 | 29.9 | 100 | This study |
| CC-Ti_PAN 10% | 10.1 | 29.5 | 100 | This study |
| SC-Ti_PAN 33% | 10.7 | 32.5 | 100 | This study |
| SC-Ti_PAN 10% | 11.1 | 34.0 | 100 | This study |
| SH-Ti_PAN 33% | 15.5 | 49.6 | 100 | This study |
| SH-Ti_PAN 10% | 17.0 | 51.7 | 100 | This study |
| Chitosan-coated sand (CCS) | 1.2 | 99.77 | 100 | [44] |
| Modified chitosan transparent thin membrane (MCTTM) | 8.5 | - | 25 | [45] |
| Pine sawdust | 1.7 | - | 300 | [46] |
| Kinetic Model | Parameters | CC-Ti_PAN 33% | CC-Ti_PAN 10% | SC-Ti_PAN 33% | SC-Ti_PAN 10% | SH-Ti_PAN 33% | SH-Ti_PAN 10% |
|---|---|---|---|---|---|---|---|
| Experimental data | qe, exp (mg/g) | 10.714 | 10.128 | 10.687 | 11.124 | 15.521 | 17.039 |
| PSO | qe22/(mg/g) k2/(g/(mg·min) R2 | 12.053 3.889 0.973 | 13.202 2.788 0.951 | 18.232 5.545 0.995 | 16.385 8.053 0.947 | 18.575 1.068 0.898 | 19.180 5.284 0.989 |
| Elovich | α (mg/(g·min) β (g/mg) R2 | 0.114 0.385 0.970 | 0.074 0.307 0.921 | 0.021 0.167 0.959 | 0.025 0.190 0.949 | 0.049 0.192 0.910 | 1.196 0.345 0.975 |
| ID | kd (mg/g·min1/2) C (mg/g) R2 | 0.275 1.351 0.902 | 0.298 0.925 0.847 | 0.275 9.900 0.958 | 0.282 9.890 0.931 | 0.373 2.284 0.922 | 0.437 4.488 0.772 |
| Sample Code | Chitosan Type (Abbreviation) | Inorganic–Organic Composite, Ti_PAN (wt. %) |
|---|---|---|
| CC-Ti_PAN 33% | Commercial chitosan (CC) | 33 |
| CC-Ti_PAN 10% | Commercial chitosan (CC) | 10 |
| SC-Ti_PAN 33% | Chitosan from commercial chitin (SC) | 33 |
| SC-Ti_PAN 10% | Chitosan from commercial chitin (SC) | 10 |
| SH-Ti_PAN 33% | Chitosan from shrimp shell (SH) | 33 |
| SH-Ti_PAN 10% | Chitosan from shrimp shell (SH) | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miron, A.; Iordache, T.-V.; Valente, A.J.M.; Durães, L.M.R.; Sarbu, A.; Ivan, G.R.; Zaharia, A.; Sandu, T.; Iovu, H.; Chiriac, A.-L. Chitosan-Based Beads Incorporating Inorganic–Organic Composites for Copper Ion Retention in Aqueous Solutions. Int. J. Mol. Sci. 2024, 25, 2411. https://doi.org/10.3390/ijms25042411
Miron A, Iordache T-V, Valente AJM, Durães LMR, Sarbu A, Ivan GR, Zaharia A, Sandu T, Iovu H, Chiriac A-L. Chitosan-Based Beads Incorporating Inorganic–Organic Composites for Copper Ion Retention in Aqueous Solutions. International Journal of Molecular Sciences. 2024; 25(4):2411. https://doi.org/10.3390/ijms25042411
Chicago/Turabian StyleMiron, Andreea, Tanta-Verona Iordache, Artur J. M. Valente, Luisa Maria Rocha Durães, Andrei Sarbu, Georgeta Ramona Ivan, Anamaria Zaharia, Teodor Sandu, Horia Iovu, and Anita-Laura Chiriac. 2024. "Chitosan-Based Beads Incorporating Inorganic–Organic Composites for Copper Ion Retention in Aqueous Solutions" International Journal of Molecular Sciences 25, no. 4: 2411. https://doi.org/10.3390/ijms25042411
APA StyleMiron, A., Iordache, T.-V., Valente, A. J. M., Durães, L. M. R., Sarbu, A., Ivan, G. R., Zaharia, A., Sandu, T., Iovu, H., & Chiriac, A.-L. (2024). Chitosan-Based Beads Incorporating Inorganic–Organic Composites for Copper Ion Retention in Aqueous Solutions. International Journal of Molecular Sciences, 25(4), 2411. https://doi.org/10.3390/ijms25042411