The Role of Glucose-6-phosphate Dehydrogenase in the Wine Yeast Hanseniaspora uvarum
Abstract
1. Introduction
2. Results
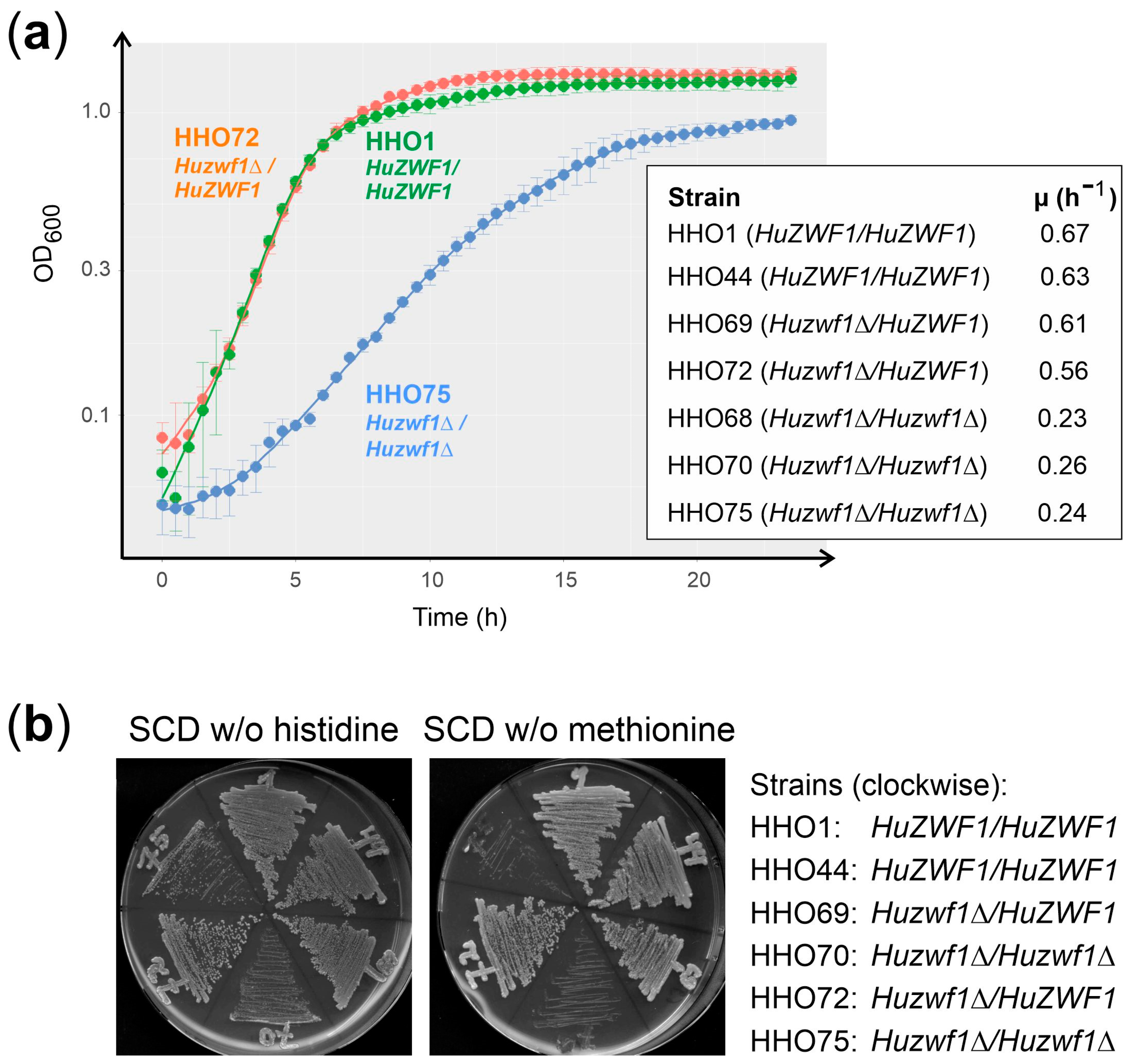
2.1. Homozygous Huzwf1 Deletion Mutants Lack Glucose-6-phosphate Dehydrogenase Activity
| Strain | Relevant ZWF1 Alleles | mU/mg Protein |
|---|---|---|
| HHO1 | Wild-type | 252 ± 11 |
| HHO44 | Wild-type | 257 ± 4 |
| HHO69 | Huzwf1Δ/HuZWF1 | 132 ± 16 |
| HHO72 | Huzwf1Δ/HuZWF1 | 123 ± 13 |
| HHO70 | Huzwf1Δ/Huzwf1Δ | b.d. |
| HHO75 | Huzwf1Δ/Huzwf1Δ | b.d. |
| HHO75/pJJH3313 | Huzwf1Δ/Huzwf1Δ Huura3::vector/HuURA3 | b.d. |
| HHO75/pJJH3320 | Huzwf1Δ/Huzwf1Δ Huura3::HuZWF1/HuURA3 | 168 ± 2 |
| HHO75/pJJH3314 | Huzwf1Δ/Huzwf1Δ Huura3::ScZWF1/HuURA3 | 11 ± 1 |
| HHO75/pJJH3315 | Huzwf1Δ/Huzwf1Δ Huura3::KlZWF1/HuURA3 | 8 ± 1 |
| HHO75/pJJH3352 | Huzwf1Δ/Huzwf1Δ Huura3::TEF1p-HuZWF1/HuURA3 | 651 ± 27 |
| HHO75/pJJH3350 | Huzwf1Δ/Huzwf1Δ Huura3::TEF1p-ScZWF1/HuURA3 | 349 ± 27 |
| HHO75/pJJH3351 | Huzwf1Δ/Huzwf1Δ Huura3::TEF1p-KlZWF1/HuURA3 | 175 ± 5 |
| HHO75/pJJH3322 | Huzwf1Δ/Huzwf1Δ Huura3::TEF1p-HsZWF1/HuURA3 | 98 ± 12 |
| Strain | Genotype | Reference |
|---|---|---|
| HHO1 | Wild-type | [36] |
| HHO44 | Huleu2::loxP/Huleu2::loxP | [36] |
| HHO68 | Huleu2::loxP/Huleu2::loxP Huzwf1::KlLEU2/Huzwf1::KlLEU2 | this work |
| HHO69 | Huleu2::loxP/Huleu2::loxP Huzwf1::KlLEU2/HuZWF1 | this work |
| HHO70 | Huleu2::loxP/Huleu2::loxP Huzwf1:: KlLEU2/Huzwf1:: KlLEU2 | this work |
| HHO71 | Huleu2::loxP/Huleu2::loxP Huzwf1:: KlLEU2/HuZWF1 | this work |
| HHO72 | Huleu2::loxP/Huleu2::loxP Huzwf1::hph/HuZWF1 | this work |
| HHO74 | Huleu2::loxP/Huleu2::loxP Huzwf1::loxP/Huzwf1::loxP | this work |
| HHO75 | Huleu2::loxP/Huleu2::loxP Huzwf1::loxP/Huzwf1::loxP | this work |
| HHO75/pJJH3313 | Huleu2::loxP/Huleu2::loxP Huzwf1::loxP/Huzwf1::loxP Huura3::hph/HuURA3 | this work |
| HHO75/pJJH3320 | Huleu2::loxP/Huleu2::loxP Huzwf1::loxP/Huzwf1::loxP Huura3::HuZWF1-hph/HuURA3 | this work |
| HHO75/pJJH3314 | Huleu2::loxP/Huleu2::loxP Huzwf1::loxP/Huzwf1::loxP Huura3::ScZWF1-hph/HuURA3 | this work |
| HHO75/pJJH3315 | Huleu2::loxP/Huleu2::loxP Huzwf1::loxP/Huzwf1::loxP Huura3::KlZWF1-hph/HuURA3 | this work |
| HHO75/pJJH3352 | Huleu2::loxP/Huleu2::loxP Huzwf1::loxP/Huzwf1::loxP Huura3::TEF1p-HuZWF1-hph/HuURA3 | this work |
| HHO75/pJJH3350 | Huleu2::loxP/Huleu2::loxP Huzwf1::loxP/Huzwf1::loxP Huura3::TEF1p-ScZWF1-hph/HuURA3 | this work |
| HHO75/pJJH3351 | Huleu2::loxP/Huleu2::loxP Huzwf1::loxP/Huzwf1::loxP Huura3::TEF1p-KlZWF1-hph/HuURA3 | this work |
| HHO75/pJJH3322 | Huleu2::loxP/Huleu2::loxP Huzwf1::loxP/Huzwf1::loxP Huura3::TEF1p-HsZWF1-hph/HuURA3 | this work |
2.2. Heterologous Genes Restore G6PDH Activity in Huzwf1 Deletion
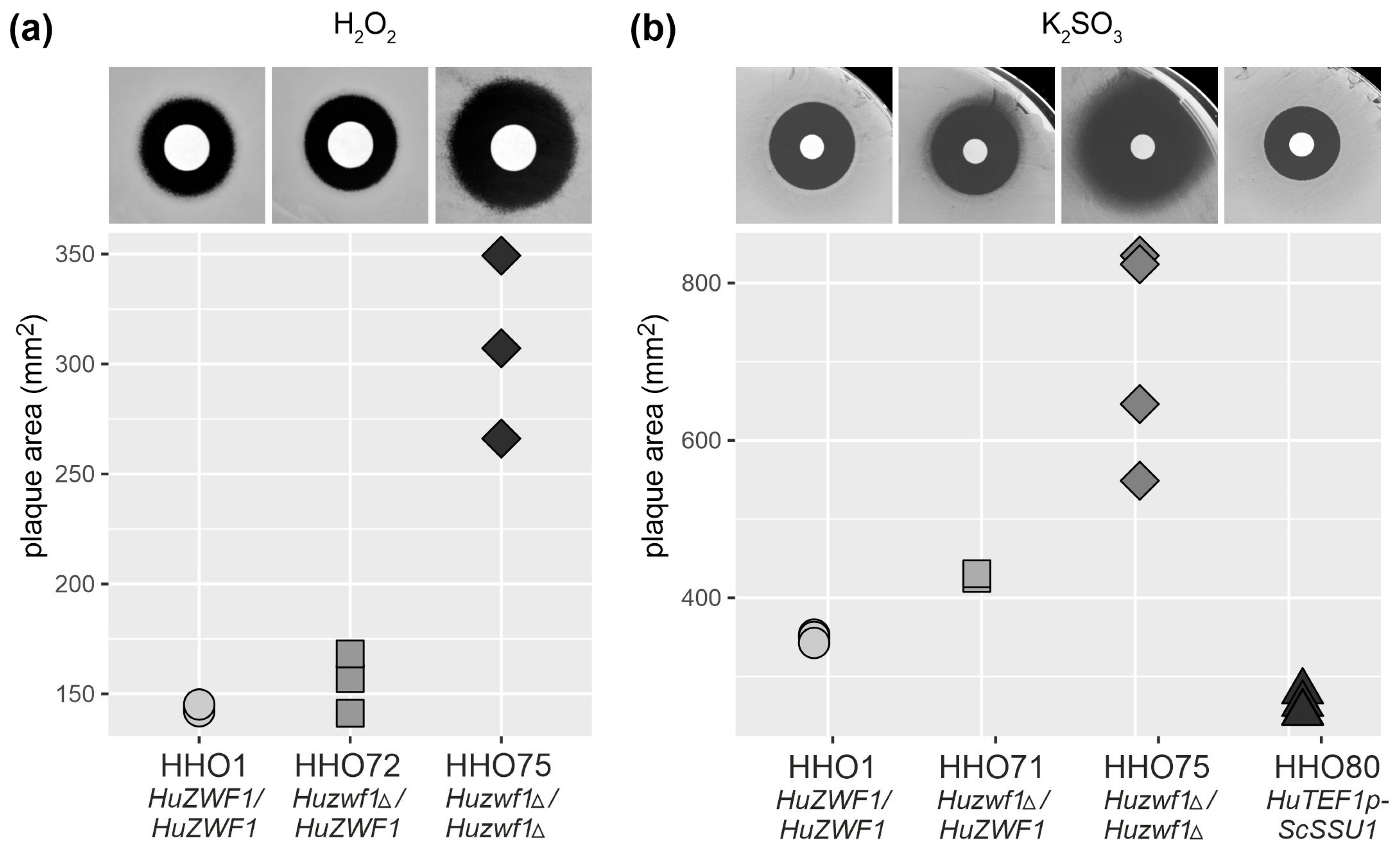
2.3. Lack of HuZwf1 Confers Methionine Auxotrophy and Hypersensitivity towards Hydrogen Peroxide and Sulfite, Whereas Heterologous Expression of a Sulfite Transporter Increases Resistance
3. Discussion
4. Materials and Methods
4.1. Media, Strains, and Culture Conditions
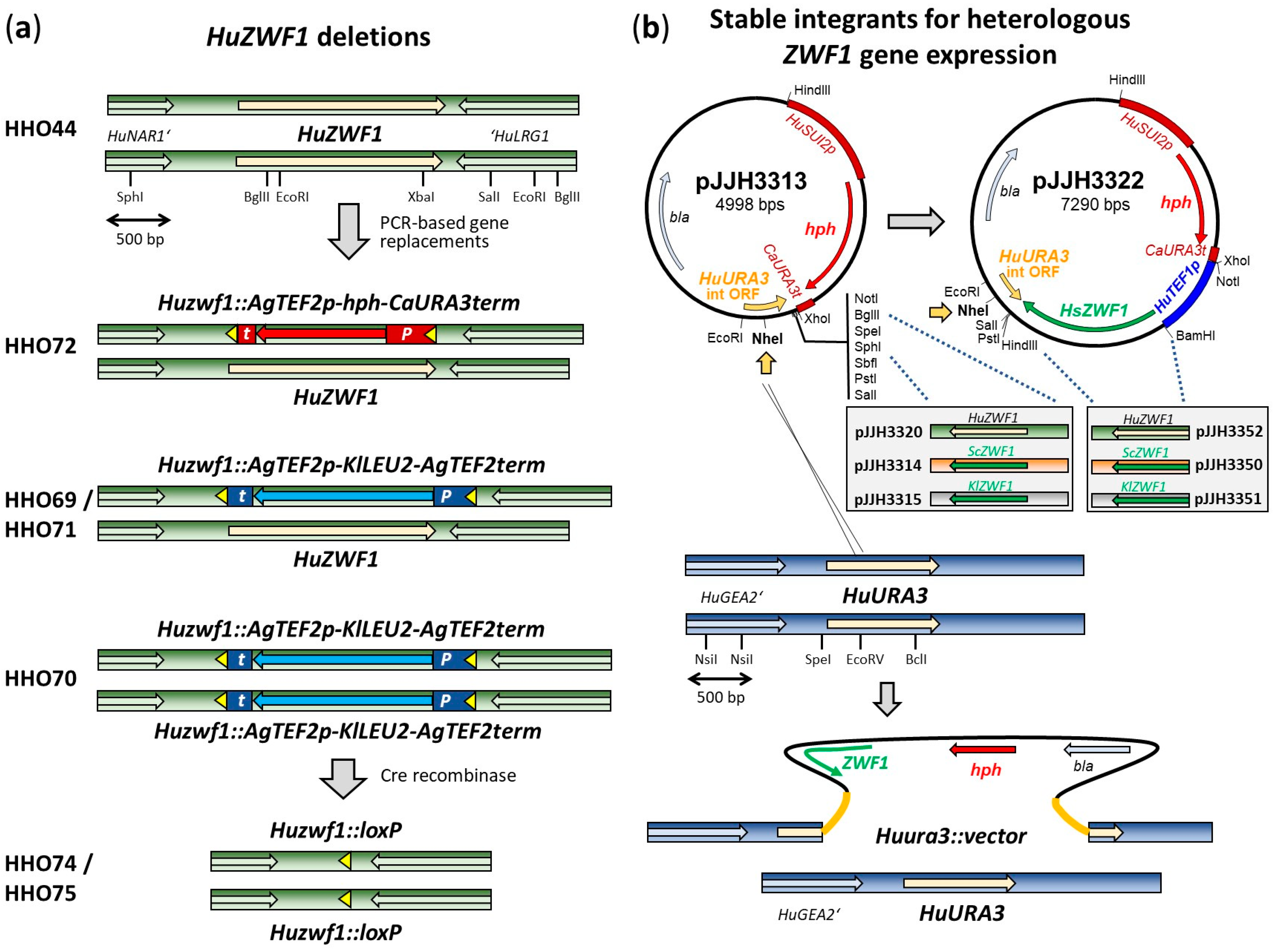
4.2. Construction of Deletion Mutants and Cloning Procedures
4.3. Growth Curves
4.4. Enzyme Assays
4.5. Inhibition Halo Assays
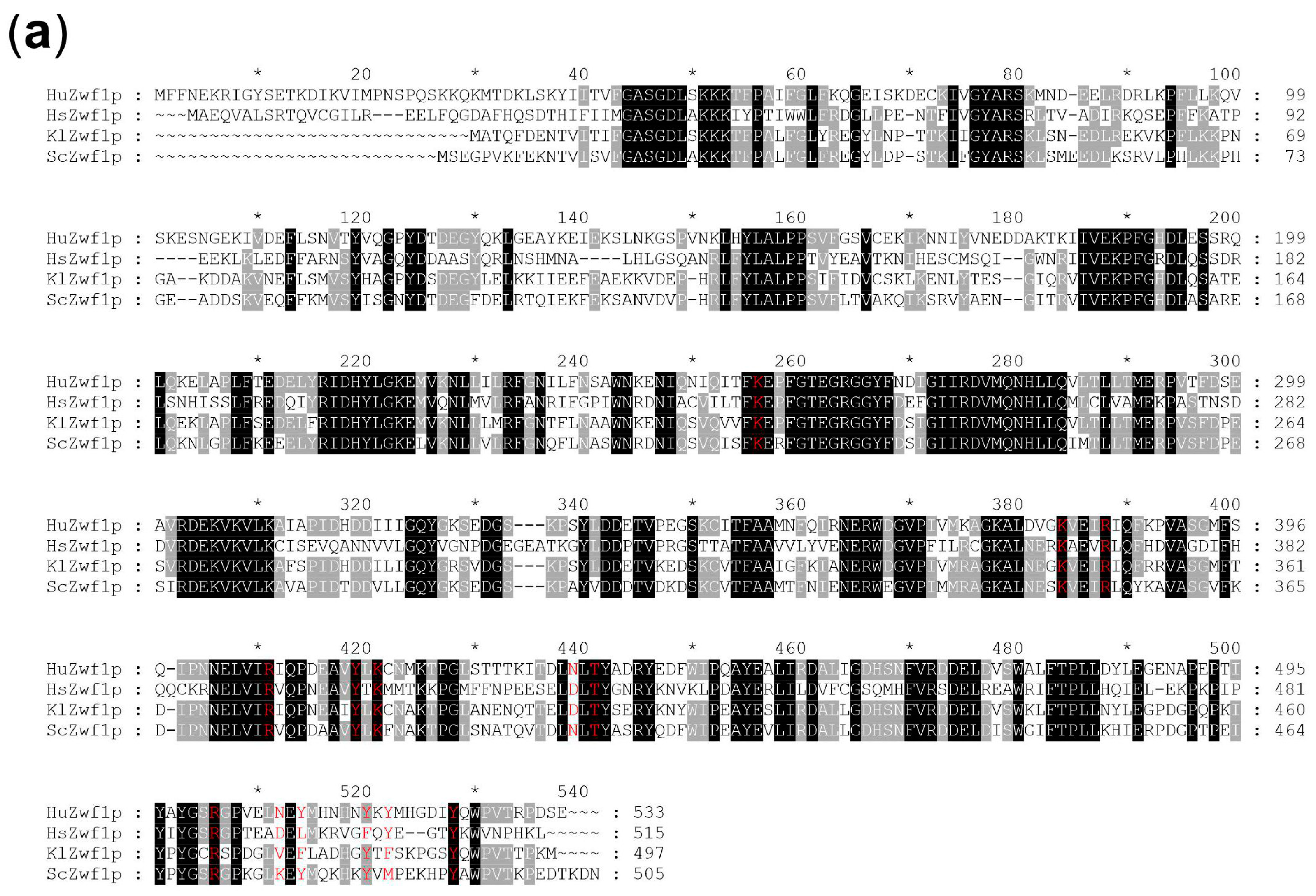
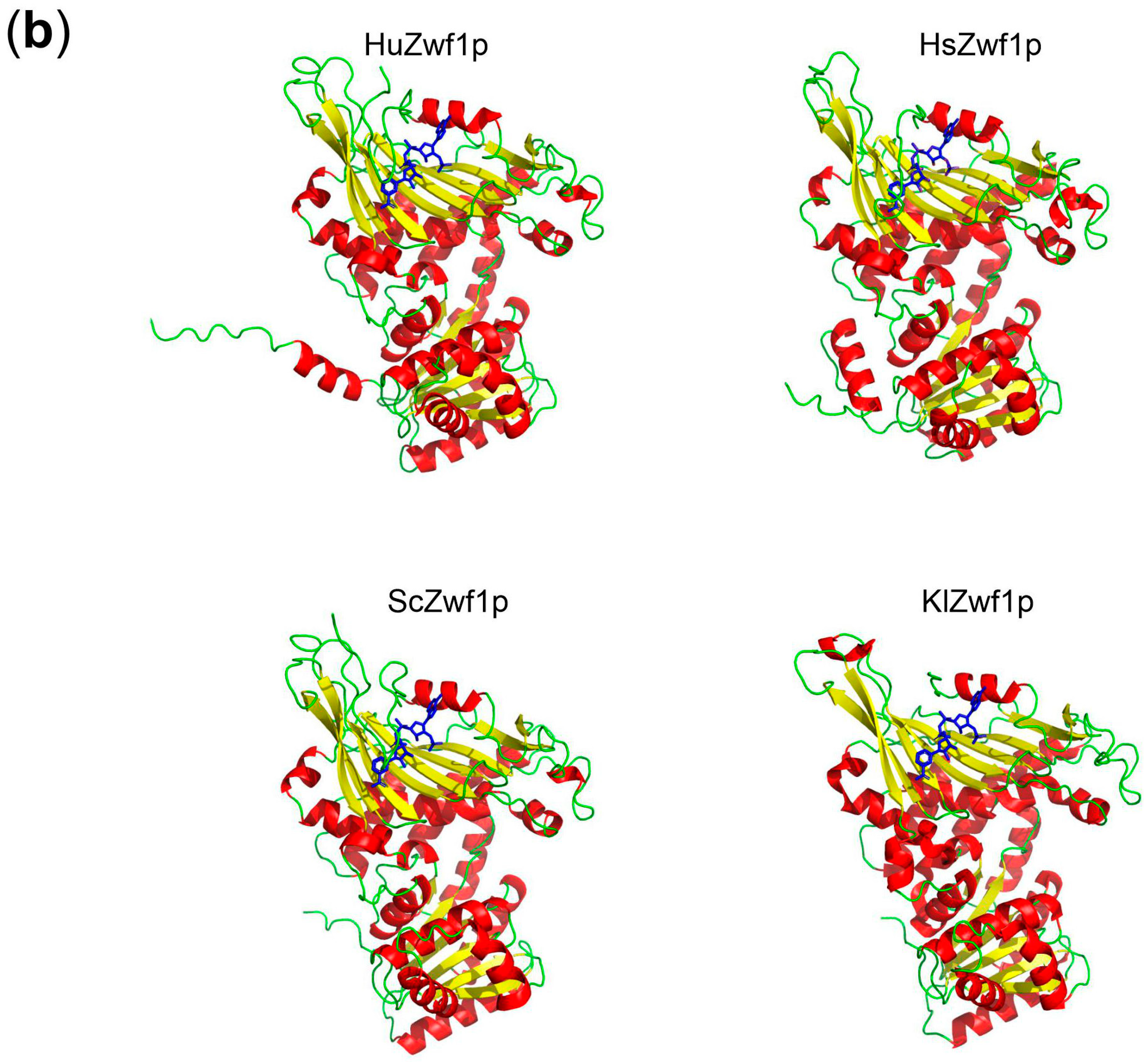
4.6. In Silico Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bertels, L.K.; Fernandez Murillo, L.; Heinisch, J.J. The pentose phosphate pathway in yeasts—More than a poor cousin of glycolysis. Biomolecules 2021, 11, 725. [Google Scholar] [CrossRef] [PubMed]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Gruning, N.M.; Kruger, A.; Tauqeer Alam, M.; et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. Camb. Philos. Soc. 2015, 90, 927–963. [Google Scholar] [CrossRef] [PubMed]
- Patra, K.C.; Hay, N. The pentose phosphate pathway and cancer. Trends Biochem. Sci. 2014, 39, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Riganti, C.; Gazzano, E.; Polimeni, M.; Aldieri, E.; Ghigo, D. The pentose phosphate pathway: An antioxidant defense and a crossroad in tumor cell fate. Free Radic. Biol. Med. 2012, 53, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.Y.; Cheng, M.L.; Chiu, D.T. Glucose-6-phosphate dehydrogenase--beyond the realm of red cell biology. Free Radic. Res. 2014, 48, 1028–1048. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Brenner, D.A.; Cui, L.; Lim, C.C.; Wang, B.; Pimentel, D.R.; Koh, S.; Sawyer, D.B.; Leopold, J.A.; Handy, D.E.; et al. Glucose-6-phosphate dehydrogenase modulates cytosolic redox status and contractile phenotype in adult cardiomyocytes. Circ. Res. 2003, 93, e9–e16. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Mason, P.J.; Vulliamy, T.J. Glucose-6-phosphate dehydrogenase deficiency. Baillieres Best Pract. Res. Clin. Haematol. 2000, 13, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Manzo, S.; Marcial-Quino, J.; Vanoye-Carlo, A.; Serrano-Posada, H.; Ortega-Cuellar, D.; Gonzalez-Valdez, A.; Castillo-Rodriguez, R.A.; Hernandez-Ochoa, B.; Sierra-Palacios, E.; Rodriguez-Bustamante, E.; et al. Glucose-6-phosphate dehydrogenase: Update and analysis of new mutations around the world. Int. J. Mol. Sci. 2016, 17, 2069. [Google Scholar] [CrossRef]
- Luzzatto, L.; Ally, M.; Notaro, R. Glucose-6-phosphate dehydrogenase deficiency. Blood 2020, 136, 1225–1240. [Google Scholar] [CrossRef]
- Ahamed, A.; Hosea, R.; Wu, S.; Kasim, V. The emerging roles of the metabolic regulator G6PD in human cancers. Int. J. Mol. Sci. 2023, 24, 17238. [Google Scholar] [CrossRef]
- Horecker, B.L. The pentose phosphate pathway. J. Biol. Chem. 2002, 277, 47965–47971. [Google Scholar] [CrossRef]
- Lobo, Z.; Maitra, P.K. Pentose phosphate pathway mutants of yeast. Mol. Gen. Genet. 1982, 185, 367–368. [Google Scholar] [CrossRef]
- Campbell, K.; Vowinckel, J.; Keller, M.A.; Ralser, M. Methionine metabolism alters oxidative stress resistance via the pentose phosphate pathway. Antioxid. Redox Signal. 2016, 24, 543–547. [Google Scholar] [CrossRef]
- Nogae, I.; Johnston, M. Isolation and characterization of the ZWF1 gene of Saccharomyces cerevisiae, encoding glucose-6-phosphate dehydrogenase. Gene 1990, 96, 161–169. [Google Scholar] [CrossRef]
- Thomas, D.; Cherest, H.; Surdin-Kerjan, Y. Identification of the structural gene for glucose-6-phosphate dehydrogenase in yeast. Inactivation leads to a nutritional requirement for organic sulfur. EMBO J. 1991, 10, 547–553. [Google Scholar] [CrossRef]
- Ralser, M.; Wamelink, M.M.; Kowald, A.; Gerisch, B.; Heeren, G.; Struys, E.A.; Klipp, E.; Jakobs, C.; Breitenbach, M.; Lehrach, H.; et al. Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. J. Biol. 2007, 6, 10. [Google Scholar] [CrossRef]
- Dick, T.P.; Ralser, M. Metabolic remodeling in times of stress: Who shoots faster than his shadow? Mol. Cell 2015, 59, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Miosga, T.; Zimmermann, F.K. Cloning and characterization of the first two genes of the non-oxidative part of the Saccharomyces cerevisiae pentose-phosphate pathway. Curr. Genet. 1996, 30, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Heinisch, J.J.; Knuesting, J.; Scheibe, R. Investigation of heterologously expressed glucose-6-phosphate dehydrogenase genes in a yeast zwf1 deletion. Microorganisms 2020, 8, 546. [Google Scholar] [CrossRef]
- Barnett, J.A.; Payne, R.W.; Yarrow, D. Yeasts: Characteristics and Identification; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Lagunas, R.; Dominguez, C.; Busturia, A.; Saez, M.J. Mechanisms of appearance of the Pasteur effect in Saccharomyces cerevisiae: Inactivation of sugar transport systems. J. Bacteriol. 1982, 152, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Heinisch, J.J.; Buchwald, U.; Gottschlich, A.; Heppeler, N.; Rodicio, R. A tool kit for molecular genetics of Kluyveromyces lactis comprising a congenic strain series and a set of versatile vectors. FEMS Yeast Res. 2010, 10, 333–342. [Google Scholar] [CrossRef]
- Rodicio, R.; Heinisch, J.J. Yeast on the milky way: Genetics, physiology and biotechnology of Kluyveromyces lactis. Yeast 2013, 30, 165–177. [Google Scholar] [CrossRef]
- Goffrini, P.; Wesolowski-Louvel, M.; Ferrero, I. A phosphoglucose isomerase gene is involved in the Rag phenotype of the yeast Kluyveromyces lactis. Mol. Gen. Genet. 1991, 228, 401–409. [Google Scholar] [CrossRef]
- Heinisch, J.; Kirchrath, L.; Liesen, T.; Vogelsang, K.; Hollenberg, C.P. Molecular genetics of phosphofructokinase in the yeast Kluyveromyces lactis. Mol. Microbiol. 1993, 8, 559–570. [Google Scholar] [CrossRef]
- Jacoby, J.; Hollenberg, C.P.; Heinisch, J.J. Transaldolase mutants in the yeast Kluyveromyces lactis provide evidence that glucose can be metabolized through the pentose phosphate pathway. Mol. Microbiol. 1993, 10, 867–876. [Google Scholar] [CrossRef]
- Saliola, M.; Scappucci, G.; De Maria, I.; Lodi, T.; Mancini, P.; Falcone, C. Deletion of the glucose-6-phosphate dehydrogenase gene KlZWF1 affects both fermentative and respiratory metabolism in Kluyveromyces lactis. Eukaryot. Cell 2007, 6, 19–27. [Google Scholar] [CrossRef]
- Saliola, M.; Tramonti, A.; Lanini, C.; Cialfi, S.; De Biase, D.; Falcone, C. Intracellular NADPH levels affect the oligomeric state of the glucose 6-phosphate dehydrogenase. Eukaryot. Cell 2012, 11, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, N.; Badura, J.; von Wallbrunn, C.; Pretorius, I.S. Exploring future applications of the apiculate yeast Hanseniaspora. Crit. Rev. Biotechnol. 2023, 44, 100–119. [Google Scholar] [CrossRef]
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Garcia-Fernandez, D.; Mas, A.; Esteve-Zarzoso, B. Fungal diversity in grape must and wine fermentation assessed by massive sequencing, quantitative PCR and DGGE. Front. Microbiol. 2015, 6, 1156. [Google Scholar] [CrossRef] [PubMed]
- Zott, K.; Miot-Sertier, C.; Claisse, O.; Lonvaud-Funel, A.; Masneuf-Pomarede, I. Dynamics and diversity of non-Saccharomyces yeasts during the early stages in winemaking. Int. J. Food Microbiol. 2008, 125, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Langenberg, A.K.; Bink, F.J.; Wolff, L.; Walter, S.; von Wallbrunn, C.; Grossmann, M.; Heinisch, J.J.; Schmitz, H.P. Glycolytic functions are conserved in the genome of the wine yeast Hanseniaspora uvarum, and pyruvate kinase limits its capacity for alcoholic fermentation. Appl. Environ. Microbiol. 2017, 83, e01580-17. [Google Scholar] [CrossRef]
- Badura, J.; van Wyk, N.; Brezina, S.; Pretorius, I.S.; Rauhut, D.; Wendland, J.; von Wallbrunn, C. Development of genetic modification tools for Hanseniaspora uvarum. Int. J. Mol. Sci. 2021, 22, 1943. [Google Scholar] [CrossRef] [PubMed]
- Badura, J.; van Wyk, N.; Zimmer, K.; Pretorius, I.S.; von Wallbrunn, C.; Wendland, J. PCR-based gene targeting in Hanseniaspora uvarum. FEMS Yeast Res. 2023, 23, foad034. [Google Scholar] [CrossRef] [PubMed]
- Heinisch, J.J.; Murra, A.; Jurgens, K.; Schmitz, H.P. A versatile toolset for genetic manipulation of the wine yeast Hanseniaspora uvarum. Int. J. Mol. Sci. 2023, 24, 1859. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–592. [Google Scholar] [CrossRef]
- Mirdita, M.; Schutze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Schehl, B.; Muller, C.; Senn, T.; Heinisch, J.J. A laboratory yeast strain suitable for spirit production. Yeast 2004, 21, 1375–1389. [Google Scholar] [CrossRef]
- Garcia-Rios, E.; Nuevalos, M.; Barrio, E.; Puig, S.; Guillamon, J.M. A new chromosomal rearrangement improves the adaptation of wine yeasts to sulfite. Environ. Microbiol. 2019, 21, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Au, S.W.; Gover, S.; Lam, V.M.; Adams, M.J. Human glucose-6-phosphate dehydrogenase: The crystal structure reveals a structural NADP(+) molecule and provides insights into enzyme deficiency. Structure 2000, 8, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.H.; Jin, C.; Chang, J.H. Structural basis for substrate recognition of glucose-6-phosphate dehydrogenase from Kluyveromyces lactis. Biochem. Biophys. Res. Commun. 2021, 553, 85–91. [Google Scholar] [CrossRef]
- Jacoby, J.J.; Heinisch, J.J. Analysis of a transketolase gene from Kluyveromyces lactis reveals that the yeast enzymes are more related to transketolases of prokaryotic origins than to those of higher eukaryotes. Curr. Genet. 1997, 31, 15–21. [Google Scholar] [CrossRef]
- Orr-Weaver, T.L.; Szostak, J.W.; Rothstein, R.J. Yeast transformation: A model system for the study of recombination. Proc. Natl. Acad. Sci. USA 1981, 78, 6354–6358. [Google Scholar] [CrossRef]
- Rodicio, R.; Koch, S.; Schmitz, H.P.; Heinisch, J.J. KlRHO1 and KlPKC1 are essential for cell integrity signalling in Kluyveromyces lactis. Microbiology 2006, 152, 2635–2649. [Google Scholar] [CrossRef]
- Jeppsson, M.; Traff, K.; Johansson, B.; Hahn-Hagerdal, B.; Gorwa-Grauslund, M.F. Effect of enhanced xylose reductase activity on xylose consumption and product distribution in xylose-fermenting recombinant Saccharomyces cerevisiae. FEMS Yeast Res. 2003, 3, 167–175. [Google Scholar] [CrossRef]
- Gold, N.D.; Gowen, C.M.; Lussier, F.X.; Cautha, S.C.; Mahadevan, R.; Martin, V.J. Metabolic engineering of a tyrosine-overproducing yeast platform using targeted metabolomics. Microb. Cell Fact. 2015, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Izawa, S.; Maeda, K.; Miki, T.; Mano, J.; Inoue, Y.; Kimura, A. Importance of glucose-6-phosphate dehydrogenase in the adaptive response to hydrogen peroxide in Saccharomyces cerevisiae. Biochem. J. 1998, 330 Pt 2, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Ogura, Y.; Monno, I.; Xu, J.; Koya, D. Effect of methionine restriction on aging: Its relationship to oxidative stress. Biomedicines 2021, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rios, E.; Guillamon, J.M. Sulfur dioxide resistance in Saccharomyces cerevisiae: Beyond SSU1. Microb. Cell 2019, 6, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Surdin-Kerjan, Y. Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 1997, 61, 503–532. [Google Scholar] [PubMed]
- Domizio, P.; Romani, C.; Lencioni, L.; Comitini, F.; Gobbi, M.; Mannazzu, I.; Ciani, M. Outlining a future for non-Saccharomyces yeasts: Selection of putative spoilage wine strains to be used in association with Saccharomyces cerevisiae for grape juice fermentation. Int. J. Food Microbiol. 2011, 147, 170–180. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Masneuf-Pomarede, I.; Bely, M.; Marullo, P.; Albertin, W. The genetics of non-conventional wine yeasts: Current knowledge and future challenges. Front. Microbiol. 2015, 6, 1563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, H.; Xia, H.; Sun, L.; Zhang, Q.; Yang, H.; Zhang, J. Wine aroma modification by Hanseniaspora uvarum: A multiple-step strategy for screening potential mixed starters. Food Chem. X 2023, 20, 100930. [Google Scholar] [CrossRef]
- Fernandes, T.; Osorio, C.; Sousa, M.J.; Franco-Duarte, R. Contributions of adaptive laboratory evolution towards the enhancement of the biotechnological potential of non-conventional yeast species. J. Fungi 2023, 9, 186. [Google Scholar] [CrossRef]
- Merritt, J.; Butz, J.A.; Ogunnaike, B.A.; Edwards, J.S. Parallel analysis of mutant human glucose 6-phosphate dehydrogenase in yeast using PCR colonies. Biotechnol. Bioeng. 2005, 92, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Kirchrath, L.; Lorberg, A.; Schmitz, H.P.; Gengenbacher, U.; Heinisch, J.J. Comparative genetic and physiological studies of the MAP kinase Mpk1p from Kluyveromyces lactis and Saccharomyces cerevisiae. J. Mol. Biol. 2000, 300, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Gietz, R.D.; Sugino, A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 1988, 74, 527–534. [Google Scholar] [CrossRef]
- Heinisch, J.J. PFK2, ISP42, ERG2 and RAD14 are located on the right arm of chromosome XIII. Yeast 1993, 9, 1103–1105. [Google Scholar] [CrossRef]
- Yanisch-Perron, C.; Vieira, J.; Messing, J. Improved M13 phage cloning vectors and host strains: Nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 1985, 33, 103–119. [Google Scholar] [CrossRef]
- Sterk, C.; Graber, L.; Schmitz, H.P.; Heinisch, J.J. Analysis of functional domains in Rho5, the yeast homolog of human Rac1 GTPase, in oxidative stress response. Int. J. Mol. Sci. 2019, 20, 5550. [Google Scholar] [CrossRef] [PubMed]
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 2 February 2024).
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Soding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, LLC. The PyMOL Molecular Graphics System, Version 1.8. 2015. Available online: https://pymol.org/2/ (accessed on 2 February 2024).
| Number | Name | Sequence (5′ → 3′) 1 |
|---|---|---|
| 20.277 | HuZWF1forBam | CTTTGgatCCAGTAGTTGTGAATTATACGG |
| 20.278 | HuZWF1revSal | TATGTGTCGACCTTTTCAAGAGG |
| 20.287 | HuZWF1startBam | gcgtggatccATGTTTTTTAACGAAAAACGAATCGG |
| 21.304 | HuURA3intforEco | TGGAaTtcGTCAACACAACAGCTGAATTATTAG |
| 21.305 | HuURA3intforEco | ggctgtcGACCCTTTAGAACTTAATTCAG |
| 22.193 | Huzwf1del5 | TTATTAGCGTTGTATATAACGACTTTGCTTGATTTTGTATGATGTTTGAATTTAT- GCTTCGTACGCTGCAGGTCGAC |
| 22.194 | Huzwf1del3 | TTTAAAACCTTTTTTTTGATATAAAAACAATTGATATTTTATATATAATATGAAGCATAGGCCACTAGTGGATCTG |
| 23.078 | HuZWF1wfor | GGAACAAGAAAACAGTTCTTTAGAACTTG |
| 23.079 | HuZWF1wrev | GGCAGGTTCTATGCATGATTTGGATAG |
| 23.080 | HuZWF1for | GTGCCTGATGAGGAATCATCTTC |
| 23.081 | HuZWF1rev | CAAGAGGTTCCAAAATGGATATAC |
| 23.089 | ScZWF1forSph | gcgtgcatgCTGGTAAGTAAGGTGTAGTTTTG |
| 23.090 | KlZWF1forSph | gcgtgcatGCGATGAAAGCCAACAGTGGTTAG |
| 23.143 | HuZWF1forBam | gtgaggatccATGTTTTTTAACGAAAAACGAATCGG |
| 20.349 | ScSSU1BamATG | gcgtggatccATGGTTGCCAATTGGGTACTTG |
| 20.350 | ScSSU1revSal | ggctgtcgacGGTTGCAGAAAGCATAAAGTTCTG |
| 99.113 | universal-47 | CGCCAGGGTTTTCCCAGTCACGAC |
| 14.111 | reverse-60 | GCTTCCGGCTCGTATGTTGTGTGG |
| 06.252 | AgTEF2term3out | TCGCCTCGACATCATCTGCCCAG |
| 10.225 | AgTEF2prom5out | CCTCAGTGGCAAATCCTAAC |
| 21.216 | Hygro5out | GACAATTGCATCAAATCAGAAACAG |
| 21.217 | Hygro3out | GTTTTGGCTGATTCTGGTAATAGAAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinisch, J.J.; Murra, A.; Fernández Murillo, L.; Schmitz, H.-P. The Role of Glucose-6-phosphate Dehydrogenase in the Wine Yeast Hanseniaspora uvarum. Int. J. Mol. Sci. 2024, 25, 2395. https://doi.org/10.3390/ijms25042395
Heinisch JJ, Murra A, Fernández Murillo L, Schmitz H-P. The Role of Glucose-6-phosphate Dehydrogenase in the Wine Yeast Hanseniaspora uvarum. International Journal of Molecular Sciences. 2024; 25(4):2395. https://doi.org/10.3390/ijms25042395
Chicago/Turabian StyleHeinisch, Jürgen J., Andrea Murra, Lucía Fernández Murillo, and Hans-Peter Schmitz. 2024. "The Role of Glucose-6-phosphate Dehydrogenase in the Wine Yeast Hanseniaspora uvarum" International Journal of Molecular Sciences 25, no. 4: 2395. https://doi.org/10.3390/ijms25042395
APA StyleHeinisch, J. J., Murra, A., Fernández Murillo, L., & Schmitz, H.-P. (2024). The Role of Glucose-6-phosphate Dehydrogenase in the Wine Yeast Hanseniaspora uvarum. International Journal of Molecular Sciences, 25(4), 2395. https://doi.org/10.3390/ijms25042395






