Association between Brain-Derived Neurotrophic Factor and Lipid Profiles in Acute Ischemic Stroke Patients
Abstract
1. Introduction
2. Results
2.1. Characteristics of Patients Based on BDNF Levels
2.2. Age and Sex-Adjusted Odds Ratios
2.3. Multivariate Logistic Regression
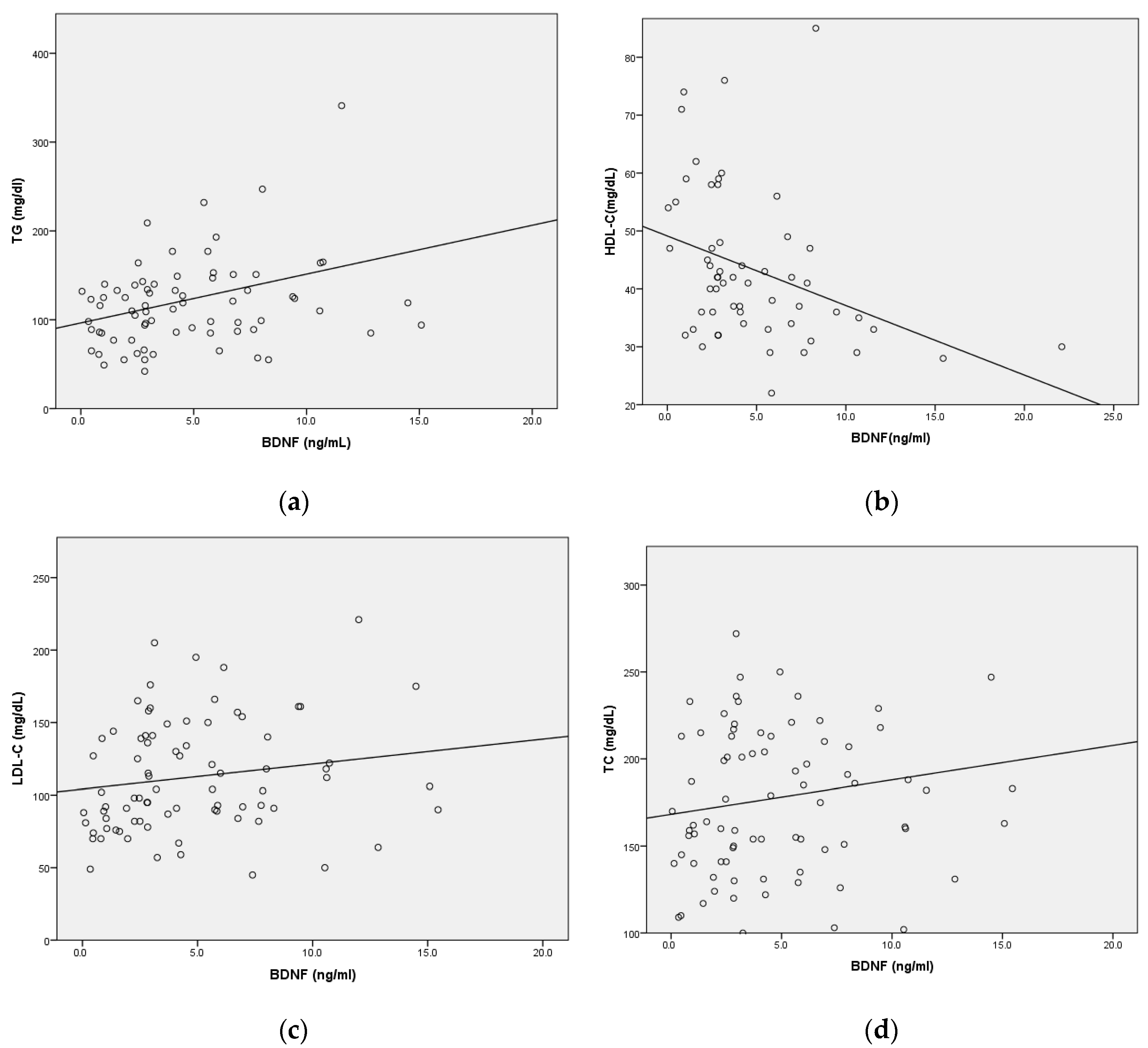
2.4. Correlation between Serum BDNF Levels and Lipid Parameters
3. Discussion
4. Materials and Methods
4.1. Blood Sampling
4.2. Blood Lipids and Glucose
4.3. BDNF Measurement
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Béjot, Y.; Daubail, B.; Giroud, M. Epidemiology of stroke and transient ischemic attacks: Current knowledge and perspectives. Rev. Neurol. 2016, 172, 59–68. [Google Scholar] [CrossRef]
- Feigin, V.L.; Lawes, C.M.; Bennett, D.A.; Barker-Collo, S.L.; Parag, V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: A systematic review. Lancet Neurol. 2009, 8, 355–369. [Google Scholar] [CrossRef]
- Glass, C.K.; Witztum, J.L. Atherosclerosis: The road ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Bowman, T.S.; Sesso, H.D.; Ma, J.; Kurth, T.; Kase, C.S.; Stampfer, M.J.; Gaziano, J.M. Cholesterol and the risk of ischemic stroke. Stroke 2003, 34, 2930–2934. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Shaper, A.G.; Ebrahim, S. HDL-cholesterol, total cholesterol, and the risk of stroke in middle-aged British men. Stroke 2000, 31, 1882–1888. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.L.; Benson, R.T.; Kargman, D.E.; Boden-Albala, B.; Tuck, C.; Lin, I.-F.; Cheng, J.F.; Paik, M.C.; Shea, S.; Berglund, L. High-Density Lipoprotein Cholesterol and Ischemic Stroke in the ElderlyThe Northern Manhattan Stroke Study. JAMA 2001, 285, 2729–2735. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.-S.; Bang, O.Y.; Kang, D.-W.; Yu, K.-H.; Bae, H.-J.; Lee, J.S.; Heo, J.H.; Kwon, S.U.; Oh, C.W.; Lee, B.-C. Stroke statistics in Korea: Part I. Epidemiology and risk factors: A report from the korean stroke society and clinical research center for stroke. J. Stroke 2013, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Amarenco, P.; Labreuche, J. Lipid management in the prevention of stroke: Review and updated meta-analysis of statins for stroke prevention. Lancet Neurol. 2009, 8, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, J.; Faraci, F.M.; Lentz, S.R.; Heistad, D.D. Cerebral vascular dysfunction during hypercholesterolemia. Stroke 2007, 38, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Nicholls, S.; Rye, K.A.; Anantharamaiah, G.M.; Navab, M.; Fogelman, A.M. Antiinflammatory properties of HDL. Circ. Res. 2004, 95, 764–772. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Merz, C.N.B.; Brewer Jr, H.B.; Clark, L.T.; Hunninghake, D.B.; Pasternak, R.C.; Smith Jr, S.C.; Stone, N.J. Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Circulation 2004, 110, 227–239. [Google Scholar] [CrossRef]
- Assmann, G.; Cullen, P.; Schulte, H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Munster (PROCAM) study. Circulation 2002, 105, 310–315. [Google Scholar] [CrossRef]
- McQueen, M.J.; Hawken, S.; Wang, X.; Ounpuu, S.; Sniderman, A.; Probstfield, J.; Steyn, K.; Sanderson, J.E.; Hasani, M.; Volkova, E. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): A case-control study. Lancet 2008, 372, 224–233. [Google Scholar] [CrossRef]
- Cui, Y.; Blumenthal, R.S.; Flaws, J.A.; Whiteman, M.K.; Langenberg, P.; Bachorik, P.S.; Bush, T.L. Non–High-Density Lipoprotein Cholesterol Level as a Predictor of Cardiovascular Disease Mortality. Arch. Intern. Med. 2001, 161, 1413–1419. [Google Scholar] [CrossRef]
- Lemieux, I.; Lamarche, B.; Couillard, C.; Pascot, A.; Cantin, B.; Bergeron, J.; Dagenais, G.R.; Després, J.-P. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: The Quebec Cardiovascular Study. Arch. Intern. Med. 2001, 161, 2685–2692. [Google Scholar] [CrossRef] [PubMed]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.; Bromberg, E.; de Vries, E.F. Brain-derived neurotrophic factor in brain disorders: Focus on neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef] [PubMed]
- Silhol, M.; Arancibia, S.; Maurice, T.; Tapia-Arancibia, L. Spatial memory training modifies the expression of brain-derived neurotrophic factor tyrosine kinase receptors in young and aged rats. Neuroscience 2007, 146, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Lee, J.; Duan, W.; Mattson, M.P. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 2002, 82, 1367–1375. [Google Scholar] [CrossRef]
- Ahuja, P.; Ng, C.F.; Pang, B.P.S.; Chan, W.S.; Tse, M.C.L.; Bi, X.; Kwan, H.-L.R.; Brobst, D.; Herlea-Pana, O.; Yang, X.; et al. Muscle-generated BDNF (brain derived neurotrophic factor) maintains mitochondrial quality control in female mice. Autophagy 2022, 18, 1367–1384. [Google Scholar] [CrossRef]
- Colardo, M.; Martella, N.; Pensabene, D.; Siteni, S.; Di Bartolomeo, S.; Pallottini, V.; Segatto, M. Neurotrophins as Key Regulators of Cell Metabolism: Implications for Cholesterol Homeostasis. Int. J. Mol. Sci. 2021, 22, 5692. [Google Scholar] [CrossRef]
- Kotlega, D.; Zembron-Lacny, A.; Morawin, B.; Golab-Janowska, M.; Nowacki, P.; Szczuko, M. Free Fatty Acids and Their Inflammatory Derivatives Affect BDNF in Stroke Patients. Mediat. Inflamm. 2020, 2020, 6676247. [Google Scholar] [CrossRef]
- Alomari, M.A.; Khalil, H.; Khabour, O.F.; Alzoubi, K.H. Lipid profile in Parkinson’s disease: The potential role of brain-derived neurotrophic factor. Life Sci. 2022, 311, 121144. [Google Scholar] [CrossRef]
- Xia, F.; Zeng, Q.; Chen, J. Circulating brain-derived neurotrophic factor dysregulation and its linkage with lipid level, stenosis degree, and inflammatory cytokines in coronary heart disease. J. Clin. Lab. Anal. 2022, 36, e24546. [Google Scholar] [CrossRef]
- Boyuk, B.; Degirmencioglu, S.; Atalay, H.; Guzel, S.; Acar, A.; Celebi, A.; Ekizoglu, I.; Simsek, C. Relationship between Levels of Brain-Derived Neurotrophic Factor and Metabolic Parameters in Patients with Type 2 Diabetes Mellitus. J. Diabetes Res. 2014, 2014, 978143. [Google Scholar] [CrossRef]
- Eyileten, C.; Zaremba, M.; Janicki, P.K.; Rosiak, M.; Cudna, A.; Kapłon-Cieślicka, A.; Opolski, G.; Filipiak, K.J.; Kosior, D.A.; Mirowska-Guzel, D. Serum brain-derived neurotrophic factor is related to platelet reactivity but not to genetic polymorphisms within BDNF encoding gene in patients with type 2 diabetes. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- Eyileten, C.; Mirowska-Guzel, D.; Milanowski, L.; Zaremba, M.; Rosiak, M.; Cudna, A.; Kaplon-Cieslicka, A.; Opolski, G.; Filipiak, K.J.; Malek, L.; et al. Serum Brain-Derived Neurotrophic Factor is Related to Platelet Reactivity and Metformin Treatment in Adult Patients With Type 2 Diabetes Mellitus. Can. J. Diabetes 2019, 43, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Beiser, A.; Kelly-Hayes, M.; Kase, C.S.; Au, R.; Kannel, W.B.; Wolf, P.A. The lifetime risk of stroke: Estimates from the Framingham Study. Stroke 2006, 37, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Mauch, D.H.; Nagler, K.; Schumacher, S.; Goritz, C.; Müller, E.-C.; Otto, A.; Pfrieger, F.W. CNS synaptogenesis promoted by glia-derived cholesterol. Science 2001, 294, 1354–1357. [Google Scholar] [CrossRef]
- Ko, M.; Zou, K.; Minagawa, H.; Yu, W.; Gong, J.-S.; Yanagisawa, K.; Michikawa, M. Cholesterol-mediated neurite outgrowth is differently regulated between cortical and hippocampal neurons. J. Biol. Chem. 2005, 280, 42759–42765. [Google Scholar] [CrossRef]
- Numakawa, T.; Suzuki, S.; Kumamaru, E.; Adachi, N.; Richards, M.; Kunugi, H. BDNF function and intracellular signaling in neurons. Histol. Histopathol. 2010, 25, 237–258. [Google Scholar]
- Guirland, C.; Suzuki, S.; Kojima, M.; Lu, B.; Zheng, J.Q. Lipid rafts mediate chemotropic guidance of nerve growth cones. Neuron 2004, 42, 51–62. [Google Scholar] [CrossRef]
- Pereira, D.B.; Chao, M.V. The tyrosine kinase Fyn determines the localization of TrkB receptors in lipid rafts. J. Neurosci. 2007, 27, 4859–4869. [Google Scholar] [CrossRef]
- Gong, X.; Chen, L.; Song, B.; Han, X.; Xu, W.; Wu, B.; Sheng, F.; Lou, M. Associations of lipid profiles with the risk of ischemic and hemorrhagic stroke: A systematic review and meta-analysis of prospective cohort studies. Front. Cardiovasc. Med. 2022, 9, 893248. [Google Scholar] [CrossRef]
- Tanne, D.; Koren-Morag, N.; Graff, E.; Goldbourt, U. Blood lipids and first-ever ischemic stroke/transient ischemic attack in the Bezafibrate Infarction Prevention (BIP) Registry: High triglycerides constitute an independent risk factor. Circulation 2001, 104, 2892–2897. [Google Scholar] [CrossRef]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin. 2017, 8, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; An, Z.; Hong, Y.; Zhou, G.; Guo, J.; Zhang, Y.; Yang, Y.; Ning, X.; Wang, J. Low total cholesterol level is the independent predictor of poor outcomes in patients with acute ischemic stroke: A hospital-based prospective study. BMC Neurol. 2016, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Lai, Y.J.; Hanneman, S.K.; Casarez, R.L.; Wang, J.; McCullough, L.D. Blood biomarkers for physical recovery in ischemic stroke: A systematic review. Am. J. Transl. Res. 2019, 11, 4603–4613. [Google Scholar] [PubMed]
- Amarenco, P.; Labreuche, J.; Touboul, P.-J. High-density lipoprotein-cholesterol and risk of stroke and carotid atherosclerosis: A systematic review. Atherosclerosis 2008, 196, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Huang, X.; Ma, W.; Yang, R.; Xu, F.; Han, D.; Huang, T.; Peng, M.; Xu, A.; Lyu, J. Associations of HDL-C/LDL-C with myocardial infarction, all-cause mortality, haemorrhagic stroke and ischaemic stroke: A longitudinal study based on 384 093 participants from the UK Biobank. Stroke Vasc. Neurol. 2023, 8, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xi, B.; Zhao, X.; Cheng, H.; Hou, D.; Wu, L.; Wang, X.; Mi, J. Common genetic variants associated with lipid profiles in a Chinese pediatric population. Hum. Genet. 2013, 132, 1275–1285. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, L.; Huang, T.; Jia, Y.; Yang, P.; Zhang, Q.; Fang, C.; Shi, M.; Guo, D.; Peng, Y. High Serum Brain-Derived Neurotrophic Factor Is Associated With Decreased Risks of Poor Prognosis After Ischemic Stroke. Stroke 2023, 54, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Z.; Chen, X.-Y.; Fu, M.; Wan, S.-F.; Zhang, X. Association between plasma immunoproteasome and 90-day prognosis after first-ever ischemic stroke. Neural Regen. Res. 2021, 16, 790. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-F.; Sudlow, C.L.M.; Anderson, N.; Jeng, J.-S. Variations of risk factors for ischemic stroke and its subtypes in Chinese patients in Taiwan. Sci. Rep. 2021, 11, 9700. [Google Scholar] [CrossRef] [PubMed]
- Venketasubramanian, N.; Yoon, B.W.; Pandian, J.; Navarro, J.C. Stroke Epidemiology in South, East, and South-East Asia: A Review. J. Stroke 2017, 19, 286–294. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Zhao, X.; Wang, D.; Wang, C.; Liu, L.; Wang, A.; Meng, X.; Li, H.; Wang, Y. Association of hypertension with stroke recurrence depends on ischemic stroke subtype. Stroke 2013, 44, 1232–1237. [Google Scholar] [CrossRef]
- Dubow, J.; Fink, M.E. Impact of hypertension on stroke. Curr. Atheroscler. Rep. 2011, 13, 298–305. [Google Scholar] [CrossRef]
| BDNF (n = 45) (≤3.227 ng/mL) N (%)/M ± SD | BDNF (n = 45) (≥3.227 ng/mL) N (%)/M ± SD | p Value | |
|---|---|---|---|
| Age (y) | 67.19 ± 13.82 | 68.21 ± 12.00 | 0.711 |
| Gender (male) | 31 (68.9) | 30 (66.7) | 1.0 |
| Hyperlipidemia | 13 (36.1) | 24 (60.0) | 0.043 * |
| Diabetes mellitus | 21 (63.6) | 23 (71.9) | 0.598 |
| Hypertension | 34 (75.6) | 30 (68.2) | 0.486 |
| Smoking | 18 (40.0) | 11 (29.7) | 0.363 |
| Alcohol | 10 (23.3) | 9 (20.0) | 0.798 |
| Systolic blood pressure (mmHg) | 165.76 ± 45.79 | 160.84 ± 38.39 | 0.585 |
| Diastolic blood pressure (mmHg) | 90.93 ± 29.99 | 90.57 ± 24.18 | 0.950 |
| White blood cells (103/uL) | 10.26 ± 4.11 | 10.54 ± 4.66 | 0.767 |
| Platelet (103/uL) | 262.98 ± 111.68 | 210.68 ± 76.98 | 0.013 * |
| Glucose (mg/dL) | 140.38 ± 56.82 | 151.65 ± 63.61 | 0.519 |
| Hemoglobin A1c (%) | 6.25 ± 1.48 | 7.06 ± 2.04 | 0.053 |
| Triglycerides (mg/dL) | 102.0 ± 36.79 | 144.08 ± 92.83 | 0.014 * |
| Total cholesterol (mg/dL) | 174.34 ± 42.91 | 181.63 ± 54.07 | 0.508 |
| High-density lipoprotein cholesterol (mg/dL) | 48.14 ± 13.04 | 38.5 ± 11.63 | 0.005 ** |
| Low-density lipoprotein cholesterol (mg/dL) | 108.0 ± 35.59 | 117.0 ± 40.86 | 0.297 |
| High-sensitivity C-reactive protein (mg/dL) | 5.28 ± 5.64 | 6.16 ± 7.14 | 0.520 |
| TC/HDL-C | 3.87 ± 1.13 | 4.70 ± 1.18 | 0.008 ** |
| LDL-C/HDL-C | 2.45 ± 1.01 | 3.05 ± 0.98 | 0.028 * |
| TG/HDL-C | 2.34 ± 1.15 | 3.88 ± 2.19 | 0.003 ** |
| Non-HDL-C | 134.76 ± 44.85 | 155.32 ± 61.80 | 0.114 |
| TyG index | 4.70 ± 0.32 | 4.83 ± 0.30 | 0.165 |
| Variable | Adjusted OR (95% CI) a | p Value |
|---|---|---|
| TC | 1.003 (0.994–1.013) | 0.496 |
| LDL-C | 1.006 (0.995–1.018) | 0.280 |
| HDL-C | 0.926 (0.876–0.978) | 0.006 ** |
| TG | 1.017 (1.003–1.030) | 0.013 * |
| TG/HDL-C | 1.903 (1.187–3.051) | 0.008 ** |
| TC/HDL-C | 1.975 (1.188–3.284) | 0.009 ** |
| LDL-C/HDL-C | 2.032 (1.113–3.711) | 0.021 * |
| Non-HDL-C | 1.008 (0.998–1.018) | 0.120 |
| TyG index | 4.757 (0.584–38.778) | 0.145 |
| GLU | 1.003 (0.993–1.013) | 0.529 |
| HbA1C | 1.386 (0.983–1.953) | 0.063 |
| DM | 1.439 (0.497–4.164) | 0.502 |
| Hyperlipidemia | 2.665 (1.050–6.765) | 0.039 * |
| SBP | 0.997 (0.987–1.007) | 0.561 |
| DBP | 1.000 (0.984–1.015) | 0.961 |
| HTN | 0.670 (0.260–1.725) | 0.407 |
| Variable | OR (95% CI) a | p Value | OR (95% CI) ᵇ | p Value | OR (95% CI) c | p Value |
|---|---|---|---|---|---|---|
| TC/HDL-C | 1.76 (1.05–2.96) | 0.034 * | 1.78 (1.06–2.99) | 0.031 * | 3.14 (1.31–7.54) | 0.010 * |
| TG/HDL-C | 1.84 (1.14–2.97) | 0.013 * | 1.78 (1.11–2.86) | 0.018 * | 2.53 (1.24–5.17) | 0.011 * |
| LDL-C/HDL-C | 1.76 (0.95–3.28) | 0.074 | 1.79 (0.96–3.35) | 0.068 | 3.02 (1.19–7.66) | 0.020 * |
| Hyperlipidemia | 1.77 (0.65–4.83) | 0.263 | 1.89 (0.67–5.33) | 0.227 | 2.06 (0.65–6.53) | 0.219 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuwar, M.N.; Chen, W.-H.; Yeh, H.-L.; Bai, C.-H. Association between Brain-Derived Neurotrophic Factor and Lipid Profiles in Acute Ischemic Stroke Patients. Int. J. Mol. Sci. 2024, 25, 2380. https://doi.org/10.3390/ijms25042380
Tuwar MN, Chen W-H, Yeh H-L, Bai C-H. Association between Brain-Derived Neurotrophic Factor and Lipid Profiles in Acute Ischemic Stroke Patients. International Journal of Molecular Sciences. 2024; 25(4):2380. https://doi.org/10.3390/ijms25042380
Chicago/Turabian StyleTuwar, Mayuri N., Wei-Hung Chen, Hsu-Ling Yeh, and Chyi-Huey Bai. 2024. "Association between Brain-Derived Neurotrophic Factor and Lipid Profiles in Acute Ischemic Stroke Patients" International Journal of Molecular Sciences 25, no. 4: 2380. https://doi.org/10.3390/ijms25042380
APA StyleTuwar, M. N., Chen, W.-H., Yeh, H.-L., & Bai, C.-H. (2024). Association between Brain-Derived Neurotrophic Factor and Lipid Profiles in Acute Ischemic Stroke Patients. International Journal of Molecular Sciences, 25(4), 2380. https://doi.org/10.3390/ijms25042380






