Statins—Their Role in Bone Tissue Metabolism and Local Applications with Different Carriers
Abstract
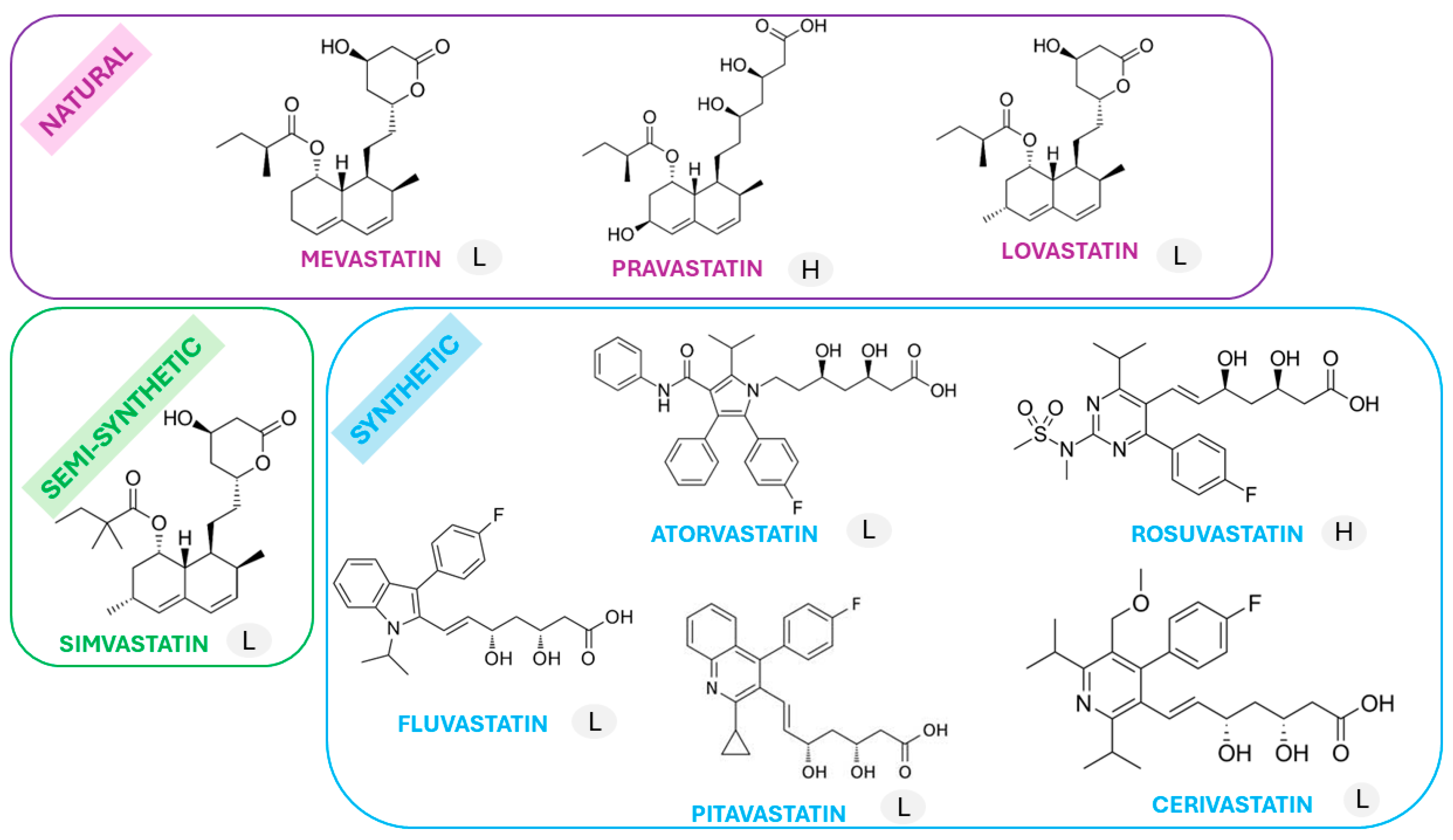
1. Introduction
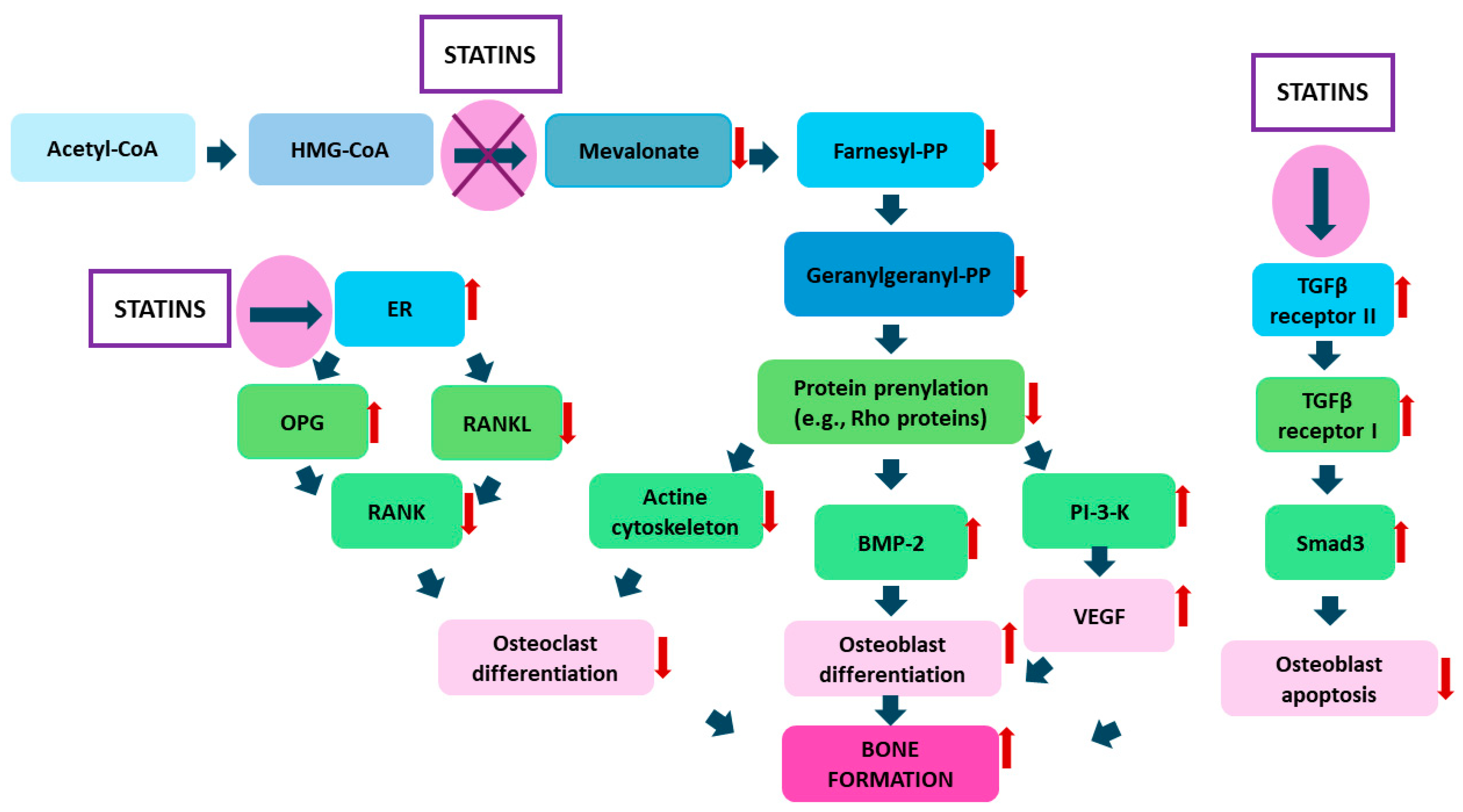
2. Statins—Their Role in Bone Tissue Metabolism
3. Statin Local Delivery Methods and Carriers
| Statin (Dose) | Carrier | Model of Study/Duration of Treatment | Findings | Reference |
|---|---|---|---|---|
| Inorganic materials | ||||
| Simvastatin (0.25 and 0.5 mg) | α-TCP (α-tricalcium phosphate) | In vivo/69 healthy Wistar adult rats; 8 weeks | Bone regeneration in rat calvarial defects was noticed | [80] |
| Simvastatin (0.1 mg) | α-TCP (α-tricalcium phosphate) | In vivo/72 healthy Wistar rats; 8 weeks | Stimulation of bone regeneration occurred | [81] |
| Simvastatin (6% concentration) | Apatite cements | In vivo/18 ovariectomized rats; 3 weeks | Bone mineral density increased | [82] |
| Simvastatin (4 mg/mL) | β-TCP (β-tricalcium phosphate) | In vitro/drug release in simulation body fluid solution; 7 days | Controlled release of the drug with a reduction of approximately 25% compared to control samples was observed | [76] |
| Simvastatin (0.1 mg) | β-TCP (β-tricalcium phosphate) | In vivo/72 healthy Wistar rats; 8 weeks | Stimulation of bone regeneration occurred | [81] |
| Simvastatin (0.1, 0.9, and 1.7 mg) | β-TCP (β-tricalcium phosphate) | In vivo/162 healthy male Sprague Dawley rats; 6 weeks | Decreased mineral apposition was observed, and after 26 weeks, increased fibrous area fraction, β-TCP area fraction, and particle size and number were noticed | [83] |
| Simvastatin (0.1 mg) | Calcium phosphate | In vivo/15 healthy female Wistar rats; 8 weeks | Bone-like tissue was formed | [84] |
| Simvastatin (1, 5, and 10% concentrations) | Calcium phosphate cement (an equimolar mixture of tetracalcium phosphate and dicalcium phosphate anhydrous) | In vivo/40 healthy New Zealand white rabbits; 4 weeks | New bone formation was observed | [85] |
| Simvastatin (0.5 and 0.25 mg/g cement) | Calcium phosphate cement | In vitro/Saos-2 cells; 7 days | Promotion of bone formation was noticed | [86] |
| Simvastatin (0.1, 0.25 and 0.5 mg/g cement) | Calcium phosphate cement (β-tricalcium phosphate and monocalcium phosphate anhydrous in molar ratio of 1:1) | In vitro/bone marrow macrophages isolated from mice; 12 days | Inhibition of osteoclastic differentiation was observed | [87] |
| Simvastatin (0.5 mg) | Calcium sulphate | In vivo/18 healthy New Zealand white rabbits; 8 weeks | An area of newly formed bone was noticed | [74] |
| Simvastatin (1 mg) | Calcium sulphate | In vivo/45 healthy male Wistar rats; 8 weeks | Stimulation of bone regeneration was observed | [75] |
| Simvastatin (0.125 mg) | Hydroxyapatite | In vivo/12 healthy New Zealand white rabbits; 8 weeks | Increased bone volume was noticed | [72] |
| Simvastatin (0.45 mg) | Hydroxyapatite | In vivo/20 adult Japanese white rabbits; 8 weeks | New bone formation was observed | [73] |
| Simvastatin (0.1 mg) | Hydroxyapatite | In vivo/72 healthy Wistar rats; 8 weeks | Stimulation of bone regeneration occurred | [88] |
| Simvastatin (10 mM) | Hydroxyapatite-coated titanium | In vitro/bone mesenchymal stem cells (BMSCs); 14 days In vivo/48 adult male Sprague Dawley rats; 6 weeks | In vitro: Enhanced osteogenesis and osteointegration occurred In vivo: Maximum forces of the Sim-Low and Sim-High groups were significantly higher than those of the Control and HA groups | [88] |
| Simvastatin (0.01 and 0.001 g/L) | Mesoporous titania thin films | In vitro/MC3T3-E1 pre-osteoblasts cells; 21 days | Incubation the formation of a complex network of pre-collagen filaments was observed | [89] |
| Simvastatin (10 mM) | Nanohydroxyapatite | In vivo/36 ovariectomized Sprague Dawley rats; 12 weeks | New bone formation around implant surfaces was noticed | [90] |
| Simvastatin (5 mg/kg) | Titanium implants | In vivo/54 ovariectomized Sprague Dawley rats; 84 days | The bone healing process was observed | [91] |
| Simvastatin (50 μg/implant) | Titanium Kirschner wires coated with PDLLA (poly(D,L-lactide)) and PUR (polyurethane) | In vivo/200 female Sprague Dawley rats; 6 weeks | Improved fracture healing was present | [92] |
| Natural and synthetic polymers and composites | ||||
| Simvastatin (5, 10, and 20 mg/15 g solutions) | 3D—PGHS (as-fabricated 3D fibrous scaffolds of poly (ε-caprolactone) poly (glycerol-sebacate) hydroxyapatite nanoparticles) | In vitro/human mesenchymal stem cells (hMSCs) and human umbilical vein endothelial cells (HUVECs); 7 days | Osteogenic differentiation and migration as well as tube formation occurred | [93] |
| Simvastatin (10 mg/mL) | ALN-CD (alendronate—β-cyclodextrin) conjugate | In vivo/44 healthy female Sprague Dawley rats; 4 weeks | The study stated that ALN-CD conjugates not only act as tissue-specific carriers but preserve new bone formation | [94] |
| Simvastatin (2.5 mg/mL dissolved in 0.2 mL water) | ACS (atelocollagen sponge) | In vivo/20 adult male Japanese white rabbits; 12 weeks | New bone formation was observed | [95] |
| Pitavastatin (0.1 μM) | β-cyclodextrin-grafted chitosan and gelatin | In vivo/40 specific-pathogen-free male Sprague Dawley rats; 4 weeks | Bone formation was observed | [63] |
| Lovastatin (1.2 mg/layer) | β-TCP/PCL (β-tricalcium phosphate/polycaprolactone) microchips and PCL nanofiber membranes | In vivo/24 ovariectomized New Zealand rabbits; 12 weeks | Bone parameters significantly improved | [60] |
| Simvastatin (0.5 μM) | BPPD (bis(PLGA-phe-PEG)-qDETA) | In vitro/bone marrow mesenchymal stem cells (BMSCs); 6 days | Promotion of osteogenesis in BMSCs was observed | [96] |
| Simvastatin (4 mg) | Chitosan | In vitro/BMSC culture; 14 days In vivo/6 healthy ovariectomized rats; 8 weeks | In vitro: A positive effect on cell proliferation was noticed In vivo: The bone regeneration process was observed | [97] |
| Simvastatin (0.25 mg) | Chitosan | In vivo/21 healthy Sprague Dawley rats; 8 weeks | No significant difference between the control and experimental groups was found | [98] |
| Simvastatin (0.05 mg) | Chitosan | In vitro/human bone marrow mesenchymal stem cells (hbMMSCs); 14 days | Chitosan scaffold is a bioactive compatible material with regenerative potential for hBMMSCs | [99] |
| Simvastatin (5 mg/0.5 mL) | Chitosan | In vivo/12 healthy male albino New Zealand rabbits; 6 weeks | The process of bone regeneration was noticed | [100] |
| Simvastatin (2.5 mg/mL) | Collagen graft | In vivo/9 healthy New Zealand white rabbits; 14 days | An osteoinductive effect was noticed | [101] |
| Simvastatin (2.5 mg/mL) | Collagen matrix | In vivo/14 healthy New Zealand white rabbits; 14 days | New bone formation was observed | [102] |
| Rosuvastain (0.1, 0.5, and 2.5 mg/mL) | Collagen sponges | In vivo/18 healthy New Zealand white female rabbits; 4 weeks | Stimulation of bone formation occurred | [64] |
| Simvastatin (1% concentration) | Gel (composed of polymer 2% HPMC K100M and 20% poloxamer 407) | In vivo/72 healthy Sprague Dawley rats; 56 days | Bone regeneration was observed | [103] |
| Simvastatin (250 μg) | Gelatin hydrogel | In vivo/60 healthy virgin female Sprague Dawley rats; 8 weeks | Acceleration of fracture healing was observed | [104] |
| Fluvastatin (1 mM) | Gelatin hydrogel | In vivo/60 healthy male Sprague Dawley rats; 4 weeks | Induced osteogenesis in rat calvarial bone was observed | [67] |
| Simvastatin (2.5 mg/mL dissolved in 0.2 mL water) | Gelatin hydrogel | In vivo/20 adult male Japanese white rabbits; 12 weeks | New bone formation was observed | [95] |
| Simvastatin (125 μg) | Gelatin hydrogel | In vivo/42 healthy mature mature Japanese rabbits; 8 weeks | Promotion of tendon–bone healing at an early stage via angiogenesis and osteogenesis occurred but did not affect the biomechanical property in the long term | [105] |
| Simvastatin (0.5 μM) | GNTS (gelatin-nanofibrillar cellulose- β tricalcium phosphate) | In vivo/30 healthy male Sprague Dawley rats; 8 weeks | Newly formed bone structures were noticed | [106] |
| Simvastatin (100 nM) | Methylated β-cyclodextrins | In vitro/MC3T3-E1 cells; 14 days | ALP production and the expression of bone sialoprotein and osteocalcin were noticed | [107] |
| Simvastatin (2.2 mg) | Methylcellulose gel and PLA (polylactide membrane) | In vivo/56 healthy female ICR Swiss mice; 44 days | An increase in bone thickness was observed | [108] |
| Simvastatin (0.5 mg) | NLC (nanostructured lipid carrier) | In vivo/20 healthy rabbits; 4 weeks | Enhanced bone formation was observed | [109] |
| Simvastatin (20 μg) | PCL (poly (ε-caprolactone)) | In vivo/90 healthy Wistar albino rats; 6 months | An increase in bone mineralization was noticed | [110] |
| Simvastatin (100 μg/mL) | PCL (poly (ε-caprolactone)) and collagen | In vitro/primary human umbilical vein endothelial cells (pHUVECs); 21 days | Enhanced osteogenic differentiation was noticed | [111] |
| Simvastatin (2.2 mg) | PCL (poly (ε-caprolactone)) fibrous sheets and structured nanofibers with a gelatin shell | In vivo/24 healthy male New Zealand white rabbits; 12 weeks | Good cell viability and effective osteoinductive and barrier properties were observed | [112] |
| Simvastatin (5% concentration) | PCL (poly (ε-caprolactone)) nanofibers loaded with polyaniline-coated titanium oxide nanoparticles (TiO2/PANI) | In vitro/MC3T3-E1 osteoblast cells; 14 days | Profound cell proliferation was observed | [113] |
| Simvastatin (dose not stated) | PCL-HA (poly(ε-caprolactone- hydroxyapatite)) microspheres | In vitro/bone marrow mesenchymal stromal cells (BMSCs); 21 days In vivo/3 healthy Sprague Dawley rats; 8 weeks | Osteogenic differentiation of BMSCs was noticed in vitro. Promotion of vascular network and functional bone formation was observed in vivo | [114] |
| Simvastatin (5% concentration) | PCL-HA (poly(ε-caprolactone-hydroxyapatite)) composite coated on biodegradable Mg alloy nanofibers | In vitro/MC3T3 mouse osteoblast cell line; 7 days | An increase in bone regeneration and control of its degradation occurred | [115] |
| Simvastatin (from 2.5 × 10−6 to 2.5 × 10−10 M) | PECL (poly (ethylene glycol))-poly(ε-caprolactone)) | In vitro/human osteoblast-like MG-63 cells; 7 days | Osteoblast differentiation and mineralization were observed | [116] |
| Fluvastatin (0.01 and 0.1 μM) | PEGDM (poly (ethylene glycol) dimethacrylate) | In vitro/human mesenchymal stem cell (hMSC); 14 days | An increase in hMSC CBFA1, ALP, and COL I gene expression was noticed, which indicated an effect on osteogenic differentiation | [68] |
| Simvastatin (0.5 mg) | PEG-PLA (polyethylene glycol- polylactic acid) polymeric nanomicelles | In vivo/6 healthy New Zealand white rabbits; 4 weeks | Osteoblasts and new capillaries around the trabecular bone were found | [117] |
| Simvastatin (0.28 and 0.31 μg/mg) | PEG-PLGA (poly (ethylene glycol))-block-poly(lactic-co-glycolic acid) | In vivo/6 healthy ovariectomized Sprague Dawley rats; 12 weeks | A bone formation effect was present | [118] |
| Simvastatin (2 mg/mL) | PEEK (polyetheretherketone)bio-composite | In vitro/MC3T3-E1 pre-osteoblasts; 14 days | Osteogenic differentiation was observed | [79] |
| Simvastatin (1 mg/mL) | PET (polyethylene terephthalate) | In vivo/36 healthy New Zealand white rabbits; 8 weeks | Bone healing was observed | [119] |
| Fluvastatin (75 μg) | PGA (propylene glycol alginate) | In vivo/60 healthy female Wistar rats; 2 weeks | An increase in bone volume was noticed | [69] |
| Fluvastatin (75 μg) | PGA (propylene glycol alginate) | In vivo/48 healthy female Wistar rats; 4 weeks | An increase in bone–implant contact and mineralized bone volume was observed | [70] |
| Lovastatin (1 mg/mL) | PGA-PEG (poly(glycolide)-poly(ethylene glycol)) | In vitro/mice; 7 days | The study showed that the maximum tolerated dose in mice can be increased | [61] |
| Simvastatin (5 mg) | PLA (polylactic acid) | In vivo/16 healthy New Zealand white rabbits; 12 weeks | High-density spots were observed and the margins of the defects were more irregular | [120] |
| Simvastatin (4mg/g PLG) | PLG (poly(lactide-co-glycolide)) | In vitro/rat bone marrow cells; 10 days | Bone cell mineralization was observed | [121] |
| Fluvastatin (0.5 and 1 mg/kg) | PLGA (poly (lactic-co-glycolic acid)) | In vivo/40 healthy Sprague Dawley rats; 4 weeks | More bone trabeculae were observed | [71] |
| Simvastatin (1 mg) | PLGA (poly (lactic-co-glycolic acid)) | In vitro/human osteoblastic cell line (hFOB); 11 days | Osteoblastic differentiation was observed | [122] |
| Simvastatin (0.6% concentration) | PLGA (poly (lactic-co-glycolic acid)) coated around titanium | In vitro/human gingival fibroblasts (HGFs) and stem cells from human exfoliated deciduous teeth (SHEDs); 7 days | High cell viability was observed | [123] |
| Simvastatin (20 mg/kg) | PLGA (poly (lactic-co-glycolic acid))-encapsulated hydroxyapatite | In vivo/24 healthy female Wistar rats; 45 days | Significant improvement in the bone surface was observed | [124] |
| Simvastatin (2, 5, and 8% concentrations) | PLGA (poly (lactide-co-glycolide)) microspheres using the electrospraying method | In vitro/human MG-63 osteoblast cells; 7 days | Good biocompatibility of the electrosprayed PLGA microspheres was observed, which increased in the presence of a statin | [125] |
| Simvastatin (5% concentration) | PLGA (poly (lactic-co-glycolic acid)) microspheres loaded into hydrogel-loaded BCP (biphasic calcium phosphate) | In vitro/MC3T3-E1 pre-osteoblast cells; 7 days | Bone remodeling gene and protein expression were observed | [126] |
| Simvastatin (3 mg of simvastatin/PLGA) | PLGA (poly (lactic-co-glycolic acid)) with a rapidly absorbable calcium sulfate | In vivo/60 healthy male Sprague Dawley rats; 12 weeks | Osteogenic and angiogenic activity and bone healing process increased | [127] |
| Simvastatin (0.5 μM) | PLGA-PEG (poly (lactic acid-co-glycolic acid)-polyethylene glycol)) | In vitro/BMSCs; 6 days | Improvement in bone healing was observed | [96] |
| Simvastatin (1 mg) | PLLA (poly-L-lactide) | In vivo/29 healthy male Sprague Dawley rats; 8 weeks | New bone formation and increased bone mineral density were observed | [128] |
| Simvastatin (~ 120 mg/kg/day) | Polyethylene particles | In vivo/21 healthy female and male C57BL/J6 mice; 14 days | New bone formation was noticed | [129] |
| Simvastatin (2.2 mg) | Poly(ethylene glycol)-block-poly(simvastatin) | In vivo/144 healthy male Sprague Dawley rats; 8 weeks | A significant osteogenic effect was noticed | [130] |
| Simvastatin (0.5 mM) | Poly (N-isopropylacrylamide) Brush-modified mesoporous hydroxyapatite | In vivo/20 ovariectomized Wistar rats; 6 weeks | Promotion of osteogenesis was observed | [131] |
| Lovastatin (200 μg/g of foam) | Polyurethane (PUR) | In vivo/6 healthy male Sprague Dawley rats; 4 weeks | An increase in the density of the newly formed bone was observed | [62] |
| Simvastatin (5 mg/mL) | Polyurethane nanofibers | In vivo/32 healthy adult male Wistar rats; 4 weeks | Induction of bone healing was noticed | [132] |
| Rosuvastatin (5 mg/mL) | PVA-SF (polyvinyl alcohol–silk fibroin) core-shell nanofibers | In vitro/Human adipose-derived stem cells (hADSCs); 21 days | Improved cell proliferation and osteogenic differentiation occurred | [65] |
| Simvastatin (2 mg) | SIM-DOME (methylcellulose gel under a polylactic acid dome membrane) | In vivo/44 healthy mature female Sprague Dawley rats; 24 days | New bone formation was observed | [94] |
| Simvastatin (0.5–1 μM) | Poly(l-lactide-co-glycolide) | In vivo/4 healthy male C57/BL/6 J mice; 12 weeks | New bone formation was observed | [133] |
| Organic non-polymer materials | ||||
| Simvastatin (10 mg) | Gelfoam soaked with normal saline | In vivo/50 humans; 12 weeks | An increase in bone density occurred | [134] |
| Simvastatin (0.1 and 1 mg) | Hyaluronic acid (HA) hydrogels | In vivo/12 healthy male New Zealand rabbits; 8 weeks | A significant influence on osteogenesis was observed | [135] |
| Simvastatin (0.01, 0.1, and 1 μM) | Injectable tissue-engineered bone (ITB) | In vitro/human adipose-derived stromal cells (hADSCs); 14 days In vivo/26 healthy BALB/C homozygous nude mice; 4 weeks | Osteoblastic differentiation in vitro and bone formation in vivo were observed | [136] |
| Rosuvastatin (3 mg/mL) | SF (silk fibroin) nanofibers | In vitro/Human adipose-derived stem cells (hADSCs); 21 days | Osteogenic gene differentiation was observed | [66] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stancu, C.; Sima, A. Statins: Mechanism of action and effects. J. Cell Mol. Med. 2001, 5, 378–387. [Google Scholar] [CrossRef]
- Maron, D.J.; Fazio, S.; Linton, M.F. Current perspectives on statins. Circulation 2000, 101, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Egom, E.E.; Hafeez, H. Biochemistry of Statins. Adv. Clin. Chem. 2016, 73, 127–168. [Google Scholar] [CrossRef] [PubMed]
- Hunninghake, D.B. HMG-CoA reductase inhibitors. Curr. Opin. Lipidol. 1992, 3, 22–28. [Google Scholar] [CrossRef]
- Watson, K.E.; Fonarow, G.C. The past, present, and future of statin therapy. Rev. Cardiovasc. Med. 2005, 6, 129–139. [Google Scholar]
- Puccetti, L.; Pasqui, A.L.; Auteri, A.; Bruni, F. Mechanisms for antiplatelet action of statins. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2005, 5, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Mohammadkhani, N.; Gharbi, S.; Rajani, H.F.; Farzaneh, A.; Mahjoob, G.; Hoseinsalari, A.; Korsching, E. Statins: Complex outcomes but increasingly helpful treatment options for patients. Eur. J. Pharmacol. 2019, 863, 172704. [Google Scholar] [CrossRef] [PubMed]
- Lahera, V.; Goicoechea, M.; de Vinuesa, S.G.; Miana, M.; de las Heras, N.; Cachofeiro, V.; Luño, J. Endothelial dysfunction, oxidative stress and inflammation in atherosclerosis: Beneficial effects of statins. Curr. Med. Chem. 2007, 14, 243–248. [Google Scholar] [CrossRef]
- Vaughan, C.J.; Gotto, A.M.; Basson, C.T. The evolving role of statins in the management of atherosclerosis. J. Am. Coll. Cardiol. 2000, 35, 1–10. [Google Scholar] [CrossRef]
- Schachter, M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: An update. Fundam. Clin. Pharmacol. 2005, 19, 117–125. [Google Scholar] [CrossRef]
- Bellosta, S.; Ferri, N.; Bernini, F.; Paoletti, R.; Corsini, A. Non-lipid-related effects of statins. Ann. Med. 2000, 32, 164–176. [Google Scholar] [CrossRef]
- Athyros, V.G.; Kakafika, A.I.; Tziomalos, K.; Karagiannis, A.; Mikhailidis, D.P. Pleiotropic effects of statins—Clinical evidence. Curr. Pharm. Des. 2009, 15, 479–489. [Google Scholar] [CrossRef]
- Rossini, E.; Biscetti, F.; Rando, M.M.; Nardella, E.; Cecchini, A.L.; Nicolazzi, M.A.; Covino, M.; Gasbarrini, A.; Massetti, M.; Flex, A. Statins in High Cardiovascular Risk Patients: Do Comorbidities and Characteristics Matter? Int. J. Mol. Sci. 2022, 23, 9326. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R. The pharmacology of statins. Pharmacol. Res. 2014, 88, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.G. Statin therapy. Curr. Pharm. Des. 2012, 18, 6284–6290. [Google Scholar] [CrossRef] [PubMed]
- Climent, E.; Benaiges, D.; Pedro-Botet, J. Hydrophilic or lipophilic statins? Front. Cardiovasc. Med. 2021, 8, 687585. [Google Scholar] [CrossRef]
- Waters, D.D. What the statin trials have taught us. Am. J. Cardiol. 2006, 98, 129–134. [Google Scholar] [CrossRef]
- McKenney, J.M. Pharmacologic characteristics of statins. Clin Cardiol. 2003, 26 (Suppl. 3), 32–38. [Google Scholar] [CrossRef] [PubMed]
- Corsini, A.; Bellosta, S.; Baetta, R.; Fumagalli, R.; Bernini, F. New insights into the pharmacodynamics and pharmacokinetic properties of statins. Pharmacol. Ther. 1999, 84, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Dagli-Hernandez, C.; Zhou, Y.; Lauschke, V.M.; Genvigir, F.D.V.; Hirata, T.D.C.; Hirata, M.H.; Hirata, R.D.C. Pharmacogenomics of statins: Lipid response and other outcomes in Brazilian cohorts. Pharmacol Rep. 2022, 74, 47–66. [Google Scholar] [CrossRef]
- Yaturu, S. Skeletal effects of statins. Endocr. Pract. 2003, 9, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Sharif, P.S.; Abdollahi, M. A systematic review on the relation between use of statins and osteoporosis. Int. J. Pharmacol. 2011, 7, 180–188. [Google Scholar] [CrossRef]
- Cruz, A.C.; Gruber, B.L. Statins and osteoporosis: Can these lipid-lowering drugs also bolster bones? Clevel. Clin. J. Med. 2002, 69, 277–288. [Google Scholar] [CrossRef]
- An, T.; Hao, J.; Sun, S.; Li, R.; Yang, M.; Cheng, G.; Zhou, M. Efficacy of statins for osteoporosis: A systematic review and meta-analysis. Osteoporos. Int. 2017, 28, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Tsartsalis, A.N.; Dokos, C.; Kaiafa, D.G.; Tsartsalis, D.N.; Kattamis, A.; Hatzitolios, A.I.; Savopoulos, C.G. Statins, bone formations and osteoporosis: Hope or hype? Hormones 2012, 11, 126–139. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liao, J.K. Current advances in statin treatment: From molecular mechanisms to clinical practice. Arch. Med. Sci. 2007, 4A, 91–96. [Google Scholar]
- Oryan, A.; Kamali, A.; Moshiri, A. Potential mechanisms and applications of statins on osteogenesis: Current modalities, conflicts and future directions. J. Controll. Rel. 2015, 215, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Sun, J.S.; Tsuang, Y.H.; Chen, M.H.; Weng, P.W.; Lin, F.H. Simvastatin promotes osteoblast viability and differentiation via Ras/Smad/Erk/ BMP-2 signalling pathway. Nutr. Res. 2010, 30, 191–199. [Google Scholar] [CrossRef]
- Montagnani, A.; Gonnelli, S.; Cepollaro, C.; Pacini, S.; Campagna, M.S.; Franci, M.B.; Lucani, B.; Gennari, C. Effect of simvastatin treatment on bone mineral density and bone turnover in hypercholesterolemic postmenopausal women: A 1-year longitudinal study. Bone 2003, 32, 427–433. [Google Scholar] [CrossRef]
- Kaji, H.; Naito, J.; Inoue, Y.; Sowa, H.; Sugimoto, T.; Chihara, K. Statin suppresses apoptosis in osteoblastic cells: Role of transforming growth factor–beta-Smad3 pathway. Horm. Metab. Res. 2008, 40, 746–751. [Google Scholar] [CrossRef]
- Moshiri, A.; Sharifi, A.M.; Oryan, A. Role of simvastatin on fracture healing and osteoporosis: A systematic review on in vivo investigation. Clin. Exp. Pharmacol. Physiol. 2016, 43, 659–684. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.R. Statins and their potential for osteoporosis. Bone 2001, 29, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Tahamtan, S.; Shirban, F.; Baghernija, M.; Johnston, T.P.; Sahebkar, A. The effects of statins on dental and oral health: A review of preclinical and clinical studies. J. Transl. Med. 2020, 18, 155. [Google Scholar] [CrossRef] [PubMed]
- Shahrezaee, M.; Oryan, A.; Bastami, F.; Hosseinpour, S.; Shahrezaee, M.H.; Kamali, A. Comparative impact of systemic delivery of atorvastatin, simvastatin, and lovastatin on bone mineral density of the ovariectomized rats. Endocrine 2018, 60, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xie, Y.; Baloch, Z.; Shi, Q.; Huo, Q.; Ma, T. The effect of atorvastatin, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor (HMG-CoA), on the prevention of osteoporosis in ovariectomized rabbits. J. Bone Miner. Metab. 2017, 35, 245–254. [Google Scholar] [CrossRef]
- Antonenko, A.; Leahy, A.; Babenko, M.; Lyons, D. Low-dose hydrophilic statins are the preferred agents for females at risk of osteoporosis. Bone Rep. 2022, 16, 101152. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Jain, G.K. Statins and osteoporosis: A new role for old drugs. J. Pharm. Pharmacol. 2006, 58, 3–18. [Google Scholar] [CrossRef]
- Shah, S.R.; Werlang, C.A.; Kasper, F.K.; Mikos, A.G. Novel applications of statins for bone regenerations. Nat. Sci. Rev. 2015, 2, 85–99. [Google Scholar] [CrossRef]
- Hong, W.; Wei, Z.; Qiu, Z.; Li, Z.; Fu, C.; Ye, Z.; Xu, X. Atorvastatin promotes bone formation in aged apoE–/– mice through the Sirt1–Runx2 axis. J. Orthop. Surg. Res. 2020, 15, 303. [Google Scholar] [CrossRef]
- Mundy, G.; Garrett, R.; Harris, S.; Chan, J.; Chen, D.; Rossini, G.; Boyce, B.; Zhao, M.; Gutierrez, G. Stimulation of bone formation in vitro and in rodents by statins. Science 1999, 286, 1946–1949. [Google Scholar] [CrossRef]
- Lee, W.S.; Lee, E.G.; Sung, M.S.; Choi, Y.J.; Yoo, W.H. Atorvastatin inhibits osteoclast differentiation by suppressing NF-Κb and MAPK signaling during IL-1 β-induced osteoclastogenesis. Korean J. Intern. Med. 2018, 33, 397–406. [Google Scholar] [CrossRef]
- Tan, J.; Yang, N.; Fu, X.; Cui, Y.; Guo, Q.; Ma, T.; Yin, X.; Leng, H.; Song, C. Single-dose local simvastatin injection improves implant fixation via increased angiogenesis and bone formation in an ovariectomized rat model. Med. Sci. Monit. 2015, 21, 1428–1439. [Google Scholar]
- Sabandal, M.M.I.; Schäfer, E.; Imper, J.; Jung, S.; Kleinheinz, J.; Sielker, S. Simvastatin Induces In Vitro Mineralization Effects of Primary Human Odontoblast-like Cells. Materials. 2020, 3, 4679. [Google Scholar] [CrossRef]
- Von Stechow, D.; Fish, S.; Yahalom, D.; Bab, I.; Chorev, M.; Müller, R.; Alexander, J.M. Does simvastatin stimulate bone formation in vivo? BMC Musculoskelet. Disord. 2003, 28, 8. [Google Scholar] [CrossRef]
- Kabra, S.; Thosar, N.R.; Malviya, N.S. Exploring the Synergistic Effect of Simvastatin in Oral Health Applications: A Literature Review. Cureus 2023, 15, e44411. [Google Scholar] [CrossRef]
- Goes, P.; Lima, A.P.; Melo, I.M.; Rêgo, R.O.; Lima, V. Effect of atorvastatin in radiographic density on alveolar bone loss in Wistar rats. Braz. Dent. J. 2010, 21, 193–198. [Google Scholar] [CrossRef] [PubMed]
- El-Nabarawi, N.; El-Wakd, M.; Salem, M. Atorvastatin, a double weapon in osteoporosis treatment: An experimental and clinical study. Drug Des. Dev. Ther. 2017, 2, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Qadir, F.; Alam, S.M.; Zehra, T.; Mehmood, A.; Siddiqi, A.Q. Role of Pitavastatin in prevention of osteopenic changes in ovariectomized rats. J. Coll. Physicians Surg. Pak. 2016, 26, 41–45. [Google Scholar] [PubMed]
- Sorial, A.K.; Anjum, S.A.; Cook, M.J.; Board, T.N.; O’Neil, T.W. Statins: Bone biology and revision arthroplasty: Review of clinical and experimental evidence. Ther. Adv. Muscoskel. Dis. 2020, 12, 1759720X20966229. [Google Scholar]
- Tang, Q.O.; Tran, G.T.; Gamie, Z.; Graham, S.; Tsialogiannis, E.; Tsiridis, E.; Linder, T.; Tsiridis, E. Statins: Under investigation for increasing bone mineral density and augmenting fracture healing. Expert Opin. Investig. Drugs 2008, 17, 1435–1463. [Google Scholar] [CrossRef]
- Leutner, M.; Butylina, M.; Matzhold, C.; Klimek, P.; Cuhaj, C.; Bellach, L.; Baumgartner-Parzer, S.; Reiter, B.; Preindl, K.; Kautzky, A.; et al. Simvastatin therapy in higher dosage deteriorates bone quality: Consistent evidence from population-wide patient data and interventional mouse studies. Biomed. Pharmacother. 2023, 158, 114089. [Google Scholar] [CrossRef]
- Uzzan, B.; Cohen, R.; Nicolas, P.; Cucherat, M.; Perret, G.Y. Effects of statins on bone mineral density: A meta-analysis of clinical studies. Bone 2007, 40, 1581–1587. [Google Scholar] [CrossRef]
- Lin, T.K.; Chou, P.; Lin, C.H.; Hung, Y.J.; Jong, G.P. Long-term effect of statins on the risk of new-onset osteoporosis: A nationwide population-based cohort study. PLoS ONE 2018, 13, e0196713. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.C.; Adams, R.H. Biology of bone: The vasculature of the skeletal system. Cold Spring Harb. Perspect. Med. 2018, 8, a031559. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, W.; Zhang, Z.; Jiang, X. Recent advances in scaffold design and material for vascularized tissue-engineered bone regeneration. Adv. Healthc. Mater. 2019, 8, 1801433. [Google Scholar] [CrossRef]
- Elavarasu, S.; Shutanthiran, T.K.; Naveen, D. Statins: A new era in local drug delivery. J. Pharm. Bioallied. Sci. 2012, 4, 248–251. [Google Scholar] [CrossRef]
- Jin, H.; Ji, Y.; Cui, Y.; Xu, L.; Liu, H.; Wang, J. Simvastatin-Incorporated Drug Delivery Systems for Bone Regeneration. ACS Biomater. Sci. Eng. 2021, 7, 2177–2191. [Google Scholar] [CrossRef]
- Anupama Devi, V.K.; Ray, S.; Arora, U.; Mitra, S.; Sionkowska, A.; Jaiswal, A.K. Dual drug delivery platforms for bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 969843. [Google Scholar]
- Kheirallah, M.; Almeshaly, H. Simvastatin, dosage and delivery system for supporting bone regeneration, an update review. J. Oral Maxillofac. Surg. Med. Pathol. 2016, 28, 205–209. [Google Scholar] [CrossRef]
- Liu, X.; Li, T.; Wang, F.; Sun, F.; Hu, J.; Ye, X.; Wang, D.; Yang, X. Controlling sustained statins release in multi-layered composite scaffolds for healing of osteoporotic bone defects. Biomater Adv. 2022, 137, 212838. [Google Scholar] [CrossRef]
- Moore, T.L.; Schreurs, A.S.; Morrison, R.A.; Jelen, E.K.; Loo, J.; Globus, R.K.; Alexis, F. Polymer-Coated Hydroxyapatite Nanoparticles for the Delivery of Statins. Nanomed. Nanotechnol. 2014, 5, 5. [Google Scholar] [CrossRef]
- Yoshii, T.; Hafeman, A.E.; Nyman, J.S.; Esparza, J.M.; Shinomiya, K.; Spengler, D.M.; Mundy, G.R.; Gutierrez, G.E.; Guelcher, S.A. A sustained release of lovastatin from biodegradable, elastomeric polyurethane scaffolds for enhanced bone regeneration. Tissue Eng. A 2010, 16, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xie, G.; Lu, Y.; Wang, J.; Feng, B.; Wang, Q.; Xu, K.; Bao, J. An improved osseointegration of metal implants by pitavastatin loaded multilayer films with osteogenic and angiogenic properties. Biomaterials 2022, 280, 121260. [Google Scholar] [CrossRef] [PubMed]
- Monjo, M.; Rubert, M.; Wohlfahrt, J.C.; Rønold, H.J.; Ellingsen, J.E.; Lyngstadaas, S.P. In vivo performance of absorbable collagen sponges with rosuvastatin in critical-size cortical bone defects. Acta Biomater. 2010, 6, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Kalani, M.M.; Babak, N.; Rahimi, A. Electrospun coresheath poly (vinyl alcohol)/silk fibroin nanofibers with rosuvastatin release functionality for enhancing osteogenesis of human adipose-derived stem cells. Mater. Sci. Eng. 2019, 99, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Kalani, M.M.; Nourmohammadi, J.; Negahdari, B. Osteogenic potential of rosuvastatin immobilized on silk fibroin nanofibers using argon plasma treatment. Biomed. Mater. 2019, 14, 025002. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Nomoto, H.; Okumori, N.; Miura, T.; Yoshinari, M. Osteogenic effect of fluvastatin combined with biodegradable gelatin-hydrogel. Dent. Mater. J. 2012, 31, 489–493. [Google Scholar] [CrossRef]
- Benoit, D.S.; Nuttelman, C.R.; Collins, S.D.; Anseth, K.S. Synthesis and characterization of a fluvastatin-releasing hydrogel delivery system to modulate hMSC differentiation and function for bone regeneration. Biomaterials 2006, 27, 6102–6110. [Google Scholar] [CrossRef]
- Moriyama, Y.; Ayukawa, Y.; Ogino, Y.; Atsuta, I.; Koyano, K. Topical application of statin affects bone healing around implants. Clin. Oral Implant. Res. 2008, 19, 600–605. [Google Scholar] [CrossRef]
- Moriyama, Y.; Ayukawa, Y.; Ogino, Y.; Atsuta, I.; Todo, M.; Takao, Y.; Koyano, K. Local application of fluvastatin improves peri-implant bone quantity and mechanical properties: A rodent study. Acta Biomater. 2010, 6, 1610–1618. [Google Scholar] [CrossRef]
- Masuzaki, T.; Ayukawa, Y.; Moriyama, Y.; Jinno, Y.; Atsuta, I.; Ogino, Y.; Koyano, K. The effect of a single remote injection of statin-impregnated poly (lactic-co-glycolic acid) microspheres on osteogenesis around titanium implants in rat tibia. Biomaterials 2010, 31, 3327–3334. [Google Scholar] [CrossRef]
- Chalisserry, E.P.; Nam, S.Y.; Anil, S. Simvastatin Loaded Nano Hydroxyapatite in Bone Regeneration: A Study in the Rabbit Femoral Condyle. Curr. Drug Deliv. 2019, 16, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Shiota, M.; Fujii, M.; Chen, K.; Shimogishi, M.; Sato, M.; Kasugai. Combination of simvastatin and hydroxyapatite fiber induce bone augmentation. Open J. Regen. Medicine. 2013, 02, 53–60. [Google Scholar] [CrossRef]
- Huang, X.; Huang, Z.; Li, W. Highly efficient release of simvastatin from simvastatin-loaded calcium sulphate scaffolds enhances segmental bone regeneration in rabbits. Mol. Med. Rep. 2014, 9, 2152–2158. [Google Scholar] [CrossRef]
- Nyan, M.; Sato, D.; Oda, M.; Machida, T.; Kobayashi, H.; Nakamura, T.; Kasugai, S. Bone formation with the combination of simvastatin and calcium sulfate in critical-sized rat calvarial defect. J. Pharmacol. Sci. 2007, 104, 384–386. [Google Scholar] [CrossRef]
- Chou, J.; Ito, T.; Bishop, D.; Otsuka, M.; Ben-Nissan, B.; Milthorpe, B. Controlled release of simvastatin from biomimetic β-TCP drug delivery system. PLoS ONE 2013, 8, e54676. [Google Scholar] [CrossRef]
- Laskus-Zakrzewska, A.; Kazimierczak, P.; Kolmas, J. Porous Composite granules with potential function on bone substitute and simvastatin releasing system: A preliminary study. Materials 2021, 14, 5068. [Google Scholar] [CrossRef] [PubMed]
- Khurana, K.; Guillem-Marti, J.; Soldera, F.; Mücklich, F.; Canal, C.; Ginebra, M.-P. Injectable calcium phosphate foams for the delivery of Pitavastatin as osteogenic and angiogenic agent. J. Biomed. Mater. Res. 2019, 108, 760–777. [Google Scholar] [CrossRef]
- Deng, L.J.; Wu, Y.L.; He, X.H.; Xie, K.N.; Xie, L.; Deng, Y. Simvastatin delivery on PEEK for bioactivity and osteogenesis enhancements. J. Biomater. Sci. Polym. Ed. 2018, 29, 2237–2251. [Google Scholar] [CrossRef] [PubMed]
- Nyan, M.; Sato, D.; Kihara, H.; Machida, T.; Ohya, K.; Kasugai, S. Effects of the combination with alpha-tricalcium phosphate and simvastatin on bone regeneration. Clin. Oral Implant. Res. 2009, 20, 280–287. [Google Scholar] [CrossRef]
- Rojbani, H.; Nyan, M.; Ohya, K.; Kasugai, S. Evaluation of the osteoconductivity of α-tricalcium phosphate, β-tricalcium phosphate, and hydroxyapatite combined with or without simvastatin in rat calvarial defect. J. Biomed. Mater. Res. A 2011, 98, 488–498. [Google Scholar] [CrossRef]
- Hamada, H.; Ohshima, H.; Otsuka, M. Dissolution medium responsive simvastatin release from biodegradable apatite cements and the therapeutic effect in osteoporosis rats. J. Appl. Biomater. Funct. Mater. 2012, 10, 22–28. [Google Scholar]
- Ma, B.; Clarke, S.A.; Brooks, R.A.; Rushton, N. The effect of simvastatin on bone formation and ceramic resorption in a peri-implant defect model. Acta Biomater. 2008, 4, 149–155. [Google Scholar] [CrossRef]
- de Santana, W.M.; de Sousa, D.N.; Ferreira, V.M.; Duarte, W.R. Simvastatin and biphasic calcium phosphate affects bone formation in critical-sized rat calvarial defects. Acta Cir. Bras. 2016, 31, 300–307. [Google Scholar] [CrossRef]
- Yin, H.; Li, Y.G.; Si, M.; Li, J.M. Simvastatin-loaded macroporous calcium phosphate cement: Preparation, in vitro characterization, and evaluation of in vivo performance. J. Biomed. Mater. Res. A 2012, 100, 2991–3000. [Google Scholar] [CrossRef]
- Montazerolghaem, M.; Engqvist, H.; Karlsson Ott, M. Sustained release of simvastatin from premixed injectable calcium phosphate cement. J. Biomed. Mater. Res. A 2014, 102, 340–347. [Google Scholar] [CrossRef]
- Montazerolghaem, M.; Rasmusson, A.; Melhus, H.; Engqvist, H.; Karlsson Ott, M. Simvastatin-doped pre-mixed calcium phosphate cement inhibits osteoclast differentiation and resorption. J. Mater. Sci. Mater. Med. 2016, 27, 83. [Google Scholar] [CrossRef]
- Sun, T.; Huang, J.; Zhang, W.; Zheng, X.; Wang, H.; Liu, J.; Leng, H.; Yuan, W.; Song, C. Simvastatin-hydroxyapatite coatings prevent biofilm formation and improve bone formation in implant-associated infections. Bioact. Mater. 2022, 13, 44–56. [Google Scholar] [CrossRef]
- López-Álvarez, M.; López-Puente, V.; Rodríguez-Valencia, C.; Angelomé, P.C.; Liz-Marzán, L.M.; Serra, J.; Pastoriza-Santos, I.; González, P. Osteogenic effects of simvastatin-loaded mesoporous titania thin films. Biomed. Mater. 2018, 13, 025017. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Zhao, S.; He, F.; Liu, L.; Yang, G. Influence of simvastatin-loaded implants on osseointegration in an ovariectomized animal model. Biomed. Res. Int. 2015, 2015, 831504. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Chen, J.; Yan, F.; Xiao, Y. Effects of Simvastatin on bone healing around titanium implants in osteoporotic rats. Clin. Oral Implant. Res. 2009, 20, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Pauly, S.; Luttosch, F.; Morawski, M.; Haas, N.P.; Schmidmaier, G.; Wildemann, B. Simvastatin locally applied from a biodegradable coating of osteosynthetic implants improves fracture healing comparable to BMP-2 application. Bone 2009, 45, 505–511. [Google Scholar] [CrossRef]
- Rezk, A.I.; Kim, J.Y.; Kim, B.S.; Park, C.H.; Kim, C.S. De novo dual functional 3D scaffold using computational simulation with controlled drug release. J. Colloid Interface Sci. 2022, 625, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Liu, X.; Nawshad, A.; Marx, D.B.; Wang, D.; Reinhardt, R.A. Role of prostaglandin pathway and alendronate-based carriers to enhance statin-induced bone. Mol. Pharm. 2011, 8, 1035–1042. [Google Scholar] [CrossRef]
- Mukozawa, A.; Ueki, K.; Marukawa, K.; Okabe, K.; Moroi, A.; Nakagawa, K. Bone healing of critical-sized nasal defects in rabbits by statins in two different carriers. Clin. Oral Implant. Res. 2011, 22, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Fu, Y.C.; Jian, S.C.; Wang, Y.H.; Liu, P.L.; Ho, M.L.; Wang, C.K. Synthesis and characterization of cationic polymeric nanoparticles as simvastatin carriers for enhancing the osteogenesis of bone marrow mesenchymal stem cells. J. Colloid Interface Sci. 2014, 432, 190–199. [Google Scholar] [CrossRef]
- Xue, Y.; Wu, M.; Liu, Z.; Song, J.; Luo, S.; Li, H.; Li, Y.; Jin, L.; Guan, B.; Lin, M.; et al. In vitro and in vivo evaluation of chitosan scaffolds combined with simvastatin-loaded nanoparticles for guided bone regeneration. J. Mater. Sci. Mater. Med. 2019, 30, 47. [Google Scholar] [CrossRef]
- Ghadri, N.; Anderson, K.M.; Adatrow, P.; Stein, S.H.; Su, H.J.; Garcia-Godoy, F.; Karydis, A.; Bumgardner, J.D. Evaluation of Bone Regeneration of Simvastatin Loaded Chitosan Nanofiber Membranes in Rodent Calvarial Defects. J. Biomater. Nanobiotechnol. 2018, 9, 210–231. [Google Scholar] [CrossRef]
- Alsawah, G.M.; Al-Obaida, M.I.; Al-Madi, E.M. Effect of a Simvastatin-Impregnated Chitosan Scaffold on Cell Growth and Osteoblastic Differentiation. Appl. Sci. 2021, 11, 5346. [Google Scholar] [CrossRef]
- Delan, W.K.; Zakaria, M.; Elsaadany, B.; ElMeshad, A.N.; Mamdouh, W.; Fares, A.R. Formulation of simvastatin chitosan nanoparticles for controlled delivery in bone regeneration: Optimization using Box-Behnken design, stability and in vivo study. Int J. Pharm. 2020, 577, 119038. [Google Scholar] [CrossRef]
- Wong, R.W.; Rabie, A.B. Statin collagen grafts used to repair defects in the parietal bone of rabbits. Br. J. Oral Maxillofac. Surg. 2013, 41, 244–248. [Google Scholar] [CrossRef]
- Wong, R.W.; Rabie, A.B. Histologic and ultrastructural study on statin graft in rabbit skulls. J. Oral Maxillofac. Surg. 2005, 63, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Yu, Y.; Guo, X.; Jiang, Q.; Luo, Y. The possibility of healing alveolar bone defects with simvastatin thermosensitive gel: In vitro/in vivo evaluation. Drug Des. Devel. Ther. 2018, 12, 1997–2003. [Google Scholar] [CrossRef] [PubMed]
- Fukui, T.; Ii, M.; Shoji, T.; Matsumoto, T.; Mifune, Y.; Kawakami, Y.; Akimaru, H.; Kawamoto, A.; Kuroda, T.; Saito, T.; et al. The therapeutic effect of local administration of low-dose simvastatin-conjugated gelatin hydrogel for fracture healing. J. Bone Miner. Res. 2012, 27, 1118–1131. [Google Scholar] [CrossRef]
- Oka, S.; Matsumoto, T.; Kubo, S.; Matsushita, T.; Sasaki, H.; Nishizawa, Y.; Matsuzaki, T.; Saito, T.; Nishida, K.; Tabata, Y.; et al. Local administration of low-dose simvastatin-conjugated gelatin hydrogel for tendon-bone healing in anterior cruciate ligament reconstruction. Tissue Eng. Part A 2013, 19, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Sukul, M.; Min, Y.K.; Lee, S.Y.; Lee, B.T. Osteogenic potential of simvastatin loaded gelatin-nanofibrillar cellulose-β tricalcium phosphate hydrogel scaffold in critical-sized rat calvarial defect. Eur. Polym. J. 2015, 73, 308–323. [Google Scholar] [CrossRef]
- Terauchi, M.; Inada, T.; Tonegawa, A.; Tamura, A.; Yamaguchi, S.; Harada, K.; Yui, N. Supramolecular inclusion complexation of simvastatin withmethylated β-cyclodextrins for promoting osteogenic differentiation. Int. J. Biol. Macromol. 2016, 93, 1492–1498. [Google Scholar] [CrossRef]
- Thylin, M.R.; McConnell, J.C.; Schmid, M.J.; Reckling, R.R.; Ojha, J.; Bhattacharyya, I.; Marx, D.B.; Reinhardt, R.A. Effects of simvastatin gels on murine calvarial bone. J. Periodontol. 2002, 73, 1141–1148. [Google Scholar] [CrossRef]
- Yue, X.; Niu, M.; Zhang, T.; Wang, C.; Wang, Z.; Wu, W.; Zhang, Q.; Lai, C.; Zhou, L. In vivo evaluation of a simvastatin-loaded nanostructured lipid carrier for bone tissue regeneration. Nanotechnology 2016, 27, 115708. [Google Scholar] [CrossRef]
- Pişkin, E.; Işoğlu, I.A.; Bölgen, N.; Vargel, I.; Griffiths, S.; Cavuşoğlu, T.; Korkusuz, P.; Güzel, E.; Cartmell, S. In vivo performance of simvastatin-loaded electrospun spiral-wound polycaprolactone scaffolds in reconstruction of cranial bone defects in the rat model. J. Biomed. Mater. Res. A 2009, 90, 1137–1151. [Google Scholar] [CrossRef]
- Saberi, A.; Kouhjani, M.; Mohammadi, M.; Hosta-Rigau, L. Novel scaffold platforms for simultaneous induction osteogenesis and angiogenesis in bone tissue engineering: A cutting-edge approach. J. Nanobiotechnol. 2023, 21, 351. [Google Scholar] [CrossRef]
- Yu, D.; Huang, C.; Jiang, C.; Zhu, H. Features of a simvastatin-loaded multi-layered co-electrospun barrier membrane for guided bone regeneration. Exp. Ther. Med. 2021, 22, 713. [Google Scholar] [CrossRef]
- Rezk, A.I.; Bhattarai, D.P.; Park, J.; Park, C.H.; Kim, C.S. Polyaniline-coated titanium oxide nanoparticles and simvastatin-loaded poly(ε-caprolactone) composite nanofibers scaffold for bone tissue regeneration application. Colloids Surf. B Biointerfaces 2020, 192, 111007. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Zhang, X.; Gao, P.; Xia, X.; Xiao, S.; Wen, J.; Guo, T.; Yang, W.; Li, J. Strontium and simvastatin dual loaded hydroxyapatite microsphere reinforced poly(ε-caprolactone) scaffolds promote vascularized bone regeneration. J. Mater. Chem. B 2023, 11, 1115–1130. [Google Scholar] [CrossRef] [PubMed]
- Rezk, A.I.; Mousa, H.M.; Lee, J.; Park, C.H.; Kim, C.S. Composite PCL/HA/simvastatin electrospun nanofiber coating on biodegradable Mg alloy for orthopedic implant application. J. Coat. Technol. Res. 2019, 16, 477–489. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Zhou, L.; Li, S.; Sun, J.; Wang, Z.; Gao, Y.; Jiang, Y.; Lu, H.; Wang, Q.; et al. Effects of simvastatin-loaded polymeric micelles on human osteoblast-like MG-63 cells. Colloids Surf. B Biointerfaces 2013, 102, 420–427. [Google Scholar] [CrossRef]
- Feng, X.; Yue, X.; Niu, M. Simvastatin-Loaded Nanomicelles Enhance the Osteogenic Effect of Simvastatin. J. Nanomaterials. 2020, 2020, 1072765. [Google Scholar] [CrossRef]
- Lin, C.W.; Lee, C.Y.; Lin, S.Y.; Kang, L.; Fu, Y.C.; Chen, C.H.; Wang, C.K. Bone-Targeting Nanoparticles of a Dendritic (Aspartic acid)3-Functionalized PEG-PLGA Biopolymer Encapsulating Simvastatin for the Treatment of Osteoporosis in Rat Models. Int. J. Mol. Sci. 2022, 23, 10530. [Google Scholar] [CrossRef]
- Zhang, P.; Han, F.; Li, Y.; Chen, J.; Chen, T.; Zhi, Y.; Jiang, J.; Lin, C.; Chen, S.; Zhao, P. Local delivery of controlled-release simvastatin to improve the biocompatibility of polyethylene terephthalate artificial ligaments for reconstruction of the anterior cruciate ligament. Int. J. Nanomed. 2016, 11, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Yueyi, C.; Xiaoguang, H.; Jingying, W.; Quansheng, S.; Jie, T.; Xin, F.; Yingsheng, X.; Chunli, S. Calvarial defect healing by recruitment of autogenous osteogenic stem cells using locally applied simvastatin. Biomaterials 2013, 34, 9373–9380. [Google Scholar] [CrossRef]
- Whang, K.; Grageda, E.; Khan, A.; McDonald, J.; Lawton, M.; Satsangi, N. A novel osteotropic biomaterial OG-PLG: In vitro efficacy. J. Biomed. Mater. Res. A 2005, 74, 247–253. [Google Scholar] [CrossRef]
- Gentile, P.; Nandagiri, V.K.; Daly, J.; Chiono, V.; Mattu, C.; Tonda-Turo, C.; Ciardelli, G.; Ramtoola, Z. Localised controlled release of simvastatin from porous chitosan-gelatin scaffolds engrafted with simvastatin loaded PLGA-microparticles for bone tissue engineering application. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 249–257. [Google Scholar] [CrossRef]
- Littuma, G.J.S.; Sordi, M.B.; Borges Curtarelli, R.; Aragonês, Á.; da Cruz, A.C.C.; Magini, R.S. Titanium coated with poly(lactic-co-glycolic) acid incorporating simvastatin: Biofunctionalization of dental prosthetic abutments. J. Periodontal. Res. 2020, 55, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Nagpal, M.; Grewal, A.K.; Chauhan, S.; Dora, C.P.; Singh, T.G. Molecular Complex of HSIM-loaded Polymeric Nanoparticles: Potential Carriers in Osteoporosis. Curr. Drug Targets 2023, 24, 1066–1078. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.D.; Son, S.; Sadiasa, A.; Min, Y.K.; Lee, B.T. Preparation and characterization of PLGA microspheres by the electrospraying method for delivering simvastatin for bone regeneration. Int. J. Pharm. 2013, 443, 87–94. [Google Scholar] [CrossRef]
- Nath, S.D.; Linh, N.T.; Sadiasa, A.; Lee, B.T. Encapsulation of simvastatin in PLGA microspheres loaded into hydrogel loaded BCP porous spongy scaffold as a controlled drug delivery system for bone tissue regeneration. J. Biomater. Appl. 2014, 28, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.C.; Wang, Y.H.; Chen, C.H.; Wang, C.K.; Wang, G.J.; Ho, M.L. Combination of calcium sulfate and simvastatin-controlled release microspheres enhances bone repair in critical-sized rat calvarial bone defects. Int. J. Nanomed. 2015, 10, 7231–7240. [Google Scholar]
- Yan, M.; Ni, J.; Shen, H.; Song, D.; Ding, M.; Huang, J. Local controlled release of simvastatin and PDGF from core/shell microspheres promotes bone regeneration in vivo. RSC Adv. 2017, 7, 19621–19629. [Google Scholar] [CrossRef]
- von Knoch, F.; Wedemeyer, C.; Heckelei, A.; Saxler, G.; Hilken, G.; Brankamp, J.; Sterner, T.; Landgraeber, S.; Henschke, F.; Löer, F.; et al. Promotion of bone formation by simvastatin in polyethylene particle-induced osteolysis. Biomaterials 2005, 26, 5783–5789. [Google Scholar] [CrossRef]
- Venkatesan, N.; Liyanage, A.D.T.; Castro-Núñez, J.; Asafo-Adjei, T.; Cunningham, L.L.; Dziubla, T.D.; Puleo, D.A. Biodegradable polymerized simvastatin stimulates bone formation. Acta Biomater. 2019, 93, 192–199. [Google Scholar] [CrossRef]
- Wu, T.; Sun, J.; Tan, L.; Yan, Q.; Li, L.; Chen, L.; Liu, X.; Bin, S. Enhanced osteogenesis and therapy of osteoporosis using simvastatin loaded hybrid system. Bioact. Mater. 2020, 5, 348–357. [Google Scholar] [CrossRef]
- Hajializade, M.; Moghtadaei, M.; Mirzaei, A.; Abdollahi Kordkandi, S.; Babaheidarian, P.; Pazoki-Toroudi, H.; Yeganeh, A. Significant effect of simvastatin and/or ezetimibe-loaded nanofibers on the healing of femoral defect: An experimental study. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110861. [Google Scholar] [CrossRef] [PubMed]
- Wadagaki, R. Osteogenic induction of bone marrow-derived stromal cells on simvastatin-releasing, biodegradable, nano-to microscale fiber scaffolds. Ann. Biomed. Eng. 2011, 39, 1872–1881. [Google Scholar] [CrossRef]
- Harsha, G.; Madhavi, S.; Arthi, S.; Haritha, S. Evaluation of efficacy of simvastatin in bone regeneration following local application in third molar extraction socket: A randomized control trial. Natl. J. Maxillofac Surg. 2023, 14, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.S.; Yang, D.H.; Lee, J.B.; Heo, D.N.; Kwon, Y.D.; Youn, I.C.; Choi, K.; Hong, J.H.; Kim, G.T.; Choi, Y.S.; et al. Photo-cured hyaluronic acid-based hydrogels containing simvastatin as a bone tissue regeneration scaffold. Biomaterials 2011, 32, 8161–8171. [Google Scholar] [CrossRef]
- Zhou, Y.; Ni, Y.; Liu, Y.; Zeng, B.; Xu, Y.; Ge, W. The role of simvastatin in the osteogenesis of injectable tissue-engineered bone based on human adipose-derived stromal cells and platelet-rich plasma. Biomaterials 2010, 31, 5325–5335. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granat, M.M.; Eifler-Zydel, J.; Kolmas, J. Statins—Their Role in Bone Tissue Metabolism and Local Applications with Different Carriers. Int. J. Mol. Sci. 2024, 25, 2378. https://doi.org/10.3390/ijms25042378
Granat MM, Eifler-Zydel J, Kolmas J. Statins—Their Role in Bone Tissue Metabolism and Local Applications with Different Carriers. International Journal of Molecular Sciences. 2024; 25(4):2378. https://doi.org/10.3390/ijms25042378
Chicago/Turabian StyleGranat, Marcin Mateusz, Joanna Eifler-Zydel, and Joanna Kolmas. 2024. "Statins—Their Role in Bone Tissue Metabolism and Local Applications with Different Carriers" International Journal of Molecular Sciences 25, no. 4: 2378. https://doi.org/10.3390/ijms25042378
APA StyleGranat, M. M., Eifler-Zydel, J., & Kolmas, J. (2024). Statins—Their Role in Bone Tissue Metabolism and Local Applications with Different Carriers. International Journal of Molecular Sciences, 25(4), 2378. https://doi.org/10.3390/ijms25042378







