Genetics and beyond: Precision Medicine Real-World Data for Patients with Cervical, Vaginal or Vulvar Cancer in a Tertiary Cancer Center
Abstract
1. Introduction
2. Results
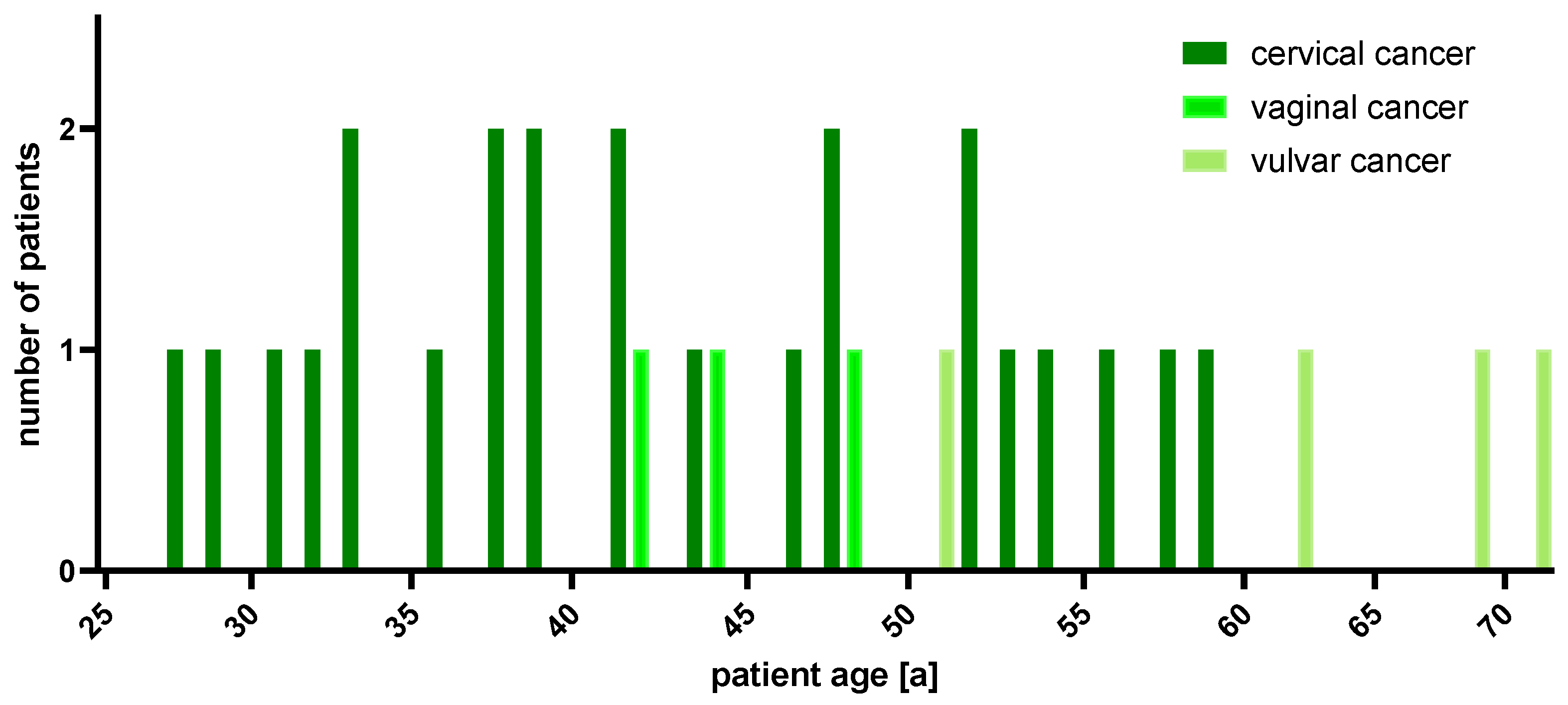
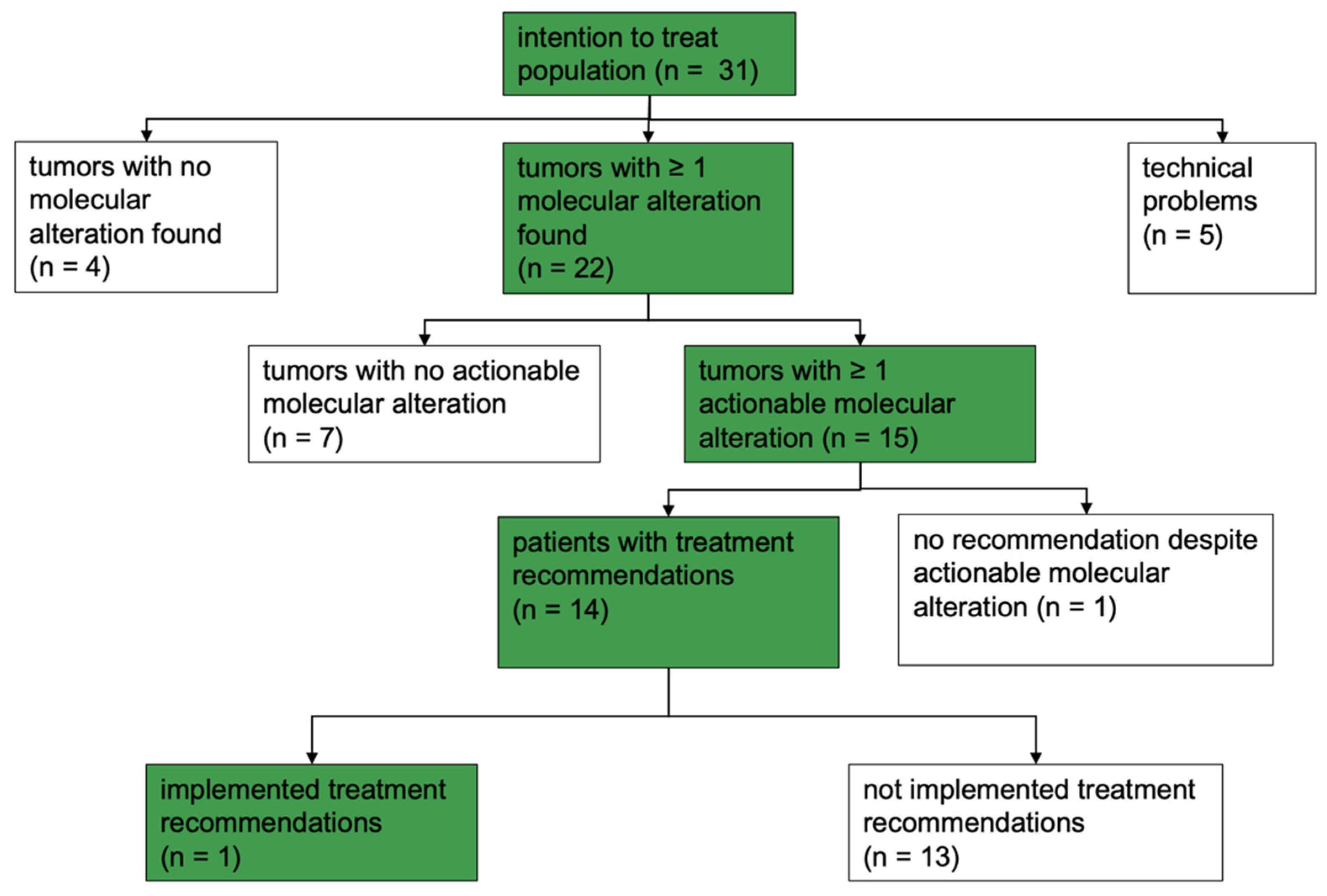
2.1. Patient Cohort
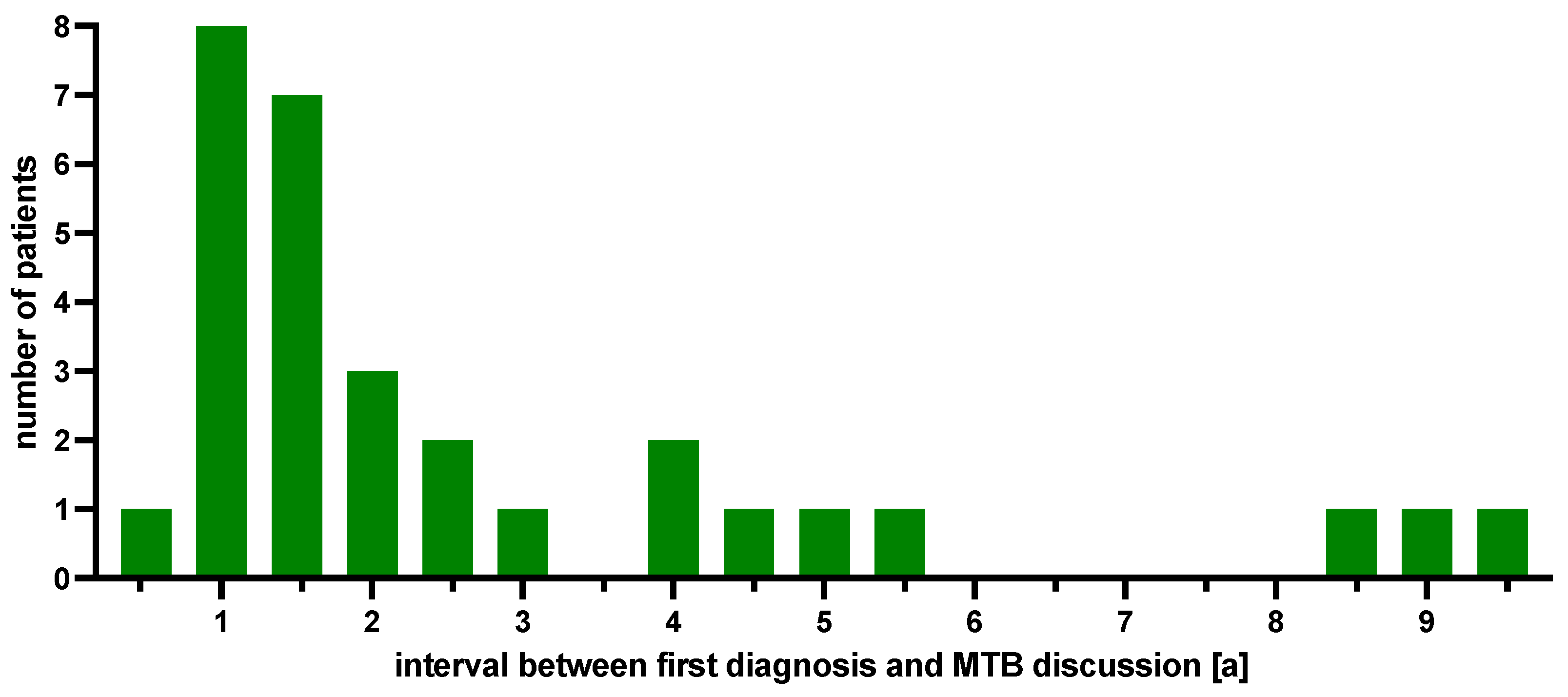
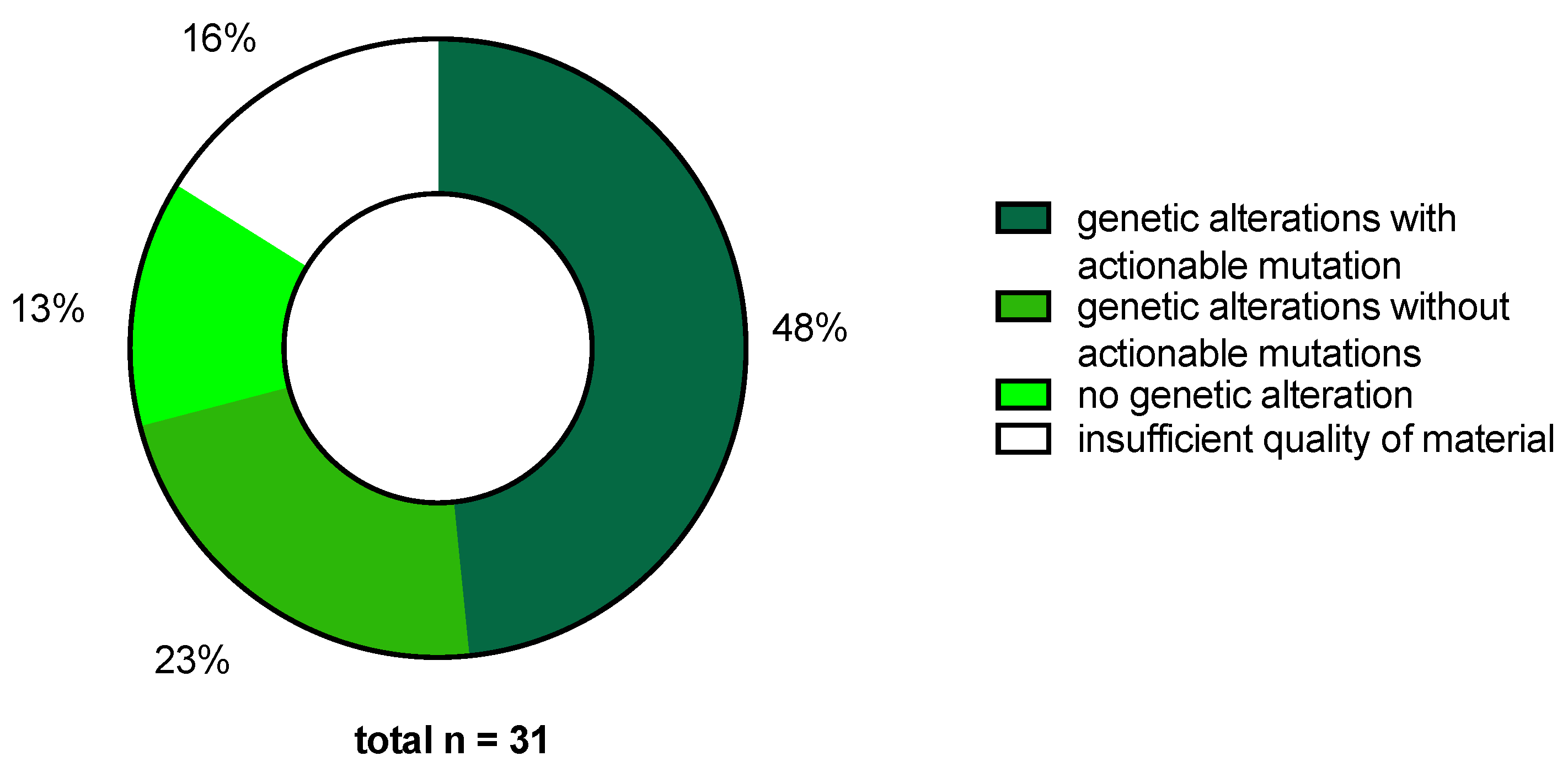
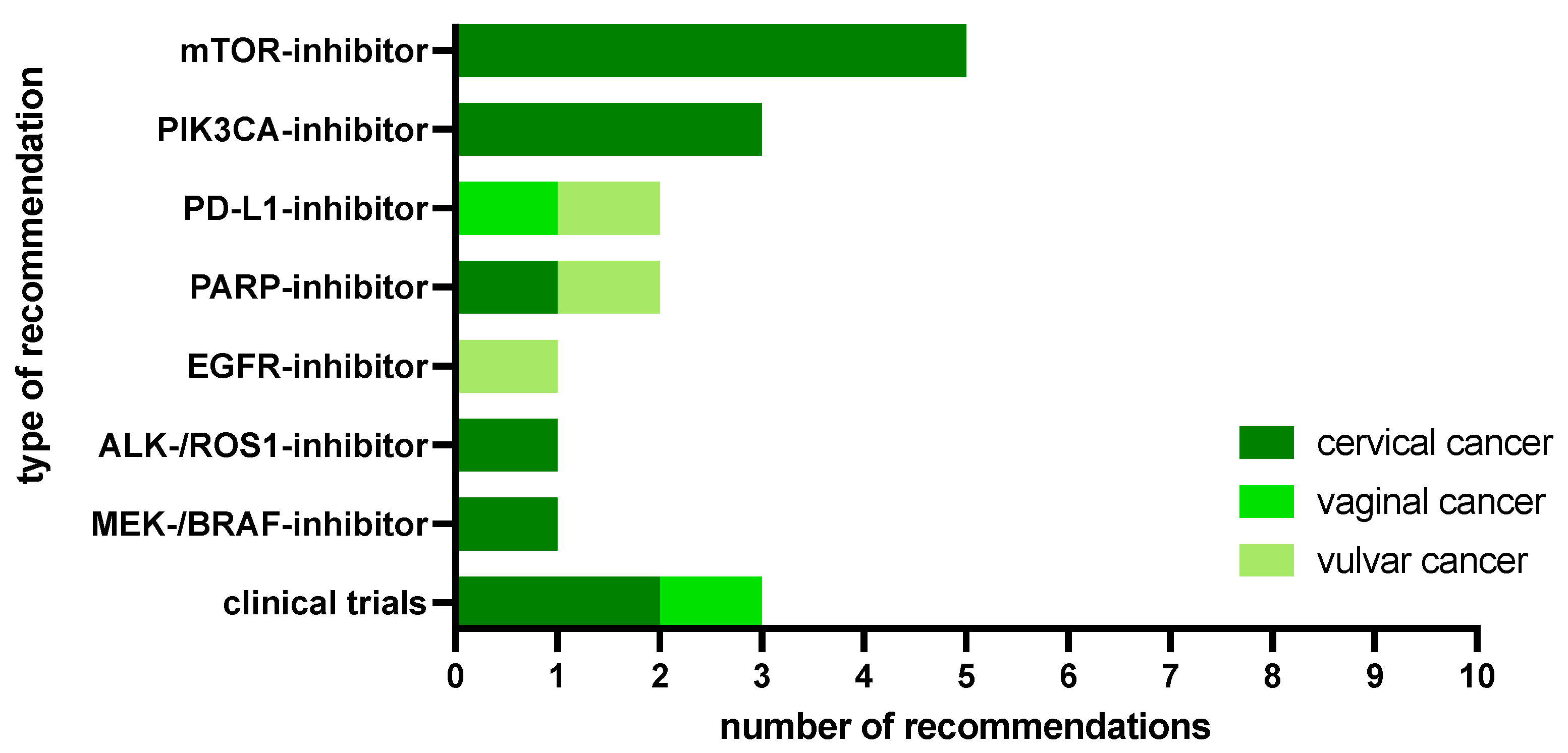
2.2. MTB Recommendations
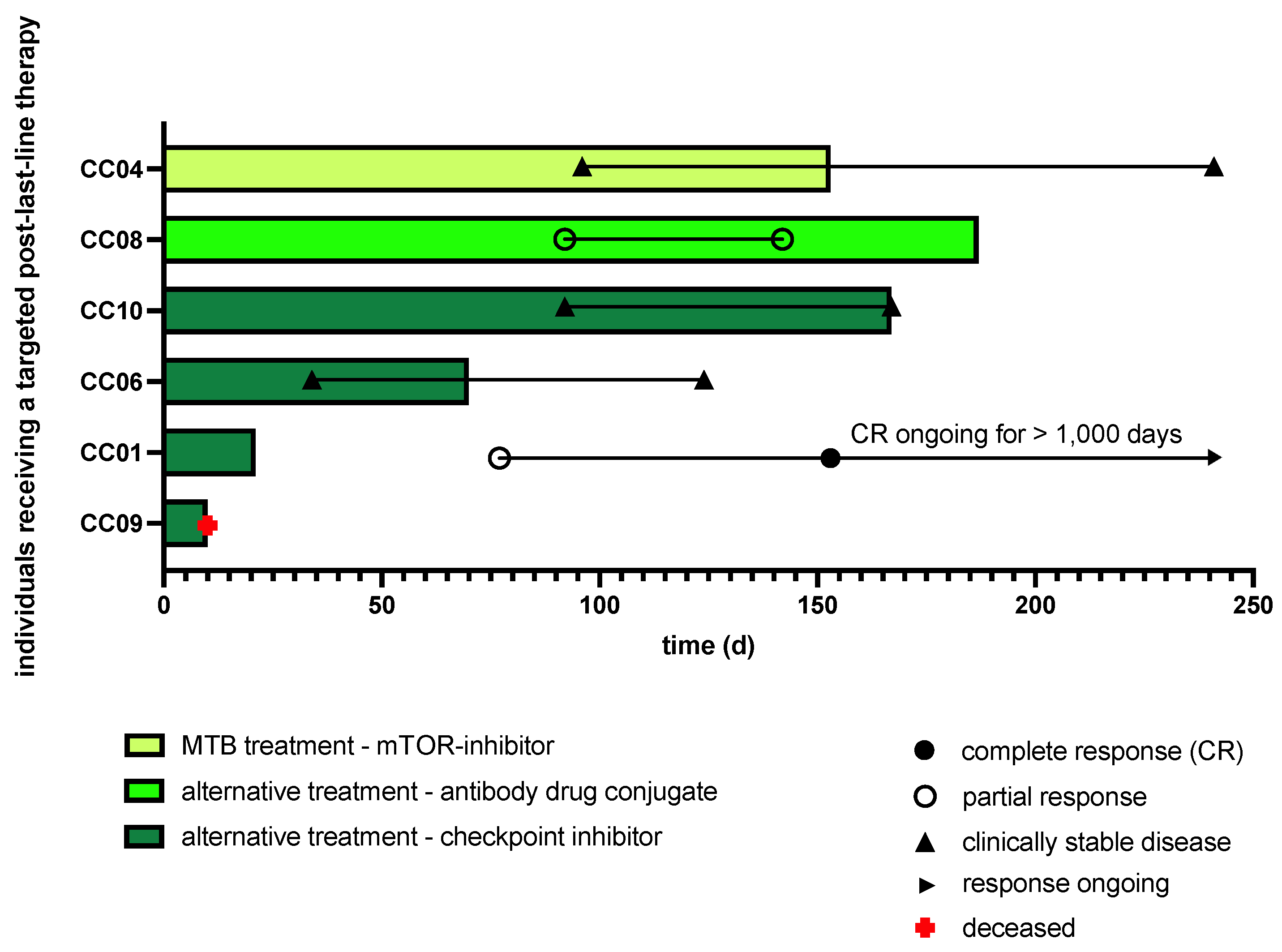
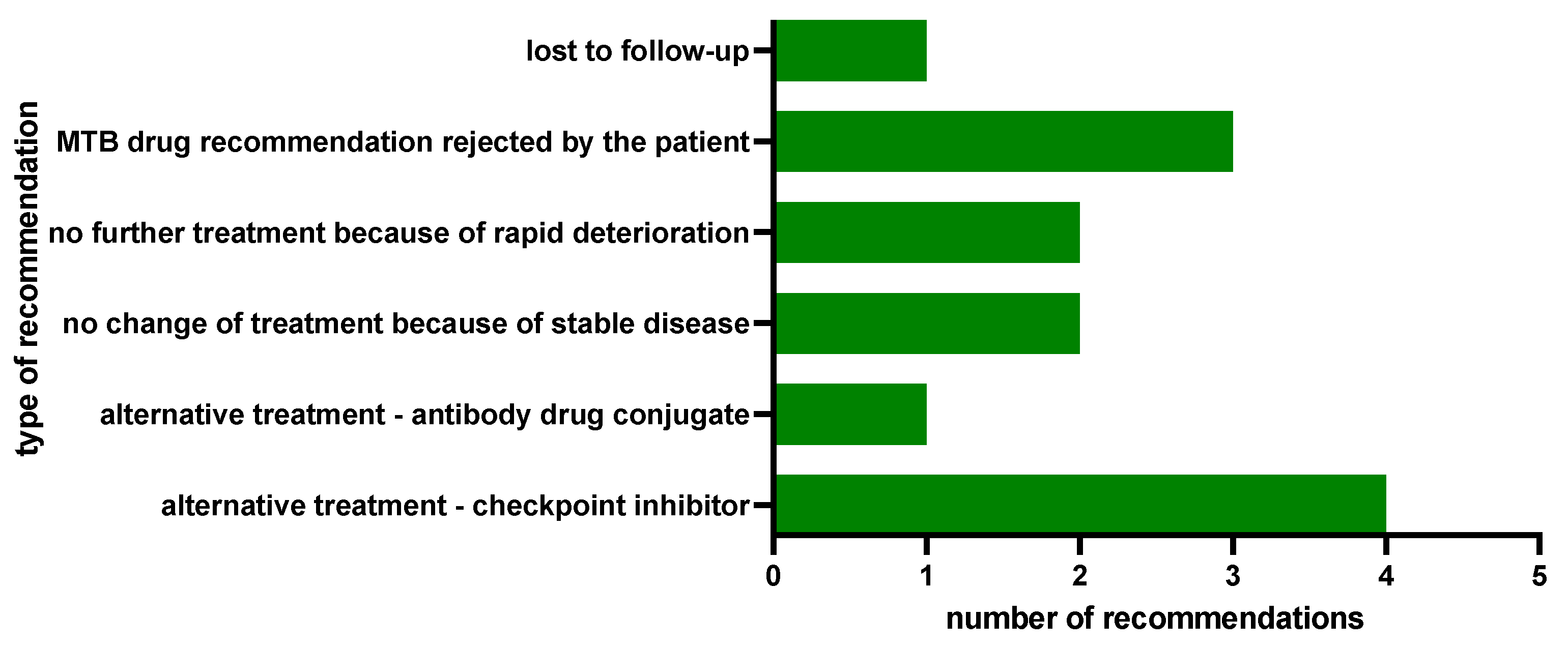
2.3. Clinical Implementation of MTB Drug Recommendations
3. Discussion
4. Materials and Methods
4.1. Precision Oncology Program
4.2. Tissue and Sequencing Results
4.3. Molecular-Genetic Analysis
4.4. Study Procedure
4.5. Software
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bruni, L.A.G.; Serrano, B.; Mena, M.; Collado, J.J.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). In Human Papillomavirus and Related Diseases in the World; Summary Report 10 March 2023; ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre): Barcelona, Spain, 2023. [Google Scholar]
- Mann, M.; Singh, V.P.; Kumar, L. Cervical cancer: A tale from HPV infection to PARP inhibitors. Genes Dis. 2023, 10, 1445–1456. [Google Scholar] [CrossRef]
- Balasubramaniam, S.D.; Balakrishnan, V.; Oon, C.E.; Kaur, G. Key Molecular Events in Cervical Cancer Development. Medicina 2019, 55, 384. [Google Scholar] [CrossRef]
- He, S.; Wang, A.; Wang, J.; Tang, Z.; Wang, X.; Wang, D.; Chen, J.; Liu, C.; Zhao, M.; Chen, H.; et al. Human papillomavirus E7 protein induces homologous recombination defects and PARPi sensitivity. J. Cancer Res. Clin. Oncol. 2024, 150, 27. [Google Scholar] [CrossRef]
- Chung, T.K.; Cheung, T.H.; Wang, V.W.; Yu, M.Y.; Wong, Y.F. Microsatellite instability, expression of hMSH2 and hMLH1 and HPV infection in cervical cancer and their clinico-pathological association. Gynecol. Obs. Investig. 2001, 52, 98–103. [Google Scholar] [CrossRef]
- Richart, R.M. Natural history of cervical intraepithelial neoplasia. Clin. Obstet. Gynecol. 1967, 10, 748–784. [Google Scholar] [CrossRef]
- Preti, M.; Igidbashian, S.; Costa, S.; Cristoforoni, P.; Mariani, L.; Origoni, M.; Sandri, M.T.; Boveri, S.; Spolti, N.; Spinaci, L.; et al. VIN usual type-from the past to the future. Ecancermedicalscience 2015, 9, 531. [Google Scholar] [CrossRef] [PubMed]
- Ao, M.; Zheng, D.; Wang, J.; Gu, X.; Xi, M. A retrospective study of cytology and HPV genotypes results of 3229 vaginal intraepithelial neoplasia patients. J. Med. Virol. 2022, 94, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Backes, D.M.; Hoots, B.E.; Kurman, R.J.; Pimenta, J.M. Human papillomavirus type-distribution in vulvar and vaginal cancers and their associated precursors. Obs. Gynecol. 2009, 113, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.M.; Cristea, M.; DeRosa, M.; Eisenhauer, E.L.; et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 191–226. [Google Scholar] [CrossRef] [PubMed]
- Borella, F.; Preti, M.; Bertero, L.; Collemi, G.; Castellano, I.; Cassoni, P.; Cosma, S.; Carosso, A.R.; Bevilacqua, F.; Gallio, N.; et al. Is There a Place for Immune Checkpoint Inhibitors in Vulvar Neoplasms? A State of the Art Review. Int. J. Mol. Sci. 2020, 22, 190. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.F.; Mardis, E.R. The emerging clinical relevance of genomics in cancer medicine. Nat. Rev. Clin. Oncol. 2018, 15, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, J. Personalizing oncology: Perspectives and prospects. J. Clin. Oncol. 2013, 31, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.S.; Sill, M.W.; Long, H.J., 3rd; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; et al. Improved survival with bevacizumab in advanced cervical cancer. N. Engl. J. Med. 2014, 370, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Hoyos Usta, E.; Yanez, E.; et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Reusser, N.M.; Downing, C.; Guidry, J.; Tyring, S.K. HPV Carcinomas in Immunocompromised Patients. J. Clin. Med. 2015, 4, 260–281. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chang, W.W.; Xia, X. Immune checkpoint inhibitors in advanced and recurrent/metastatic cervical cancer. Front. Oncol. 2022, 12, 996495. [Google Scholar] [CrossRef] [PubMed]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef]
- Oaknin, A.; Gladieff, L.; Martínez-García, J.; Villacampa, G.; Takekuma, M.; De Giorgi, U.; Lindemann, K.; Woelber, L.; Colombo, N.; Duska, L.; et al. Atezolizumab plus bevacizumab and chemotherapy for metastatic, persistent, or recurrent cervical cancer (BEATcc): A randomised, open-label, phase 3 trial. Lancet 2023, 403, 31–43. [Google Scholar] [CrossRef]
- Coleman, R.L.; Lorusso, D.; Gennigens, C.; Gonzalez-Martin, A.; Randall, L.; Cibula, D.; Lund, B.; Woelber, L.; Pignata, S.; Forget, F.; et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021, 22, 609–619. [Google Scholar] [CrossRef]
- Vergote, I.B.; Gonzalez Martin, A.; Fujiwara, K.; Kalbacher, E.; Bagameri, A.; Ghamande, S.; Lee, J.Y.; Banerjee, S.; Maluf, F.C.; Lorusso, D.; et al. LBA9 innovaTV 301/ENGOT-cx12/GOG-3057: A global, randomized, open-label, phase III study of tisotumab vedotin vs investigator’s choice of chemotherapy in 2L or 3L recurrent or metastatic cervical cancer. Ann. Oncol. 2023, 34, S1276–S1277. [Google Scholar] [CrossRef]
- Kraus, F.B.T.; Topalov, N.E.; Deuster, E.; Hysenaj, I.; Mayr, D.; Chelariu-Raicu, A.; Beyer, S.; Kolben, T.; Burges, A.; Mahner, S.; et al. Expression pattern and prognostic potential of histamine receptors in epithelial ovarian cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 2501–2511. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research, N.; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Schwaederle, M.; Zhao, M.; Lee, J.J.; Eggermont, A.M.; Schilsky, R.L.; Mendelsohn, J.; Lazar, V.; Kurzrock, R. Impact of Precision Medicine in Diverse Cancers: A Meta-Analysis of Phase II Clinical Trials. J. Clin. Oncol. 2015, 33, 3817–3825. [Google Scholar] [CrossRef] [PubMed]
- Massard, C.; Michiels, S.; Ferte, C.; Le Deley, M.C.; Lacroix, L.; Hollebecque, A.; Verlingue, L.; Ileana, E.; Rosellini, S.; Ammari, S.; et al. High-Throughput Genomics and Clinical Outcome in Hard-to-Treat Advanced Cancers: Results of the MOSCATO 01 Trial. Cancer Discov. 2017, 7, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, J.D.; Meric-Bernstam, F.; Swanton, C.; Hurwitz, H.; Spigel, D.R.; Sweeney, C.; Burris, H.; Bose, R.; Yoo, B.; Stein, A.; et al. Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results From MyPathway, an Open-Label, Phase IIa Multiple Basket Study. J. Clin. Oncol. 2018, 36, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, C.; Delord, J.P.; Goncalves, A.; Gavoille, C.; Dubot, C.; Isambert, N.; Campone, M.; Tredan, O.; Massiani, M.A.; Mauborgne, C.; et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015, 16, 1324–1334. [Google Scholar] [CrossRef]
- Aust, S.; Schwameis, R.; Gagic, T.; Mullauer, L.; Langthaler, E.; Prager, G.; Grech, C.; Reinthaller, A.; Krainer, M.; Pils, D.; et al. Precision Medicine Tumor Boards: Clinical Applicability of Personalized Treatment Concepts in Ovarian Cancer. Cancers 2020, 12, 548. [Google Scholar] [CrossRef]
- Gunderson, C.C.; Rowland, M.R.; Wright, D.L.; Andrade, K.L.; Mannel, R.S.; McMeekin, D.S.; Moore, K.N. Initiation of a formalized precision medicine program in gynecologic oncology. Gynecol. Oncol. 2016, 141, 24–28. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, L.; Hirshfield, K.M.; Rojas, V.; DiPaola, R.S.; Gibbon, D.; Hellmann, M.; Isani, S.; Leiser, A.; Riedlinger, G.M.; Wagreich, A.; et al. Use of comprehensive genomic profiling to direct point-of-care management of patients with gynecologic cancers. Gynecol. Oncol. 2016, 141, 2–9. [Google Scholar] [CrossRef]
- Taghizadeh, H.; Mader, R.M.; Mullauer, L.; Aust, S.; Polterauer, S.; Kolbl, H.; Seebacher, V.; Grimm, C.; Reinthaller, A.; Prager, G.W. Molecular Guided Treatments in Gynecologic Oncology: Analysis of a Real-World Precision Cancer Medicine Platform. Oncologist 2020, 25, e1060–e1069. [Google Scholar] [CrossRef]
- Prieto-Potin, I.; Idrovo, F.; Suarez-Gauthier, A.; Diaz-Blazquez, M.; Astilleros-Blanco de Cordova, L.; Chamizo, C.; Zazo, S.; Carvajal, N.; Lopez-Sanchez, A.; Perez-Buira, S.; et al. Comprehensive Approach to Genomic and Immune Profiling: Insights of a Real-World Experience in Gynecological Tumors. Diagnostics 2022, 12, 1903. [Google Scholar] [CrossRef]
- Heinrich, K.; Miller-Phillips, L.; Ziemann, F.; Hasselmann, K.; Ruhlmann, K.; Flach, M.; Biro, D.; von Bergwelt-Baildon, M.; Holch, J.; Herold, T.; et al. Lessons learned: The first consecutive 1000 patients of the CCCMunich(LMU) Molecular Tumor Board. J. Cancer Res. Clin. Oncol. 2023, 149, 1905–1915. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Gray, R.J.; Chen, A.P.; Li, S.; McShane, L.M.; Patton, D.; Hamilton, S.R.; Williams, P.M.; Iafrate, A.J.; Sklar, J.; et al. Molecular Landscape and Actionable Alterations in a Genomically Guided Cancer Clinical Trial: National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH). J. Clin. Oncol. 2020, 38, 3883–3894. [Google Scholar] [CrossRef]
- Maruthi, V.K.; Khazaeli, M.; Jeyachandran, D.; Desouki, M.M. The Clinical Utility and Impact of Next Generation Sequencing in Gynecologic Cancers. Cancers 2022, 14, 1352. [Google Scholar] [CrossRef] [PubMed]
- Tannock, I.F.; Hickman, J.A. Molecular screening to select therapy for advanced cancer? Ann. Oncol. 2019, 30, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, T.S.; Singh, N.; Walker, T.D.J.; Newton, C.; Evans, D.G.R.; Crosbie, E.J.; Ryan, N.A.J. Immune checkpoint inhibitors efficacy across solid cancers and the utility of PD-L1 as a biomarker of response: A systematic review and meta-analysis. Front. Med. 2023, 10, 1192762. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.; Rodrigues, B.; Giudice, F.; Baiocchi, G.; Soares, F.A.; Martins, V.; Rocha, R.M. Abstract 2662: A dual PI3K/mTOR inhibitor as potential therapeutic option for vulvar cancer. Cancer Res. 2015, 75 (Suppl. S15), 2662. [Google Scholar] [CrossRef]
- de Melo, A.C.; Grazziotin-Reisner, R.; Erlich, F.; Fontes Dias, M.S.; Moralez, G.; Carneiro, M.; Ingles Garces, A.H.; Guerra Alves, F.V.; Novaes Neto, B.; Fuchshuber-Moraes, M.; et al. A phase I study of mTOR inhibitor everolimus in association with cisplatin and radiotherapy for the treatment of locally advanced cervix cancer: PHOENIX I. Cancer Chemother. Pharmacol. 2016, 78, 101–109. [Google Scholar] [CrossRef]
- Gross, M.; Spencer, R.J. Recurrent Cervical Cancer Treated Successfully with Single-Agent PARP-Inhibitor, Olaparib. Case Rep. Obs. Gynecol. 2022, 2022, 6579715. [Google Scholar] [CrossRef]
- Crafton, S.M.; Bixel, K.; Hays, J.L. PARP inhibition and gynecologic malignancies: A review of current literature and on-going trials. Gynecol. Oncol. 2016, 142, 588–596. [Google Scholar] [CrossRef]
- Venkatesan, S.; Swanton, C.; Taylor, B.S.; Costello, J.F. Treatment-Induced Mutagenesis and Selective Pressures Sculpt Cancer Evolution. Cold Spring Harb. Perspect. Med. 2017, 7, a026617. [Google Scholar] [CrossRef]
- Tan, S.H.; Sapari, N.S.; Miao, H.; Hartman, M.; Loh, M.; Chng, W.J.; Iau, P.; Buhari, S.A.; Soong, R.; Lee, S.C. High-Throughput Mutation Profiling Changes before and 3 Weeks after Chemotherapy in Newly Diagnosed Breast Cancer Patients. PLoS ONE 2015, 10, e0142466. [Google Scholar] [CrossRef]
- Sakamoto, H.; Attiyeh, M.A.; Gerold, J.M.; Makohon-Moore, A.P.; Hayashi, A.; Hong, J.; Kappagantula, R.; Zhang, L.; Melchor, J.P.; Reiter, J.G.; et al. The Evolutionary Origins of Recurrent Pancreatic Cancer. Cancer Discov. 2020, 10, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Levy, D. Modeling the Dynamics of Heterogeneity of Solid Tumors in Response to Chemotherapy. Bull. Math. Biol. 2017, 79, 2986–3012. [Google Scholar] [CrossRef] [PubMed]
- Maynard, A.; McCoach, C.E.; Rotow, J.K.; Harris, L.; Haderk, F.; Kerr, D.L.; Yu, E.A.; Schenk, E.L.; Tan, W.; Zee, A.; et al. Therapy-Induced Evolution of Human Lung Cancer Revealed by Single-Cell RNA Sequencing. Cell 2020, 182, 1232–1251.e1222. [Google Scholar] [CrossRef] [PubMed]
- França, G.S.; Baron, M.; Pour, M.; King, B.R.; Rao, A.; Misirlioglu, S.; Barkley, D.; Dolgalev, I.; Ho-Tang, K.; Avital, G.; et al. Drug-induced adaptation along a resistance continuum in cancer cells. bioRxiv 2022. [Google Scholar] [CrossRef]
- Hoogstrate, Y.; Draaisma, K.; Ghisai, S.A.; van Hijfte, L.; Barin, N.; de Heer, I.; Coppieters, W.; van den Bosch, T.P.P.; Bolleboom, A.; Gao, Z.; et al. Transcriptome analysis reveals tumor microenvironment changes in glioblastoma. Cancer Cell 2023, 41, 678–692.e677. [Google Scholar] [CrossRef]
- Challagundla, K.B.; Wise, P.M.; Neviani, P.; Chava, H.; Murtadha, M.; Xu, T.; Kennedy, R.; Ivan, C.; Zhang, X.; Vannini, I.; et al. Exosome-Mediated Transfer of microRNAs Within the Tumor Microenvironment and Neuroblastoma Resistance to Chemotherapy. JNCI J. Natl. Cancer Inst. 2015, 107, djv135. [Google Scholar] [CrossRef]
- Casolino, R.; Beer, P.A.; Chakravarty, D.; Davis, M.B.; Malapelle, U.; Mazzarella, L.; Normanno, N.; Pauli, C.; Subbiah, V.; Turnbull, C.; et al. Interpreting and integrating genomic tests results in clinical cancer care: Overview and practical guidance. CA Cancer J. Clin. 2024, 1–22. [Google Scholar] [CrossRef]
- Crimini, E.; Repetto, M.; Tarantino, P.; Ascione, L.; Antonarelli, G.; Rocco, E.G.; Barberis, M.; Mazzarella, L.; Curigliano, G. Challenges and Obstacles in Applying Therapeutical Indications Formulated in Molecular Tumor Boards. Cancers 2022, 14, 3193. [Google Scholar] [CrossRef] [PubMed]
- Sultova, E.; Westphalen, C.B.; Jung, A.; Kumbrink, J.; Kirchner, T.; Mayr, D.; Rudelius, M.; Ormanns, S.; Heinemann, V.; Metzeler, K.H.; et al. Implementation of Precision Oncology for Patients with Metastatic Breast Cancer in an Interdisciplinary MTB Setting. Diagnostics 2021, 11, 733. [Google Scholar] [CrossRef]
- Hirshfield, K.M.; Tolkunov, D.; Zhong, H.; Ali, S.M.; Stein, M.N.; Murphy, S.; Vig, H.; Vazquez, A.; Glod, J.; Moss, R.A.; et al. Clinical Actionability of Comprehensive Genomic Profiling for Management of Rare or Refractory Cancers. Oncologist 2016, 21, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Arend, R.C.; Goel, N.; Roane, B.M.; Foxall, M.E.; Dholakia, J.; Londono, A.I.; Wall, J.A.; Leath, C.A., 3rd; Huh, W.K. Systematic Next Generation Sequencing is feasible in clinical practice and identifies opportunities for targeted therapy in women with uterine cancer: Results from a prospective cohort study. Gynecol. Oncol. 2021, 163, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.C.; Guinigundo, A.S. The Role of Biomarkers in Guiding Clinical Decision-Making in Oncology. J. Adv. Pract. Oncol. 2023, 14 (Suppl. S1), 15–37. [Google Scholar] [CrossRef] [PubMed]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid biopsy: Current technology and clinical applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef]
- Pascual, J.; Attard, G.; Bidard, F.C.; Curigliano, G.; De Mattos-Arruda, L.; Diehn, M.; Italiano, A.; Lindberg, J.; Merker, J.D.; Montagut, C.; et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2022, 33, 750–768. [Google Scholar] [CrossRef]
- Fu, Y.; Li, J.; Feng, M.X.; Yang, X.M.; Wang, Y.H.; Zhang, Y.L.; Qin, W.; Xia, Q.; Zhang, Z.G. Cytohesin-3 is upregulated in hepatocellular carcinoma and contributes to tumor growth and vascular invasion. Int. J. Clin. Exp. Pathol. 2014, 7, 2123–2132. [Google Scholar]
- Mateo, J.; Chakravarty, D.; Dienstmann, R.; Jezdic, S.; Gonzalez-Perez, A.; Lopez-Bigas, N.; Ng, C.K.Y.; Bedard, P.L.; Tortora, G.; Douillard, J.Y.; et al. A framework to rank genomic alterations as targets for cancer precision medicine: The ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann. Oncol. 2018, 29, 1895–1902. [Google Scholar] [CrossRef]
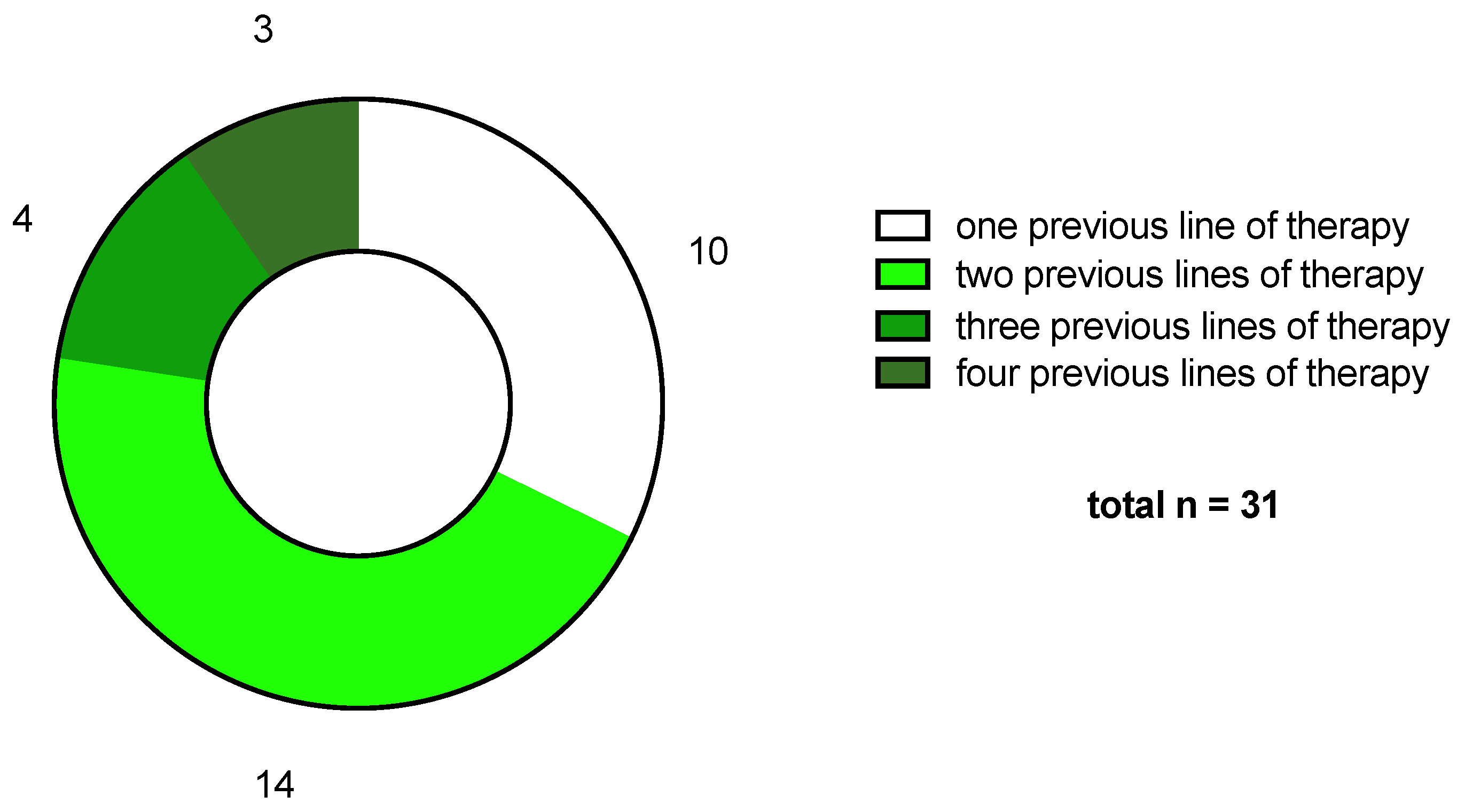


| Assay/Panel | DNA Alterations (Gene Number) | RNA/Gene Fusions (Gene Number) | CNV (Gene Number) | TMB | MSI | Sequencing Technology/Provider | Cases (N) |
|---|---|---|---|---|---|---|---|
| Oncomine Focus | 52 | 23 | 19 | - | - | IonTorrent, Thermo Fisher, Waltham, MA, USA | 4 |
| Oncomine comprehensive v3 | 161 | 51 | 43 | - | - | IonTorrent, Thermo Fisher, Waltham, MA, USA | 3 |
| AmpliSeq for Illumina Comprehensive Panel v3 | 161 | 51 | 43 | - | - | Illumina, San Diego, CA, USA | 4 |
| Oncomine tumor mutation load | 409 | - | - | Yes | - | IonTorrent, Thermo Fisher, Waltham, MA, USA | 5 |
| Oncomine comprehensive + Tumor mutation load | 161 | 51 | 43 | Yes | - | IonTorrent, Thermo Fisher, Waltham, MA, USA; Illumina, San Diego, CA, USA | 9 |
| Oncomine comprehensive plus | 391 | 51 | Yes | Yes | IonTorrent, Thermo Fisher, Waltham, MA, USA | 7 | |
| TrueSightOncology (TSO) 500 | 523 | 55 | 59 | Yes | Yes | Illumina, San Diego, CA, USA | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraus, F.B.T.; Sultova, E.; Heinrich, K.; Jung, A.; Westphalen, C.B.; Tauber, C.V.; Kumbrink, J.; Rudelius, M.; Klauschen, F.; Greif, P.A.; et al. Genetics and beyond: Precision Medicine Real-World Data for Patients with Cervical, Vaginal or Vulvar Cancer in a Tertiary Cancer Center. Int. J. Mol. Sci. 2024, 25, 2345. https://doi.org/10.3390/ijms25042345
Kraus FBT, Sultova E, Heinrich K, Jung A, Westphalen CB, Tauber CV, Kumbrink J, Rudelius M, Klauschen F, Greif PA, et al. Genetics and beyond: Precision Medicine Real-World Data for Patients with Cervical, Vaginal or Vulvar Cancer in a Tertiary Cancer Center. International Journal of Molecular Sciences. 2024; 25(4):2345. https://doi.org/10.3390/ijms25042345
Chicago/Turabian StyleKraus, Fabian B. T., Elena Sultova, Kathrin Heinrich, Andreas Jung, C. Benedikt Westphalen, Christina V. Tauber, Jörg Kumbrink, Martina Rudelius, Frederick Klauschen, Philipp A. Greif, and et al. 2024. "Genetics and beyond: Precision Medicine Real-World Data for Patients with Cervical, Vaginal or Vulvar Cancer in a Tertiary Cancer Center" International Journal of Molecular Sciences 25, no. 4: 2345. https://doi.org/10.3390/ijms25042345
APA StyleKraus, F. B. T., Sultova, E., Heinrich, K., Jung, A., Westphalen, C. B., Tauber, C. V., Kumbrink, J., Rudelius, M., Klauschen, F., Greif, P. A., König, A., Chelariu-Raicu, A., Czogalla, B., Burges, A., Mahner, S., Wuerstlein, R., & Trillsch, F. (2024). Genetics and beyond: Precision Medicine Real-World Data for Patients with Cervical, Vaginal or Vulvar Cancer in a Tertiary Cancer Center. International Journal of Molecular Sciences, 25(4), 2345. https://doi.org/10.3390/ijms25042345






