The Role of the Kinin System and the Effect of Des-Arginine9-Bradykinin on Coagulation and Platelet Function in Critically Ill COVID-19 Patients: A Secondary Analysis of a Prospective Observational Study
Abstract
1. Introduction
2. Results
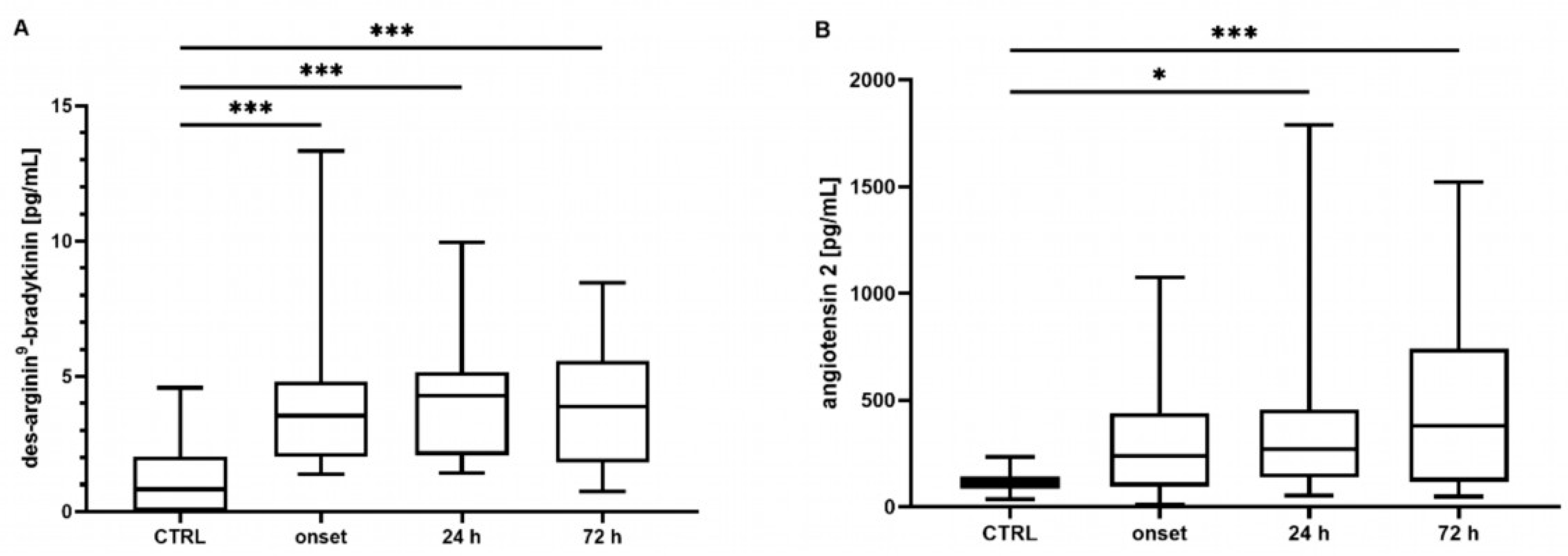
2.1. Bradykinin, Des-Arginine9-Bradykinin, and Angiotensin 2 Concentrations
2.2. Coagulatory Function in Patients with COVID-19
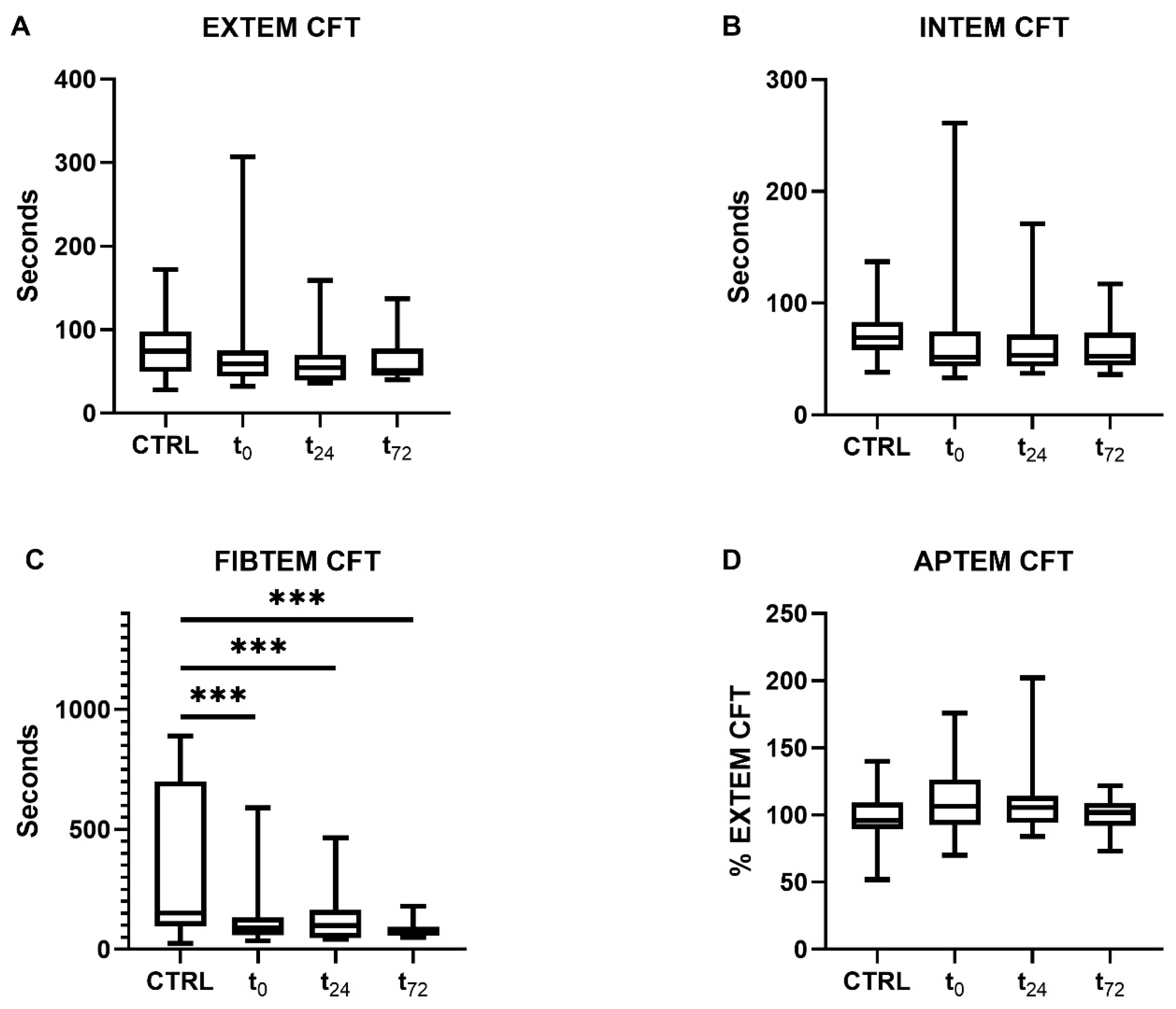
2.2.1. Clot Formation and Stability
2.2.2. Platelet Function
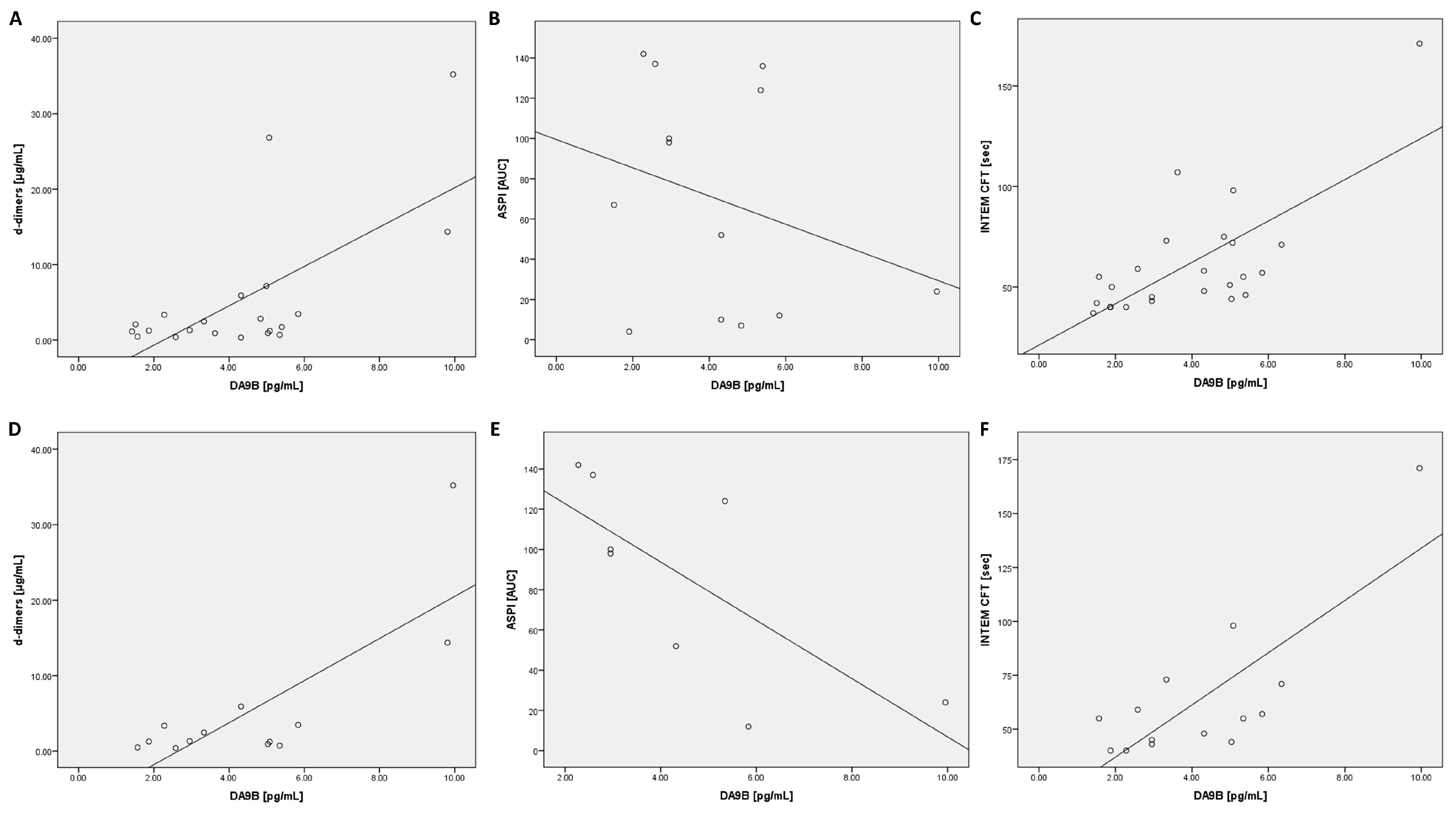
2.3. Correlation Analysis of Des-Arginine9-Bradykinin in All COVID-19 Patients
2.4. Correlation Analysis of Des-Arginine9-Bradykinin in ARDS COVID-19 Patients
2.5. Correlation Analysis of Angiotensin 2 in All COVID-19 Patients
2.6. Correlation Analysis of Angiotensin 2 in ARDS COVID-19 Patients
2.7. Correlation Analysis of Impedance Aggregometry with Des-Arginine9-Bradykinin and Angiotensin 2
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Sample Processing
4.3. Coagulation Analysis
4.4. Laboratory Analysis
4.5. ELISA
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. Coronavirus Disease (COVID-19) Weekly Epidemiological Update. Available online: https://www.who.int/publications/m/item/covid-19-epidemiological-update (accessed on 29 September 2023).
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef]
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc. Diabetol. 2021, 20, 172. [Google Scholar] [CrossRef]
- Root-Bernstein, R. COVID-19 coagulopathies: Human blood proteins mimic SARS-CoV-2 virus, vaccine proteins and bacterial co-infections inducing autoimmunity: Combinations of bacteria and SARS-CoV-2 synergize to induce autoantibodies targeting cardiolipin, cardiolipin-binding proteins, platelet factor 4, prothrombin, and coagulation factors. Bioessays 2021, 43, e2100158. [Google Scholar] [CrossRef]
- Taha, M.; Samavati, L. Antiphospholipid antibodies in COVID-19: A meta-analysis and systematic review. RMD Open 2021, 7, e001580. [Google Scholar] [CrossRef]
- Szturmowicz, M.; Demkow, U. Neutrophil Extracellular Traps (NETs) in Severe SARS-CoV-2 Lung Disease. Int. J. Mol. Sci. 2021, 22, 8854. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, N.; Colucci, M. The Prothrombotic State Associated with SARS-CoV-2 Infection: Pathophysiological Aspects. Mediterr. J. Hematol. Infect. Dis. 2021, 13, e2021045. [Google Scholar] [CrossRef] [PubMed]
- Bekassy, Z.; Lopatko Fagerström, I.; Bader, M.; Karpman, D. Crosstalk between the renin-angiotensin, complement and kallikrein-kinin systems in inflammation. Nat. Rev. Immunol. 2022, 22, 411–428. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.C.; Rainger, G.E.; Mason, J.C.; Guzik, T.J.; Osto, E.; Stamataki, Z.; Neil, D.; Hoefer, I.E.; Fragiadaki, M.; Waltenberger, J.; et al. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020, 116, 2177–2184. [Google Scholar] [CrossRef]
- Nägele, M.P.; Haubner, B.; Tanner, F.C.; Ruschitzka, F.; Flammer, A.J. Endothelial dysfunction in COVID-19: Current findings and therapeutic implications. Atherosclerosis 2020, 314, 58–62. [Google Scholar] [CrossRef]
- Zhang, J.; Tecson, K.M.; McCullough, P.A. Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Rev. Cardiovasc. Med. 2020, 21, 315–319. [Google Scholar] [CrossRef]
- Mahmudpour, M.; Roozbeh, J.; Keshavarz, M.; Farrokhi, S.; Nabipour, I. COVID-19 cytokine storm: The anger of inflammation. Cytokine 2020, 133, 155151. [Google Scholar] [CrossRef]
- Kerr, R.; Stirling, D.; Ludlam, C.A. Interleukin 6 and haemostasis. Br. J. Haematol. 2001, 115, 3–12. [Google Scholar] [CrossRef]
- Balzanelli, M.G.; Distratis, P.; Dipalma, G.; Vimercati, L.; Inchingolo, A.D.; Lazzaro, R.; Aityan, S.K.; Maggiore, M.E.; Mancini, A.; Laforgia, R.; et al. SARS-CoV-2 Virus Infection May Interfere CD34+ Hematopoietic Stem Cells and Megakaryocyte-Erythroid Progenitors Differentiation Contributing to Platelet Defection towards Insurgence of Thrombocytopenia and Thrombophilia. Microorganisms 2021, 9, 1632. [Google Scholar] [CrossRef]
- Datta, P.K.; Liu, F.; Fischer, T.; Rappaport, J.; Qin, X. SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics 2020, 10, 7448–7464. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Zhang, C.; Huang, F.; Wang, F.; Yuan, J.; Wang, Z.; Li, J.; Li, J.; Feng, C.; et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020, 63, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hu, R.; Zhang, C.; Ren, W.; Yu, A.; Zhou, X. Elevation of plasma angiotensin II level is a potential pathogenesis for the critically ill COVID-19 patients. Crit. Care 2020, 24, 290. [Google Scholar] [CrossRef] [PubMed]
- El-Arif, G.; Farhat, A.; Khazaal, S.; Annweiler, C.; Kovacic, H.; Wu, Y.; Cao, Z.; Fajloun, Z.; Khattar, Z.A.; Sabatier, J.M. The Renin-Angiotensin System: A Key Role in SARS-CoV-2-Induced COVID-19. Molecules 2021, 26, 6945. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, I.A.; Kaplan, A.P. Studies of the digestion of bradykin, lys-bradykinin, and des-Arg9-bradykinin by angiotensin converting enzyme. Biochem. Pharmacol. 1986, 35, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, E.; Díaz-García, E.; García-Tovar, S.; Zamarrón, E.; Mangas, A.; Galera, R.; Nanwani-Nanwani, K.; Pérez-de-Diego, R.; López-Collazo, E.; García-Río, F.; et al. Impaired Kallikrein-Kinin System in COVID-19 Patients’ Severity. Front. Immunol. 2022, 13, 909342. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.M.d.M.; Do Nascimento, I.J.B.; Marazzi-Diniz, P.H.; Da Silveira, I.B.; Itaborahy, M.F.; Viana, L.E.; Silva, F.A.; Santana, M.F.; Pinto, R.A.; Dutra, B.G.; et al. The des-Arg9-bradykinin/B1R axis: Hepatic damage in COVID-19. Front. Physiol. 2022, 13, 1080837. [Google Scholar] [CrossRef] [PubMed]
- Marceau, F.; Bachelard, H.; Bouthillier, J.; Fortin, J.-P.; Morissette, G.; Bawolak, M.-T.; Charest-Morin, X.; Gera, L. Bradykinin receptors: Agonists, antagonists, expression, signaling, and adaptation to sustained stimulation. Int. Immunopharmacol. 2020, 82, 106305. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.C.; Voetsch, B.; Loscalzo, J. Endogenous mechanisms of inhibition of platelet function. Microcirculation 2005, 12, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Bertolin, A.J.; Dalçóquio, T.F.; Salsoso, R.; de M Furtado, R.H.; Kalil-Filho, R.; Hajjar, L.A.; Siciliano, R.F.; Kallás, E.G.; Baracioli, L.M.; Lima, F.G.; et al. Platelet Reactivity and Coagulation Markers in Patients with COVID-19. Adv. Ther. 2021, 38, 3911–3923. [Google Scholar] [CrossRef] [PubMed]
- Heinz, C.; Miesbach, W.; Herrmann, E.; Sonntagbauer, M.; Raimann, F.J.; Zacharowski, K.; Weber, C.F.; Adam, E.H. Greater Fibrinolysis Resistance but No Greater Platelet Aggregation in Critically Ill COVID-19 Patients. Anesthesiology 2021, 134, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Notz, Q.; Schlesinger, T.; Stumpner, J.; Kredel, M.; Sitter, M.; Schmid, B.; Kranke, P.; Schulze, H.; Meybohm, P.; et al. Point of care diagnostic of hypercoagulability and platelet function in COVID-19 induced acute respiratory distress syndrome: A retrospective observational study. Thromb. J. 2021, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Edinger, F.; Edinger, S.; Koch, C.; Markmann, M.; Hecker, M.; Sander, M.; Schneck, E. Peak Plasma Levels of mtDNA Serve as a Predictive Biomarker for COVID-19 in-Hospital Mortality. J. Clin. Med. 2022, 11, 7161. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Meini, S.; Zanichelli, A.; Sbrojavacca, R.; Iuri, F.; Roberts, A.T.; Suffritti, C.; Tascini, C. Understanding the Pathophysiology of COVID-19: Could the Contact System Be the Key? Front. Immunol. 2020, 11, 2014. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, S.; Zhang, Y.; Zhi, Y. Does hereditary angioedema make COVID-19 worse? World Allergy Organ. J. 2020, 13, 100454. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- De Azambuja Pias Weber, A.; Viero, F.T.; Pillat, M.M.; de Lima Gonçalves, T. Changes in markers of inflammation and their correlation with death in patients with COVID-19 in the intensive care unit. Cytokine 2024, 175, 156509. [Google Scholar] [CrossRef] [PubMed]
- De Maistre, E.; Savard, P.; Guinot, P.-G. COVID-19 and the Concept of Thrombo-Inflammation: Review of the Relationship between Immune Response, Endothelium and Coagulation. J. Clin. Med. 2023, 12, 7245. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, O.H.M.; Lengquist, M.; Spångfors, M.; Annborn, M.; Bergmann, D.; Schulte, J.; Levin, H.; Melander, O.; Frigyesi, A.; Friberg, H. Circulating bioactive adrenomedullin as a marker of sepsis, septic shock and critical illness. Crit. Care 2020, 24, 636. [Google Scholar] [CrossRef] [PubMed]
- Schmaier, A.H. The contact activation and kallikrein/kinin systems: Pathophysiologic and physiologic activities. J. Thromb. Haemost. 2016, 14, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Cahill, M.; Fishman, J.B.; Polgar, P. Effect of des arginine9-bradykinin and other bradykinin fragments on the synthesis of prostacyclin and the binding of bradykinin by vascular cells in culture. Agents Actions 1988, 24, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.Z.; Raslan, Z.; Atkinson, L.; Aburima, A.; Thomas, S.G.; Naseem, K.M.; Calaminus, S.D.J. Prostacyclin reverses platelet stress fibre formation causing platelet aggregate instability. Sci. Rep. 2017, 7, 5582. [Google Scholar] [CrossRef]
- Ghevaert, C.; Salsmann, A.; Watkins, N.A.; Schaffner-Reckinger, E.; Rankin, A.; Garner, S.F.; Stephens, J.; Smith, G.A.; Debili, N.; Vainchenker, W.; et al. A nonsynonymous SNP in the ITGB3 gene disrupts the conserved membrane-proximal cytoplasmic salt bridge in the alphaIIbbeta3 integrin and cosegregates dominantly with abnormal proplatelet formation and macrothrombocytopenia. Blood 2008, 111, 3407–3414. [Google Scholar] [CrossRef]
- He, M.; He, X.; Xie, Q.; Chen, F.; He, S. Angiotensin II induces the expression of tissue factor and its mechanism in human monocytes. Thromb. Res. 2006, 117, 579–590. [Google Scholar] [CrossRef]
- Mogielnicki, A.; Chabielska, E.; Pawlak, R.; Szemraj, J.; Buczko, W. Angiotensin II enhances thrombosis development in renovascular hypertensive rats. Thromb. Haemost. 2005, 93, 1069–1076. [Google Scholar] [CrossRef]
- Cyr, M.; Lepage, Y.; Blais, C.; Gervais, N.; Cugno, M.; Rouleau, J.L.; Adam, A. Bradykinin and des-Arg(9)-bradykinin metabolic pathways and kinetics of activation of human plasma. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H275–H283. [Google Scholar] [CrossRef] [PubMed]
- Den Heijer, M.; Lewington, S.; Clarke, R. Homocysteine, MTHFR and risk of venous thrombosis: A meta-analysis of published epidemiological studies. J. Thromb. Haemost. 2005, 3, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Wassel, C.L.; Lange, L.A.; Keating, B.J.; Taylor, K.C.; Johnson, A.D.; Palmer, C.; Ho, L.A.; Smith, N.L.; Lange, E.M.; Li, Y.; et al. Association of genomic loci from a cardiovascular gene SNP array with fibrinogen levels in European Americans and African-Americans from six cohort studies: The Candidate Gene Association Resource (CARe). Blood 2011, 117, 268–275. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Patients with COVID-19 (n = 29) | Controls (n = 29) | ||
|---|---|---|---|
| General characteristics | |||
| Age (years) | 70 [59–80] | 70 [58–79] | |
| Male sex (%) | 65.5 | 65.5 | |
| BMI (kg/m2) | 28.4 [24.2–30.5] | 29.7 [26.8–32.0] | |
| In-hospital mortality | 16 (55.2%) | 0 (0.0%) | |
| ARDS | Admission | 7 (24.1%), 7 (24.1%), 9 (31.0%), 6 (20.7%) | NA |
| No, mild | 24 h | 5 (20.0%), 3 (12.0%), 13 (52.0%), 4 (16.0%) | NA |
| Moderate, severe | 72 h | 5 (20.8%), 4 (16.7%), 13 (54.1%), 2 (8.3%) | NA |
| Murray score | Admission | 1.9 [1.3–2.5] | NA |
| 24 h | 1.8 [1.3–2.5] | NA | |
| 72 h | 2.3 [1.7–2.6] | NA | |
| SOFA score | Admission | 7.0 [5.0–9.0] | NA |
| 24 h | 6.0 [5.0–8.0] | NA | |
| 72 h | 6.5 [4.0–8.3] | NA | |
| Pre-existing diseases | |||
| CAD | 8 (27.6%) | 8 (27.6%) | |
| Arterial hypertension | 25 (86.2%) | 25 (86.2%) | |
| Diabetes mellitus | 14 (48.3%) | 14 (48.3%) | |
| Chronic kidney disease | 5 (17.2%) | 5 (17.2%) | |
| Anti-coagulation | |||
| Prophylactic | Admission | 16 (55.2%) | 0 (0.0%) |
| 24 h | 9 (36.0%) | NA | |
| 72 h | 8 (33.3%) | NA | |
| Therapeutic | Admission | 13 (44.8%) | 0 (0.0%) |
| 24 h | 16 (64.0%) | NA | |
| 72 h | 13 (54.2%) | NA | |
| Heparin | Admission | 5.7 [5.0–10.0] | 0 [0–0] |
| (I.U./kg/h) | 24 h | 7.3 [4.8–11.4] | NA |
| 72 h | 8.7 [4.3–12.3] | NA | |
| Enoxaparin | Admission | 1.1 [0.8–1.5] | 0 [0–0] |
| (mg/kg/d) | 24 h | 1.4 [1.0–1.8] | NA |
| 72 h | 1.4 [1.1–1.9] | NA | |
| t0 | t24 | t72 | ||
|---|---|---|---|---|
| Laboratory parameters | d-dimers | r = 0.40 * p = 0.033 | r = 0.45 * p = 0.043 | r = 0.55 * p = 0.015 |
| ferritin | r = 0.40 * p = 0.036 | r = 0.33 p = 0.139 | r = 0.44 p = 0.055 | |
| GOT | r = 0.42 * p = 0.022 | r = 0.38 p = 0.065 | r = 0.25 p = 0.241 | |
| LDH | r = 0.53 ** p = 0.004 | r = 0.23 p = 0.269 | r = 0.46 * p = 0.047 | |
| Thromboelastographic parameters | EXTEM CFT | r = 0.40 p = 0.053 | r = 0.48 * p = 0.018 | r = 0.12 p = 0.934 |
| INTEM CFT | r = 0.58 ** p = 0.003 | r = 0.60 ** p = 0.002 | r = 0.33 p = 0.129 | |
| APTEM CFT | r = −0.04 p = 0.869 | r = −0.47 * p = 0.019 | r = 0.06 p = 0.790 |
| t0 | t24 | t72 | ||
|---|---|---|---|---|
| Laboratory parameters | d-dimers | r = 0.79 * p = 0.001 | r = 0.538 p = 0.058 | r = 0.74 * p = 0.010 |
| platelets | r = −0.37 p = 0.216 | r = −0.53 * p = 0.044 | r = −0.58 * p = 0.037 | |
| fibrinogen | r = −0.42 p = 0.153 | r = −0.53 * p = 0.050 | r = −0.51 p = 0.092 | |
| PCT | r = 0.70 ** p = 0.008 | r = 0.52 * p = 0.046 | r = 0.59 p = 0.056 | |
| ferritin | r = 0.73 ** p = 0.005 | r = 0.65 * p = 0.017 | r = 0.23 p = 0.470 | |
| GOT | r = 0.50 p = 0.079 | r = 0.52 * p = 0.048 | r = 0.15 p = 0.629 | |
| LDH | r = 0.76 ** p = 0.003 | r = 0.32 p = 0.242 | r = 0.16 p = 0.650 | |
| Thromboelastographic parameters | EXTEM CFT | r = 0.59 p = 0.055 | r = 0.48 p = 0.084 | r = 0.33 p = 0.303 |
| INTEM CFT | r = 0.82 ** p = 0.002 | r = 0.61 * p = 0.020 | r = 0.54 p = 0.085 | |
| INTEM A20 | r = −0.70 * p = 0.025 | r = −0.63 * p = 0.016 | r = −0.61 * p = 0.046 | |
| INTEM MCF | r = −0.593 p = 0.054 | r = −0.04 p = 0.904 | r = −0.60 * p = 0.049 | |
| APTEM A20 | r = 0.13 p = 0.699 | r = 0.64 * p = 0.018 | r = 0.31 p = 0.327 | |
| APTEM MCF | r = −0.14 p = 0.689 | r = 0.63 * p = 0.023 | r = 0.25 p = 0.440 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edinger, F.; Edinger, S.; Schmidt, G.; Koch, C.; Sander, M.; Schneck, E. The Role of the Kinin System and the Effect of Des-Arginine9-Bradykinin on Coagulation and Platelet Function in Critically Ill COVID-19 Patients: A Secondary Analysis of a Prospective Observational Study. Int. J. Mol. Sci. 2024, 25, 2342. https://doi.org/10.3390/ijms25042342
Edinger F, Edinger S, Schmidt G, Koch C, Sander M, Schneck E. The Role of the Kinin System and the Effect of Des-Arginine9-Bradykinin on Coagulation and Platelet Function in Critically Ill COVID-19 Patients: A Secondary Analysis of a Prospective Observational Study. International Journal of Molecular Sciences. 2024; 25(4):2342. https://doi.org/10.3390/ijms25042342
Chicago/Turabian StyleEdinger, Fabian, Sophia Edinger, Götz Schmidt, Christian Koch, Michael Sander, and Emmanuel Schneck. 2024. "The Role of the Kinin System and the Effect of Des-Arginine9-Bradykinin on Coagulation and Platelet Function in Critically Ill COVID-19 Patients: A Secondary Analysis of a Prospective Observational Study" International Journal of Molecular Sciences 25, no. 4: 2342. https://doi.org/10.3390/ijms25042342
APA StyleEdinger, F., Edinger, S., Schmidt, G., Koch, C., Sander, M., & Schneck, E. (2024). The Role of the Kinin System and the Effect of Des-Arginine9-Bradykinin on Coagulation and Platelet Function in Critically Ill COVID-19 Patients: A Secondary Analysis of a Prospective Observational Study. International Journal of Molecular Sciences, 25(4), 2342. https://doi.org/10.3390/ijms25042342








