Role of Leptin in Obesity, Cardiovascular Disease, and Type 2 Diabetes
Abstract
1. Introduction
2. Obesity and CVD
3. Obesity and Diabetes
4. Diabetes and CVD
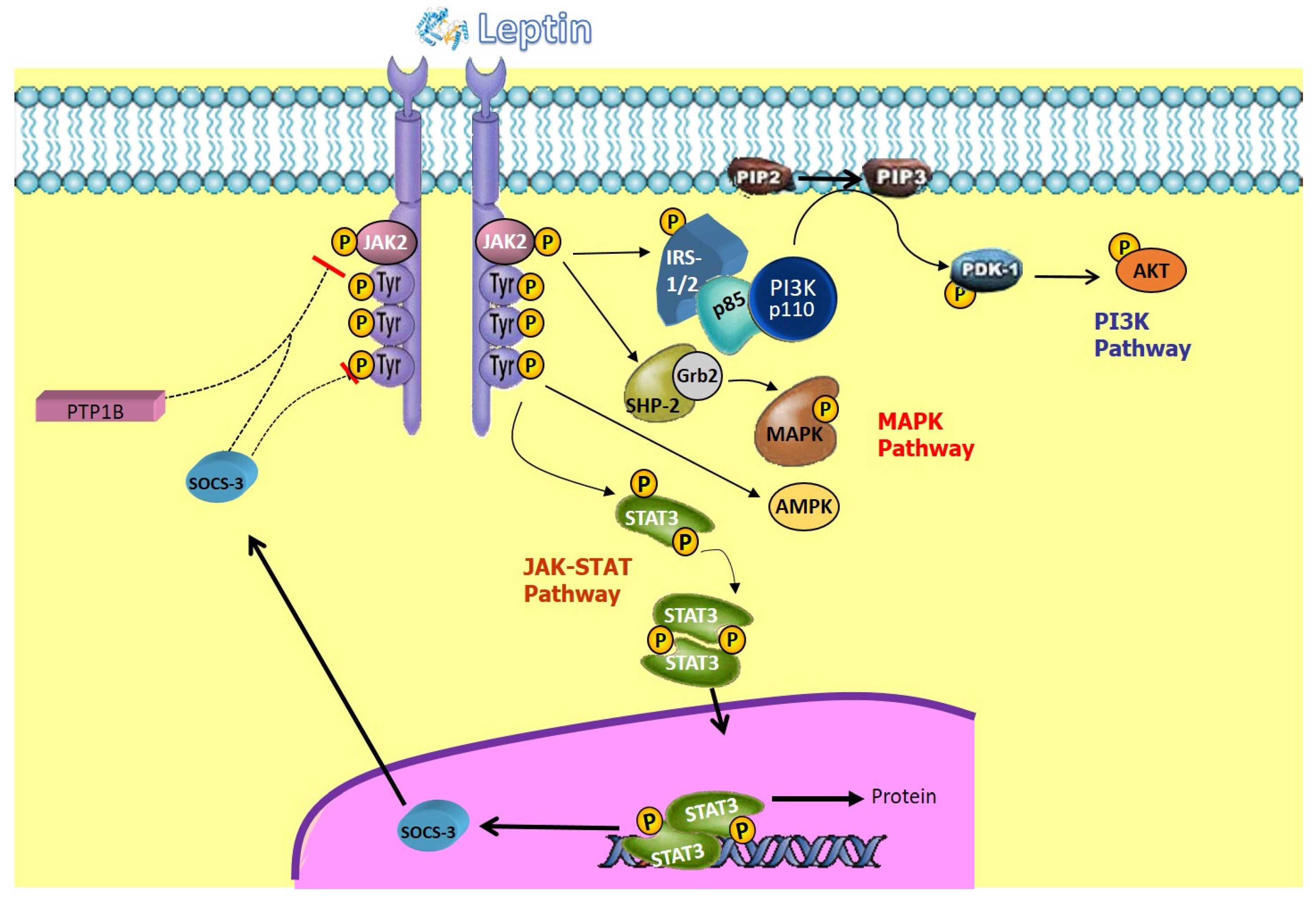
5. Leptin
6. Leptin and Obesity
7. Leptin and T2DM
8. Leptin and CVD
9. Leptin as a Therapeutic Target
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Correction Statement
References
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef]
- Kerner, W.; Brückel, J. Definition, classification and diagnosis of diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 2014, 122, 384–386. [Google Scholar] [CrossRef]
- Coraci, D.; Giovannini, S.; Loreti, C.; Pecchioli, C.; Piccinini, G.; Padua, L. The past encounters the future: “old” diagnostic methods to check innovative treatments for carpal tunnel syndrome. Comment on: “Treatment of carpal tunnel syndrome: From ultrasonography to ultrasound surgery” by Petrover and Richette. Joint Bone Spine 2017 https://doi.org/10.1016/j.jbspin.2017.11.003. Jt. Bone Spine 2018, 85, 783–784. [Google Scholar] [CrossRef]
- Endalifer, M.L.; Diress, G. Epidemiology, Predisposing Factors, Biomarkers, and Prevention Mechanism of Obesity: A Systematic Review. J. Obes. 2020, 2020, 6134362. [Google Scholar] [CrossRef] [PubMed]
- Chobot, A.; Górowska-Kowolik, K.; Sokołowska, M.; Jarosz-Chobot, P. Obesity and diabetes-Not only a simple link between two epidemics. Diabetes. Metab. Res. Rev. 2018, 34, e3042. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ. Res. 2016, 118, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- La Sala, L.; Pontiroli, A.E. Prevention of Diabetes and Cardiovascular Disease in Obesity. Int. J. Mol. Sci. 2020, 21, 8178. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M. Leptin, leptin receptors, and the control of body weight. Nutr. Rev. 1998, 56, S38–S46. [Google Scholar] [CrossRef] [PubMed]
- Considine, R.V.; Caro, J.F. Leptin and the regulation of body weight. Int. J. Biochem. Cell Biol. 1997, 29, 1255–1272. [Google Scholar] [CrossRef]
- White, D.W.; Tartaglia, L.A. Leptin and OB-R: Body weight regulation by a cytokine receptor. Cytokine Growth Factor Rev. 1996, 7, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; Sánchez-Margalet, V. Role of Leptin in Inflammation and Vice Versa. Int. J. Mol. Sci. 2020, 21, 5887. [Google Scholar] [CrossRef]
- Wang, C.; Chang, L.; Wang, J.; Xia, L.; Cao, L.; Wang, W.; Xu, J.; Gao, H. Leptin and risk factors for atherosclerosis: A review. Medicine 2023, 102, E36076. [Google Scholar] [CrossRef]
- Raman, P.; Khanal, S. Leptin in Atherosclerosis: Focus on Macrophages, Endothelial and Smooth Muscle Cells. Int. J. Mol. Sci. 2021, 22, 5446. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Romero-Corral, A.; Montori, V.M.; Somers, V.K.; Korinek, J.; Thomas, R.J.; Allison, T.G.; Mookadam, F.; Lopez-Jimenez, F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: A systematic review of cohort studies. Lancet 2006, 368, 666–678. [Google Scholar] [CrossRef]
- Kang, K.W.; Ok, M.; Lee, S.K. Leptin as a Key between Obesity and Cardiovascular Disease. J. Obes. Metab. Syndr. 2020, 29, 248–259. [Google Scholar] [CrossRef]
- Bombelli, M.; Facchetti, R.; Sega, R.; Carugo, S.; Fodri, D.; Brambilla, G.; Giannattasio, C.; Grassi, G.; Mancia, G. Impact of body mass index and waist circumference on the long-term risk of diabetes mellitus, hypertension, and cardiac organ damage. Hypertension 2011, 58, 1029–1035. [Google Scholar] [CrossRef]
- Wensveen, F.M.; Valentić, S.; Šestan, M.; Turk Wensveen, T.; Polić, B. The “Big Bang” in obese fat: Events initiating obesity-induced adipose tissue inflammation. Eur. J. Immunol. 2015, 45, 2446–2456. [Google Scholar] [CrossRef]
- Martin, S.S.; Qasim, A.; Reilly, M.P. Leptin resistance: A possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J. Am. Coll. Cardiol. 2008, 52, 1201–1210. [Google Scholar] [CrossRef]
- Recinella, L.; Orlando, G.; Ferrante, C.; Chiavaroli, A.; Brunetti, L.; Leone, S. Adipokines: New Potential Therapeutic Target for Obesity and Metabolic, Rheumatic, and Cardiovascular Diseases. Front. Physiol. 2020, 11, 578966. [Google Scholar] [CrossRef]
- Carbone, S.; Canada, J.M.; Billingsley, H.E.; Siddiqui, M.S.; Elagizi, A.; Lavie, C.J. Obesity paradox in cardiovascular disease: Where do we stand? Vasc. Health Risk Manag. 2019, 15, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Metabolically Healthy Obesity. Endocr. Rev. 2020, 41, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Huang, R.; Jiang, M.; Wang, W.; Chai, Y.; Liu, Q.; Zhang, W.; Han, Y.; Yan, F.; Lu, Q.; et al. Myocardial Tissue-Level Characteristics of Adults with Metabolically Healthy Obesity. JACC Cardiovasc. Imaging 2023, 16, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, P.; Henriksson, H.; Tynelius, P.; Berglind, D.; Löf, M.; Lee, I.M.; Shiroma, E.J.; Ortega, F.B. Fitness and Body Mass Index During Adolescence and Disability Later in Life: A Cohort Study. Ann. Intern. Med. 2019, 170, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, H.; Henriksson, P.; Tynelius, P.; Ekstedt, M.; Berglind, D.; Labayen, I.; Ruiz, J.R.; Lavie, C.J.; Ortega, F.B. Cardiorespiratory fitness, muscular strength, and obesity in adolescence and later chronic disability due to cardiovascular disease: A cohort study of 1 million men. Eur. Heart J. 2020, 41, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Boutari, C.; DeMarsilis, A.; Mantzoros, C.S. Obesity and diabetes. Diabetes Res. Clin. Pract. 2023, 202, 110773. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef]
- Hocking, S.; Samocha-Bonet, D.; Milner, K.L.; Greenfield, J.R.; Chisholm, D.J. Adiposity and insulin resistance in humans: The role of the different tissue and cellular lipid depots. Endocr. Rev. 2013, 34, 463–500. [Google Scholar] [CrossRef]
- Sinha, R.; Fisch, G.; Teague, B.; Tamborlane, W.V.; Banyas, B.; Allen, K.; Savoye, M.; Rieger, V.; Taksali, S.; Barbetta, G.; et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N. Engl. J. Med. 2002, 346, 802–810. [Google Scholar] [CrossRef]
- D’Adamo, E.; Cali, A.M.G.; Weiss, R.; Santoro, N.; Pierpont, B.; Northrup, V.; Caprio, S. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care 2010, 33, 1817–1822. [Google Scholar] [CrossRef]
- Kahkoska, A.R.; Pokaprakarn, T.; Rumay Alexander, G.; Crume, T.L.; Dabelea, D.; Divers, J.; Dolan, L.M.; Jensen, E.T.; Lawrence, J.M.; Marcovina, S.; et al. The Impact of Racial and Ethnic Health Disparities in Diabetes Management on Clinical Outcomes: A Reinforcement Learning Analysis of Health Inequity Among Youth and Young Adults in the SEARCH for Diabetes in Youth Study. Diabetes Care 2022, 45, 108–118. [Google Scholar] [CrossRef]
- Al Amiri, E.; Abdullatif, M.; Abdulle, A.; Al Bitar, N.; Afandi, E.Z.; Parish, M.; Darwiche, G. The prevalence, risk factors, and screening measure for prediabetes and diabetes among Emirati overweight/obese children and adolescents. BMC Public Health 2015, 15, 1298. [Google Scholar] [CrossRef] [PubMed]
- Deeb, A.; Salima, A.; Samia, M.; Ghada, E.; Abubaker, E. Insulin Resistance, Impaired fasting, Glucose Intolerance and Type II Diabetes Mellitus in Overweight and Obese Children in Abu Dhabi. J. Diabetes Obes. 2017, 4, 1–8. [Google Scholar] [CrossRef][Green Version]
- Dilworth, L.; Facey, A.; Omoruyi, F. Diabetes Mellitus and Its Metabolic Complications: The Role of Adipose Tissues. Int. J. Mol. Sci. 2021, 22, 7644. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mclaughlin, T.; Ackerman, S.E.; Shen, L.; Engleman, E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Investig. 2017, 127, 5–13. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Vilariño-García, T.; Fernández-Riejos, P.; Martín-González, J.; Segura-Egea, J.J.; Sánchez-Margalet, V. Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev. 2017, 35, 71–84. [Google Scholar] [CrossRef]
- Sánchez-Margalet, V.; Martín-Romero, C.; Santos-Alvarez, J.; Goberna, R.; Najib, S.; Gonzalez-Yanes, C. Role of leptin as an immunomodulator of blood mononuclear cells: Mechanisms of action. Clin. Exp. Immunol. 2003, 133, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Margalet, V.; Fernández-Riejos, P.; Najib, S.; Santos-Alvarez, J.; Martín-Romero, C.; Pérez-Pérez, A.; González-Yanes, C. Role of leptin in the activation of immune cells. Mediat. Inflamm. 2010, 2010, 568343. [Google Scholar] [CrossRef]
- Bonamichi, B.D.S.F.; Lee, J. Unusual Suspects in the Development of Obesity-Induced Inflammation and Insulin Resistance: NK cells, iNKT cells, and ILCs. Diabetes Metab. J. 2017, 41, 229–250. [Google Scholar] [CrossRef]
- Wouters, K.; Kusters, Y.H.A.M.; Bijnen, M.; Wetzels, S.; Zhang, X.; Linssen, P.B.C.; Gaens, K.; Houben, A.J.H.M.; Joris, P.J.; Plat, J.; et al. NK cells in human visceral adipose tissue contribute to obesity-associated insulin resistance through low-grade inflammation. Clin. Transl. Med. 2020, 10, e192. [Google Scholar] [CrossRef]
- Caussy, C.; Aubin, A.; Loomba, R. The Relationship Between Type 2 Diabetes, NAFLD, and Cardiovascular Risk. Curr. Diab. Rep. 2021, 21, 15. [Google Scholar] [CrossRef]
- Naito, R.; Kasai, T. Coronary artery disease in type 2 diabetes mellitus: Recent treatment strategies and future perspectives. World J. Cardiol. 2015, 7, 119. [Google Scholar] [CrossRef]
- Shah, A.D.; Langenberg, C.; Rapsomaniki, E.; Denaxas, S.; Pujades-Rodriguez, M.; Gale, C.P.; Deanfield, J.; Smeeth, L.; Timmis, A.; Hemingway, H. Type 2 diabetes and incidence of cardiovascular diseases: A cohort study in 1·9 million people. Lancet Diabetes Endocrinol. 2015, 3, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Punthakee, Z.; Goldenberg, R.; Katz, P. Definition, Classification and Diagnosis of Diabetes, Prediabetes and Metabolic Syndrome. Can. J. Diabetes 2018, 42 (Suppl. S1), S10–S15. [Google Scholar] [CrossRef] [PubMed]
- Prandi, F.R.; Evangelista, I.; Sergi, D.; Palazzuoli, A.; Romeo, F. Mechanisms of cardiac dysfunction in diabetic cardiomyopathy: Molecular abnormalities and phenotypical variants. Heart Fail. Rev. 2023, 28, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Pietz, K.; Battleman, D.S.; Beyth, R.J. Prevalence of Comorbid Hypertension and Dyslipidemia and Associated Cardiovascular Disease. Heart Dis. 2004, 2, 3. [Google Scholar]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Barrett-Connor, E. Hypercholesterolemia predicts early death from coronary heart disease in elderly men but not women. The Rancho Bernardo Study. Ann. Epidemiol. 1992, 2, 77–83. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, C.; Hong, J.; Zeng, J.; Lai, S.; Lv, A.; Su, Q.; Dong, Y.; Zhou, Z.; Tang, W.; et al. Lipid profiling reveals different therapeutic effects of metformin and glipizide in patients with type 2 diabetes and coronary artery disease. Diabetes Care 2014, 37, 2804–2812. [Google Scholar] [CrossRef]
- Nelson, A.J.; Pagidipati, N.J.; Aroda, V.R.; Cavender, M.A.; Green, J.B.; Lopes, R.D.; Al-Khalidi, H.; Gaynor, T.; Kaltenbach, L.A.; Kirk, J.K.; et al. Incorporating SGLT2i and GLP-1RA for Cardiovascular and Kidney Disease Risk Reduction: Call for Action to the Cardiology Community. Circulation 2021, 144, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Strain, W.D.; Paldánius, P.M. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc. Diabetol. 2018, 17, 57. [Google Scholar] [CrossRef]
- Halaas, J.L.; Gajiwala, K.S.; Maffei, M.; Cohen, S.L.; Chait, B.T.; Rabinowitz, D.; Lallone, R.L.; Burley, S.K.; Friedman, J.M. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 1995, 269, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef]
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef]
- Wajchenberg, B.L. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr. Rev. 2000, 21, 697–738. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Maymó, J.; Gambino, Y.; Guadix, P.; Dueñas, J.L.; Varone, C.; Sánchez-Margalet, V. Insulin enhances leptin expression in human trophoblastic cells. Biol. Reprod. 2013, 89, 20. [Google Scholar] [CrossRef] [PubMed]
- Miell, J.P.; Englaro, P.; Blum, W.F. Dexamethasone induces an acute and sustained rise in circulating leptin levels in normal human subjects. Horm. Metab. Res. 1996, 28, 704–707. [Google Scholar] [CrossRef]
- De Vos, P.; Saladin, R.; Auwerx, J.; Staels, B. Induction of ob gene expression by corticosteroids is accompanied by body weight loss and reduced food intake. J. Biol. Chem. 1995, 270, 15958–15961. [Google Scholar] [CrossRef]
- Ahima, R.S.; Flier, J.S. Leptin. Annu. Rev. Physiol. 2000, 62, 413–437. [Google Scholar] [CrossRef]
- Margetic, S.; Gazzola, C.; Pegg, G.G.; Hill, R.A. Leptin: A review of its peripheral actions and interactions. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1407–1433. [Google Scholar] [CrossRef]
- Mantzoros, C.S.; Magkos, F.; Brinkoetter, M.; Sienkiewicz, E.; Dardeno, T.A.; Kim, S.Y.; Hamnvik, O.P.R.; Koniaris, A. Leptin in human physiology and pathophysiology. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E567–E584. [Google Scholar] [CrossRef]
- Hickey, M.S.; Israel, R.G.; Gardiner, S.N.; Considine, R.V.; McCammon, M.R.; Tyndall, G.L.; Houmard, J.A.; Marks, R.H.L.; Caro, J.F. Gender Differences in Serum Leptin Levels in Humans. Biochem. Mol. Med. 1996, 59, 1–6. [Google Scholar] [CrossRef]
- Arch, J.R.S.; Stock, M.J.; Trayhurn, P. Leptin resistance in obese humans: Does it exist and what does it mean? Int. J. Obes. Relat. Metab. Disord. 1998, 22, 1159–1163. [Google Scholar] [CrossRef]
- Knight, Z.A.; Hannan, K.S.; Greenberg, M.L.; Friedman, J.M. Hyperleptinemia is required for the development of leptin resistance. PLoS ONE 2010, 5, e11376. [Google Scholar] [CrossRef] [PubMed]
- Poetsch, M.S.; Strano, A.; Guan, K. Role of Leptin in Cardiovascular Diseases. Front. Endocrinol. 2020, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Ren, J. Leptin and hyperleptinemia—From friend to foe for cardiovascular function. J. Endocrinol. 2004, 181, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin, Leptin and Cardiovascular Disorders. Circ. Res. 2021, 128, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.J.; Clifton, D.K.; Steiner, R.A. Leptin’s actions on the reproductive axis: Perspectives and mechanisms. Biol. Reprod. 1999, 60, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. Obesity and reproduction. Hum. Reprod. 1997, 12 (Suppl. S1), 26–32. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.L.; Nagatani, S. Physiological perspectives on leptin as a regulator of reproduction: Role in timing puberty. Biol. Reprod. 1999, 60, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Maymó, J.; Dueñas, J.L.; Varone, C.; Sánchez-Margalet, V. Role of leptin in female reproduction. Clin. Chem. Lab. Med. 2015, 53, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Toro, A.; Vilariño-García, T.; Maymó, J.; Guadix, P.; Dueñas, J.L.; Fernández-Sánchez, M.; Varone, C.; Sánchez-Margalet, V. Leptin action in normal and pathological pregnancies. J. Cell. Mol. Med. 2018, 22, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.B.S. Leptin—Much more than a satiety signal. Annu. Rev. Nutr. 2000, 20, 45–75. [Google Scholar] [CrossRef]
- Meister, B. Control of food intake via leptin receptors in the hypothalamus. Vitam. Horm. 2000, 59, 265–304. [Google Scholar] [CrossRef]
- Sanchez-Margalet, V.; Martin-Romero, C. Human leptin signaling in human peripheral blood mononuclear cells: Activation of the JAK-STAT pathway. Cell. Immunol. 2001, 211, 30–36. [Google Scholar] [CrossRef]
- Villanueva, E.C.; Myers, M.G. Leptin receptor signaling and the regulation of mammalian physiology. Int. J. Obes. 2008, 32 (Suppl. S7), S8–S12. [Google Scholar] [CrossRef]
- Bates, S.H.; Myers, M.G. The role of leptin receptor signaling in feeding and neuroendocrine function. Trends Endocrinol. Metab. 2003, 14, 447–452. [Google Scholar] [CrossRef]
- Bjørbaek, C.; Kahn, B.B. Leptin signaling in the central nervous system and the periphery. Recent Prog. Horm. Res. 2004, 59, 305–331. [Google Scholar] [CrossRef] [PubMed]
- Jéquier, E. Leptin signaling, adiposity, and energy balance. Ann. N. Y. Acad. Sci. 2002, 967, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Murakami, T.; Otani, S.; Kuwajima, M.; Shima, K. Leptin receptor signal transduction: OBRa and OBRb of fa type. Biochem. Biophys. Res. Commun. 1998, 246, 752–759. [Google Scholar] [CrossRef]
- Takahashi, Y.; Okimura, Y.; Mizuno, I.; Iida, K.; Takahashi, T.; Kaji, H.; Abe, H.; Chihara, K. Leptin induces mitogen-activated protein kinase-dependent proliferation of C3H10T1/2 cells. J. Biol. Chem. 1997, 272, 12897–12900. [Google Scholar] [CrossRef] [PubMed]
- Bjørbæk, C.; Uotani, S.; Da Silva, B.; Flier, J.S. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J. Biol. Chem. 1997, 272, 32686–32695. [Google Scholar] [CrossRef] [PubMed]
- Baumann, H.; Morella, K.K.; White, D.W.; Dembski, M.; Bailon, P.S.; Kim, H.; Lai, C.F.; Tartaglia, L.A. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc. Natl. Acad. Sci. USA 1996, 93, 8374–8378. [Google Scholar] [CrossRef] [PubMed]
- Bjørbæk, C.; El-Haschimi, K.; Frantz, J.D.; Flier, J.S. The role of SOCS-3 in leptin signaling and leptin resistance. J. Biol. Chem. 1999, 274, 30059–30065. [Google Scholar] [CrossRef]
- Zabolotny, J.M.; Bence-Hanulec, K.K.; Stricker-Krongrad, A.; Haj, F.; Wang, Y.; Minokoshi, Y.; Kim, Y.B.; Elmquist, J.K.; Tartaglia, L.A.; Kahn, B.B.; et al. PTP1B regulates leptin signal transduction in vivo. Dev. Cell 2002, 2, 489–495. [Google Scholar] [CrossRef]
- Martín-Romero, C.; Sánchez-Margalet, V. Human leptin activates PI3K and MAPK pathways in human peripheral blood mononuclear cells: Possible role of Sam68. Cell. Immunol. 2001, 212, 83–91. [Google Scholar] [CrossRef]
- Ren, D.; Li, M.; Duan, C.; Rui, L. Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metab. 2005, 2, 95–104. [Google Scholar] [CrossRef]
- Liu, J.; Lai, F.; Hou, Y.; Zheng, R. Leptin signaling and leptin resistance. Med. Rev. 2022, 2, 363–384. [Google Scholar] [CrossRef]
- Minokoshi, Y.; Alquier, T.; Furukawa, H.; Kim, Y.B.; Lee, A.; Xue, B.; Mu, J.; Foufelle, F.; Ferré, P.; Birnbaum, M.J.; et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004, 428, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Minokoshi, Y.; Kim, Y.B.; Peroni, O.D.; Fryer, L.G.D.; Müller, C.; Carling, D.; Kahn, B.B. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 2002, 415, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-activated protein kinase: A key regulator of energy balance with many roles in human disease. J. Intern. Med. 2014, 276, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Tomaszuk, A.; Simpson, C.; Williams, G. Neuropeptide Y, the hypothalamus and the regulation of energy homeostasis. Horm. Res. 1996, 46, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Seeley, R.J.; Yagaloff, K.A.; Fisher, S.L.; Burn, P.; Thiele, T.E.; Van Dijk, G.; Baskin, D.G.; Schwartz, M.W. Melanocortin receptors in leptin effects. Nature 1997, 390, 349. [Google Scholar] [CrossRef] [PubMed]
- Matson, C.A.; Wiater, M.F.; Kuijper, J.L.; Weigle, D.S. Synergy between leptin and cholecystokinin (CCK) to control daily caloric intake. Peptides 1997, 18, 1275–1278. [Google Scholar] [CrossRef] [PubMed]
- Ellacott, K.L.J.; Halatchev, I.G.; Cone, R.D. Interactions between gut peptides and the central melanocortin system in the regulation of energy homeostasis. Peptides 2006, 27, 340–349. [Google Scholar] [CrossRef]
- Klein, S.; Coppack, S.W.; Mohamed-Ali, V.; Landt, M. Adipose tissue leptin production and plasma leptin kinetics in humans. Diabetes 1996, 45, 984–987. [Google Scholar] [CrossRef]
- Niskanen, L.; Haffner, S.; Karhunen, L.J.; Turpeinen, A.K.; Miettinen, H.; Uusitupa, M.I.J. Serum leptin in relation to resting energy expenditure and fuel metabolism in obese subjects. Int. J. Obes. Relat. Metab. Disord. 1997, 21, 309–313. [Google Scholar] [CrossRef][Green Version]
- Frederich, R.C.; Hamann, A.; Anderson, S.; Löllmann, B.; Lowell, B.B.; Flier, J.S. Leptin levels reflect body lipid content in mice: Evidence for diet-induced resistance to leptin action. Nat. Med. 1995, 1, 1311–1314. [Google Scholar] [CrossRef]
- Myers, M.G.; Heymsfield, S.B.; Haft, C.; Kahn, B.B.; Laughlin, M.; Leibel, R.L.; Tschöp, M.H.; Yanovski, J.A. Challenges and opportunities of defining clinical leptin resistance. Cell Metab. 2012, 15, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Wabitsch, M.; Funcke, J.-B.; Lennerz, B.; Kuhnle-Krahl, U.; Lahr, G.; Debatin, K.-M.; Vatter, P.; Gierschik, P.; Moepps, B.; Fischer-Posovszky, P. Biologically inactive leptin and early-onset extreme obesity. N. Engl. J. Med. 2015, 372, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Mantzoros, C.S. The role of leptin in human obesity and disease: A review of current evidence. Ann. Intern. Med. 1999, 130, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Mark, A.L. Selective leptin resistance revisited. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R566–R581. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J. 20 years of leptin: Leptin at 20: An overview. J. Endocrinol. 2014, 223, T1–T8. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wong, J.; Brooks, V.L. Obesity: Sex and sympathetics. Biol. Sex Differ. 2020, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Oliveira, V.; Câmara, N.O.S.; Moraes-Vieira, P.M. Adipokines as drug targets in diabetes and underlying disturbances. J. Diabetes Res. 2015, 2015, 681612. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Seeley, R.J.; Campfield, L.A.; Burn, P.; Baskin, D.G. Identification of targets of leptin action in rat hypothalamus. J. Clin. Investig. 1996, 98, 1101–1106. [Google Scholar] [CrossRef]
- German, J.P.; Thaler, J.P.; Wisse, B.E.; Oh-I, S.; Sarruf, D.A.; Matsen, M.E.; Fischer, J.D.; Taborsky, G.J.; Schwartz, M.W.; Morton, G.J. Leptin activates a novel CNS mechanism for insulin-independent normalization of severe diabetic hyperglycemia. Endocrinology 2011, 152, 394–404. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Wang, R.; Zhang, J.P. The Effect of Sitagliptin on Obese Patients with Insulin Treatment-Induced Diabetes Mellitus. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3490–3495. [Google Scholar] [PubMed]
- Tang, X.; Li, J.; Xiang, W.; Cui, Y.; Xie, B.; Wang, X.; Xu, Z.; Gan, L. Metformin increases hepatic leptin receptor and decreases steatosis in mice. J. Endocrinol. 2016, 230, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Farooq, R.; Amin, S.; Hayat Bhat, M.; Malik, R.; Wani, H.A.; Majid, S. Type 2 diabetes and metabolic syndrome—Adipokine levels and effect of drugs. Gynecol. Endocrinol. 2017, 33, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.J.M.; Ware, D.P.; Lefer, D.J. Myocardial infarction and heart failure in the db/db diabetic mouse. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H146–H153. [Google Scholar] [CrossRef] [PubMed]
- Belke, D.D.; Larsen, T.S.; Gibbs, E.M.; Severson, D.L. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1104–E1113. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Rodrigues, B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1489–H1506. [Google Scholar] [CrossRef] [PubMed]
- Karmazyn, M.; Purdham, D.M.; Rajapurohitam, V.; Zeidan, A. Leptin as a cardiac hypertrophic factor: A potential target for therapeutics. Trends Cardiovasc. Med. 2007, 17, 206–211. [Google Scholar] [CrossRef]
- Katsiki, N.; Mikhailidis, D.P.; Banach, M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacol. Sin. 2018, 39, 1176–1188. [Google Scholar] [CrossRef]
- Lieb, W.; Sullivan, L.M.; Harris, T.B.; Roubenoff, R.; Benjamin, E.; Levy, D.; Fox, C.S.; Wang, T.J.; Wilson, P.W.; Kannel, W.B.; et al. Plasma leptin levels and incidence of heart failure, cardiovascular disease, and total mortality in elderly individuals. Diabetes Care 2009, 32, 612–616. [Google Scholar] [CrossRef]
- Rajapurohitam, V.; Izaddoustdar, F.; Martinez-Abundis, E.; Karmazyn, M. Leptin-induced cardiomyocyte hypertrophy reveals both calcium-dependent and calcium-independent/RhoA-dependent calcineurin activation and NFAT nuclear translocation. Cell. Signal. 2012, 24, 2283–2290. [Google Scholar] [CrossRef]
- Hall, M.E.; Harmancey, R.; Stec, D.E. Lean heart: Role of leptin in cardiac hypertrophy and metabolism. World J. Cardiol. 2015, 7, 511. [Google Scholar] [CrossRef] [PubMed]
- Leifheit-Nestler, M.; Wagner, N.M.; Gogiraju, R.; Didié, M.; Konstantinides, S.; Hasenfuss, G.; Schäfer, K. Importance of leptin signaling and signal transducer and activator of transcription-3 activation in mediating the cardiac hypertrophy associated with obesity. J. Transl. Med. 2013, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Matthes, J.; Schuster, I.; Valdivia, H.H.; Herzig, S.; Richard, S.; Gómez, A.M. Mechanisms of [Ca2+]i transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes 2006, 55, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Kho, C.; Lee, A.; Hajjar, R.J. Altered sarcoplasmic reticulum calcium cycling—targets for heart failure therapy. Nat. Rev. Cardiol. 2012, 9, 717–733. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Gustafsson, Å.B. Role of apoptosis in cardiovascular disease. Apoptosis 2009, 14, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhao, Y.; Xu, S.; Jin, C.; Wang, M.; Fu, G. Leptin confers protection against TNF-α-induced apoptosis in rat cardiomyocytes. Biochem. Biophys. Res. Commun. 2014, 455, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Abundis, E.; Rajapurohitam, V.; Haist, J.V.; Gan, X.T.; Karmazyn, M. The obesity-related peptide leptin sensitizes cardiac mitochondria to calcium-induced permeability transition pore opening and apoptosis. PLoS ONE 2012, 7, e41612. [Google Scholar] [CrossRef][Green Version]
- Dixon, R.A.; Davidson, S.M.; Wynne, A.M.; Yellon, D.M.; Smith, C.C.T. The cardioprotective actions of leptin are lost in the Zucker obese (fa/fa) rat. J. Cardiovasc. Pharmacol. 2009, 53, 311–317. [Google Scholar] [CrossRef]
- McGaffin, K.R.; Witham, W.G.; Yester, K.A.; Romano, L.C.; Odoherty, R.M.; McTiernan, C.F.; Odonnell, C.P. Cardiac-specific leptin receptor deletion exacerbates ischaemic heart failure in mice. Cardiovasc. Res. 2011, 89, 60–71. [Google Scholar] [CrossRef]
- Procopio, C.; Andreozzi, F.; Laratta, E.; Cassese, A.; Beguinot, F.; Arturi, F.; Hribal, M.L.; Perticone, F.; Sesti, G. Leptin-stimulated endothelial nitric-oxide synthase via an adenosine 5’-monophosphate-activated protein kinase/Akt signaling pathway is attenuated by interaction with C-reactive protein. Endocrinology 2009, 150, 3584–3593. [Google Scholar] [CrossRef]
- Bełtowski, J.; Wójcicka, G.; Jamroz-Wiśniewska, A.; Borkowska, E. Role of PI3K and PKB/Akt in acute natriuretic and NO-mimetic effects of leptin. Regul. Pept. 2007, 140, 168–177. [Google Scholar] [CrossRef]
- Jamroz-Wiśniewska, A.; Gertler, A.; Solomon, G.; Wood, M.E.; Whiteman, M.; Beltowski, J. Leptin-induced endothelium-dependent vasorelaxation of peripheral arteries in lean and obese rats: Role of nitric oxide and hydrogen sulfide. PLoS ONE 2014, 9, e86744. [Google Scholar] [CrossRef]
- Rahmouni, K.; Morgan, D.A.; Morgan, G.M.; Mark, A.L.; Haynes, W.G. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes 2005, 54, 2012–2018. [Google Scholar] [CrossRef]
- Bell, B.B.; Rahmouni, K. Leptin as a Mediator of Obesity-Induced Hypertension. Curr. Obes. Rep. 2016, 5, 397–404. [Google Scholar] [CrossRef]
- Faulkner, J.L.; Bruder-Nascimento, T.; Belin De Chantemèle, E.J. The regulation of aldosterone secretion by leptin: Implications in obesity-related cardiovascular disease. Curr. Opin. Nephrol. Hypertens. 2018, 27, 63–69. [Google Scholar] [CrossRef]
- Beldhuis, I.E.; Myhre, P.L.; Bristow, M.; Claggett, B.; Damman, K.; Fang, J.C.; Fleg, J.L.; McKinlay, S.; Lewis, E.F.; O’Meara, E.; et al. Spironolactone in Patients With Heart Failure, Preserved Ejection Fraction, and Worsening Renal Function. J. Am. Coll. Cardiol. 2021, 77, 1211–1221. [Google Scholar] [CrossRef]
- Beltowski, J. Leptin and atherosclerosis. Atherosclerosis 2006, 189, 47–60. [Google Scholar] [CrossRef] [PubMed]
- VanPatten, S.; Karkanias, G.B.; Rossetti, L.; Cohen, D.E. Intracerebroventricular leptin regulates hepatic cholesterol metabolism. Biochem. J. 2004, 379, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.K.; Steinberg, G.R. AMPK and the Endocrine Control of Metabolism. Endocr. Rev. 2023, 44, 910–933. [Google Scholar] [CrossRef] [PubMed]
- Tommerdahl, K.L.; Baumgartner, K.; Schäfer, M.; Bjornstad, P.; Melena, I.; Hegemann, S.; Baumgartner, A.D.; Pyle, L.; Cree-Green, M.; Truong, U.; et al. Impact of Obesity on Measures of Cardiovascular and Kidney Health in Youth with Type 1 Diabetes as Compared with Youth with Type 2 Diabetes. Diabetes Care 2021, 44, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Bruder-Nascimento, T.; Kress, T.C.; Belin De Chantemele, E.J. Recent advances in understanding lipodystrophy: A focus on lipodystrophy-associated cardiovascular disease and potential effects of leptin therapy on cardiovascular function. F1000Research 2019, 8, F1000. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.G.; Crujeiras, A.B.; Casanueva, F.F.; Carreira, M.C. Leptin, Obesity, and Leptin Resistance: Where Are We 25 Years Later? Nutrients 2019, 11, 2704. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Mattace Raso, G.; Meli, R. Drug targeting of leptin resistance. Life Sci. 2015, 140, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.D.; Roland, B.L.; Cole, R.L.; Trevaskis, J.L.; Weyer, C.; Koda, J.E.; Anderson, C.M.; Parkes, D.G.; Baron, A.D. Leptin responsiveness restored by amylin agonism in diet-induced obesity: Evidence from nonclinical and clinical studies. Proc. Natl. Acad. Sci. USA 2008, 105, 7257–7262. [Google Scholar] [CrossRef]
- Trevaskis, J.L.; Coffey, T.; Cole, R.; Lei, C.; Wittmer, C.; Walsh, B.; Weyer, C.; Koda, J.; Baron, A.D.; Parkes, D.G.; et al. Amylin-mediated restoration of leptin responsiveness in diet-induced obesity: Magnitude and mechanisms. Endocrinology 2008, 149, 5679–5687. [Google Scholar] [CrossRef]
- Liu, J.; Lee, J.; Hernandez, M.A.S.; Mazitschek, R.; Ozcan, U. Treatment of obesity with celastrol. Cell 2015, 161, 999–1011. [Google Scholar] [CrossRef]
- Feng, X.; Guan, D.; Auen, T.; Choi, J.W.; Salazar Hernández, M.A.; Lee, J.; Chun, H.; Faruk, F.; Kaplun, E.; Herbert, Z.; et al. IL1R1 is required for celastrol’s leptin-sensitization and antiobesity effects. Nat. Med. 2019, 25, 575–582. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Duan, X.; Zhao, G.; Zhang, M. Celastrol: A Promising Agent Fighting against Cardiovascular Diseases. Antioxidants 2022, 11, 1597. [Google Scholar] [CrossRef]
- Berglund, E.D.; Grobe, J.L.; Rahmouni, K.; Cui, H.; Saito, K.; Davis, K.C.; Morgan, D.A.; Toth, B.A.; Jiang, J.; Singh, U. Celastrol Reduces Obesity in MC4R Deficiency and Stimulates Sympathetic Nerve Activity Affecting Metabolic and Cardiovascular Functions. Diabetes 2019, 68, 1210–1220. [Google Scholar] [CrossRef]
- Chakhtoura, M.; Haber, R.; Ghezzawi, M.; Rhayem, C.; Tcheroyan, R.; Mantzoros, C.S. Pharmacotherapy of obesity: An update on the available medications and drugs under investigation. eClinicalMedicine 2023, 58, 101882. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilariño-García, T.; Polonio-González, M.L.; Pérez-Pérez, A.; Ribalta, J.; Arrieta, F.; Aguilar, M.; Obaya, J.C.; Gimeno-Orna, J.A.; Iglesias, P.; Navarro, J.; et al. Role of Leptin in Obesity, Cardiovascular Disease, and Type 2 Diabetes. Int. J. Mol. Sci. 2024, 25, 2338. https://doi.org/10.3390/ijms25042338
Vilariño-García T, Polonio-González ML, Pérez-Pérez A, Ribalta J, Arrieta F, Aguilar M, Obaya JC, Gimeno-Orna JA, Iglesias P, Navarro J, et al. Role of Leptin in Obesity, Cardiovascular Disease, and Type 2 Diabetes. International Journal of Molecular Sciences. 2024; 25(4):2338. https://doi.org/10.3390/ijms25042338
Chicago/Turabian StyleVilariño-García, Teresa, María L. Polonio-González, Antonio Pérez-Pérez, Josep Ribalta, Francisco Arrieta, Manuel Aguilar, Juan C. Obaya, José A. Gimeno-Orna, Pedro Iglesias, Jorge Navarro, and et al. 2024. "Role of Leptin in Obesity, Cardiovascular Disease, and Type 2 Diabetes" International Journal of Molecular Sciences 25, no. 4: 2338. https://doi.org/10.3390/ijms25042338
APA StyleVilariño-García, T., Polonio-González, M. L., Pérez-Pérez, A., Ribalta, J., Arrieta, F., Aguilar, M., Obaya, J. C., Gimeno-Orna, J. A., Iglesias, P., Navarro, J., Durán, S., Pedro-Botet, J., & Sánchez-Margalet, V. (2024). Role of Leptin in Obesity, Cardiovascular Disease, and Type 2 Diabetes. International Journal of Molecular Sciences, 25(4), 2338. https://doi.org/10.3390/ijms25042338







