Serotonergic and Adrenergic Neuroreceptor Manipulation Ameliorates Core Symptoms of ADHD through Modulating Dopaminergic Receptors in Spontaneously Hypertensive Rats
Abstract
1. Introduction
2. Results
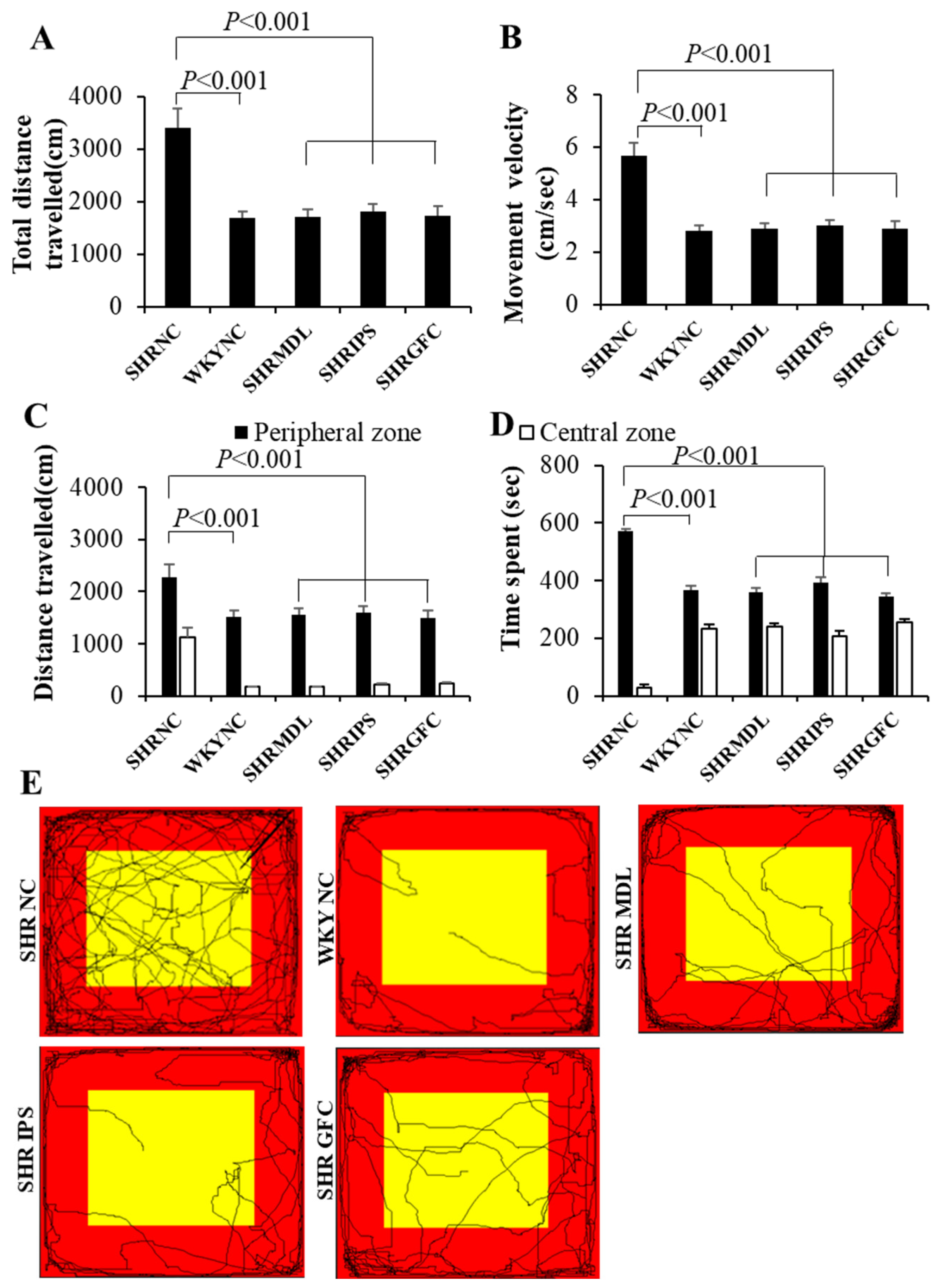
2.1. General Locomotor and Exploratory Behavior
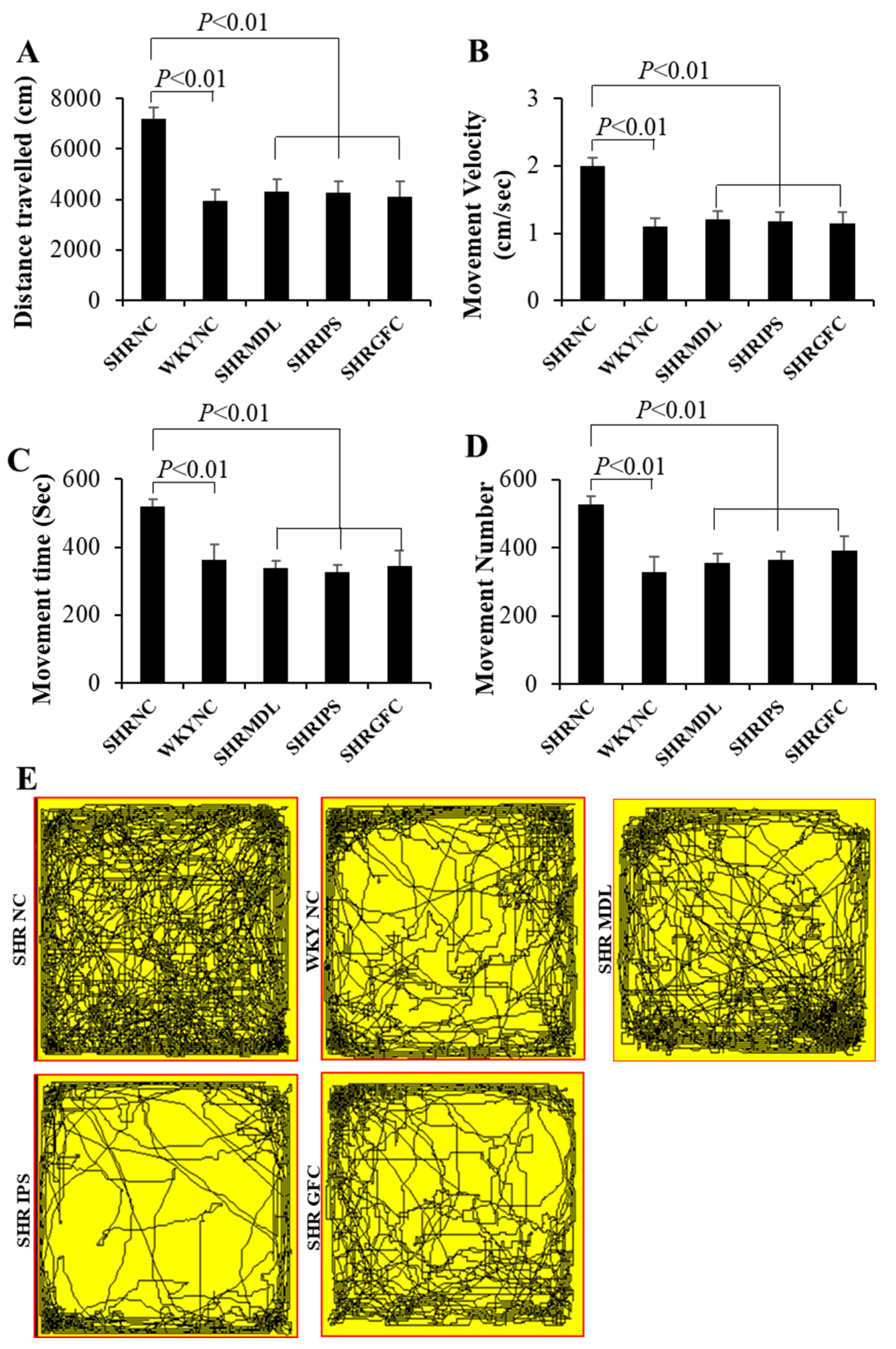
2.2. Locomotor Hyperactivity
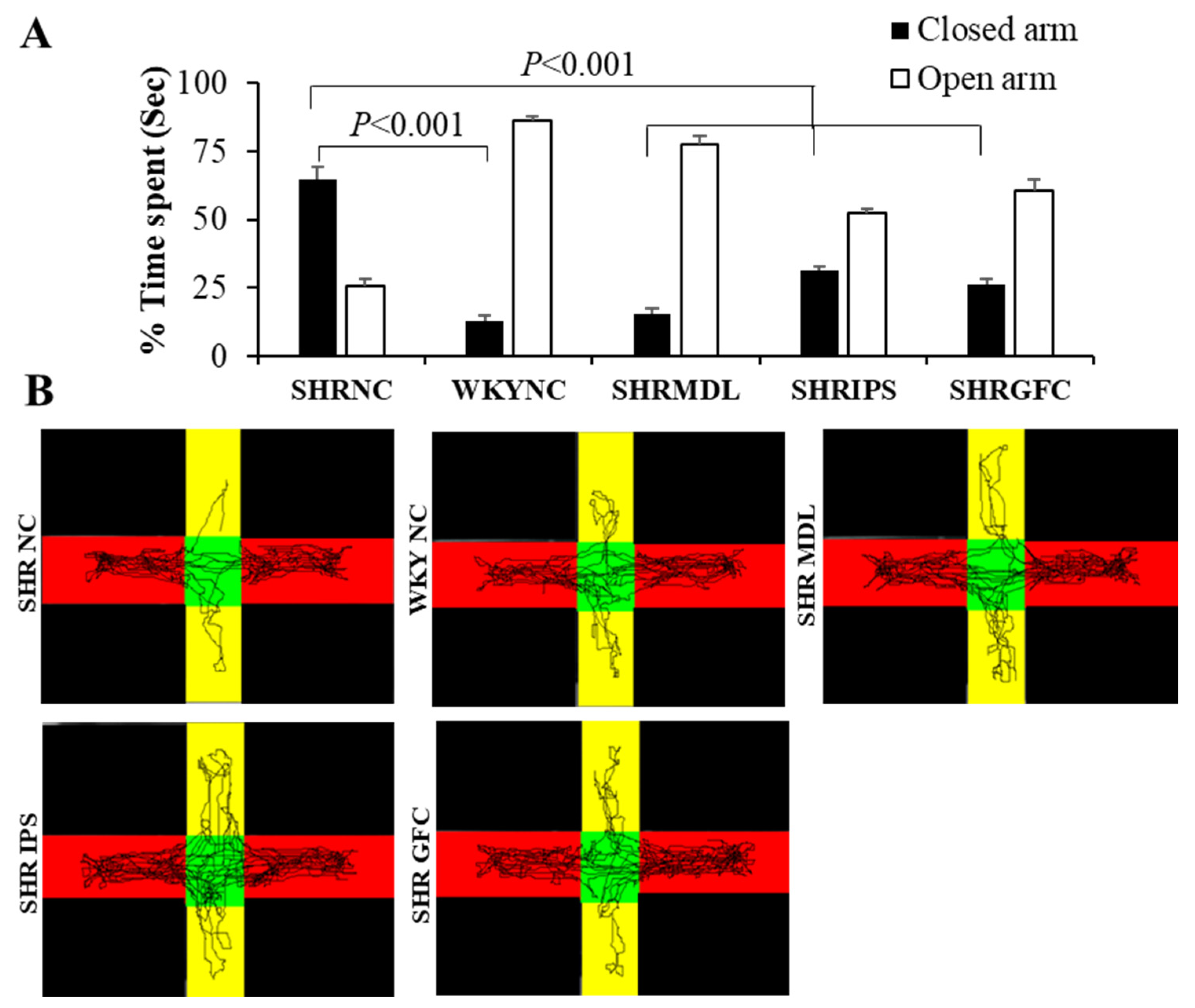
2.3. Anxiety Level
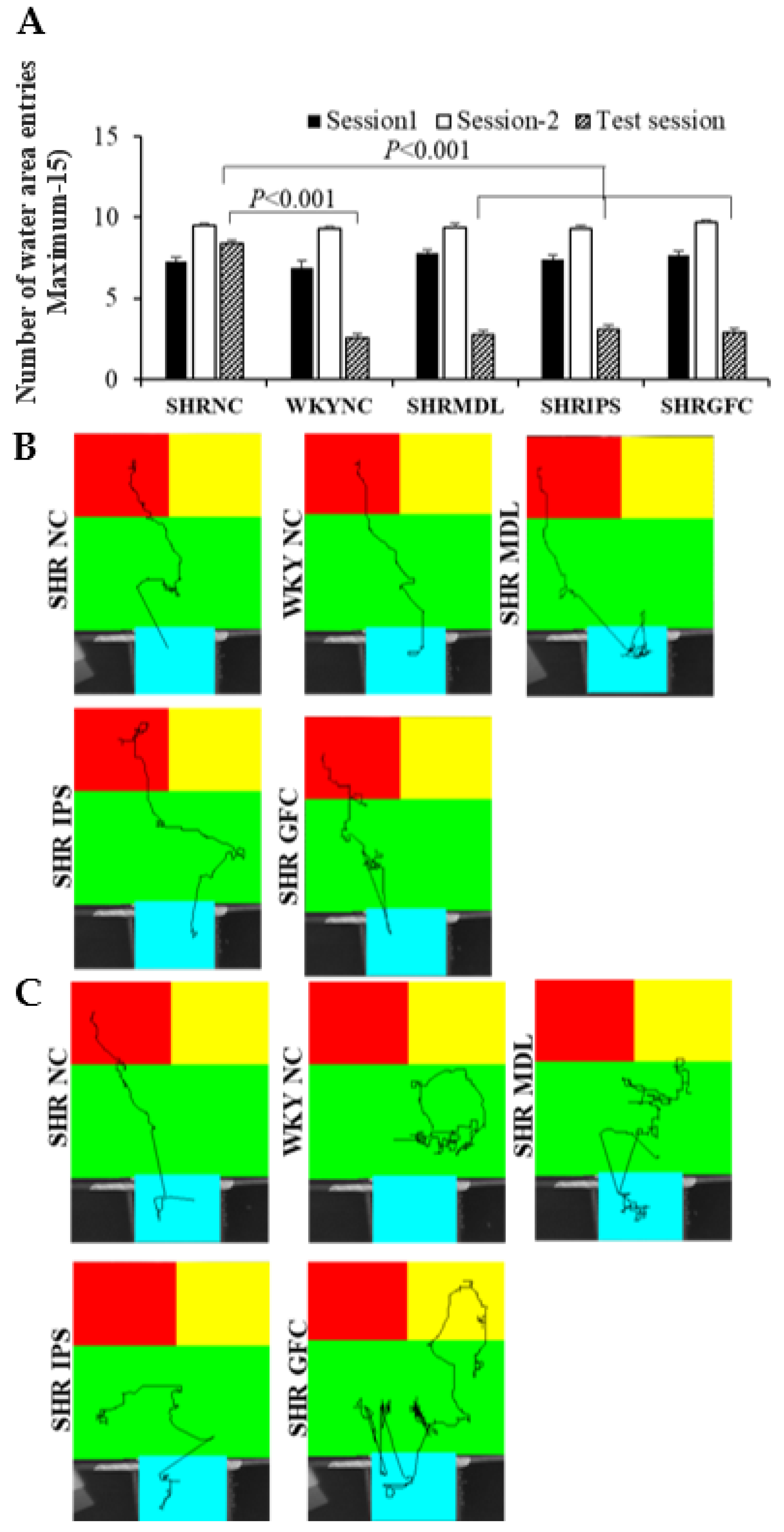
2.4. Impulsivity (Tolerance Delay) in Modified T-Maze Test
2.5. Impulsive Water Drinking (in Aversive Electro Foot Shock Apparatus)
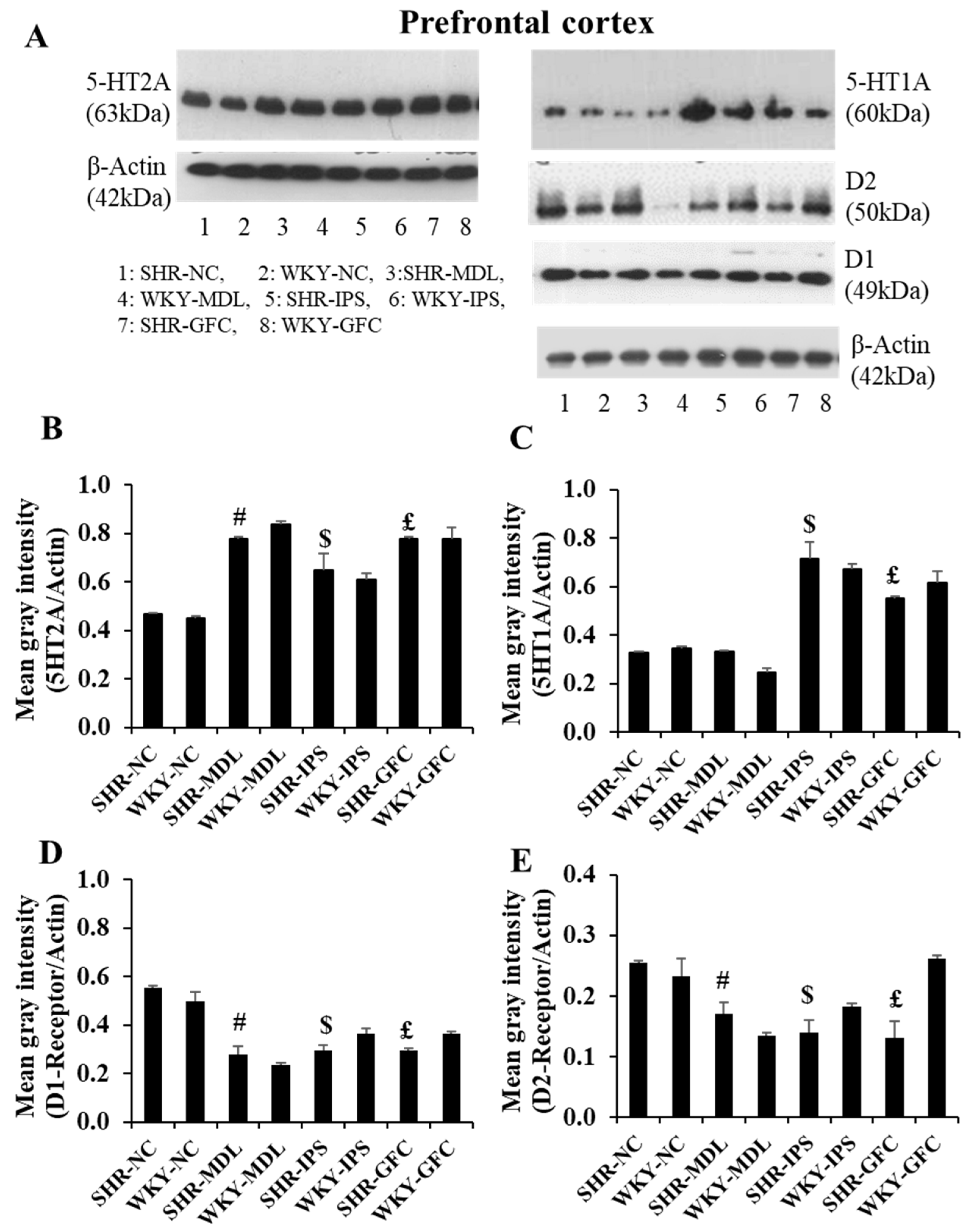
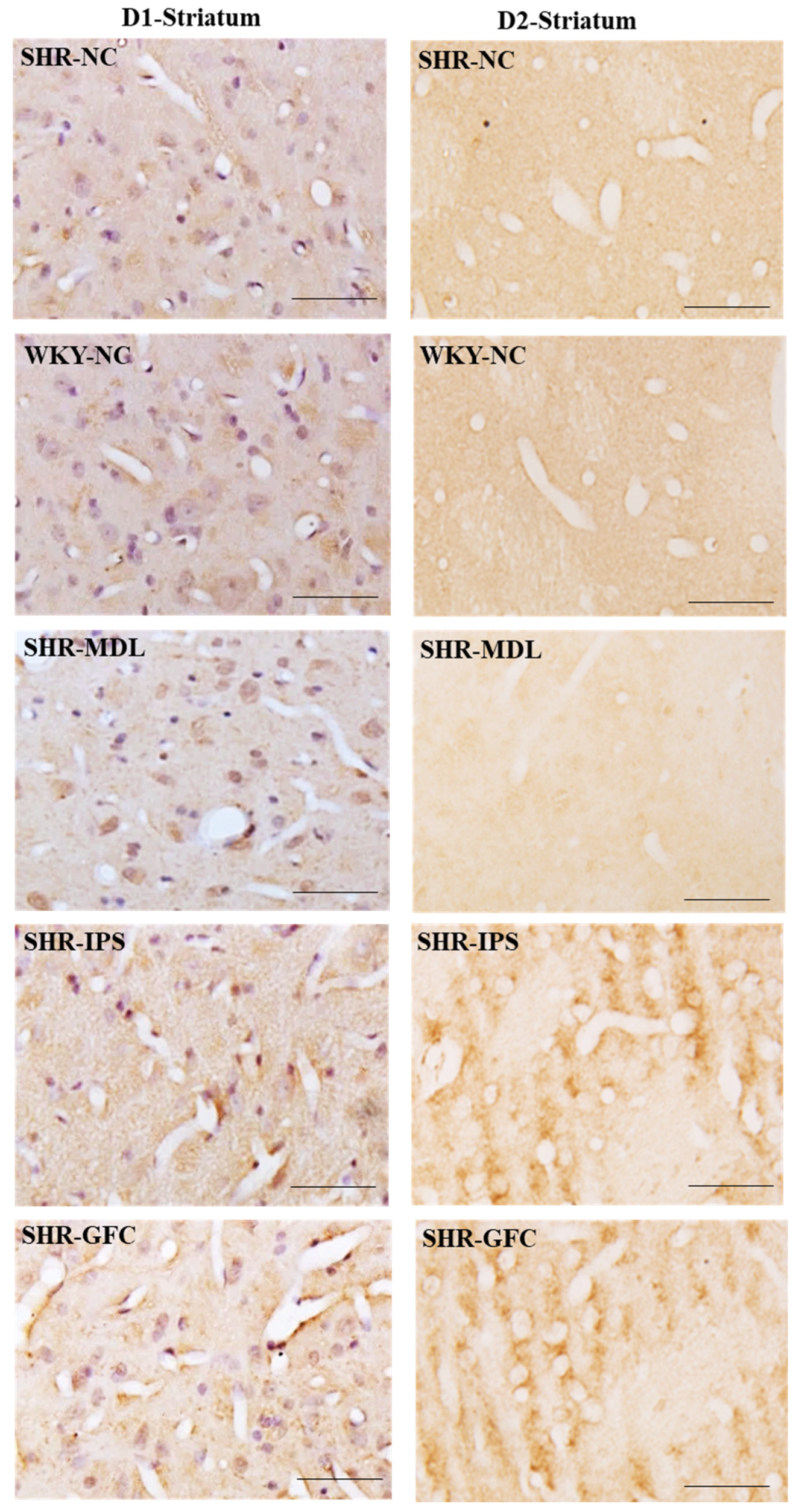
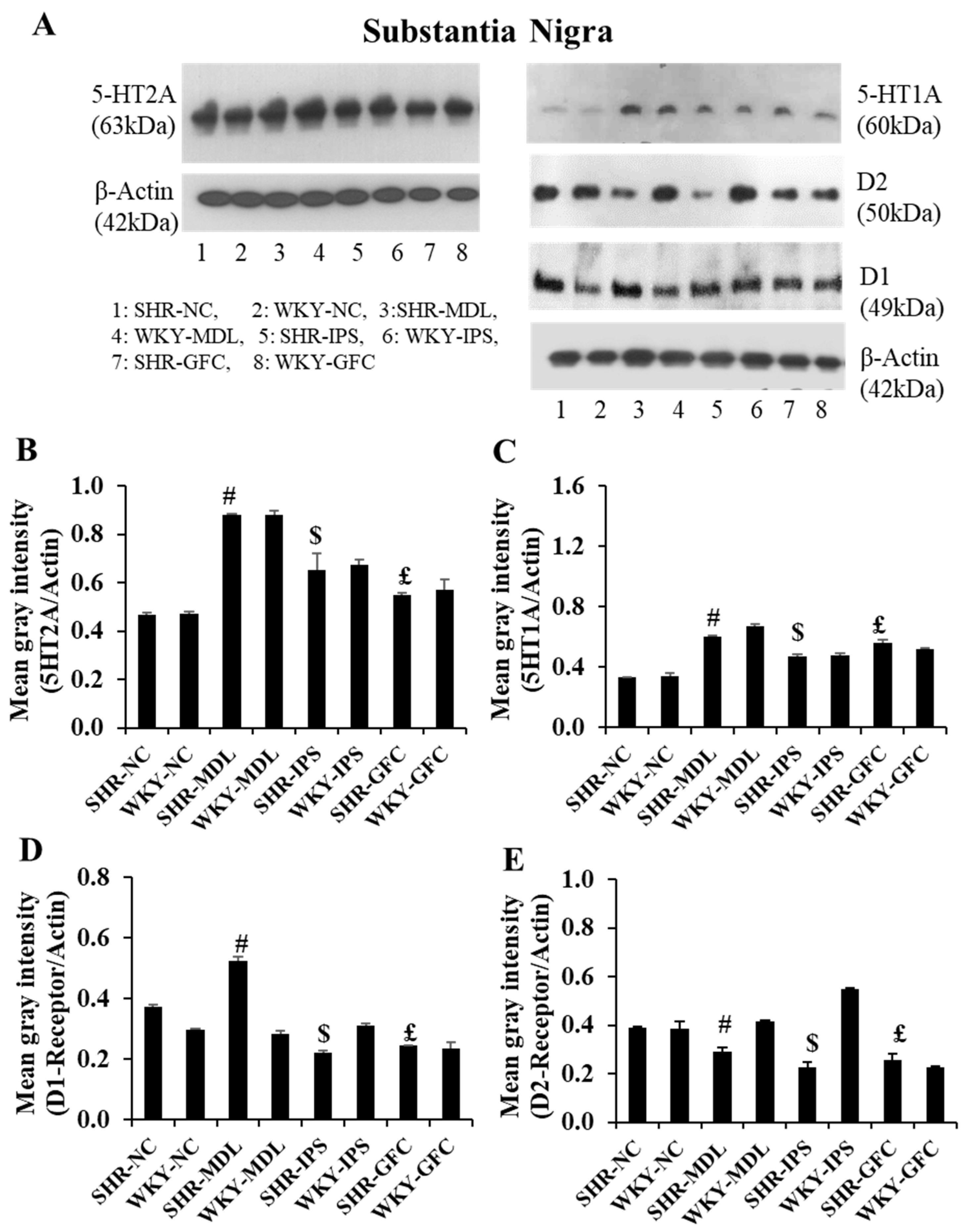
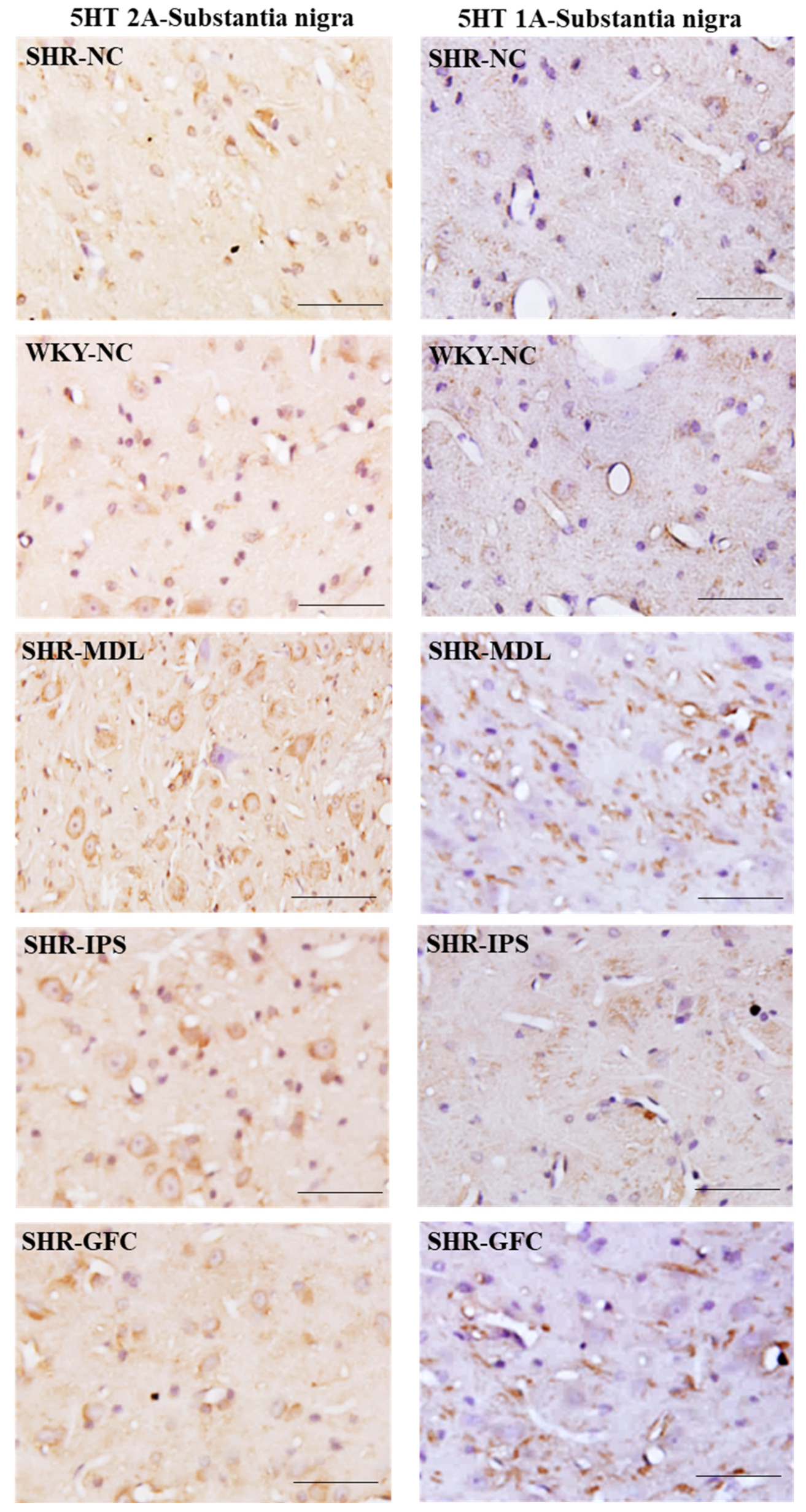
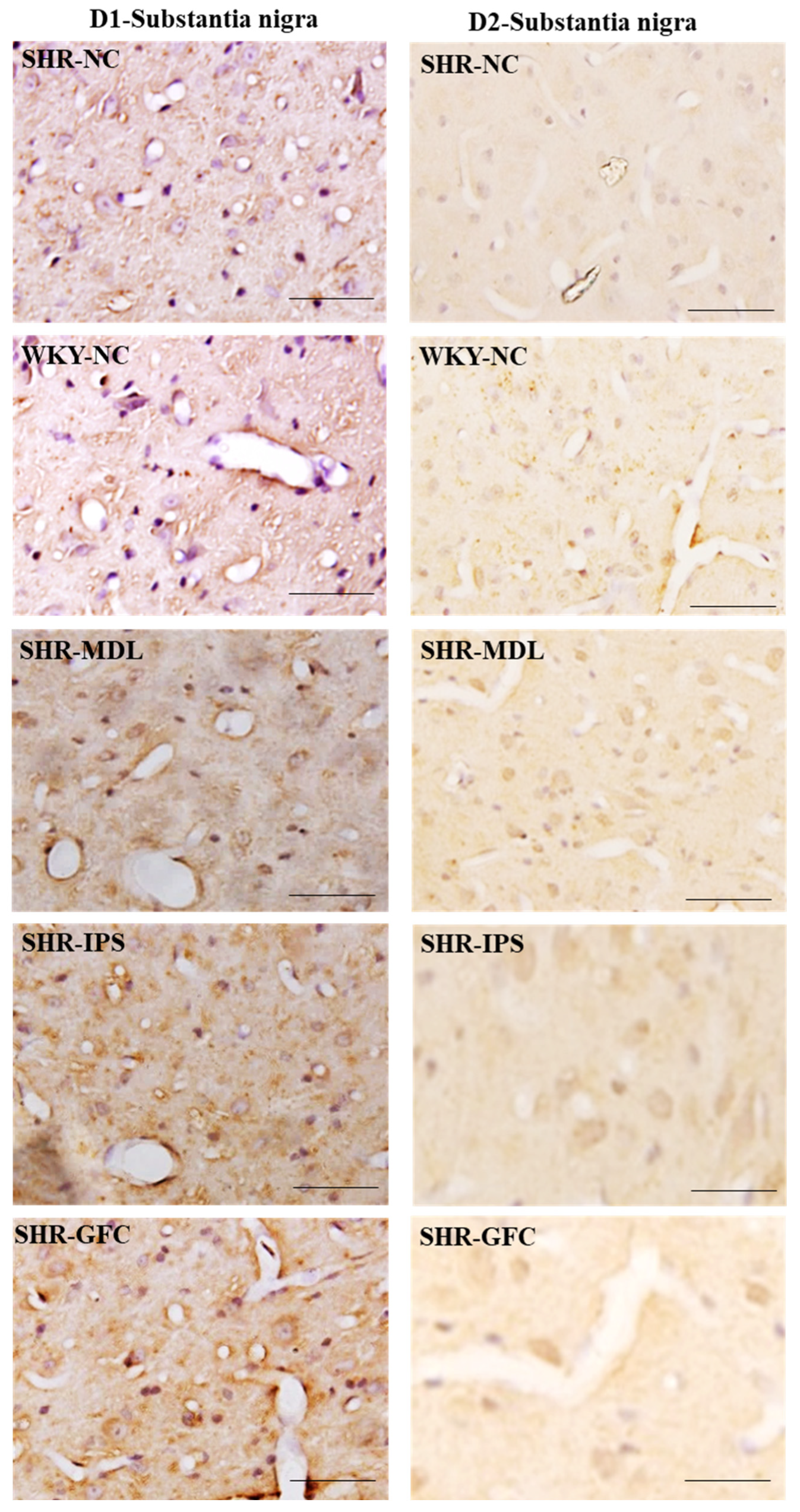
2.6. Neuroreceptor Quantity and Expression in Prefrontal Cortex, Striatum, and Substantia Nigra
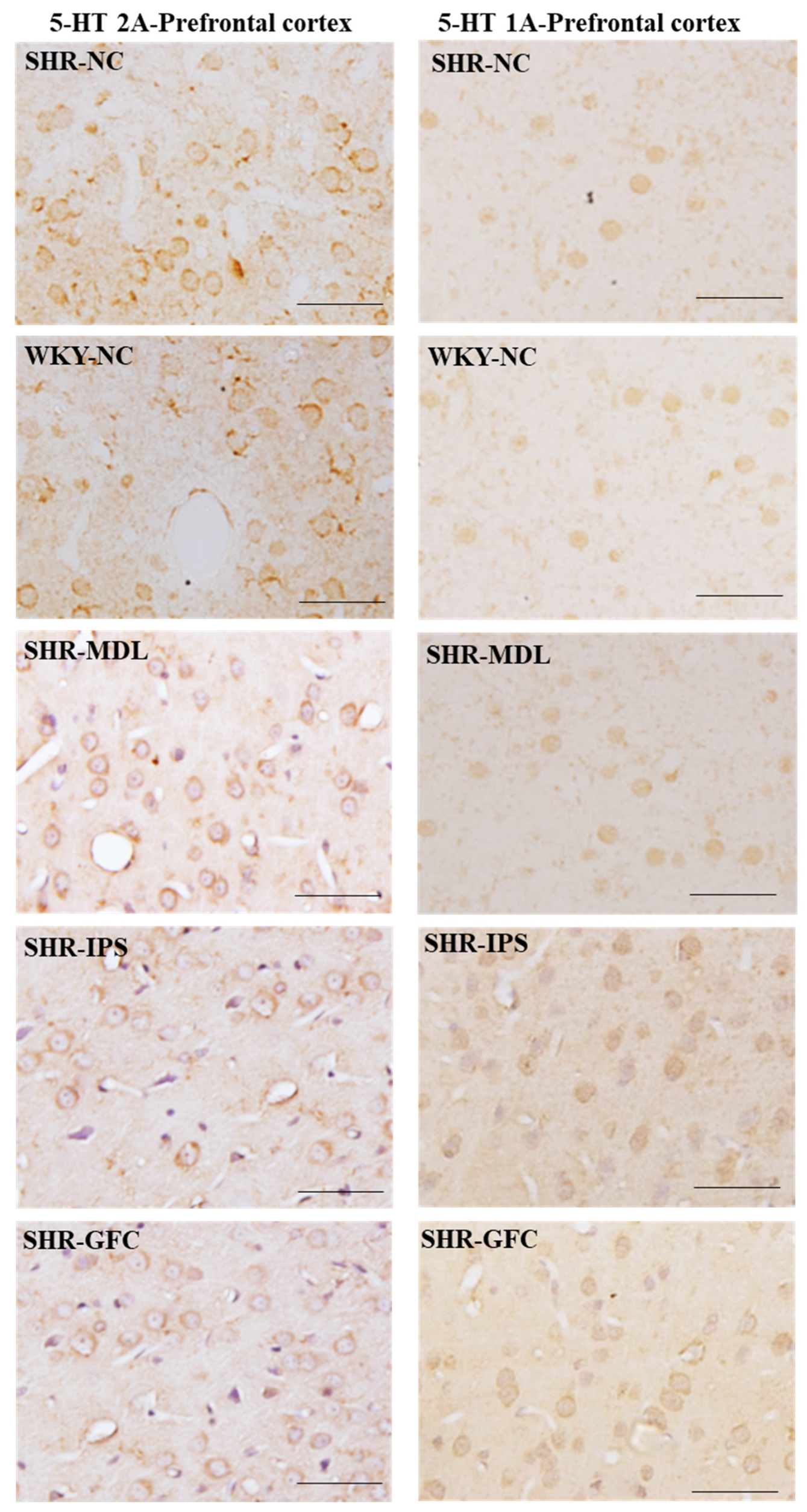
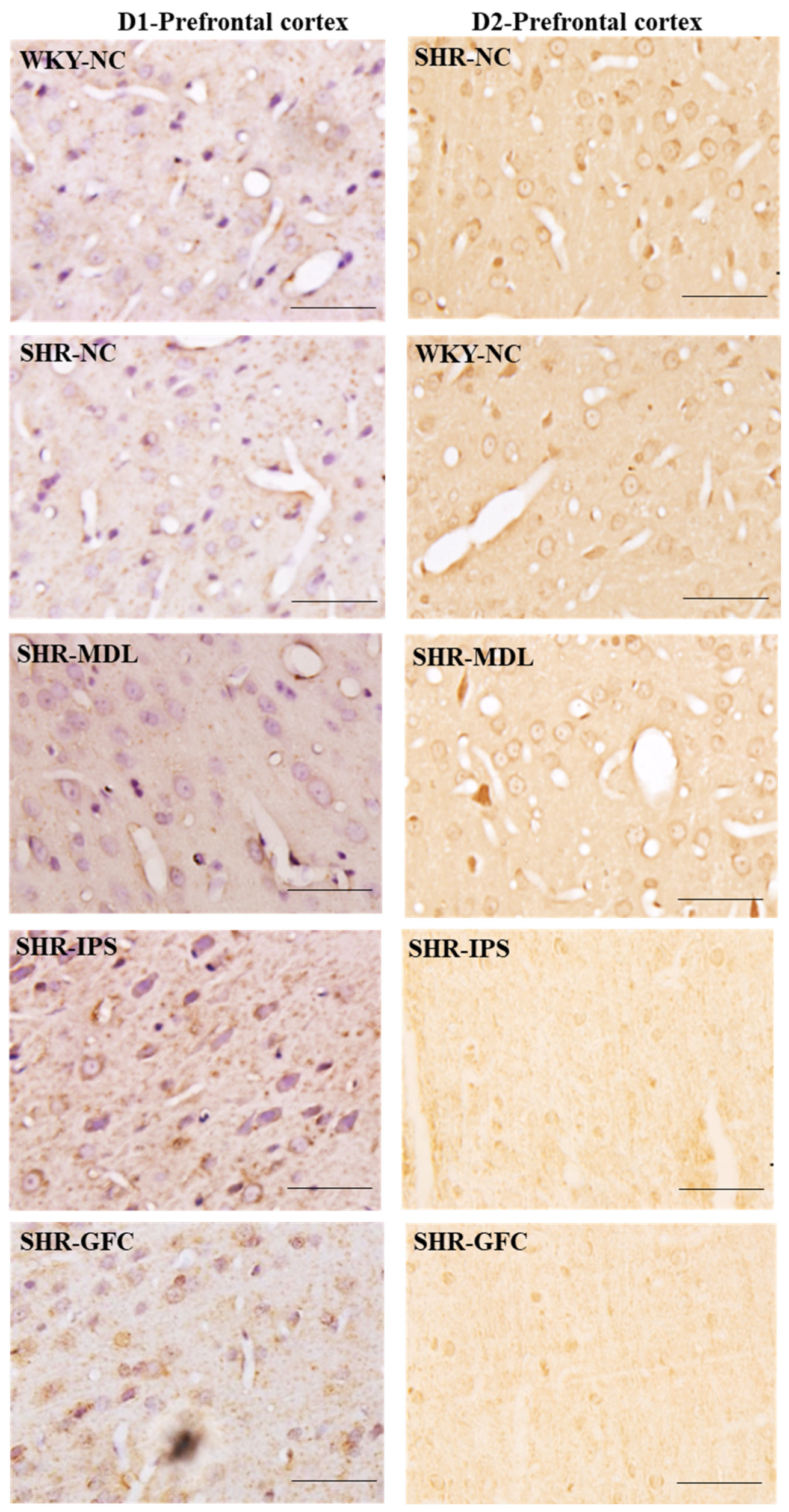
2.6.1. Prefrontal Cortex
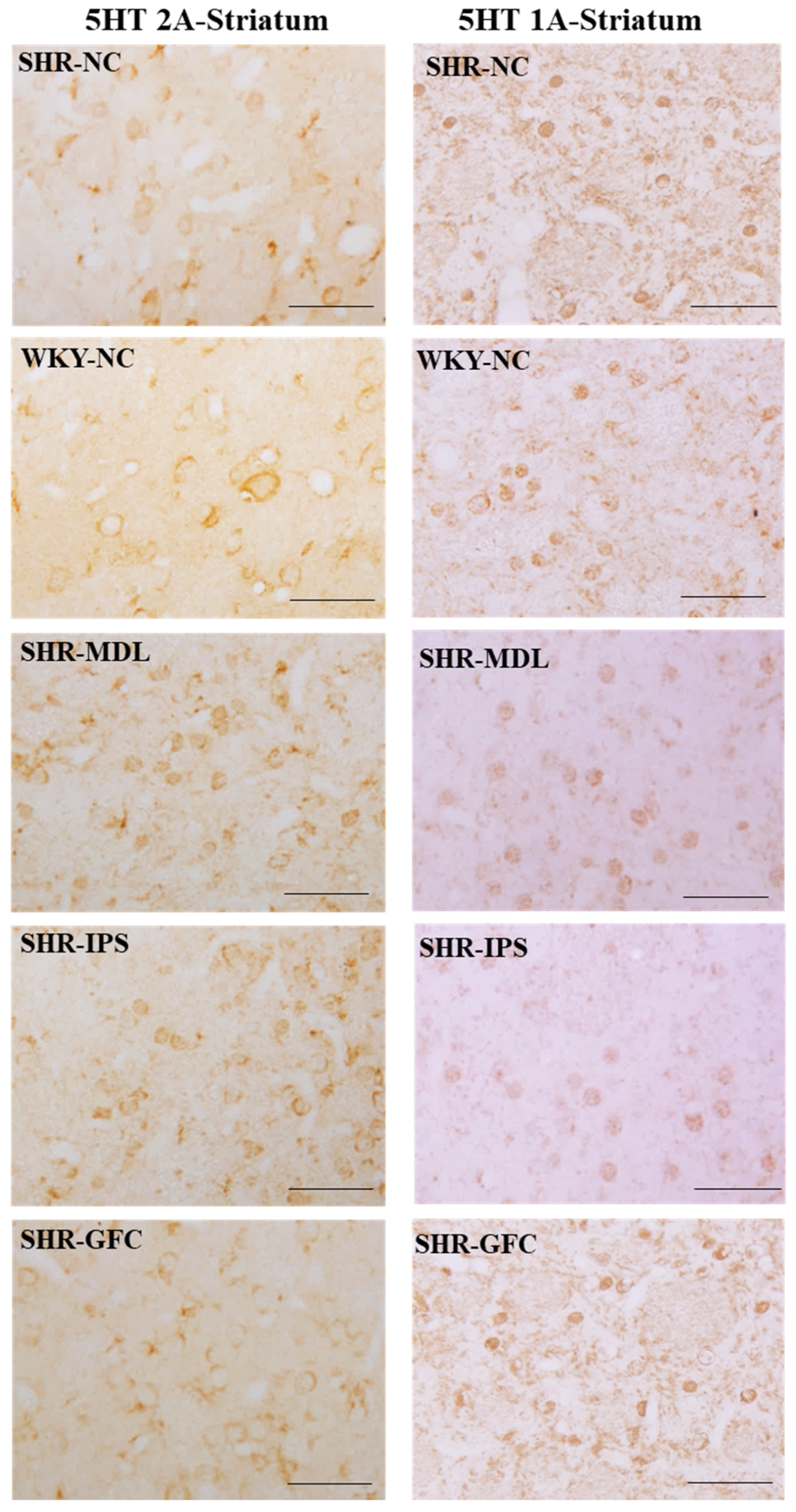
2.6.2. Striatum
2.7. Substantia Nigra
3. Discussion
3.1. 5-HT1A Agonist Effects
3.2. 5-HT2A Antagonism Effects
3.3. Alpha-2 Agonist Effects
3.4. Expression of DA Receptors
3.5. Involvement of the Striatum, Prefrontal Cortex, and the Substantia Nigra in ADHD
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Agonist and Antagonist Dose and Preparation
4.4. Behavioral Tests (for Assessment of ADHD)
4.4.1. General Locomotor and Exploratory Behavior in a Novel Environment
4.4.2. Locomotor Hyperactivity Assessment
4.4.3. Anxiety-like Behavior Assessment in Elevated plus Maze
4.4.4. Impulsivity Analysis in Modified T-Maze
4.4.5. Impulsivity Analysis in Electro Foot Shock Aversive Water Drinking Test (EFSDT)
4.5. Western Blotting
4.6. Immunohistochemistry
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Visser, S.; Danielson, M.; Bitsko, R.; Holbrook, J.; Kogan, M.; Ghandour, R.; Perou, R.; Blumberg, S. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. J. Am. Acad Child Adolesc. Psychiatry 2014, 53, 34–46. [Google Scholar] [CrossRef]
- Levy, F. Dopamine vs. noradrenaline: Inverted-U effects and ADHD theories. Aust. N. Z. J. Psychiatry 2009, 43, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Oades, R.D. Dopamine-serotonin interactions in attention-deficit hyperactivity disorder. Prog. Brain Res. 2008, 172, 543–565. [Google Scholar] [PubMed]
- Pattij, T.; Schoffelmeer, A.N. Serotonin and inhibitory response control: Focusing on the role of 5-HT(1A) receptors. Eur. J. Pharmacol. 2015, 15, 140–145. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. NICE Guideline (2020)—Attention Deficit Hyperactivity Disorder: Diagnosis and Management. Available online: https://www.nice.org.uk/guidance/ng87/resources/attention-deficit-hyperactivity-disorder-diagnosis-and-management-pdf-1837699732933 (accessed on 1 December 2022).
- Faraone, S.V. The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci. Biobehav. Rev. 2018, 87, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Banaschewski, T.; Johnson, M.; Lecendreux, M.; Zuddas, A.; Adeyi, B.; Hodgkins, P.; Squires, L.A.; Coghill, D.R. Health-related quality of life and functional outcomes from a randomized-withdrawal study of long-term lisdexamfetamine dimesylate treatment in children and adolescents with attention-deficit/hyperactivity disorder. CNS Drugs. 2014, 28, 1191–1203. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Man, K.K.C.; Coghill, D.; Chan, E.W.; Lau, W.C.Y.; Hollis, C.; Liddle, E.; Banaschewski, T.; McCarthy, S.; Neubert, A.; Sayal, K.; et al. Association of Risk of Suicide Attempts With Methylphenidate Treatment. JAMA Psychiatry 2017, 74, 1048–1055. [Google Scholar] [CrossRef]
- Childress, A.; Sallee, F. Pozanicline for the treatment of attention deficit/hyperactivity disorder. Expert. Opin. Investig. Drugs. 2014, 23, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M. Stahl’s Essential Psychopharmacology; Cambridge University Press: New York, NY, USA, 2008; pp. 878–879. [Google Scholar]
- Moore, K.E. The actions of amphetamine on neurotransmitters: A brief review. Biol. Psychiatry 1977, 12, 451–462. [Google Scholar]
- Pehek, A.E. Comparison of effects of Haloperidol administration on amphetamine-stimulated dopamine release in the rat medial prefrontal cortex and dorsal striatum. J. Pharmacol. Exp. Therapeut. 1999, 289, 14–23. [Google Scholar]
- Nakahra, T.; Hashimoto, K.; Hondo, H.; Tsutsumi, T.; Motomura, K.; Ueki, H.; Hirano, M.; Uchimura, H. Effect of atypical antipsychotics on phencyclidine-induced expression of arc in rat brain. Neuroreport 2000, 11, 551–555. [Google Scholar] [CrossRef]
- Kargieman, L.; Santana, N.; Mengod, G.; Celada, P.; Artigas, F. NMDA antagonist and antipsychotic actions in cortico-subcortical circuits. Neurotox. Res. 2008, 14, 129–140. [Google Scholar] [CrossRef]
- Bymaster, F.; Katner, J.S.; Nelson, D.L.; Hemrick-Luecke, S.K.; Threlkeld, P.G.; Heiligenstein, J.H.; Morin, S.M.; Gehlert, D.R.; Perry, K.W. Atomoxetine Increases Extracellular Levels of Norepinephrine and Dopamine in Prefrontal Cortex of Rat: A Potential Mechanism for Efficacy in Attention Deficit/Hyperactivity Disorder. Neuropsychopharmacology 2002, 27, 699–711. [Google Scholar] [CrossRef]
- Bolea-Alamañac, B.; Nutt, D.J.; Adamou, M.; Asherson, P.; Bazire, S.; Coghill, D.; Heal, D.; Müller, U.; Nash, J.; Santosh, P.; et al. Evidence-based guidelines for the pharmacological management of attention deficit hyperactivity disorder: Update on recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 2014, 28, 179–203. [Google Scholar] [CrossRef]
- Callahan, P.M.; Plagenhoef, M.R.; Blake, D.T.; Terry, A.V., Jr. Atomoxetine improves memory and other components of executive function in young-adult rats and aged rhesus monkeys. Neuropharmacology 2019, 155, 65–75. [Google Scholar] [CrossRef]
- Fu, D.; Wu, D.D.; Guo, H.L.; Hu, Y.H.; Xia, Y.; Ji, X.; Fang, W.R.; Li, Y.M.; Xu, J.; Chen, F.; et al. The Mechanism, Clinical Efficacy, Safety, and Dosage Regimen of Atomoxetine for ADHD Therapy in Children: A Narrative Review. Front. Psychiatry 2022, 12, 780921. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.; Bangs, M.; Trzepacz, P.; Jin, L.; Zhang, S.; Witte, M.M.; Ball, S.G.; Spencer, T.J. Safety and tolerability of atomoxetine over 3 to 4 years in children and adolescents with ADHD. J. Am. Acad. Child. Adolesc. Psychiatry 2009, 48, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Lee, T.D.; Lombroso, P.J.; King, R.A. Atomoxetine and tics in ADHD. J. Am. Acad. Child. Adolesc. Psychiatry 2004, 43, 1068–1069. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, M. Atomoxetine use associated with onset of a motor tic. J. Child. Adolesc. Psychopharmacol. 2005, 15, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Garnock-Jones, K.P.; Keating, G.M. Atomoxetine: A review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr. Drugs. 2009, 11, 203–226. [Google Scholar] [CrossRef] [PubMed]
- Huss, M.; Chen, W.; Ludolph, A.G. Guanfacine Extended Release: A New Pharmacological Treatment Option in Europe. Clin. Drug Investig. 2016, 36, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V.; McBurnett, K.; Sallee, F.R.; Steeber, J.; López, F.A. Guanfacine extended release: A novel treatment for attention-deficit/hyperactivity disorder in children and adolescents. Clin. Ther. 2013, 35, 1778–1793. [Google Scholar] [CrossRef] [PubMed]
- Cantwell, D.P.; Swanson, J.; Connor, D.F. Case study: Adverse response to clonidine. J. Am. Acad. Child. Adolesc. Psychiatry 1997, 36, 539–544. [Google Scholar] [CrossRef] [PubMed]
- McNeil, S.E.; Gibbons, J.R.; Cogburn, M. Risperidone. In Stat. Pearls [Internet]; Stat. Pearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459313/ (accessed on 1 December 2022).
- Eapen, V.; Gururaj, A.K. Risperidone treatment in 12 children with developmental disorders and attention-deficit/hyperactivity disorder. Prim. Care Companion J. Clin. Psychiatry 2005, 7, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Martsenkovsky, I.; Martsenkovska, I.; Martsenkovskyi, D. Risperidone and Atomoxetine in the Treatment of Several and Challenging Behaviors in Children with PDD. European Psychiatry 2015, 30, 195. [Google Scholar] [CrossRef]
- Correll, C.U.; Carlson, H.E. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J. Am. Acad. Child. Adolesc. Psychiatry 2006, 45, 771–791. [Google Scholar] [CrossRef] [PubMed]
- Safer, D.J. A comparison of risperidone-induced weight gain across the age span. J. Clin. Psychopharmacol. 2004, 24, 429–435. [Google Scholar] [CrossRef]
- Findling, R.L.; Kusumakar, V.; Daneman, D.; Moshang, T.; Desmedt, G.; Binder, C. Prolactin levels during long-term risperidone treatment in children and adolescents. J. Clin. Psychiatry 2003, 64, 1362–1369. [Google Scholar] [CrossRef]
- Maan, J.S.; Ershadi, M.; Khan, I.; Saadabadi, A. Quetiapine. 22. In Stat Pearls [Internet]; Stat Pearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://pubmed.ncbi.nlm.nih.gov/29083706/ (accessed on 1 December 2022).
- Artigas, F. Developments in the field of antidepressants, where do we go now? Eur. Neuropsychopharmacol. 2013, 25, 657–670. [Google Scholar] [CrossRef]
- Ortega-Ruiz, M.; Soria-Chacartegui, P.; Villapalos-García, G.; Abad-Santo, F.; Zubiaur, P. The Pharmacogenetics of Treatment with Quetiapine. Future Pharmacol. 2022, 2, 276–286. [Google Scholar] [CrossRef]
- Kronenberger, W.G.; Giauque, A.L.; Lafata, D.E.; Bohnstedt, B.N.; Maxey, L.E.; Dunn, D.W. Quetiapine addition in methylphenidate treatment-resistant adolescents with comorbid ADHD, conduct/oppositional-defiant disorder, and aggression: A prospective, open-label study. J. Child. Adolesc. Psychopharmacol. 2007, 17, 334–347. [Google Scholar] [CrossRef]
- Naguy, A. Low-dose quetiapine complements stimulant response in attention deficit hyperactivity disorder and more. Ther. Adv. Psychopharmacol. 2016, 6, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Park, P.; Caballero, J.; Omidian, H. Use of Serotonin Norepinephrine Reuptake Inhibitors in the Treatment of Attention-Deficit Hyperactivity Disorder in Pediatrics. Ann. Pharmacother. 2014, 48, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Wankerl, B.; Hauser, J.; Makulska-Gertruda, E.; Reibmann, A.; Sontag, T.; Tucha, O.; Lange, K. Neurobiology of attention deficit hyperactivity disorder. Fortschritte Der Neurol.-Psychiatrie 2014, 82, 9–29. [Google Scholar] [CrossRef]
- Riley, T.B.; Overton, P.G. Enhancing the efficacy of 5-HT uptake inhibitors in the treatment of attention deficit hyperactivity disorder. Med. Hypotheses 2019, 133, 109407. [Google Scholar] [CrossRef]
- Aznar, S.; Hervig, M.-S. The 5-HT2A serotonin receptor in executive function: Implications for neuropsychiatric and neurodegenerative diseases. Neurosci. Biobehav. Rev. 2016, 64, 63–82. [Google Scholar] [CrossRef]
- Wingen, M.; Kuypers, K.P.; Ramaekers, J.G. The role of 5-HT1a and 5-HT2a receptors in attention and motor control: A mechanistic study in healthy volunteers. Psychopharmacology 2007, 190, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.; Michael, K. Effects of acute intra-cerebral administration of the 5-HT2A/C receptor ligands DOI and ketanserin on impulse control in rats. Behav. Brain Res. 2009, 204, 88–92. [Google Scholar] [CrossRef]
- Wischhof, L.; Hollensteiner, K.J.; Koch, M. Impulsive behaviour in rats induced by intracortical DOI infusions is antagonized by co-administration of an mGlu2/3 receptor agonist. Behav. Pharmacol. 2011, 22, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, J.; Meltzer, H.Y. Effect of antidepressants on striatal and accumbens extracellular dopamine levels. Eur. J. Pharmacol. 1995, 281, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Bymaster, F.P.; Zhang, W.; Carter, P.A.; Shaw, J.; Chernet, E.; Phebus, L.; Wong, D.T.; Perry, K.W. Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology 2002, 160, 353–361. [Google Scholar] [CrossRef]
- Bortolozzi, A.; Díaz-Mataix, L.; Scorza, M.C.; Celada, P.; Artigas, F. The activation of 5-HT receptors in prefrontal cortex enhances dopaminergic activity. J. Neurochem. 2005, 95, 1597–1607. [Google Scholar] [CrossRef]
- Amiri, S.; Farhang, S.; Ghoreishizadeh, M.A.; Malek, A.; Mohammadzadeh, S. Double-blind controlled trial of venlafaxine for treatment of adults with attention deficit/hyperactivity disorder. Hum. Psychopharmacol. 2012, 27, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Davari-Ashtiani, R.; Eslami Shahrbabaki, M.; Razjouyan, K.; Amini, H.; Mazhabdar, H. Buspirone Versus Methylphenidate in the Treatment of Attention Deficit Hyperactivity Disorder: A Double-Blind and Randomized Trial. Child. Psychiatry Hum. Dev. 2010, 41, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Pehrson, A.L.; Bondi, C.O.; Totah, N.K.; and Moghaddam, B. The influence of NMDA and GABA(A) receptors and glutamic acid decarboxylase (GAD) activity on attention. Psychopharmacology 2013, 225, 31–39. [Google Scholar] [CrossRef][Green Version]
- Ochiai, Y.; Fujita, M.; Hagino, Y.; Kobayashi, K.; Okiyama, R.; Takahashi, K.; Ikeda, K. Therapeutic Effects of Quetiapine and 5-HT1A Receptor Agonism on Hyperactivity in Dopamine-Deficient Mice. Int. J. Mol. Sci. 2022, 23, 7436. [Google Scholar] [CrossRef] [PubMed]
- Winstanley, C.A.; Theobald, D.E.; Dalley, J.W.; Robbins, T.W. Fractionating impulsivity: Contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology 2004, 29, 1331–1343. [Google Scholar] [CrossRef]
- Worbe, Y.; Savulich, G.; Voon, V.; Fernandez-Egea, E.; Robbins, T.W. Serotonin depletion induces ‘waiting impulsivity’ on the human four-choice serial reaction time task: Cross-species translational significance. Neuropsychopharmacology 2014, 39, 1519–1526. [Google Scholar] [CrossRef]
- Robinson, E.S.; Dalley, J.W.; Theobald, D.E.; Glennon, J.C.; Pezze, M.A.; Murphy, E.R.; Robbins, T.W. Opposing roles for 5-HT2A and 5-HT2C receptors in the nucleus accumbens on inhibitory response control in the 5-choice serial reaction time task. Neuropsychopharmacology 2008, 33, 2398–2406. [Google Scholar] [CrossRef]
- Macoveanu, J.; Rowe, J.B.; Hornboll, B.; Elliott, R.; Paulson, O.B.; Knudsen, G.M.; Siebner, H.R. Serotonin 2A receptors contribute to the regulation of risk-averse decisions. Neuroimage 2013, 83, 35–44. [Google Scholar] [CrossRef]
- Raote, I.; Bhattacharya, A.; Panicker, M.M. Serotonin 2A (5-HT2A) Receptor Function: Ligand-Dependent Mechanisms and Pathways. In Serotonin Receptors in Neurobiology; Chapter 6; Chattopadhyay, A., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007. Available online: https://pubmed.ncbi.nlm.nih.gov/21204452/ (accessed on 1 December 2022).
- Lin, X.; Huang, L.; Huang, H.; Ke, Z.; Chen, Y. Disturbed relationship between glucocorticoid receptor and 5-HT1AR/5-HT2AR in ADHD rats: A correlation study. Front. Neurosci. 2022, 16, 1064369. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Shintani, N.; Hashimoto, H.; Kawagishi, N.; Ago, Y.; Matsuda, T.; Hashimoto, R.; Kunugi, H.; Yamamoto, A.; Kawaguchi, C.; et al. Psychostimulant-induced attenuation of hyperactivity and prepulse inhibition deficits in Adcyap1-deficient mice. J. Neurosci. 2006, 26, 5091–5107. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.I.; Adler, D.N.; Temporini, H.D.; Kemether, E.; Harvey, P.D.; White, L.; Parrella, M.; Davis, K.L. Guanfacine treatment of cognitive impairment in schizophrenia. Neuropsychopharmacology 2001, 25, 402–429. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scheinin, M.; Lomasney, J.W.; Hayden-Hixson, D.M.; Schambra, U.B.; Caron, M.G.; Lefkowitz, R.J.; Fremeau, R.T., Jr. Distribution of alpha 2-adrenergic receptor subtype gene expression in rat brain. Brain Res. Mol. Brain Res. 1994, 21, 133–149. [Google Scholar] [CrossRef]
- Wang, M.; Ramos, B.P.; Paspalas, C.D.; Shu, Y.; Simen, A.; Duque, A.; Vijayraghavan, S.; Brennan, A.; Dudley, A.; Nou, E.; et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell 2007, 129, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Fukui, R.; Hieda, E.; Kuroiwa, M.; Bateup, H.S.; Kano, T.; Greengard, P.; Nishi, A. Role of adrenoceptors in the regulation of dopamine/DARPP-32 signaling in neostriatal neurons. J. Neurochem. 2010, 113, 1046–1059. [Google Scholar] [CrossRef]
- Zuo, L.; Saba, L.; Lin, X.; Tan, Y.; Wang, K.; Krystal, J.H.; Tabakoff, B.; Luo, X. Significant association between rare IPO11-HTR1A variants and attention deficit hyperactivity disorder in Caucasians. Am. J. Med. Genet. B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2015, 168, 544–556. [Google Scholar] [CrossRef]
- Basura, G.J.; Walker, P.D. Serotonin 2A receptor mRNA levels in the neonatal dopamine-depleted rat striatum remain upregulated following suppression of serotonin hyperinnervation. Brain Res. Dev. Brain Res. 1999, 116, 111–117. [Google Scholar] [CrossRef]
- Navandar, M.; Martín-García, E.; Maldonado, R.; Lutz, B.; Gerber, S.; and Ruiz de Azua, I. Transcriptional signatures in prefrontal cortex confer vulnerability versus resilience to food and cocaine addiction-like behavior. Sci. Rep. 2021, 11, 9076. [Google Scholar] [CrossRef] [PubMed]
- Satoh, H.; Suzuki, H.; Saitow, F. Downregulation of Dopamine D1-like Receptor Pathways of GABAergic Interneurons in the Anterior Cingulate Cortex of Spontaneously Hypertensive Rats. Neuroscience 2018, 394, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Wulaer, B.; Kunisawa, K.; Tanabe, M.; Yanagawa, A.; Saito, K.; Mouri, A.; Nabeshima, T. Pharmacological blockade of dopamine D1- or D2-receptor in the prefrontal cortex induces attentional impairment in the object-based attention test through different neuronal circuits in mice. Mol. Brain 2021, 14, 43. [Google Scholar] [CrossRef]
- Usiello, A.; Baik, J.H.; Rougé-Pont, F.; Picetti, R.; Dierich, A.; LeMeur, M.; Piazza, P.V.; Borrelli, E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature 2000, 408, 199–203. [Google Scholar] [CrossRef]
- Smiley, J.F.; Levey, A.I.; Ciliax, B.J.; Goldman-Rakic, P.S. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: Predominant and extrasynaptic localization in dendritic spines. Proc. Natl. Acad. Sci. USA 1994, 91, 5720–5724. [Google Scholar] [CrossRef]
- Beaulieu, J.-M.; Sotnikova, T.D.; Yao, W.-D.; Kockeritz, L.; Woodgett, J.R.; Gainetdinov, R.R.; Caron, M.G. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc. Natl. Acad. Sci. USA 2004, 101, 5099–5104. [Google Scholar] [CrossRef]
- Ma, X.M.; Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009, 10, 307. [Google Scholar] [CrossRef]
- Bach, M.E.; Simpson, E.H.; Kahn, L.; Marshall, J.J.; Kandel, E.R.; Kellendonk, C. Transient and selective overexpression of D2 receptors in the striatum causes persistent deficits in conditional associative learning. Proc. Natl. Acad. Sci. USA 2008, 105, 16027–16032. [Google Scholar] [CrossRef]
- Maldonado, R.; Saiardi, A.; Valverde, O.; Samad, T.A.; Roques, B.P.; Borrelli, E. Absence of opiate rewarding effects in mice lacking dopamine D2 receptors. Nature 1997, 388, 586–589. [Google Scholar] [CrossRef]
- Cho, H.S.; Baek, D.J.; Baek, S.S. Effect of exercise on hyperactivity, impulsivity and dopamine D2 receptor expression in the substantia nigra and striatum of spontaneous hypertensive rats. J. Exerc. Nutr. Biochem. 2014, 18, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Morales, M. The brain on drugs: From reward to addiction. Cell 2015, 162, 712–725. [Google Scholar] [CrossRef] [PubMed]
- van Ewijk, H.; Heslenfeld, D.J.; Zwiers, M.P.; Buitelaar, J.K.; Oosterlaan, J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2012, 36, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology. CNS Drugs 2009, 23, 33–41. [Google Scholar] [CrossRef]
- Puig, M.V.; Santana, N.; Celada, P.; Mengod, G.; Artigas, F. In vivo excitation of GABA interneurons in the medial prefrontal cortex through 5-HT3 receptors. Cereb. Cortex. 2004, 14, 1365–1375. [Google Scholar] [CrossRef]
- Andreollo, N.A.; Santos, E.F.; Araujo, M.R.; Lopes, L.R. Rat’s age versus human’s age: What is the relationship? Arq. Bras. Cir. Dig. 2012, 25, 49–51. [Google Scholar] [CrossRef]
- Minabe, Y.; Hashimoto, K.; Watanabe, K.I.; Ashby, C.R., Jr. Acute and repeated administration of the selective 5-HT(2A) receptor antagonist M100907 significantly alters the activity of midbrain dopamine neurons: An in vivo electrophysiological study. Synapse 2001, 40, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Sagvolden, T. The alpha-2A adrenoceptor agonist guanfacine improves sustained attention and reduces overactivity and impulsiveness in an animal model of Attention-Deficit/Hyperactivity Disorder (ADHD). Behav. Brain Funct. 2006, 2, 41. [Google Scholar] [CrossRef]
- Kim, P.; Choi, I.; Pena, I.C.; Kim, H.J.; Kwon, K.J.; Park, J.H.; Han, S.H.; Ryu, J.H.; Cheong, J.H.; Shin, C.Y. A simple behavioral paradigm to measure impulsive behavior in an animal model of attention deficit hyperactivity disorder (ADHD) of the spontaneously hypertensive rats. Biomol. Ther. 2012, 20, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Bairy, K.L.; Madhyastha, S.; Ashok, K.P.; Bairy, I.; Malini, S. Developmental and behavioral consequences of prenatal fluoxetine. Pharmacology 2007, 79, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bizot, J.C.; Chenault, N.; Houze, B.; Herpin, A.; David, S.; Pothion, S.; Trovero, F. Methylphenidate reduces impulsive behaviour in juvenile Wistar rats, but not in adult Wistar, SHR and WKY rats. Psychopharmacology 2007, 193, 215–223. [Google Scholar] [CrossRef]
- Bizot, J.C.; David, S.; Trovero, F. Effects of atomoxetine, desipramine, d-amphetamine and methylphenidate on impulsivity in juvenile rats, measured in a T-maze procedure. Neurosci. Lett. 2011, 489, 20–24. [Google Scholar] [CrossRef]
- Szlachta, M.; Kuśmider, M.; Pabian, P.; Solich, J.; Kolasa, M.; Żurawek, D.; Dziedzicka-Wasylewska, M.; Faron-Górecka, A. Repeated Clozapine Increases the Level of Serotonin 5-HT1AR Heterodimerization with 5-HT2A or Dopamine D2 Receptors in the Mouse Cortex. Front. Mol. Neurosci. 2018, 11, 40. [Google Scholar] [CrossRef]
- Savignac, H.M.; Couch, Y.; Stratford, M.; Bannerman, D.M.; Tzortzis, G.; Anthony, D.C.; Burnet, P.W.J. Prebiotic administration normalizes lipopolysaccharide (LPS)-induced anxiety and cortical 5-HT2A receptor and IL1-β levels in male mice. Brain Behav. Immun. 2016, 52, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Natesan, S.; Reckless, G.E.; Nobrega, J.N.; Fletcher, P.J.; Kapur, S. Dissociation between in vivo occupancy and functional antagonism of dopamine D2 receptors: Comparing aripiprazole to other antipsychotics in animal models. Neuropsychopharmacology 2006, 31, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.-F.; Han, F.; Shi, Y.-X. Changes in 5-HT1A receptor in the dorsal raphe nucleus in a rat model of post-traumatic stress disorder. Mol. Med. Rep. 2011, 4, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Verdurand, M.; Chauveau, F.; Daoust, A.; Morel, A.L.; Bonnefoi, F.; Liger, F.; Bérod, A.; Zimmer, L. Differential effects of amyloid-beta 1-40 and 1-42 fibrils on 5-HT1A serotonin receptors in rat brain. Neurobiol. Aging 2016, 40, 11–21. [Google Scholar] [CrossRef]
- McDonald, A.J.; Mascagni, F. Neuronal localization of 5-HT type 2A receptor immunoreactivity in the rat basolateral amygdala. Neuroscience 2007, 146, 306–320. [Google Scholar] [CrossRef]
- Romero-Fernandez, W.; Borroto-Escuela, D.; Vargas-Barroso, V.; Narváez, M.; Di Palma, M.; Agnati, L.; Sahd, J.L.; Fuxe, K. Dopamine D1 and D2 receptor immunoreactivities in the arcuate-median eminence complex and their link to the tubero-infundibular dopamine neurons. Eur. J. Histochem. 2014, 58, 2400. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madhyastha, S.; Rao, M.S.; Renno, W.M. Serotonergic and Adrenergic Neuroreceptor Manipulation Ameliorates Core Symptoms of ADHD through Modulating Dopaminergic Receptors in Spontaneously Hypertensive Rats. Int. J. Mol. Sci. 2024, 25, 2300. https://doi.org/10.3390/ijms25042300
Madhyastha S, Rao MS, Renno WM. Serotonergic and Adrenergic Neuroreceptor Manipulation Ameliorates Core Symptoms of ADHD through Modulating Dopaminergic Receptors in Spontaneously Hypertensive Rats. International Journal of Molecular Sciences. 2024; 25(4):2300. https://doi.org/10.3390/ijms25042300
Chicago/Turabian StyleMadhyastha, Sampath, Muddanna S. Rao, and Waleed M. Renno. 2024. "Serotonergic and Adrenergic Neuroreceptor Manipulation Ameliorates Core Symptoms of ADHD through Modulating Dopaminergic Receptors in Spontaneously Hypertensive Rats" International Journal of Molecular Sciences 25, no. 4: 2300. https://doi.org/10.3390/ijms25042300
APA StyleMadhyastha, S., Rao, M. S., & Renno, W. M. (2024). Serotonergic and Adrenergic Neuroreceptor Manipulation Ameliorates Core Symptoms of ADHD through Modulating Dopaminergic Receptors in Spontaneously Hypertensive Rats. International Journal of Molecular Sciences, 25(4), 2300. https://doi.org/10.3390/ijms25042300




