CHST4 Gene as a Potential Predictor of Clinical Outcome in Malignant Pleural Mesothelioma
Abstract
1. Introduction
2. Results
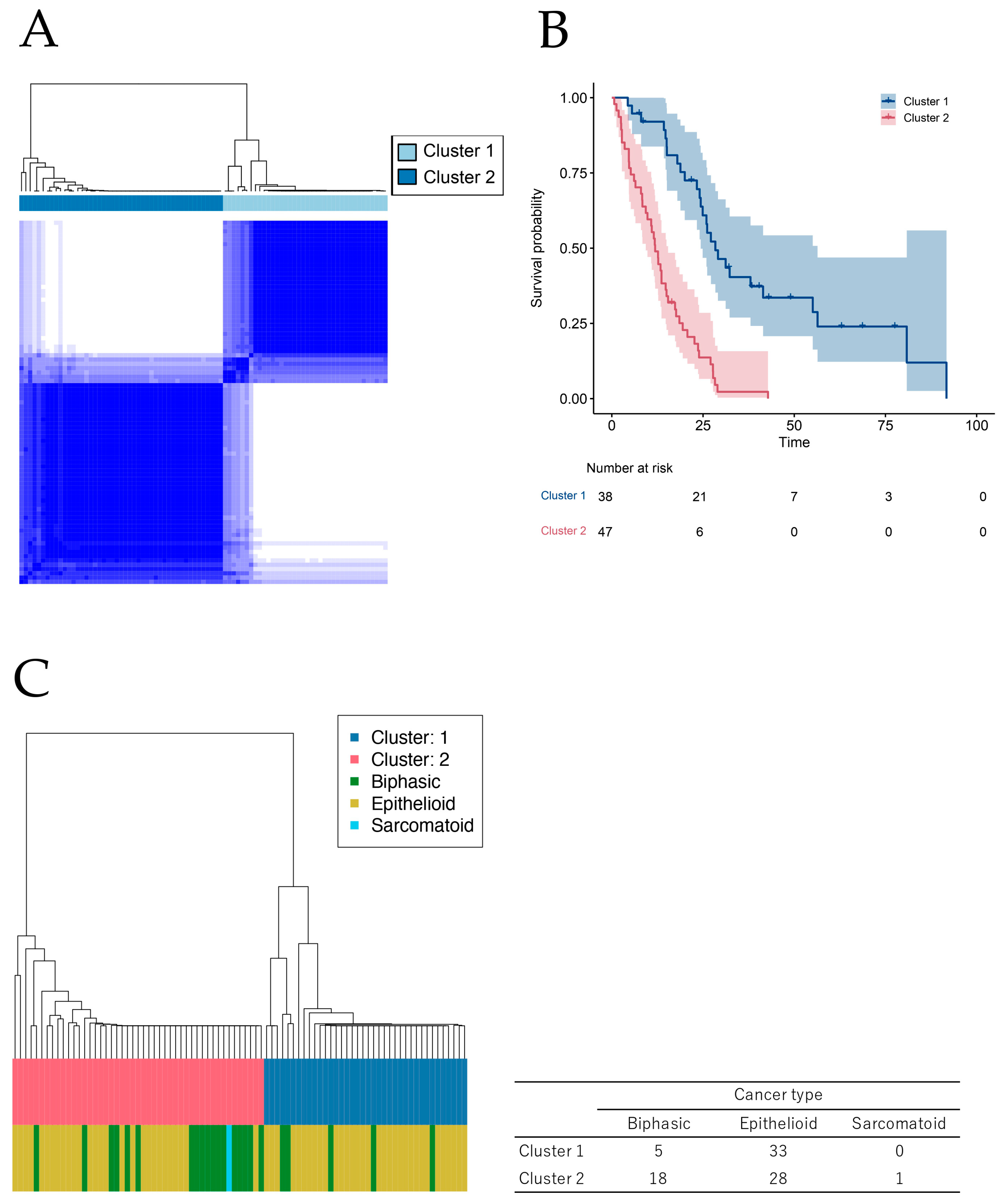
2.1. Novel Classification of MPM Based on Comprehensive Survival Analysis of Cancer Genome Database
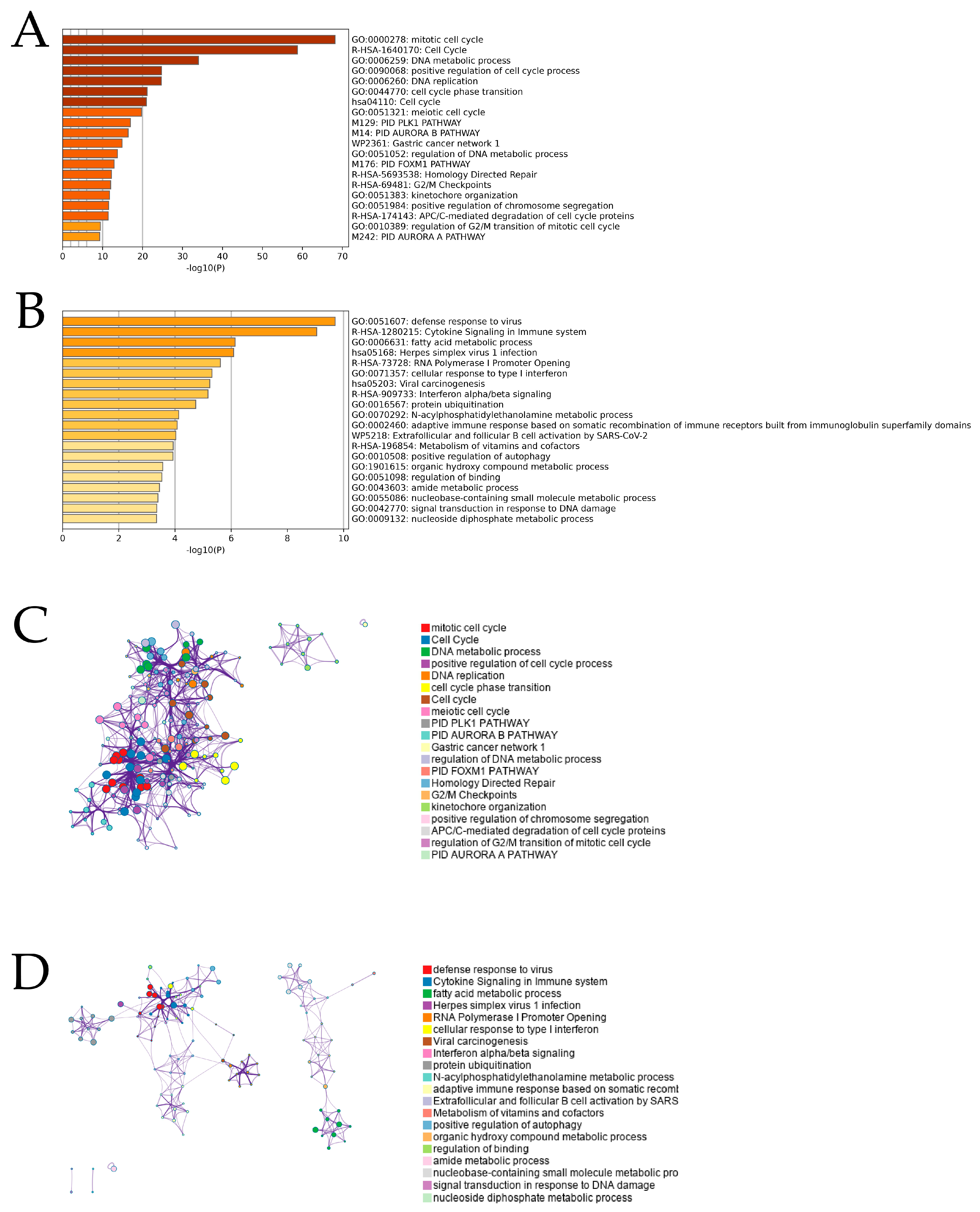
2.2. Enrichment Analysis for Prognostic Genes of MPM
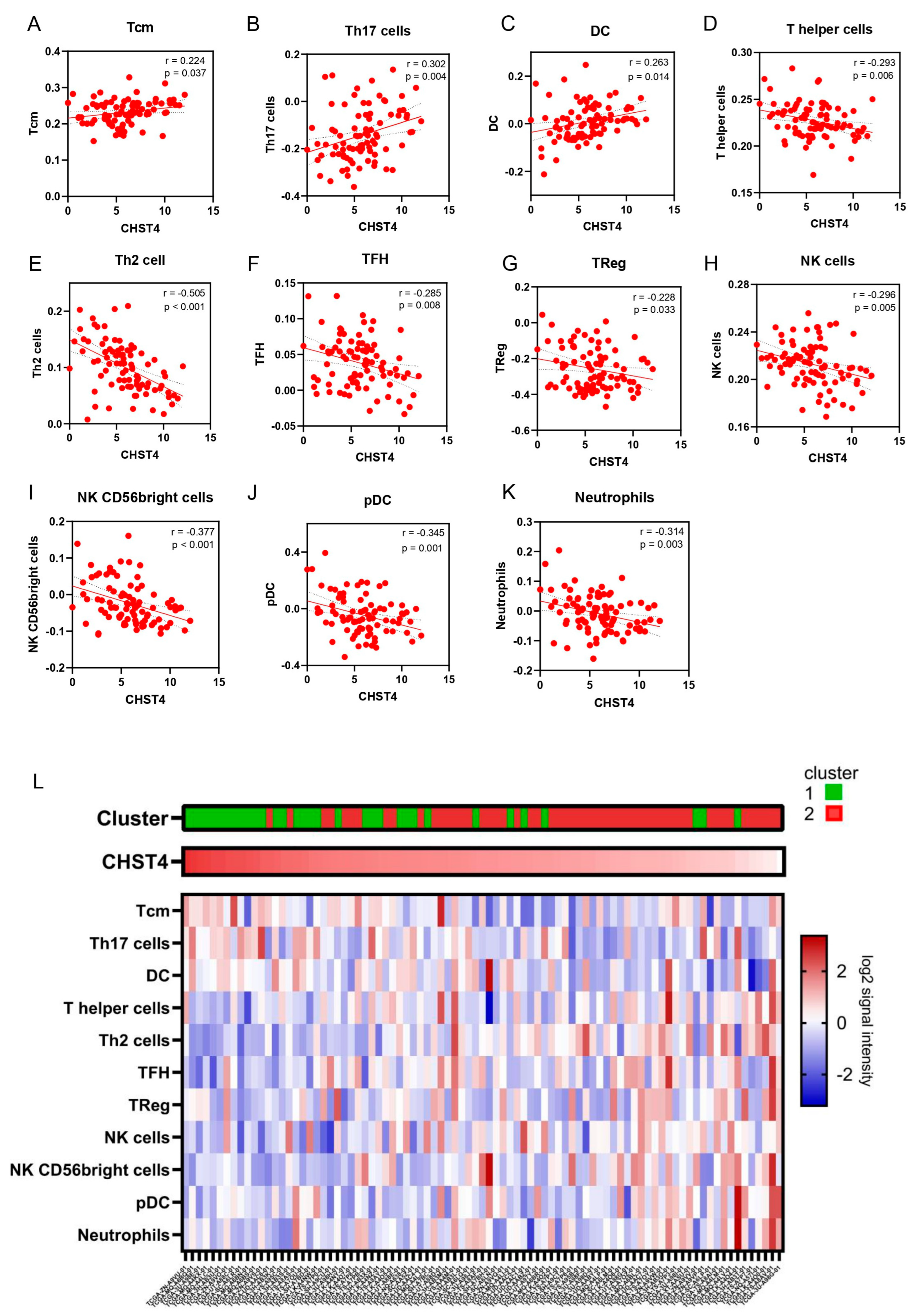
2.3. CHST4 as a Prognostic Gene of MPM and the Relationship with Immune-Related Gene Signatures
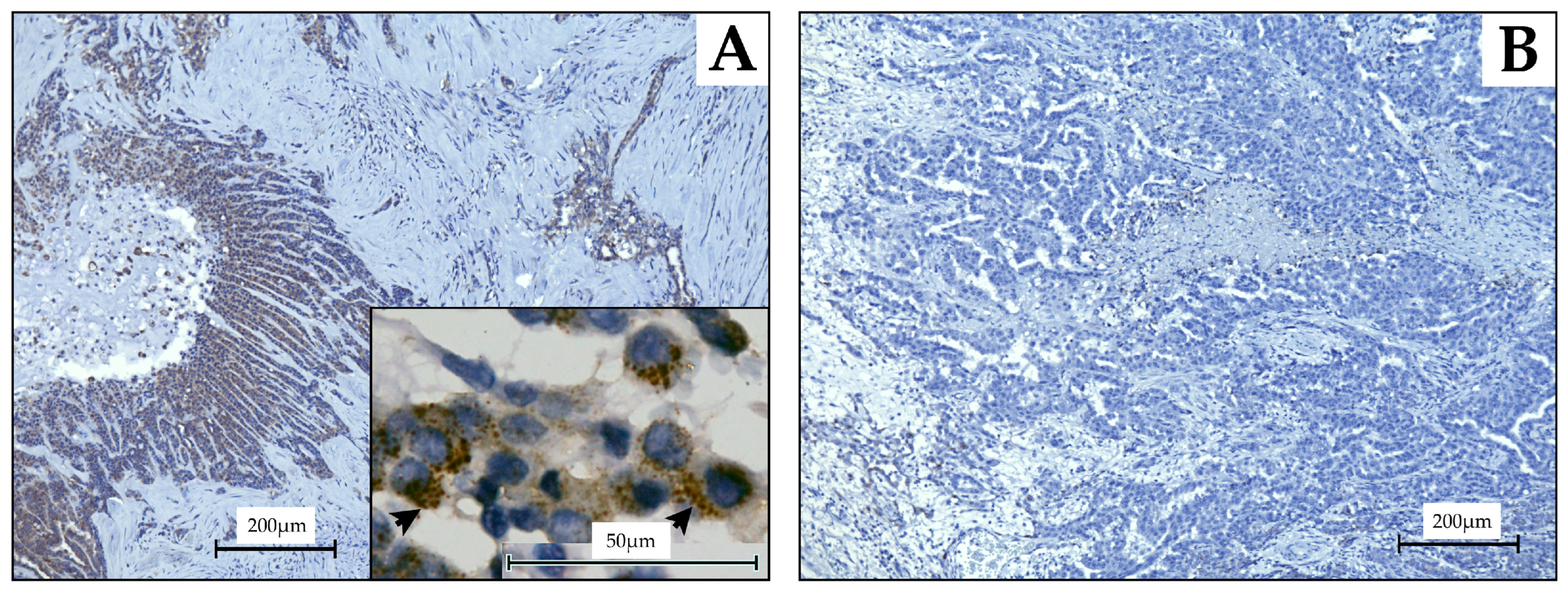
2.4. Evaluation Using MPM Tissue Samples of CHST4 Expression
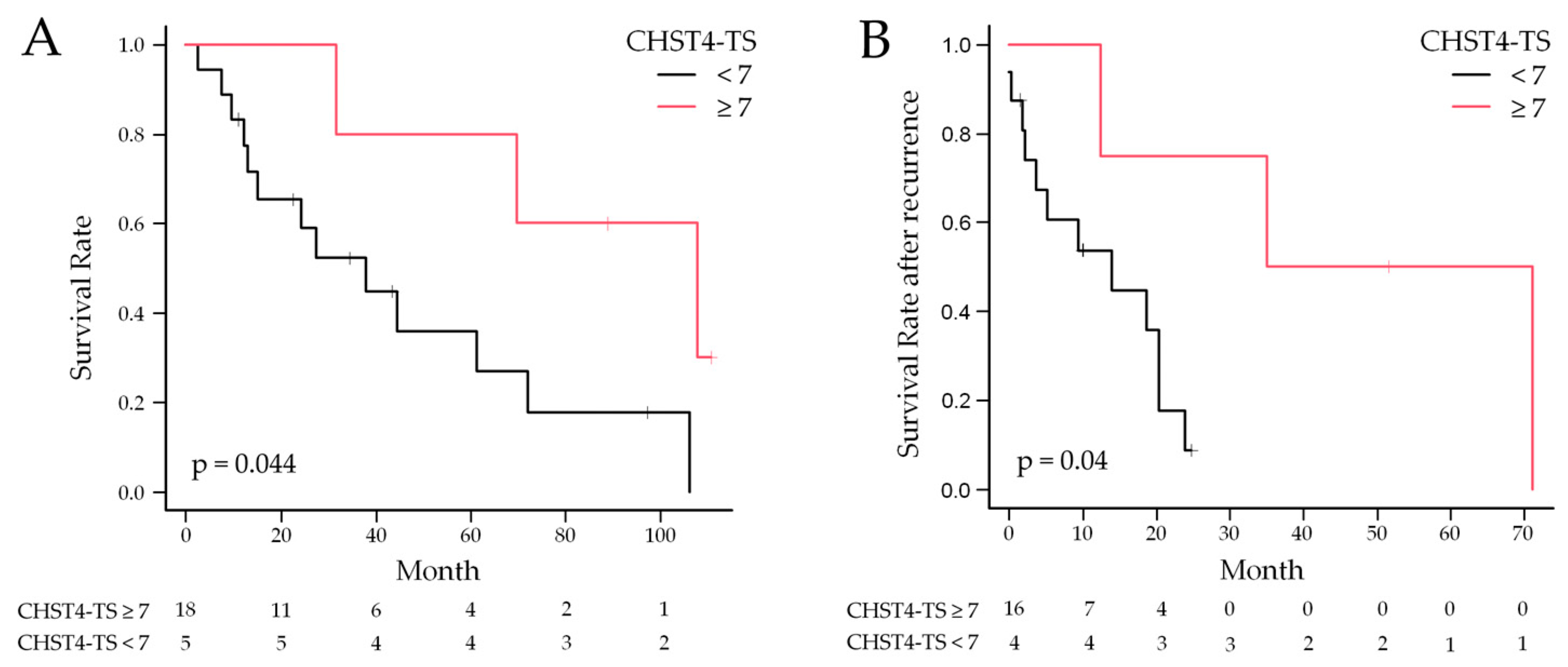
2.5. Statistical Analysis of CHST4 Protein Expression Intensity and Prognosis
3. Discussion
4. Materials and Methods
4.1. Discovery of Prognostic Genes and Consensus Clustering
4.2. Overall Enrichment Analysis
4.3. Calculation of the Immune-Gene Signature
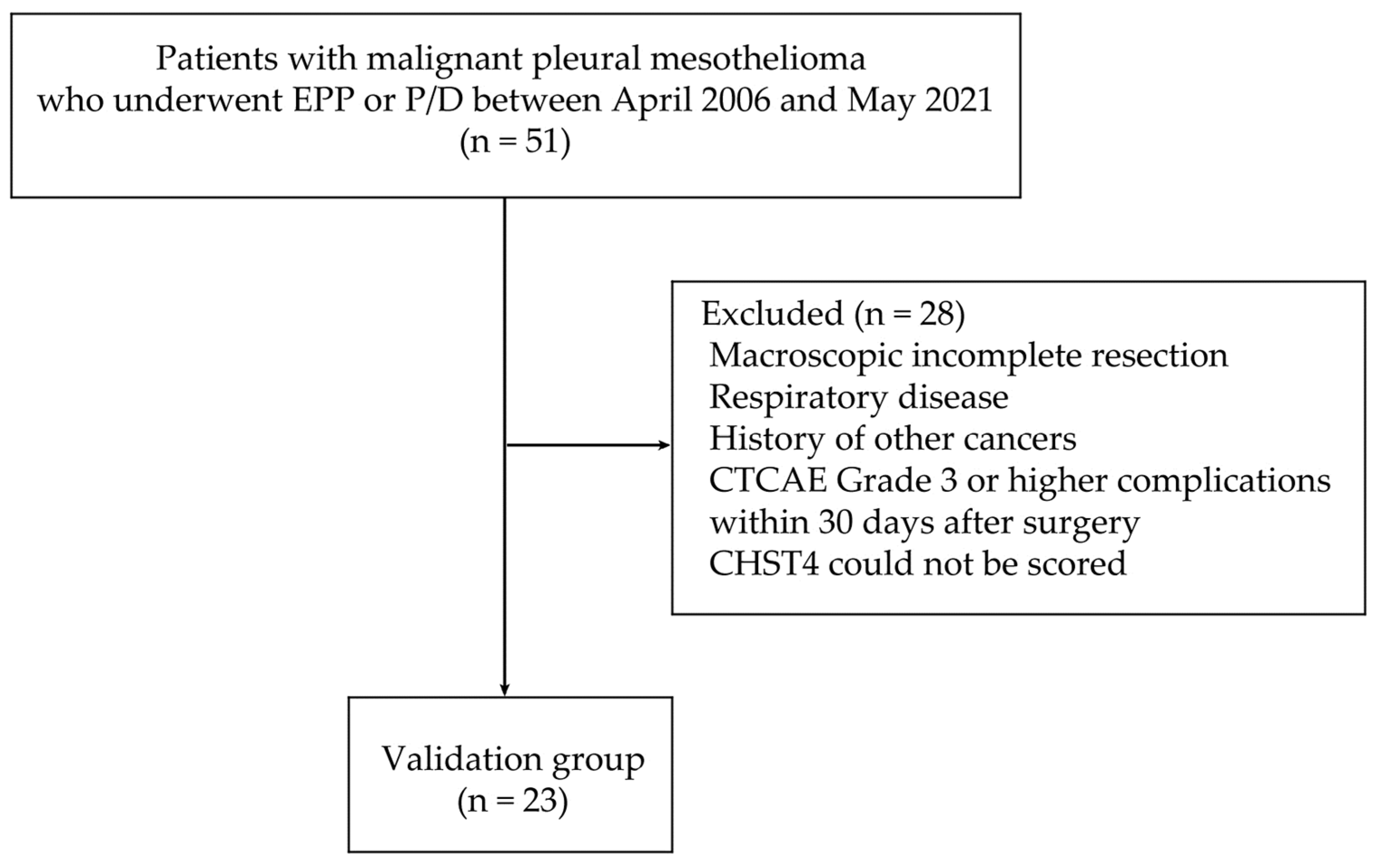
4.4. Patient Data and Tumor Material
4.5. Immunohistochemistry and Evaluation of Stained Slides
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhuo, M.; Zheng, Q. Survival analysis via nomogram of surgical patients with malignant pleural mesothelioma in the Surveillance, Epidemiology, and End Results database. Thorac. Cancer 2019, 10, 1193–1202. [Google Scholar] [CrossRef]
- Bovolato, P.; Casadio, C. Does surgery improve survival of patients with malignant pleural mesothelioma? A multicenter retrospective analysis of 1365 consecutive patients. J. Thorac. Oncol. 2014, 9, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Larose, F.; Quigley, N. Malignant pleural mesothelioma: Comparison of surgery-based trimodality therapy to medical therapy at two tertiary academic institutions. Lung Cancer 2021, 156, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Fennell, D.A.; Dulloo, S. Immunotherapy approaches for malignant pleural mesothelioma. Nat. Rev. Clin. Oncol. 2022, 19, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Huang, W. Exploring TCGA database for identification of potential prognostic genes in stomach adenocarcinoma. Cancer Cell. Int. 2020, 20, 264. [Google Scholar] [CrossRef] [PubMed]
- Pablos, J.L.; Santiago, B. A HEV-restricted sulfotransferase is expressed in rheumatoid arthritis synovium and is induced by lymphotoxin-alpha/beta and TNF-alpha in cultured endothelial cells. BMC Immunol. 2005, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Bentley, K. Transgenic LacZ under control of Hec-6st regulatory sequences recapitulates endogenous gene expression on high endothelial venules. Proc. Natl. Acad. Sci. USA 2007, 104, 4577–4582. [Google Scholar] [CrossRef]
- Veerman, K.; Tardiveau, C. Single-Cell Analysis Reveals Heterogeneity of High Endothelial Venules and Different Regulation of Genes Controlling Lymphocyte Entry to Lymph Nodes. Cell. Rep. 2019, 26, 3116–3131.e5. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, Y. Carbohydrate Sulfotransferase 4 Inhibits the Progression of Hepatitis B Virus-Related Hepatocellular Carcinoma and Is a Potential Prognostic Marker in Several Tumors. Front. Oncol. 2020, 10, 554331. [Google Scholar] [CrossRef]
- Yu, S.Y.; Hsiao, C.T. Distinct substrate specificities of human GlcNAc-6-sulfotransferases revealed by mass spectrometry-based sulfoglycomic analysis. J. Biol. Chem. 2018, 293, 15163–15177. [Google Scholar] [CrossRef]
- Hu, B.; Ma, X. The mRNA-miRNA-lncRNA Regulatory Network and Factors Associated with Prognosis Prediction of Hepatocellular Carcinoma. Genom. Proteom. Bioinform. 2021, 19, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Vogelzang, N.J.; Rusthoven, J.J. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Okada, M. Trimodality strategy for treating malignant pleural mesothelioma: Results of a feasibility study of induction pemetrexed plus cisplatin followed by extrapleural pneumonectomy and postoperative hemithoracic radiation (Japan Mesothelioma Interest Group 0601 Trial). Int. J. Clin. Oncol. 2016, 21, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Krug, L.M.; Pass, H.I. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J. Clin. Oncol. 2009, 27, 3007–3013. [Google Scholar] [CrossRef]
- Van Schil, P.E.; Baas, P. Trimodality therapy for malignant pleural mesothelioma: Results from an EORTC phase II multicentre trial. Eur. Respir. J. 2010, 36, 1362–1369. [Google Scholar] [CrossRef]
- Bölükbas, S.; Manegold, C. Survival after trimodality therapy for malignant pleural mesothelioma: Radical Pleurectomy, chemotherapy with Cisplatin/Pemetrexed and radiotherapy. Lung Cancer 2011, 71, 75–81. [Google Scholar] [CrossRef]
- Lim, E.; Darlison, L. Mesothelioma and Radical Surgery 2 (MARS 2): Protocol for a multicentre randomised trial comparing (extended) pleurectomy decortication versus no (extended) pleurectomy decortication for patients with malignant pleural mesothelioma. BMJ Open 2020, 10, e038892. [Google Scholar] [CrossRef]
- Baas, P.; Scherpereel, A. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Jang, H.J. A Phase II Window of Opportunity Study of Neoadjuvant PD-L1 versus PD-L1 plus CTLA-4 Blockade for Patients with Malignant Pleural Mesothelioma. Clin. Cancer Res. 2023, 29, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Bueno, R.; Stawiski, E.W. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat. Genet. 2016, 48, 407–416. [Google Scholar] [CrossRef]
- Hmeljak, J.; Sanchez-Vega, F. Integrative Molecular Characterization of Malignant Pleural Mesothelioma. Cancer Discov. 2018, 8, 1548–1565. [Google Scholar] [CrossRef] [PubMed]
- Mangiante, L.; Alcala, N. Multiomic analysis of malignant pleural mesothelioma identifies molecular axes and specialized tumor profiles driving intertumor heterogeneity. Nat. Genet. 2023, 55, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, H. The LINC00452/miR-204/CHST4 Axis Regulating Thymic Tregs Might Be Involved in the Progression of Thymoma-Associated Myasthenia Gravis. Front. Neurol. 2022, 13, 828970. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, Y. Response Stratification in the First-Line Combined Immunotherapy of Hepatocellular Carcinoma at Genomic, Transcriptional and Immune Repertoire Levels. J. Hepatocell. Carcinoma 2021, 8, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zou, D. Combined mRNAs and clinical factors model on predicting prognosis in patients with triple-negative breast cancer. PLoS ONE 2021, 16, e0260811. [Google Scholar] [CrossRef]
- Nowak, A.K.; Chansky, K. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J. Thorac. Oncol. 2016, 11, 2089–2099. [Google Scholar] [CrossRef]
- Lauk, O.; Patella, M. Implementing CT tumor volume and CT pleural thickness into future staging systems for malignant pleural mesothelioma. Cancer Imaging 2021, 21, 48. [Google Scholar] [CrossRef]
- Ito, T.; Nakamura, S. Impact of Pleural Thickness on Occurrence of Postoperative Complications in Patients with Malignant Pleural Mesothelioma. Ann. Surg. Oncol. 2023, 30, 1574–1583. [Google Scholar] [CrossRef]
- Michele, C.; Prasad, S.A. Mesothelioma: Scientific clues for prevention, diagnosis, and therapy. CA Cancer J. Clin. 2019, 69, 402–429. [Google Scholar] [CrossRef]
- Zhenying, G.; Li, S. Aurora Kinase A as a Diagnostic and Prognostic Marker of Malignant Mesothelioma. Front. Oncol. 2021, 11, 789244. [Google Scholar] [CrossRef]
- Monti, S.; Tamayo, P. Consensus Clustering: A Resampling-Based Method for Class Discovery and Visualization of Gene Expression Microarray Data. Mach. Learn. 2003, 52, 91–118. [Google Scholar] [CrossRef]
- Golkar-Narenji, A.; Antosik, P. Gene Ontology Groups and Signaling Pathways Regulating the Process of Avian Satellite Cell Differentiation. Genes 2022, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Aran, D.; Hu, Z. xCell: Digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017, 18, 220. [Google Scholar] [CrossRef]
- Xu, L.; Deng, C. TIP: A Web Server for Resolving Tumor Immunophenotype Profiling. Cancer Res. 2018, 78, 6575–6580. [Google Scholar] [CrossRef] [PubMed]
- Issa, R.M.; Lebeau, A. Estrogen receptor gene amplification occurs rarely in ovarian cancer. Mod. Pathol. 2009, 22, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.H.; Gao, H.W. Amongst Women Stratified to Receive Endocrine Therapy on the Basis of Their Tumor Estrogen and Progesterone Receptor Levels, Those with Higher Tumor Progesterone Receptor Levels Had a Better Outcome Than Those with Lower Levels of Tumor Progesterone Receptor. Cancers 2021, 13, 905. [Google Scholar] [CrossRef]
- Ahn, S.G.; Nam, S.J. Clinical Outcomes Following Letrozole Treatment according to Estrogen Receptor Expression in Postmenopausal Women: LETTER Study (KBCSG-006). J. Breast Cancer 2021, 24, 164–174. [Google Scholar] [CrossRef]
- Dave, R.V.; Elsberger, B. Bridging pre-surgical endocrine therapy for breast cancer during the COVID-19 pandemic: Outcomes from the B-MaP-C study. Breast Cancer Res. Treat. 2023, 199, 265–279. [Google Scholar] [CrossRef]
- Therneau, T. A Package for Survival Analysis in R—R Package Version 3.5–7. 2023. Available online: https://CRAN.R-project.org/package=survival (accessed on 13 February 2024).
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000; ISBN 0-387-98784-3. [Google Scholar]
- Storey, J.D.; Bass, A.J. Qvalue: Q-Value Estimation for False Discovery Rate Control—R Package Version 2.34.0. 2023. Available online: https://bioconductor.org/packages/qvalue (accessed on 13 February 2024).
- Wilkerson, D.M.; Hayes, N.D. ConsensusClusterPlus: A class discovery tool with confidence assessments and item tracking. Bioinformatics 2010, 26, 1572–1573. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| Hugo Symbol | Hazard Ratio (95% CI) | p-Value | Hugo Symbol | Hazard Ratio (95% CI) | p-Value |
| CHST4 | 0.37 (0.21–0.65) | 0.0007 | FOXO4 | 0.22 (0.09–0.52) | 0.0006 |
| EMB | 0.38 (0.23–0.61) | <0.0001 | CACHD1 | 0.31 (0.18–0.53) | <0.0001 |
| CACHD1 | 0.39 (0.24–0.63) | 0.0002 | CHST4 | 0.32 (0.17–0.60) | 0.0003 |
| EPB41L4A | 0.41 (0.25–0.66) | 0.0002 | EMB | 0.35 (0.21–0.58) | <0.0001 |
| ATP8A1 | 0.42 (0.26–0.68) | 0.0004 | PRIMA1 | 0.36 (0.20–0.66) | 0.0008 |
| GHR | 0.42 (0.27–0.64) | <0.0001 | GHR | 0.36 (0.23–0.56) | <0.0001 |
| EMBP1 | 0.42 (0.28–0.64) | <0.0001 | PLEKHH1 | 0.39 (0.24–0.63) | 0.0001 |
| TNFSF13 | 0.43 (0.30–0.60) | <0.0001 | EMBP1 | 0.39 (0.25–0.61) | <0.0001 |
| BTN3A3 | 0.44 (0.30–0.65) | <0.0001 | EPB41L4A | 0.40 (0.25–0.64) | 0.0002 |
| HIST1H2AC | 0.45 (0.31–0.64) | <0.0001 | ADH1B | 0.41 (0.25–0.68) | 0.0005 |
| RTP4 | 0.45 (0.31–0.66) | <0.0001 | THTPA | 0.41 (0.29–0.59) | <0.0001 |
| KLHL9 | 0.45 (0.34–0.59) | <0.0001 | HIST1H2AC | 0.41 (0.28–0.58) | <0.0001 |
| THTPA | 0.46 (0.32–0.65) | <0.0001 | RICH2 | 0.41 (0.28–0.61) | <0.0001 |
| NUDT7 | 0.46 (0.32–0.67) | <0.0001 | TNFSF13 | 0.41 (0.29–0.58) | <0.0001 |
| HIST1H2BD | 0.46 (0.32–0.67) | <0.0001 | KLHL9 | 0.41 (0.30–0.55) | <0.0001 |
| C5orf4 | 0.47 (0.33–0.66) | <0.0001 | BTN3A3 | 0.42 (0.28–0.63) | <0.0001 |
| FLJ11235 | 0.47 (0.34–0.65) | <0.0001 | ATP8A1 | 0.42 (0.25–0.69) | 0.0006 |
| DCAF11 | 0.47 (0.35–0.63) | <0.0001 | RTP4 | 0.42 (0.29–0.61) | <0.0001 |
| TMCO4 | 0.47 (0.35–0.63) | <0.0001 | HIST1H2BC | 0.42 (0.26–0.67) | 0.0003 |
| SH3BGRL | 0.47 (0.35–0.64) | <0.0001 | HIST1H2BD | 0.42 (0.29–0.60) | <0.0001 |
| Clinicopathological Feature | n = 23 | |
|---|---|---|
| Sex | ||
| Male | 19 (82.6%) | |
| Female | 4 (17.4%) | |
| Age, years (median, range) | 61 (46–73) | |
| Histological type | ||
| Epithelioid mesothelioma | 18 (78.3%) | |
| Biphasic mesothelioma | 5 (21.7%) | |
| Pleural thickness, mm (median, range) | 29.4 (11.1–128.8) | |
| Surgical procedure | ||
| Extrapleural pneumonectomy | 15 (65.2%) | |
| Pleurectomy/decortication | 8 (34.8%) | |
| pStage | ||
| I | 15 (65.2%) | |
| II | 0 (0.0%) | |
| III | 7 (30.4%) | |
| IV | 1 (4.3%) | |
| Proportion of Positively Stained at Perinuclear Cytoplasm | Proportion Score (PS) | Average Intensity of Positively Stained at Perinuclear Cytoplasm | Intensity Score (IS) |
|---|---|---|---|
| None | 0 | None | 0 |
| <1/100 | 1 | Weak | 1 |
| 1/100 to 1/10 | 2 | Moderate/medium | 2 |
| 1/10 to 1/3 | 3 | Strong | 3 |
| 1/3 to 2/3 | 4 | ||
| >2/3 | 5 | ||
| Variable | Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |||
| Lower | Upper | Lower | Upper | |||||
| Age | 0.98 | 0.93 | 1.05 | 0.65 | 1.00 | 0.91 | 1.10 | 0.98 |
| Sex (male) | 2.26 | 0.62 | 8.20 | 0.22 | 3.91 | 0.51 | 29.8 | 0.19 |
| pStage | 1.47 | 0.97 | 2.22 | 0.07 | 3.06 | 1.48 | 6.33 | 0.003 |
| Histological type (epithelioid) | 0.46 | 0.16 | 1.36 | 0.16 | 0.67 | 0.15 | 3.01 | 0.60 |
| Pleural thickness | 1.02 | 1.01 | 1.04 | 0.01 | 1.01 | 0.99 | 1.03 | 0.28 |
| CHST4-TS (≥7) | 0.23 | 0.05 | 1.07 | 0.06 | 0.12 | 0.01 | 1.15 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okado, S.; Kato, T.; Hanamatsu, Y.; Emoto, R.; Imamura, Y.; Watanabe, H.; Kawasumi, Y.; Kadomatsu, Y.; Ueno, H.; Nakamura, S.; et al. CHST4 Gene as a Potential Predictor of Clinical Outcome in Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2024, 25, 2270. https://doi.org/10.3390/ijms25042270
Okado S, Kato T, Hanamatsu Y, Emoto R, Imamura Y, Watanabe H, Kawasumi Y, Kadomatsu Y, Ueno H, Nakamura S, et al. CHST4 Gene as a Potential Predictor of Clinical Outcome in Malignant Pleural Mesothelioma. International Journal of Molecular Sciences. 2024; 25(4):2270. https://doi.org/10.3390/ijms25042270
Chicago/Turabian StyleOkado, Shoji, Taketo Kato, Yuki Hanamatsu, Ryo Emoto, Yoshito Imamura, Hiroki Watanabe, Yuta Kawasumi, Yuka Kadomatsu, Harushi Ueno, Shota Nakamura, and et al. 2024. "CHST4 Gene as a Potential Predictor of Clinical Outcome in Malignant Pleural Mesothelioma" International Journal of Molecular Sciences 25, no. 4: 2270. https://doi.org/10.3390/ijms25042270
APA StyleOkado, S., Kato, T., Hanamatsu, Y., Emoto, R., Imamura, Y., Watanabe, H., Kawasumi, Y., Kadomatsu, Y., Ueno, H., Nakamura, S., Mizuno, T., Takeuchi, T., Matsui, S., & Chen-Yoshikawa, T. F. (2024). CHST4 Gene as a Potential Predictor of Clinical Outcome in Malignant Pleural Mesothelioma. International Journal of Molecular Sciences, 25(4), 2270. https://doi.org/10.3390/ijms25042270






