Evaluating the Effects of Kidney Preservation at 10 °C with Hemopure and Sodium Thiosulfate in a Rat Model of Syngeneic Orthotopic Kidney Transplantation
Abstract
1. Introduction
2. Results
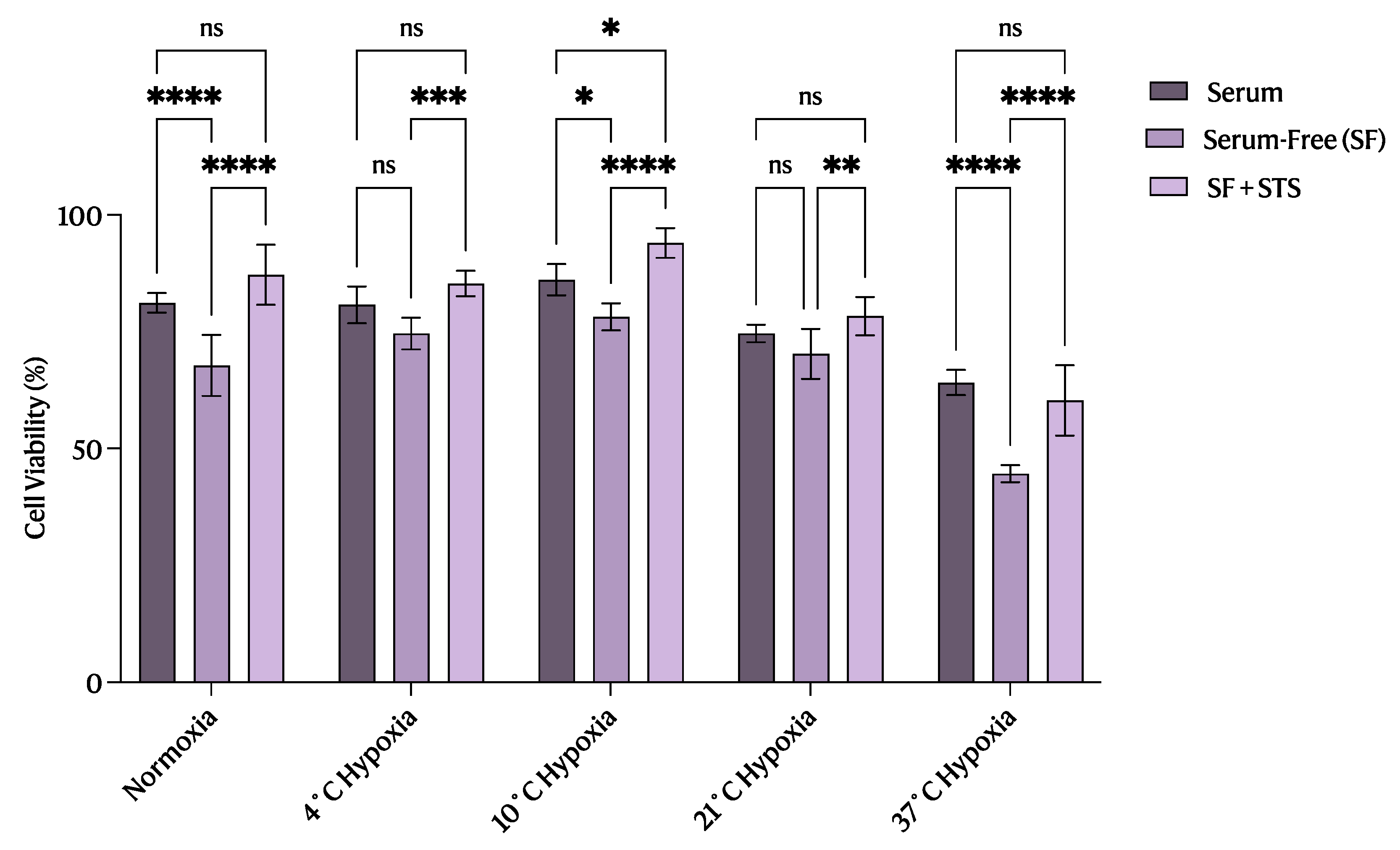
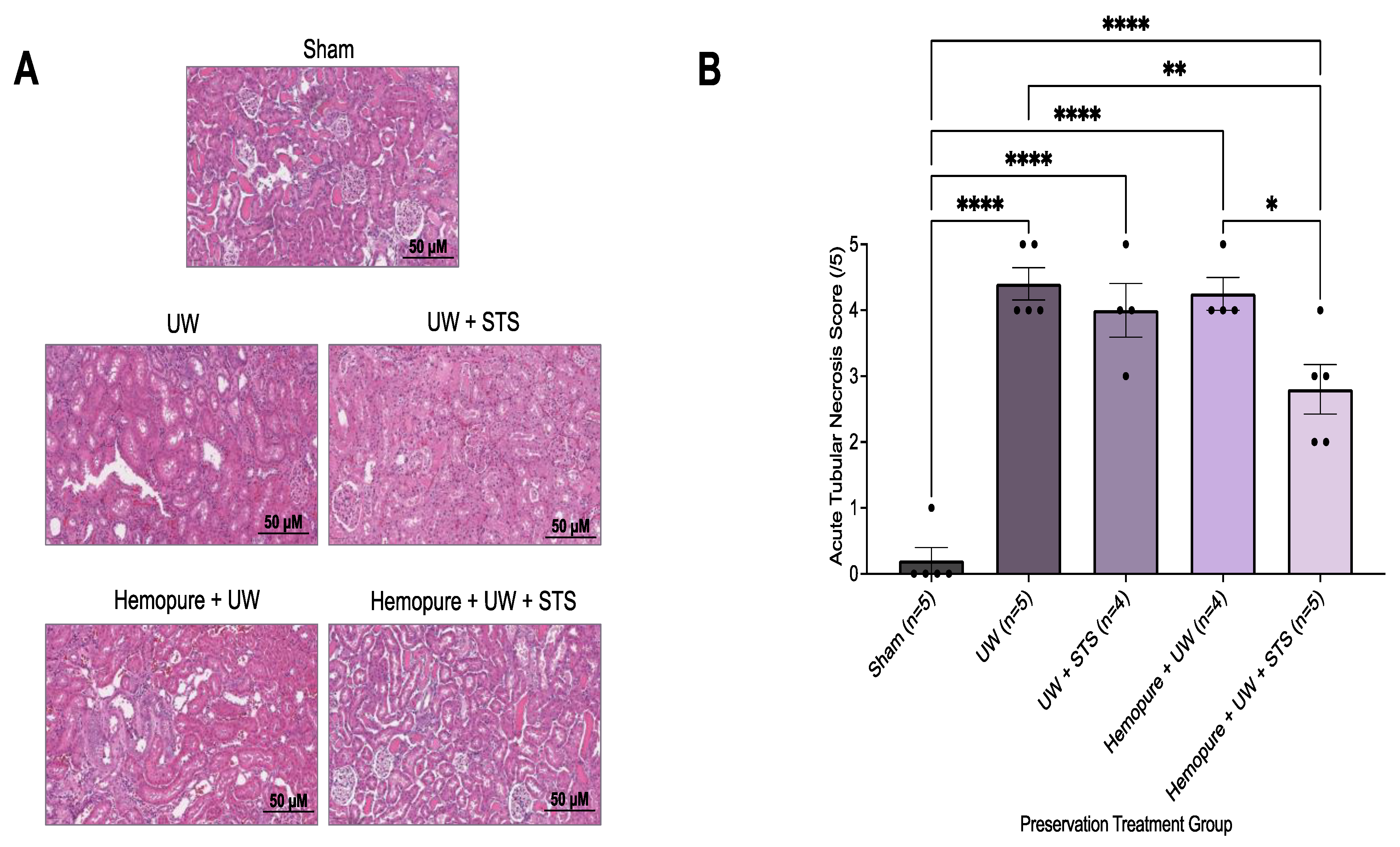
2.1. 10 °C STS Treatment Improved Proximal Tubular Epithelial Cell Survival
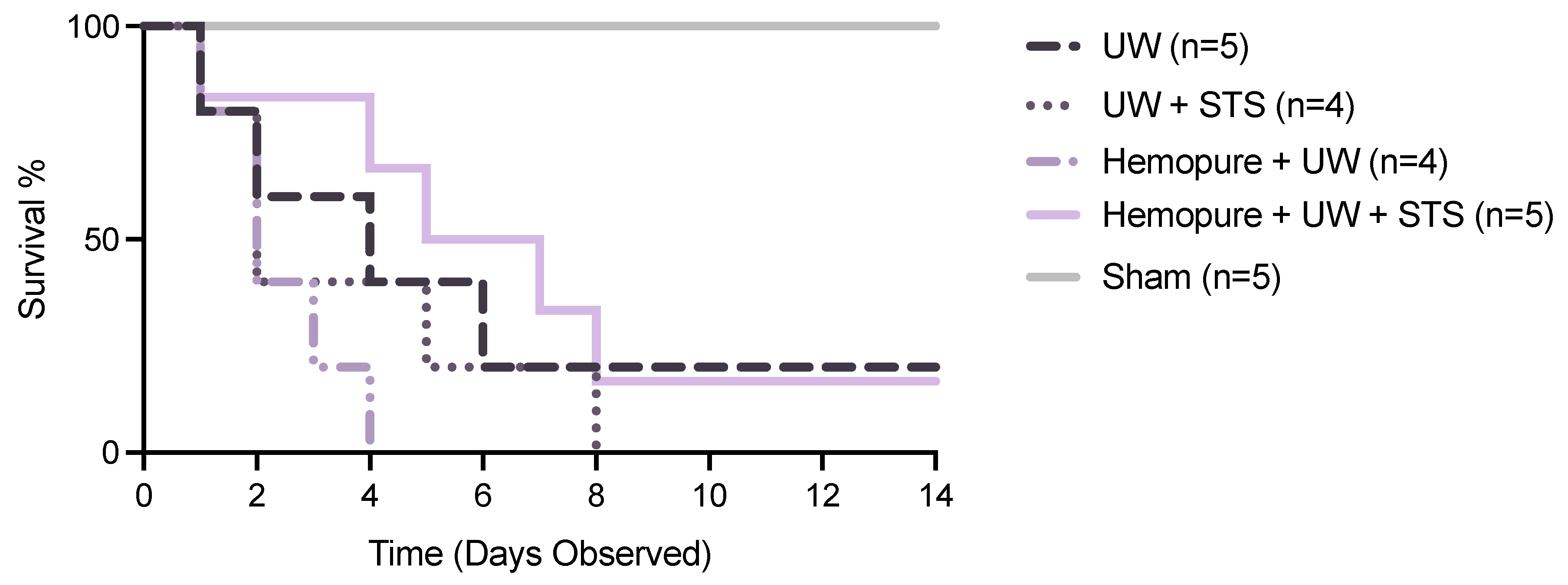
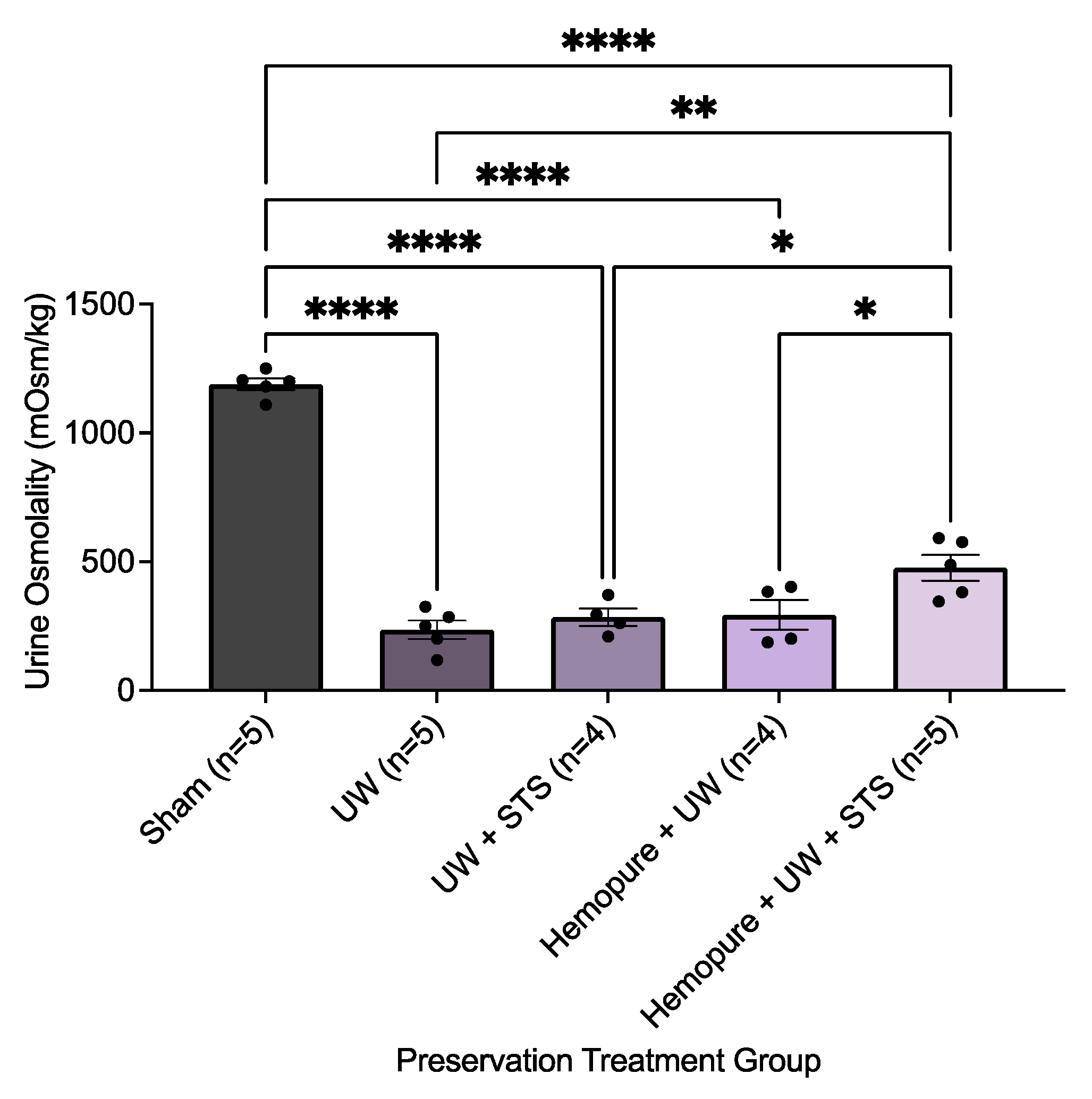
2.2. Hemopure and STS-Supplemented Preservation Solution Improved Early Renal Graft Function
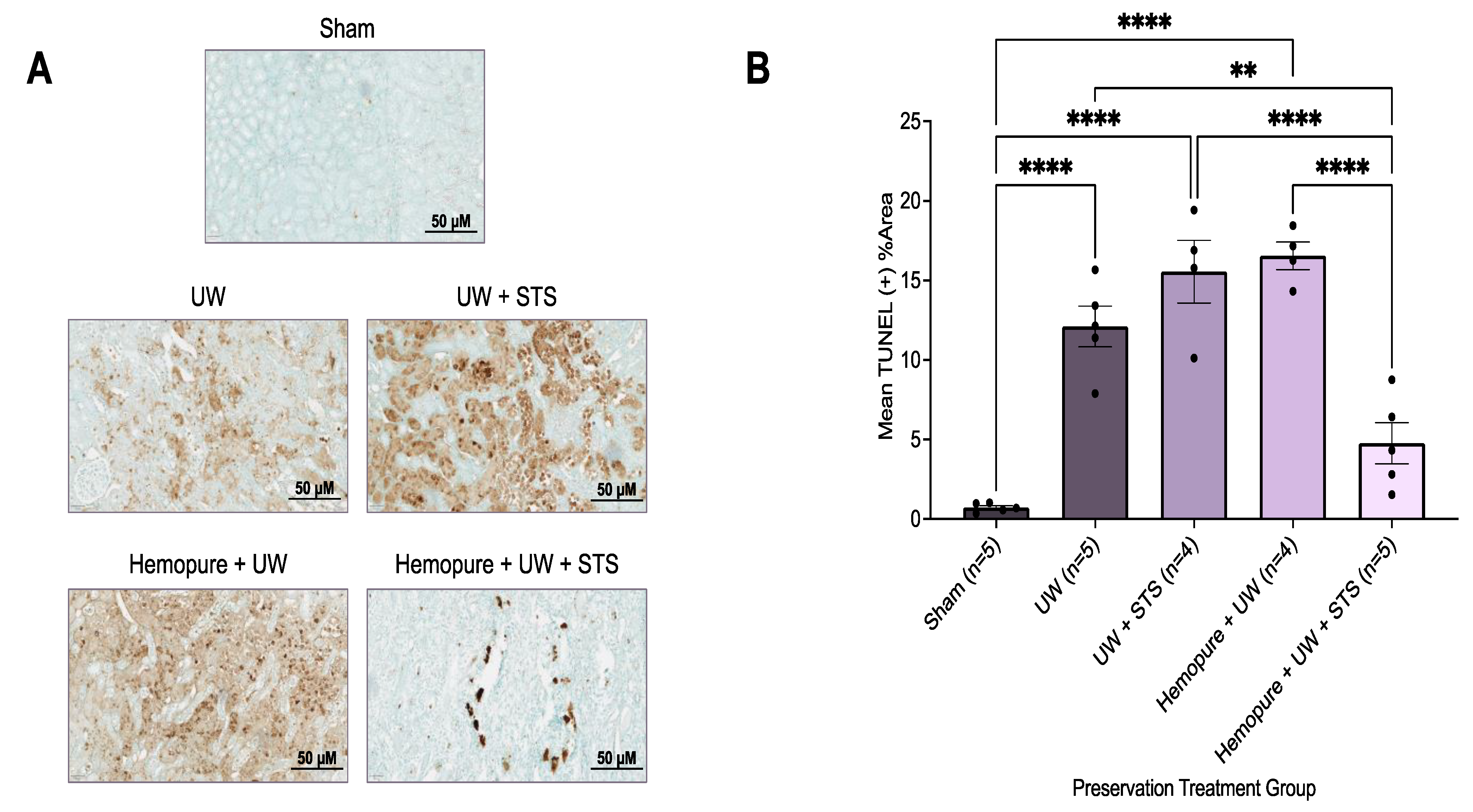
2.3. Hemopure and STS-Supplemented Preservation Solution Reduced Donor Kidney Apoptosis and Necrosis after Kidney Transplantation
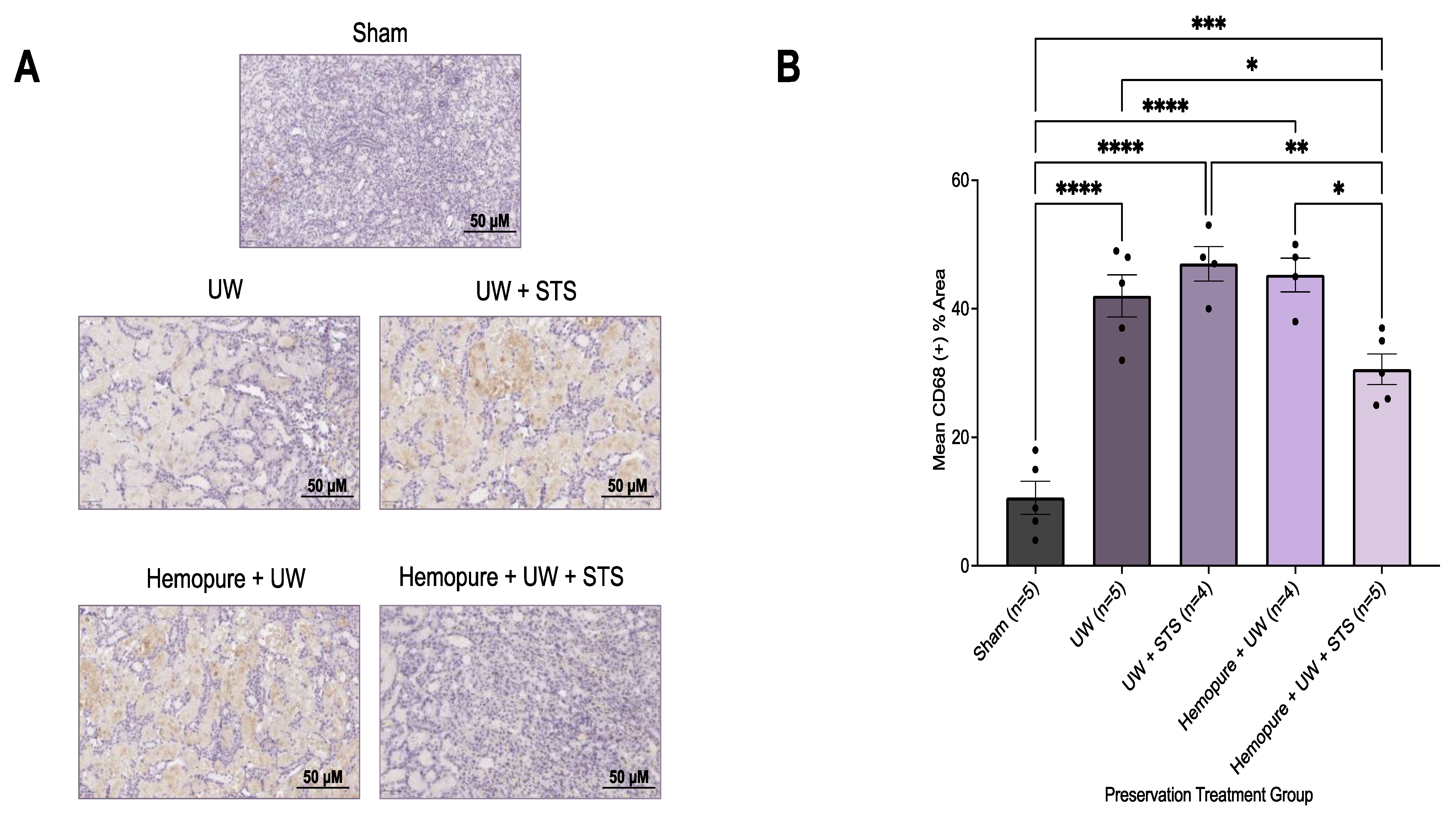
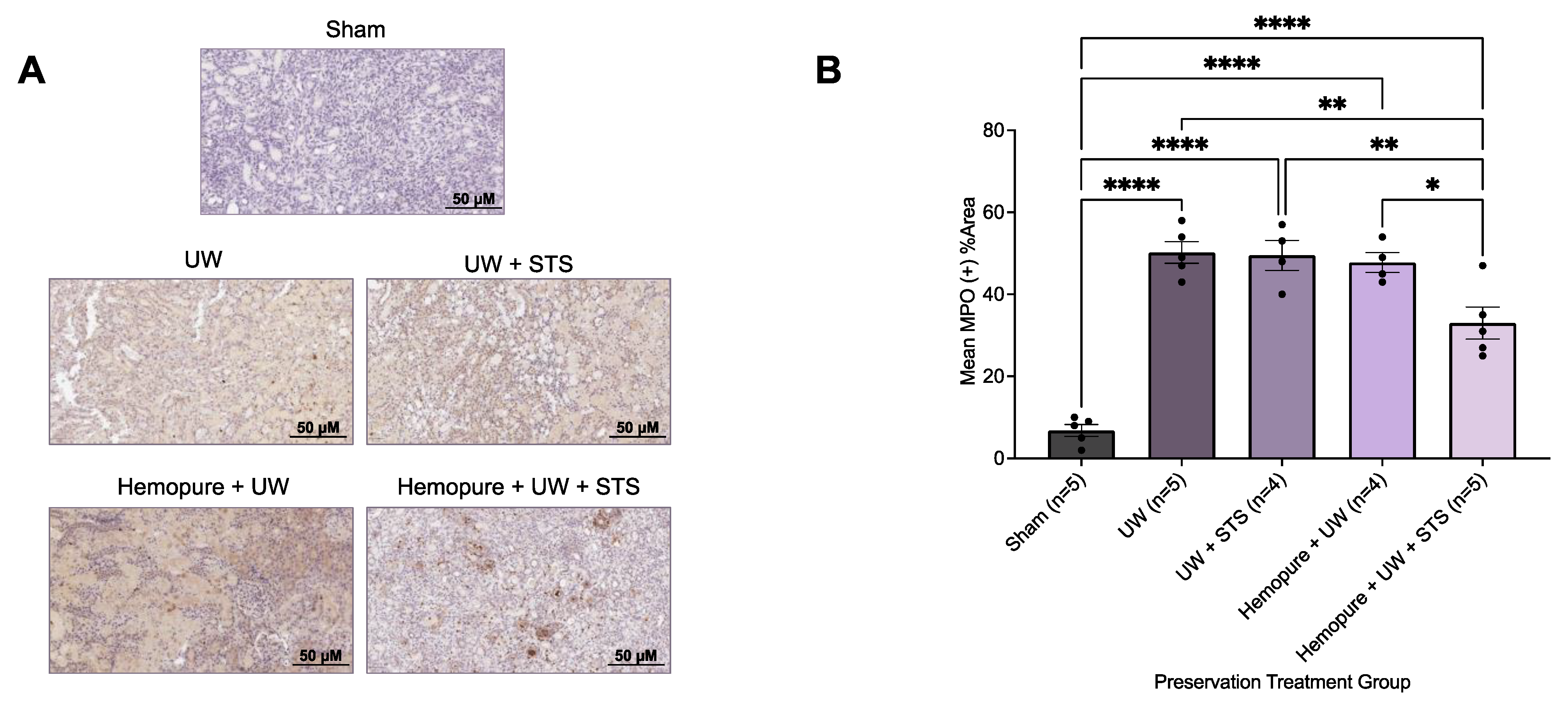
2.4. Hemopure and STS-Supplemented Preservation Solution Decreased Donor Kidney Injury Markers and Inflammatory Infiltrate after Kidney Transplantation
3. Discussion
Limitations
4. Methods
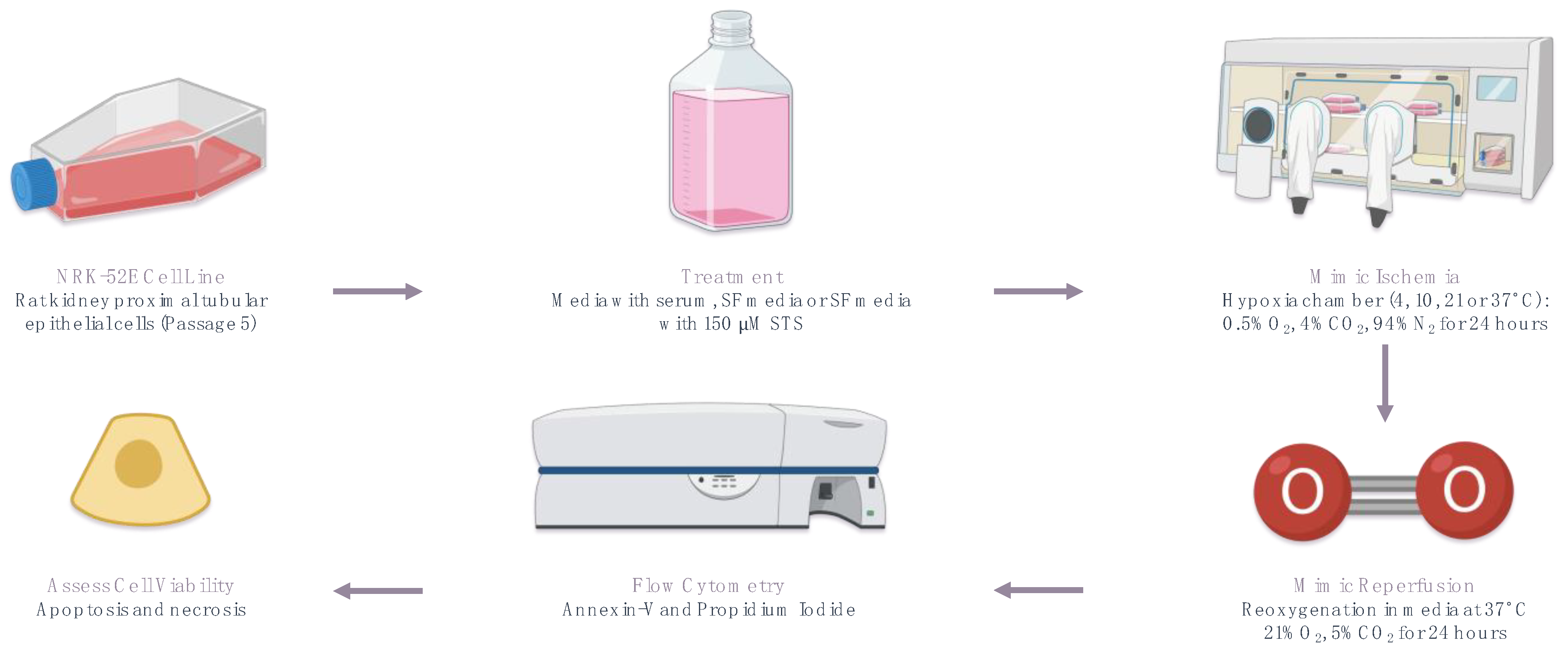
4.1. In Vitro Model of Rat Renal IRI
4.2. Analysis of Cell Viability, Apoptosis, and Necrosis
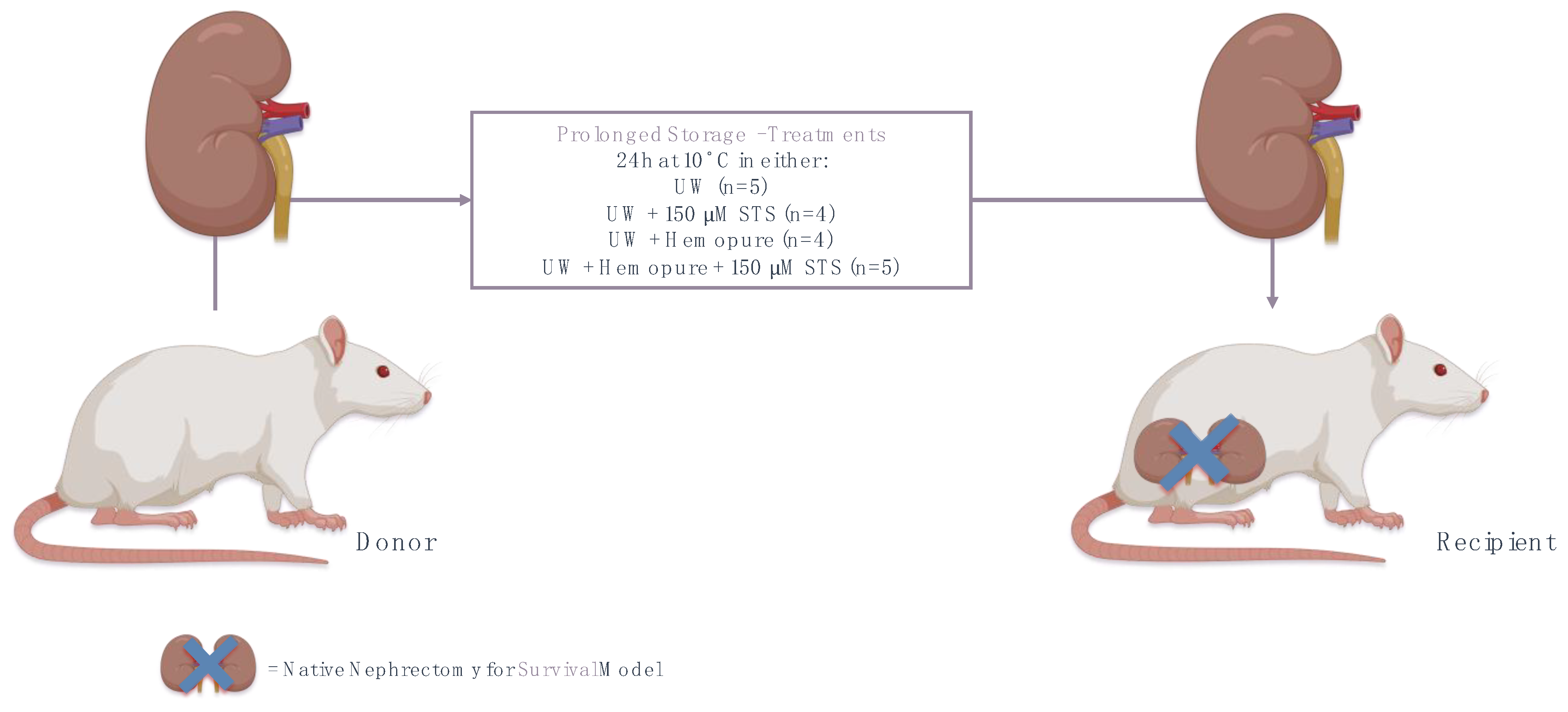
4.3. Experimental Animals
4.4. Syngeneic Orthotopic Kidney Transplant Model
4.5. Creatinine and Urine Assays
4.6. Histopathological Imaging and Quantification
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thurlow, J.S.; Joshi, M.; Yan, G.; Norris, K.C.; Agodoa, L.Y.; Yuan, C.M.; Nee, R. Global Epidemiology of End-Stage Kidney Disease and Disparities in Kidney Replacement Therapy. Am. J. Nephrol. 2021, 52, 98–107. [Google Scholar] [CrossRef]
- Deng, Y.; Li, N.; Wu, Y.; Wang, M.; Yang, S.; Zheng, Y.; Deng, X.; Xiang, D.; Zhu, Y.; Xu, P.; et al. Global, Regional, and National Burden of Diabetes-Related Chronic Kidney Disease From 1990 to 2019. Front. Endocrinol. 2021, 12, 672350. [Google Scholar] [CrossRef]
- Lacroix, J.D.; Mahoney, J.E.; Knoll, G.A. Renal transplantation using non-heart-beating donors: A potential solution to the organ donor shortage in Canada. Can. J. Surg. 2004, 47, 10–14. [Google Scholar] [PubMed]
- Himmelfarb, J.; Vanholder, R.; Mehrotra, R.; Tonelli, M. The current and future landscape of dialysis. Nat. Rev. Nephrol. 2020, 16, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Israni, A.K.; Danovitch, G. Long-Term Survival after Kidney Transplantation. N. Engl. J. Med. 2021, 385, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Steichen, C.; Giraud, S.; Bon, D.; Barrou, B.; Badet, L.; Salamé, E.; Kerforne, T.; Allain, G.; Roumy, J.; Jayle, C.; et al. Barriers and Advances in Kidney Preservation. BioMed Res. Int. 2018, 2018, 9206257. [Google Scholar] [CrossRef]
- van Eck van der Sluijs, A.; Vonk, S.; van Jaarsveld, B.C.; Bonenkamp, A.A.; Abrahams, A.C. Good practices for dialysis education, treatment, and eHealth: A scoping review. PLoS ONE 2021, 16, e0255734. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Knoll, G.; Bello, A.; Browne, S.; Jadhav, D.; Klarenbach, S.; Gill, J. Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am. J. Transplant. 2011, 11, 2093–2109. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, J.; Xia, T.C.; Xu, R.; He, X.; Xia, Y. Preservation Solutions for Kidney Transplantation: History, Advances and Mechanisms. Cell Transplant. 2019, 28, 1472–1489. [Google Scholar] [CrossRef] [PubMed]
- Guibert, E.E.; Petrenko, A.Y.; Balaban, C.L.; Somov, A.Y.; Rodriguez, J.V.; Fuller, B.J. Organ Preservation: Current Concepts and New Strategies for the Next Decade. Transfus. Med. Hemother. 2011, 38, 125–142. [Google Scholar] [CrossRef]
- van’t Hoff, M.J.H. Etudes de dynamique chimique. Recl. Trav. Chim. Pays-Bas 2010, 3, 333–336. [Google Scholar] [CrossRef]
- Lee, C.M.; Carter, J.T.; Alfrey, E.J.; Ascher, N.L.; Roberts, J.P.; Freise, C.E. Prolonged cold ischemia time obviates the benefits of 0 HLA mismatches in renal transplantation. Arch. Surg. 2000, 135, 1016–1020. [Google Scholar] [CrossRef]
- Debout, A.; Foucher, Y.; Trébern-Launay, K.; Legendre, C.; Kreis, H.; Mourad, G.; Garrigue, V.; Morelon, E.; Buron, F.; Rostaing, L.; et al. Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int. 2015, 87, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Ojo, A.O.; Wolfe, R.A.; Held, P.J.; Port, F.K.; Schmouder, R.L. Delayed graft function: Risk factors and implications for renal allograft survival. Transplantation 1997, 63, 968–974. [Google Scholar] [CrossRef]
- Barba, J.; Zudaire, J.J.; Robles, J.E.; Tienza, A.; Rosell, D.; Berián, J.M.; Pascual, I. Is there a safe cold ischemia time interval for the renal graft? Actas Urol. Esp. 2011, 35, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.R.; Wong, G.; Chapman, J.R.; Coates, P.T.; Russ, G.R.; Pleass, H.; Russell, C.; He, B.; Lim, W.H. Prolonged Ischemic Time, Delayed Graft Function, and Graft and Patient Outcomes in Live Donor Kidney Transplant Recipients. Am. J. Transplant. 2016, 16, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, D.P.; Gallinat, A.; Swoboda, S.; Wohlschläger, J.; Rauen, U.; Paul, A.; Minor, T. Subnormothermic machine perfusion for preservation of porcine kidneys in a donation after circulatory death model. Transpl. Int. 2014, 27, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Stubenitsky, B.M.; Booster, M.H.; Brasile, L.; Araneda, D.; Haisch, C.E.; Kootstra, G. Exsanguinous metabolic support perfusion—A new strategy to improve graft function after kidney transplantation. Transplantation 2000, 70, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.S.; Chatterjee, P.K.; Di Paola, R.; Mazzon, E.; Britti, D.; De Sarro, A.; Cuzzocrea, S.; Thiemermann, C. Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. J. Pharmacol. Exp. Ther. 2005, 312, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Michel, S.G.; La Muraglia GM 2nd Madariaga, M.L.; Titus, J.S.; Selig, M.K.; Farkash, E.A.; Allan, J.S.; Anderson, L.M.; Madsen, J.C. Twelve-Hour Hypothermic Machine Perfusion for Donor Heart Preservation Leads to Improved Ultrastructural Characteristics Compared to Conventional Cold Storage. Ann. Transplant. 2015, 20, 461–468. [Google Scholar] [CrossRef]
- Sage, A.T.; Richard-Greenblatt, M.; Zhong, K.; Bai, X.H.; Snow, M.B.; Babits, M.; Ali, A.; Baciu, C.; Yeung, J.C.; Liu, M.; et al. Prediction of donor related lung injury in clinical lung transplantation using a validated ex vivo lung perfusion inflammation score. J. Heart Lung Transplant. 2021, 40, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Wang, A.; Ribeiro, R.V.P.; Beroncal, E.L.; Baciu, C.; Galasso, M.; Gomes, B.; Mariscal, A.; Hough, O.; Brambate, E.; et al. Static lung storage at 10 °C maintains mitochondrial health and preserves donor organ function. Sci. Transl. Med. 2021, 13, eabf7601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Dugbartey, G.J.; Juriasingani, S.; Akbari, M.; Liu, W.; Haig, A.; McLeod, P.; Arp, J.; Sener, A. Sodium thiosulfate-supplemented UW solution protects renal grafts against prolonged cold ischemia-reperfusion injury in a murine model of syngeneic kidney transplantation. Biomed. Pharmacother. 2022, 145, 112435. [Google Scholar] [CrossRef] [PubMed]
- Juriasingani, S.; Ruthirakanthan, A.; Richard-Mohamed, M.; Akbari, M.; Aquil, S.; Patel, S.; Al-Ogaili, R.; Whiteman, M.; Luke, P.; Sener, A. Subnormothermic Perfusion with H2S Donor AP39 Improves DCD Porcine Renal Graft Outcomes in an Ex Vivo Model of Kidney Preservation and Reperfusion. Biomolecules 2021, 11, 446. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, R.L. The proximal tubule is the primary target of injury and progression of kidney disease: Role of the glomerulotubular junction. Am. J. Physiol.-Ren. Physiol. 2016, 311, F145–F161. [Google Scholar] [CrossRef] [PubMed]
- Besseling, P.J.; Pieters, T.T.; Nguyen, I.T.N.; de Bree, P.M.; Willekes, N.; Dijk, A.H.; Bovée, D.M.; Hoorn, E.J.; Rookmaaker, M.B.; Gerritsen, K.G.; et al. A plasma creatinine- and urea-based equation to estimate glomerular filtration rate in rats. Am. J. Physiol.-Ren. Physiol. 2021, 320, F518–F524. [Google Scholar] [CrossRef] [PubMed]
- Cima, L.; Nacchia, F.; Ghimenton, C.; Valotto, G.; Boschiero, L.; Gobbo, S.; Zaza, G.; Neil, D.; Mescoli, C.; Vanzo, F.; et al. Histopathology and Long-Term Outcome of Kidneys Transplanted from Donors with Severe Acute Kidney Injury. Prog. Transplant. 2019, 29, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Dugbartey, G.J.; Juriasingani, S.; Sener, A. Hydrogen Sulfide Metabolite, Sodium Thiosulfate: Clinical Applications and Underlying Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 6452. [Google Scholar] [CrossRef]
- Lobb, I.; Mok, A.; Lan, Z.; Liu, W.; Garcia, B.; Sener, A. Supplemental hydrogen sulphide protects transplant kidney function and prolongs recipient survival after prolonged cold ischaemia-reperfusion injury by mitigating renal graft apoptosis and inflammation. BJU Int. 2012, 110, E1187–E1195. [Google Scholar] [CrossRef]
- Lobb, I.; Davison, M.; Carter, D.; Liu, W.; Haig, A.; Gunaratnam, L.; Sener, A. Hydrogen Sulfide Treatment Mitigates Renal Allograft Ischemia-Reperfusion Injury during Cold Storage and Improves Early Transplant Kidney Function and Survival Following Allogeneic Renal Transplantation. J. Urol. 2015, 194, 1806–1815. [Google Scholar] [CrossRef]
- Lobb, I.; Jiang, J.; Lian, D.; Liu, W.; Haig, A.; Saha, M.N.; Torregrossa, R.; Wood, M.E.; Whiteman, M.; Sener, A. Hydrogen Sulfide Protects Renal Grafts Against Prolonged Cold Ischemia-Reperfusion Injury via Specific Mitochondrial Actions. Am. J. Transplant. 2017, 17, 341–352. [Google Scholar] [CrossRef]
- Juriasingani, S.; Akbari, M.; Chan, J.Y.; Whiteman, M.; Sener, A. H2S supplementation: A novel method for successful organ preservation at subnormothermic temperatures. Nitric Oxide 2018, 81, 57–66. [Google Scholar] [CrossRef]
- Adams, T.D.; Hosgood, S.A.; Nicholson, M.L. Physiological effects of altering oxygenation during kidney normothermic machine perfusion. Am. J. Physiol.-Ren. Physiol. 2019, 316, F823–F829. [Google Scholar] [CrossRef]
- Elliott, T.R.; Nicholson, M.L.; Hosgood, S.A. Normothermic kidney perfusion: An overview of protocols and strategies. Am. J. Transplant. 2021, 21, 1382–1390. [Google Scholar] [CrossRef]
- Boteon, Y.L.; Hessheimer, A.J.; Brüggenwirth, I.M.A.; Boteon, A.P.C.S.; Padilla, M.; de Meijer, V.E.; Domínguez-Gil, B.; Porte, R.J.; Perera, M.T.P.R.; Martins, P.N. The economic impact of machine perfusion technology in liver transplantation. Artif. Organs 2022, 46, 191–200. [Google Scholar] [CrossRef]
- Wight, J.; Chilcott, J.; Holmes, M.; Brewer, N. The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors. Health Technol. Assess. 2003, 7, 1–94. [Google Scholar] [CrossRef]
- Webb, A.N.; Lester, E.L.W.; Shapiro, A.M.J.; Eurich, D.T.; Bigam, D.L. Cost-utility analysis of normothermic machine perfusion compared to static cold storage in liver transplantation in the Canadian setting. Am. J. Transplant. 2022, 22, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Tabibzadeh, N.; Wagner, S.; Metzger, M.; Flamant, M.; Houillier, P.; Boffa, J.J.; Vrtovsnik, F.; Thervet, E.; Stengel, B.; Haymann, J.P.; et al. Fasting Urinary Osmolality, CKD Progression, and Mortality: A Prospective Observational Study. Am. J. Kidney Dis. 2019, 73, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Devuyst, O.; Chapman, A.B.; Gansevoort, R.T.; Higashihara, E.; Perrone, R.D.; Torres, V.E.; Blais, J.D.; Zhou, W.; Ouyang, J.; Czerwiec, F.S. Urine Osmolality, Response to Tolvaptan, and Outcome in Autosomal Dominant Polycystic Kidney Disease: Results from the TEMPO 3:4 Trial. J. Am. Soc. Nephrol. 2017, 28, 1592–1602. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Nakayama, M.; Sakoh, T.; Yoshitomi, R.; Fukui, A.; Katafuchi, E.; Tsuda, S.; Nakano, T.; Tsuruya, K.; Kitazono, T. Blood urea nitrogen is independently associated with renal outcomes in Japanese patients with stage 3–5 chronic kidney disease: A prospective observational study. BMC Nephrol. 2019, 20, 115. [Google Scholar] [CrossRef] [PubMed]
- Dragun, D.; Hoff, U.; Park, J.K.; Qun, Y.; Schneider, W.; Luft, F.C.; Haller, H. Prolonged cold preservation augments vascular injury independent of renal transplant immunogenicity and function. Kidney Int. 2001, 60, 1173–1181. [Google Scholar] [CrossRef]
- Tozzi, M.; Franchin, M.; Soldini, G.; Ietto, G.; Chiappa, C.; Maritan, E.; Villa, F.; Carcano, G.; Dionigi, R. Impact of static cold storage VS hypothermic machine preservation on ischemic kidney graft: Inflammatory cytokines and adhesion molecules as markers of ischemia/reperfusion tissue damage. Our preliminary results. Int. J. Surg. 2013, 11 (Suppl. 1), S110–S114. [Google Scholar] [CrossRef]
- Hua, Y.; Ying, X.; Qian, Y.; Liu, H.; Lan, Y.; Xie, A.; Zhu, X. Physiological and pathological impact of AQP1 knockout in mice. Biosci. Rep. 2019, 39, BSR20182303. [Google Scholar] [CrossRef]
- Luo, R.; Hu, S.; Liu, Q.; Han, M.; Wang, F.; Qiu, M.; Li, S.; Li, X.; Yang, T.; Fu, X.; et al. Hydrogen sulfide upregulates renal AQP-2 protein expression and promotes urine concentration. FASEB J. 2019, 33, 469–483. [Google Scholar] [CrossRef]
- Hunter, J.P.; Hosgood, S.A.; Patel, M.; Rose, R.; Read, K.; Nicholson, M.L. Effects of hydrogen sulphide in an experimental model of renal ischaemia-reperfusion injury. Br. J. Surg. 2012, 99, 1665–1671. [Google Scholar] [CrossRef]
- Dursun, M.; Otunctemur, A.; Ozbek, E.; Sahin, S.; Besiroglu, H.; Ozsoy, O.D.; Cekmen, M.; Somay, A.; Ozbay, N. Protective effect of hydrogen sulfide on renal injury in the experimental unilateral ureteral obstruction. Int. Braz. J. Urol. 2015, 41, 1185–1193. [Google Scholar] [CrossRef]
- Burne, M.J.; Elghandour, A.; Haq, M.; Saba, S.R.; Norman, J.; Condon, T.; Bennett, F.; Rabb, H. IL-1 and TNF independent pathways mediate ICAM-1/VCAM-1 up-regulation in ischemia reperfusion injury. J. Leukoc. Biol. 2001, 70, 192–198. [Google Scholar] [CrossRef]
- Fan, L.; Gao, W.; Nguyen, B.V.; Jefferson, J.R.; Liu, Y.; Fan, F.; Roman, R.J. Impaired renal hemodynamics and glomerular hyperfiltration contribute to hypertension-induced renal injury. Am. J. Physiol.-Ren. Physiol. 2020, 319, F624–F635. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.L.; Chen, Y.S.; Chung, S.D.; Lin, S.C.; Chien, C.T. Sodium Thiosulfate Ameliorates Renovascular Hypertension-Induced Renal Dysfunction and Injury in Rats. Kidney Blood Press. Res. 2021, 46, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.N.; Patel, S.V.B.; Sun, Q.; Jiang, L.; Richard-Mohamed, M.; Ruthirakanthan, A.; Aquil, S.; Al-Ogaili, R.; Juriasingani, S.; Sener, A.; et al. Renal Protection Against Ischemia Reperfusion Injury: Hemoglobin-based Oxygen Carrier-201 Versus Blood as an Oxygen Carrier in Ex Vivo Subnormothermic Machine Perfusion. Transplantation 2020, 104, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jin, S.; Teng, X.; Hu, Z.; Zhang, Z.; Qiu, X.; Tian, D.; Wu, Y. Hydrogen Sulfide Attenuates LPS-Induced Acute Kidney Injury by Inhibiting Inflammation and Oxidative Stress. Oxid. Med. Cell. Longev. 2018, 2018, 6717212. [Google Scholar] [CrossRef] [PubMed]
- Minor, T.; von Horn, C.; Gallinat, A.; Kaths, M.; Kribben, A.; Treckmann, J.; Paul, A. First-in-man controlled rewarming and normothermic perfusion with cell-free solution of a kidney prior to transplantation. Am. J. Transplant. 2020, 20, 1192–1195. [Google Scholar] [CrossRef] [PubMed]
- Bonventre, J.V. Kidney injury molecule-1 (KIM-1): A urinary biomarker and much more. Nephrol. Dial. Transplant. 2009, 24, 3265–3268. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Ismail, O.Z.; Zhang, X.; Haig, A.; Lian, D.; Gunaratnam, L. Donor kidney injury molecule-1 promotes graft recovery by regulating systemic necroinflammation. Am. J. Transplant. 2018, 18, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Czigany, Z.; Lurje, I.; Schmelzle, M.; Schöning, W.; Öllinger, R.; Raschzok, N.; Sauer, I.M.; Tacke, F.; Strnad, P.; Trautwein, C.; et al. Ischemia-Reperfusion Injury in Marginal Liver Grafts and the Role of Hypothermic Machine Perfusion: Molecular Mechanisms and Clinical Implications. J. Clin. Med. 2020, 9, 846. [Google Scholar] [CrossRef] [PubMed]
- Khalid, U.; Pino-Chavez, G.; Nesargikar, P.; Jenkins, R.H.; Bowen, T.; Fraser, D.J.; Chavez, R. Kidney ischaemia reperfusion injury in the rat: The EGTI scoring system as a valid and reliable tool for histological assessment. J. Histol. Histopathol. 2016, 3, 1. [Google Scholar] [CrossRef]
- Ali, A.; Alzamly, A.; Greish, Y.E.; Bakiro, M.; Nguyen, H.L.; Mahmoud, S.T. A Highly Sensitive and Flexible Metal-Organic Framework Polymer-Based H2S Gas Sensor. ACS Omega 2021, 6, 17690–17697. [Google Scholar] [CrossRef]
- Moretti, R.; Papale, P. On the oxidation state and volatile behavior in multicomponent gas-melt equilibria. Chem. Geol. 2004, 213, 265–280. [Google Scholar] [CrossRef]
- Legeai, C.; Durand, L.; Savoye, E.; Macher, M.A.; Bastien, O. Effect of preservation solutions for static cold storage on kidney transplantation outcomes: A National Registry Study. Am. J. Transplant. 2020, 20, 3426–3442. [Google Scholar] [CrossRef]
- Schroeder, R.J., 2nd; Audlin, J.; Luo, J.; Nicholas, B.D. Pharmacokinetics of sodium thiosulfate in Guinea pig perilymph following middle ear application. J. Otol. 2018, 13, 54–58. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou Taka, M.; Dugbartey, G.J.; Richard-Mohamed, M.; McLeod, P.; Jiang, J.; Major, S.; Arp, J.; O’Neil, C.; Liu, W.; Gabril, M.; et al. Evaluating the Effects of Kidney Preservation at 10 °C with Hemopure and Sodium Thiosulfate in a Rat Model of Syngeneic Orthotopic Kidney Transplantation. Int. J. Mol. Sci. 2024, 25, 2210. https://doi.org/10.3390/ijms25042210
Abou Taka M, Dugbartey GJ, Richard-Mohamed M, McLeod P, Jiang J, Major S, Arp J, O’Neil C, Liu W, Gabril M, et al. Evaluating the Effects of Kidney Preservation at 10 °C with Hemopure and Sodium Thiosulfate in a Rat Model of Syngeneic Orthotopic Kidney Transplantation. International Journal of Molecular Sciences. 2024; 25(4):2210. https://doi.org/10.3390/ijms25042210
Chicago/Turabian StyleAbou Taka, Maria, George J. Dugbartey, Mahms Richard-Mohamed, Patrick McLeod, Jifu Jiang, Sally Major, Jacqueline Arp, Caroline O’Neil, Winnie Liu, Manal Gabril, and et al. 2024. "Evaluating the Effects of Kidney Preservation at 10 °C with Hemopure and Sodium Thiosulfate in a Rat Model of Syngeneic Orthotopic Kidney Transplantation" International Journal of Molecular Sciences 25, no. 4: 2210. https://doi.org/10.3390/ijms25042210
APA StyleAbou Taka, M., Dugbartey, G. J., Richard-Mohamed, M., McLeod, P., Jiang, J., Major, S., Arp, J., O’Neil, C., Liu, W., Gabril, M., Moussa, M., Luke, P., & Sener, A. (2024). Evaluating the Effects of Kidney Preservation at 10 °C with Hemopure and Sodium Thiosulfate in a Rat Model of Syngeneic Orthotopic Kidney Transplantation. International Journal of Molecular Sciences, 25(4), 2210. https://doi.org/10.3390/ijms25042210





