Theoretical Analysis and Expression Profiling of 17β-Hydroxysteroid Dehydrogenase Genes in Gonadal Development and Steroidogenesis of Leopard Coral Grouper (Plectropomus leopardus)
Abstract
1. Introduction
2. Results
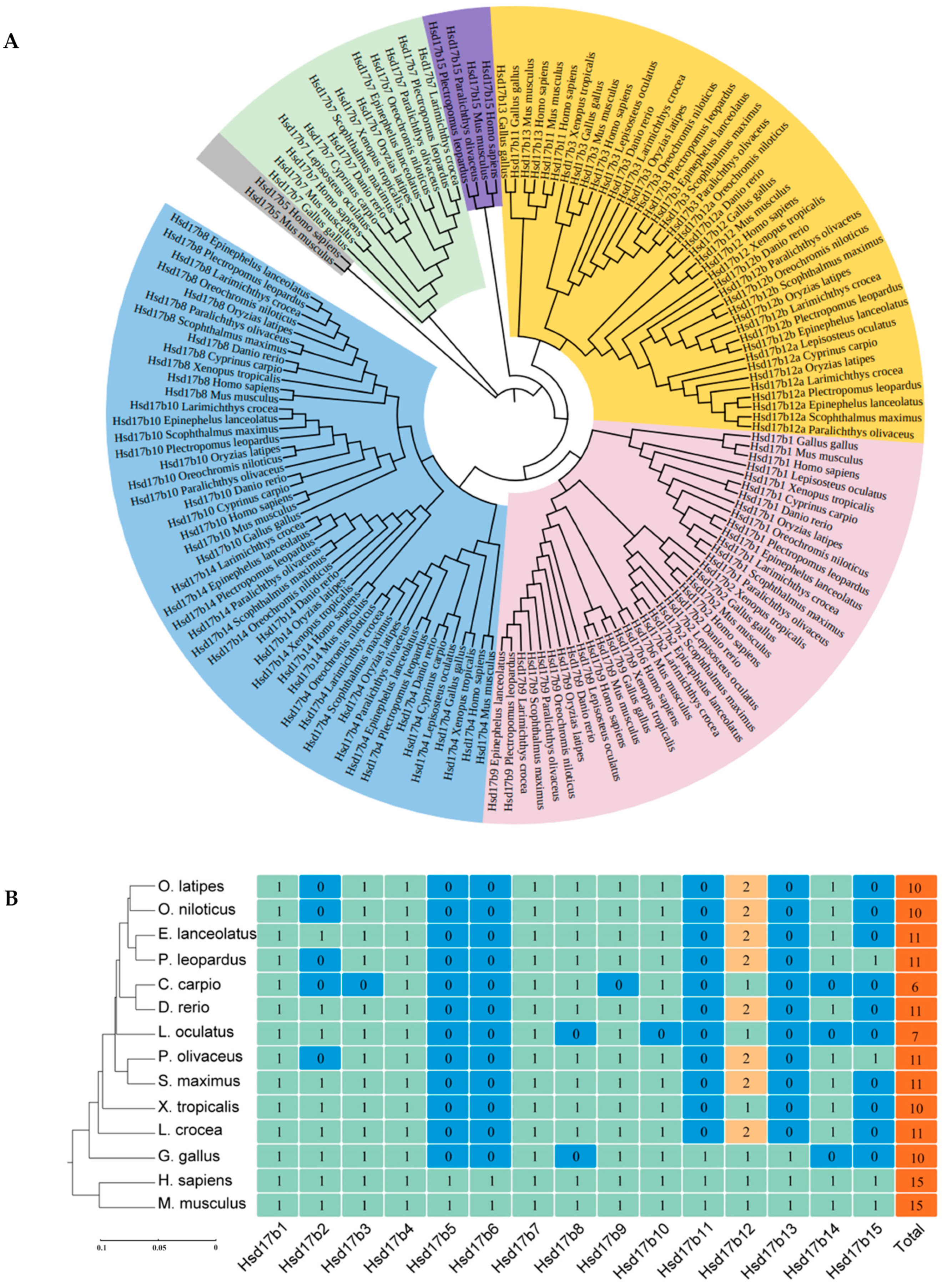
2.1. Amino Acid Sequence Alignment and Phylogenetic Analysis of the Hsd17b Gene Family
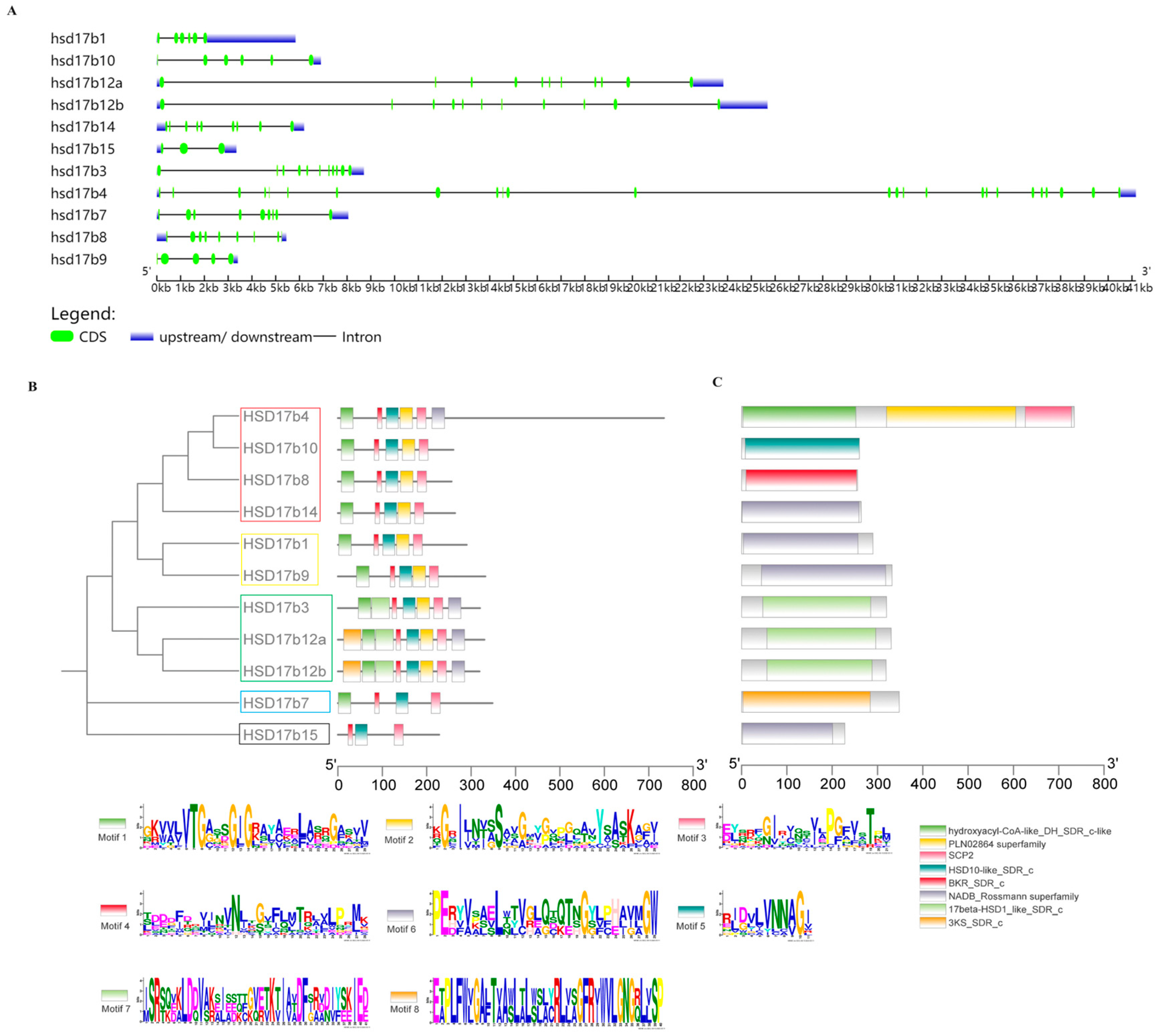
2.2. Gene Structure Analysis
2.3. Protein Structure and Biochemical Properties Prediction
2.4. Subcellular Localization
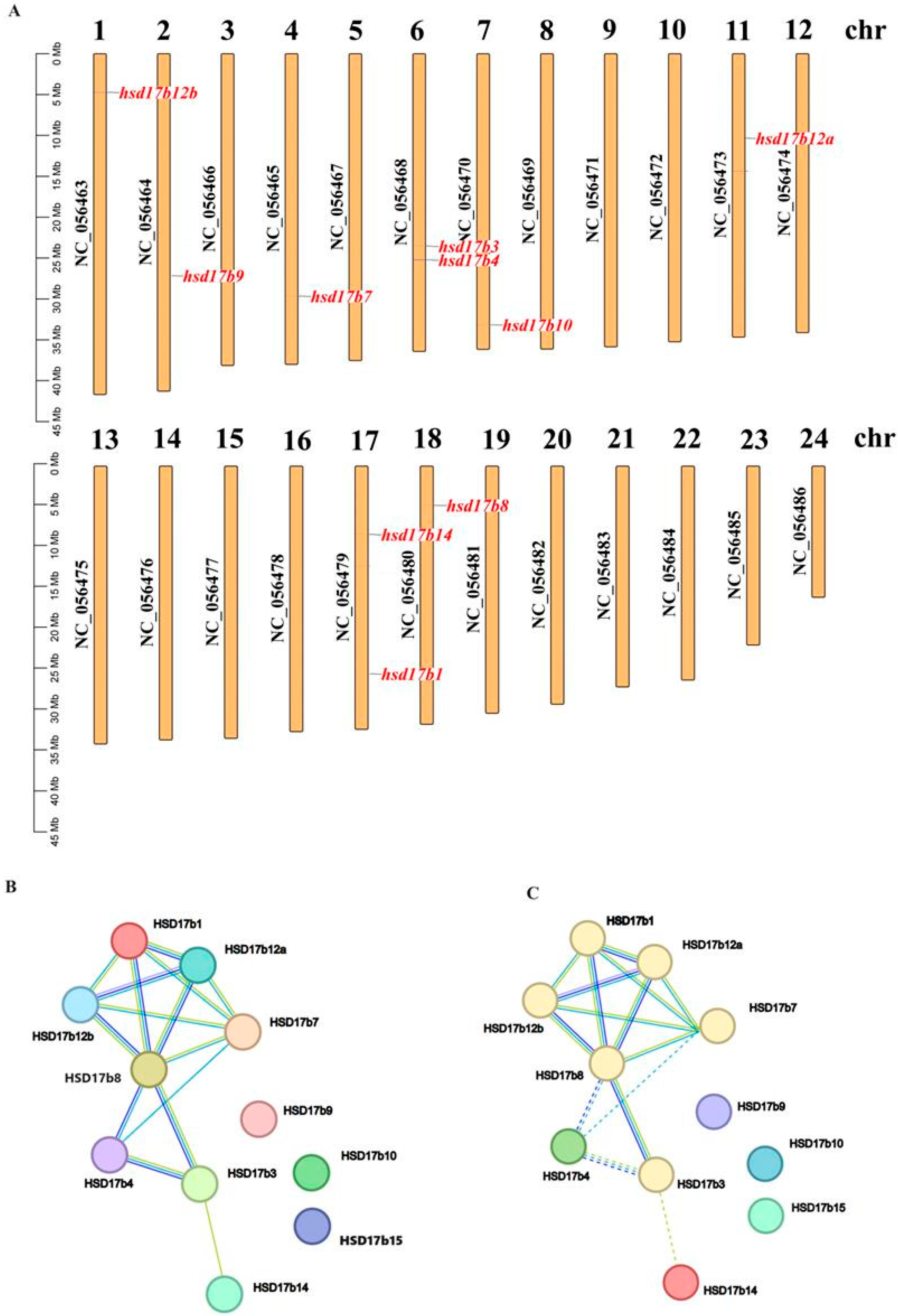
2.5. Chromosomal Distribution and Protein Interaction Prediction
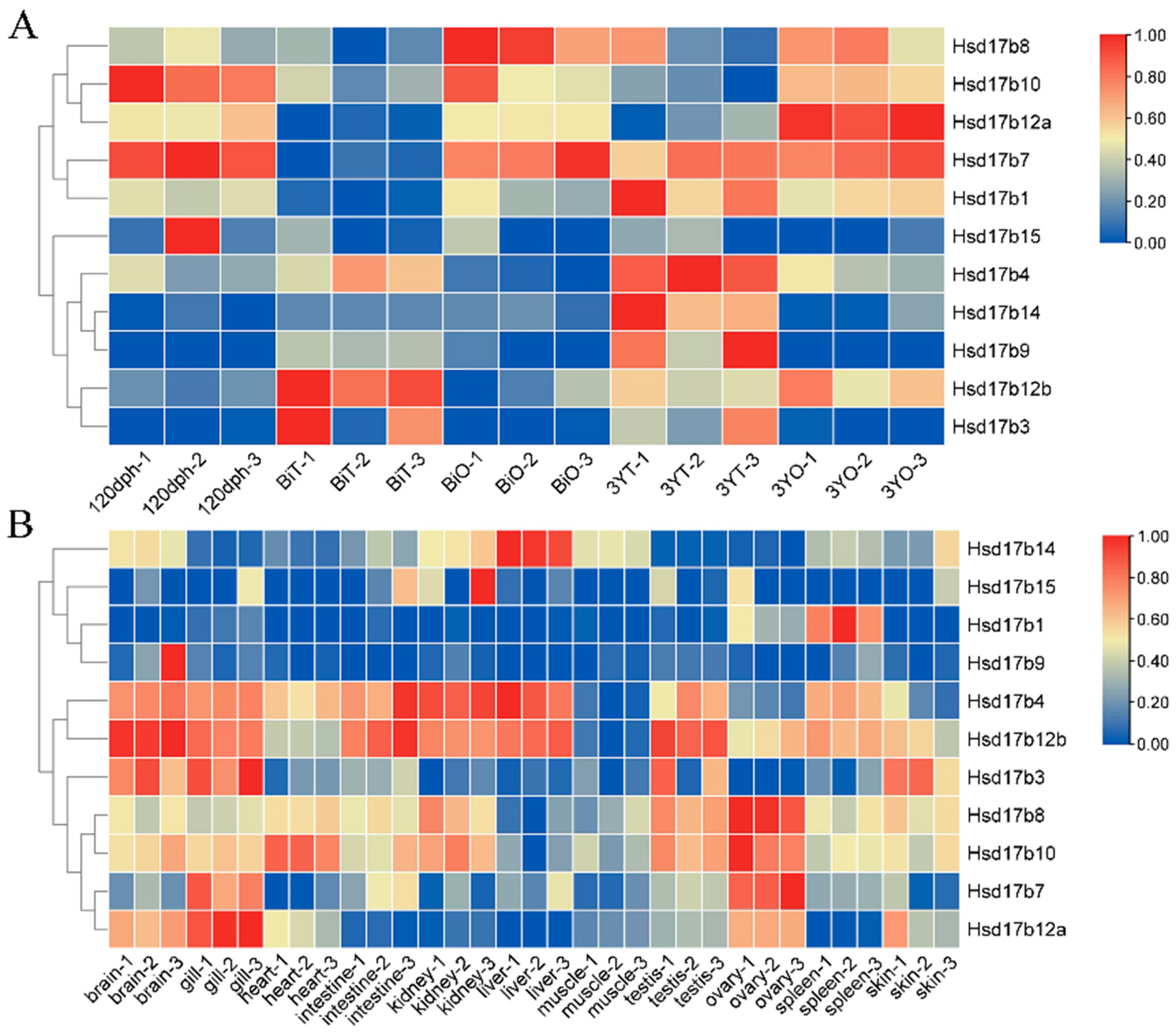
2.6. Expression Levels of Hsd17b Family Genes in Male and Female Gonads at Different Stages
2.7. Expression Levels of Hsd17b Family Genes in Different Tissues
2.8. HE Staining Observation and Specific Expression of Hsd17b4 and -12a in Gonads
3. Discussion
4. Materials and Methods
4.1. Experimental Fish and Sample Collection
4.2. Identification and Phylogenetic Analysis of Hsd17b Family Members
4.3. Physicochemical Properties Analysis of Hsd17b Family Members
4.4. Transcriptome-Based Expression Analysis of Hsd17b
4.5. Histological Observation
4.6. Fluorescence In Situ Hybridization (FISH)
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swain, A.; Lovell-Badge, R. Mammalian sex determination: A molecular drama. Genes Dev. 1999, 13, 755–767. [Google Scholar] [CrossRef]
- Butka, E.G.; Freedberg, S. Population structure leads to male-biased population sex ratios under environmental sex determination, Evolution. Int. J. Org. Evol. 2019, 73, 99–110. [Google Scholar]
- Vega-Frutis, R.; Macías-Ordóñez, R.; Guevara, R.; Fromhage, L. Sex change in plants and animals: A unified perspective. J. Evol. Biol. 2014, 27, 667–675. [Google Scholar] [CrossRef]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Nakamoto, M.; Shibata, Y.; Ohno, K.; Usami, T.; Kamei, Y.; Taniguchi, Y.; Todo, T.; Sakamoto, T.; Young, G.; Swanson, P.; et al. Ovarian aromatase loss-of-function mutant medaka undergo ovary degeneration and partial female-to-male sex reversal after puberty. Mol. Cell. Endocrinol. 2018, 460, 104–122. [Google Scholar] [CrossRef]
- Nakamura, M.; Kobayashi, T.; Chang, X.-T.; Nagahama, Y. Gonadal sex differentiation in teleost fish. J. Exp. Zool. 1998, 281, 362–372. [Google Scholar] [CrossRef]
- Qin, M.; Zhang, Z.; Song, W.; Wong, Q.W.-L.; Chen, W.; Shirgaonkar, N.; Ge, W. Roles of Figla/figla in Juvenile Ovary Development and Follicle Formation during Zebrafish Gonadogenesis. Endocrinology 2018, 159, 3699–3722. [Google Scholar] [CrossRef]
- Strüssmann, C.A.; Nakamura, M. Morphology, endocrinology, and environmental modulation of gonadal sex differentiation in teleost fishes. Fish Physiol. Biochem. 2002, 26, 13–29. [Google Scholar] [CrossRef]
- Hinfray, N.; Sohm, F.; Caulier, M.; Chadili, E.; Piccini, B.; Torchy, C.; Porcher, J.M.; Guiguen, Y.; Brion, F. Dynamic and differential expression of the gonadal aromatase during the process of sexual differentiation in a novel transgenic cyp19a1a-eGFP zebrafish line. Gen. Comp. Endocrinol. 2018, 261, 179–189. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, H.; Xiang, Y.; Jia, K.; Luo, M.; Yi, M. A novel germline and somatic cell expression of two sexual differentiation genes, Dmrt1 and Foxl2 in marbled goby (Oxyeleotris marmorata). Aquaculture 2020, 516, 734619. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, M.; Wang, L.; Yu, Z.; Wang, J.; Yu, Q.; Xiao, L.; Lu, M.; Li, S.; Zhang, Y.; et al. Overexpression of Anti-müllerian Hormone Gene in vivo Affects Gonad Sex Differentiation in Undifferentiated Orange-Spotted Groupers (Epinephelus coioides). Front. Endocrinol. 2019, 10, 210. [Google Scholar] [CrossRef]
- Peng, Y.-X.; Huang, Y.-Q.; Zhong, J.; Jiang, Z.-T.; Fan, S.; Shi, H.-J.; Chen, H.-P.; Deng, S.-P.; Li, G.-L.; Jiang, D.-N. Identification of sex-linked marker and candidate sex determination gene in ornamental fish, African scat (Scatophagus tetracanthus). Aquaculture 2023, 563, 739023. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, G.; Li, M.; Zhu, F.; Liu, Q.; Naruse, K.; Herpin, A.; Nagahama, Y.; Li, J.; Hong, Y. Autosomal gsdf acts as a male sex initiator in the fish medaka. Sci. Rep. 2016, 6, 19738. [Google Scholar] [CrossRef]
- Godwin, J. Neuroendocrinology of sexual plasticity in teleost fishes. Front. Neuroendocrinol. 2010, 31, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.-O. 3 Sex Differentiation. In Fish Physiology; Hoar, W.S., Randall, D.J., Eds.; Academic Press: Cambridge, MA, USA, 1969; pp. 117–175. [Google Scholar]
- Li, M.; Sun, L.; Wang, D. Roles of estrogens in fish sexual plasticity and sex differentiation. Gen. Comp. Endocrinol. 2019, 277, 9–16. [Google Scholar] [CrossRef]
- Miller, K.A.; Kenter, L.W.; Breton, T.S.; Berlinsky, D.L. The effects of stress, cortisol administration and cortisol inhibition on black sea bass (Centropristis striata) sex differentiation. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 227, 154–160. [Google Scholar] [CrossRef]
- Peltoketo, H.; Luu-The, V.; Simard, J.; Adamski, J. 17beta-hydroxysteroid dehydrogenase (HSD)/17-ketosteroid reductase (KSR) family; nomenclature and main characteristics of the 17HSD/KSR enzymes. J. Mol. Endocrinol. 1999, 23, 1–11. [Google Scholar] [CrossRef]
- Steckelbroeck, S.; Watzka, M.; Reissinger, A.; Wegener-Toper, P.; Bidlingmaier, F.; Bliesener, N.; Hans, V.H.; Clusmann, H.; Ludwig, M.; Siekmann, L.; et al. Characterisation of estrogenic 17beta-hydroxysteroid dehydrogenase (17beta-HSD) activity in the human brain. J. Steroid Biochem. Mol. Biol. 2003, 86, 79–92. [Google Scholar] [CrossRef]
- Kazeto, Y.; Ijiri, S.; Matsubara, H.; Adachi, S.; Yamauchi, K. Cloning of 17beta-hydroxysteroid dehydrogenase-I cDNAs from Japanese eel ovary. Biochem. Biophys. Res. Commun. 2000, 279, 451–456. [Google Scholar] [CrossRef]
- Rajakumar, A.; Senthilkumaran, B. Molecular cloning and expression analysis of 17β-hydroxysteroid dehydrogenase 1 and 12 during gonadal development, recrudescence and after in vivo hCG induction in catfish, Clarias batrachus. Steroids 2014, 92, 81–89. [Google Scholar] [CrossRef]
- Akhavan, S.R.; Salati, A.P.; Jalali, A.H.; Falahatkar, B. Expression profile of star and cyp19 and plasma sex steroid during gonad development from previtellogenesis to early atresia in captive Sterlet sturgeon, Acipenser ruthenus. J. Appl. Ichthyol. 2019, 35, 249–256. [Google Scholar] [CrossRef]
- Liang, D.; Fan, Z.; Weng, S.; Jiao, S.; Wu, Z.; Zou, Y.; Tan, X.; Li, J.; Zhang, P.; You, F. Characterization and expression of StAR2a and StAR2b in the olive flounder Paralichthys Olivaceus. Gene 2017, 626, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Yu, H.; Ni, F.; Niu, J.; Liu, X.; Wang, X. Roles of two cyp11 genes in sex hormone biosynthesis in Japanese flounder (Paralichthys olivaceus). Mol. Reprod. Dev. 2020, 87, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Moeller, G.; Adamski, J. Integrated view on 17beta-hydroxysteroid dehydrogenases. Mol. Cell. Endocrinol. 2009, 301, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Guo, X.; Huang, Y.; Gao, H.; Xu, H.; Liu, S.; Zheng, W.; Zhang, T.; Tian, C.; Zhu, C.; et al. De novo sequencing and chromosomal-scale genome assembly of leopard coral grouper, Plectropomus leopardus. Mol. Ecol. Resour. 2020, 20, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Fabinyi, M. Historical, cultural and social perspectives on luxury seafood consumption in China. Environ. Conserv. 2012, 39, 83–92. [Google Scholar] [CrossRef]
- Wang, T.; Wu, X.; Song, L.; Yang, Y.; Gong, S.; Zeng, L.; Tao, Y.; Zhong, C.; Meng, Z.; Liu, X. Identification of candidate growth-related SNPs and genes using GWAS and transcriptome analyses in leopard coral grouper (Plectropomus leopardus). Aquaculture 2023, 574, 739677. [Google Scholar] [CrossRef]
- Hao, R.; Zhu, X.; Tian, C.; Zhu, C.; Li, G. Analysis of body color formation of leopard coral grouper Plectropomus leopardus. Front. Mar. Sci. 2022, 9, 964774. [Google Scholar] [CrossRef]
- Zhu, X.; Hao, R.; Zhang, J.; Tian, C.; Hong, Y.; Zhu, C.; Li, G. Dietary astaxanthin improves the antioxidant capacity, immunity and disease resistance of coral trout (Plectropomus leopardus). Fish Shellfish Immunol. 2022, 122, 38–47. [Google Scholar] [CrossRef]
- Gai, C.; Liu, J.; Zheng, X.; Xu, L.; Ye, H. Identification of Vibrio ponticus as a bacterial pathogen of coral trout Plectropomus leopardus. Front. Cell. Infect. Microbiol. 2022, 12, 1925. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Ijiri, S.; Surugaya, R.; Sakai, R.; Adachi, S. 17β-hydroxysteroid dehydrogenase type 12-like is associated with maturation-inducing steroid synthesis during induced oocyte maturation and ovulation in sturgeons. Aquaculture 2022, 546, 737238. [Google Scholar] [CrossRef]
- Zou, C.; Wang, L.; Zou, Y.; Wu, Z.; Wang, W.; Liang, S.; Wang, L.; You, F. Characteristics and sex dimorphism of 17β-hydroxysteroid dehydrogenase family genes in the olive flounder Paralichthys olivaceus. J. Steroid Biochem. Mol. Biol. 2020, 199, 105597. [Google Scholar] [CrossRef]
- Oppermann, U.; Filling, C.; Hult, M.; Shafqat, N.; Wu, X.; Lindh, M.; Shafqat, J.; Nordling, E.; Kallberg, Y.; Persson, B.; et al. Short-chain dehydrogenases/reductases (SDR): The 2002 update. Chem. Biol. Interact. 2003, 143–144, 247–253. [Google Scholar] [CrossRef]
- London, S.E.; Clayton, D.F. Genomic and neural analysis of the estradiol-synthetic pathway in the zebra finch. BMC Neurosci. 2010, 11, 46. [Google Scholar] [CrossRef]
- Mindnich, R.; Deluca, D.; Adamski, J. Identification and characterization of 17β-hydroxysteroid dehydrogenases in the zebrafish, Danio Rerio. Mol. Cell. Endocrinol. 2004, 215, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Fomitcheva, J.; Baker, M.E.; Anderson, E.; Lee, G.Y.; Aziz, N. Characterization of Ke 6, a New 17β-Hydroxysteroid Dehydrogenase, and Its Expression in Gonadal Tissues*. J. Biol. Chem. 1998, 273, 22664–22671. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.H.; Hales, D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef] [PubMed]
- Samson, M.; Labrie, F.; Luu-The, V. Specific estradiol biosynthetic pathway in choriocarcinoma (JEG-3) cell line. J. Steroid Biochem. Mol. Biol. 2009, 116, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Kemiläinen, H.; Adam, M.; Mäki-Jouppila, J.; Damdimopoulou, P.; Damdimopoulos, A.E.; Kere, J.; Hovatta, O.; Laajala, T.D.; Aittokallio, T.; Adamski, J.; et al. The Hydroxysteroid (17β) Dehydrogenase Family Gene HSD17B12 Is Involved in the Prostaglandin Synthesis Pathway, the Ovarian Function, and Regulation of Fertility. Endocrinology 2016, 157, 3719–3730. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.M.; Mustonen, M.V.; Poutanen, M.H.; Isomaa, V.V.; Vihko, R.K. Human 17 beta-hydroxysteroid dehydrogenase type 1 and type 2 isoenzymes have opposite activities in cultured cells and characteristic cell- and tissue-specific expression. Biochem. J. 1996, 314 Pt 3, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Belyaeva, O.V.; Brown, P.M.; Fujita, K.; Valles, K.; Karki, S.; de Boer, Y.S.; Koh, C.; Chen, Y.; Du, X.; et al. 17-Beta Hydroxysteroid Dehydrogenase 13 Is a Hepatic Retinol Dehydrogenase Associated with Histological Features of Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 1504–1519. [Google Scholar] [CrossRef]
- Oduwole, O.O.; Li, Y.; Isomaa, V.V.; Mäntyniemi, A.; Pulkka, A.E.; Soini, Y.; Vihko, P.T. 17beta-hydroxysteroid dehydrogenase type 1 is an independent prognostic marker in breast cancer. Cancer Res. 2004, 64, 7604–7609. [Google Scholar] [CrossRef] [PubMed]
- He, X.-Y.; Wen, G.-Y.; Merz, G.; Lin, D.; Yang, Y.-Z.; Mehta, P.; Schulz, H.; Yang, S.-Y. Abundant type 10 17β-hydroxysteroid dehydrogenase in the hippocampus of mouse Alzheimer’s disease model. Mol. Brain Res. 2002, 99, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L. Gene Expression and Enzyme Activity Characterization of Three Types 17p-Hydroxysteroid Dehydrogenases (17P-HSD1, 17P-HSD3, 17P-HSD8) From Nile Tilapia, Oreochromis niloticus; Southwest China Normal University: Chongqing, China, 2004; 17p. [Google Scholar]
- Qiao, Q.; Le Manach, S.; Sotton, B.; Huet, H.; Duvernois-Berthet, E.; Paris, A.; Duval, C.; Ponger, L.; Marie, A.; Blond, A.; et al. Deep sexual dimorphism in adult medaka fish liver highlighted by multi-omic approach. Sci. Rep. 2016, 6, 32459. [Google Scholar] [CrossRef] [PubMed]
- Leguen, I.; Carlsson, C.; Perdu-Durand, E.; Prunet, P.; Pärt, P.; Cravedi, J.P. Xenobiotic and steroid biotransformation activities in rainbow trout gill epithelial cells in culture. Aquat. Toxicol. 2000, 48, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Sunami, Y.; Rebelo, A.; Kleeff, J. Lipid Droplet-Associated Factors, PNPLA3, TM6SF2, and HSD17B Proteins in Hepatopancreatobiliary Cancer. Cancers 2021, 13, 4391. [Google Scholar] [CrossRef] [PubMed]
- Pierce, S.B.; Walsh, T.; Chisholm, K.M.; Lee, M.K.; Thornton, A.M.; Fiumara, A.; Opitz, J.M.; Levy-Lahad, E.; Klevit, R.E.; King, M.C. Mutations in the DBP-deficiency protein HSD17B4 cause ovarian dysgenesis, hearing loss, and ataxia of Perrault Syndrome. Am. J. Hum. Genet. 2010, 87, 282–288. [Google Scholar] [CrossRef]
- Rasiah, K.K.; Gardiner-Garden, M.; Padilla, E.J.D.; Möller, G.; Kench, J.G.; Alles, M.C.; Eggleton, S.A.; Stricker, P.D.; Adamski, J.; Sutherland, R.L.; et al. HSD17B4 overexpression, an independent biomarker of poor patient outcome in prostate cancer. Mol. Cell. Endocrinol. 2009, 301, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.-Y.; Yao, C.-B.; Li, J.-T.; Zhao, X.-N.; Yang, H.-B.; Zhang, M.; Yin, M.; Chen, J.; Lei, Q.-Y. Acetylation targets HSD17B4 for degradation via the CMA pathway in response to estrone. Autophagy 2017, 13, 538–553. [Google Scholar] [CrossRef]
- Pyun, J.A.; Kim, S.; Cha, D.H.; Ko, J.J.; Kwack, K. Epistasis between the HSD17B4 and TG polymorphisms is associated with premature ovarian failure. Fertil. Steril. 2012, 97, 968–973. [Google Scholar] [CrossRef]
- De Launoit, Y.; Adamski, J. Unique multifunctional HSD17B4 gene product: 17beta-hydroxysteroid dehydrogenase 4 and D-3-hydroxyacyl-coenzyme A dehydrogenase/hydratase involved in Zellweger syndrome. J. Mol. Endocrinol. 1999, 22, 227–240. [Google Scholar] [CrossRef]
- Abdelmoneim, A.; Abdu, A.; Chen, S.; Sepúlveda, M.S. Molecular signaling pathways elicited by 17α-ethinylestradiol in Japanese medaka male larvae undergoing gonadal differentiation. Aquat. Toxicol. 2019, 208, 187–195. [Google Scholar] [CrossRef]
- Heikelä, H.; Ruohonen, S.T.; Adam, M.; Viitanen, R.; Liljenbäck, H.; Eskola, O.; Gabriel, M.; Mairinoja, L.; Pessia, A.; Velagapudi, V.; et al. Hydroxysteroid (17β) dehydrogenase 12 is essential for metabolic homeostasis in adult mice. Am. J. Physiol. -Endocrinol. Metab. 2020, 319, E494–E508. [Google Scholar] [CrossRef]
- Bellemare, V.; Phaneuf, D.; Luu-The, V. Target deletion of the bifunctional type 12 17β-hydroxysteroid dehydrogenase in mice results in reduction of androgen and estrogen levels in heterozygotes and embryonic lethality in homozygotes. Horm. Mol. Biol. Clin. Investig. 2010, 2, 311–318. [Google Scholar] [CrossRef]
- Laplante, Y.; Rancourt, C.; Poirier, D. Relative involvement of three 17beta-hydroxysteroid dehydrogenases (types 1, 7 and 12) in the formation of estradiol in various breast cancer cell lines using selective inhibitors. Mol. Cell. Endocrinol. 2009, 301, 146–153. [Google Scholar] [CrossRef]
- Mindnich, R.; Adamski, J. Zebrafish 17beta-hydroxysteroid dehydrogenases: An evolutionary perspective. Mol. Cell. Endocrinol. 2009, 301, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Wang, M.; Gao, C.; Lin, X.; Liu, Y.; Han, M.; Wang, X.; Zhang, Q.; He, Y. Genome-wide identification and characterization of COMMD family genes reveals their function on innate immune response in Paralichthys olivaceus. Aquaculture 2021, 536, 736498. [Google Scholar] [CrossRef]
- Hall, B.G. Building phylogenetic trees from molecular data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-J.; Gao, J.; Guo, H.-Y.; Zhu, K.-C.; Liu, B.-S.; Zhang, N.; Sun, J.-H.; Zhang, D.-C. Transcriptomics Reveal the Effects of Breeding Temperature on Growth and Metabolism in the Early Developmental Stage of Platax teira. Biology 2023, 12, 1161. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, X.; Zhang, Q. Evolutionary Conservation of pou5f3 Genomic Organization and Its Dynamic Distribution during Embryogenesis and in Adult Gonads in Japanese Flounder Paralichthys Olivaceus. Int. J. Mol. Sci. 2017, 18, 231. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ding, H.; Wu, S.; Wang, M.; Wei, C.; Wang, B.; Bao, Z.; Hu, J. Vasa Is a Potential Germ Cell Marker in Leopard Coral Grouper (Plectropomus leopardus). Genes 2022, 13, 1077. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Ding, H.; Jin, C.; Wang, M.; Li, P.; Bao, Z.; Wang, B.; Hu, J. Theoretical Analysis and Expression Profiling of 17β-Hydroxysteroid Dehydrogenase Genes in Gonadal Development and Steroidogenesis of Leopard Coral Grouper (Plectropomus leopardus). Int. J. Mol. Sci. 2024, 25, 2180. https://doi.org/10.3390/ijms25042180
Liu M, Ding H, Jin C, Wang M, Li P, Bao Z, Wang B, Hu J. Theoretical Analysis and Expression Profiling of 17β-Hydroxysteroid Dehydrogenase Genes in Gonadal Development and Steroidogenesis of Leopard Coral Grouper (Plectropomus leopardus). International Journal of Molecular Sciences. 2024; 25(4):2180. https://doi.org/10.3390/ijms25042180
Chicago/Turabian StyleLiu, Mingjian, Hui Ding, Chaofan Jin, Mingyi Wang, Peiyu Li, Zhenmin Bao, Bo Wang, and Jingjie Hu. 2024. "Theoretical Analysis and Expression Profiling of 17β-Hydroxysteroid Dehydrogenase Genes in Gonadal Development and Steroidogenesis of Leopard Coral Grouper (Plectropomus leopardus)" International Journal of Molecular Sciences 25, no. 4: 2180. https://doi.org/10.3390/ijms25042180
APA StyleLiu, M., Ding, H., Jin, C., Wang, M., Li, P., Bao, Z., Wang, B., & Hu, J. (2024). Theoretical Analysis and Expression Profiling of 17β-Hydroxysteroid Dehydrogenase Genes in Gonadal Development and Steroidogenesis of Leopard Coral Grouper (Plectropomus leopardus). International Journal of Molecular Sciences, 25(4), 2180. https://doi.org/10.3390/ijms25042180





