The Role of the Vascular System in Degenerative Diseases: Mechanisms and Implications
Abstract
1. Introduction
- Neurodegenerative Diseases: There is growing evidence linking vascular health to neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease [10]. Research suggests that compromised blood flow to the brain, often due to conditions like hypertension and atherosclerosis, can contribute to cognitive decline and neurodegeneration.
- Cardiovascular Diseases and Metabolic Syndrome: The relationship between cardiovascular diseases and metabolic syndrome (a cluster of conditions like obesity, high blood pressure, high blood sugar, and abnormal cholesterol levels) is well-established [11]. These conditions are often intertwined and can collectively contribute to the progression of degenerative diseases.
- Age-Related Macular Degeneration (AMD): AMD is a leading cause of vision loss in the elderly. Studies have revealed associations between vascular factors, such as hypertension and atherosclerosis, and an increased risk of AMD [14].
- Aging and Vascular Dysfunction: As people age, their blood vessels can undergo structural and functional changes, which can contribute to the development of various degenerative conditions [16]. Understanding the mechanisms behind age-related vascular dysfunction is a key area of research.
- Inflammation and Endothelial Dysfunction: Endothelial cells lining blood vessels play a crucial role in regulating vascular health. Dysfunction of these cells can lead to chronic inflammation and contribute to the development of degenerative diseases [17].
2. Vascular System: Anatomy and Functions
2.1. Anatomy
- Arteries: Arteries are thick-walled blood vessels that carry oxygenated blood away from the heart and distribute it to various tissues. They have a strong muscular layer that allows them to withstand the force generated by the heart’s contractions. The largest artery, the aorta, emerges directly from the heart and branches into smaller arteries that further divide into arterioles [18,19].
- Veins: Veins are blood vessels responsible for transporting deoxygenated blood back to the heart for oxygenation. Unlike arteries, veins have thinner walls and less muscular tissue. They use one-way valves to prevent blood from flowing backward and rely on skeletal muscle contractions to assist in pushing blood against gravity, particularly in the limbs. Veins gradually merge into larger vessels, ultimately forming the superior and inferior vena cava, which return blood to the heart [18,19].
- Capillaries: Capillaries are the smallest and most numerous blood vessels, connecting arteries and veins within tissues. They facilitate the exchange of gases, nutrients, and waste products between the blood and surrounding cells. Capillary walls consist of a single layer of endothelial cells, allowing for efficient diffusion of substances. Importantly, there are structural differences in the capillaries of different organs. For example, capillaries of the CNS possess tight-junctions, which allows them to be highly selective to the molecules that can enter CNS parenchyma. Oxygen and nutrients pass from capillaries into tissues, while waste products enter the capillaries for eventual elimination [18,19].
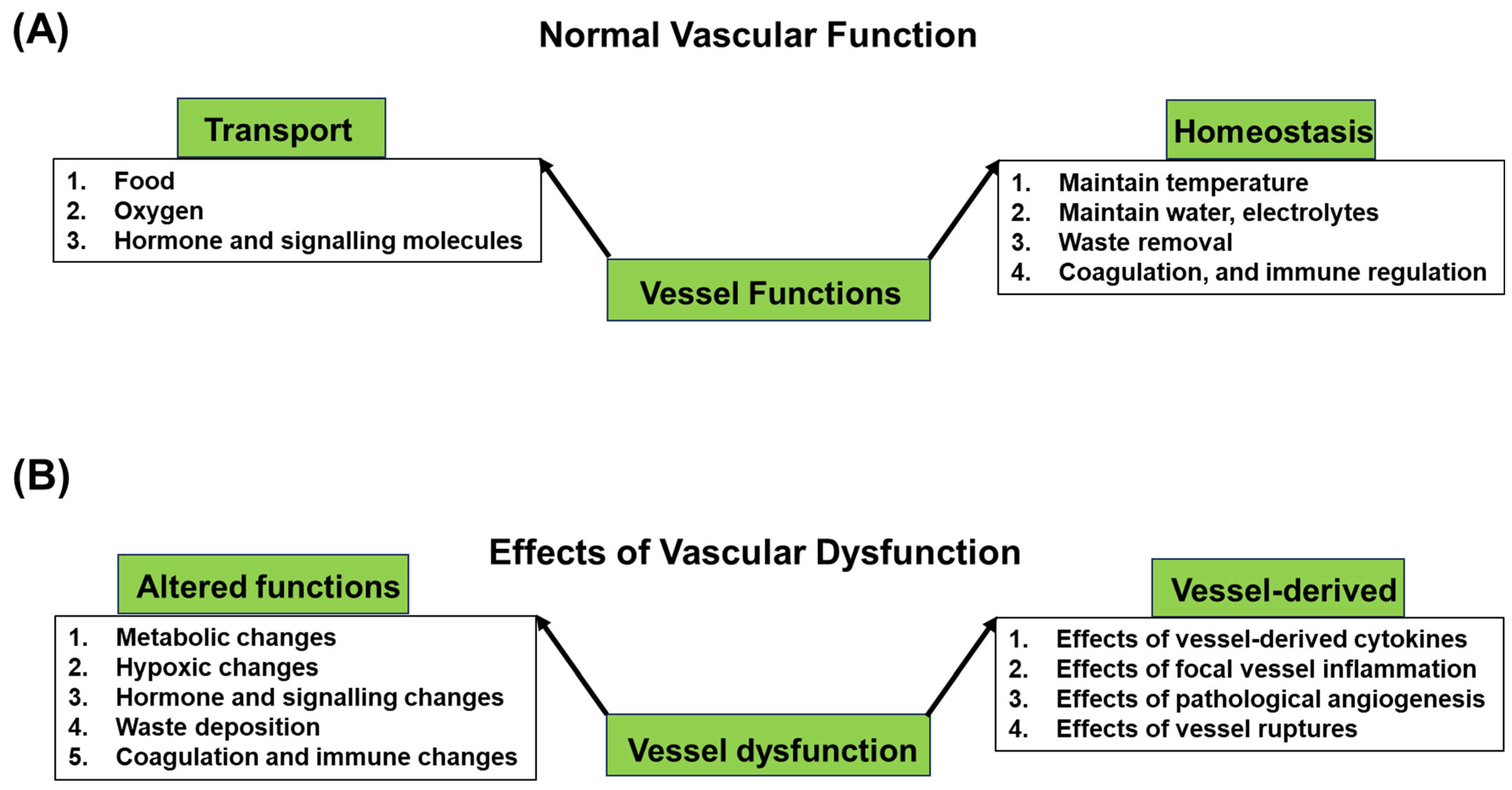
2.2. Functions
- (i)
- Transporting Oxygen, Nutrients, and Waste Products: One of the primary functions of the vascular system is to facilitate the transportation of oxygen and nutrients to the tissues and organs. In the tissue capillaries, oxygen and nutrients diffuse from the blood into the surrounding cells, providing energy for cellular processes. Conversely, waste products, including carbon dioxide and metabolic byproducts, are exchanged at the capillary bed and flow back to the heart, where they are then pumped to the lungs for oxygenation and to other elimination organs for waste removal.
- (ii)
- Maintaining Homeostasis: The vascular system plays a crucial role in maintaining homeostasis through the regulation of body temperature by redistributing blood flow to dissipate or conserve heat. It also contributes to fluid balance by controlling the movement of water and electrolytes between blood and tissues. In response to injuries, the vascular system initiates clotting processes to prevent excessive bleeding. Additionally, blood vessels participate in immune responses by transporting immune cells to areas of infection or injury, contributing to the body’s defense mechanisms.
3. Vascular Dysfunction in Degenerative Diseases
3.1. Cardiovascular System
- (i)
- Hypertension: Vascular dysfunction is a key contributor to the onset and maintenance of hypertension. Endothelial dysfunction is frequently seen in hypertensive conditions [24], where it results in narrowed blood vessels and elevated systemic resistance, causing blood pressure to increase [30]. Additionally, vascular dysfunction and the renin–angiotensin–aldosterone system, a hormonal pathway that regulates blood pressure, can affect each other bidirectionally. Dysregulation of this system due to impaired vascular function can lead to sustained high blood pressure levels, further damaging blood vessel walls and promoting hypertensive complications [31,32].
- (ii)
- Atherosclerosis: Impaired vascular function is intimately linked to the development of atherosclerosis, a condition characterized by focal inflammation and the accumulation of oxidized-LDL-laden plaques within arterial walls [33]. Endothelial dysfunction triggers inflammatory processes, attracting immune cells, including macrophages and T-cells, that contribute to plaque formation [23,34]. As plaques grow, the arterial lumen narrows, reducing blood flow and causing turbulence that further enhances the endothelial inflammatory condition. This cycle of inflammation, plaque buildup, and arterial narrowing creates a hostile environment conducive to blood clot formation, increasing the risk of myocardial infarction and strokes.
- (iii)
- Heart Failure: Vascular dysfunction significantly contributes to the progression of heart failure [25]. Chronic hypertension and atherosclerosis, driven by impaired vascular function, strain the heart by requiring it to pump against increased resistance, leading to cardiac enlargement, especially left ventricular hypertrophy. Additionally, vascular dysfunctions impact the blood vessels within the heart, which can lead to reduced oxygen and nutrient delivery, further weakening cardiac function [35]. The increased workload, impaired coronary blood flow, and inflammatory signals can also contribute to the development of heart failure with preserved ejection fraction (HFpEF), a type of heart failure characterized by impaired relaxation of the heart’s chambers [36].
3.2. Nervous System
- (i)
- Alzheimer’s Disease: Vascular dysfunction is closely linked to the pathogenesis of Alzheimer’s disease (AD), a neurodegenerative disorder characterized by cognitive decline and memory loss. Deposition of aggregated amyloid β (Aβ) peptide is considered the main cause of AD [37]. In normal brains, Aβ is cleared mainly through perivascular pathways [38]. Dysfunctional blood vessels contribute to reduced Aβ clearance, leading to increased aggregation and deposition [5]. Such deposited Aβ further impairs cerebral blood flow and causes pathological angiogenesis, blood–brain barrier disruption, neuroinflammation, and impaired clearance of toxic protein aggregates, including aggregated Aβ peptide [5,6,39].
- (ii)
- Stroke: Impaired vascular function significantly increases the risk of stroke. Endothelial dysfunction compromises blood vessel dilation and responsiveness, promoting the development of blood clots [40]. Atherosclerosis, driven by vascular dysfunction, can lead to plaque rupture, triggering clot formation and embolic strokes [33]. Furthermore, chronic hypertension could cause sclerosis and damage the blood vessels, increasing the likelihood of vessel rupture and hemorrhagic strokes [41]. The role of vascular dysfunction in both ischemic and hemorrhagic stroke underscores its impact on overall brain health.
- (iii)
- Cerebral Small Vessel Diseases: Cerebral small vessel diseases encompass a group of conditions that affect the small blood vessels within the brain. Impaired vascular function contributes to the development of these diseases, including arteriolosclerosis and cerebral microbleeds [42]. Endothelial dysfunction, inflammation, and oxidative stress compromise the structural integrity of these small vessels, leading to vessel wall thickening, narrowing, and increased fragility [42,43,44]. The cumulative effect of vascular dysfunction in cerebral small vessel diseases disrupts cerebral blood flow, which can result in lacunar infarcts and contribute to cognitive impairment, mobility issues, and other neurological deficits.
- (iv)
- Parkinson’s Disease: Vascular dysfunction also holds implications for Parkinson’s disease (PD), a neurodegenerative disorder characterized by motor symptoms like tremors and rigidity. In PD, the primary pathology involves the degeneration of dopaminergic neurons in the substantia nigra region of the midbrain. The key diagnostic histopathological features of PD are intraneuronal Lewy bodies containing aggregated α-synuclein, leading to the belief that such aggregation is the primary cause of neurodegeneration and, consequently, the disease [45]. In the context of aggregation, an altered protein quality control system, especially the ubiquitin-proteasome pathway, is thought to play a crucial role in PD [46,47]. Numerous studies have implicated vascular changes, including impaired blood-brain barrier function and pathological angiogenesis, in the development of the pathological changes seen in PD [48,49,50,51]. These changes result in hypoperfusion. Given that hypoperfusion can impact the protein quality control system, including the ubiquitin-proteasome system, it is plausible that decreased blood flow to the substantia nigra may contribute to α-synuclein aggregation. Moreover, hypoperfusion can subject cells to stress, potentially triggering the aggregation process of α-synuclein. Furthermore, impaired vascular health disrupts the supply of oxygen and nutrients, which can expedite neuronal damage in this vulnerable region.
3.3. Kidney System
- (i)
- Chronic Kidney Disease (CKD): Impaired vascular function contributes to the pathogenesis and progression of CKD. The disease is a long-term medical condition characterized by the gradual and irreversible deterioration of kidney function over an extended period, typically months to years. CKD is often categorized into different stages based on the level of kidney function, with stage 1 being the mildest and stage 5 representing end-stage renal disease (ESRD), where kidney function is severely impaired, and patients usually require dialysis or a kidney transplant to survive [52]. Common causes of CKD include diabetes, hypertension, glomerulonephritis, polycystic kidney disease, and certain other medical conditions [53]. Hypertension can lead to increased resistance to blood flow. This can damage the delicate blood vessels in the kidneys over time, impairing their ability to filter waste and regulate fluids [54]. Additionally, atherosclerosis and endothelial dysfunction are often seen in individuals with CKD, which affect the kidney perfusion and ability to regulate blood flow and maintain an appropriate balance of vasodilation and constriction [54,55]. This results in reduced blood flow to the kidneys. Moreover, chronic inflammation associated with vascular dysfunction can promote fibrosis and scarring within the kidney tissues. As a result, kidney function gradually declines over time.
- (ii)
- Diabetic Kidney Disease: Diabetic kidney disease, also referred to as diabetic nephropathy, represents a prevalent complication of diabetes. Poorly controlled diabetes can directly inflict harm upon the glomeruli and renal blood vessels [56]. Additionally, advanced glycation end products (AGEs) can harm blood vessels by triggering the generation of reactive oxygen species (ROS) and fostering inflammation [56,57]. Elevated blood sugar levels result in injury to the blood vessels that provide the kidneys, ultimately causing endothelial dysfunction and inflammation.
- (iii)
- Hypertensive Nephropathy: Hypertensive nephropathy results from chronic hypertension damaging the blood vessels within the kidneys [58]. Vascular dysfunction plays a key role in its progression. Elevated blood pressure causes structural changes in the small blood vessels, leading to arteriosclerosis and narrowing of the renal arteries [59]. These changes reduce blood flow to the kidneys, triggering a cascade of events that contribute to kidney damage. The decreased blood flow prompts the kidneys to release hormones that raise blood pressure further, creating a vicious cycle [58,59]. The compromised blood vessels also impair the kidneys’ ability to filter waste and maintain fluid and electrolyte balance, ultimately leading to kidney dysfunction.
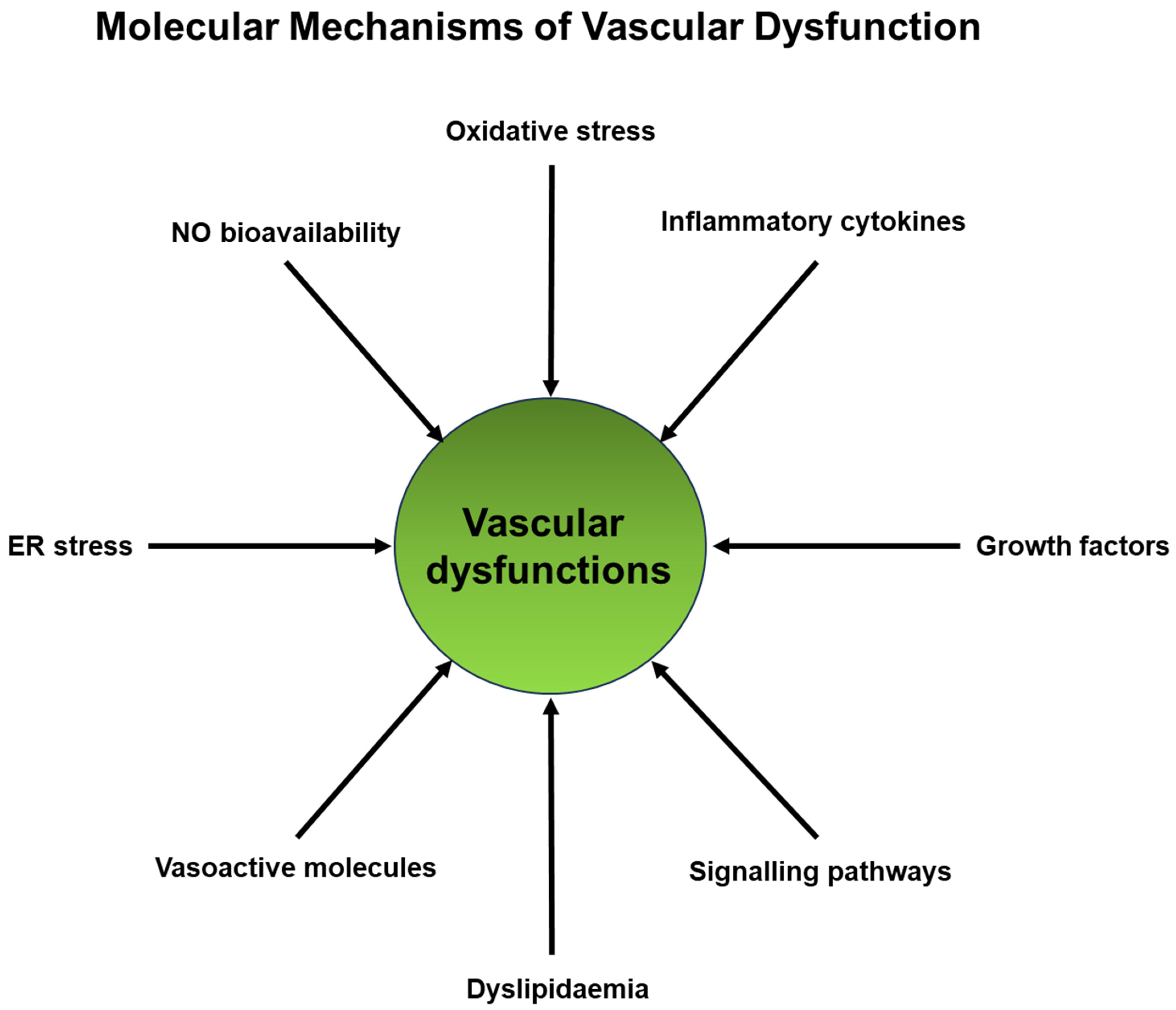
4. Molecular Mechanisms Linking Vascular Dysfunction and Degenerative Diseases
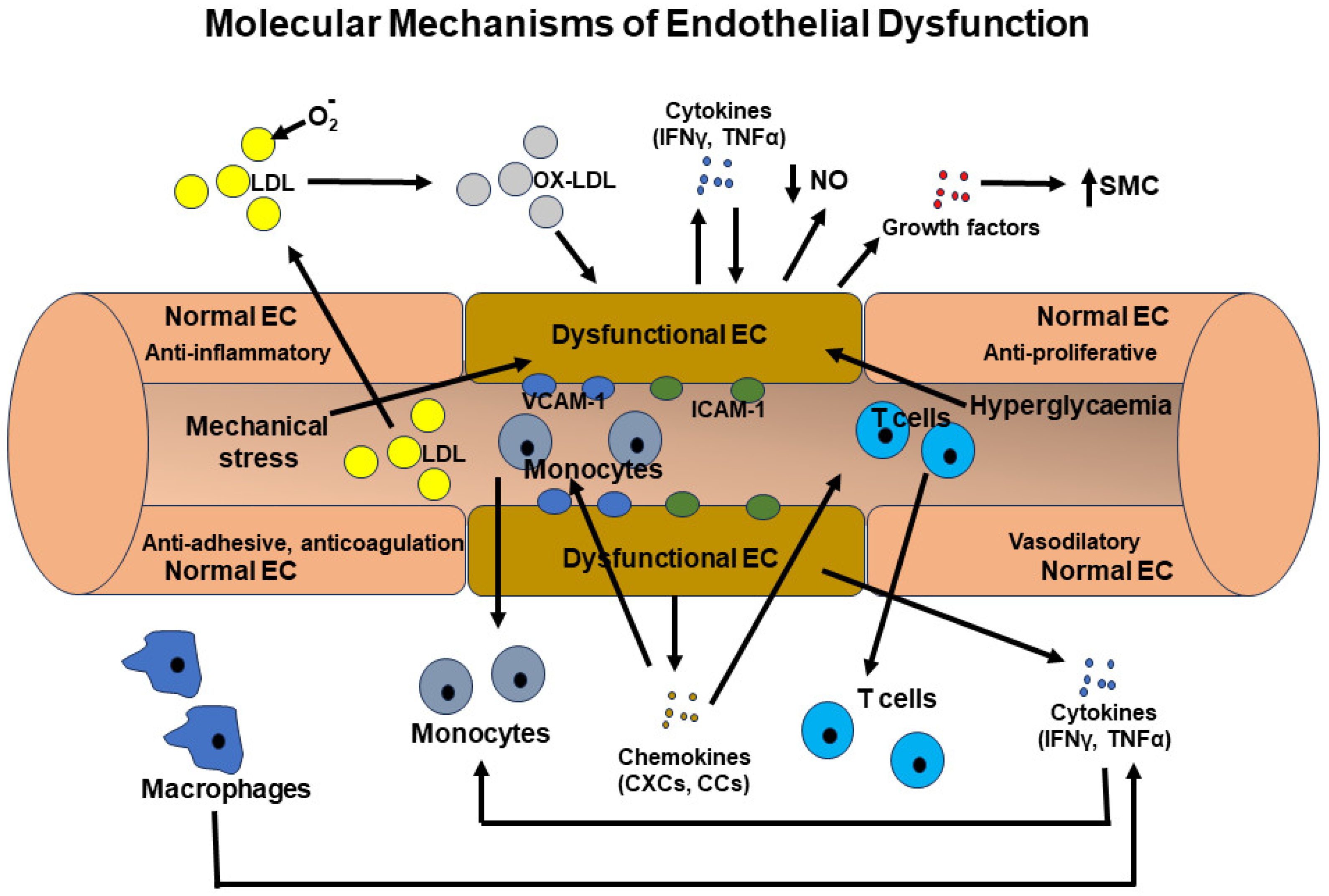
4.1. Endothelial Dysfunction
- (i)
- Oxidative Stress: Excessive production of reactive oxygen species (ROS), such as the superoxide anion (O2·−) and hydrogen peroxide (H2O2), can inflict damage upon endothelial cells and impair the bioactivity of nitric oxide (NO) [64]. Nitric oxide is a molecule that fosters vasodilation, thereby promoting vascular health [64]. Also, superoxide can interact with NO and generate peroxynitrite (ONOO−), which can similarly inflict harm upon these cells [65]. The activation of NADPH oxidases (NOX) and xanthine oxidase is commonly implicated in the generation of these ROS species, a phenomenon observed within endothelial cells in numerous degenerative diseases [66,67].
- (ii)
- Inflammation: Chronic inflammation, characterized by elevated levels of proinflammatory cytokines (such as TNF-α, IL-1β), chemokines (MCP-1), adhesion molecules (VCAM-1, ICAM-1), and inflammatory receptors (Toll-like receptors, TNF receptors), can be induced in inflammatory conditions [17,21]. Through the action of adhesion molecules, chemokines, and their receptors, proinflammatory cells are recruited to the affected area. Inflammatory cytokines and their receptors typically activate proinflammatory transcription systems, including NF-κB, AP-1, and STATs [68]. This activation leads to the production of more proinflammatory cytokines, chemokines, and growth factors in these cells, as well as in endothelial cells, disrupting their normal function. Additionally, inflammatory mediators can impair the synthesis of nitric oxide (NO) and promote vasoconstriction [69]. These effects create a vicious cycle that profoundly alters endothelial cell properties. Furthermore, low-density lipoprotein (LDL) cholesterol can undergo oxidation by reactive oxygen species (ROS) and infiltrate the endothelium. This triggers an inflammatory response and the formation of atherosclerotic plaques, which can narrow and stiffen blood vessels [33].
- (iii)
- Nitric Oxide (NO) Bioavailability: Nitric oxide (NO), synthesized by endothelial nitric oxide synthase (eNOS), is a key endogenous vasodilator crucial for maintaining proper vascular function [70]. Dysregulation of eNOS activity, whether through mechanisms like eNOS uncoupling or reduced eNOS expression, results in reduced NO bioavailability [69,70,71]. This, in turn, hinders vasodilation and promotes vasoconstriction.
- (iv)
- Endoplasmic Reticulum Stress: Accumulating evidence suggests that endoplasmic reticulum (ER) stress is one of the primary causes of endothelial dysfunction [72]. The buildup of misfolded proteins within the ER triggers unfolded protein response (UPR) pathways, which in turn contribute to endothelial dysfunction, inflammation, and apoptosis [72].
4.2. Atherosclerosis
- (i)
- (ii)
- (iii)
- Endothelial Dysfunction and Impaired Nitric Oxide (NO) Bioavailability: Atherosclerosis can lead to endothelial dysfunction, reducing the production and availability of NO, a molecule important for vasodilation and maintaining vascular health [70,71]. This endothelial dysfunction can affect blood flow throughout the body.
- (iv)
- (v)
- Platelet Activation and Thrombosis: Atherosclerotic plaques can rupture, leading to the exposure of pro-thrombotic substances. Platelets can become activated and contribute to thrombosis, potentially causing strokes or myocardial infarctions [75].
4.3. Hypertension
- (i)
- Renin–Angiotensin–Aldosterone System (RAAS): The RAAS is a central regulator of blood pressure and fluid balance [76]. In hypertension, there is often an overactivation of the RAAS. This system involves the release of renin from the kidneys, which leads to the conversion of angiotensinogen to angiotensin I and then angiotensin II. Angiotensin II is a potent vasoconstrictor and can promote inflammation and oxidative stress, contributing to vascular damage, atherosclerosis, and organ damage. Angiotensin II also stimulates the release of aldosterone, which can lead to sodium and water retention, further increasing blood pressure and strain on the cardiovascular system [76,77,78].
- (ii)
- Inflammation Signaling: Hypertension is associated with chronic low-grade inflammation [17,79,80]. Molecular signaling pathways involving cytokines, chemokines, and inflammatory mediators, such as IL-1β, NF-kB, and TNF-α, play a role in the inflammatory response [80,81]. This chronic inflammation can contribute to the development and progression of degenerative diseases by damaging tissues and promoting atherosclerosis.
- (iii)
- Oxidative Stress: Increased blood pressure can lead to increased production of reactive oxygen species (ROS) in blood vessels and tissues. Molecular pathways related to oxidative stress, such as those involving NADPH oxidase and superoxide dismutase, are implicated [82]. Oxidative stress can damage cellular components, including DNA, lipids, and proteins, contributing to cellular dysfunction and degenerative diseases.
- (iv)
- Endothelial Dysfunction: Hypertension can impair the function of endothelial cells that line blood vessels [24,30,32]. Signaling pathways involving nitric oxide (NO), which is a vasodilator, and endothelin-1 (ET-1), a vasoconstrictor, are altered in endothelial dysfunction. This dysfunction can lead to vasoconstriction, inflammation, and atherosclerosis.
- (v)
- Cellular Growth and Hypertrophy: Chronic high blood pressure can lead to hypertrophy of cardiac muscle cells through signaling pathways involving proteins like mTOR (mammalian target of rapamycin) and calcineurin [83]. Cardiac hypertrophy can eventually lead to heart failure and increase the risk of cardiac degenerative diseases.
- (vi)
- Neuroinflammation and Brain Damage: In the context of hypertension-related brain damage and neurodegenerative diseases, signaling pathways involving microglia activation, cytokines, and amyloid-beta protein processing are implicated [84]. Chronic hypertension can promote neuroinflammation and contribute to the pathogenesis of conditions like Alzheimer’s disease and vascular dementia [84,85].
4.4. Environment and Endocrine Disruptors
5. Common Risk Factors That Affect Vascular System and Degenerative Diseases
- Aging: The aging process itself is a significant risk factor for both vascular dysfunction and degenerative diseases [97,98]. As people age, their blood vessels naturally undergo structural and functional changes, becoming less elastic and more susceptible to damage [99]. This contributes to conditions like arterial stiffness and an increased risk of atherosclerosis, which can affect both the heart and other organs.
- Hypertension: Hypertension is a common risk factor for both vascular dysfunction and degenerative diseases [24,30,41,43,54]. Chronic high blood pressure can damage the walls of arteries, making them less elastic and more prone to atherosclerosis. It also increases the risk of conditions like stroke, heart disease, and kidney disease.
- Smoking: Smoking is a major risk factor for vascular dysfunction and degenerative diseases [99,100]. It constricts blood vessels, increases oxidative stress, increases blood coagulability, reduces oxygen delivery to tissues, and promotes the development of atherosclerosis. Smoking is strongly associated with cardiovascular diseases, kidney diseases, lung diseases, and cancers.
- Dyslipidemia: An imbalance in blood lipid levels, particularly elevated levels of low-density lipoprotein (LDL) cholesterol and reduced levels of high-density lipoprotein (HDL) cholesterol, is also considered a risk factor for various health conditions, including vascular dysfunction, atherosclerosis, nervous system diseases such as cerebral small vessel diseases, and diabetes [33,101,102]. Dyslipidemia, characterized by these lipid abnormalities, poses a significant risk for atherosclerosis, a condition that can affect arteries throughout the body and contribute to vascular dysfunction, as well as degenerative diseases like heart disease and stroke. Additionally, dyslipidemia is known to increase overall levels of inflammation and oxidative stress, negatively impacting not only the health of blood vessels but also the well-being of other organs.
- Diabetes: Diabetes, especially type 2 diabetes, is a common risk factor for both vascular dysfunction and degenerative diseases [29,51,62,103]. High blood sugar levels can damage blood vessels (diabetic vasculopathy), contribute to atherosclerosis, and increase the risk of heart disease, stroke, and peripheral vascular disease, along with degenerative diseases of the nervous system, including Alzheimer’s disease.
- Obesity: Obesity is associated with several health issues, including insulin resistance, dyslipidemia, inflammation, and increased blood pressure [104]. These factors collectively contribute to the development of vascular dysfunction and degenerative diseases, such as heart disease, type 2 diabetes, and osteoarthritis.
- Physical Inactivity: A sedentary lifestyle is a risk factor for both vascular dysfunction and degenerative diseases [105]. Lack of physical activity can lead to weight gain, worsen insulin sensitivity and diabetes, and promote the development of atherosclerosis. Regular exercise, on the other hand, can help improve vascular health and reduce the risk of many degenerative diseases.
- Poor Diet: A diet high in saturated and trans fats, refined sugars, and processed foods can contribute to obesity, dyslipidemia, diabetes, and inflammation [106]. These dietary factors are associated with an increased risk of both vascular dysfunction and degenerative diseases.
- Chronic Inflammation: Chronic inflammation is a common factor underlying many degenerative diseases and can also contribute to vascular dysfunction [15,21,34]. Inflammatory processes can damage blood vessel walls, promote atherosclerosis, and increase the risk of conditions like rheumatoid arthritis and cardiovascular disease.
6. The Impact of Degenerative Diseases on Vascular Dysfunction
- Diabetes: Diabetes, particularly type 2 diabetes, can lead to vascular dysfunction [62]. High blood sugar levels can damage the endothelial cells lining blood vessels, promoting atherosclerosis and increasing the risk of heart disease, stroke, and peripheral vascular disease.
- Inflammation: Chronic inflammation, a hallmark of many degenerative diseases (e.g., rheumatoid arthritis, inflammatory bowel disease), can directly impact the vascular system [108,109]. Inflammatory processes can damage the endothelium, leading to endothelial dysfunction and increased risk of atherosclerosis and cardiovascular events.
- CKD: CKD is both a cause and consequence of vascular dysfunction [52,53,54,55]. Kidney damage can result from prolonged exposure to risk factors like high blood pressure and diabetes, which also contribute to vascular impairment. In turn, kidney dysfunction can lead to imbalances in electrolytes and hormones, affecting blood vessel tone and function.
- Cerebral Small Vessel Diseases: CSVD primarily affects the small blood vessels in the brain, leading to white matter lesions, lacunar infarcts, and microbleeds [42,43,44]. These vascular abnormalities are closely associated with cognitive decline, and they can disrupt the brain’s ability to function properly. CSVD can lead to both ischemic and hemorrhagic events in the brain. These events contribute to vascular dysfunction and can result in stroke-like symptoms and cognitive impairment. Degenerative changes in small blood vessels can lead to reduced blood flow, increasing the risk of strokes and vascular dementia.
- Alzheimer’s Disease: In AD, cerebral vessel integrity is compromised due to the deposition of Aβ, resulting in cerebral amyloid angiopathy [27,42]. These abnormal protein deposits can affect blood vessels, reducing cerebral blood flow. Reduced blood flow deprives brain cells of oxygen and nutrients, contributing to cognitive decline and memory loss. Some individuals with Alzheimer’s disease also experience vascular cognitive impairment (VCI), where vascular dysfunction in the brain exacerbates cognitive deficits. This can occur alongside the neurodegenerative processes in Alzheimer’s disease.
7. Therapeutic Implications
- Blood Pressure Control: Maintaining optimal blood pressure is crucial in managing vascular dysfunction. Antihypertensive medications, such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), are often used to control hypertension and reduce the strain on blood vessels [110]. They can also help control CKD [111]. Although antihypertensives are not the primary treatment for CSVD, associated hypertension may worsen the disease, and hypertension control can show beneficial effects. Some studies have suggested a link between hypertension and an increased risk of developing Alzheimer’s disease [112,113]. Hypertension may contribute to vascular dysfunction in the brain, which can exacerbate AD-related cognitive decline.
- RAAS Inhibition: Inhibition of the renin–angiotensin–aldosterone system (RAAS) is a common therapeutic strategy to protect vascular health in conditions like CKD. Medications like ACE inhibitors and ARBs not only lower blood pressure but also have direct vascular protective effects by reducing vasoconstriction and inflammation [111,114].
- Antioxidant Therapy: Since oxidative stress plays an important role in most degenerative diseases and vascular dysfunction, antioxidant therapy could be a good adjunct to the main therapy of a specific degenerative disease [64,66,67]. Antioxidant supplements or diets rich in antioxidants may help combat oxidative stress, which is a common contributor to vascular dysfunction. Antioxidants like vitamin C, vitamin E, and coenzyme Q10 may have beneficial effects in vascular diseases as well as neurodegenerative diseases, including Alzheimer’s disease.
- Anti-Inflammatory Medications: Since inflammation plays a significant role in vascular dysfunction, nonsteroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory medications may be used to reduce inflammation and alleviate vascular damage [74,79]. Indeed, aspirin has long been used as a preventive measure for cardiovascular diseases due to its anti-inflammatory and anticoagulative effects [115].
- Lifestyle Modifications: Lifestyle changes, such as adopting a heart-healthy diet, increasing physical activity, and quitting smoking, can improve vascular function and reduce the risk of degenerative diseases [116].
- Exercise Programs: Regular exercise has a positive impact on vascular health. Exercise can improve endothelial function, increase nitric oxide production, and enhance blood vessel flexibility [116].
- Stem cell therapy: In recent years, stem cell therapy has emerged as a potential treatment option for degenerative diseases of the future. Stem cells, including embryonic stem cells, induced pluripotent cells, and tissue-specific stem cells, possess remarkable regenerative properties. These properties enable them to repair damaged tissues caused by degenerative diseases and restore the full functionality of organs and tissues. Additionally, numerous stem cell types exhibit immunomodulatory, angiogenic, and cell-protective properties, which could contribute to the restoration of blood vessel integrity and tissue health [120,121].
8. Future Directions and Research Opportunities
- Mechanisms of Vascular Dysfunction: While we know that vascular dysfunction is a common feature in many degenerative diseases, the precise mechanisms underlying this dysfunction remain incompletely understood. Elucidating the specific molecular and cellular pathways involved in vascular damage and their crosstalk with degenerative disease pathology is crucial for developing targeted therapies.
- Biomarkers for Vascular Dysfunction: Identifying reliable biomarkers that can detect early signs of vascular dysfunction and degenerative diseases is an ongoing challenge. Research efforts are needed to discover and validate biomarkers that can predict the risk of degenerative diseases and monitor vascular health.
- Interplay with Genetics: The influence of genetic factors on vascular dysfunction in degenerative diseases is not fully understood. Further research is required to unravel the genetic predispositions that may make some individuals more susceptible to vascular complications.
- Role of the Microbiome: Emerging evidence suggests that the gut microbiome may play a role in many degenerative diseases [122]. Investigating the interactions between gut microbiota and the vascular system in the context of a degenerative disease could provide insights into novel therapeutic approaches.
- Age-Related Changes: Aging is a significant risk factor for both degenerative diseases and vascular dysfunction [16,17]. Research is needed to understand how age-related changes in vessels and tissues interact with each other in the context of structural and functional alterations that contribute to disease progression.
- Plasticity and Repair: A comprehensive understanding of the natural mechanisms for repair and plasticity in the context of vessels and specific tissue is essential for a deeper understanding of vascular dysfunction and degenerative disease [109]. Such an understanding is pivotal for gaining deeper insights into vascular dysfunction and degenerative diseases. Additional research is imperative in this field.
- Impact of Environmental Factors: The role of environmental factors, such as pollution, diet, lifestyle, and climate change, in vascular dysfunction and degenerative diseases needs further exploration [123]. Understanding how these factors interact with genetic and biological mechanisms is vital.
- Long-Term Outcomes: Many studies focus on short-term outcomes of vascular interventions. There is a need for long-term follow-up to assess the durability and sustained benefits of vascular-focused therapies in degenerative diseases.
- Integration of Data: Combining data from various sources, such as genomics, proteomics, and clinical records, can provide a comprehensive view of vascular dysfunction in degenerative diseases. Advanced data integration techniques and artificial intelligence can help uncover hidden patterns and relationships.
- Ethnic and Racial Disparities: Investigating ethnic and racial disparities in the prevalence and impact of degenerative diseases and vascular dysfunction is essential for addressing health inequities and tailoring treatments to diverse populations.
- Translational Research: Bridging the gap between basic science research and clinical application is crucial. Further efforts are needed to translate promising laboratory findings into effective treatments for patients with degenerative diseases.
9. Concluding Remarks
Funding
Conflicts of Interest
References
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef]
- Aïdoud, A.; Gana, W.; Poitau, F.; Debacq, C.; Leroy, V.; Nkodo, J.; Poupin, P.; Angoulvant, D.; Fougère, B. High Prevalence of Geriatric Conditions Among Older Adults with Cardiovascular Disease. J. Am. Heart Assoc. 2023, 12, e026850. [Google Scholar] [CrossRef] [PubMed]
- Couser, W.G.; Remuzzi, G.; Mendis, S.; Tonelli, M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011, 80, 1258–1270. [Google Scholar] [CrossRef]
- Guyton, A.C.; Hall, J.E. Text Book of Medical Physiology, 11th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2006. [Google Scholar]
- Shibly, A.Z.; Sheikh, A.M.; Michikawa, M.; Tabassum, S.; Azad, A.K.; Zhou, X.; Zhang, Y.; Yano, S.; Nagai, A. Analysis of Cerebral Small Vessel Changes in AD Model Mice. Biomedicines 2022, 11, 50. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Yano, S.; Tabassum, S.; Mitaki, S.; Michikawa, M.; Nagai, A. Alzheimer’s Amyloid β Peptide Induces Angiogenesis in an Alzheimer’s Disease Model Mouse through Placental Growth Factor and Angiopoietin 2 Expressions. Int. J. Mol. Sci. 2023, 24, 4510. [Google Scholar] [CrossRef]
- Satoh, M. Endothelial dysfunction as an underlying pathophysiological condition of chronic kidney disease. Clin. Exp. Nephrol. 2012, 16, 518–521. [Google Scholar] [CrossRef]
- Schiffrin, E.L.; Lipman, M.L.; Mann, J.F. Chronic Kidney Disease: Effects on the cardiovascular system. Circulation 2007, 116, 85–97. [Google Scholar] [CrossRef]
- Otsubo, R.; Higuchi, M.d.L.; Gutierrez, P.S.; Benvenuti, L.A.; Massarollo, P.C.B.; Costa, A.L.; Ramires, J.A.F. Influence of chronic liver disease on coronary atherosclerosis vulnerability features. Int. J. Cardiol. 2006, 109, 387–391. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- Sebastian, S.A.; Padda, I.; Johal, G. Cardiovascular-Kidney-Metabolic (CKM) syndrome: A state-of-the-art review. Curr. Probl. Cardiol. 2023, 49, 102344. [Google Scholar] [CrossRef]
- Hussain, S.M.; Dawson, C.; Wang, Y.; Tonkin, A.M.; Chou, L.; Wluka, A.E.; Cicuttini, F.M. Vascular Pathology and Osteoarthritis: A Systematic Review. J. Rheumatol. 2020, 47, 748–760. [Google Scholar] [CrossRef]
- Wang, H.; Bai, J.; He, B.; Hu, X.; Liu, D. Osteoarthritis and the risk of cardiovascular disease: A meta-analysis of observational studies. Sci. Rep. 2016, 6, 39672. [Google Scholar] [CrossRef]
- Bucan, K.; Lukic, M.; Bosnar, D.; Kopic, A.; Jukic, T.; Konjevoda, S.; Glavadanovic, S.; Antunica, A.G. Analysis of association of risk factors for age-related macular degeneration. Eur. J. Ophthalmol. 2022, 32, 410–416. [Google Scholar] [CrossRef]
- Shi, A.; Mansour, S.G. The Role of Vascular Biomarkers in Outcomes of Patients with Kidney Disease. Nephron 2023, 147, 778–781. [Google Scholar] [CrossRef]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef]
- Pacinella, G.; Ciaccio, A.M.; Tuttolomondo, A. Endothelial Dysfunction and Chronic Inflammation: The Cornerstones of Vascular Alterations in Age-Related Diseases. Int. J. Mol. Sci. 2022, 23, 15722. [Google Scholar] [CrossRef]
- Standring, S. (Ed.) Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 41st ed.; Elsevier Saunders: Philadelphia, PA, USA, 2015. [Google Scholar]
- Fleischer, S.; Tavakol, D.N.; Vunjak-Novakovic, G. From Arteries to Capillaries: Approaches to Engineering Human Vasculature. Adv. Funct. Mater. 2020, 30, 1910811. [Google Scholar] [CrossRef]
- Matjuda, E.N.; Engwa, G.A.; Sewani-Rusike, C.R.; Nkeh-Chungag, B.N. An Overview of Vascular Dysfunction and Determi-nants: The Case of Children of African Ancestry. Front. Pediatr. 2021, 9, 769589. [Google Scholar] [CrossRef]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Kiss, T.; Wren, J.D.; Giles, C.B.; Griffin, C.T.; Murfee, W.L.; Pacher, P.; Csiszar, A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 2018, 15, 555–565. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, III27–III32. [Google Scholar] [CrossRef]
- Brandes, R.P. Endothelial Dysfunction and Hypertension. Hypertension 2014, 64, 924–928. [Google Scholar] [CrossRef]
- Zuchi, C.; Tritto, I.; Carluccio, E.; Mattei, C.; Cattadori, G.; Ambrosio, G. Role of endothelial dysfunction in heart failure. Heart Fail. Rev. 2020, 25, 21–30. [Google Scholar] [CrossRef]
- Kress, B.T.; Iliff, J.J.; Xia, M.; Wang, M.; Wei, H.S.; Zeppenfeld, D.; Xie, L.; Kang, H.; Xu, Q.; Liew, J.A.; et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014, 76, 845–861. [Google Scholar] [CrossRef]
- Badimon, A.; Torrente, D.; Norris, E.H. Vascular Dysfunction in Alzheimer’s Disease: Alterations in the Plasma Contact and Fibrinolytic Systems. Int. J. Mol. Sci. 2023, 24, 7046. [Google Scholar] [CrossRef]
- Li, L.-P.; Tan, H.; Thacker, J.M.; Li, W.; Zhou, Y.; Kohn, O.; Sprague, S.M.; Prasad, P.V. Evaluation of Renal Blood Flow in Chronic Kidney Disease Using Arterial Spin Labeling Perfusion Magnetic Resonance Imaging. Kidney Int. Rep. 2017, 2, 36–43. [Google Scholar] [CrossRef]
- Zitouni, K.; Steyn, M.; Lyka, E.; Kelly, F.J.; Cook, P.; Ster, I.C.; Earle, K.A. Derepression of glomerular filtration, renal blood flow and antioxidant defence in patients with type 2 diabetes at high-risk of cardiorenal disease. Free Radic. Biol. Med. 2020, 161, 283–289. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics—2023 Update: A Report From the American Heart Association. Circulation 2023, 147, E93–E621. [Google Scholar] [CrossRef]
- World Heart Report 2023: Confronting the World’s Number One Killer; World Heart Federation: Geneva, Switzerland, 2023; Available online: https://world-heart-federation.org/wp-content/uploads/World-Heart-Report-2023.pdf (accessed on 27 December 2023).
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Dunbar, S.B.; Khavjou, O.A.; Bakas, T.; Hunt, G.; Kirch, R.A.; Leib, A.R.; Morrison, R.S.; Poehler, D.C.; Roger, V.L.; Whitsel, L.P.; et al. Projected Costs of Informal Caregiving for Cardiovascular Disease: 2015 to 2035: A Policy Statement From the American Heart Association. Circulation 2018, 137, E558–E577. [Google Scholar] [CrossRef]
- Olsen, M.H.; Wachtell, K.; Aalkjaer, C.; Devereux, R.B.; Dige-Petersen, H.; Ibsen, H. Endothelial dysfunction in resistance arteries is related to high blood pressure and circulating low density lipoproteins in previously treated hypertension. Am. J. Hypertens. 2001, 14, 861–867. [Google Scholar] [CrossRef]
- Noureddine, F.Y.; Altara, R.; Fan, F.; Yabluchanskiy, A.; Booz, G.W.; Zouein, F.A. Impact of the Renin–Angiotensin System on the Endothelium in Vascular Dementia: Unresolved Issues and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 4268. [Google Scholar] [CrossRef]
- Goto, K.; Fujii, K.; Onaka, U.; Abe, I.; Fujishima, M. Renin-Angiotensin System Blockade Improves Endothelial Dysfunction in Hypertension. Hypertension 2000, 36, 575–580. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis as an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Ap-proaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef]
- Giannitsi, S.; Bougiakli, M.; Bechlioulis, A.; Naka, K. Endothelial dysfunction and heart failure: A review of the existing bibli-ography with emphasis on flow mediated dilation. JRSM Cardiovasc. Dis. 2019, 8, 2048004019843047. [Google Scholar] [CrossRef]
- Saavedra-Alvarez, A.; Pereyra, K.V.; Toledo, C.; Iturriaga, R.; Del Rio, R. Vascular dysfunction in HFpEF: Potential role in the development, maintenance, and progression of the disease. Front. Cardiovasc. Med. 2022, 9, 1070935. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Ueno, M.; Chiba, Y.; Matsumoto, K.; Nakagawa, T.; Miyanaka, H. Clearance of Beta-Amyloid in the Brain. Curr. Med. Chem. 2014, 21, 4085–4090. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.; Parbo, P.; Madsen, L.S.; Hansen, A.K.; Hansen, K.V.; Schaldemose, J.L.; Kjeldsen, P.L.; Stokholm, M.G.; Gottrup, H.; Eskildsen, S.F.; et al. The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer’s disease: A longitudinal PET study. J. Neuroinflammation 2020, 17, 151. [Google Scholar] [CrossRef]
- Lopes, A.A.; Caramurú, L.H.; Maeda, N.Y. Endothelial Dysfunction Associated with Chronic Intravascular Coagulation in Secondary Pulmonary Hypertension. Clin. Appl. Thromb. Hemost. 2002, 8, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.J.; Werring, D.J. New Insights Into Cerebrovascular Pathophysiology and Hypertension. Stroke 2022, 53, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Markus, H.S.; de Leeuw, F.E. Alzheimer’s vessel disease: Recent advances and future directions. Int. J. Stroke 2023, 18, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Guy, R.; Volkman, R.; Wilczynski, E.; Yagil, C.; Yagil, Y.; Findler, M.; Auriel, E.; Nevo, U.; Offen, D. A Novel Rodent Model of Hypertensive Cerebral Small Vessel Disease with White Matter Hyperintensities and Peripheral Oxidative Stress. Int. J. Mol. Sci. 2022, 23, 5915. [Google Scholar] [CrossRef]
- Low, A.; Mak, E.; Rowe, J.B.; Markus, H.S.; O’brien, J.T. Inflammation and cerebral small vessel disease: A systematic review. Ageing Res. Rev. 2019, 53, 100916. [Google Scholar] [CrossRef]
- Lotharius, J.; Brundin, P. Pathogenesis of parkinson’s disease: Dopamine, vesicles and alpha-synuclein. Nat. Rev. Neurosci. 2002, 3, 932–942. [Google Scholar] [CrossRef]
- Kurup, P.K.; Xu, J.; Videira, R.A.; Ononenyi, C.; Baltazar, G.; Lombroso, P.J.; Nairn, A.C. STEP 61 is a substrate of the E3 ligase parkin and is upregulated in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2015, 112, 1202–1207. [Google Scholar] [CrossRef]
- McNaught, K.S.P.; Olanow, C.W.; Halliwell, B.; Isacson, O.; Jenner, P. Failure of the ubiquitin–proteasome system in Parkinson’s disease. Nat. Rev. Neurosci. 2001, 2, 589–594. [Google Scholar] [CrossRef]
- Bradaric, B.D.; Patel, A.; Schneider, J.A.; Carvey, P.M.; Hendey, B. Evidence for angiogenesis in Parkinson’s disease, incidental Lewy body disease, and progressive supranuclear palsy. J. Neural Transm. 2011, 119, 59–71. [Google Scholar] [CrossRef]
- Carvey, P.M.; Zhao, C.H.; Hendey, B.; Lum, H.; Trachtenberg, J.; Desai, B.S.; Snyder, J.; Zhu, Y.G.; Ling, Z.D. 6-Hydroxydopamine-induced alterations in blood–brain barrier permeability. Eur. J. Neurosci. 2005, 22, 1158–1168. [Google Scholar] [CrossRef]
- Barcia, C.; Bautista, V.; Sánchez-Bahillo, A.; Fernández-Villalba, E.; Faucheux, B.; Poza y Poza, M.; Fernandez Barreiro, A.; Hirsch, E.C.; Herrero, M.T. Changes in vascularization in substantia nigra pars compacta of monkeys rendered parkinsonian. J. Neural Transm. 2005, 112, 1237–1248. [Google Scholar] [CrossRef]
- Elabi, O.F.; Cunha, J.P.M.C.M.; Gaceb, A.; Fex, M.; Paul, G. High-fat diet-induced diabetes leads to vascular alterations, pericyte reduction, and perivascular depletion of microglia in a 6-OHDA toxin model of Parkinson disease. J. Neuroinflam. 2021, 18, 175. [Google Scholar] [CrossRef]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef]
- Romagnani, P.; Remuzzi, G.; Glassock, R.; Levin, A.; Jager, K.J.; Tonelli, M.; Massy, Z.; Wanner, C.; Anders, H.-J. Chronic kidney disease. Nat. Rev. Dis. Primers 2017, 3, 17088. [Google Scholar] [CrossRef]
- Burnier, M.; Damianaki, A. Hypertension as Cardiovascular Risk Factor in Chronic Kidney Disease. Circ. Res. 2023, 132, 1050–1063. [Google Scholar] [CrossRef]
- Iwai, T.; Kataoka, Y.; Otsuka, F.; Asaumi, Y.; Nicholls, S.J.; Noguchi, T.; Yasuda, S. Chronic kidney disease and coronary atherosclerosis: Evidences from intravascular imaging. Expert Rev. Cardiovasc. Ther. 2019, 17, 707–716. [Google Scholar] [CrossRef]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.M.; Zoungas, S.; Rossing, P.; Groop, P.-H.; Cooper, M.E. Diabetic kidney disease. Nat. Rev. Dis. Primers 2015, 1, 15018. [Google Scholar] [CrossRef]
- Williams, M.E. Clinical studies of advanced glycation end product inhibitors and diabetic kidney disease. Curr. Diabetes Rep. 2004, 4, 441–446. [Google Scholar] [CrossRef]
- Lucero, C.M.; Prieto-Villalobos, J.; Marambio-Ruiz, L.; Balmazabal, J.; Alvear, T.F.; Vega, M.; Barra, P.; Retamal, M.A.; Orellana, J.A.; Gómez, G.I. Hypertensive Nephropathy: Unveiling the Possible Involvement of Hemichannels and Pannexons. Int. J. Mol. Sci. 2022, 23, 15936. [Google Scholar] [CrossRef]
- Ameer, O.Z. Hypertension in chronic kidney disease: What lies behind the scene. Front. Pharmacol. 2022, 13, 949260. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial Dysfunction: A marker of atherosclerotic risk. Arter. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef]
- Ding, H.; Triggle, C.R. Endothelial cell dysfunction and the vascular complications associated with type 2 diabetes: Assessing the health of the endothelium. Vasc. Health Risk Manag. 2005, 1, 55–71. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Higashi, Y.; Noma, K.; Yoshizumi, M.; Kihara, Y. Endothelial Function and Oxidative Stress in Cardiovascular Diseases. Circ. J. 2009, 73, 411–418. [Google Scholar] [CrossRef]
- Lin, K.-T.; Xue, J.-Y.; Sun, F.F.; Wong, P.Y.-K. Reactive Oxygen Species Participate in Peroxynitrite-Induced Apoptosis in HL-60 Cells. Biochem. Biophys. Res. Commun. 1997, 230, 115–119. [Google Scholar] [CrossRef]
- Yang, K.-J.; Choi, W.J.; Chang, Y.-K.; Park, C.W.; Kim, S.Y.; Hong, Y.A. Inhibition of Xanthine Oxidase Protects against Diabetic Kidney Disease through the Amelioration of Oxidative Stress via VEGF/VEGFR Axis and NOX-FoxO3a-eNOS Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 3807. [Google Scholar] [CrossRef]
- Basuroy, S.; Bhattacharya, S.; Leffler, C.W.; Parfenova, H.; Tejero, J.; Shiva, S.; Gladwin, M.T.; Pourcyrous, M.; Fedinec, A.L.; Liu, J.; et al. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-α in cerebral vascular endothelial cells. Am. J. Physiol. Cell Physiol. 2009, 296, C422–C432. [Google Scholar] [CrossRef]
- Groten, S.A.; Smit, E.R.; Janssen, E.F.J.; Eshof, B.L.v.D.; van Alphen, F.P.J.; van der Zwaan, C.; Meijer, A.B.; Hoogendijk, A.J.; Biggelaar, M.v.D. Multi-omics delineation of cytokine-induced endothelial inflammatory states. Commun. Biol. 2023, 6, 525. [Google Scholar] [CrossRef]
- Rosenkranz-Weiss, P.; Sessa, W.C.; Milstien, S.; Kaufman, S.; A Watson, C.; Pober, J.S. Regulation of nitric oxide synthesis by proinflammatory cytokines in human umbilical vein endothelial cells. Elevations in tetrahydrobiopterin levels enhance endo-thelial nitric oxide synthase specific activity. J. Clin. Investig. 1994, 93, 2236–2243. [Google Scholar] [CrossRef]
- Tran, N.; Garcia, T.; Aniqa, M.; Ali, S.; Ally, A.; Nauli, S.M. Endothelial Nitric Oxide Synthase (eNOS) and the Cardiovascular System: In Physiology and in Disease States. Am. J. Biomed. Sci. Res. 2022, 15, 153–177. [Google Scholar] [PubMed]
- Janaszak-Jasiecka, A.; Płoska, A.; Wierońska, J.M.; Dobrucki, L.W.; Kalinowski, L. Endothelial dysfunction due to eNOS uncoupling: Molecular mechanisms as potential therapeutic targets. Cell. Mol. Biol. Lett. 2023, 28, 21. [Google Scholar] [CrossRef] [PubMed]
- Lenna, S.; Han, R.; Trojanowska, M. Endoplasmic reticulum stress and endothelial dysfunction. IUBMB Life 2014, 66, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Persson, J.; Nilsson, J.; Lindholm, M.W. Interleukin-1beta and tumour necrosis factor-alpha impede neutral lipid turnover in macrophage-derived foam cells. BMC Immunol. 2008, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and Atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Wang, L.; Tang, C. Targeting Platelet in Atherosclerosis Plaque Formation: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 9760. [Google Scholar] [CrossRef]
- Goldfarb, D.A. The renin-angiotensin system. New concepts in regulation of blood pressure and renal function. Urol. Clin. N. Am. 1994, 21, 187–194. [Google Scholar] [CrossRef]
- Nishiyama, A.; Kim-Mitsuyama, S. New Approaches to Blockade of the Renin–Angiotensin–Aldosterone System: Overview of Regulation of the Renin–Angiotensin–Aldosterone System. J. Pharmacol. Sci. 2010, 113, 289–291. [Google Scholar] [CrossRef]
- Nakagawa, P.; Sigmund, C.D. How Is the Brain Renin–Angiotensin System Regulated? Hypertension 2017, 70, 10–18. [Google Scholar] [CrossRef]
- Patrick, D.M.; Van Beusecum, J.P.; Kirabo, A. The role of inflammation in hypertension: Novel concepts. Curr. Opin. Physiol. 2021, 19, 92–98. [Google Scholar] [CrossRef]
- Rothman, A.M.; MacFadyen, J.; Thuren, T.; Webb, A.; Harrison, D.G.; Guzik, T.J.; Libby, P.; Glynn, R.J.; Ridker, P.M. Effects of Interleukin-1β Inhibition on Blood Pressure, Incident Hypertension, and Residual Inflammatory Risk: A Secondary Analysis of CANTOS. Hypertension 2020, 75, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.-W.; Tian, N.; Shparago, M.; Tan, W.; Bailey, A.P.; Manning, R.D. Renal NF-kappaB activation and TNF-alpha upregulation correlate with salt-sensitive hypertension in Dahl salt-sensitive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1817–R1824. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A. Environmental Determinants of Cardiovascular Disease. Circ. Res. 2017, 121, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.I.; Lorigo, M.; Cairrao, E. Endocrine-Disrupting Effects of Bisphenol A on the Cardiovascular System: A Review. J. Xenobiotics 2022, 12, 181–213. [Google Scholar] [CrossRef] [PubMed]
- Easson, S.; Singh, R.D.; Connors, L.; Scheidl, T.; Baker, L.; Jadli, A.; Zhu, H.-L.; Thompson, J. Exploring oxidative stress and endothelial dysfunction as a mechanism linking bisphenol S exposure to vascular disease in human umbilical vein endothelial cells and a mouse model of postnatal exposure. Environ. Int. 2022, 170, 107603. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, H.; Hwang, B.; Kim, S.; Choi, Y.H.; Kim, W.-J.; Moon, S.-K. Bisphenol A exposure inhibits vascular smooth muscle cell responses: Involvement of proliferation, migration, and invasion. Environ. Toxicol. Pharmacol. 2023, 98, 104060. [Google Scholar] [CrossRef]
- Costa, H.E.; Cairrao, E. Effect of bisphenol A on the neurological system: A review update. Arch. Toxicol. 2024, 98, 1–73. [Google Scholar] [CrossRef]
- Inadera, H. Neurological Effects of Bisphenol A and its Analogues. Int. J. Med. Sci. 2015, 12, 926–936. [Google Scholar] [CrossRef]
- Engin, A.B.; Engin, A. Risk of Alzheimer’s disease and environmental bisphenol A exposure. Curr. Opin. Toxicol. 2021, 25, 36–41. [Google Scholar] [CrossRef]
- Calaf, G.M.; Ponce-Cusi, R.; Aguayo, F.; Muñoz, J.P.; Bleak, T.C. Endocrine disruptors from the environment affecting breast cancer. Oncol. Lett. 2020, 20, 19–32. [Google Scholar] [CrossRef]
- Migliaccio, S.; Bimonte, V.M.; Besharat, Z.M.; Sabato, C.; Lenzi, A.; Crescioli, C.; Ferretti, E. Environmental Contaminants Acting as Endocrine Disruptors Modulate Atherogenic Processes: New Risk Factors for Cardiovascular Diseases in Women? Biomolecules 2021, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Marroqui, L.; Tudurí, E.; Alonso-Magdalena, P.; Quesada, I.; Nadal, Á.; dos Santos, R.S. Mitochondria as target of endo-crine-disrupting chemicals: Implications for type 2 diabetes. J. Endocrinol. 2018, 239, R27–R45. [Google Scholar] [CrossRef] [PubMed]
- Maradonna, F.; Carnevali, O. Lipid Metabolism Alteration by Endocrine Disruptors in Animal Models: An Overview. Front. Endocrinol. 2018, 9, 654. [Google Scholar] [CrossRef]
- Sabbatinelli, J.; Ramini, D.; Giuliani, A.; Recchioni, R.; Spazzafumo, L.; Olivieri, F. Connecting vascular aging and frailty in Alzheimer’s disease. Mech. Ageing Dev. 2021, 195, 111444. [Google Scholar] [CrossRef]
- Xu, X.; Wang, B.; Ren, C.; Hu, J.; Greenberg, D.A.; Chen, T.; Xie, L.; Jin, K. Age-related Impairment of Vascular Structure and Functions. Aging Dis. 2017, 8, 590–610. [Google Scholar] [CrossRef]
- Rajesh, N.; Moudgil-Joshi, J.; Kaliaperumal, C. Smoking and degenerative spinal disease: A systematic review. Brain Spine 2022, 2, 100916. [Google Scholar] [CrossRef]
- Kondo, T.; Nakano, Y.; Adachi, S.; Murohara, T. Effects of Tobacco Smoking on Cardiovascular Disease. Circ. J. 2019, 83, 1980–1985. [Google Scholar] [CrossRef]
- Mooradian, A.D. Dyslipidemia in type 2 diabetes mellitus. Nat. Clin. Pract. Endocrinol. Metab. 2009, 5, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Wee, J.; Sukudom, S.; Bhat, S.; Marklund, M.; Peiris, N.J.; Hoyos, C.M.; Patel, S.; Naismith, S.L.; Dwivedi, G.; Misra, A. The relationship between midlife dyslipidemia and lifetime incidence of dementia: A systematic review and meta-analysis of cohort studies. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2023, 15, e12395. [Google Scholar] [CrossRef]
- Schubert, M. The Correlation between Diabetes Mellitus and Neurodegenerative Diseases. Klin. Monbl. Augenheilkd. 2023, 240, 130–135. [Google Scholar] [CrossRef]
- Luli, M.; Yeo, G.; Farrell, E.; Ogden, J.; Parretti, H.; Frew, E.; Bevan, S.; Brown, A.; Logue, J.; Menon, V.; et al. The implications of defining obesity as a disease: A report from the Association for the Study of Obesity 2021 annual conference. eClinicalMedicine 2023, 58, 101962. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.A.; Potashkin, J.A. Physical activity and lifestyle modifications in the treatment of neurodegenerative diseases. Front. Aging Neurosci. 2023, 15, 1185671. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; De Lorenzo, A. Diet, Nutrition and Chronic Degenerative Diseases. Nutrients 2021, 13, 1372. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, E.; Nenic, K.; Milanovic, V.; Knezevic, N.N. The Role of Cortisol in Chronic Stress, Neurodegenerative Diseases, and Psychological Disorders. Cells 2023, 12, 2726. [Google Scholar] [CrossRef] [PubMed]
- Crowson, C.S.; Liao, K.P.; Davis, J.M., 3rd; Solomon, D.H.; Matteson, E.L.; Knutson, K.L.; Hlatky, M.A.; Gabriel, S.E. Rheumatoid arthritis and cardiovascular disease. Am. Heart J. 2013, 166, 622–628.e1. [Google Scholar] [CrossRef]
- Chen, B.; Collen, L.V.; Mowat, C.; Isaacs, K.L.; Singh, S.; Kane, S.V.; Farraye, F.A.; Snapper, S.; Jneid, H.; Lavie, C.J.; et al. In-flammatory Bowel Disease and Cardiovascular Diseases. Am. J. Med. 2022, 135, 1453–1460. [Google Scholar] [CrossRef]
- Jameson, J.L.; Kasper, D.L.; Longo, D.L.; Fauci, A.S.; Hauser, S.L.; Loscalzo, J. Harrison’s Principles of Internal Medicine, 20th ed.; McGrew Hill: New York, NY, USA, 2018; pp. 1901–1902. [Google Scholar]
- Xie, X.; Liu, Y.; Perkovic, V.; Li, X.; Ninomiya, T.; Hou, W.; Zhao, N.; Liu, L.; Lv, J.; Zhang, H.; et al. Renin-Angiotensin System Inhibitors and Kidney and Cardiovascular Outcomes in Patients With CKD: A Bayesian Network Meta-analysis of Randomized Clinical Trials. Am. J. Kidney Dis. 2016, 67, 728–741. [Google Scholar] [CrossRef]
- Klegeris, A.; Bajwa, E. Neuroinflammation as a mechanism linking hypertension with the increased risk of Alzheimer’s disease. Neural Regen. Res. 2022, 17, 2342–2346. [Google Scholar] [CrossRef]
- Solé-Guardia, G.; Custers, E.; de Lange, A.; Clijncke, E.; Geenen, B.; Gutierrez, J.; Küsters, B.; Claassen, J.A.H.R.; de Leeuw, F.-E.; Wiesmann, M.; et al. Association between hypertension and neurovascular inflammation in both normal-appearing white matter and white matter hyperintensities. Acta Neuropathol. Commun. 2023, 11, 2. [Google Scholar] [CrossRef]
- Gaudreault-Tremblay, M.-M.; Foster, B.J. Benefits of Continuing RAAS Inhibitors in Advanced CKD. Clin. J. Am. Soc. Nephrol. 2020, 15, 592–593. [Google Scholar] [CrossRef]
- Ittaman, S.V.; VanWormer, J.J.; Rezkalla, S.H. The Role of Aspirin in the Prevention of Cardiovascular Disease. Clin. Med. Res. 2014, 12, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Zyriax, B.-C.; Windler, E. Lifestyle changes to prevent cardio- and cerebrovascular disease at midlife: A systematic review. Maturitas 2023, 167, 60–65. [Google Scholar] [CrossRef]
- Pinal-Fernandez, I.; Casal-Dominguez, M.; Mammen, A.L. Statins: Pros and cons. Med. Clin. 2018, 150, 398–402. [Google Scholar] [CrossRef]
- Passacquale, G.; Sharma, P.; Perera, D.; Ferro, A. Antiplatelet therapy in cardiovascular disease: Current status and future directions. Br. J. Clin. Pharmacol. 2022, 88, 2686–2699. [Google Scholar] [CrossRef]
- Baradaran, A.; Nasri, H.; Rafieian-Kopaei, M. Oxidative stress and hypertension: Possibility of hypertension therapy with antioxidants. J. Res. Med. Sci. 2014, 19, 358–367. [Google Scholar] [PubMed]
- Sheikh, A.M.; Yano, S.; Mitaki, S.; Haque, A.; Yamaguchi, S.; Nagai, A. A Mesenchymal stem cell line (B10) increases angiogenesis in a rat MCAO model. Exp. Neurol. 2019, 311, 182–193. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Nagai, A.; Wakabayashi, K.; Narantuya, D.; Kobayashi, S.; Yamaguchi, S.; Kim, S.U. Mesenchymal stem cell transplantation modulates neuroinflammation in focal cerebral ischemia: Contribution of fractalkine and IL-5. Neurobiol. Dis. 2011, 41, 717–724. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z. Gut microbiome and cardiovascular disease. Curr. Opin. Cardiol. 2020, 35, 207–218. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheikh, A.M.; Yano, S.; Tabassum, S.; Nagai, A. The Role of the Vascular System in Degenerative Diseases: Mechanisms and Implications. Int. J. Mol. Sci. 2024, 25, 2169. https://doi.org/10.3390/ijms25042169
Sheikh AM, Yano S, Tabassum S, Nagai A. The Role of the Vascular System in Degenerative Diseases: Mechanisms and Implications. International Journal of Molecular Sciences. 2024; 25(4):2169. https://doi.org/10.3390/ijms25042169
Chicago/Turabian StyleSheikh, Abdullah Md., Shozo Yano, Shatera Tabassum, and Atsushi Nagai. 2024. "The Role of the Vascular System in Degenerative Diseases: Mechanisms and Implications" International Journal of Molecular Sciences 25, no. 4: 2169. https://doi.org/10.3390/ijms25042169
APA StyleSheikh, A. M., Yano, S., Tabassum, S., & Nagai, A. (2024). The Role of the Vascular System in Degenerative Diseases: Mechanisms and Implications. International Journal of Molecular Sciences, 25(4), 2169. https://doi.org/10.3390/ijms25042169





