Network Pharmacology and Experimental Verifications to Discover Scutellaria baicalensis Georgi’s Effects on Joint Inflammation, Destruction, and Pain in Osteoarthritis
Abstract
1. Introduction
2. Results
2.1. Network Pharmacology of SB against OA
2.1.1. Screening of Potential Active Compounds in SB for OA
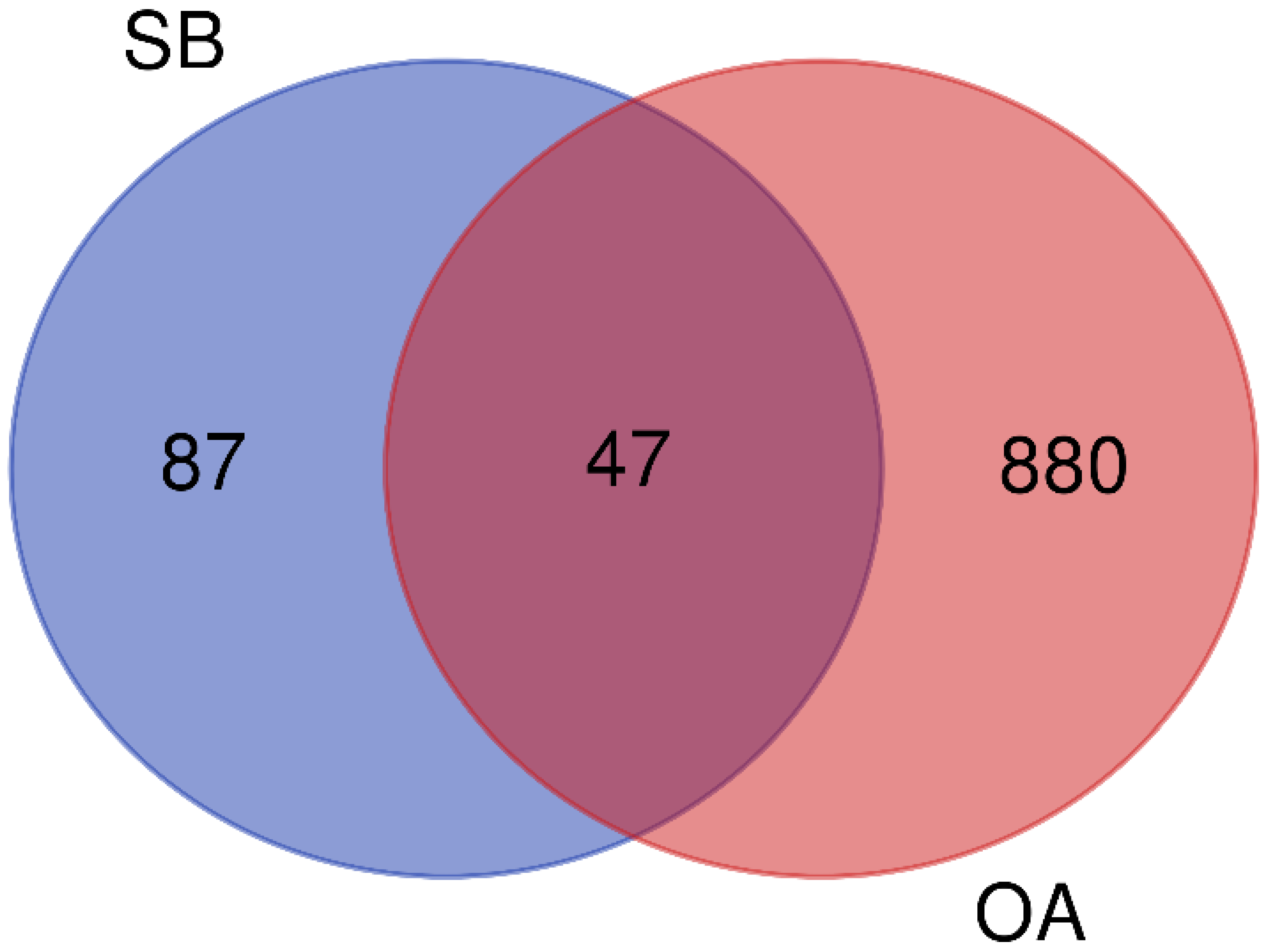
2.1.2. Construction of D-C-T-D Network
2.1.3. PPI Network Analysis
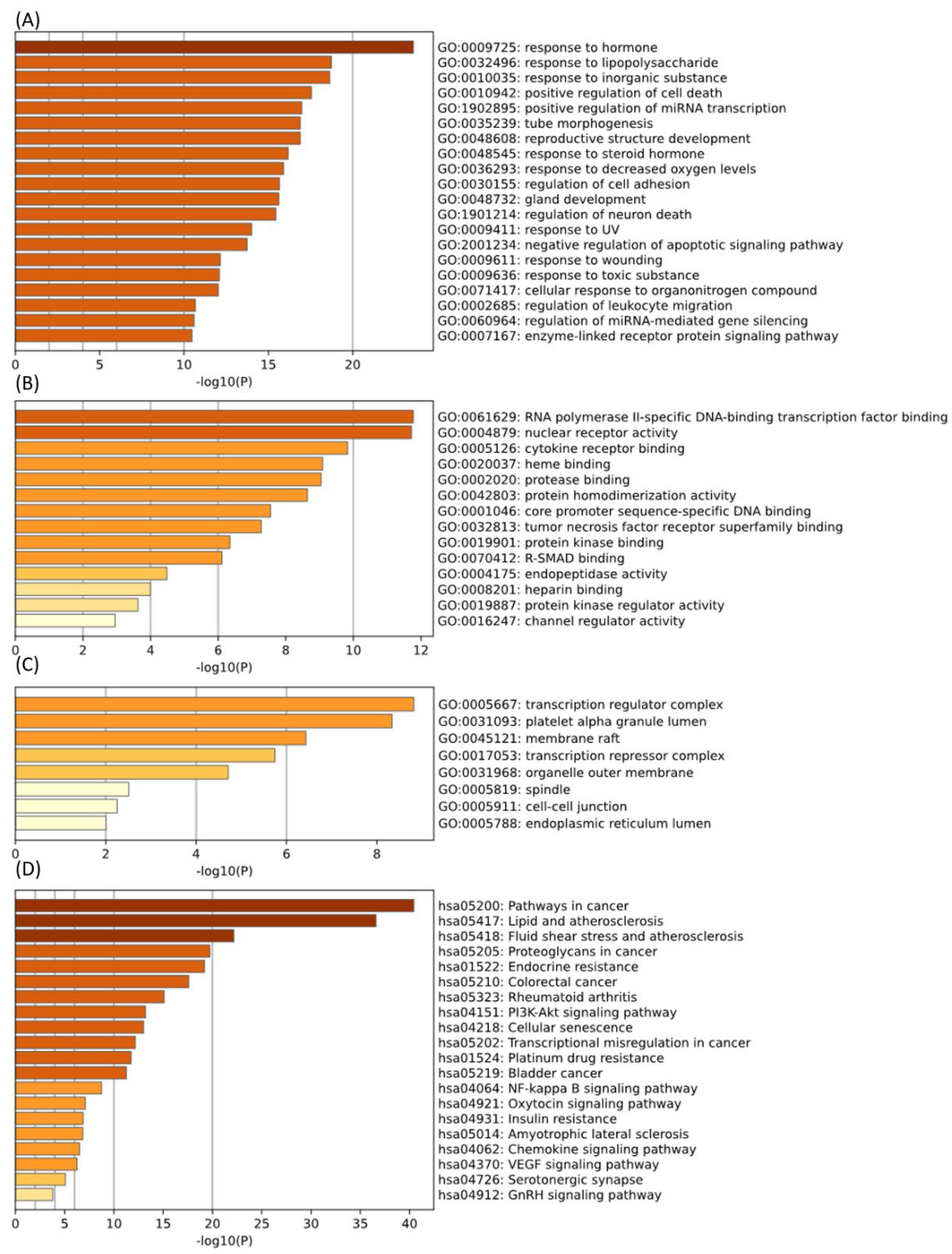
2.1.4. GO and KEGG Enrichment Analysis
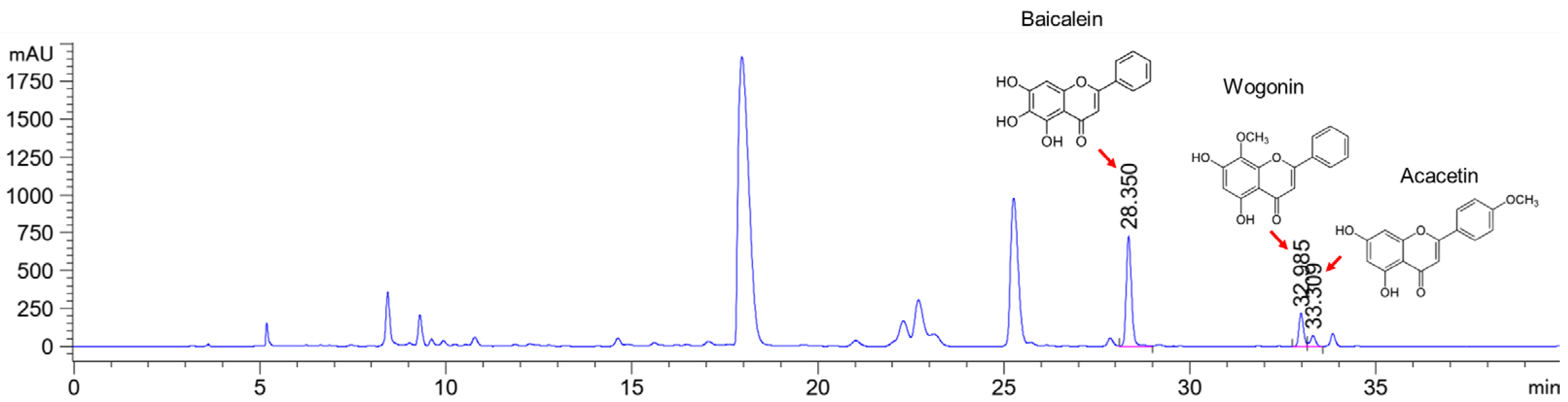
2.2. HPLC Analysis
2.3. Effects on the Weight-Bearing Arrangement in MIA Rat Models
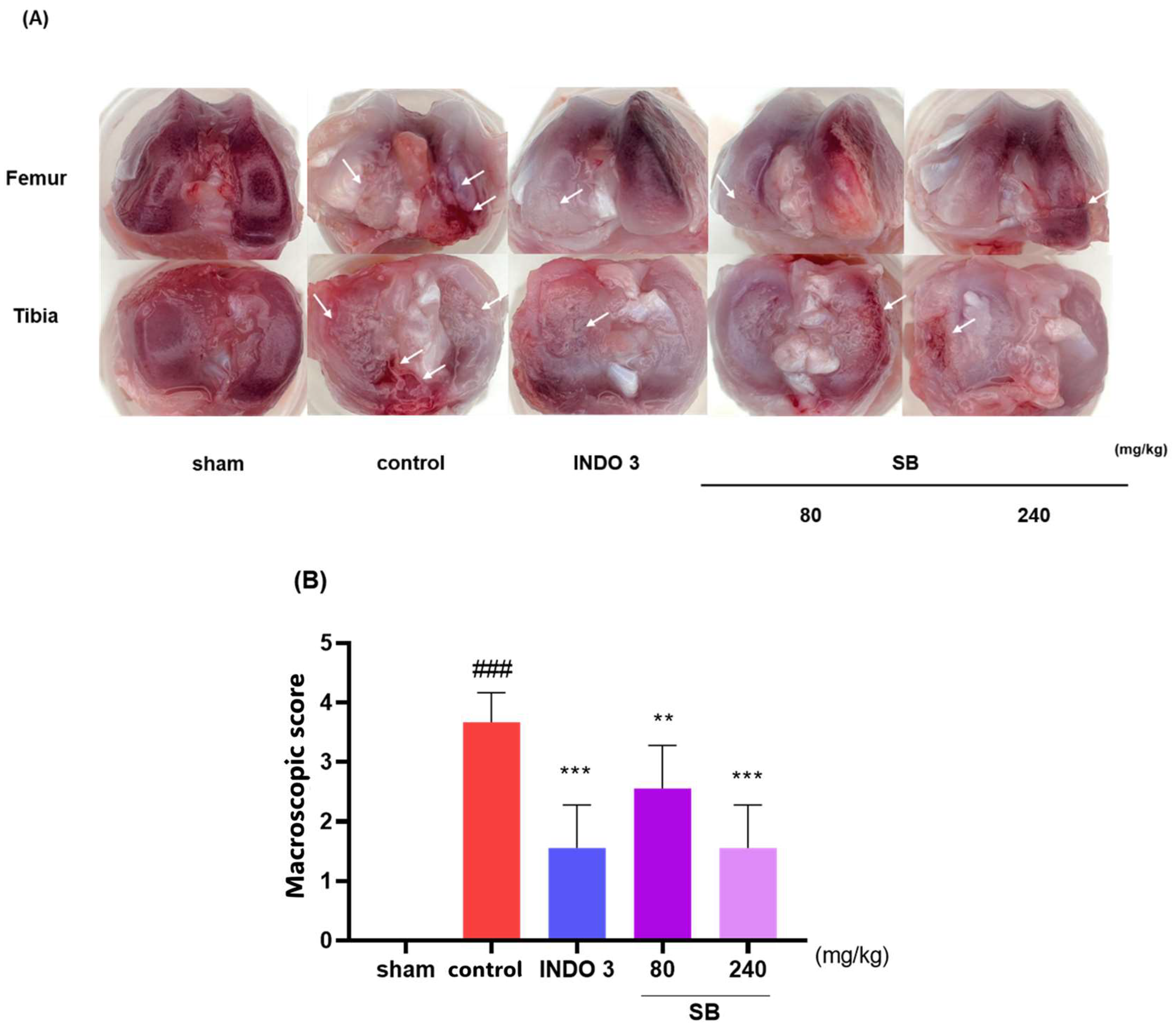
2.4. Cartilage Damage in the MIA Model
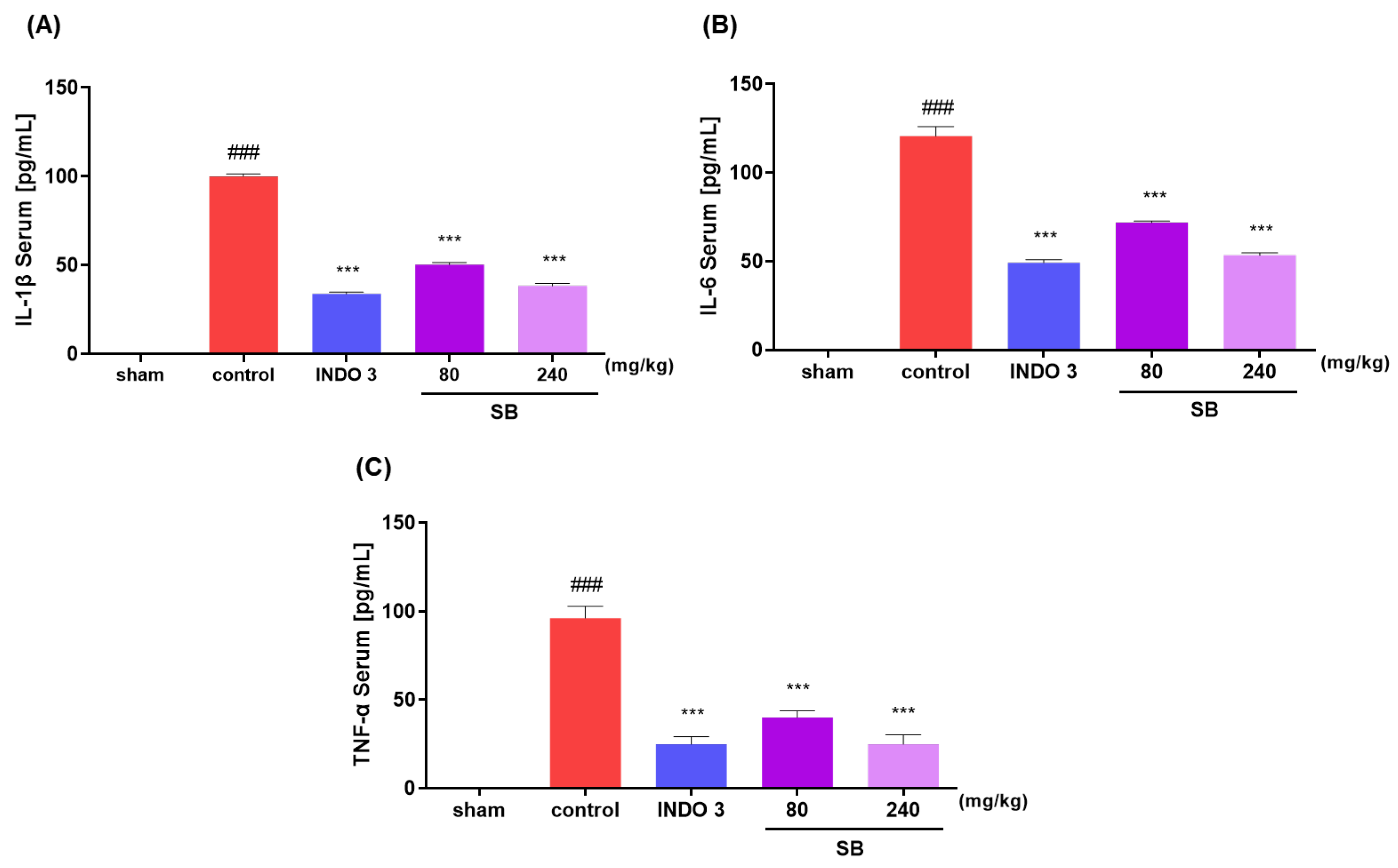
2.5. Inflammatory Cytokine Levels in MIA Rats
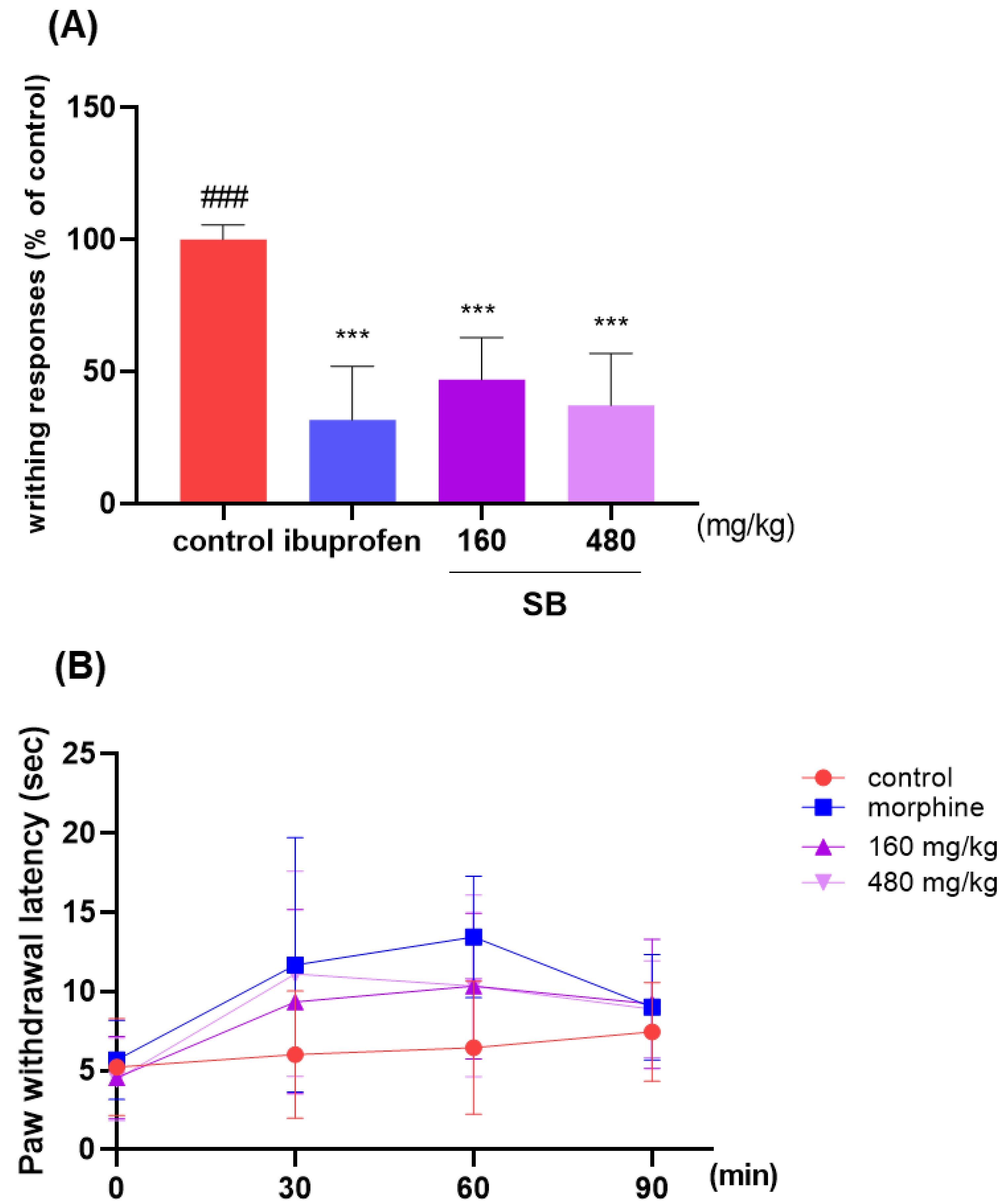
2.6. Effect on Analgesic Responses
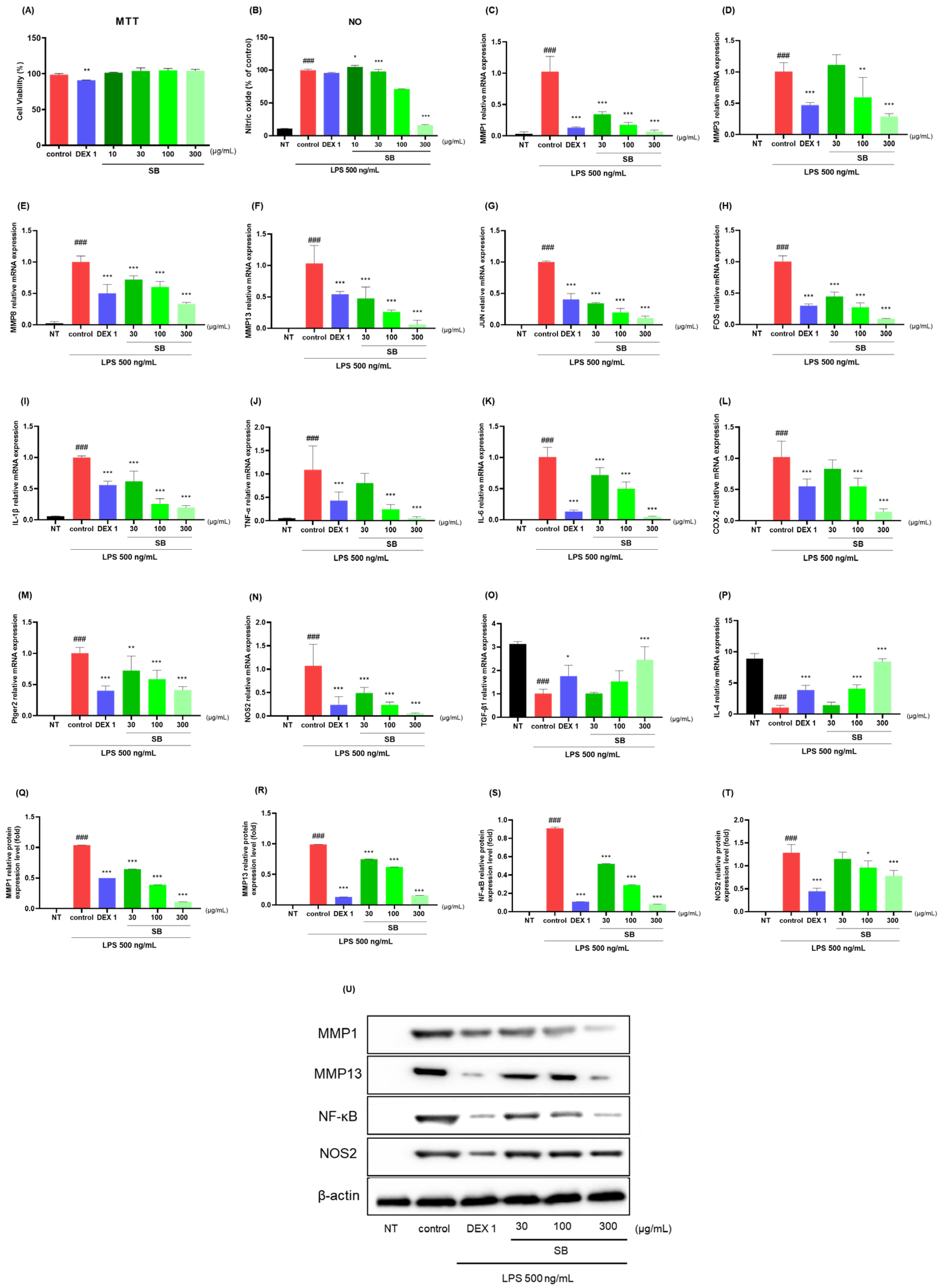
2.7. Anti-Inflammatory Effects in LPS-Stimulated RAW264.7 Cells
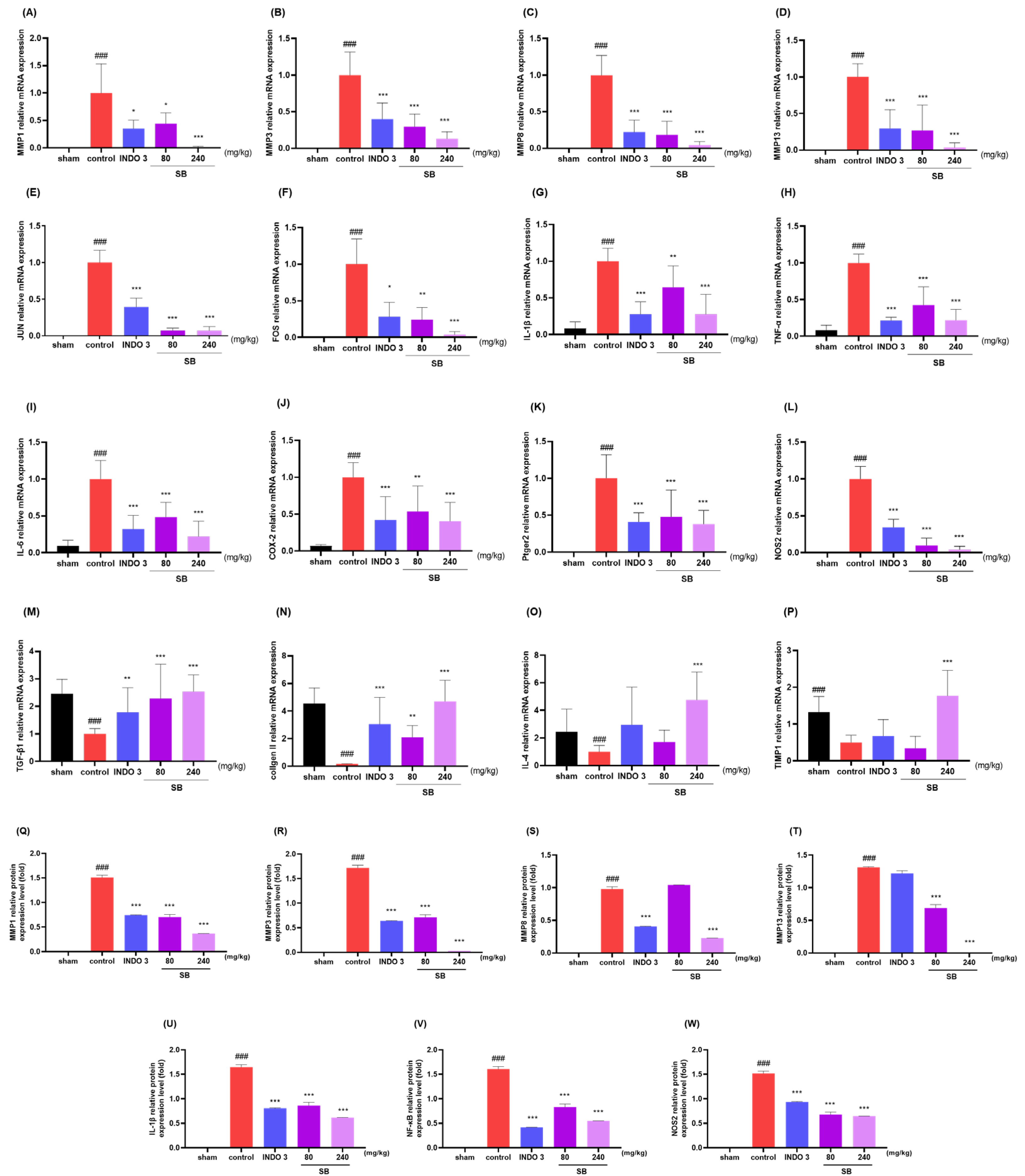
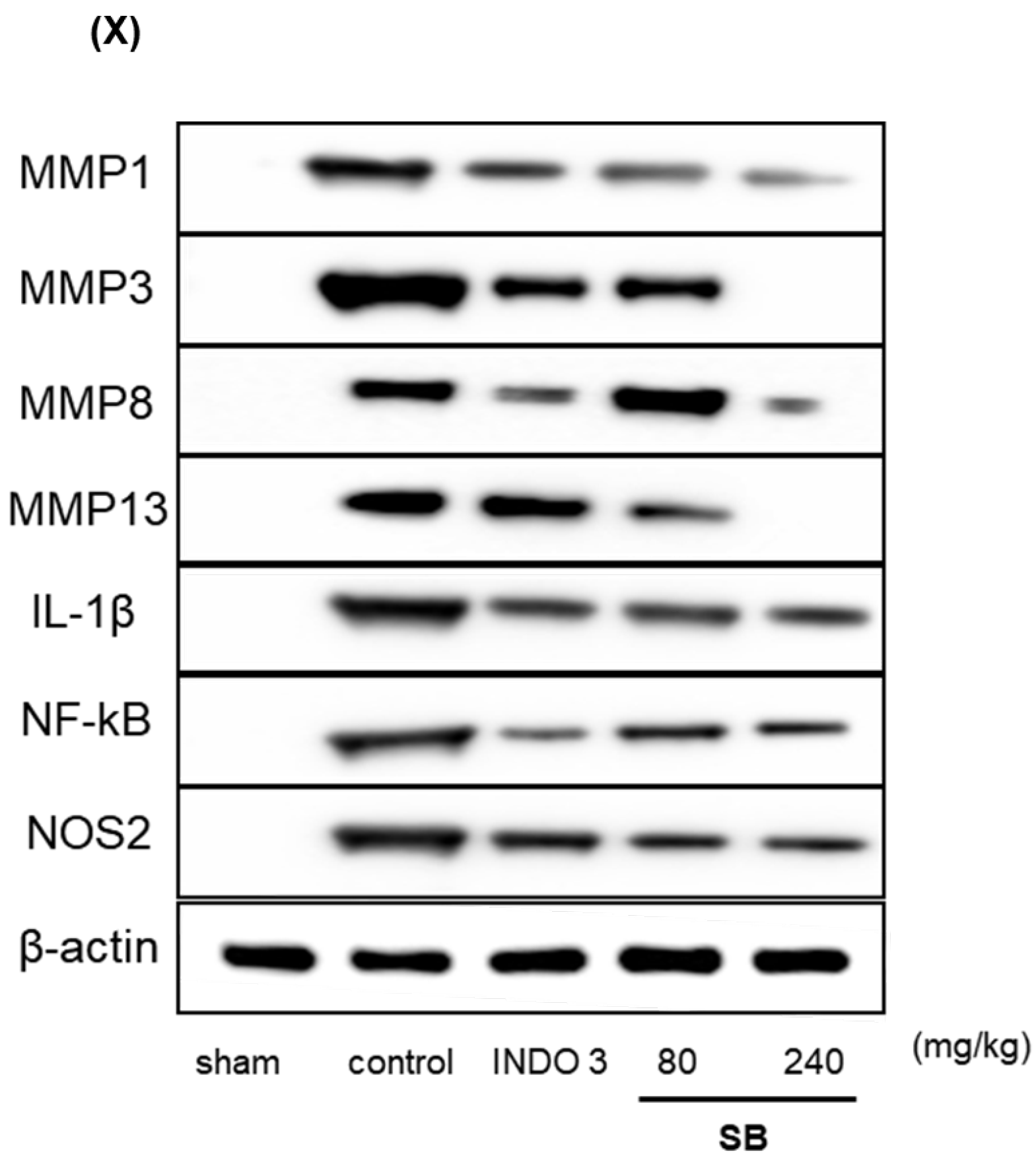
2.8. Effects on Cytokine Responses in Knee Joint Cartilage Tissue
3. Discussion
4. Materials and Methods
4.1. Network Pharmacology of SB for OA
4.1.1. Active Compounds of SB- and OA-Related Target Genes
4.1.2. Common Target Acquisition
4.1.3. Protein–Protein Interaction (PPI) Network Construction
4.1.4. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Analysis
4.2. Scutellaria baicalensis Georgi Extract Preparations
4.3. High-Performance Liquid Chromatography
4.4. Animal
4.5. Monosodium Iodoacetate Injection and Diet Preparation
4.6. Hind Limb Weight-Bearing Measurement
4.7. Cartilage Degradation Evaluation
4.8. Monosodium Iodoacetateserum Concentration Analysis
4.9. Writhing Test
4.10. Hot Plate Test
4.11. RAW264.7 Cell Culture
4.12. Nitric Oxide (NO) Production and Cell Toxicity Evaluation
4.13. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
4.14. Protein Expression Analysis
4.15. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, D.; Zhang, H.; Liang, J.; Feng, X.; Zhao, J.; Sun, L. Incidence Trend of Five Common Musculoskeletal Disorders from 1990 to 2017 at the Global, Regional and National Level: Results from the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2020, 79, 1014–1022. [Google Scholar] [CrossRef]
- Global Burden of Disease Study 2013 Collaborators. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 301 Acute and Chronic Diseases and Injuries in 188 Countries, 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Runhaar, J.; Bierma-Zeinstra, S.; Roos, E.M. A Lifespan Approach to Osteoarthritis Prevention. Osteoarthr. Cartil. 2021, 29, 1638–1653. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F.; Wallace, I.J.; Lieberman, D.E.; Felson, D.T. Modern-Day Environmental Factors in the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2018, 14, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Greene, M.A.; Loeser, R.F. Aging-Related Inflammation in Osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Batt, M. An Update on the Pathophysiology of Osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Yu, H.; Huang, X.; Shen, J.; Xiao, G.; Chen, L.; Wang, H.; Xing, L.; Chen, D. Current Understanding of Osteoarthritis Pathogenesis and Relevant New Approaches. Bone Res. 2022, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Rannou, F.; Pelletier, J.-P.; Martel-Pelletier, J. Efficacy and Safety of Topical NSAIDs in the Management of Osteoarthritis: Evidence from Real-Life Setting Trials and Surveys. Semin. Arthritis Rheum. 2016, 45, S18–S21. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Robertson, W.B.; Zhao, J.; Chen, W.; Xu, J. Emerging Trend in the Pharmacotherapy of Osteoarthritis. Front. Endocrinol. 2019, 10, 431. [Google Scholar] [CrossRef]
- Jo, H.-G.; Lee, G.-Y.; Baek, C.Y.; Song, H.S.; Lee, D. Analgesic and Anti-Inflammatory Effects of Aucklandia lappa Root Extracts on Acetic Acid-Induced Writhing in Mice and Monosodium Iodoacetate-Induced Osteoarthritis in Rats. Plants 2020, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.-G.; Baek, C.Y.; Kim, D.; Lee, D.; Song, H.S. Stem of Sorbus commixta Hedl. Extract Inhibits Cartilage Degradation and Arthritic Pain in Experimental Model via Anti-Inflammatory Activity. Nutrients 2023, 15, 3774. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-M.; Hui, K.-K.; Perng, W.-T.; Wang, Y.-H.; Wei, J.C.-C. Chinese Herbal Medicine Might Be Associated with a Lower Rate of Joint Replacement in Patients with Osteoarthritis: A 12-Year Population-Based Matched Cohort Analysis. J. Ethnopharmacol. 2021, 280, 114419. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology and Toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Wang, S.; Kuang, Y.; Hu, Z.-M.; Qiao, X.; Ye, M. A Comprehensive Review on Phytochemistry, Pharmacology, and Flavonoid Biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef]
- Liao, H.; Ye, J.; Gao, L.; Liu, Y. The Main Bioactive Compounds of Scutellaria baicalensis Georgi. for Alleviation of Inflammatory Cytokines: A Comprehensive Review. Biomed. Pharmacother. 2021, 133, 110917. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Askari, V.R.; Hosseinzadeh, H. Promising Influences of Scutellaria baicalensis and Its Two Active Constituents, Baicalin, and Baicalein, against Metabolic Syndrome: A Review. Phytother. Res. 2021, 35, 3558–3574. [Google Scholar] [CrossRef]
- EghbaliFeriz, S.; Taleghani, A.; Tayarani-Najaran, Z. Scutellaria: Debates on the Anticancer Property. Biomed. Pharmacother. 2018, 105, 1299–1310. [Google Scholar] [CrossRef]
- EghbaliFeriz, S.; Taleghani, A.; Tayarani-Najaran, Z. Central Nervous System Diseases and Scutellaria: A Review of Current Mechanism Studies. Biomed. Pharmacother. 2018, 102, 185–195. [Google Scholar] [CrossRef]
- Liu, W.-J.; Chen, W.-W.; Chen, J.-Y.; Sun, Y.-B.; Chang, D.; Wang, C.-X.; Xie, J.-D.; Lin, W.; Li, S.-H.; Xu, W.; et al. Baicalin Attenuated Metabolic Dysfunction-Associated Fatty Liver Disease by Suppressing Oxidative Stress and Inflammation via the P62-Keap1-Nrf2 Signalling Pathway in Db/Db Mice. Phytother. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, C.; Cai, L.; Xie, H.; Hu, W.; Wang, T.; Lu, D.; Chen, H. Baicalin Suppresses IL-1β-Induced Expression of Inflammatory Cytokines via Blocking NF-κB in Human Osteoarthritis Chondrocytes and Shows Protective Effect in Mice Osteoarthritis Models. Int. Immunopharmacol. 2017, 52, 218–226. [Google Scholar] [CrossRef]
- Chan, E.; Wong, C.Y.-K.; Wan, C.-W.; Kwok, C.-Y.; Wu, J.-H.; Ng, K.-M.; So, C.-H.; Au, A.L.-S.; Poon, C.C.-W.; Seto, S.-W.; et al. Evaluation of Anti-Oxidant Capacity of Root of Scutellaria baicalensis Georgi, in Comparison with Roots of Polygonum Multiflorum thunb and Panax Ginseng CA Meyer. Am. J. Chin. Med. 2010, 38, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, M.; Stompor-Gorący, M. Promising Role of the Scutellaria baicalensis Root Hydroxyflavone-Baicalein in the Prevention and Treatment of Human Diseases. Int. J. Mol. Sci. 2023, 24, 4732. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, S.; Zhang, J.; Wu, J. Scutellaria baicalensis Georgi Is a Promising Candidate for the Treatment of Autoimmune Diseases. Front. Pharmacol. 2022, 13, 946030. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.-K.; Bae, J.-S. Anti-Inflammatory Effects of Baicalin, Baicalein, and Wogonin in Vitro and in Vivo. Inflammation 2015, 38, 110–125. [Google Scholar] [CrossRef]
- Tan, Y.-Q.; Lin, F.; Ding, Y.-K.; Dai, S.; Liang, Y.-X.; Zhang, Y.-S.; Li, J.; Chen, H.-W. Pharmacological Properties of Total Flavonoids in Scutellaria baicalensis for the Treatment of Cardiovascular Diseases. Phytomedicine 2022, 107, 154458. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, D.; Zhou, W.; Wang, L.; Wang, B.; Zhang, T.; Li, S. Network Pharmacology: Towards the Artificial Intelligence-Based Precision Traditional Chinese Medicine. Brief. Bioinform. 2023, 25, bbad518. [Google Scholar] [CrossRef]
- Jo, H.-G.; Baek, E.; Lee, D. Comparative Efficacy of East Asian Herbal Formulae Containing Astragali Radix-Cinnamomi Ramulus Herb-Pair against Diabetic Peripheral Neuropathy and Mechanism Prediction: A Bayesian Network Meta-Analysis Integrated with Network Pharmacology. Pharmaceutics 2023, 15, 1361. [Google Scholar] [CrossRef]
- Jo, H.-G.; Kim, H.; Baek, E.; Lee, D.; Hwang, J.H. Efficacy and Key Materials of East Asian Herbal Medicine Combined with Conventional Medicine on Inflammatory Skin Lesion in Patients with Psoriasis Vulgaris: A Meta-Analysis, Integrated Data Mining, and Network Pharmacology. Pharmaceuticals 2023, 16, 1160. [Google Scholar] [CrossRef]
- Ren, J.-L.; Yang, L.; Qiu, S.; Zhang, A.-H.; Wang, X.-J. Efficacy Evaluation, Active Ingredients, and Multitarget Exploration of Herbal Medicine. Trends Endocrinol. Metab. 2023, 34, 146–157. [Google Scholar] [CrossRef]
- Noor, F.; Asif, M.; Ashfaq, U.A.; Qasim, M.; Tahir Ul Qamar, M. Machine Learning for Synergistic Network Pharmacology: A Comprehensive Overview. Brief. Bioinform. 2023, 24, bbad120. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Li, N.; Chen, J.; Xu, H.; Wang, Y.; Liang, Q. Network Pharmacology, a Promising Approach to Reveal the Pharmacology Mechanism of Chinese Medicine Formula. J. Ethnopharmacol. 2023, 309, 116306. [Google Scholar] [CrossRef]
- Jo, H.G.; Baek, C.Y.; Kim, D.; Kim, S.; Han, Y.; Park, C.; Song, H.S.; Lee, D. Network Analysis, in Vivo, and in Vitro Experiments Identified the Mechanisms by Which Piper longum L. [Piperaceae] Alleviates Cartilage Destruction, Joint Inflammation, and Arthritic Pain. Front Pharmacol 2023, 14, 1282943. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.M.; Haseeb, A.; Ansari, M.Y.; Devarapalli, P.; Haynie, S.; Haqqi, T.M. Wogonin, a Plant Derived Small Molecule, Exerts Potent Anti-Inflammatory and Chondroprotective Effects through the Activation of ROS/ERK/Nrf2 Signaling Pathways in Human Osteoarthritis Chondrocytes. Free Radic. Biol. Med. 2017, 106, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Sirong, S.; Yang, C.; Taoran, T.; Songhang, L.; Shiyu, L.; Yuxin, Z.; Xiaoru, S.; Tao, Z.; Yunfeng, L.; Xiaoxiao, C. Effects of Tetrahedral Framework Nucleic Acid/Wogonin Complexes on Osteoarthritis. Bone Res. 2020, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Shen, K.; Yu, H.; Fan, W. Baicalein Limits Osteoarthritis Development by Inhibiting Chondrocyte Ferroptosis. Free Radic. Biol. Med. 2023, 196, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Yi, N.; Mi, Y.; Xu, X.; Li, N.; Zeng, F.; Yan, K.; Tan, K.; Kuang, G.; Lu, M. Baicalein Alleviates Osteoarthritis Progression in Mice by Protecting Subchondral Bone and Suppressing Chondrocyte Apoptosis Based on Network Pharmacology. Front. Pharmacol. 2021, 12, 788392. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, C.; Huang, K.; Chen, S.; Ma, Y. Acacetin Suppresses IL-1β-Induced Expression of Matrix Metalloproteinases in Chondrocytes and Protects against Osteoarthritis in a Mouse Model by Inhibiting NF-κB Signaling Pathways. BioMed Res. Int. 2020, 2020, 2328401. [Google Scholar] [CrossRef]
- Liu, L.; Yang, J.; Zu, B.; Wang, J.; Sheng, K.; Zhao, L.; Xu, W. Acacetin Regulated the Reciprocal Differentiation of Th17 Cells and Treg Cells and Mitigated the Symptoms of Collagen-Induced Arthritis in Mice. Scand. J. Immunol. 2018, 88, e12712. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial Inflammation in Osteoarthritis Progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ke, C.; Zhou, Z.; Xu, K.; Wang, Y.; Liu, Y.; Tu, J. Scutellaria baicalensis Pith-Decayed Root Inhibits Macrophage-Related Inflammation Through the NF-κB/NLRP3 Pathway to Alleviate LPS-Induced Acute Lung Injury. Planta Med. 2022, 89, 493–507. [Google Scholar] [CrossRef]
- Domitrović, R.; Potočnjak, I. A Comprehensive Overview of Hepatoprotective Natural Compounds: Mechanism of Action and Clinical Perspectives. Arch. Toxicol. 2016, 90, 39–79. [Google Scholar] [CrossRef]
- Chan, E.; Liu, X.-X.; Guo, D.-J.; Kwan, Y.-W.; Leung, G.P.-H.; Lee, S.M.-Y.; Chan, S.-W. Extract of Scutellaria baicalensis Georgi Root Exerts Protection against Myocardial Ischemia-Reperfusion Injury in Rats. Am. J. Chin. Med. 2011, 39, 693–704. [Google Scholar] [CrossRef]
- Liu, M.; Huang, Q.; Zhu, Y.; Chen, L.; Li, Y.; Gong, Z.; Ai, K. Harnessing Reactive Oxygen/Nitrogen Species and Inflammation: Nanodrugs for Liver Injury. Mater. Today Bio 2022, 13, 100215. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, W.; Sui, L.; Huang, Q.; Nan, Y.; Liu, J.; Ai, K. Reactive Oxygen Species-Based Nanomaterials for the Treatment of Myocardial Ischemia Reperfusion Injuries. Bioact. Mater. 2022, 7, 47–72. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Cao, L.; Song, R.; Yang, X.-F.; Li, J.-L.; Yang, H.-T.; Zhou, H.-X.; Fan, H.-T. Glutamine Exerts a Protective Effect on Osteoarthritis Development by Inhibiting the Jun N-Terminal Kinase and Nuclear Factor Kappa-B Signaling Pathways. Sci. Rep. 2022, 12, 11957. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Tuerxunyiming, M.; Xu, J.; Wu, Z.; Wang, W.; Liu, H.; Lin, L.; Liu, Q. Triptolide Ameliorates Osteoarthritis by Regulating Nuclear Factor Kappa B-Mediated Inflammatory Response. J. Pharm. Pharmacol. 2022, 74, rgab182. [Google Scholar] [CrossRef]
- Cai, T.; Ye, H.; Jiang, H.; Lin, C.; Lou, C.; Wang, W.; Yan, Z.; Xue, X.; Pan, X.; Lin, J. Stevioside Targets the NF-κB and MAPK Pathways for Inhibiting Inflammation and Apoptosis of Chondrocytes and Ameliorates Osteoarthritis in Vivo. Int. Immunopharmacol. 2023, 115, 109683. [Google Scholar] [CrossRef]
- Yang, G.; Wang, K.; Song, H.; Zhu, R.; Ding, S.; Yang, H.; Sun, J.; Wen, X.; Sun, L. Celastrol Ameliorates Osteoarthritis via Regulating TLR2/NF-κB Signaling Pathway. Front. Pharmacol. 2022, 13, 963506. [Google Scholar] [CrossRef]
- Yang, Z.; Feng, L.; Huang, J.; Zhang, X.; Lin, W.; Wang, B.; Cui, L.; Lin, S.; Li, G. Asiatic Acid Protects Articular Cartilage through Promoting Chondrogenesis and Inhibiting Inflammation and Hypertrophy in Osteoarthritis. Eur. J. Pharmacol. 2021, 907, 174265. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, H.; Pan, J.; Hu, Z.; Liu, L.; Liu, Y.; Yu, X.; Bai, X.; Cai, D.; Zhang, H. Fargesin Ameliorates Osteoarthritis via Macrophage Reprogramming by Downregulating MAPK and NF-κB Pathways. Arthritis Res. Ther. 2021, 23, 142. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A Database of Systems Pharmacology for Drug Discovery from Herbal Medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Xue, R.; Fang, Z.; Zhang, M.; Yi, Z.; Wen, C.; Shi, T. TCMID: Traditional Chinese Medicine Integrative Database for Herb Molecular Mechanism Analysis. Nucleic Acids Res. 2013, 41, D1089–D1095. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-Y.; Zhang, Y.-Q.; Liu, Z.-M.; Chen, T.; Lv, C.-Y.; Tang, S.-H.; Zhang, X.-B.; Zhang, W.; Li, Z.-Y.; Zhou, R.-R.; et al. ETCM: An Encyclopaedia of Traditional Chinese Medicine. Nucleic Acids Res. 2019, 47, D976–D982. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Dong, L.; Liu, L.; Guo, J.; Zhao, L.; Zhang, J.; Bu, D.; Liu, X.; Huo, P.; Cao, W.; et al. HERB: A High-Throughput Experiment- and Reference-Guided Database of Traditional Chinese Medicine. Nucleic Acids Res. 2021, 49, D1197–D1206. [Google Scholar] [CrossRef]
- The UniProt Consortium UniProt: The Universal Protein Knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape Provides a Biologist-Oriented Resource for the Analysis of Systems-Level Datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Jhun, J.; Cho, K.-H.; Lee, D.-H.; Kwon, J.Y.; Woo, J.S.; Kim, J.; Na, H.S.; Park, S.-H.; Kim, S.J.; Cho, M.-L. Oral Administration of Lactobacillus Rhamnosus Ameliorates the Progression of Osteoarthritis by Inhibiting Joint Pain and Inflammation. Cells 2021, 10, 1057. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-R.; Jeong, Y.-J.; Lee, J.-W.; Jhun, J.; Na, H.S.; Cho, K.-H.; Kim, S.J.; Cho, M.-L.; Heo, T.-H. Tannic Acid, an IL-1β-Direct Binding Compound, Ameliorates IL-1β-Induced Inflammation and Cartilage Degradation by Hindering IL-1β-IL-1R1 Interaction. PLoS ONE 2023, 18, e0281834. [Google Scholar] [CrossRef] [PubMed]
- Guingamp, C.; Gegout-Pottie, P.; Philippe, L.; Terlain, B.; Netter, P.; Gillet, P. Mono-iodoacetate-induced Experimental Osteoarthritis. A Dose-response Study of Loss of Mobility, Morphology, and Biochemistry. Arthritis Rheum. 1997, 40, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Onodera, T.; Terkawi, M.A.; Iwasaki, K.; Hishimura, R.; Liang, D.; Miyazaki, T.; Iwasaki, N. Local Administration of Low-Dose Nerve Growth Factor Antibody Reduced Pain in a Rat Osteoarthritis Model. Int. J. Mol. Sci. 2021, 22, 2552. [Google Scholar] [CrossRef]



| Pubchem ID | Compound Name | Structure | OB (%) | DL |
|---|---|---|---|---|
| 6782 | Diisobutyl phthalate | 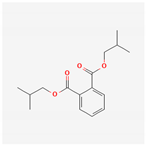 | 43.59 | 0.35 |
| 31161 | Pedalitin | 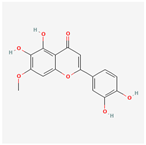 | 34.02 | 0.31 |
| 33934 | Diisooctyl phthalate | 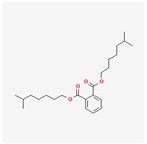 | 43.59 | 0.39 |
| 64982 | Baicalin | 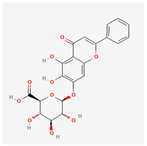 | 40.12 | 0.75 |
| 72322 | Coptisine | 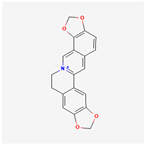 | 30.67 | 0.86 |
| 72323 | Jatrorrhizine | 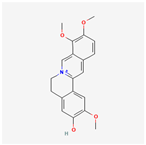 | 30.44 | 0.75 |
| 124211 | Skullcapflavone II | 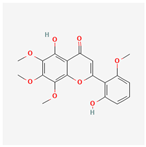 | 69.51 | 0.44 |
| 156992 | 5,8,2′-Trihydroxy-7-methoxyflavone | 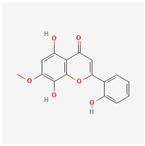 | 37.01 | 0.27 |
| 159029 | Tenaxin I | 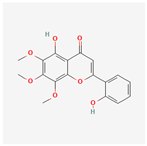 | 31.71 | 0.35 |
| 160876 | Epiberberine | 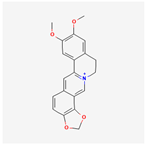 | 43.09 | 0.78 |
| 161271 | Salvigenin | 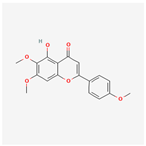 | 49.07 | 0.33 |
| 182232 | (+)-Epicatechin | 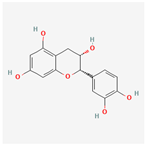 | 48.96 | 0.24 |
| 188308 | Carthamidin | 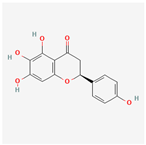 | 33.23 | 0.24 |
| 188316 | 5-Hydroxy-7,8-dimethoxyflavone | 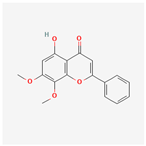 | 44.09 | 0.25 |
| 222284 | beta-Sitosterol | 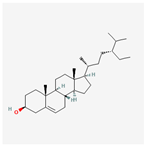 | 36.91 | 0.75 |
| 373261 | Eriodyctiol (flavanone) | 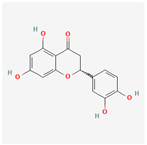 | 41.35 | 0.24 |
| 440735 | Eriodictyol | 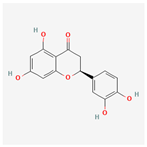 | 71.79 | 0.24 |
| 457801 | Clionasterol | 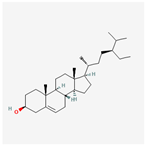 | 36.91 | 0.75 |
| 471719 | Negletein | 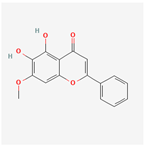 | 41.16 | 0.23 |
| 5280442 | Acacetin | 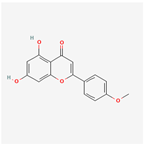 | 34.97 | 0.24 |
| 5280666 | Chrysoeriol | 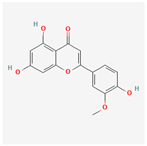 | 35.85 | 0.27 |
| 5280794 | Stigmasterol | 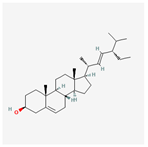 | 43.83 | 0.76 |
| 5281330 | Poriferasterol | 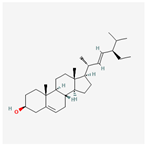 | 43.83 | 0.76 |
| 5281605 | Baicalein | 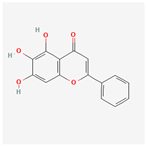 | 33.52 | 0.21 |
| 5281674 | Norwogonin | 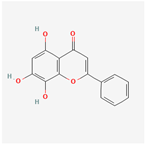 | 39.4 | 0.21 |
| 5281703 | Wogonin | 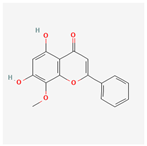 | 30.68 | 0.23 |
| 5283637 | 22,23-Dihydrobrassicasterol | 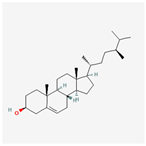 | 37.58 | 0.71 |
| 5320315 | Oroxylin A | 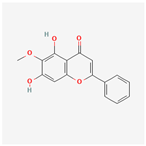 | 41.37 | 0.23 |
| 5320399 | Skullcapflavone I | 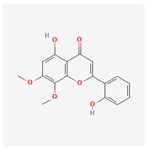 | 76.26 | 0.29 |
| 5321865 | 5,7,2′,6′-Tetrahydroxyflavone | 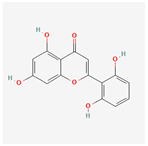 | 37.01 | 0.24 |
| 5322059 | Viscidulin II | 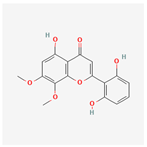 | 45.05 | 0.33 |
| 42608119 | 5,7,4′-trihydroxy-8-methoxyflavanone | 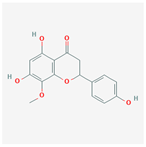 | 74.24 | 0.26 |
| 5322078 | 4′-Hydroxywogonin | 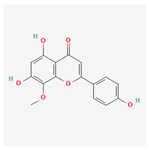 | 36.56 | 0.27 |
| 5365674 | 11,13-Eicosadienoic acid, methyl ester |  | 39.28 | 0.23 |
| 5367328 | 1-Monolinolenoyl-rac-glycerol | 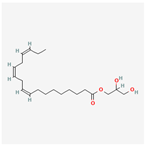 | 38.14 | 0.31 |
| 9601691 | Glucobrassicin | 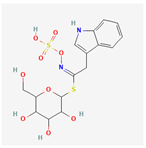 | 66.02 | 0.48 |
| 12303645 | 3-epi-beta-Sitosterol |  | 36.91 | 0.75 |
| 13889022 | Rivularin (flavone) |  | 37.94 | 0.37 |
| 14135323 | (2S)-dihydrobaicalein |  | 40.04 | 0.21 |
| 25721350 | Dihydrooroxylin |  | 66.06 | 0.23 |
| 26213330 | (2R)-5,7-dihydroxy-2-(4-hydroxyphenyl)-6-methoxy-2,3-dihydrochromen-4-one | 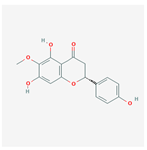 | 36.63 | 0.27 |
| 44258628 | 5,7,3′,6′-Tetrahydroxy-6,8,2′-trimethoxyflavone | 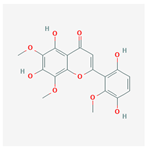 | 33.82 | 0.45 |
| 141457867 | 5-Hydroxy-2-(2-hydroxy-5-methoxyphenyl)-6,7,8-trimethoxychromen-4-one | 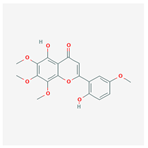 | 104.34 | 0.44 |
| 162988960 | Carthamidin | 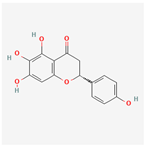 | 41.15 | 0.24 |
| Compound Name | Degree Centrality | Betweenness Centrality | Closeness Centrality |
|---|---|---|---|
| Wogonin | 26 | 0.094 | 0.509 |
| Baicalein | 19 | 0.062 | 0.471 |
| Acacetin | 16 | 0.035 | 0.456 |
| beta-Sitosterol | 13 | 0.030 | 0.442 |
| 5-Hydroxy-7,8-dimethoxyflavone | 12 | 0.011 | 0.438 |
| Oroxylin A | 12 | 0.017 | 0.438 |
| 4′-Hydroxywogonin | 11 | 0.009 | 0.433 |
| Chrysoeriol | 11 | 0.009 | 0.433 |
| 5-Hydroxy-2-(2-hydroxy-5-methoxyphenyl)-6,7,8-trimethoxychromen-4-one | 10 | 0.010 | 0.429 |
| Rivularin (flavone) | 9 | 0.007 | 0.425 |
| Jatrorrhizine | 8 | 0.004 | 0.421 |
| Salvigenin | 8 | 0.004 | 0.421 |
| Skullcapflavone I | 8 | 0.003 | 0.421 |
| Tenaxin I | 8 | 0.003 | 0.421 |
| Viscidulin II | 8 | 0.003 | 0.421 |
| Gene Name | Degree Centrality | Betweenness Centrality | Closeness Centrality | Average Shortest Path Length |
|---|---|---|---|---|
| JUN | 22 | 0.209 | 0.560 | 1.786 |
| RELA | 16 | 0.086 | 0.519 | 1.929 |
| FOS | 15 | 0.060 | 0.494 | 2.024 |
| TP53 | 15 | 0.222 | 0.525 | 1.905 |
| MAPK14 | 13 | 0.058 | 0.500 | 2.000 |
| ESR1 | 13 | 0.111 | 0.472 | 2.119 |
| TNF | 12 | 0.078 | 0.483 | 2.071 |
| IL6 | 11 | 0.112 | 0.477 | 2.095 |
| AKT1 | 10 | 0.067 | 0.457 | 2.190 |
| NR3C1 | 9 | 0.008 | 0.457 | 2.190 |
| AR | 8 | 0.015 | 0.412 | 2.429 |
| CCND1 | 7 | 0.004 | 0.438 | 2.286 |
| CXCL8 | 7 | 0.027 | 0.457 | 2.190 |
| TGFB1 | 7 | 0.033 | 0.429 | 2.333 |
| Group | OA Inducer (50 μL, Intra-Articular) | Sample (10 mL/kg, P.O.) |
|---|---|---|
| Sham | Saline | DW |
| Control | MIA 40 mg/mL | DW |
| Indomethacin | MIA 40 mg/mL | indomethacin 200 mg/kg |
| SB (low dose) | MIA 40 mg/mL | SB 80 mg/kg |
| SB (high dose) | MIA 40 mg/mL | SB 240 mg/kg |
| Grade | Cartilage Appearance |
|---|---|
| 0 | Normal appearance in cartilage surface |
| 1 | Slight yellowish discoloration of the surface or slight fibrillation |
| 2 | Erosion reaching the superficial or middle layers of the cartilage |
| 3 | Extensive erosions reaching down to the subchondral bone |
| 4 | Massive erosions with extensive exposure of subchondral bone |
| MMP-1 | F | AACTTGGGTGAAGACGTCCA |
| R | TCCTGTCACTTTCAGCCCAA | |
| MMP-3 | F | GTACGGCTGTGTGCTCATCC |
| R | TCAGCCCAAGGAACTTCTGC | |
| MMP-8 | F | TCTGTTCTTCTTCCACACACAG |
| R | GCAATCATAGTGGCATTCCT | |
| MMP-13 | F | ACCTTCTTCTTGTTGAGTTGGA |
| R | CTGCATTTCTCGGAGTCTA | |
| JUN | F | CCAACCAACGTGAGTGCAAG |
| R | GAG GGCATCGTCGTAGAAGG | |
| FOS | F | TACTACCATTCCCCAGCCGA |
| R | GCGTATCTGTCAGCTCCCTC | |
| IL-1β | F | AACTCAACTGTGAAATAGCAGC |
| R | TCCACAGCCACAATGAGTG | |
| TNF-α | F | GCATGATCCGAGATGTGGAA |
| R | GATGAGAGGGAGCCCATTTG | |
| IL-6 | F | TCCGCAAGAGACTTCCAGC |
| R | CCTCCGACTTGTGAAGTGG | |
| COX-2 | F | GTTCCAACCCATGTCAAAAC |
| R | TGTCAGGAATCTCGGCGTAG | |
| Ptger2 | F | TGTGTGTACTGTCCGTCTGC |
| R | CAGGGATCCAGTCTCGGTGT | |
| TGF-β1 | F | AGGAGACGGAATACAGGGCT |
| R | CCACGTAGTAGACGATGGGC | |
| Type II collagen | F | TGGCCTTGGTGGAGGAAA |
| R | AGGACCAGGGAGGCCTCTTT | |
| IL-4 | F | CGTGATGTACCTCCGTGCTT |
| R | GTGAGTTCAGACCGCTGACA | |
| TIMP-1 | F | TTTCCCTGTTCAGCCATCCC |
| R | TAGCCCTTCTCAGAGCCCAT | |
| GAPDH | F | CTTGTGACAAAGTGGACATTGTT |
| R | TGACCAGCTTCCCATTCTC |
| MMP-1 | F | ATGCCTAGCCTTCCTTTGCT |
| R | TTCCAGGTATTTCCAGACTG | |
| MMP-3 | F | AAGTTCCTCGGGTTGGAGAT |
| R | ACCAACATCAGGAACACCAC | |
| MMP-8 | F | CAATCAATTCCGGTCTTCGA |
| R | GGTTAGCAAGAAATCACCAGA | |
| MMP-13 | F | AACCAAGATGTGGAGTGCCT |
| R | GACCAGACCTTGAAGGCTTT | |
| JUN | F | ACAGAGCATGACCTTGAACCT |
| R | GTGATGTGCCCATTGCTGGA | |
| FOS | F | GGACTTTTGCGCAGATCTGT |
| R | GGTGGGGAGTCCGTAAGGAT | |
| IL-1β | F | CCAGCTTCAAATCTCGCAGC |
| R | GTGCTCATGTCCTCATCCTGG | |
| TNF-α | F | GAGAAGTTCCCAAATGGCCT |
| R | AGCCACTCCAGCTGCTCCT | |
| IL-6 | F | CACTTCACAAGTCGGAGGCT |
| R | CAAGTGCATCATCGTTGTTC | |
| COX-2 | F | ATCCATGTCAAAACCGTGGG |
| R | TTGGGGTGGGCTTCAGCAG | |
| Ptger2 | F | CTGGTAACGGAATTGGTGC |
| R | TGGCCAGACTAAAGAAGGTC | |
| NOS2 | F | ACCAAGATGGCCTGGAGGAA |
| R | CCGACCTGATGTTGCCATTG | |
| TGF-β1 | F | GGACTCTCCACCTGCAAGAC |
| R | TGTTGTACAAAGCGAGCACC | |
| IL-4 | F | ACGGAGATGGATGTGCCAA |
| R | TGCGAAGCACCTTGGAAGC | |
| GAPDH | F | ATGGTGAAGGTCGGTGTG |
| R | GCCGTGAGTGGAGTCATAC |
| Antibody | Dilution Rate | Company |
|---|---|---|
| MMP-1 | 1:700 | Proteintech |
| MMP-3 | 1:1000 | Abcam |
| MMP-8 | 1:1000 | Abcam |
| MMP-13 | 1:2000 | Proteintech |
| IL-1β | 1:1000 | Abcam |
| NF-κB p65 | 1:1000 | Cell Signaling |
| NOS2 | 1:1000 | Abcam |
| β-actin | 1:1000 | Cell Signaling |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.-G.; Baek, C.-Y.; Song, H.S.; Lee, D. Network Pharmacology and Experimental Verifications to Discover Scutellaria baicalensis Georgi’s Effects on Joint Inflammation, Destruction, and Pain in Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 2127. https://doi.org/10.3390/ijms25042127
Jo H-G, Baek C-Y, Song HS, Lee D. Network Pharmacology and Experimental Verifications to Discover Scutellaria baicalensis Georgi’s Effects on Joint Inflammation, Destruction, and Pain in Osteoarthritis. International Journal of Molecular Sciences. 2024; 25(4):2127. https://doi.org/10.3390/ijms25042127
Chicago/Turabian StyleJo, Hee-Geun, Chae-Yun Baek, Ho Sueb Song, and Donghun Lee. 2024. "Network Pharmacology and Experimental Verifications to Discover Scutellaria baicalensis Georgi’s Effects on Joint Inflammation, Destruction, and Pain in Osteoarthritis" International Journal of Molecular Sciences 25, no. 4: 2127. https://doi.org/10.3390/ijms25042127
APA StyleJo, H.-G., Baek, C.-Y., Song, H. S., & Lee, D. (2024). Network Pharmacology and Experimental Verifications to Discover Scutellaria baicalensis Georgi’s Effects on Joint Inflammation, Destruction, and Pain in Osteoarthritis. International Journal of Molecular Sciences, 25(4), 2127. https://doi.org/10.3390/ijms25042127








